Abstract
An efficient, regioselective and convergent method for the ring expansion and rearrangement of 1-sulfonyl-1,2,3-triazoles under rhodium(II)-catalyzed conditions is described. These denitrogenative reactions form substituted enaminone and olefin-based products, which in the former case can be further functionalized to unique products rendering the sulfonyl triazole traceless.
Keywords: rhodium(II) catalysis, click chemistry, ring expansions, rearrangement reactions, heterocyclic synthesis
The ring expansion and rearrangement of diazo compounds, specifically rhodium carbenes (derived from the corresponding diazo species), is an efficient and operationally simple method for the construction of structurally unique frameworks.[1] Products derived from these reactions normally consist of large carbocyclic rings (7, 8 and 9 membered) and multiply substituted olefins, which are ubiquitous in natural products and drug molecules.[2] Indeed, these robust methods have found widespread use in organic synthesis, both for the synthesis of complex natural products and in pharmaceutical research.[3] Bolstered by our recent success utilizing readily available and stable 1-sulfonyl-1,2,3-triazoles 1 as direct precursors to rhodium(II) azavinyl carbenes 2 (Scheme 1),[4,5] we hypothesized that these diazo progenitors could be effective in these ring expansion/rearrangement reactions, delivering unique products that are not accessible via conventional diazo compounds. Herein we report the rhodium(II)-catalyzed ring expansion and rearrangement reactions of azavinyl carbenes (2), to access various enaminones and substituted olefins, which, in the case of the expanded enaminones can be further reacted to form a variety of heterocycles and ketone-based products.[6–7]
Scheme 1.
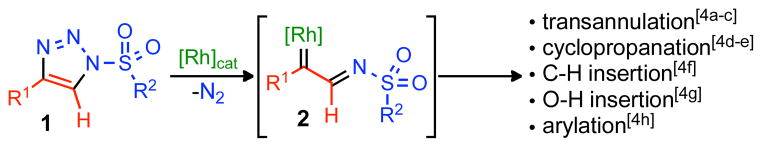
Utility of Rhodium(II) Azavinyl Carbenes.
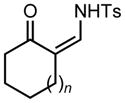
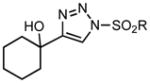
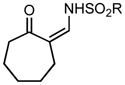
We began our investigations by the synthesis of various 1-sulfonyl triazoles bearing cyclic, tertiary alcohols at the 4-position (Table 1, entry 1 – 10) from the corresponding sulfonyl azide and commercial or easily synthesized propargylic alcohols.[8] These unique triazoles species (3) were submitted to a rhodium-catalyzed denitrogenative ring expansion reaction to form the homologated product. To this end, we reacted triazole 3a with 1 mol% rhodium(II) octanoate dimer in chloroform. When the reaction was heated to a minimum of 70 °C, a rapid evolution of gas occurred and reaction proceeded to completion within 15 min. The ring-expanded product 5a was formed in 91% yield (Table 1, entry 1). Upon isolation and characterization a Z-substituted enaminone (5) was exclusively formed due to a facile but selective tautomerization of the acidic alpha-proton (the tautomeric keto-imine 4 was not observed). Of note, no reaction was observed without the addition of a rhodium(II) catalyst, even under forcing conditions (>100 °C).
Table 1.
Ring expansion of various 1-sulfonyl 4-tertiary alcohol triazoles 3 yielding products 5. General reaction conditions: 1-sulfonyl triazole (1.0 mmol, 1.0 equiv.), rhodium(II) octanoate (0.5 mol%), CHCl3 (2.0 mL), 70 °C, mw, 5 – 60 min. All values shown represent isolated yields.
| Entry | Triazole | [°C/min] | Product | Yield |
|---|---|---|---|---|
|
|
|||
| 1 | 3a: n = 1 | 70/15 | 5a: n = 1 | 91% |
| 2 | 3b: n = 2 | 70/15 | 5b: n = 2 | 93% |
| 3 | 3c: n = 3 | 70/15 | 5c: n = 3 | 94% |
|
|
|||
| 4 | 3d: R = p-OMePh | 70/60 | 5d: R = p-OMePh | 95% |
| 5 | 3e: R = p-IPh | 70/5 | 5e: R = p-IPh | 71% |
| 6 | 3f: R = iPr | 70/20 | 5f: R = iPr | 66% |
| 7 |
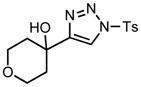 3g |
70/30 |
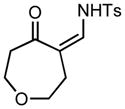 5g |
92% |
| 8 |
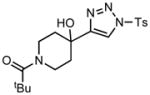 3h |
70/45 |
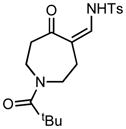 5h |
98% |
| 9 |
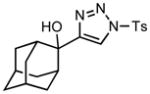 3i |
70/60 |
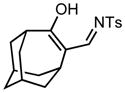 5i |
89% |
| 10 |
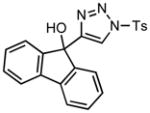 3j |
70/30 |
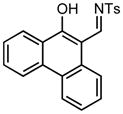 5j |
95% |
Applying these conditions to different sulfonyl triazoles yielded similarly satisfying results (Table 1, yields 66% – 98%). Various cyclic substituents were smoothly and rapidly (15 min) converted to the expanded enaminone products, yielding 6, 7 and 8-membered rings (5a – 5c, 91% – 94%). Triazoles bearing electron withdrawing, donating, and aliphatic sulfonyl groups easily underwent this homologation process to obtain the enaminone products (5d – 5f, 66% – 95%) in good to excellent yields. Furthermore, heteroatoms within the ring structure did not affect the efficiency of this reaction; both ether 3g and amide 3h yielded the expected products in excellent yields (5g and 5h, 92% – 98%). Interestingly, the strained adamantane 3i and fluorene 3j triazoles underwent this homologation reaction effectively (89% and 95% respectively) but instead of the expected enaminone moiety, they delivered peculiar enone/imine products 5i and 5j respectively. It should be noted that due to the stability of the 1-sulfonyl triazoles in Table 1 and the robust nature of the process, no special precautions were taken regarding the exclusion of air and moisture. Performing these reactions in a microwave reactor allows for the reaction progress to be monitored by the expulsion of dinitrogen; conventional heating is also effective for this process.
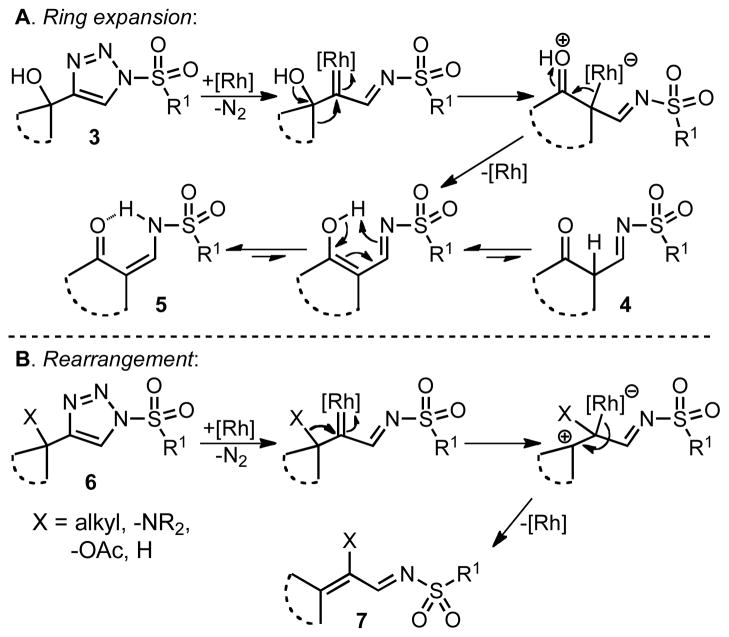
Having shown that 1-sulfonyl triazoles bearing a tertiary alcohol at the 4-position (e.g. 3) can readily undergo ring expansion reactions, we sought to explore the use of azavinyl carbenes in rhodium-catalyzed rearrangements.[1g–n] We believed that 1-sulfonyl triazoles bearing a quaternary center at C-4 (e.g. 6) would undergo rearrangement to the corresponding substituted olefin (7) upon formation of the rhodium(II) azavinyl carbene. To investigate this hypothesis, we synthesized 1-sulfonyl triazole 6a bearing the requisite tert-butyl group at C-4 and submitted it to the general reaction conditions. Gratifyingly, upon heating to 70 °C in the presence of 1 mol% rhodium(II) octanoate, the rapid evolution of gas occurred (20 min) and 6a was smoothly converted to the expected tetra-substituted olefin 7a in 91% yield (Table 2, entry 1).
Table 2.
Rearrangements of 1-sulfonyl triazoles 6 to give substituted alkenes 7. General reaction conditions: 1-sulfonyl triazole (1.0 mmol, 1.0 equiv.), rhodium(II) octanoate (0.5 mol%), CHCl3 (2.0 mL), 70 °C, mw, 20 – 80 min. All values shown represent isolated yields.
| Entry | Triazole | [°C/min] | Product | Yield |
|---|---|---|---|---|
| 1 |
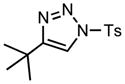 6a |
70/20 |
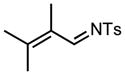 7a |
91% |
| 2 |
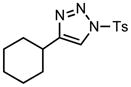 6b |
70/60 |
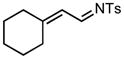 7b |
96% |
| 3 |
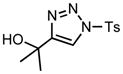 6c |
70/30 |
 7c |
92% |
| 4 |
 6d |
70/30 |
 7d |
96% |
| 5 |
 6e |
70/60 |
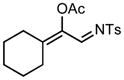 7e |
92% |
| 6 |
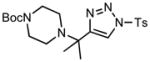 6f |
70/80 |
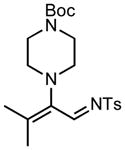 7f |
71% |
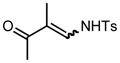
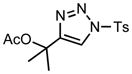
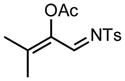
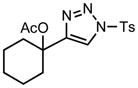
We next examined the migratory aptitude of different atoms and functional groups. When cyclohexyl triazole 6b was submitted to the reaction conditions, a facile hydride shift occurred, leading to the formation of alkene 7b in 96% yield. In contrast to triazoles 3a-e, triazole 6c bearing a tertiary non-tethered alcohol at the 4-position led to the non-regioselective migration of the alkyl group giving enaminone 7c in a 3:1 ratio of isomers (Z/E ratio). Furthermore, acetoxy triazoles 6d and 6e led to the exclusive migration of the acetoxy group, yielding the acetyl enols 7d and 7e in excellent yields (96% and 92% respectively). Finally and most interestingly, reaction of piperazine triazole 6f under our general conditions led to the selective migration of the nitrogen group giving the stable imine-enamine 7f in good yield (71%). We believe that this selective nitrogen transfer is likely to proceed via an attack of the nitrogen lone pair of the pendant piperazine onto the rhodium carbene center, forming an aziridinium ylide intermediate, which upon loss of the rhodium catalyst leads to the observed product. This rearrangement is, to the best of our knowledge, unknown, which we account to the difficulty in preparation of the diazo starting material. All of the triazoles shown in Table 2 are formed from the commercial or easily prepared alkynes and tosyl azide, allowing for the simple synthesis of active diazo progenitors, which would be inaccessible utilizing traditional diazo chemistry.
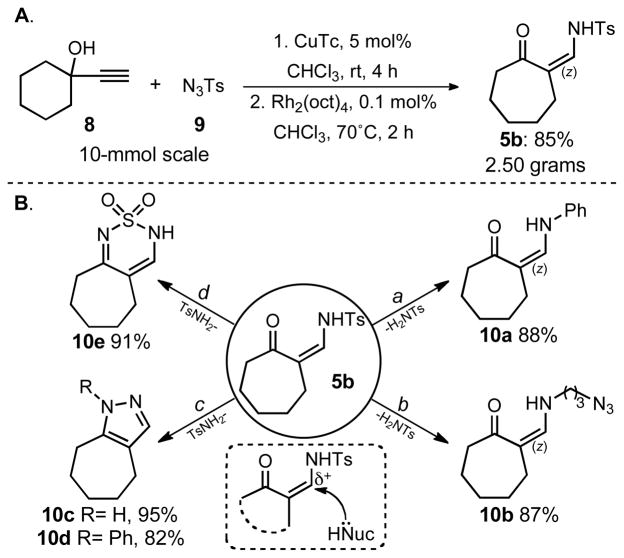
The copper(I)-catalyzed synthesis of 1-sulfonyl triazoles and their subsequent rhodium(II)-catalyzed ring expansion or rearrangement can be combined into a sequential one-pot procedure, eliminating the requirement for purification of the starting 1-sulfonyl triazole. This efficient procedure was demonstrated on a 10-mmol scale for the conversion of commercial alkyne 8 and tosyl azide 9 into the 7-membered enaminone 5b, delivering 2.5 g of material in an overall 85% yield using only 0.1 mol% of the rhodium catalyst for the ring expansion step (Scheme 3a). With this unique enaminone (5b) building block in hand, we sought to transform it into useful ketone and heterocyclic-based products. Due to enaminones being well-studied and versatile intermediates in organic synthesis,[9] we found the subsequent functionalization to be easily accomplished. Indeed, enaminone 5b can easily undergo transamination by the addition of an amine nucleophile with loss of tosyl amine, giving products 10a and 10b at room temperature in excellent yields (88% and 87% respectively). When enaminone 5b was reacted with hydrazines, pyrazoles 10c and 10d were formed as a single regioisomers, at room temperature and in high yields (95% and 82% respectively). Additionally, this transamination–cyclization procedure was further demonstrated by the reaction of enaminone 5b with sulfamide to obtain heterocycle 10e in 91% yield (Scheme 3b).
Scheme 3.
(A) One-pot large-scale synthesis of 5b. Reaction conditions: 8 (10 mmol, 1 equiv.), 9 (10 mmol, 1 equiv.), CuTc (5 mol%), CHCl3 (15 mL), rt, 4 h; then rhodium(II) octanoate (0.1 mol%), 70 °C, 2 h. (B) Functionalization of 5b. a. PhNH2 (3 equiv), MeOH, rt, 6 h; b. 3-azidopropan-1-amine (2 equiv.), MeOH, rt, 2 h; c. hydrazine-hydrate (5 equiv), MeOH, rt, 1 h; d. Phenyl hydrazine hydrochloride (1.1 equiv), MeOH, rt, 5 h; e. Sulfamide (1.1 equiv), p-TsOH (10 mol%), MeOH, 70 °C, 36 h; See supporting information for reaction details.
The facile ring expansion and rearrangement reactions of 4-substituted 1-sulfonyl-1,2,3 triazoles employing a rhodium(II) catalyst shown here are experimentally simple and useful transformations for the synthesis of enaminones and olefins in high yield and selectivity.[10] Furthermore, the exocyclic enaminone products can be readily transformed to various heterocycles and ketone derivatives.
Supplementary Material
Scheme 2.
Rhodium(II)-catalyzed ring expansion and rearrangement of azavinyl carbenes.
Acknowledgments
This work was supported by the National Science Foundation (CHE-0848982) and the National Institute of General Medicine Sciences, National Institutes of Health (GM-08762). N.S. also acknowledges a postdoctoral fellowship from the Swedish Research Council (VR). Dr. Jessica Raushel’s contributions to the initial ring-expansion studies is gratefully acknowledged.
Footnotes
Supporting information for this article is available on the WWW under http://www.angewandte.org or from the author.
References
- 1.Rhodium(II)-catalyzed ring expansions: Padwa A, Kulkarni YS, Zhang Z. J Org Chem. 1990;55:4144.Pellicciari R, Natalini B, Sadeghpour BM, Marinozzi M, Snyder JP, Williamson BL, Kuethe JT, Padwa A. J Am Chem Soc. 1996;118:1.Damour D, Renaudon A, Mignani S. Synlett. 1995:111.Chen S, Zhao Y, Wang J. Synthesis. 2006:1705.Xu H, Zhang W, Shu D, Werness JB, Tang W. Angew Chem Int Ed. 2008;47:8933. doi: 10.1002/anie.200803910.For a recent example of a chiral Lewis acid-catalyzed ring expansion see: Hashimoto T, Naganawa Y, Maruoka K. J Am Chem Soc. 2011;133:8834. doi: 10.1021/ja202070j.Rhodium(II)-catalyzed rearrangements: Nagao K, Chiba M, Kim S-W. Synthesis. 1983:197.Nagao K, Yoshimura I, Chiba M, Kim S-W. Chem Pharm Bull. 1983;31:114.Pellicciari R, Natalini B, Cecchetti S, Fringuelli R. Steroids. 1987;49:433. doi: 10.1016/0039-128x(87)90016-x.Baudouy R, Core J, Ruest L. Tetrahedron. 1987;43:1099.Ye T, McKervey MA. Tetrahedron. 1992;48:8007.Kanemasa S, Kanai T, Araki T, Wada E. Tetrahedron Lett. 1999;49:5055.Vitale M, Lecourt T, Sheldon CG, Aggarwal VK. J Am Chem Soc. 2006;128:2524. doi: 10.1021/ja057739z.Shi W, Xiao F, Wang J. J Org Chem. 2005;70:4318. doi: 10.1021/jo050173c.
- 2.a) Yet L. Chem Rev. 2000;100:2963. doi: 10.1021/cr990407q. [DOI] [PubMed] [Google Scholar]; b) Wang YF, Shi QW, Dong M, Kiyota H, Gu Y-C, Cong B. Chem Rev. 2011;111:7652. doi: 10.1021/cr100147u. [DOI] [PubMed] [Google Scholar]
- 3.Recent uses of rhodium-catalyzed ring expansions in synthesis: Wender P, Deschamps N, Sun R. Angew Chem Int Ed. 2006;3957 doi: 10.1002/anie.200600806.Frey B, Wells AP, Rogers DH, Mander LN. J Am Chem Soc. 1998;120:1914.Kane JL, Shea KM, Crombie AL, Danheiser RL. Org Lett. 2001;3:1081. doi: 10.1021/ol0156897.Liu R, Zhang M, Wyche TP, Winston-McPherson GN, Bugni TS, Tang W. Angew Chem Int Ed. 2012;51:7503. doi: 10.1002/anie.201203379.
- 4.Recent uses of rhodium(II) azavinyl carbenes: Horneff T, Chuprakov S, Chernyak N, Gevorgyan V, Fokin VV. Am Chem Soc J. 2008;130:14972. doi: 10.1021/ja805079v.Miura T, Yamauchi M, Murakami M. Chem Comm. 2009;12:1359. doi: 10.1039/b819162j.Chattopadhyay B, Gevorgyan V. Org Lett. 2011;13:3746. doi: 10.1021/ol2014347.Chuprakov S, Kwok SW, Zhang L, Lercher L, Fokin VV. J Am Chem Soc. 2009;131:18034. doi: 10.1021/ja908075u.Grimster NP, Zhang L, Fokin VV. J Am Chem Soc. 2010;13:4870. doi: 10.1021/ja910187s.Chuprakov S, Malik JA, Zibinsky M, Fokin VV. J Am Chem Soc. 2011;133:10352. doi: 10.1021/ja202969z.Miura T, Biyajima T, Fujii T, Murakami M. J Am Chem Soc. 2012;134:194. doi: 10.1021/ja2104203.Selander N, Worrell BT, Chuprakov S, Velaparthi S, Fokin VV. J Am Chem Soc. 2012;134:14670. doi: 10.1021/ja3062453.
- 5.For a recent review of denitrogenative reactions of 1-sulfonyl triazoles see: Chattopadhyay B, Gevorgyan V. Angew Chem Int Ed. 2012;51:862. doi: 10.1002/anie.201104807.
- 6.Initial experiments were performed by Raushel Jessica, Raushel J. Diss Abstr Int, B. 2009;69:4763.PhD Dissertation. The Scripps Research Institute; La Jolla, CA: 2009.
- 7.For a recent study of the ring expansion of 4-cyclopropyl-1-sulfonyl-1,2,3-triazoles to substituted cyclobutenes under silver-catalyzed conditions see: Liu R, Zhang M, Winston-McPherson G, Tang W. Chem Commun. 2012 doi: 10.1039/c2cc34609e.
- 8.Raushel J, Fokin VV. Org Lett. 2010;12:4952. doi: 10.1021/ol102087r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Selected applications of enaminones in synthesis: Greenhill JV. Chem Soc Rev. 1977;6:277.Elassar ZA, El-Dhair AA. Tetrahedron. 2003;59:8463.Stanovnik B, Svete J. Chem Rev. 2004;104:2433. doi: 10.1021/cr020093y.Hansen KB, Hsiao Y, Xu F, Rivera N, Clausen A, Kubryk M, Krska S, Rosner T, Simmons B, Balsells J, Ikemoto N, Sun Y, Spindler F, Malan C, Grabowski EJJ, Armstrong JD., III J Am Chem Soc. 2009;131:8798. doi: 10.1021/ja902462q.
- 10.While this manuscript was under review, the following account describing a similar transformation of 1-sulfonyl triazoles to substituted enaminones was published: Miura T, Funakoshi Y, Morimoto M, Biyajima T, Murakami M. J Am Chem Soc. 2012 doi: 10.1021/ja308285r.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.