Abstract
Spatial and temporal patterns of bone morphogenetic protein (BMP) signaling are crucial to the assembly of appropriately positioned and shaped bones of the face and head. This review advances the hypothesis that reconstitution of such patterns with cutting-edge gene therapies will transform the clinical management of craniofacial bone defects attributed to trauma, disease, or surgical resection. Gradients in BMP signaling within developing limbs and orofacial primordia regulate proliferation and differentiation of mesenchymal progenitors. Similarly, vascular and mesenchymal cells express BMPs in various places and at various times during normal fracture healing. In non-healing fractures of long bones, BMP signaling is severely attenuated. Devices that release recombinant BMPs promote healing of bone in spinal fusions and, in some cases, of open fractures, but cannot control the timing and localization of BMP release. Gene therapies with regulated expression systems may provide substantial improvements in efficacy and safety compared with protein-based therapies. Synthetic gene switches, activated by pharmacologics or light or hyperthermic stimuli, provide several avenues for the non-invasive regulation of the expression of BMP transgenes in both time and space. Through new gene therapy platforms such as these, active control over BMP signaling can be achieved to accelerate bone regeneration.
Keywords: osteogenesis, gene expression regulation, bone morphogenetic proteins, growth & development, heat shock proteins, tissue engineering
Introduction
Since the cloning of the bone morphogenetic protein 2 (BMP2) gene nearly 25 years ago, both academic and commercial research laboratories made major commitments to the development of BMP-based therapies for the repair of bone defects. At the same time, molecular analysis of spatial and temporal patterns of BMP gene expression revealed intriguing similarities between skeletal development and fracture healing. Based on this analysis, it is likely that the highly dynamic spatial and temporal patterns of BMP expression are critical to its function. This review will summarize what is known about BMP expression during bone development and fracture healing and then describe state-of-the-art BMP gene therapies, including scaffold- and inducible expression-based technologies. Finally, methods for reconstituting developmental patterns of BMP expression in both time and space will be discussed. Such technologies represent the next frontier in BMP gene therapy as the research community aims to provide tightly regulated and site-specific doses of these potent osteogenic molecules.
Patterns of BMP Expression in Skeletal and Craniofacial Development
BMPs have important roles during skeletal patterning and bone formation. Much of our understanding of this area comes from studies on murine and avian limb buds where BMP2 and BMP7 mRNAs were localized to the apical ectodermal ridge (AER) (Lyons et al., 1995). Later in development, BMP2 was also found in pre-cartilaginous condensations and the interdigital mesenchyme. These observations provided the first indications that morphogen functions of BMPs require spatially and temporally restricted patterns of expression. More recently, the dynamics of embryonic BMP signaling activity were investigated through the use of transgenic mice and zebrafish harboring a lacZ or eGFP reporter driven by a synthetic BMP-responsive element (BRE) (Monteiro et al., 2008; Alexander et al., 2011; Javier et al., 2012). Consistent with the previously described patterns of BMP ligand distribution, BMP signaling was restricted to the AER early in limb development and later was localized to pre-cartilage condensations and sites of endochondral ossification throughout the skeleton. In addition, BRE activity in the interdigital mesenchyme of the limbs was associated with the developing vasculature. Collectively, these data elegantly capture ways in which spatially and temporally restricted patterns of BMP signaling coincide with specific morphogenic events during embryonic development of cartilage and bone.
BMPs also regulate patterning of bones in the face and head (Fig. 1) by maintaining cell plasticity, directing migration, or inducing differentiation of mesenchymal progenitors leading to the formation of the neural crest and subsequent dorsal-ventral patterning of the pharyngeal arches (embryonic structures that give rise to craniofacial bones) (Nie et al., 2006). At very early times in zebrafish embryogenesis (i.e., gastrulation and formation of the neural crest), BMP gradients are important for generating spatially distinct patterns of differentiation in the ectoderm, and for directing formation of neural crest progenitors (Schumacher et al., 2011). BMPs 2 and 4 play critical roles in establishing morphologic features of the palate and mandible, since conditional knockout of either or both of these BMPs leads to dramatic disruption of bone formation (Bonilla-Claudio et al., 2012). Conversely, application of recombinant BMPs at ectopic sites disrupts normal patterning of facial primordia in the chick (Barlow and Francis-West, 1997). Initially, BMPs and endothelin operate to provide ventralizing signals originating in the pharyngeal ectoderm. Later in development, BMPs become more restricted to the ventral (e.g., mandibular) and anterior (e.g., palate) bones of the face (Alexander et al., 2011; Jumlongras et al., 2012). Spatially and temporally dynamic BMP and Wnt signals in the developing mouse molar comprise a feedback circuit that regulates the epithelial-mesenchymal interactions necessary for odontogenesis (O’Connell et al., 2012). Expression of BMP4 mRNA is localized to tooth formation areas early in mouse development (embryonic day 11.5), spreads to the oral epithelium by day 12, and then increases close to Meckel’s cartilage and ossification sites in the mandible, maxillae, and the frontonasal process by day 12.5 (Paiva et al., 2010). The timing of bone formation in the mandible is regulated by temporally restricted expression of BMP4 within the presumptive mesenchyme. Interestingly, evolutionary shifts in the timing, localization, and level of BMP expression are manifest in the wide range of mandibular morphologies observed across species (Hu et al., 2008; Merrill et al., 2008). Restriction of BMP signaling to anterior aspects of the palatal mesenchyme is also required for normal palatogenesis (He et al., 2010). Also, sutures between the parietal bones in the skull exhibit spatially and temporally restricted patterns of BMP expression during development, with BMPs 2 and 4 being localized to the interparietal fronts of mineralization through embryonic day 18 in the mouse and diminishing at later times (Kim et al., 1998).
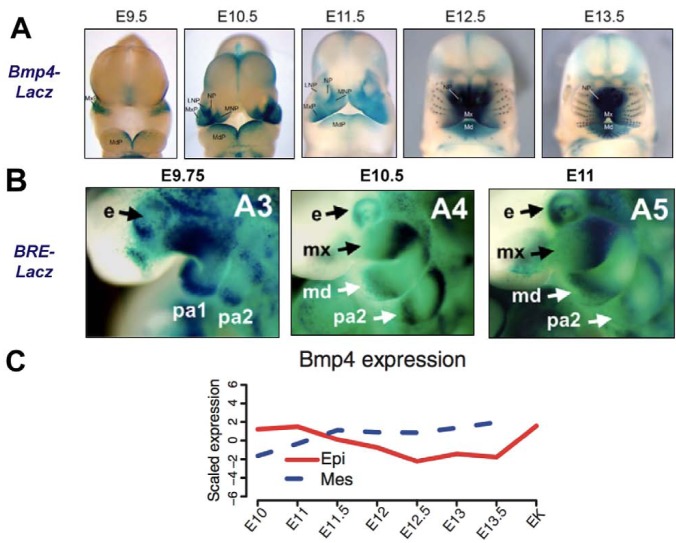
Figure 1.
Spatially and temporally restricted patterns of BMP expression and signaling during mouse craniofacial development. (A) Timecourse of Bmp4 transcription in the presumptive mandible, maxilla, and other orofacial structures during embryonic development (adapted from Jumlongras et al., 2012). The staining for β-galactosidase activity denotes regions of Bmp4 expression. Abbreviations: LNP, lateral nasal process; Md, mandible; Mx, maxilla, MdP, mandibular process; MxP, maxillary process; MNP, medial nasal process; NP, nasal pit. (B) Timecourse of BMP signaling activity in the presumptive mandible, maxilla, and other orofacial structures during embryonic development (Javier et al., 2012). The staining for β-galactosidase activity denotes regions of BMP-responsive element (BRE) promoter activity. Abbreviations: e, eye; md, mandible; mx, maxilla; pa, pharyngeal arch. (C) Quantification of Bmp4 transcription in the epithelial (Epi) and mesenchymal (Mes) compartments of the murine molar tooth primordium during embryonic development through formation of the enamel knot (EK) at E14.5 (adapted from O’Connell et al., 2012).
Patterns of BMP Expression During Bone Healing
Many elements of the embryonic developmental program are reactivated during wound healing (Vortkamp et al., 1998; Grimes et al., 2011). Thus, new bone at a fracture site forms through a combination of endochondral and primary ossification processes and requires BMPs (Retting et al., 2009). Interestingly, whereas BMP2 is dispensable for bone development, deletion of BMP2 potently inhibited even early phases of fracture repair, despite compensatory up-regulation of BMPs 4 and 7 (Tsuji et al., 2006). Subsequent studies showed that BMP2 does not recruit mesenchymal progenitors to the osteogenic lineage, yet provides necessary signals for post-natal mineralization, in part through regulation of the osteogenic transcription factor, Runx2 (Bais et al., 2009; Yu et al., 2010a). Thus, fracture healing reactivates many features of bone development (e.g., patterns of cell growth and differentiation), but exhibits some distinctions at the molecular level.
As in development, BMPs exhibit temporally and spatially restricted patterns of expression throughout fracture healing (Fig. 2). In mouse long bones, BMP2 mRNA is induced shortly after fracture, transiently decreases, and then exhibits a second peak when the cartilage callus undergoes mineralization. BMP2 expression then returns to baseline levels as the wound-healing process resolves. BMP2 3, 4, 7, and 8, in contrast, exhibit a single peak coincident with the second peak in BMP2 expression (Cho et al., 2002). At the protein level, BMPs exhibit temporal fluctuations consistent with transcriptional studies, and, in both mouse and human tissue, BMPs are found primarily in chondrocytes, osteoblasts, and periosteum of the fracture callus (Kloen et al., 2003). Significantly, BMP expression is also detected in granulation tissue and endothelial cells at the regeneration site (Yu et al., 2010b). In a different model of post-natal bone formation, distraction osteogenesis, BMPs 2 and 4 were not expressed until approximately 5 days following distraction, peaked at day 7, and declined at 2 weeks (Sojo et al., 2005). In this model, the neovascular tissues emanating from the periosteum and surrounding soft tissue were found to be a primary source of BMP2 (Matsubara et al., 2012). Localization of BMPs to the neovasculature during bone regeneration may have implications for the identification of mesenchymal precursors contributing to bone formation, since pre-osteoblastic cells in the perivascular compartment co-migrate with endothelial cells into the callus and have the capacity to initiate ossification (Maes et al., 2010). Indeed, the spatially and temporally restricted patterns of BMP expression during bone regeneration appear to be necessary for determining the site(s) of coupled angiogenesis and osteogenesis that underlie bone regeneration. In related work, BMP signaling was recently shown to drive bone resorption in BMP receptor knockout mice (Kamiya et al., 2008) and non-human primate bone defects (Seeherman et al., 2010), indicating that BMPs can have anabolic and catabolic effects on bone formation during both development and regeneration.
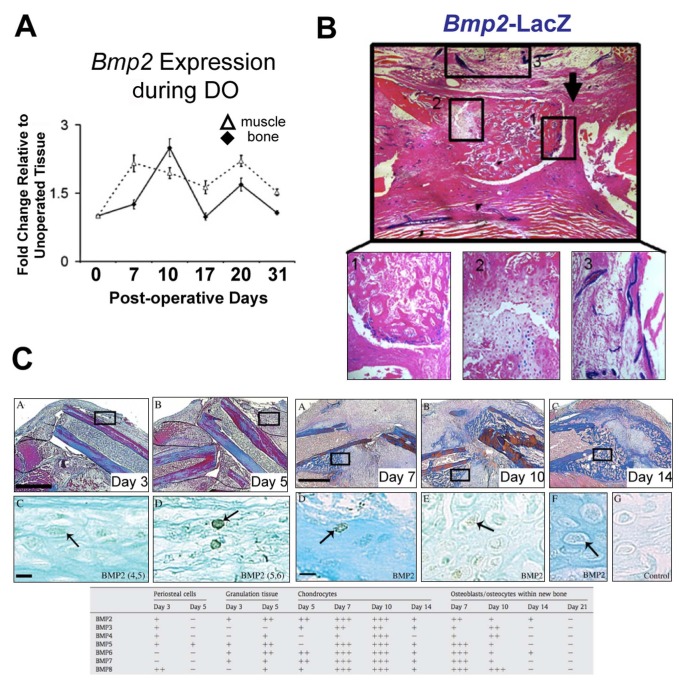
Figure 2.
Spatially and temporally restricted patterns of BMP expression during bone healing. (A) Timecourse of Bmp2 mRNA expression in gap region bone and surrounding muscle in a distraction osteogenesis model of murine bone regeneration. (B) Localization of Bmp2 transcription during distraction osteogenesis (post-operative day 20). The staining for β-galactosidase activity denotes regions of Bmp2 expression (A and B adapted from Matsubara et al., 2012). (C) Timecourse of BMP2 localization in a non-stabilized long bone fracture in the mouse. Upper panels show Milligan’s Trichrome staining of the fracture site, and the lower panels show immunohistochemical localization of intra- and peri-cellular BMP2. The table below summarizes immunohistochemically determined BMP expression in various cell types at different stages of bone healing (adapted from Yu et al., 2010b).
BMP signaling in non-union or delayed healing fractures differs from signaling in fractures that heal normally. Although BMPs 2, 4, 7, and 14, BMP receptors, and phosphorylated Smad1 have all been detected in non-healing fractures (Kwong et al., 2009), subsequent studies have demonstrated a clear imbalance between BMPs and their inhibitors such as noggin, gremlin, and chordin. Whereas the cartilaginous regions of human non-unions exhibited expression levels of BMP inhibitors similar to those in the fracture calluses, BMPs levels were substantially reduced (Kwong et al., 2009; Kloen et al., 2012). One consequence of this imbalance is a net attenuation of BMP activity. This is consistent with the clinical outcome of diminished, disorganized bone formation in non-unions, and suggests that therapies able to restore the BMP:BMP-antagonist ratio in the wound bed will improve bone healing.
The significance of spatially and temporally restricted expression of BMPs in bone healing is further supported by studies on mammalian digit regeneration. In children and mice, digit tips undergo limited wound healing analogous to the exceptionally robust regenerative responses of urodeles like the salamander. Provided amputation is limited to about half of the terminal phalanx, digits exhibit a near-complete regeneration that occurs through the formation of a blastema, or condensation of undifferentiated and proliferating cells (Fernando et al., 2011). This process is mediated by BMP4, which is up-regulated in a band of subdermal mesenchyme at the amputation stump by day 4 post-amputation (Han et al., 2003). BMP2 and BMP7 also exhibit dynamic patterns of expression in the amputated digit tip, and distally targeted application of exogenous BMP2 or BMP7 stimulated the regeneration of more proximal amputations that do not heal spontaneously (L Yu et al., 2010). Collectively, these findings demonstrate the potential for BMP-based therapies to mediate endogenous regenerative responses and underscore the significance of restricting delivery of BMPs to the appropriate times and locations within the wound bed.
Current Approaches for Regulating BMP Transgene Expression
Advantages of Gene vs. Protein Therapy for BMP Delivery
Controlling the release of exogenous BMP for therapeutic applications was initially motivated by early studies demonstrating that the in vivo half-life of the protein is very short (on the order of minutes). Collagen-based carriers were the earliest platforms used to localize and prolong BMP release and continue to be used in the clinic. Recently, more sophisticated materials have been developed to “tune” the timing and localization of BMP release (Kolambkar et al., 2011). Nevertheless, protein-carrier devices ultimately rely on passive diffusion of the growth factor into the wound bed and therefore have a limited capacity for reconstituting the highly dynamic spatial and temporal patterns of BMP expression described above. In addition, high (mg) doses of recombinant protein are required to elicit durable osteogenic responses in humans. Gene therapy approaches for the delivery of BMPs have the potential to overcome these limitations, especially when we consider state-of-the-art regulated expression systems (Kimelman-Bleich et al., 2012). In contrast to constitutive promoter-driven expression constructs, which have some of the same limitations as protein-carrier devices, chemically or physically activated expression systems provide substantial control over the level, duration, and spatial localization of BMPs. With the continued development of safe and efficient vectors (Ishihara et al., 2012), emergence of “same day” ex vivo gene delivery (Virk et al., 2011), and evaluation in large immunocompetent animal models (Ishihara et al., 2010), the technologies described below have tremendous potential to improve the clinical outcomes associated with BMP therapy.
Controlling the Timing of BMP Transgene Expression
A simple method to define temporal patterns of transgene expression in bone healing was explored with viral vectors. Delaying delivery of a BMP adenovirus by 5 to 10 days in a rat critical-size femoral defect led to more new bone formation than in defects exposed to AdBMP at the time of surgery (Betz et al., 2007). Although the mechanisms behind this effect were not determined, it is possible that diminished inflammation, increased adenovirus receptor expression, or formation of a stable hematoma that enhances the retention of viral particles at the wound site increased the effectiveness of the viral transduction at later times. However, it cannot be excluded that aligning the peak in adenoviral transgene expression with the endogenous wound-healing responses may also have been important for enhancing bone formation.
More sophisticated approaches to controlling the timing of BMP transgene expression involve the use of inducible expression systems. Such platforms have the potential to overcome limitations of commonly used constitutively active viral promoters, which provide minimal control over the magnitude, timing, duration, and spatial localization of BMP production. Best known are the tetracycline-dependent systems (Tet) that rely on 2 components, a tet-regulatable transactivator (tTA) and a tTA-dependent promoter that controls the expression of a downstream gene of interest. The TetON system is induced in the presence of tetracycline or its analog doxycycline (Dox) to trigger transgene expression. In contrast, the transactivator in the TetOFF system cannot bind its target in the presence of antibiotic, thereby inhibiting expression. A murine MSC line engineered to express BMP2 under the control of the TetOFF system promoted healing of a non-union radius fracture in mice (Moutsatsos et al., 2001). However, excess bone formation was observed in some samples, which was attributed to expression of BMP beyond the desired period. When BMP2 regulated by a TetON system was delivered in vivo to hBMSCs implanted in a critical-sized calvarial defect, the addition of Dox to the animal’s drinking water led to the expression of BMP2 and eventual closure of the defect (Gafni et al., 2004). However, a major concern with the clinical use of Tet systems is the risk that patients may develop resistance to tetracycline. Also, because tetracycline/Dox are bone-seeking drugs, these compounds may accumulate in bone and interfere with regulated expression. The recently described findings that Dox counteracts BMP2-induced osteogenic mediators in human periodontal ligament cells suggest that Tet systems may be particularly problematic in the regulation of BMP2 expression (Muthukuru and Sun, 2013). Finally, Tet-regulated systems can be “leaky” in that they express significant amount of transgene in the uninduced state (Gould and Chernajovsky, 2004). Therefore, more stringent gene expression systems suitable for bone regeneration are required.
Dimerizer-based gene switches use heterodimeric transcription factors composed of separate DNA-binding and activation domains that interact only in the presence of a small dimerizer molecule such as rapamycin to form a functional transactivator (Rivera, 1998). Because only the dimerized factor is capable of functioning as a transcription factor, this system provides stringent regulation of target gene expression. A major improvement was made by the development of rapalogs, non-immunosuppressive analogs of rapamycin that retain the ability to function as dimerizers. We tested the ability of a rapamycin/rapalog-based system to regulate BMP2 expression and heal a critical-size calvarial defect (Fig. 3) (Koh et al., 2006). Rapamycin tightly regulated the in vivo production of the growth factor; the system exhibited clear dose dependence, and BMP levels were shown to decline rapidly 4-6 days after a single rapamycin injection. Repeated rapamycin treatment over several weeks led to uniform new bone formation in the defect. New bone was fully integrated with the host and showed no signs of overgrowth. In contrast, when cells were transduced with an adenovirus encoding BMP2 under the control of a constitutive promoter, the new bone was highly irregular and discontinuous with the surrounding tissue. These differences may be attributed to the dynamics of BMP2 secretion driven by the inducible system, which provided sustained low-level delivery of BMP over time vs. the high (but transient) levels of transgene production with adenovirus. By this approach, precise temporal control over BMP delivery was achieved, a key factor for successful fracture healing and bone formation.
Figure 3.
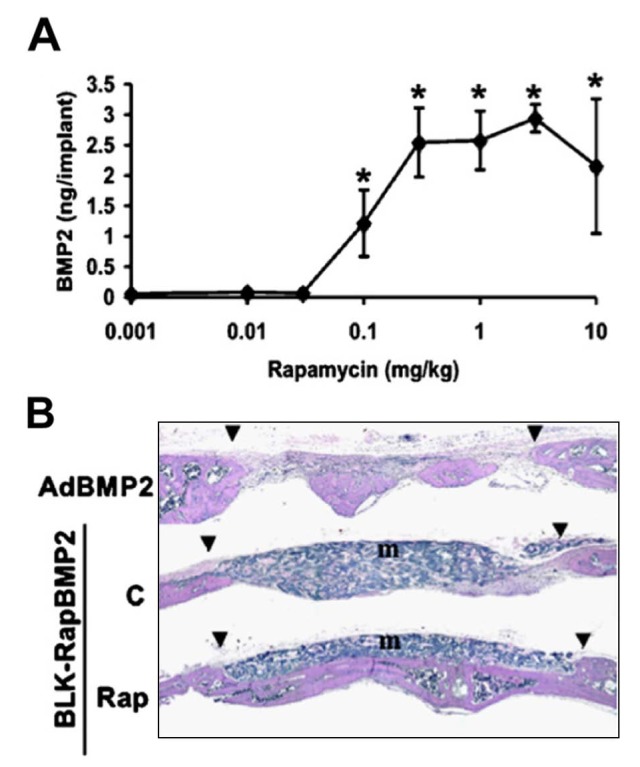
In vivo performance of an inducible BMP2 gene expression system. (A) Dose-response curve demonstrating the rapamycin-inducible expression of BMP2 in engineered cells subcutaneously implanted into mice. (B) Rapamycin-induced expression of BMP2 led to the formation and integration of new bone in a critical-sized calvarial defect. In contrast, expression of BMP2 under the control of a constitutive viral promoter harbored by an adenoviral vector (AdBMP2) led to the overgrowth and disorganization of repaired tissue (adapted from Koh et al., 2006). Legend: triangles, margins of defect; m, marrow.
Defining the Spatial Localization of BMP Transgene Expression
Natural and synthetic scaffolds serve as flexible and often highly effective platforms for the restriction of BMP-transgene expression in space. Scaffolds for delivering viruses, plasmids, or genetically engineered cells include devitalized bone (Yazici et al., 2011), biopolymers such as collagen and alginate (Wegman et al., 2011), and biodegradable synthetic materials including poly-lactic-co-glycolic acid (PLGA). Adsorbed vectors or cells provide localized expression of a BMP transgene at the implant site. Creating well-defined gradients and other physiologic patterns of expression, however, remains a substantial challenge. Graded immobilization of retroviral vectors encoding Runx2 in collagen scaffolds was achieved via controlled deposition of poly(L-lysine). Ectopic implantation of these scaffolds resulted in spatial patterns of transcription factor expression, osteogenic differentiation, and mineralization (Phillips et al., 2008). Chemical vapor deposition of reactive polymer coatings and wax-masking were applied in porous poly-caprolactone scaffolds to spatially pattern sites of adenoviral conjugation and restrict transgene expression to predefined subvolumes (Hu et al., 2009). Localized adsorption of DNA lipoplexes to a PLGA scaffold coated with fibronectin generated sub-millimeter scale patterns of transgene expression in vitro and in vivo (De Laporte et al., 2009), and lentiviruses adsorbed to PLGA microspheres doped with phosphatidylserine generated tissue-scale patterns of transduction (Shin et al., 2010). Micropatterning cell-scale topographical features into the surface of the scaffold and subsequent adsorption of DNA lipoplexes also promoted localized transfection of neurons (Houchin-Ray et al., 2009). These scaffolds exhibit impressive capacities for establishing polarity in cell growth in vitro and cell infiltration in vivo.
New frontiers in Regulated Gene Expression: BMP Expression in 4D
Analysis of empirical data and computational models unambiguously demonstrated that quantitative variations in the timing and localization of BMP expression are necessary for morphogenesis of cartilage and bone. Thus, regulating the expression of BMPs in 4 dimensions (3 spatial and time) pre- and post-implantation may substantially improve our ability to guide bone formation. At present, there are few technologies capable of providing both spatial and temporal control over transgene expression. Through synthetic biology, however, we now have a growing number of genetic tools capable of providing 4D control of transgene expression in vivo (Fig. 4).
Figure 4.
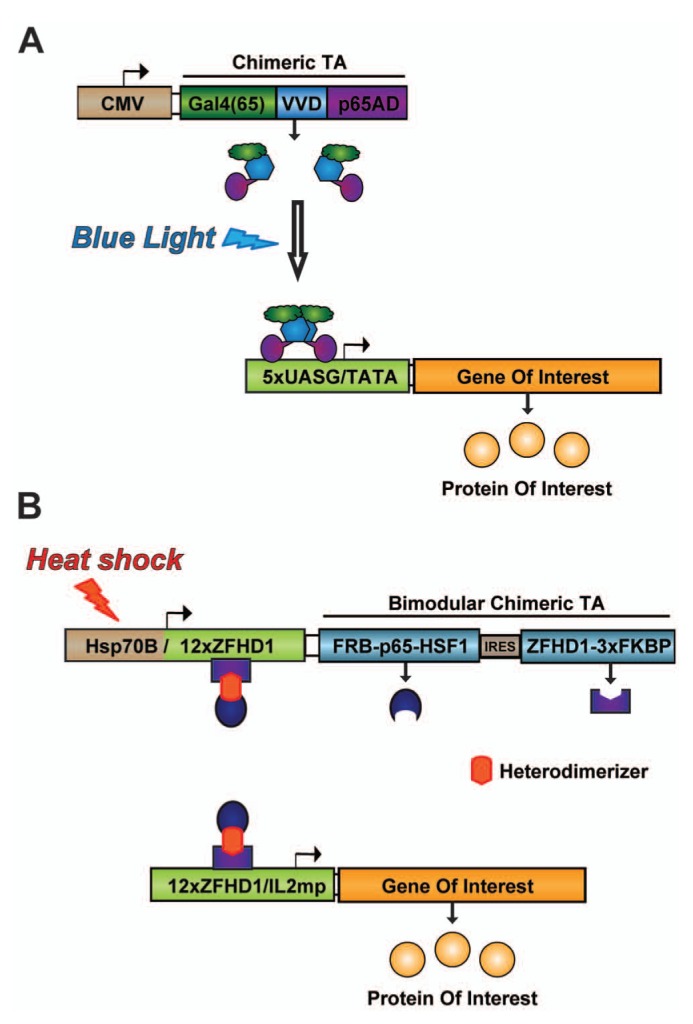
Next-generation synthetic gene circuits for the spatial and temporal regulation of transgene expression. (A) A light-switchable transgene system consists of a light-switchable transactivator (TA) that, upon exposure to blue-light, dimerizes and initiates transcription of the gene of interest. The TA contains a Gal4 DNA-binding domain, the light-oxygen-voltage domain Vivid (VVD), and the activation domain of the human p65 protein, a component of the NF-κB complex (Wang et al., 2012). (B) A dual-requirement gene circuit is activated following a hyperthermic stimulus in the presence of rapamycin or its analogues (rapalogs). The activated TA consists of two modules heterodimerized by rapamycin/rapalog through FRB and FKBP (distinct rapamycin-binding domains). DNA binding occurs through zinc finger homeodomain 1 (ZFHD1), and activation domains from the p65 subunit of human NF-κB and human heat-shock factor 1 (HSF1) drive transcription of the gene of interest. Transient heat shock triggers expression of the TA modules, and an autoregulatory loop sustains expression of the TA in the presence of the heterodimerizer.
Optogenetics systems exploit the ability of certain proteins to be activated by light. A light-inducible synthetic gene switch was recently described (Wang et al., 2012). Upon exposure to blue light, the transactivator of this system binds synthetic promoters that rapidly initiate transcription of target transgenes. Withdrawal from light leads to the eventual inactivation of the transactivator and, thus, to gene silencing. A second synthetic signaling cascade was developed for blue-light-inducible transgene expression that uses the light-sensitive molecule, melanopsin. Blue light triggers an increase in intracellular calcium, which stimulates calcineurin-mediated mobilization of the transcription factor, nuclear factor of active T-cells or NFAT (Ye et al., 2011). Induction of antihyperglycemic hormones (insulin, glucagon-like peptide 1) was achieved even in internal organs (e.g., liver). One challenge to the use of these systems in vivo is related to difficulties in focusing light deep within the body. Light scattering, particularly of short wavelengths, substantially attenuates the ability of light to penetrate tissues. For this reason, it would be very difficult to activate transgene expression at deep tissue sites without also activating more proximal cells within the light path.
Inducible systems based on promoters that are activated by externally directed physical stimuli may be more generally useful for the generation of 4D patterns of transgene expression (reviewed in Vilaboa et al., 2011). Promoters of this type include heat-shock protein (hsp) gene promoters and radiation-induced promoters that can be activated by heat and directed ionizing radiation (IR), respectively. Both IR and heat administration can be focused, but due to its intrinsic toxicity, IR should be utilized only in the context of cancer or ablative therapies. In contrast, localized heating of tissues can be achieved by multiple safe and non-invasive methods including ultrasound, microwave, or infrared radiation. Ultrasound is currently the most promising approach, because it exhibits low attenuation in biologic media over organ-scale distances and can be focused to generate mm3-cm3 subvolumes of hyperthermia deep within the body. Localized, focused ultrasound (FUS)-induced expression of target genes with the human hsp70B promoter has been reported in in vivo models, even in deep-seated organs such as the liver (Rome et al., 2005). Image-guided spatial and temporal control over transgene expression can be achieved by the coupling of focused ultrasound with magnetic resonance imaging (MRI)-based thermography. MRI-guided FUS has been able to mediate localized in vivo expression of a reporter gene controlled by the hsp70B promoter without inducing tissue damage (Deckers et al., 2009). In view of the successful performance and potential of this technology, a relatively clear path to translation can be envisioned, since commercially available MRI-FUS instruments are now in clinical use. To date, FUS-induced transgene expression has not been used for controlling the delivery of growth factors in regenerative medicine applications.
The heat-inducible systems described above may prove useful for temporal and spatial control of BMP expression, but the kinetics of activation and subsequent de-activation of hsp promoters are defined by endogenous signaling mechanisms. Owing to autoregulation, hsp promoters do not remain active for periods exceeding a few hours. In addition, hsp promoter-controlled gene therapies are susceptible to non-specific activation by hyperthermia associated with disease, local inflammation, strenuous exercise, pharmacological interventions, or ischemic events. To overcome this problem, synthetic gene circuits were designed that combine an hsp70B promoter and a small molecule-dependent transactivator (Vilaboa et al., 2005). These gene switches consist of (a) a ligand-activated transactivator expressed under the dual control of the hsp70B promoter and (b) a promoter that is responsive to the transactivator to control the gene of interest. Steroid receptor-derived and dimerizer-controlled gene switches have been built and tested. These switches were shown to stringently regulate the expression of reporter molecules, such as luciferase, and soluble factors including VEGF. We observed that brief application of FUS to cells harboring these switches in a fibrin scaffold led to dose-dependent induction of a reporter transgene (Fig. 5). Furthermore, this activation can be restricted to ~30 mm3 subvolumes and can be used to create gradients. In ongoing studies, this approach to 4D regulation of transgene expression is being adapted for in vivo applications. With this technology, we envision patterning the expression of BMP transgenes to establish physiologically relevant distributions of BMP signaling to promote the formation of bone having site-specific composition and geometry. This approach is predicated on the notion that morphogenic/regenerative signals induced by BMPs rely on their localization, persistence, and amplitude, and that such “context” for exogenous BMP activity will be critical to defining regions of bone formation and integration with surrounding tissue, particularly in high-volume bone defects. Of interest for bone regeneration applications, heat-activated gene switches based on different ligand-activated transactivators may be used in combination for independent control of multiple transgenes.
Figure 5.
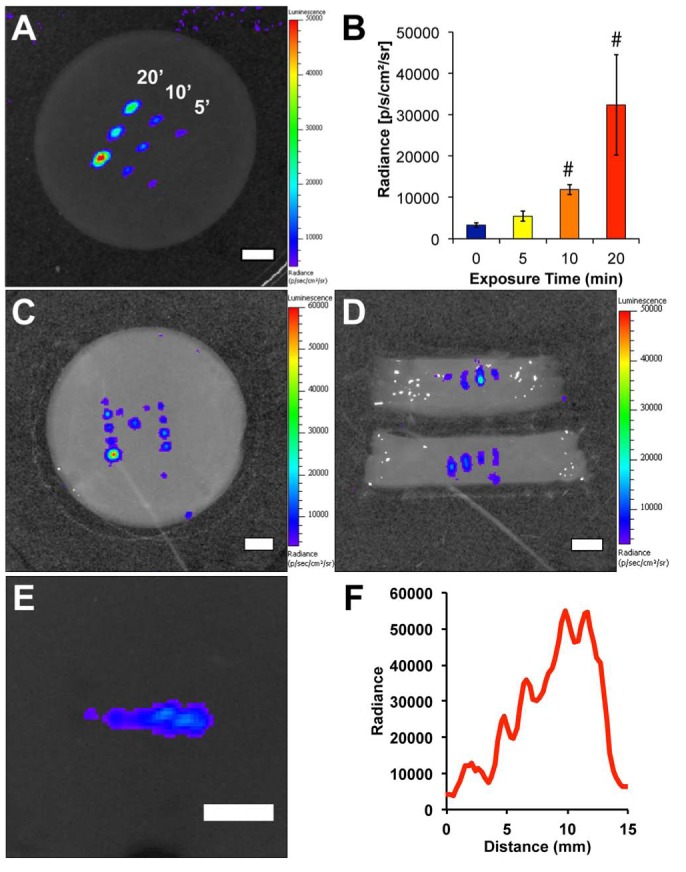
Patterning the expression of a transgene with a heat-shock- and ligand-dependent gene circuit with focused ultrasound (FUS). (A) Spatially restricted activation of reporter gene expression. Cells harboring a heat-shock- and rapamycin-dependent gene circuit for regulating the expression of firefly luciferase (fLuc) were suspended in a fibrin scaffold and exposed to 0 to 20 minutes of FUS. (B) Quantification of fLuc activity demonstrated dose-dependent increases in transgene expression after ultrasound energy delivery. # p < .05 vs. 0 minute controls. (C) Complex patterns of transgene expression can be achieved with this methodology. Here, a block “M” was patterned through several exposures to FUS. (D) A cross-sectional view of the activated regions in the right arm of the M indicates that fLuc activity is also restricted in the third spatial dimension. (E) A physiologically relevant pattern, here a gradient can be generated by varying the focal position and duration of FUS exposure to tune levels of transgene expression. (F) Quantification of fLuc activity demonstrates the gradient in transgene expression along the activated region in E. Scale bars = 5 mm (Wilson et al., unpublished observations).
Summary
We have described the spatial and temporal patterns of BMP signaling observed during skeletal development and post-natal bone regeneration, as well as a variety of gene-based therapies having the potential to recapitulate aspects of the morphogenic cascade that are absent in non-unions and massive tissue injuries. We expect that continued research and development at the confluence of developmental biology, synthetic biology, and gene- and scaffold-engineering will not only lead to the identification of spatio-temporal patterns of BMP transgene expression that drive regeneration, but also provide the experimental and clinical tools for generating those patterns in vivo.
Footnotes
CGW was supported by NIH training grant T32DE007057-34; NV and FMM-S are supported by program I3SNS and Sara Borrell, respectively, from Fondo de Investigaciones Sanitarias (Spain). Research was supported by Department of Defense grant OR090134 (RTF) and NIH grant R01DE013386-09 (RTF) and by Fondo de Investigaciones Sanitarias (Spain) grant PI12/01698 (NV).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Alexander C, Zuniga E, Blitz IL, Wada N, Le Pabic P, Javidan Y, et al. (2011). Combinatorial roles for BMPs and Endothelin 1 in patterning the dorsal-ventral axis of the craniofacial skeleton. Development 138:5135-5146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bais MV, Wigner N, Young M, Toholka R, Graves DT, Morgan EF, et al. (2009). BMP2 is essential for post natal osteogenesis but not for recruitment of osteogenic stem cells. Bone 45:254-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barlow AJ, Francis-West PH. (1997). Ectopic application of recombinant BMP-2 and BMP-4 can change patterning of developing chick facial primordia. Development 124:391-398. [DOI] [PubMed] [Google Scholar]
- Betz OB, Betz VM, Nazarian A, Egermann M, Gerstenfeld LC, Einhorn TA, et al. (2007). Delayed administration of adenoviral BMP-2 vector improves the formation of bone in osseous defects. Gene Ther 14:1039-1044. [DOI] [PubMed] [Google Scholar]
- Bonilla-Claudio M, Wang J, Bai Y, Klysik E, Selever J, Martin JF. (2012). Bmp signaling regulates a dose-dependent transcriptional program to control facial skeletal development. Development 139:709-719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho TJ, Gerstenfeld LC, Einhorn TA. (2002). Differential temporal expression of members of the transforming growth factor beta superfamily during murine fracture healing. J Bone Miner Res 17:513-520. [DOI] [PubMed] [Google Scholar]
- De Laporte L, Yan AL, Shea LD. (2009). Local gene delivery from ECM-coated poly(lactide-co-glycolide) multiple channel bridges after spinal cord injury. Biomaterials 30:2361-2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deckers R, Quesson B, Arsaut J, Eimer S, Couillaud F, Moonen CT. (2009). Image-guided, noninvasive, spatiotemporal control of gene expression. Proc Natl Acad Sci USA 106:1175-1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando WA, Leininger E, Simkin J, Li N, Malcom CA, Sathyamoorthi S, et al. (2011). Wound healing and blastema formation in regenerating digit tips of adult mice. Dev Biol 350:301-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gafni Y, Pelled G, Zilberman Y, Turgeman G, Apparailly F, Yotvat H, et al. (2004). Gene therapy platform for bone regeneration using an exogenously regulated, AAV-2-based gene expression system. Mol Ther 9:587-595. [DOI] [PubMed] [Google Scholar]
- Gould DJ, Chernajovsky Y. (2004). Endogenous GATA factors bind the core sequence of the tetO and influence gene regulation with the tetracycline system. Mol Ther 10:127-138. [DOI] [PubMed] [Google Scholar]
- Grimes R, Jepsen KJ, Fitch JL, Einhorn TA, Gerstenfeld LC. (2011). The transcriptome of fracture healing defines mechanisms of coordination of skeletal and vascular development during endochondral bone formation. J Bone Miner Res 26:2597-2609. [DOI] [PubMed] [Google Scholar]
- Han M, Yang X, Farrington JE, Muneoka K. (2003). Digit regeneration is regulated by Msx1 and BMP4 in fetal mice. Development 130:5123-5132. [DOI] [PubMed] [Google Scholar]
- He F, Xiong W, Wang Y, Matsui M, Yu X, Chai Y, et al. (2010). Modulation of BMP signaling by Noggin is required for the maintenance of palatal epithelial integrity during palatogenesis. Dev Biol 347:109-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houchin-Ray T, Huang A, West ER, Zelivyanskaya M, Shea LD. (2009). Spatially patterned gene expression for guided neurite extension. J Neurosci Res 87:844-856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu D, Colnot C, Marcucio RS. (2008). Effect of bone morphogenetic protein signaling on development of the jaw skeleton. Dev Dyn 237:3727-3737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu WW, Elkasabi Y, Chen HY, Zhang Y, Lahann J, Hollister SJ, et al. (2009). The use of reactive polymer coatings to facilitate gene delivery from poly (epsilon-caprolactone) scaffolds. Biomaterials 30:5785-5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara A, Zekas LJ, Litsky AS, Weisbrode SE, Bertone AL. (2010). Dermal fibroblast-mediated BMP2 therapy to accelerate bone healing in an equine osteotomy model. J Orthop Res 28:403-411. [DOI] [PubMed] [Google Scholar]
- Ishihara A, Bartlett JS, Bertone AL. (2012). Inflammation and immune response of intra-articular serotype 2 adeno-associated virus or adenovirus vectors in a large animal model. Arthritis 2012:735472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Javier AL, Doan LT, Luong M, Reyes de, Mochel NS, Sun A, Monuki ES, et al. (2012). Bmp indicator mice reveal dynamic regulation of transcriptional response. PLoS ONE 7:e42566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jumlongras D, Lachke SA, O’Connell DJ, Aboukhalil A, Li X, Choe SE, et al. (2012). An evolutionarily conserved enhancer regulates Bmp4 expression in developing incisor and limb bud. PLoS ONE 7:e38568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya N, Ye L, Kobayashi T, Lucas DJ, Mochida Y, Yamauchi M, et al. (2008). Disruption of BMP signaling in osteoblasts through type IA receptor (BMPRIA) increases bone mass. J Bone Miner Res 23:2007-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HJ, Rice DP, Kettunen PJ, Thesleff I. (1998). FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development 125:1241-1251. [DOI] [PubMed] [Google Scholar]
- Kimelman-Bleich N, Kallai I, Lieberman JR, Schwarz EM, Pelled G, Gazit D. (2012). Gene therapy approaches to regenerating bone. Adv Drug Deliv Rev 64:1320-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloen P, Di Paola M, Borens O, Richmond J, Perino G, Helfet DL, et al. (2003). BMP signaling components are expressed in human fracture callus. Bone 33:362-371. [DOI] [PubMed] [Google Scholar]
- Kloen P, Lauzier D, Hamdy RC. (2012). Co-expression of BMPs and BMP-inhibitors in human fractures and non-unions. Bone 51:59-68. [DOI] [PubMed] [Google Scholar]
- Koh JT, Ge C, Zhao M, Wang Z, Krebsbach PH, Zhao Z, et al. (2006). Use of a stringent dimerizer-regulated gene expression system for controlled BMP2 delivery. Mol Ther 14:684-691. [DOI] [PubMed] [Google Scholar]
- Kolambkar YM, Boerckel JD, Dupont KM, Bajin M, Huebsch N, Mooney DJ, et al. (2011). Spatiotemporal delivery of bone morphogenetic protein enhances functional repair of segmental bone defects. Bone 49:485-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong FN, Hoyland JA, Evans CH, Freemont AJ. (2009a). Regional and cellular localisation of BMPs and their inhibitors’ expression in human fractures. Int Orthop (SICOT) 33:281-288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwong FN, Hoyland JA, Freemont AJ, Evans CH. (2009b). Altered relative expression of BMPs and BMP inhibitors in cartilaginous areas of human fractures progressing towards nonunion. J Orthop Res 27:752-757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyons KM, Hogan BL, Robertson EJ. (1995). Colocalization of BMP 7 and BMP 2 RNAs suggests that these factors cooperatively mediate tissue interactions during murine development. Mech Dev 50:71-83. [DOI] [PubMed] [Google Scholar]
- Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, et al. (2010). Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell 19:329-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsubara H, Hogan DE, Morgan EF, Mortlock DP, Einhorn TA, Gerstenfeld LC. (2012). Vascular tissues are a primary source of BMP2 expression during bone formation induced by distraction osteogenesis. Bone 51:168-180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrill AE, Eames BF, Weston SJ, Heath T, Schneider RA. (2008). Mesenchyme-dependent BMP signaling directs the timing of mandibular osteogenesis. Development 135:1223-1234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro RM, de Sousa Lopes SM, Bialecka M, de Boer S, Zwijsen A, Mummery CL. (2008). Real time monitoring of BMP Smads transcriptional activity during mouse development. Genesis 46:335-346. [DOI] [PubMed] [Google Scholar]
- Moutsatsos IK, Turgeman G, Zhou S, Kurkalli BG, Pelled G, Tzur L, et al. (2001). Exogenously regulated stem cell-mediated gene therapy for bone regeneration. Mol Ther 3:449-461. [DOI] [PubMed] [Google Scholar]
- Muthukuru M, Sun J. (2013). Doxycycline counteracts Bone Morphogenic Protein-2 induced osteogenic mediators. J Periodontol [Epub ahead of print 7/16/2012] (in press). [DOI] [PubMed] [Google Scholar]
- Nie X, Luukko K, Kettunen P. (2006). BMP signalling in craniofacial development. Int J Dev Biol 50:511-521. [DOI] [PubMed] [Google Scholar]
- O’Connell DJ, Ho JW, Mammoto T, Turbe-Doan A, O’Connell JT, Haseley PS, et al. (2012). A Wnt-bmp feedback circuit controls intertissue signaling dynamics in tooth organogenesis. Sci Signal 5:ra4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva KB, Silva-Valenzuela MD, Massironi SM, Ko GM, Siqueira FM, Nunes FD. (2010). Differential Shh, Bmp and Wnt gene expressions during craniofacial development in mice. Acta Histochem 112:508-517. [DOI] [PubMed] [Google Scholar]
- Phillips JE, Burns KL, Le Doux JM, Guldberg RE, García AJ. (2008). Engineering graded tissue interfaces. Proc Natl Acad Sci USA 105:12170-12175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retting KN, Song B, Yoon BS, Lyons KM. (2009). BMP canonical Smad signaling through Smad1 and Smad5 is required for endochondral bone formation. Development 136:1093-1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivera VM. (1998). Controlling gene expression using synthetic ligands. Methods 14:421-429. [DOI] [PubMed] [Google Scholar]
- Rome C, Couillaud F, Moonen CT. (2005). Spatial and temporal control of expression of therapeutic genes using heat shock protein promoters. Methods 35:188-198. [DOI] [PubMed] [Google Scholar]
- Schumacher JA, Hashiguchi M, Nguyen VH, Mullins MC. (2011). An intermediate level of BMP signaling directly specifies cranial neural crest progenitor cells in zebrafish. PLoS ONE 6:e27403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seeherman HJ, Li XJ, Bouxsein ML, Wozney JM. (2010). rhBMP-2 induces transient bone resorption followed by bone formation in a nonhuman primate core-defect model. J Bone Joint Surg Am 92:411-426. [DOI] [PubMed] [Google Scholar]
- Shin S, Tuinstra HM, Salvay DM, Shea LD. (2010). Phosphatidylserine immobilization of lentivirus for localized gene transfer. Biomaterials 31:4353-4359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sojo K, Sawaki Y, Hattori H, Mizutani H, Ueda M. (2005). Immunohistochemical study of vascular endothelial growth factor (VEGF) and bone morphogenetic protein-2, -4 (BMP-2, -4) on lengthened rat femurs. J Craniomaxillofac Surg 33:238-245. [DOI] [PubMed] [Google Scholar]
- Tsuji K, Bandyopadhyay A, Harfe BD, Cox K, Kakar S, Gerstenfeld L, et al. (2006). BMP2 activity, although dispensable for bone formation, is required for the initiation of fracture healing. Nat Genet 38:1424-1429. [DOI] [PubMed] [Google Scholar]
- Vilaboa N, Fenna M, Munson J, Roberts SM, Voellmy R. (2005). Novel gene switches for targeted and timed expression of proteins of interest. Mol Ther 12:290-298. [DOI] [PubMed] [Google Scholar]
- Vilaboa N, Boellmann F, Voellmy R. (2011). Gene switches for deliberate regulation of transgene expression: recent advances in system development and uses. J Genet Syndr Gene Ther 2:107. [Google Scholar]
- Virk MS, Sugiyama O, Park SH, Gambhir SS, Adams DJ, Drissi H, et al. (2011). “Same day” ex-vivo regional gene therapy: a novel strategy to enhance bone repair. Mol Ther 19:960-968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vortkamp A, Pathi S, Peretti GM, Caruso EM, Zaleske DJ, Tabin CJ. (1998). Recapitulation of signals regulating embryonic bone formation during postnatal growth and in fracture repair. Mech Dev 71:65-76. [DOI] [PubMed] [Google Scholar]
- Wang X, Chen X, Yang Y. (2012). Spatiotemporal control of gene expression by a light-switchable transgene system. Nat Methods 9:266-269. [DOI] [PubMed] [Google Scholar]
- Wegman F, Bijenhof A, Schuijff L, Oner FC, Dhert WJ, Alblas J. (2011). Osteogenic differentiation as a result of BMP-2 plasmid DNA based gene therapy in vitro and in vivo. Eur Cell Mater 21:230-242. [DOI] [PubMed] [Google Scholar]
- Yazici C, Takahata M, Reynolds DG, Xie C, Samulski RJ, Samulski J, et al. (2011). Self-complementary AAV2.5-BMP2-coated femoral allografts mediated superior bone healing versus live autografts in mice with equivalent biomechanics to unfractured femur. Mol Ther 19:1416-1425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Daoud-El Baba M, Peng RW, Fussenegger M. (2011). A synthetic optogenetic transcription device enhances blood-glucose homeostasis in mice. Science 332:1565-1568. [DOI] [PubMed] [Google Scholar]
- Yu L, Han M, Yan M, Lee EC, Lee J, Muneoka K. (2010). BMP signaling induces digit regeneration in neonatal mice. Development 137:551-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YY, Lieu S, Lu C, Colnot C. (2010a). Bone morphogenetic protein 2 stimulates endochondral ossification by regulating periosteal cell fate during bone repair. Bone 47:65-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YY, Lieu S, Lu C, Miclau T, Marcucio RS, Colnot C. (2010b). Immunolocalization of BMPs, BMP antagonists, receptors, and effectors during fracture repair. Bone 46:841-851. [DOI] [PubMed] [Google Scholar]