Abstract
African Americans have increased hemodynamic responses to both physiologic and pharmacologic adrenergic stimulation compared to Caucasians, and this may contribute to the greater prevalence of hypertension in this ethnic group. A small study suggested enhanced α1-adrenoreceptor-mediated arterial vasoconstriction in the forearm vasculature of African Americans compared to Caucasians, but it is unknown whether this reflects a generalized vascular phenomenon. The objective of this study was to examine the hypothesis that there are ethnic differences in venous α1-adrenoreceptor responsiveness. Using a linear variable differential transformer, we measured local dorsal hand vein responses to increasing doses of the selective α1-adrenoreceptor agonist, phenylephrine, in 106 subjects (64 Caucasians and 42 African Americans). There was wide interindividual variability in responses to phenylephrine. The dose that produced 50% of maximal constriction (ED50) ranged from 11 to 5442 ng/min, and maximal venoconstriction (Emax) ranged from 13.5% to 100%. African Americans (geometric mean ED50=172 ng/min; 95% CI, 115 to 256 ng/min) were more sensitive to phenylephrine than Caucasians (310 ng/min; 95% CI, 222 to 434 ng/min; unadjusted P=0.026; adjusted P=0.003). Median Emax was slightly higher in African Americans (89%; IQR, 82% to 98%) compared to Caucasians (85%; IQR, 75% to 95%; P=0.07). Taken together with previous findings in arterial vessels, our results suggest a generalized increased sensitivity to α1-adrenoreceptor-mediated vasoconstriction in African Americans. Increased vascular α-adrenoreceptor sensitivity could predispose to hypertension, and future studies addressing this mechanism’s contribution to ethnic differences in the prevalence of hypertension will be of interest.
Keywords: Alpha-1 adrenergic receptor, vasoconstriction, phenylephrine, ethnicity, hypertension
Introduction
The sympathetic nervous system is an important regulator of blood pressure and vascular resistance. Its effects are mediated through adrenergic receptors (AR). Vascular α1-and α2-ARs contribute to blood pressure regulation by maintaining vascular tone through vascular smooth muscle contraction, both at rest and in response to various intrinsic and extrinsic stimuli.1, 2 Vascular α1-ARs are expressed in arterial resistance and venous capacitance vessels, where they act as prime mediators for smooth muscle contraction.3, 4
The marked prevalence of hypertension in African Americans has led to studies to better understand ethnic differences in the regulation of blood pressure.5, 6 A consistent finding has been increased cardiovascular responses to both physiologic and pharmacologic sympathetic stimulation in persons of African descent.7, 8 The mechanisms underlying increased blood pressure responses to stress in African Americans are unclear. Earlier studies found increased sympathetic activation, measured as muscle sympathetic nervous system activity, during the cold pressor test in blacks compared to whites, but this was restricted to normotensive subjectswith a family history of hypertension.7, 9 In a previous study, sympathetic activation at rest and in response to stress (cold pressor test), assessed as norepinephrine release, was similar in African Americans and Caucasians.10 Thus, increased vascular sensitivity to endogenous catecholamines in African Americans has been postulated.10, 11
Since vascular α1-ARs are the principal mediators of vasoconstriction, studies of ethnic differences in vascular sensitivity have focused on responses to α1-AR agonists. After the systemic administration of phenylephrine, a selective α1-AR-agonist, the increase in blood pressure was greater in African Americans than Caucasians, but this could be confounded by systemic reflex responses.12, 13 After local infusion of phenylephrine into the brachial artery, vasoconstrictor responses were greater in 10 African Americans than 10 Caucasians.10 However, it is unknown whether these preliminary findings are limited to a specific vascular bed or reflect a generalized ethnic difference in vascular α1-AR mediated responsiveness.
We therefore studied responses to phenylephrine using the dorsal hand vein model in a large cohort of healthy Caucasian and African American subjects. The dorsal hand vein model is an established model for the study of agonist-specific local venous responses that elicits only minimal systemic cardiovascular changes and resulting counter-regulatory responses.14 Our objective was to determine whether the ethnic difference in α1-AR mediated arterial sensitivity is also present in veins.
METHODS
Subjects
The Institutional Review Board of Vanderbilt University Medical Center approved the study protocol. Subjects were recruited by advertisement within the Vanderbilt University Campus and through the Vanderbilt University Clinical Research Center study volunteer database.15 Healthy, normotensive male and non-pregnant female Caucasians and African Americans aged 18–45 years were eligible for the study. After providing written consent, eligible volunteers attended a screening visit that included a detailed medical questionnaire, physical exam, electrocardiogram (ECG), and laboratory tests. Subjects were excluded if they had any clinically significant abnormalities according to medical history, physical examination, ECG, or routine laboratory testing. Ethnicity and family history of hypertension were determined by self-report. Subjects were classified as African Americans or Caucasians if both parents and at least three grandparents were of the same ethnicity. Body mass index (BMI) was calculated as weight/height2 [kg/m2]. Subjects took no medications for at least 2 weeks, and abstained from alcohol and caffeine for at least 5 days before the study. Each participant received a diet containing 150 mmol/day of sodium, 70 mmol/day of potassium, and 600 mmol/day of calcium for at least 4 days prior to the study day. Studies were performed in the morning after an overnight fast in a temperature-controlled room at the Vanderbilt Clinical Research center.
Measurement of vascular responses
Vascular responses were measured in a dorsal hand vein with a linear variable differential transformer (LVDT) as previously described.16 This is an established model to assess the effects of vasoactive substances on a superficial vein in vivo without eliciting confounding systemic responses, yielding highly reproducible results.17 In brief, a 24-gauge intravenous cannula was inserted into a suitable right dorsal hand vein and kept patent with saline solution infused at a flow rate of 0.4 mL/min. A LVDT (MHR 100; Shaevitz Engineering, Pennsaken, NJ) was mounted on the dorsum of the subject’s hand with its movable central core centered over the vein approximately 1 cm proximal to the cannula tip. A second intravenous cannula was inserted for blood sampling into the antecubital vein of the contralateral arm. After 30 minutes of saline infusion, a blood sample was taken for the determination of baseline plasma catecholamines. Then, we determined the baseline vein diameter while a sphygmomanometer cuff around the upper arm was inflated to 50 mm Hg to induce venous filling. After 3 stable baseline measurements, we assessed vein constriction in response to increasing doses of phenylephrine. Phenylephrine (Elkins-Sinn, Cherry Hill, NJ) was infused through the cannula with a syringe infusion pump (Harvard Apparatus, Holliston, MA) at increasing dose rates (range, 12–9,600 ng/min). The infusion at each dose rate lasted 7 minutes, and the vein diameter was measured during the last two minutes of the infusion. The total flow rate through the vein was kept constant at 0.4 mL/min throughout the various phenylephrine dilutions. Subjects remained in supine position throughout the study. Heart rate and blood pressure were continuously monitored with a bedside cardiac monitor (Dinamap MPS; Johnson and Johnson Medical, Tampa, FL).
Analysis of hand vein response to phenylephrine
Venoconstriction was expressed as the percentage reduction in vein diameter from baseline maximum dilation, plotted against dose rates in individual semi-logarithm dose–response graphs, and analyzed using a sigmoid dose–response model with variable slope (GraphPad Prism 4.03, GraphPad, La Jolla, CA). The dose that produced 50% of maximal constriction (ED50, representing sensitivity to the drug) and the maximal venoconstriction (Emax, representing maximum response) were determined for each subject. Analyses were performed by a single investigator unaware of the subject’s ethnicity.
Determination of plasma catecholamine concentrations
Blood was collected into cooled heparinized tubes that were placed on ice until centrifuged at 4°C for 10 minutes at 3000 rpm. Plasma was separated and stored at −20°C in tubes containing 40μL of reduced glutathione (6%) until assayed. Norepinephrine and epinephrine concentrations were measured by high-performance liquid chromatography using electrochemical detection with dihydroxybenzylamine as internal standard.18
Statistical analysis
Our cohort was a convenience sample consisting of participants in previous studies,19 and the sample size was not predetermined by sample size calculations. ED50 values were not normally distributed and were therefore log-transformed for analysis and expressed as geometric means with 95% confidence intervals (CIs). Other data are expressed as median and interquartile range (IQR) or mean and standard deviation (SD) as appropriate. Variables were compared between the two ethnic groups using an independent t-test, χ2 test, or Mann-Whitney U test, as appropriate. We compared sensitivity (LogED50) to phenylephrine between African Americans and Caucasians first by independent t-test and then by multiple linear regression analysis to adjust for potential confounders (age, sex, BMI, family history of hypertension, baseline systolic and diastolic blood pressure, baseline heart rate, resting norepinephrine and epinephrine concentrations). The measure of efficacy (Emax) was not normally distributed, and we therefore compared Emax between African Americans and Caucasians using non-parametric analysis (Mann-Whitney U test). All analyses were two-tailed, and a P-value < 0.05 was considered significant. Statistical analyses were performed using SPSS software (v. 19, IBM® SPSS® Inc., Chicago, IL).
RESULTS
Subjects
We studied 106 subjects (64 Caucasians and 42 African Americans). Table 1 shows demographic and baseline cardiovascular parameters. Compared to Caucasians, African Americans had higher heart rate and BMI. There were no differences between the groups in resting plasma norepinephrine or epinephrine concentrations.
Table 1.
Demographic characteristics of 106 subjects
| Parameter | Caucasians n = 64 | African Americans n = 42 | P value |
|---|---|---|---|
| Age, years | 26.9 ± 6.8 | 28.1 ± 8.0 | 0.42 |
| Female sex, n (%) | 31 (48%) | 18 (43%) | 0.69 |
| Family history of Hypertension, n (%) | 25 (39%) | 20 (48%) | 0.55 |
| Systolic blood pressure, mmHg | 110.3 ± 11 | 111.1 ± 9.5 | 0.70 |
| Diastolic blood pressure, mmHg | 60.7 ± 6.8 | 63.4 ± 8.0 | 0.065 |
| Heart rate, bpm | 59.0 ± 8.2 | 63.7 ± 8.0 | 0.005 |
| Plasma norepinephrine, pg/ml | 162.3 ± 58.0 | 179.5 ± 70.8 | 0.21 |
| Plasma epinephrine, pg/ml | 18.5 ± 12.5 | 21.0 ± 15.6 | 0.39 |
| BMI, kg/m2 | 24.3 ± 3.8 | 26.8 ± 5.4 | 0.007 |
Data presented as mean ± SD.
Vascular and systemic responses
There was wide interindividual variability in response to phenylephrine, with the range of ED50 spanning three log units (11 to 5442 ng/min; geometric mean, 245 ng/min; 95% CI, 190 to 318 ng/min). The Emax ranged from 13.5% to 100%, with a median of 87% (IQR, 76% to 96%). Compared to baseline, after the highest phenylephrine dose, systolic blood pressure increased by 4.3±8.0 mmHg (P<0.001), diastolic blood pressure by 2.6±5.9 mmHg (P<0.001), and heart rate decreased by 1.4±6.3 bpm (P=0.025). For all these parameters, changes after phenylephrine were similar between races (all P>0.58).
Ethnic differences in sensitivity
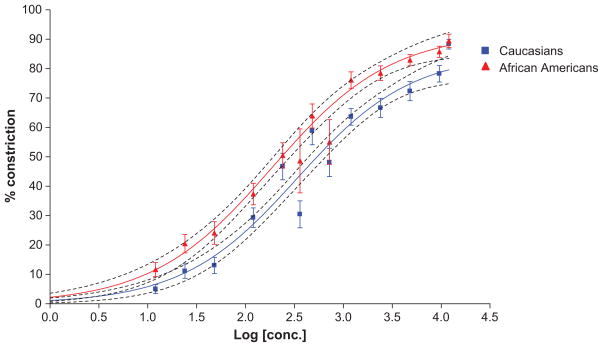
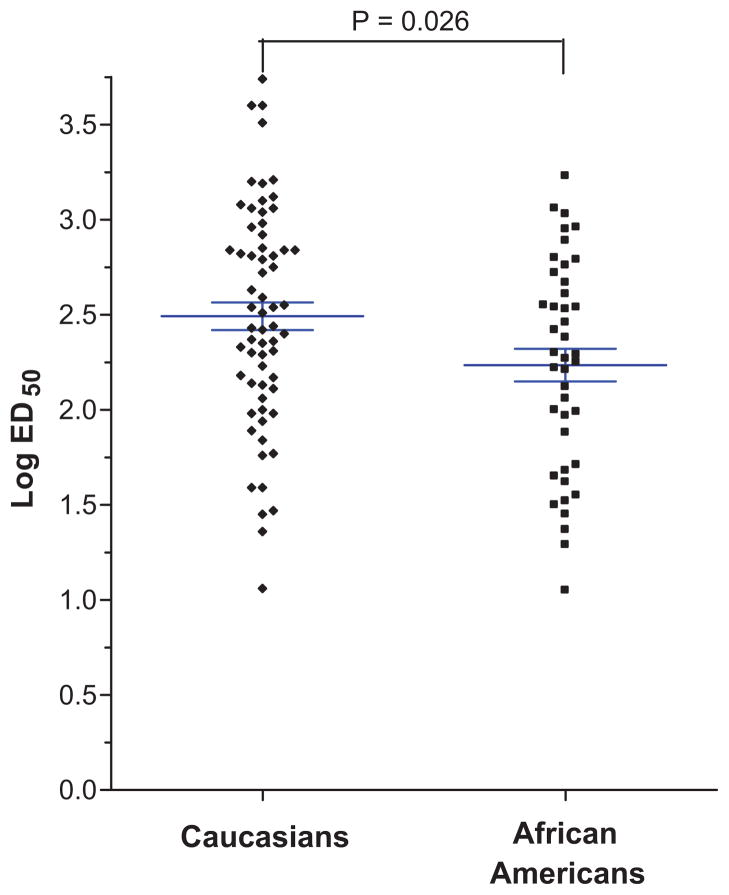
Compared to Caucasians, the dose-response for phenylephrine-induced venoconstriction showed a leftward shift in African Americans, reflecting increased sensitivity to phenylephrine-induced venoconstriction (Figure 1). Accordingly, geometric mean ED50 was 45% lower in African Americans than in Caucasians (172 ng/min and 310 ng/min in African Americans and Caucasians, respectively; P=0.026; Table 2, Figure 2). After adjusting for age, sex, BMI, family history of hypertension, baseline systolic and diastolic blood pressure, baseline heart rate, baseline norepinephrine and epinephrine values, this difference was more pronounced (P = 0.003). In this model, ethnicity accounted for 8.5% of the interindividual variation in LogED50, while none of the other covariates was significantly associated with logED50.
Figure 1.
Dose-response curves in Caucasians (blue, n=64) and African Americans (red, n=42) based on averaged hand vein responses to phenylephrine. African Americans have a leftward shift in the dose-response curve indicating increased sensitivity with a significantly lower ED50 and a trend to increased efficacy (increased Emax). The dotted lines represent the 95% CI.
Table 2.
Ethnic differences in sensitivity of phenylephrine
| Outcome | Caucasians | African Americans | P-value | |
|---|---|---|---|---|
| ED50, ng/min, mean (95% CI) | All | 310 (222 to 434) | 172 (115 to 256) | 0.026 |
| n=64 | n=42 | *0.003 | ||
| Males | 376 (245–578) | 160 (96–268) | 0.011 | |
| n=33 | n=24 | *0.001 | ||
| Females | 253 (148–432) | 188 (95–374) | 0.49 | |
| n=31 | n=18 | *0.20 |
After adjustment for age, BMI, family history of hypertension, baseline systolic and diastolic blood pressure, baseline heart rate, baseline norepinephrine and epinephrine values
Figure 2.
Vascular sensitivity to phenylephrine (expressed as Log ED50) in Caucasians and African Americans. The horizontal lines represent the mean, and the whiskers represent the standard error of the mean. African Americans were more sensitive to phenylephrine than Caucasians (unadjusted p=0.026; adjusted p=0.003).
An exploratory subgroup analysis separated by sex revealed that in both males and females, African Americans had greater sensitivity to phenylephrine (Table 2). Race differences were enhanced in males (adjusted P=0.001) but not statistically significant in females (adjusted P=0.20; Table 2).
Ethnic differences in efficacy
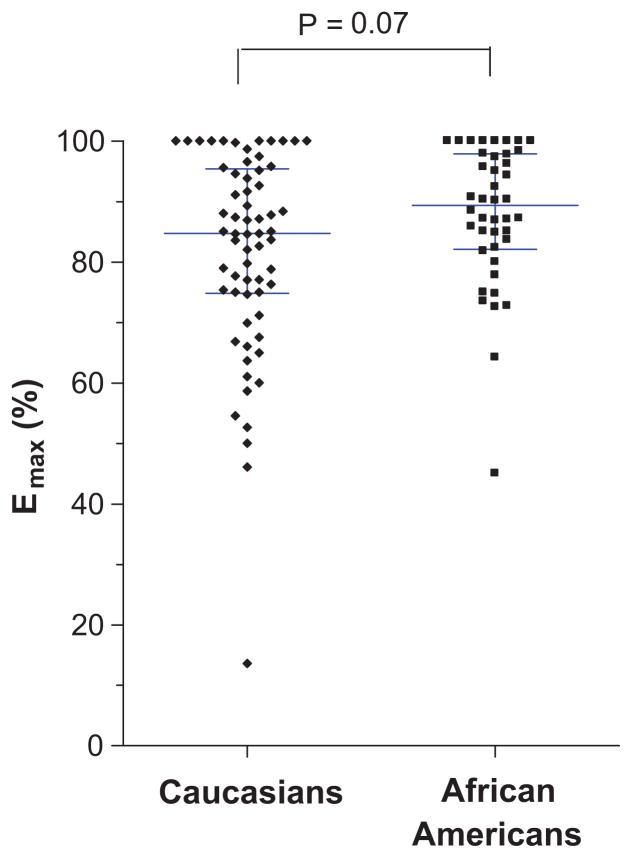
Median Emax was slightly higher in African Americans (89%) compared to Caucasians (85%) but this difference did not reach statistical significance (P = 0.07; Table 3, Figure 3). A subgroup analysis of Emax separated by gender revealed that African American males had a significantly higher median Emax (90%; IQR, 82 to 99%) compared to Caucasian males (83%; IQR, 71 to 95%; P = 0.045) but there were no significant differences in females (P = 0.65; Table 3).
Table 3.
Ethnic differences in efficacy of phenylephrine
| Outcome | Caucasians | African Americans | P-value | |
|---|---|---|---|---|
| Emax, %, median (IQR) | All | 85 (75 to 95) | 89 (82 to 98) | 0.07 |
| n=64 | n=42 | |||
| Males | 83 (71–95) | 90 (82–99) | 0.045 | |
| n=33 | n=24 | |||
| Females | 87 (75–96) | 87 (81–97) | 0.65 | |
| n=31 | n=18 |
Figure 3.
Efficacy of phenylephrine (expressed as Emax) in Caucasians and African Americans. The horizontal lines represent the median, and the whiskers represent the interquartile range.
DISCUSSION
The major finding of this study is that on average African Americans appear more sensitive to α1-AR mediated venoconstriction than Caucasians. Taken together with our earlier study in an arterial vascular bed, these findings suggest that African Americans may have a generally enhanced sensitivity to α1-AR mediated vasoconstriction which is not restricted to a specific vascular bed.
A number of studies have examined ethnic differences in vascular sensitivity to α1-AR agonists. African Americans had increased blood pressure response to systemic infusion of phenylephrine.12, 13 However, this experimental setting may not reflect vascular sensitivity to α1-agonists, since the systemic administration of phenylephrine elicits a large pressor response and, inherently, strong counter-regulatory neuro-humoral responses that may confound the findings. In a preliminary previous study, we therefore administered phenylephrine directly into the brachial artery to study local α1-AR mediated arterial constriction and its effects on forearm blood flow without attending changes in systemic blood pressure. Using this model, forearm blood flow decreased to a significantly greater extent in ten healthy African Americans compared to ten Caucasians.10 Little is known about ethnic differences in α1-AR mediated vasoconstriction in venous vessels.
Using the dorsal hand vein model, Dachman et al. found lower maximal venoconstriction (Emax) in response to phenylephrine among ten Mexican Americans compared to ten white Americans but no significant difference in sensitivity (ED50).20 In a study among 24 white and black Americans, Eichler et al. found a lower Emax in African Americans compared to Caucasians and a lower ED50 (245 and 342 ng/min for African Americans and Caucasians, respectively), although the latter was not statistically significant (P=0.50).21 In contrast, in this present study, using a much larger cohort we found a similar, but statistically significant racial difference in ED50, and additionally a trend to increased Emax in African Americans compared to Caucasians. In addition to sample size, other differences in study design, e.g. in maximal phenylephrine dose rates and dietary salt restrictions, may have contributed to the discordant findings on Emax.
The dorsal hand vein model is a commonly used and reproducible experimental model for the study of vascular responses to vasoactive agents in vivo, allowing direct infusion of the study drug into a superficial vein while eliciting only minimal counter-regulatory reflex effects.16, 17, 22 Although less invasive than experimental models requiring arterial cannulation, it is not clear to what extent findings derived from a superficial vein can be extrapolated to other vascular beds, in particular arteries, since receptor expression and regulation of vascular tone may differ substantially among different vascular beds. For instance, there was no correlation between the sensitivity in arterial and venous vascular beds for β2-AR mediated vasodilation in response to isoproterenol.23 In contrast, for α1-AR mediated vasoconstriction, there was a significant correlation between the phenylephrine responses in the dorsal hand vein and blood pressure responses following systemic infusion.24 Thus, for the study of α1-AR mediated responses, dorsal hand vein responses appear to reflect findings in arterial vascular beds. This conclusion is supported by our current findings of greater α1-mediated hand vein sensitivity in African American subjects, extending our previous preliminary findings in the arterial bed to venous vessels.
In addition to α1-ARs, vasoconstriction is also mediated by vascular α2-ARs. Interestingly, we previously found no ethnic differences for α2-AR mediated vascular responses.14, 25 African Americans and Caucasians had comparable hand vein constriction in response to the selective α2-AR agonist, dexmedetomidine, in the hand vein model,14 and also comparable blood pressure response after systemic administration.25 Thus, ethnic differences in α1-mediated vasoconstriction are specific to the α1-AR signaling pathway, and not a reflection of the ethnic differences that exist in vasodilating mechanisms (e.g. reduced β2-mediated vasodilation in African Americans compared to Caucasians) 10, 26, 27 or in intracellular signal transduction and vasoconstrictor mechanisms shared by α1- and α2-AR pathways. This conclusion is also supported by the lack of correlation between vascular sensitivities to α1- and α2-mediated venoconstriction, respectively. 19, 28
In an exploratory subgroup analysis, we found more pronounced ethnic differences in sensitivity to phenylephrine and its efficacy in male subjects, but in female subjects this difference did not reach statistical significance. A number of reasons may account for this finding: In women, vascular reactivity may be affected by hormonal changes during the menstrual cycle, and there is some evidence that such hormonal effects may affect white and black women differently.29 In our study, we did not account for the participant’s menstrual cycle or use of hormonal contraceptives. Thus, hormonal effects on venous reactivity may have confounded our findings in the female subgroup. In addition, the number of women in our cohort, in particular among African Americans, was rather small, reducing our chances to find racial differences.
Taken together with our previous findings of racial differences in arterial vasoconstriction in response to phenylephrine,10 the current study suggests a generally increased sensitivity in African Americans to α1-AR mediated vasoconstriction in various vascular beds. Such a globally enhanced sensitivity may have important clinical implications. α1-ARs are prime mediators of vasoconstriction in the human vasculature3, 4 and thus affect arterial and venous vascular tone, peripheral resistance and blood pressure, and cardiac filling.1 Thus, increased α1-AR mediated sensitivity may contribute to the enhanced cardiovascular responses to physical and mental stress observed in black subjects compared to Caucasians,7, 8, 30, 31 as well as to the increased prevalence of hypertension.5, 6
Our study had a number of limitations. Human veins express the three α1-AR subtypes, α 1A, α1B, and α1D.3, 32 Phenylephrine is selective for α1-ARs but not for a particular subtype, so is not clear whether the ethnic difference in sensitivity is mediated by a specific receptor subtype, all subtypes, or a combination. However, subtype-selective α1-AR agonists are currently not available for use in humans. Secondly, our findings are derived from dorsal hand veins and cannot automatically be extrapolated to other vascular beds. However, similar findings in the brachial artery suggest that the enhanced sensitivity to α1-AR mediated vasoconstriction in African Americans is not limited to a particular vascular bed but represents a more widespread phenomenon. Studies in other arterial and venous vessels would be of interest but are more difficult to perform in view of their invasive nature. Thirdly, we did not study the female participants at predefined periods of their menstrual cycle or account for use of hormonal contraceptives. Some but not all studies showed that hormonal changes during the menstrual cycle affect α1-AR-mediated vascular reactivity.29, 33 As discussed earlier, confounding by differences with respect to the menstrual cycle may have contributed to the lack of ethnic differences in phenylephrine responses in female subjects. Finally, our study does not shed light on the mechanisms of enhanced α1-AR mediated vascular sensitivity in African Americans. Future studies focused on genetic and environmental factors associated with altered sensitivity will be informative.
Perspectives.
Using the dorsal hand vein model, we found an increased venoconstrictive response to the α1-AR agonist phenylephrine in African Americans compared to Caucasians. Taken together with previous findings in arterial vessels, these findings suggest an increased sensitivity to α1-AR mediated vasoconstriction in different vascular beds in African American subjects, potentially contributing to the higher incidence of hypertension in this group. Future studies addressing the mechanisms for these ethnic differences, including genetic variation in components of the α1-AR mediated vasoconstriction pathway, will be of interest.
NOVELTY AND SIGNIFICANCE.
What is new?
Compared to Caucasians, African Americans are more sensitive to the venoconstricting effect of the α1-adrenoreceptor agonist phenylephrine in the dorsal hand-vein model.
Taken together with previous preliminary findings in arterial vessels, our findings suggest an increased sensitivity to α1-adrenoreceptor- mediated vasoconstriction in various vascular beds in African Americans compared to Caucasians.
What is relevant?
Compared to Caucasians, African Americans have a greater blood pressure response to mental and physical stress which is not fully explained by differences in sympathetic activation. Our findings of enhanced sensitivity to α1-adrenoreceptor- mediated vasoconstriction could provide an explanation for this phenomenon.
Moreover, increased vascular sensitivity to α1-adrenoreceptor- mediated vasoconstriction could potentially contribute to the increased prevalence of hypertension in African Americans.
Summary
We measured venoconstriction in response to increasing doses of the α1-adrenoreceptor agonist phenylephrine in the dorsal hand vein model in 106 Caucasians and African Americans. African Americans had a higher sensitivity to phenylephrine-induced venoconstriction compared to Caucasians. These findings may contribute to the greater blood pressure response after physical and mental stresses and to the greater prevalence of hypertension in African Americans.
Acknowledgments
Sources of Funding: This study was supported by Vanderbilt CTSA grant 1 UL1 TR000445 from the National Center for Research Resources and P01 HL56693 from the National Institutes of Health. Dr. Stein is the recipient of the Dan May Chair in Medicine.
Footnotes
Disclosures: None
References
- 1.Docherty JR. Subtypes of functional alpha1-adrenoceptor. Cell Mol Life Sci. 2010;67:405–417. doi: 10.1007/s00018-009-0174-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tanoue A, Koshimizu TA, Tsujimoto G. Transgenic studies of alpha(1)-adrenergic receptor subtype function. Life Sci. 2002;71:2207–2215. doi: 10.1016/s0024-3205(02)02012-x. [DOI] [PubMed] [Google Scholar]
- 3.Rudner XL, Berkowitz DE, Booth JV, Funk BL, Cozart KL, D’Amico EB, El-Moalem H, Page SO, Richardson CD, Winters B, Marucci L, Schwinn DA. Subtype specific regulation of human vascular alpha(1)-adrenergic receptors by vessel bed and age. Circulation. 1999;100:2336–2343. doi: 10.1161/01.cir.100.23.2336. [DOI] [PubMed] [Google Scholar]
- 4.Leech CJ, Faber JE. Different alpha-adrenoceptor subtypes mediate constriction of arterioles and venules. Am J Physiol. 1996;270:H710–H722. doi: 10.1152/ajpheart.1996.270.2.H710. [DOI] [PubMed] [Google Scholar]
- 5.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carson AP, Howard G, Burke GL, Shea S, Levitan EB, Muntner P. Ethnic differences in hypertension incidence among middle-aged and older adults: the multi-ethnic study of atherosclerosis. Hypertension. 2011;57:1101–1107. doi: 10.1161/HYPERTENSIONAHA.110.168005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun DA, Mutinga ML, Collins AS, Wyss JM, Oparil S. Normotensive blacks have heightened sympathetic response to cold pressor test. Hypertension. 1993;22:801–805. doi: 10.1161/01.hyp.22.6.801. [DOI] [PubMed] [Google Scholar]
- 8.Light KC, Obrist PA, Sherwood A, James SA, Strogatz DS. Effects of race and marginally elevated blood pressure on responses to stress. Hypertension. 1987;10:555–563. doi: 10.1161/01.hyp.10.6.555. [DOI] [PubMed] [Google Scholar]
- 9.Calhoun DA, Mutinga ML. Race, family history of hypertension, and sympathetic response to cold pressor testing. Blood Press. 1997;6:209–213. doi: 10.3109/08037059709062071. [DOI] [PubMed] [Google Scholar]
- 10.Stein CM, Lang CC, Singh I, He HB, Wood AJ. Increased vascular adrenergic vasoconstriction and decreased vasodilation in blacks. Additive mechanisms leading to enhanced vascular reactivity. Hypertension. 2000;36:945–951. doi: 10.1161/01.hyp.36.6.945. [DOI] [PubMed] [Google Scholar]
- 11.Stein CM, Lang CC, Xie HG, Wood AJ. Hypertension in black people: study of specific genotypes and phenotypes will provide a greater understanding of interindividual and interethnic variability in blood pressure regulation than studies based on race. Pharmacogenetics. 2001;11:95–110. doi: 10.1097/00008571-200103000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Sherwood A, Hinderliter AL. Responsiveness to alpha- and beta-adrenergic receptor agonists. Effects of race in borderline hypertensive compared to normotensive men. Am J Hypertens. 1993;6:630–635. doi: 10.1093/ajh/6.7.630. [DOI] [PubMed] [Google Scholar]
- 13.Thomas KS, Nelesen RA, Malcarne VL, Ziegler MG, Dimsdale JE. Ethnicity, perceived discrimination, and vascular reactivity to phenylephrine. Psychosom Med. 2006;68:692–697. doi: 10.1097/01.psy.0000238214.80871.e6. [DOI] [PubMed] [Google Scholar]
- 14.Muszkat M, Sofowora GG, Wood AJ, Stein CM. Alpha2-adrenergic receptor-induced vascular constriction in blacks and whites. Hypertension. 2004;43:31–35. doi: 10.1161/01.HYP.0000103694.30164.C7. [DOI] [PubMed] [Google Scholar]
- 15.Harris PA, Lane L, Biaggioni I. Clinical research subject recruitment: the Volunteer for Vanderbilt Research Program. J Am Med Inform Assoc. 2005;12:608–613. doi: 10.1197/jamia.M1722. www.volunteer.mc.vanderbilt.edu. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sofowora GG, Dishy V, Landau R, Xie HG, Prasad HC, Byrne DW, Smiley RM, Kim RB, Wood AJ, Stein CM. Alpha 1A-adrenergic receptor polymorphism and vascular response. Clin Pharmacol Ther. 2004;75:539–545. doi: 10.1016/j.clpt.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Schindler C, Grossmann M, Dobrev D, Francke K, Ravens U, Kirch W. Reproducibility of dorsal hand vein responses to phenylephrine and prostaglandin F2 alpha using the dorsal hand vein compliance method. J Clin Pharmacol. 2003;43:228–236. doi: 10.1177/0091270002251004. [DOI] [PubMed] [Google Scholar]
- 18.He HB, Deegan RJ, Wood M, Wood AJ. Optimization of high-performance liquid chromatographic assay for catecholamines. Determination of optimal mobile phase composition and elimination of species-dependent differences in extraction recovery of 3,4-dihydroxybenzylamine. J Chromatogr. 1992;574:213–218. [PubMed] [Google Scholar]
- 19.Muszkat M, Kurnik D, Sofowora GG, Wood AJ, Stein CM. Independent regulation of alpha1 and alpha2 adrenergic receptor-mediated vasoconstriction in vivo. J Hypertens. 2011;29:251–256. doi: 10.1097/HJH.0b013e3283407ffd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dachman WD, Adubofour KO, Kapoor C, Rackleff D. Variability in vascular responsiveness between Mexican-Americans and White Americans. J Hum Hypertens. 1998;12:167–171. doi: 10.1038/sj.jhh.1000577. [DOI] [PubMed] [Google Scholar]
- 21.Eichler HG, Blaschke TF, Hoffman BB. Decreased responsiveness of superficial hand veins to phenylephrine in black normotensive males. J Cardiovasc Pharmacol. 1990;16:177–181. doi: 10.1097/00005344-199008000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Alradi AO, Carruthers SG. Evaluation and application of the linear variable differential transformer technique for the assessment of human dorsal hand vein alpha-receptor activity. Clin Pharmacol Ther. 1985;38:495–502. doi: 10.1038/clpt.1985.214. [DOI] [PubMed] [Google Scholar]
- 23.Stein CM, Deegan R, Wood AJ. Lack of correlation between arterial and venous beta-adrenergic receptor sensitivity. Hypertension. 1997;29:1273–1277. doi: 10.1161/01.hyp.29.6.1273. [DOI] [PubMed] [Google Scholar]
- 24.Vincent J, Blaschke TF, Hoffman BB. Vascular reactivity to phenylephrine and angiotensin II: comparison of direct venous and systemic vascular responses. Clin Pharmacol Ther. 1992;51:68–75. doi: 10.1038/clpt.1992.9. [DOI] [PubMed] [Google Scholar]
- 25.Kurnik D, Muszkat M, Sofowora GG, Friedman EA, Dupont WD, Scheinin M, Wood AJ, Stein CM. Ethnic and genetic determinants of cardiovascular response to the selective alpha 2-adrenoceptor agonist dexmedetomidine. Hypertension. 2008;51:406–411. doi: 10.1161/HYPERTENSIONAHA.107.098939. [DOI] [PubMed] [Google Scholar]
- 26.Cardillo C, Kilcoyne CM, Cannon RO, III, Panza JA. Attenuation of cyclic nucleotide-mediated smooth muscle relaxation in blacks as a cause of racial differences in vasodilator function. Circulation. 1999;99:90–95. doi: 10.1161/01.cir.99.1.90. [DOI] [PubMed] [Google Scholar]
- 27.Lang CC, Stein CM, Brown RM, Deegan R, Nelson R, He HB, Wood M, Wood AJ. Attenuation of isoproterenol-mediated vasodilatation in blacks. N Engl J Med. 1995;333:155–160. doi: 10.1056/NEJM199507203330304. [DOI] [PubMed] [Google Scholar]
- 28.Posti JP, Valve L, Ruohonen S, Akkila J, Scheinin M, Snapir A. Dorsal hand vein responses to the alpha-adrenoceptor agonist phenylephrine do not predict responses to the alpha-adrenoceptor agonist dexmedetomidine. Eur J Pharmacol. 2011;653(1–3):70–74. doi: 10.1016/j.ejphar.2010.11.024. [DOI] [PubMed] [Google Scholar]
- 29.Freedman RR, Girgis R. Effects of menstrual cycle and race on peripheral vascular alpha-adrenergic responsiveness. Hypertension. 2000;35:795–799. doi: 10.1161/01.hyp.35.3.795. [DOI] [PubMed] [Google Scholar]
- 30.Ekelund LG, Suchindran CM, Karon JM, McMahon RP, Tyroler HA. Black-white differences in exercise blood pressure. The Lipid Research Clinics Program Prevalence Study. Circulation. 1990;81:1568–1574. doi: 10.1161/01.cir.81.5.1568. [DOI] [PubMed] [Google Scholar]
- 31.Duey WJ, Bassett DR, Jr, Walker AJ, Torok DJ, Howley ET, Ely D, Pease MO. Cardiovascular and plasma catecholamine response to static exercise in normotensive blacks and whites. Ethn Health. 1997;2:127–136. doi: 10.1080/13557858.1997.9961821. [DOI] [PubMed] [Google Scholar]
- 32.Yan M, Sun J, Bird PI, Liu DL, Grigg M, Lim YL. Alpha1A- and alpha1B-adrenoceptors are the major subtypes in human saphenous vein. Life Sci. 2001;68:1191–1198. doi: 10.1016/s0024-3205(00)01027-4. [DOI] [PubMed] [Google Scholar]
- 33.Limberg JK, Eldridge MW, Proctor LT, Sebranek JJ, Schrage WG. Alpha-adrenergic control of blood flow during exercise: effect of sex and menstrual phase. J Appl Physiol. 2010;109:1360–1368. doi: 10.1152/japplphysiol.00518.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]