Abstract
Amino acids are essential nutrients for living organisms. There are genetic differences in voluntary consumption of amino acids among mouse strains. In two-bottle preference tests, C57BL/6ByJ (B6) mice consume more glutamate (Glu) and glycine (Gly) solutions than do 129P3/J (129) mice. To examine the role of postingestive metabolism of these amino acids in regulation of their intake, we compared metabolism of orally administered Glu and Gly in B6 and 129 mice. After administration of Glu, there were increases in circulating glucose and insulin in B6 mice, whereas 129 mice had elevated blood alanine and body temperature. After ingestion of Gly, B6 mice had increases in blood glucose, whereas there was an elevation of body temperature in 129 mice. These data suggest that B6 mice preferentially convert ingested Glu and Gly to glucose in contrast to 129 mice, which preferentially use them for thermogenesis. This study strongly supports the hypothesis that the metabolic fate of a nutrient plays an important regulatory role in control of its intake. This is the first detailed study of mouse strain differences in amino acid metabolism.
Keywords: gluconeogenesis, thermogenesis, blood glucose, insulin, liver
amino acids are essential to all live organisms. They serve primarily as building blocks of protein but also play many important roles in a free form [e.g., glutamic acid (Glu) and glycine (Gly) are neurotransmitters]. The major source of amino acids for animals is protein contained in ingested food. Once ingested proteins are hydrolyzed by digestive proteases, released amino acids are extensively metabolized in the small intestine (3, 13, 28, 46). Absorbed amino acids are transported by circulation to various tissues for protein synthesis. Amino acids not used for protein synthesis are usually metabolized by the liver, where they either serve as a source of energy or are converted to glucose or ketones (5). Amino acids usually provide ~10% of the energy requirement in normal adult humans. If carbohydrate intake is insufficient or carbohydrates cannot be metabolized (e.g., because of diabetes mellitus), amino acids become a more important energy source.
Not all amino acids are metabolized the same way. For example, ingested Glu is extensively metabolized in the small intestine into CO2, lactate, glutathione, glutamine (Gln), alanine (Ala), and several other amino acids (28, 46). Under normal conditions, e.g., after consumption of proteins or monosodium glutamate (MSG) mixed with food, blood Glu does not rise because of its utilization in the intestine and liver (28, 35). However, blood Glu may increase under artificial experimental conditions, when large amounts of concentrated water solutions of Glu are administered to the digestive tract. Ingested Gly is metabolized by the intestine to a lesser degree compared with Glu (46). It is used for intestinal protein synthesis and serves as an energy source and a precursor for glutathione synthesis. Dietary Glu or Gly that is not metabolized in the intestine is released into the circulation, most of which is further metabolized in the liver. Both Glu and Gly are gluconeogenic amino acids. In the liver, along with their intestinal metabolites Ala and lactate, they can be converted to glucose, which is then either released back into circulation or stored in the liver in the form of glycogen. Alternatively, their carbon skeletons can be oxidized through the tricarboxylic acid cycle to generate energy. Nitrogen in the amino acids is converted to urea, which is excreted in urine.
Although key enzymes involved in amino acid metabolism are well studied, little is known about genetic differences in amino acid metabolism. A few studies published so far indicate that amino acid metabolism is under genetic control in mice (11, 24, 33). We therefore have begun studies to examine genetic variation in amino acid metabolism.
We chose for this study two strains that differ in voluntary consumption of Glu and Gly. Our previous studies showed (1, 2) that in two-bottle preference tests mice from the C57BL/6ByJ (B6) inbred strain drink more MSG and Gly solutions than mice from the 129P3/J (129) inbred strain. Like many free amino acids, Glu and Gly have distinct tastes to humans and other animals. Glu salts, including MSG, have umami (savory meaty) taste, whereas Gly has sweet taste (Refs. 15, 22, 26; Murata Y, Bachmanov AA, Beauchamp GK, unpublished observations). This may suggest that strain differences in Glu and Gly consumption are mediated by differences in taste responsiveness. However, gustatory neural responses to MSG and Gly were similar in B6 and 129 mice (16, 17), and there were no or only minor differences between these two strains in qualitative taste perception of MSG and Gly (Ref. 22; Murata Y, Bachmanov AA, Beauchamp GK, unpublished observations). This indicates that mechanisms other than taste perception underlie strain differences in consumption of these amino acids. Consistent with this, intakes of MSG and Gly appear to be influenced by exposure to these amino acids, which suggests the role of postingestive mechanisms. First, during 4-day two-bottle tests, B6 mice increased MSG consumption while 129 mice decreased it. As a result, the strain difference in MSG consumption was larger during the last 2 days of testing than during the first 2 days (1). Second, while in the 48-h two-bottle tests B6 mice consumed more Gly than 129 mice (2), lick rates in brief-access tests tended to be lower in B6 mice compared with 129 mice (9).
This study was carried out to test the hypothesis that the strain differences in Glu and Gly consumption depend on postingestive metabolism of these amino acids. To examine postingestive metabolism of orally administered Glu and Gly in B6 and 129 mice, we measured changes in blood contents of Glu, Gly, their major metabolites, insulin, liver glycogen, urine nitrogen excretion, and body temperature. By comparing postingestive metabolism of Glu and Gly in B6 and 129 mice with different Glu and Gly consumption, we attempted to determine whether metabolic differences underlie voluntary intake of these amino acids.
MATERIALS AND METHODS
Animals and diet
All protocols involving animals were approved by the Institutional Animal Care and Use Committee at the Monell Center before the experiments. Male B6 and 129 mice at the age of 8 wk were purchased from the Jackson Laboratory (Bar Harbor, ME). They were housed individually in plastic shoe box cages with wood chip bedding and stainless steel wire tops. Ambient temperature was kept at 22–23°C, and lights were set to be on at 7 AM and off at 7 PM. Mice had access to Teklad Rodent Diet 8604 and deionized water at all time unless otherwise mentioned below. They were allowed to recover from the shipping trip from the vendor and to adapt to the laboratory environment for at least 1 wk before experiments started.
Experiment 1: postingestive metabolites
The experiment was conducted with 16 B6 and 16 129 mice divided into 2 groups (with 8 mice from each strain in each group). Mice from group 1 were gavaged with MSG. Mice from group 2 were gavaged with Gly.
At 9 AM, mouse body weights were recorded and food was removed. At 10 AM, 50 μl of tail blood was collected from each mouse into a heparinized capillary tube, and mice from group 1 were gavaged with 0.591 M (10% wt/vol) MSG at a volume of 1 ml/100 g body wt. This dose (1 g/kg body wt) is similar to the average amount of MSG voluntarily consumed in 1 h by an 129 mouse (B6 mice consume almost twice as much; Ref. 1) and had been used in previous studies (35, 37). Mice from group 2 were gavaged with 0.591 M (4.4% wt/vol) Gly at a volume of 1 ml/100 g body wt. This dose (0.44 g/kg body wt) is similar to the average amount of Gly voluntarily consumed in 1 h by a B6 mouse (2). Blood collection was repeated at 15, 30, 45, 60, and 120 min after the gavage. The blood sample was immediately mixed with 500 μl of ice-cold 0.4 M HClO4 and vortexed. The mixture was centrifuged at 12,000 g for 30 s at 4°C. An aliquot of 100 μl of clear supernatant (acid extract) was transferred into a clean tube. Another aliquot of 350 μl was transferred and neutralized with 2 M KHCO3. After centrifugation at 12,000 g for 10 min at 4°C, the neutralized supernatant (neutralized acid extract) was transferred into a clean tube. After the final blood sampling, mice were killed by exposure to CO2 and their livers were immediately excised and clamped with a pair of aluminum tongs prechilled in liquid nitrogen. Blood extracts and livers were stored at −80°C until further analysis.
Gavaging and blood and tissue collection were conducted over 4 days. Each day, four B6 and four 129 mice were used. On days 1 and 2, mice from group 1 were gavaged with MSG. On days 3 and 4, mice from group 2 were gavaged with Gly.
Experiment 2: postingestive insulin
Serial blood collection was repeated with a separate group of 12 B6 and 12 129 mice, with 6 from each strain given MSG and the other 6 given Gly. Plasma was prepared within 15 min of blood collection and used for determination of insulin levels.
Experiment 3: postingestive thermogenesis
Under anesthesia with halothane, 10 B6 and 10 129 mice were implanted with IPTT-300 programmable temperature transponders (Bio Medic Data Systems, Seaford, DE) in subcutaneous interscapular tissue. During the next 4 days, mice were put into metabolic cages individually for 2 h/day to allow them to adapt to the cages. The metabolic cages were made out of 3-liter plastic soda bottles with bottoms cut out. They were mounted on stands upside down with metal clamps. A plastic mesh was fitted above the narrow neck of the bottle to let a mouse stay on and another finer mesh underneath to separate feces from urine. Five days after implantation, five B6 and five 129 mice were placed individually in metabolic cages at 9 AM with only access to deionized water. Body temperature of each mouse was recorded at 9:30 AM, 9:40 AM, and 9:50 AM with a WRS-6007 wireless IPTT reader. At 10:00 AM, each mouse was gavaged with 0.591 M NaCl at the volume of 1 ml/100 g body wt. Body temperature was recorded again for 4 h at 10-min intervals. Urine excreted during the 7 h after gavage was collected into a tube and stored frozen until further analysis. After 7 h, mice were returned to their home cages.
The experiment was repeated the following day with the remaining five B6 and five 129 mice and 0.591 M glucose. On the third day the first batch of mice was used to repeat the experiment with 0.591 M MSG, and on the fourth day the second batch was used to repeat the experiment with 0.591 M Gly.
After a 1-wk interval, all mice were tested again in a counterbalanced fashion. However, one 129 mouse lost the transponder during the interval and was not used for glucose and Gly experiments. Therefore, at the end, all mice except one were tested with all four solutions.
Biochemical analyses
Acid extracts of blood samples were used to determine concentrations of lactate and alanine with enzymatic assays described by Maughan (23). Gly concentration was also determined in acid extracts of blood samples collected from mice gavaged with Gly by EZ:faast Free Amino Acid Analysis (Phenomenex, Torrance, CA) with a HP 6890 series GC system (Hewlett Packard, Wilmington, DE) according to the protocol specified by Phenomenex. Quantification was achieved with a Gly standard and an internal standard of known concentrations provided by Phenomenex.
Neutralized acid extracts of blood samples were used to determine blood concentrations of glucose (with a kit from Waco Chemicals USA, Richmond, VA), total (oxidized plus reduced) glutathione (GSH) (with the Glutathione Assay Kit from Cayman Biochemical, Ann Arbor, MI), urea (with the QuantiChrom Urea Assay Kit from Bioassay Systems, Hayward, CA), and ammonia [with a standard enzymatic method (4)]. Concentrations of Glu and Gln in neutralized acid extracts from blood samples collected from mice gavaged with Glu were determined with the Glutamine/Glutamate Determination Kit from Sigma (St. Louis, MO).
Concentrations of insulin in plasma samples were determined by ELISA at the Radioimmunoassy Core Facility in the Diabetes Center of the University of Pennsylvania. Glycogen content in frozen liver samples was assessed by a standard method (21). Concentrations of ammonia and urea in urine samples were quantified with the methods described above. Total nitrogen excreted in urine was calculated from the ammonia and urea values.
Statistical analyses
Two-way ANOVA was used to compare the values of the two strains when repeated measures were made over time (i.e., blood metabolites and body temperature). When a significant strain difference was found, Tukey least significant difference post hoc tests were used to find significant differences at individual time points. Follow-up one-way repeated-measures ANOVA was used to determine whether there were significant changes over time for each strain. In experiment 3, differences between temperature measured after nutrient (Glu, Gly, or glucose) ingestion and after NaCl ingestion were also analyzed with a one-sample hypothesis test and a 95% confidence level to assess whether they were significantly different from zero. For liver glycogen contents and total urine nitrogen, Student’s t-tests were used to compare the two strains. Significance level was set at P ≤ 0.05.
RESULTS
Postingestive metabolism of Glu
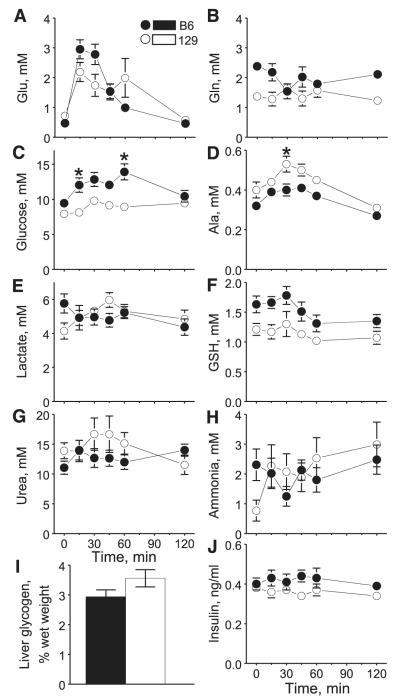
Concentrations of plasma Glu rose significantly after Glu ingestion in both B6 and 129 mice, as suggested by the significant time effect of two-way ANOVA (see results of 2-way ANOVA in Table 1), with peaks at 15 min after Glu administration (Fig. 1A). Although there was no significant strain difference, there was significant interaction between effects of strain and time: compared with 129 mice, the Glu peak in B6 mice was higher but waned faster. Glu returned to basal levels (0 min) by 120 min in both strains. Plasma Gln levels were significantly higher in B6 mice than in 129 mice (Fig. 1B). However, the effect seemed to be the result of a strain difference in baseline levels; Glu ingestion did not have any significant effect on Gln levels in either strain.
Table 1.
F-values of two-way ANOVA analyses of metabolic measures after ingestion of either Glu or Gly
| After Glu Ingestion |
After Gly Ingestion |
|||||
|---|---|---|---|---|---|---|
| Measures | Time F(11,5) | Strain F(11,1) | Time × Strain F(11,5) | Time F(11,5) | Strain F(11,1) | Time × Strain F(11,5) |
| Glu | 16.00* | 0.20 | 3.08* | |||
| Gln | 0.35 | 21.58* | 2.01 | |||
| Gly | 28.89* | 0.47 | 2.05 | |||
| Glucose | 3.12* | 37.80* | 1.66 | 10.34* | 30.92* | 3.49* |
| Ala | 13.96* | 30.85* | 0.97 | 12.49* | 1.37 | 0.27 |
| Lactate | 0.42 | 0.40 | 2.43* | 3.98* | 7.77* | 2.47* |
| Total GSH | 2.44* | 27.40* | 0.24 | 4.22* | 3.03 | 0.61 |
| Urea | 0.54 | 3.42 | 1.08 | 2.59* | 4.72* | 1.43 |
| Ammonia | 0.12 | 0.86 | 0.09 | 0.90 | 0.82 | 0.32 |
| Insulin | 0.38 | 10.65* | 0.34 | 1.04 | 2.82 | 1.83 |
Glu, glutamate; Gly, glycine; Gln, glutamine; Ala, alanine; GSH, glutathione.
Significance at P ≤ 0.05.
Fig. 1.
Mean ± SE plasma levels of metabolites and insulin and liver glycogen content after C57BL/6ByJ (B6) and 129P3/J (129) mice were gavaged with monosodium glutamate (MSG). A–I: data from experiment 1 (n = 8 for each strain). Glu, glutamate; Gly, glycine; Ala, alanine; GSH, total glutathione (oxidized + reduced). Glycogen was determined at the end of the experiment (at 120 min). J: data from experiment 2 (n = 6 for each strain). *Significant difference between 129 and B6 strains at a specific time point (P ≤ 0.05).
Concentrations of plasma glucose were significantly higher in B6 mice than in 129 mice after Glu ingestion (Fig. 1C). The difference was due to a significant postingestive increase. A follow-up analysis revealed that the increase occurred in B6 mice (P < 0.05, 1-way ANOVA) but not in 129 mice. There were no strain differences in plasma levels of glucose at 0 and 120 min, but the levels were significantly higher in B6 than in 129 mice at 15 and 60 min. An opposite pattern was seen for plasma Ala levels (Fig. 1D). Postingestive Ala was significantly higher in 129 mice than in B6 mice because of a postingestive increase in 129 mice (P = 6.0 × 10−5) but not in B6 mice. A significant strain difference was seen at 30 min after Glu ingestion. There were no strain differences in plasma lactate levels and no effect of Glu ingestion (Fig. 1E). Plasma GSH levels were higher in B6 than in 129 mice, but this appears to be the result of a strain difference in baseline levels (Fig. 1F), and post hoc analysis revealed no significant differences at individual time points. There were no significant changes in plasma urea (Fig. 1G) or ammonia (Fig. 1H) concentrations after Glu ingestion, nor were there strain differences. Liver content of glycogen did not differ between the two strains at 120 min after Glu ingestion (Fig. 1I). Overall postingestive insulin levels were significantly higher in B6 mice than in 129 mice (Fig. 1J). However, no significant differences at individual time points were detected by post hoc analysis.
Postingestive metabolism of Gly
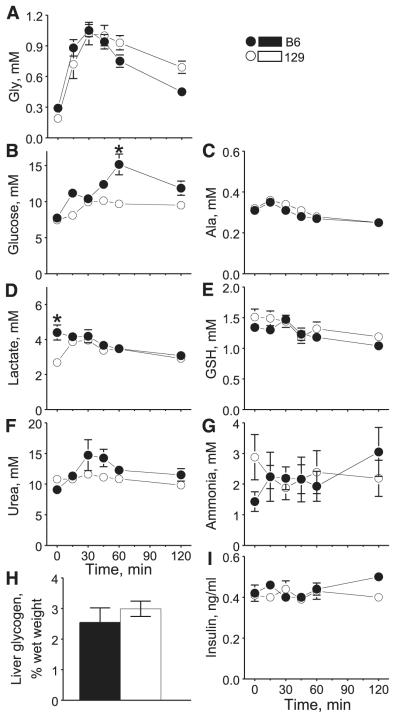
Plasma Gly levels rose significantly in both strains of mice after Gly ingestion (Fig. 2A). They peaked at 30 min and waned afterward. There were no strain differences in postingestive levels of plasma Gly. However, unlike after ingestion of Glu, plasma Gly did not return to its basal levels at 120 min after ingestion of Gly. There was a significant strain difference in postingestive levels of plasma glucose (Fig. 2B). Post hoc analysis revealed significantly higher glucose concentrations in B6 mice than in 129 mice at 60 min. The differences were due to larger postingestive increases in B6 than in 129 mice. Plasma Ala levels did not differ between strains and were not affected by Gly ingestion (Fig. 2C). Basal levels of plasma lactate were higher in B6 mice than 129 mice (Fig. 2D), leading to overall significantly higher lactate levels in B6 mice than in 129 mice. The levels steadily decreased after Gly ingestion in B6 mice but increased initially then decreased in 129 mice, resulting in a significant strain × time interaction. There was a significant change in plasma GSH after Gly ingestion, but the two strains did not differ (Fig. 2E). Levels of plasma urea changed significantly after ingestion of Gly, and there was a significant strain effect (Fig. 2F). Nevertheless, post hoc analysis did not reveal any significant differences at individual time points. There were no significant changes in plasma ammonia concentrations after Gly ingestion, nor were there strain differences (Fig. 2G). Liver glycogen content at 120 min after Gly ingestion did not differ between the strains (Fig. 2H). Overall, there were no significant changes over time or a strain difference in postingestive levels of plasma insulin, but B6 mice tended to have higher insulin levels at the end of testing (Fig. 2I).
Fig. 2.
Mean ± SE plasma levels of metabolites and insulin and liver glycogen content after B6 and 129 mice were gavaged with Gly. A–H: data from experiment 1 (n = 8 for each strain). GSH, total glutathione (oxidized + reduced). Glycogen was determined at the end of the experiment (at 120 min). I: data from experiment 2 (n = 6 for each strain). *Significant difference between 129 and B6 strains at a specific time point (P ≤ 0.05).
Postingestive thermogenesis
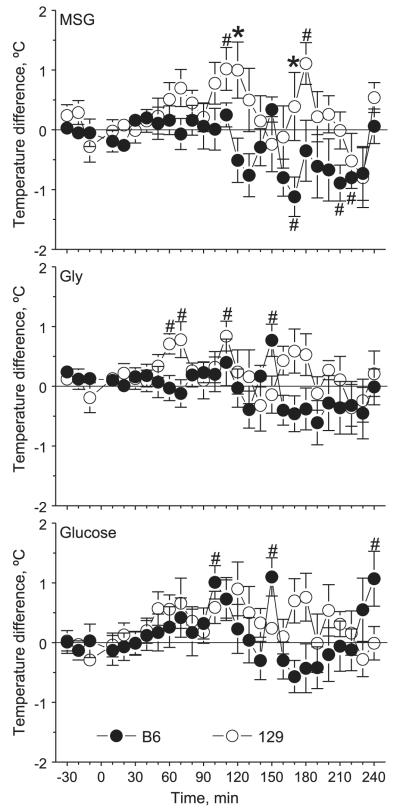
In a pilot experiment, we found that simple handling of mice resulted in elevation of body temperature and that a mock gavage produced increase in body temperature, which remained elevated for a long time (data not shown). To separate these nonspecific effects from thermogenic effects of nutrient administration, we subtracted body temperatures after MSG, Gly, or glucose ingestion by those after NaCl ingestion for each mouse at each corresponding time point. NaCl ingestion does not have thermogenic effects (34), and we found no strain differences in body temperature after NaCl ingestion (data not shown). Thus the resulting values allowed us to estimate the temperature changes due to nutrient metabolism, excluding the nonspecific effects of handling. Therefore, the results presented here are the changes in body temperature due to nutrient ingestion beyond those caused by NaCl ingestion. Body temperature after glucose ingestion was measured to compare effects of the amino acids with effects of another nutrient.
After MSG ingestion, there was a significant strain difference [F(,153) = 30.4, P = 5.8 × 10−8] in body temperature changes, with significantly higher values in 129 mice than in B6 mice at 120 and 170 min (Fig. 3, top). In both strains, there were significant changes in temperature over the 4-h period after gavage. In 129 mice, temperature was mostly above the baseline (0) level, with significant elevations above 0 at 110 and 180 min. In B6 mice, temperature was mostly below 0, with significant drops below 0 at 170, 210, and 220 min.
Fig. 3.
Mean ± SE relative changes in body temperature of B6 (n = 10) and 129 (n = 9 or 10) mice after they were gavaged with MSG (top), Gly (middle), or glucose (bottom) in experiment 3. Mice were gavaged at time 0. Values presented are deviations in body temperature from those recorded after gavage with NaCl. The horizontal 0 line represents no additional change in body temperature due to ingestion of a nutrient (i.e., relative to temperature after gavage with NaCl). *Significant difference between B6 and 129 mice at a specific time point (P < 0.05); #temperature level significantly different from zero (P ≤ 0.05).
After Gly ingestion, there was also an overall significant strain difference [F(,153) = 8.6, P = 0.004] in body temperature changes, but post hoc analysis did not reveal any significant differences between the strains at individual time points (Fig. 3, middle). One-way ANOVAs did not detect significant variation in postingestive body temperature over time in any strain, but in 129 mice temperature was mostly above 0 level, with significant elevations above 0 at 60, 70, and 110 min. In B6 mice, temperature was mostly below 0, but at 150 min it was significantly elevated above 0.
Therefore, up to 120 min after ingestion of either MSG or Gly, when circulating levels of Glu or Gly returned or were close to basal levels, body temperature of B6 mice did not change beyond that caused by gavaging of NaCl, whereas body temperature of 129 mice had a significant increase beyond that caused by gavaging of NaCl.
After glucose ingestion, there were significant changes in body temperature over time [F(,2653) = 1.56, P = 0.04] due to large variations in B6 mice seen during a period past 90 min after gavage, with significant elevations above 0 at 100, 150, and 240 min (Fig. 3, bottom). However, there were no significant strain differences.
In experiment 3, we also measured total nitrogen (ammonia plus urea) excretion in urine over 7 h after the ingestion of Glu, Gly, glucose, or NaCl and found no strain differences (data not shown).
DISCUSSION
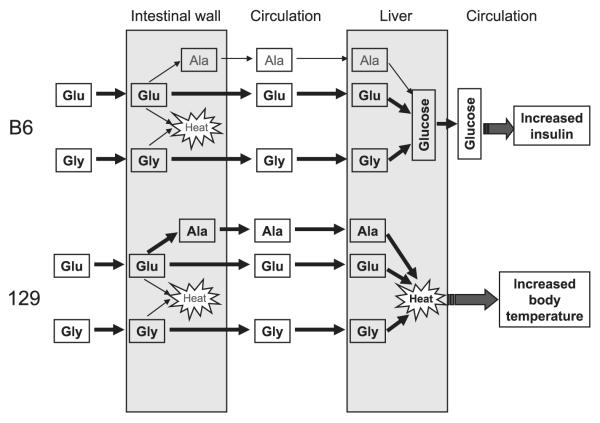
We uncovered some significant strain differences in postingestive metabolism of Glu and Gly between B6 and 129 mice. During 2 h after ingestion of either amino acid, B6 mice had higher increases in blood glucose levels compared with 129 mice. During the same period, body temperature increased in 129 but not B6 mice. Levels of blood Ala were also significantly more elevated in 129 mice than in B6 mice after Glu ingestion. Consistent with higher plasma glucose levels in B6 mice, plasma insulin levels after Glu ingestion were also higher in these mice than in 129 mice. These data suggest that B6 and 129 mice metabolize ingested amino acids differently (Fig. 4). These metabolic differences may explain their differential consumption of amino acids.
Fig. 4.
Differences in Glu and Gly metabolism between B6 and 129 mice. Thick arrows and bold text indicate predominant metabolic pathways in each strain. Thin arrows and shaded text indicate relatively minor pathways.
Postingestive metabolism of Glu and Gly
When mice were given a dose of Glu that was equivalent to the amount voluntarily consumed by a 129 mouse in 1 h, blood Glu levels increased rapidly, peaked at 15 min, and then returned to basal levels within 2 h in both strains. Such a time course is similar to those reported earlier for mice that were orally administered similarly high doses of water solutions of MSG (35, 37). Contrasting this rapid rise in blood Glu, there was no change in blood Gln, suggesting that little ingested Glu was converted to Gln and then released into the circulation by either mouse strain. On the other hand, there was a rapid rise in blood glucose in B6 mice, an increase in blood Ala in 129 mice, and little change in blood lactate concentration in either strain. These results imply that administered Glu is rapidly absorbed into the circulation in both mouse strains. On the basis of time of the peak levels of blood Glu and Ala, it seems that a portion of ingested Glu is metabolized in the intestine by 129 mice to Ala, which is subsequently released into circulation, but much less so by B6 mice. An increase in blood glucose was only seen in B6 mice, suggesting that absorbed Glu was converted to glucose in the liver of these mice through the gluconeogenic pathway and then released back into the circulation. On the other hand, 129 mice did not show a significant increase in blood glucose; instead, they had a significant increase in body temperature, which was not seen in B6 mice. Lack of a strain difference in liver glycogen after Glu ingestion excluded the possibility of newly produced glucose being stored as liver glycogen. Therefore, these observations indicate that the metabolic fate of oral Glu differs between B6 and 129 mice as the former tend to use it as a gluconeogenic precursor in the liver whereas the latter convert it to Ala in the intestine; then Ala and possibly Glu are further used for thermogenesis (Fig. 4).
After ingestion of Gly, blood Gly levels peaked at 30 min and waned slowly thereafter in both strains of mice. Clearance of Gly in blood seemed to take a longer time than clearance of Glu. Similar to the effects of Glu ingestion, there was a substantial increase in blood glucose in B6 mice after Gly ingestion but at a seemingly slower rate with a smaller peak compared with that after Glu ingestion. Rise in blood glucose in 129 mice was smaller than in B6 mice, suggesting that B6 mice converted more ingested Gly to glucose than 129 mice. Unlike after ingestion of Glu, there were no strain differences in blood Ala after Gly ingestion, which suggests that Ala is not a significant metabolite of Gly in the intestine. Like after ingestion of Glu, body temperature of 129 mice was elevated significantly, whereas body temperature of B6 mice remained mostly unchanged after Gly ingestion. These data suggest that strain differences in Gly metabolism seem to exist in the liver, where B6 mice use it for gluconeogenesis but 129 mice use it for thermogenesis (Fig. 4).
The time courses for appearance of Glu, Gly, and some of their metabolites in the circulation suggest that metabolism of a single oral dose of the amino acid was mostly completed within 2 h. The extent of the rise in blood glucose was smaller after Gly ingestion than after Glu ingestion. This was likely due to the smaller carbon skeleton of Gly compared with Glu but equal molar doses of the amino acids administered. Consistent with the higher levels of blood glucose in B6 mice after ingestion of either amino acid, blood insulin was also elevated in B6 mice compared with 129 mice. The time courses of blood glucose and insulin show that the rise in insulin followed the rise in glucose in B6 mice, suggesting that release of insulin was triggered by rising blood glucose. The lack of consistent effects of Glu or Gly ingestion on, or strain differences in, levels of other metabolites indicates that both Glu and Gly are not significant precursors for synthesis of lactate, glutathione, or liver glycogen. Nitrogen in Glu and Gly was rapidly converted to urea, which was actively excreted in urine, because we observed little change in blood ammonia or urea after amino acid ingestion.
It appears that B6 mice preferentially convert a significant portion of ingested Glu and Gly to glucose, whereas 129 mice preferentially convert it to heat. Although mice increase their temperature easily due to handling stress, using NaCl as a control enabled us to estimate body temperature change due to ingestion of a nutrient after excluding the effect of handling stress. The data presented here show that administration of amino acids resulted in a significant difference in thermogenesis between 129 and B6 mice. Up to 120 min after ingestion of either MSG or Gly, when circulating levels of Glu or Gly returned or were close to basal levels, the body temperature of B6 mice did not change beyond that caused by gavaging of NaCl, whereas during the same period the body temperature of 129 mice had a significant increase beyond that caused by gavaging of NaCl. The most pronounced changes in blood amino acid metabolites (including those synthesized in the intestine, e.g., Ala) occurred within the first hour after gavage, but the most pronounced changes in thermogenesis in 129 mice occurred later, within the second hour after amino acid administration. This delayed thermogenic response suggests that the thermogenesis mainly occurred not in the intestine but in the liver and/or other tissues. We implanted temperature transponders in the interscapular region that contains brown adipose tissue, which was shown to be involved in MSG-induced thermogenesis in rats (34). However, our data do not allow us to determine whether liver, brown adipose tissue, or other organs were sites of thermogenesis. Thus additional studies would be needed to find out in which organs Glu- or Gly-induced thermogenesis occurs. Similar to a finding in rats (34), there were no strain differences in body temperature change after glucose ingestion, suggesting that the strain differences in thermogenesis were specific to Glu and Gly but not to nutrients in general. This genetic difference further suggests that Glu and Gly act more as an energy source in 129 mice than in B6 mice, which use them as gluconeogenic precursors.
Although in this experiment we intended to approximate conditions that mice experience during 24-h two-bottle tests, the delivery of amino acids was not identical in these two types of tests. First, we calculated doses of Glu and Gly based on 24-h intakes of 0.3 M MSG and 0.3 M Gly (the most preferred concentrations) in two-bottle tests with water, assuming that most of the intake occurred during the 12-h dark period. We delivered these hourly ingested amounts of amino acids as a single bolus of 0.591 M solutions. Therefore, the time course of the amino acid delivery was much faster in this experiment than in the 24-h two-bottle tests. Second, during two-bottle tests, animals consume some water in addition to the amino acid solutions, which dilutes the concentrations of consumed amino acids. Therefore, in this experiment we administered more than twice higher concentrations of amino acids compared with their concentrations in the stomach during the two-bottle tests with the most preferred solutions. All this likely resulted in faster intestinal absorption rates and higher blood levels of Glu and Gly in this experiment compared with 24-h two-bottle tests. Nevertheless, the strain differences in postingestive metabolism of Glu and Gly found in this study are likely to also influence postingestive processing of these amino acids delivered under different regimens, including those occurring during long-term two-bottle tests.
Amino acid metabolism and intake
The observations presented here suggest that in mice intake of an amino acid may be regulated by its metabolic fate. B6 mice prefer and consume large quantities of both amino acids (1, 2), possibly because they channel amino acids into the gluconeogenic pathway, resulting in elevations of blood glucose and then insulin. In contrast, 129 mice prefer and consume less of either amino acid, possibly because they use them mostly for thermogenesis. Although the present study did not provide a causal relationship between intake and postingestive thermogenesis or gluconeogenesis, previous studies have shown evidence to support such a relationship.
Numerous studies indicate that changes in body temperature are inversely related to feeding. People exposed to cold climates increase food intake (10). Energy intake is inversely correlated with local temperature (20). An increase in core temperature decreases food intake (43), whereas a decrease in core temperature promotes overeating (44). In addition, diet-induced thermogenesis positively correlates with satiety (42). Recently, the effectiveness of a low-carbohydrate dieting regime has been attributed to appetite suppression by thermogenesis of protein usually consumed in large quantities in this dieting regime (41). It is therefore likely that in 129 mice thermogenesis as the preferred metabolic fate of Glu or Gly raises body temperature, which subsequently limits intake.
Although the present study was not designed to examine whether Glu- or Gly-induced thermogenesis occurs in the intestine, liver, or other tissues, our data suggest that the liver could be a major site for thermogenesis in 129 mice. This process involves oxidation of carbon skeletons through the tricarboxylic acid cycle in mitochondria and buildup of a proton gradient across mitochondrial membrane. The gradient then drives the synthesis of ATP to provide energy for numerous cell functions or produces heat if uncoupled. Heat can also be generated from breakdown of ATP when it is used. Therefore, thermogenesis induced by amino acids is likely an indication of increased cellular energy status. Several studies have demonstrated that hepatic energy status regulates appetite, because a decrease in energy status promotes food intake (12, 19, 27) and an increase produces satiety (18). Protein is the most satiating among the three macronutrients (36, 40). The present study provides further evidence to support the underlying mechanism for protein’s ability to induce thermogenesis and the role of energy metabolism in regulation of energy intake in general. It will be interesting to see whether Glu or Gly suppresses energy intake in 129 mice more than in B6 mice.
Compared with 129 mice, B6 mice consume more Glu and Gly, and also have higher blood glucose after ingestion of either amino acid, which suggests that rising blood glucose may have a rewarding effect that promotes feeding. It has been shown previously that raising blood glucose generates a satiety signal. For example, intravenous infusion of glucose suppresses intake in baboons (45) and rats (6, 39). This satiety signal, similar to thermogenesis in 129 mice, potentially could be associated with reduced amino acid consumption by B6 mice. However, satiating effects of nutrients appear to be distinct from their postingestive reward effects (30). For example, intragastric and hepatic portal glucose infusions were shown to condition flavor preferences in rats (29, 38). Intragastric carbohydrate infusions also condition flavor preferences in mice (31, 32). Although systemic intravenous glucose administration has rewarding properties in hungry animals under certain conditions (7, 8), it was not effective to condition flavor preferences (14, 38). Thus the rewarding signal of amino acid ingestion in B6 mice may be initiated by glucose generated during gluconeogenesis in the liver before it enters the circulation. However, additional studies are needed to examine this hypothesis.
Summary
The present study uncovered strain differences in postingestive metabolism of Glu and Gly between B6 and 129 mice. Metabolic fates of the two amino acids are characterized by gluconeogenesis and elevations in blood glucose and insulin in B6 mice and by thermogenesis in 129 mice. These data provide strong evidence that postingestive metabolism plays an important regulatory role in higher intake of these amino acids in B6 mice compared with 129 mice. The findings presented here provide the first detailed characterization of genetic variation in amino acid metabolism.
ACKNOWLEDGMENTS
We thank Maria Theodorides, Zakiyyah Smith, and Mauricio Avigdor for technical assistance.
GRANTS Funding for this research was provided by the Ajinomoto Amino Acid Research Program.
REFERENCES
- 1.Bachmanov AA, Tordoff MG, Beauchamp GK. Intake of umamitasting solutions by mice: a genetic analysis. J Nutr. 2000;130:935S–941S. doi: 10.1093/jn/130.4.935S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bachmanov AA, Tordoff MG, Beauchamp GK. Sweetener preference of C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:905–913. doi: 10.1093/chemse/26.7.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baracos VE. Animal models of amino acid metabolism: a focus on the intestine. J Nutr. 2004;134:1656S–1659S. doi: 10.1093/jn/134.6.1656S. [DOI] [PubMed] [Google Scholar]
- 4.Bergmeyer HU, Beutler HO. Ammonia. In: Bergmeyer HU, Bergmeyer J, Grassl M, editors. Methods of Enzymatic Analysis. Verlag Chemie; Deerfield Beach, FL: 1983. pp. 454–461. [Google Scholar]
- 5.Brosnan JT. Interorgan amino acid transport and its regulation. J Nutr. 2003;133:2068S–2072S. doi: 10.1093/jn/133.6.2068S. [DOI] [PubMed] [Google Scholar]
- 6.Burggraf KK, Willing AE, Koopmans HS. The effects of glucose or lipid infused intravenously or intragastrically on voluntary food intake in the rat. Physiol Behav. 1997;61:787–793. doi: 10.1016/s0031-9384(96)00531-8. [DOI] [PubMed] [Google Scholar]
- 7.Chambers RM. Effects on intravenous glucose injections on learning, general activity, and hunger drive. J Comp Physiol Psychol. 1956;49:558–564. doi: 10.1037/h0047138. [DOI] [PubMed] [Google Scholar]
- 8.Coppock HW, Chambers RM. Reinforcement of position preference by automatic intravenous injections of glucose. J Comp Physiol Psychol. 1954;47:355–357. doi: 10.1037/h0061319. [DOI] [PubMed] [Google Scholar]
- 9.Dotson CD, Spector AC. The relative affective potency of glycine, l-serine and sucrose as assessed by a brief-access taste test in inbred strains of mice. Chem Senses. 2004;29:489–498. doi: 10.1093/chemse/bjh051. [DOI] [PubMed] [Google Scholar]
- 10.Edholm OG, Goldsmith R. Food intakes and weight changes in climatic extremes. Proc Nutr Soc. 1966;25:113–119. doi: 10.1079/pns19660025. [DOI] [PubMed] [Google Scholar]
- 11.Ernest S, Hosack A, O’Brien WE, Rosenblatt DS, Nadeau JH. Homocysteine levels in A/J and C57BL/6J mice: genetic, diet, gender, and parental effects. Physiol Genomics. 2005;21:404–410. doi: 10.1152/physiolgenomics.00199.2004. [DOI] [PubMed] [Google Scholar]
- 12.Friedman MI, Harris RB, Ji H, Ramirez I, Tordoff MG. Fatty acid oxidation affects food intake by altering hepatic energy status. Am J Physiol Regul Integr Comp Physiol. 1999;276:R1046–R1053. doi: 10.1152/ajpregu.1999.276.4.R1046. [DOI] [PubMed] [Google Scholar]
- 13.Fuller MF, Reeds PJ. Nitrogen cycling in the gut. Annu Rev Nutr. 1998;18:385–411. doi: 10.1146/annurev.nutr.18.1.385. [DOI] [PubMed] [Google Scholar]
- 14.Gowans SE, Weingarten HP. Elevations of plasma glucose do not support taste-to-postingestive consequence learning. Am J Physiol Regul Integr Comp Physiol. 1991;261:R1409–R1417. doi: 10.1152/ajpregu.1991.261.6.R1409. [DOI] [PubMed] [Google Scholar]
- 15.Hayakawa Y, Kawai M. Taste properties of l-amino acid solutions at suprathreshold concentration. Jpn J Taste Smell Res. 2003;10:463–466. [Google Scholar]
- 16.Inoue M, Beauchamp GK, Bachmanov AA. Gustatory neural responses to umami taste stimuli in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2004;29:789–795. doi: 10.1093/chemse/bjh083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inoue M, McCaughey SA, Bachmanov AA, Beauchamp GK. Whole-nerve chorda tympani responses to sweeteners in C57BL/6ByJ and 129P3/J mice. Chem Senses. 2001;26:915–923. doi: 10.1093/chemse/26.7.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ji H, Friedman MI. Compensatory hyperphagia after fasting tracks recovery of liver energy status. Physiol Behav. 1999;68:181–186. doi: 10.1016/s0031-9384(99)00173-0. [DOI] [PubMed] [Google Scholar]
- 19.Ji H, Graczyk-Milbrandt G, Friedman MI. Metabolic inhibitors synergistically decrease hepatic energy status and increase food intake. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1579–R1582. doi: 10.1152/ajpregu.2000.278.6.R1579. [DOI] [PubMed] [Google Scholar]
- 20.Johnson RE, Kark RM. Environment and food intake in man. Science. 1947;105:378–379. doi: 10.1126/science.105.2728.378. [DOI] [PubMed] [Google Scholar]
- 21.Keppler D, Decker K. Glycogen. In: Bergmeyer HU, Bergmeyer J, Grassl M, editors. Methods of Enzymatic Analysis. Verlag Chemie; Deerfield Beach, FL: 1983. pp. 11–18. [Google Scholar]
- 22.Manita S, Bachmanov AA, Li X, Beauchamp GK, Inoue M. Is glycine “sweet” to mice? Mouse strain differences in perception of glycine taste. Chem Senses. 2006;31:785–793. doi: 10.1093/chemse/bjl020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maughan RJ. A simple, rapid method for the determination of glucose, lactate, pyruvate, alanine, 3-hydroxybutyrate and acetoacetate on a single 20-μl blood sample. Clin Chim Acta. 1982;122:231–240. doi: 10.1016/0009-8981(82)90282-0. [DOI] [PubMed] [Google Scholar]
- 24.McDonald JD, Andriolo M, Cali F, Mirisola M, Puglisi-Allegra S, Romano V, Sarkissian CN, Smith CB. The phenylketonuria mouse model: a meeting review. Mol Genet Metab. 2002;76:256–261. doi: 10.1016/s1096-7192(02)00115-4. [DOI] [PubMed] [Google Scholar]
- 26.Ninomiya Y, Funakoshi M. Behavioural discrimination between glutamate and the four basic taste substances in mice. Comp Biochem Physiol A. 1989;92:365–370. doi: 10.1016/0300-9629(89)90577-x. [DOI] [PubMed] [Google Scholar]
- 27.Rawson NE, Blum H, Osbakken MD, Friedman MI. Hepatic phosphate trapping, decreased ATP, and increased feeding after 2,5-anhydro-d-mannitol. Am J Physiol Regul Integr Comp Physiol. 1994;266:R112–R117. doi: 10.1152/ajpregu.1994.266.1.R112. [DOI] [PubMed] [Google Scholar]
- 28.Reeds PJ, Burrin DG, Stoll B, Jahoor F. Intestinal glutamate metabolism. J Nutr. 2000;130:978S–982S. doi: 10.1093/jn/130.4.978S. [DOI] [PubMed] [Google Scholar]
- 29.Sclafani A. Oral and postoral determinants of food reward. Physiol Behav. 2004;81:773–779. doi: 10.1016/j.physbeh.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 30.Sclafani A, Ackroff K. The relationship between food reward and satiation revisited. Physiol Behav. 2004;82:89–95. doi: 10.1016/j.physbeh.2004.04.045. [DOI] [PubMed] [Google Scholar]
- 31.Sclafani A, Glendinning JI. Flavor preferences conditioned in C57BL/6 mice by intragastric carbohydrate self-infusion. Physiol Behav. 2003;79:783–788. doi: 10.1016/s0031-9384(03)00174-4. [DOI] [PubMed] [Google Scholar]
- 32.Sclafani A, Glendinning JI. Sugar and fat conditioned flavor preferences in C57BL/6J and 129 mice: oral and postoral interactions. Am J Physiol Regul Integr Comp Physiol. 2005;289:R712–R720. doi: 10.1152/ajpregu.00176.2005. [DOI] [PubMed] [Google Scholar]
- 33.Singer JB, Hill AE, Burrage LC, Olszens KR, Song J, Justice M, O’Brien WE, Conti DV, Witte JS, Lander ES, Nadeau JH. Genetic dissection of complex traits with chromosome substitution strains of mice. Science. 2004;304:445–448. doi: 10.1126/science.1093139. [DOI] [PubMed] [Google Scholar]
- 34.Smriga M, Murakami H, Mori M, Torii K. Use of thermal photography to explore the age-dependent effect of monosodium glutamate, NaCl and glucose on brown adipose tissue thermogenesis. Physiol Behav. 2000;71:403–407. doi: 10.1016/s0031-9384(00)00350-4. [DOI] [PubMed] [Google Scholar]
- 35.Stegink LD, Ann Reynolds W, Filer LJ, Baker GL, Daabees TT, Pitkin RM. Comparative metabolism of glutamate in the mouse, monkey, and man. In: Filer LJ, editor. Glutamic Acid: Advances in Biochemistry and Physiology. Raven; New York: 1979. pp. 85–102. [Google Scholar]
- 36.Stubbs RJ. Dietary macronutrients and glucostatic control of feeding. Proc Nutr Soc. 1996;55:467–483. doi: 10.1079/pns19960042. [DOI] [PubMed] [Google Scholar]
- 37.Takasaki Y, Matsuzawa Y, Iwata S, O’hara Y, Yonetani S, Ichimura M. Toxicological studies of monosodium l-glutamate in rodents: relationship between routes of administration and neurotoxicity. In: Filer LJ, editor. Glutamic Acid: Advances in Biochemistry and Physiology. Raven; New York: 1979. pp. 255–275. [Google Scholar]
- 38.Tordoff MG, Friedman MI. Hepatic portal glucose infusions decrease food intake and increase food preference. Am J Physiol Regul Integr Comp Physiol. 1986;251:R192–R196. doi: 10.1152/ajpregu.1986.251.1.R192. [DOI] [PubMed] [Google Scholar]
- 39.Walls EK, Koopmans HS. Differential effects of intravenous glucose, amino acids, and lipid on daily food intake in rats. Am J Physiol Regul Integr Comp Physiol. 1992;262:R225–R234. doi: 10.1152/ajpregu.1992.262.2.R225. [DOI] [PubMed] [Google Scholar]
- 40.Westerterp-Plantenga MS. The significance of protein in food intake and body weight regulation. Curr Opin Clin Nutr Metab Care. 2003;6:635–638. doi: 10.1097/00075197-200311000-00005. [DOI] [PubMed] [Google Scholar]
- 41.Westerterp-Plantenga MS, Lejeune MP. Protein intake and body-weight regulation. Appetite. 2005;45:187–190. doi: 10.1016/j.appet.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 42.Westerterp-Plantenga MS, Rolland V, Wilson SA, Westerterp KR. Satiety related to 24 h diet-induced thermogenesis during high protein/carbohydrate vs. high fat diets measured in a respiration chamber. Eur J Clin Nutr. 1999;53:495–502. doi: 10.1038/sj.ejcn.1600782. [DOI] [PubMed] [Google Scholar]
- 43.Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Cilissen C, Top S. Energy metabolism in women during short exposure to the thermoneutral zone. Physiol Behav. 2002;75:227–235. doi: 10.1016/s0031-9384(01)00649-7. [DOI] [PubMed] [Google Scholar]
- 44.Westerterp-Plantenga MS, van Marken Lichtenbelt WD, Strobbe H, Schrauwen P. Energy metabolism in humans at a lowered ambient temperature. Eur J Clin Nutr. 2002;56:288–296. doi: 10.1038/sj.ejcn.1601308. [DOI] [PubMed] [Google Scholar]
- 45.Woods SC, Stein LJ, McKay LD, Porte D., Jr Suppression of food intake by intravenous nutrients and insulin in the baboon. Am J Physiol Regul Integr Comp Physiol. 1984;247:R393–R401. doi: 10.1152/ajpregu.1984.247.2.R393. [DOI] [PubMed] [Google Scholar]
- 46.Wu G. Intestinal mucosal amino acid catabolism. J Nutr. 1998;128:1249–1252. doi: 10.1093/jn/128.8.1249. [DOI] [PubMed] [Google Scholar]