Abstract
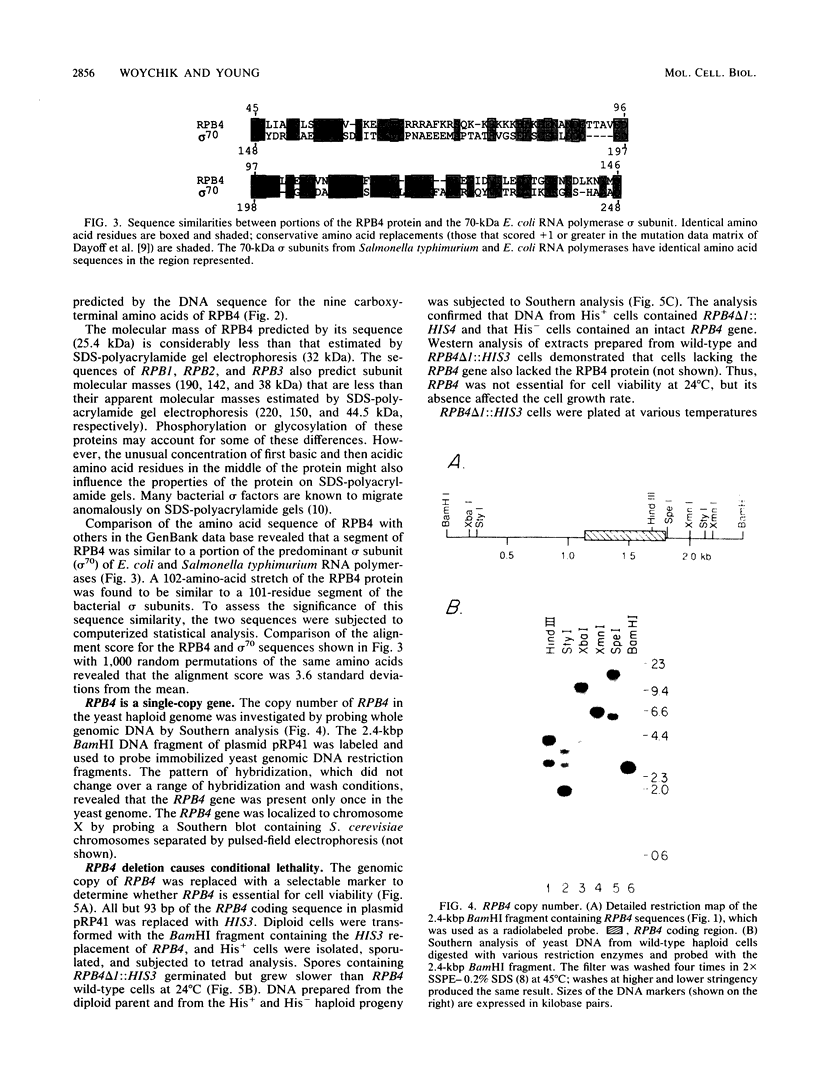
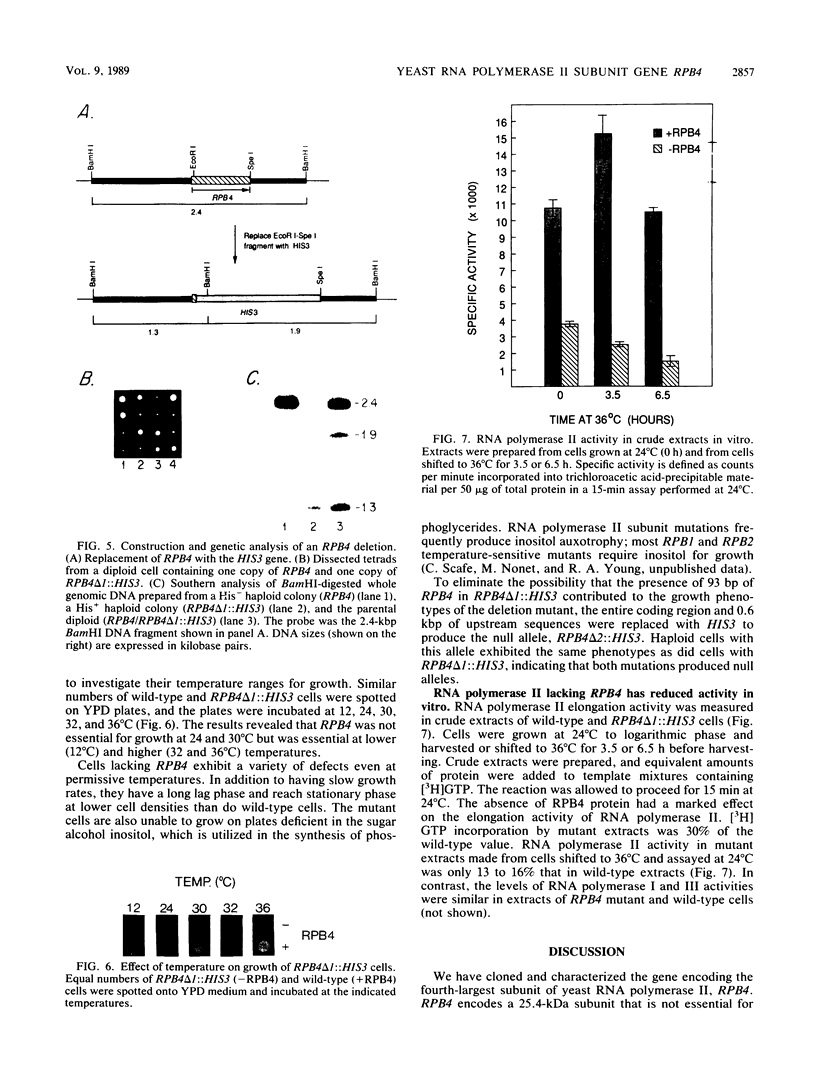
RPB4 encodes the fourth-largest RNA polymerase II subunit in Saccharomyces cerevisiae. The RPB4 gene was cloned and sequenced, and its identity was confirmed by amino acid sequence analysis of tryptic peptides from the purified subunit. The RPB4 DNA sequence predicted a protein of 221 amino acids with a molecular mass of 25,414 daltons. The central 100 amino acids of the RPB4 protein were found to be similar to a segment of the major sigma subunit in Escherichia coli RNA polymerase. Deletion of RPB4 produced cells that were heat and cold sensitive but could grow, albeit slowly, at intermediate temperatures. RNA polymerase II lacking the RPB4 subunit exhibited markedly reduced activity in crude extracts in vitro. The RPB4 subunit, although not essential for mRNA synthesis or enzyme assembly, was essential for normal levels of RNA polymerase II activity and indispensable for cell viability over a wide temperature range.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebersold R. H., Leavitt J., Saavedra R. A., Hood L. E., Kent S. B. Internal amino acid sequence analysis of proteins separated by one- or two-dimensional gel electrophoresis after in situ protease digestion on nitrocellulose. Proc Natl Acad Sci U S A. 1987 Oct;84(20):6970–6974. doi: 10.1073/pnas.84.20.6970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison L. A., Moyle M., Shales M., Ingles C. J. Extensive homology among the largest subunits of eukaryotic and prokaryotic RNA polymerases. Cell. 1985 Sep;42(2):599–610. doi: 10.1016/0092-8674(85)90117-5. [DOI] [PubMed] [Google Scholar]
- Buhler J. M., Huet J., Davies K. E., Sentenac A., Fromageot P. Immunological studies of yeast nuclear RNA polymerases at the subunit level. J Biol Chem. 1980 Oct 25;255(20):9949–9954. [PubMed] [Google Scholar]
- Carroll S. B., Stollar B. D. Conservation of a DNA-binding site in the largest subunit of eukaryotic RNA polymerase II. J Mol Biol. 1983 Nov 5;170(3):777–790. doi: 10.1016/s0022-2836(83)80131-4. [DOI] [PubMed] [Google Scholar]
- Cho J. M., Kimball A. P. Probes of eukaryotic DNA-dependent RNA polymerase II-I. Binding of 9-beta-D-arabinofuranosyl-6-mercaptopurine to the elongation subsite. Biochem Pharmacol. 1982 Aug 15;31(16):2575–2581. doi: 10.1016/0006-2952(82)90703-1. [DOI] [PubMed] [Google Scholar]
- Dayhoff M. O., Barker W. C., Hunt L. T. Establishing homologies in protein sequences. Methods Enzymol. 1983;91:524–545. doi: 10.1016/s0076-6879(83)91049-2. [DOI] [PubMed] [Google Scholar]
- Helmann J. D., Chamberlin M. J. Structure and function of bacterial sigma factors. Annu Rev Biochem. 1988;57:839–872. doi: 10.1146/annurev.bi.57.070188.004203. [DOI] [PubMed] [Google Scholar]
- Ishihama A., Shimamoto N., Aiba H., Kawakami K., Nashimoto H., Tsugawa A., Uchida H. Temperature-sensitive mutations in the alpha subunit gene of Escherichia coli RNA polymerase. J Mol Biol. 1980 Feb 25;137(2):137–150. doi: 10.1016/0022-2836(80)90321-6. [DOI] [PubMed] [Google Scholar]
- Kawakami K., Ishihama A. Defective assembly of ribonucleic acid polymerase subunits in a temperature-sensitive alpha-subunit mutant of Escherichia coli. Biochemistry. 1980 Jul 22;19(15):3491–3495. doi: 10.1021/bi00556a013. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Nonet M., Scafe C., Sexton J., Young R. Eucaryotic RNA polymerase conditional mutant that rapidly ceases mRNA synthesis. Mol Cell Biol. 1987 May;7(5):1602–1611. doi: 10.1128/mcb.7.5.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nonet M., Sweetser D., Young R. A. Functional redundancy and structural polymorphism in the large subunit of RNA polymerase II. Cell. 1987 Sep 11;50(6):909–915. doi: 10.1016/0092-8674(87)90517-4. [DOI] [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein R. J. One-step gene disruption in yeast. Methods Enzymol. 1983;101:202–211. doi: 10.1016/0076-6879(83)01015-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sentenac A. Eukaryotic RNA polymerases. CRC Crit Rev Biochem. 1985;18(1):31–90. doi: 10.3109/10409238509082539. [DOI] [PubMed] [Google Scholar]
- Sweetser D., Nonet M., Young R. A. Prokaryotic and eukaryotic RNA polymerases have homologous core subunits. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1192–1196. doi: 10.1073/pnas.84.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valenzuela P., Bell G. I., Weinberg F., Rutter W. J. Isolation and assay of eukaryotic DNA-dependent RNA polymerases. Methods Cell Biol. 1978;19:1–26. doi: 10.1016/s0091-679x(08)60006-0. [DOI] [PubMed] [Google Scholar]
- Vilamitjana J., Baltz T., Baltz D., Barreau C. Probing eukaryotic RNA polymerases B with monoclonal antibodies. FEBS Lett. 1983 Jul 25;158(2):343–348. doi: 10.1016/0014-5793(83)80610-3. [DOI] [PubMed] [Google Scholar]
- Vilamitjana J., Barreau C. A monoclonal antibody directed against a small subunit of RNA polymerase B blocks the initiation step. Eur J Biochem. 1987 Jan 15;162(2):317–323. doi: 10.1111/j.1432-1033.1987.tb10603.x. [DOI] [PubMed] [Google Scholar]
- Young R. A., Bloom B. R., Grosskinsky C. M., Ivanyi J., Thomas D., Davis R. W. Dissection of Mycobacterium tuberculosis antigens using recombinant DNA. Proc Natl Acad Sci U S A. 1985 May;82(9):2583–2587. doi: 10.1073/pnas.82.9.2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Efficient isolation of genes by using antibody probes. Proc Natl Acad Sci U S A. 1983 Mar;80(5):1194–1198. doi: 10.1073/pnas.80.5.1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Yura T., Ishihama A. Genetics of bacterial RNA polymerases. Annu Rev Genet. 1979;13:59–97. doi: 10.1146/annurev.ge.13.120179.000423. [DOI] [PubMed] [Google Scholar]