Abstract
We tested the hypotheses that glass-ceramic veneers and overglazes degrade by ion exchange in an acidic environment, and that they degrade by breakdown of the silica network in a basic environment. Disk specimens of glass-ceramic veneer and glaze were fabricated and immersed in pH 2, 7, or 10 buffer solutions, for 1, 3, 5, 10, 15, and 30 days. Each specimen was placed in a shaker bath containing de-ionized distilled water at 80°C. Concentrations of Al3+, Ca2+, Zn2+, Li2+, and Si4+ were analyzed by means of inductively coupled plasma atomic emission spectrometry (ICP/AES). Statistical analyses were performed by factorial ANOVA. Significant differences occurred among leached ion concentrations as a function of material type, solution pH, and exposure time. A substantial release of Si occurred at pH 10 over time, leading to a breakdown of the glass phase. At pH 2, dissolution was controlled by an ionic exchange mechanism. We conclude that ceramic veneers and glazes may be susceptible to considerable degradation in low- and high-pH buffer solutions.
Keywords: dissolution, chemical stability, acidic pH, basic pH, ion release, surface degradation
Introduction
In vitro studies have shown that ceramics are susceptible to corrosion in the range of oral fluid pH (Anusavice, 1992; Milleding et al., 1999b, 2002; Butler et al., 2004; Ccahuana et al., 2010; Junpoom et al., 2010; Kukiattrakoon et al., 2010b, c). The dissolution potentials of ceramics in acidic liquids such as soda pop (pH 2.525-4.038 for common brands) (Jain et al., 2007) and fruit juices (pH 2.0-5.50) differ considerably with the variation in buffering capacity of the patient’s saliva and diet. Some basic substances, such as lima beans or soy beans (pH 12), spinach (pH 8.3), and antacids (pH 10-14), can also cause dissolution of glass-phase ceramics. ISO standard 6872 for dental ceramics requires evidence of minimal chemical solubility for dental ceramic materials that are exposed to a 4% HAc solution.
Dietary habits and the buffering capacity of saliva can have a significant effect on the pH of the oral environment (Bartlett et al., 2011) and on the chemical durability of ceramic-based restorations. Moreover, ceramic corrosion can affect the fracture strength of these materials (Drummond et al., 1991; Pinto et al., 2008) and increase surface roughness (Milleding et al., 1999a), which can affect the survival of restorations and damage to adjacent oral structures as a result of increased plaque accumulation. In addition, rougher surfaces are more susceptible to adhesive and abrasive wear from the opposing dentition. Moreover, humans experience discomfort when the roughness of restorations (Ra) is 0.50 µm or more (Jones et al., 2004). The ceramic microstructure has been shown to influence the susceptibility of the prosthesis surface to pH changes, i.e., reduced chemical durability (Anusavice and Zhang, 1997; Milleding et al., 2003). Also, the cytotoxicity potential of leached ions increases during surface degradation (Mackert, 1992; Messer et al., 2003).
Previous clinical studies reported by the authors revealed clinical degradation and roughening of ceramic surfaces between years 1 and 2 (Esquivel-Upshaw et al., 2006, 2012, 2013). These crowns were made from a non-veneered (glazed) lithium disilicate glass-ceramic core ceramic and a lithium disilicate glass-ceramic core that was veneered with a glass-ceramic. The study participants reported this condition as “rough”. Based on these clinical observations, we performed the present in vitro investigation to determine the cause of the roughening process and the mechanism of surface degradation.
The objectives of this research were:
(a) to test the hypothesis that glass-ceramic veneers and their respective overglazes degrade by ion exchange of protons for alkali ions and alkaline earth oxides in an acidic environment (pH 2); and
(b) to test the hypothesis that glass-ceramic veneers and their respective overglazes degrade by breakdown of the silica network by hydrolysis of Si-O-Si bonds in a basic environment (pH 10).
Materials & Methods
Thirty-six disks of glass-ceramic veneer (GCV) (IPS Eris for Empress 2 glass-ceramic core) and 36 disks of glaze (GL) (e.max® Ceram glaze, Ivoclar Vivadent, Schaan, Liechtenstein) were fabricated with a diameter of 12 mm and a thickness of 1.0 mm. Porcelain powder was mixed with the liquid recommended by the manufacturer and poured into a metal mold. The densities of randomly selected disks were measured to ensure structural consistency prior to immersion. A programmable dental furnace (Radiance Multi-Stage MSL Furnace, Jelrus International®, Melville, NY, USA) was used to sinter the specimens according to the manufacturer’s recommendations.
The specimens were pre-dried for 7 min, dried for 2 min, heated to 403°C at a rate of 60°C/min under full vacuum, then to 724°C for 1.5 min, and subsequently cooled for 2 min. The GL and GCV specimens were processed identically, except that GL specimens were fired once and GCV specimens were fired twice. Specimens were polished after being fired through 600-grit abrasive, rinsed, and dried.
Each disk was washed 3 times in ethyl alcohol, dried, weighed (Mettler Toledo BB240, Toledo, OH, USA, precision 0.001 gm), and sealed in 15-mL polyethylene corrosion jars (Nalgene, Rochester, NY, USA) by means of 12.5-mm-wide Teflon tape. The disks were immersed in one of the following test solutions: pH 2, pH 7, and pH 10 buffer solutions for 1, 3, 5, 10, 15, and 30 days. The disks were placed vertically in the containers, allowing for exposure of both sides to the solution. Solution pH was measured before and after immersion (Mettler Toledo SevenMulti pH Conductivity Meter). The buffer solutions were certified as high purity (Fisher Scientific, Philadelphia, PA, USA; SB 108-500 Buffer Solution pH 7.00; SB96-500 Buffer Solution pH 2.00; SB116-500 Buffer Solution pH 10.00). The ratio of the specimen surface area to solution volume (A/V) was maintained at 2.333 to standardize corrosion rates. Each specimen was sealed in a polyethylene jar and placed in a shaker bath (Model 6300, Water Bath Shaker, Eberbach Corp., Ann Arbor, MI, USA) containing de-ionized-distilled water at a temperature of 80°C and a vibrating speed of 50 oscillations per min. The immersion process was performed at a temperature of 80°C in pH 2 or pH 10 buffer to accelerate degradation of the ceramic to simulate years of exposure in vivo at 37°C. This temperature is required to test the chemical durability of ceramics according to ISO 6872.
The disk surfaces were rinsed with ethyl alcohol and dried for 40 min at room temperature (25°C). Concentrations of Al3+, Ca2+, Zn2+, Li2+, and Si4+ in the corrosion solutions were analyzed by means of an inductively coupled plasma atomic emission spectrometer (Perkin-Elmer 3200RL, Downers Grove, IL, USA). Prior to analysis, specific elements were tested against known concentrations of each element to determine the accuracy of elemental detection. The instrument was calibrated to an accuracy of 1.5 mg/L at 1 ppm. Each element was measured 5 times to ensure reproducibility of results. Disk surfaces that were exposed to the test solutions for 1, 3, 5, 10, 15, and 30 days were examined by scanning electron microscopy (JEOL JSM-6400 Scanning Electron Microscope, JEOL Ltd., Tokyo, Japan).
To obtain the ratio of ions leached from the ceramic specimens, we placed one disk each of GCV and GL ceramic in 10% hydrofluoric acid until totally dissolved. The solutions were analyzed under ICP for concentration of analytes.
Statistical Analysis
Statistical analyses were performed with an SAS statistical software program (V 9.2) for factorial analysis of variance (ANOVA). The natural log of elemental concentration (Al3+, Ca2+, Zn2+, Li2+, and Si4+) was chosen as our response variable. Material type (GCV or GL), pH level (2, 7, or 10), and number of days (1, 3, 5, 10, 15, or 30), and all two-way interactions among them, were chosen as fixed factors.
Results
Weight Change
There was no change in the pH of the solution before and after immersion of specimens. There were significant effects of material and pH on the weight change of the disks (Fig. 1). Weight differences after exposure for GCV were greater across all days and pH levels, particularly at pH 7 and 10. Also, the higher the pH level, the greater the pre- and post-weight differences across all days and both groups. This was particularly significant at pH = 10. Although the effect of exposure time was not significant, there was a significant overall effect of material (p = .004), a marginally significant overall effect of days (p = .089), and a significant effect of pH (p = .003).
Figure 1.
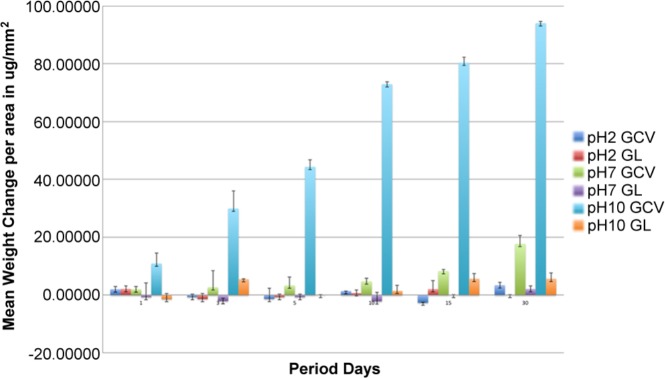
Mean weight change (weight loss) per area for glass-ceramic veneer (GCV) and glaze (GL) disk specimens (µg/mm2).
Ions in Solution
These results reveal that there was a significant difference in release rates among the material type, solution pH, and exposure time. These results suggest that, at a pH of 10, there is a substantial release of Si over time (see Appendix), leading to a breakdown of the glass phase. At the low pH, analysis of the data suggests that the release of ions is similar to an ionic exchange mechanism or selective leaching of ions.
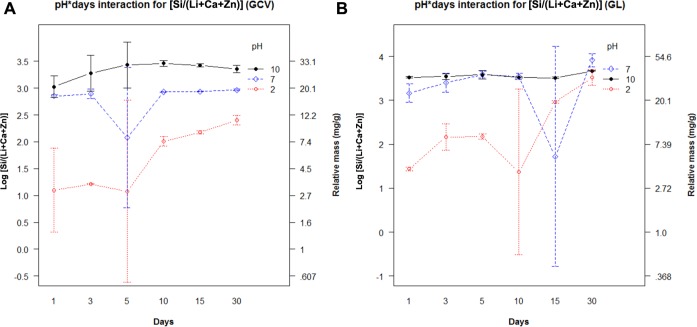
Total dissolution of both disks was calculated for the ratio of network former to network modifier [Si/(Li+Ca+Zn)]. The ratio for GCV was 1.6, and the ratio for GL was 8.0. Comparable ratios were found for the concentrations of ions at different pH levels and time periods (Figs. 2A, 2B).
Figure 2.
(A) Ions leached from glass-ceramic veneer (GCV) for ‘network former to modifier’ ratio as a function of pH and time. (B) Ions leached over time from glaze (GL) for ‘network former to modifier’ ratio as a function of pH and time.
SEM Analysis
After exposure to pH 10 buffer solution, the GCV specimens exhibited significant pitting and loss of surface structure, which suggests a breakdown of the glass phase (Fig. 3). At the low pH for the same time period, SEM images revealed some pitting but at isolated areas, which suggests a release of ions occurred in a manner similar to an ionic exchange mechanism (Fig. 4).
Figure 3.
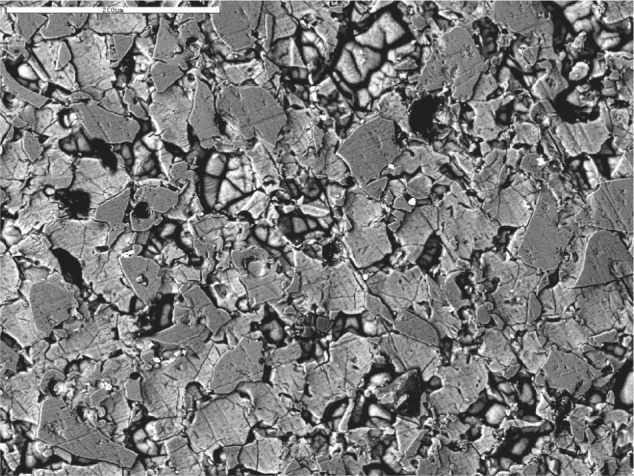
SEM backscattered image of glass-ceramic veneer (GCV) and pH 10, 30 days.
Figure 4.
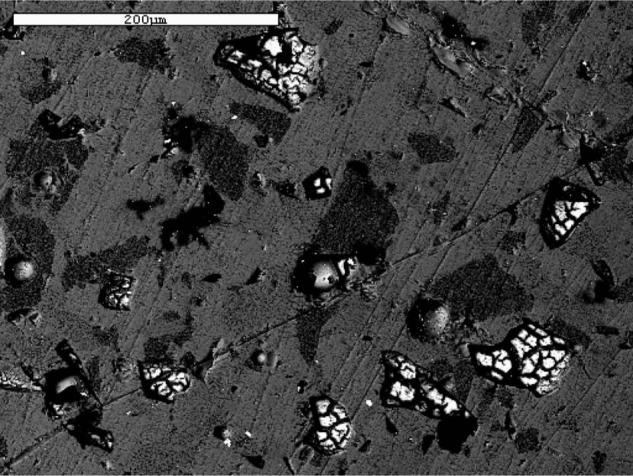
SEM backscattered image of glass-ceramic veneer (GCV) after exposure to pH 2 buffer solution for 30 days.
Discussion
This study focused on disks made of GCV and GL that were weighed and examined after immersion, and the types and concentrations of ions leached into the buffering solutions were determined. Network modifiers (Ca, Zn, Li) and network formers (Si, Al) were measured to determine the mechanism involved in leaching at different pH levels.
Based on the weight change data, the weight loss in the GCV group was, on average, 4.8 times greater than that of the GL group (95% CI = [1.7, 8.0]). The effect of pH (p = .003) was significant, as evidenced by an increase in weight loss across both groups and all days by 0.75 for each unit increase in pH (CI = 0.271, 1.22). The overall effect of exposure time in days (p = 0.089) was marginal, and there was an estimated weight loss of 0.14 mg increase per day (CI = -0.022, 0.30) from both groups at each pH. This weight loss was confirmed by the quantity of ions released in the solutions. At all times and for each pH, there was a significantly greater ion release from GCV than from GL, often an order of magnitude more. This included both network modifiers (Ca, Zn, Li) as well as Si+4, the primary network former.
SEM micrographs of the 2 material surfaces revealed a more generalized dissolution for GL and a rougher topography for GCV, which likely occurred as glass dissolution released crystals. These observations have resulted in a reformulation of the composition of GCV by the manufacturer by adding a network former to reduce ion release (personal communication, P. Oehri, October, 2011).
The ion release rate for Ca, Li, Zn, and Si from the glaze was the greatest for all time periods at pH 10. The concentrations of Li, Zn, and Si released from the glaze were similar at both pH 2 and pH 7. The release of Ca from the glaze did not follow this trend. In fact, the release rate of Ca at a pH of 2 was comparable with the release rate of Si.
The release rate trends from GCV were not as consistent as those from the glaze, with 2 exceptions. Ion release from GCV was at a maximum at a pH of 10. Also, the amount of ion release from GCV at all times and pH levels was considerably higher than that from the glaze.
The corrosion of silicate-based glasses can occur either by selective leaching or complete dissolution, but it usually involves a combination of the 2 mechanisms. In general, the process leads to the formation of a thin film or gel on the exposed glass surface, with the composition of the gel being significantly different from that of the non-corroded glass (Pantano et al., 1974; Clark, 1975; Clark et al., 1976). The composition and profile of the gel layer are usually a direct measure of the durability of the glass. Studies of binary soda-silica and lithia-silica glasses have established that the corrosion resistance is maximized when the reactions at the glass surface form a thin gel with a high surface silica concentration (Hench and Clark, 1978). In an acidic environment, the predominant reaction is an ion exchange between the protons in solution and network modifiers in the bulk. In a basic environment, the hydroxyl ions in solution attack the glass structure, breaking silicon-oxygen-silicon bonds, thereby resulting in generalized dissolution. At a neutral pH, a combination of the 2 reactions occurs. Corrosion of glass-ceramics is more complex since both glassy and crystalline phases are present. Glass dissolution leads to release of crystals into solution, which affects the kinetics of ion release.
Several in vitro studies (Milleding et al., 2002, 2003; Kukiattrakoon et al., 2010a, 2011) have analyzed ion release and surface compositional and roughness changes that occurred as a function of time, temperature, and pH. Most of these studies were limited to acidic conditions. With the high silica and alumina content of many of these materials, basic conditions (high hydroxyl content) will present a more severe environment, producing hydrolysis of the silica network and the resulting breakdown of the entire structure. The results of this study support this hypothesis. Ion release for all measured ions (both network formers and network modifiers) is greater at all time periods at a pH of 10 compared with a pH of 7 or 2.
This study confirms that exposure times at various pH levels in saliva is likely to affect ion release as well as surface changes. Furthermore, occlusal interactions between ceramic materials and natural tooth structure will also play a role in the degradation process. Further research is recommended to analyze leaching effects by cycling the exposure at both high- and low-pH levels, as occurs in the oral cavity, with longer intermittent periods at the neutral pH. This approach should more closely mimic in vivo conditions and monitor material stability.
This study investigated the mechanisms of ceramic degradation as a function of time and pH levels. Exposure in a basic pH buffer resulted in a complete breakdown of the silica network as compared with exposure to an acidic pH buffer, which resulted in selective ionic leaching of the glass matrix. Future studies should include cyclic events of exposure over a variable pH range to mimic the chemical durability of different materials under simulated intra-oral conditions.
Supplementary Material
Footnotes
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
This study was supported by the University of Florida Office of Research and by NIH/NIDCR Grants K23 DE018414 and R01 DE06672. Ceramic materials were provided by Ivoclar (Ivoclar, Vivadent, Schaan, Liechtenstein).
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
References
- Anusavice KJ. (1992). Degradability of dental ceramics. Adv Dent Res 6:82-89. [DOI] [PubMed] [Google Scholar]
- Anusavice KJ, Zhang NZ. (1997). Chemical durability of Dicor and lithia-based glass-ceramics. Dent Mater 13:13-19. [DOI] [PubMed] [Google Scholar]
- Bartlett DW, Fares J, Shirodaria S, Chiu K, Ahmad N, Sherriff M. (2011). The association of tooth wear, diet and dietary habits in adults aged 18-30 years old. J Dent 39:811-816. [DOI] [PubMed] [Google Scholar]
- Butler CJ, Masri R, Driscoll CF, Thompson GA, Runyan DA, von Fraunhofer AJ. (2004). Effect of fluoride and 10% carbamide peroxide on the surface roughness of low-fusing and ultra low-fusing porcelain. J Prosthet Dent 92:179-183. [DOI] [PubMed] [Google Scholar]
- Ccahuana VZ, Ozcan M, Mesquita AM, Nishioka RS, Kimpara ET, Bottino MA. (2010). Surface degradation of glass ceramics after exposure to acidulated phosphate fluoride. J Appl Oral Sci 18:155-165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark A. (1975). Solubility and biocompatibility of glass [dissertation]. Gainesville, FL: University of Florida. [Google Scholar]
- Clark AE, Pantano CG, Hench LL. (1976). Auger spectroscopic analysis of bioglass surfaces. J Am Ceram Soc 59:37-39. [Google Scholar]
- Drummond JL, Novickas D, Lenke JW. (1991). Physiological aging of an all-ceramic restorative material. Dent Mater 7:133-137. [DOI] [PubMed] [Google Scholar]
- Esquivel-Upshaw JF, Young H, Jones J, Yang M, Anusavice KJ. (2006). In vivo wear of enamel by a lithia disilicate-based core ceramic used for posterior fixed partial dentures: first-year results. Int J Prosthodont 19:391-396. [PubMed] [Google Scholar]
- Esquivel-Upshaw JF, Rose W.F., Barrett AA, Oliveira ER, Yang MC, Clark AE, et al. (2012). Three years in vivo wear: core-ceramic, veneers, and enamel antagonists. Dent Mater 28:615-621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esquivel-Upshaw J, Rose W, Oliveira E, Yang M, Clark AE, Anusavice K. (2013). Randomized, controlled clinical trial of bilayer-ceramic and metal-ceramic crown performance. J Prosthodont [Epub ahead of print 9/14/2012] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hench LL, Clark D. (1978). Physical chemistry of glass surfaces. J Non-Cryst Solids 28:83-105. [Google Scholar]
- Jain P, Nihill P, Sobkowski J, Agustin MZ. (2007). Commercial soft drinks: pH and in vitro dissolution of enamel. Gen Dent 55:150-154. [PubMed] [Google Scholar]
- Jones CS, Billington RW, Pearson GJ. (2004). The in vivo perception of roughness of restorations. Br Dent J 196:42-45. [DOI] [PubMed] [Google Scholar]
- Junpoom P, Kukiattrakoon B, Hengtrakool C. (2010). Surface characteristic changes of dental ceramics after cyclic immersion in acidic agents and titratable acidity. Eur J Prosthodont Restor Dent 18:177-184. [PubMed] [Google Scholar]
- Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. (2010a). Chemical durability and microhardness of dental ceramics immersed in acidic agents. Acta Odontol Scand 68:1-10. [DOI] [PubMed] [Google Scholar]
- Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. (2010b). Degradability of fluorapatite-leucite ceramics in naturally acidic agents. Dent Mater J 29:502-511. [DOI] [PubMed] [Google Scholar]
- Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. (2010c). The effect of acidic agents on surface ion leaching and surface characteristics of dental porcelains. J Prosthet Dent 103:148-162. [DOI] [PubMed] [Google Scholar]
- Kukiattrakoon B, Hengtrakool C, Kedjarune-Leggat U. (2011). Effect of acidic agents on surface roughness of dental ceramics. Dent Res J (Isfahan) 8:6-15. [PMC free article] [PubMed] [Google Scholar]
- Mackert JR., Jr (1992). Side-effects of dental ceramics. Adv Dent Res 6:90-93. [DOI] [PubMed] [Google Scholar]
- Messer RL, Lockwood PE, Wataha JC, Lewis JB, Norris S, Bouillaguet S. (2003). In vitro cytotoxicity of traditional versus contemporary dental ceramics. J Prosthet Dent 90:452-458. [DOI] [PubMed] [Google Scholar]
- Milleding P, Gerdes S, Holmberg K, Karlsson S. (1999a). Surface energy of non-corroded and corroded dental ceramic materials before and after contact with salivary proteins. Eur J Oral Sci 107:384-392. [DOI] [PubMed] [Google Scholar]
- Milleding P, Wennerberg A, Alaeddin S, Karlsson S, Simon E. (1999b). Surface corrosion of dental ceramics in vitro. Biomaterials 20:733-746. [DOI] [PubMed] [Google Scholar]
- Milleding P, Haraldsson C, Karlsson S. (2002). Ion leaching from dental ceramics during static in vitro corrosion testing. J Biomed Mater Res 61:541-550. [DOI] [PubMed] [Google Scholar]
- Milleding P, Karlsson S, Nyborg L. (2003). On the surface elemental composition of non-corroded and corroded dental ceramic materials in vitro. J Mater Sci Mater Med 14:557-566. [DOI] [PubMed] [Google Scholar]
- Pantano CG, Clark AE, Hench LL. (1974). Multilayer corrosion films on bioglass surfaces. J Am Ceram Soc 57:412-413. [Google Scholar]
- Pinto MM, Cesar PF, Rosa V, Yoshimura HN. (2008). Influence of pH on slow crack growth of dental porcelains. Dent Mater 24:814-823. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.