Abstract
The purpose of this study was to characterize methylmercury (MeHg)–induced dopamine (DA) release from undifferentiated pheochromocytoma (PC12) cells and to examine the potential role for DA synthesis in this process. MeHg caused a significant increase in DA release that was both concentration- and time-dependent. DA release was significantly increased by 2µM MeHg at 60min and by 5µM MeHg at 30min; 1µM MeHg was without effect. Because DA release induced by 5µM MeHg was associated with a significant percentage of cell death at 60 and 120min, 2µM MeHg was chosen for further characterization of release mechanisms. MeHg-induced DA release was attenuated but not abolished in the absence of extracellular calcium, whereas the vesicular content depleting drug reserpine (50nM) abolished release. Thus, MeHg-induced DA release requires vesicular exocytosis but not extracellular calcium. MeHg also increased intracellular DA and the rate of DA storage utilization, suggesting a role for DA synthesis in MeHg-induced DA release. The tyrosine hydroxylase inhibitor α-methyltyrosine (300µM, 24h) completely abolished MeHg-induced DA release. MeHg significantly increased DA precursor accumulation in cells treated with 3-hydroxybenzylhydrazine (10µM), revealing that MeHg increases tyrosine hydroxylase activity. Overall, these data demonstrate that MeHg facilitates DA synthesis, increases intracellular DA, and augments vesicular exocytosis.
Key Words: methylmercury-induced neurotoxicity, dopamine release, dopamine synthesis, PC12 cell, tyrosine hydroxylase.
Methylmercury (MeHg) is an environmental pollutant with potent neurological effects including paresthesia, ataxia, muscle weakness, and impairment of vision and hearing (Takeuchi et al., 1962). Human exposure to MeHg has been correlated with increased incidence of neurodegenerative diseases, including Parkinson disease (Ngim and Devathasan, 1989; Wermuth et al., 2000). MeHg induces cell-specific neurotoxicity, but the underlying mechanism by which specific cell types exhibit selective sensitivity is not known.
Neurotoxicity associated with MeHg exposure is multifaceted and involves perturbation of intracellular calcium (Ca2+) concentration, production of free radicals due to altered mitochondrial function, and impaired protein, DNA, and RNA biosynthesis (Ceccatelli et al., 2010; Limke et al., 2004). MeHg also stimulates spontaneous transmitter release but impairs depolarization-evoked transmitter release (Atchison et al., 1984). This effect has been demonstrated in many neurotransmitter systems, which include the dopaminergic system (Minnema et al., 1989). MeHg increases spontaneous dopamine (DA) release in a concentration-dependent manner through a presynaptic action (Dreiem et al., 2009; Faro et al., 2002; Kalisch and Racz, 1996).
In the mammalian central nervous system, MeHg accumulates mainly in the cortex, striatum, cerebellum, brain stem, and spinal cord (Møller-Madsen, 1994). This pattern of distribution could contribute to the presentation of motor symptoms associated with MeHg intoxication. Because proper basal ganglia function to modulate voluntary movement could be impaired by MeHg accumulation, the striatum has been a major target of research related to MeHg toxicity.
DA release occurs predominantly via Ca2+-dependent vesicular exocytosis; however, it can also be induced through alternative pathways, including reversal of the DA transporter (Leviel, 2011). Additionally, DA release is tightly coupled to synthesis, whereby end-product feedback inhibition decreases the rate of DA synthesis, and phosphorylation of tyrosine hydroxylase (TH) increases it (Dunkley et al., 2004).
Results of studies investigating the mechanisms underlying the stimulatory effects of MeHg on dopaminergic neuronal transmission have been inconsistent with respect to the underlying mechanisms responsible for MeHg-induced DA release. A role for DA transporter reversal has been proposed based on the finding that DA transporter inhibition or stimulation of transporter-mediated release produces an effect similar to that of MeHg alone (Faro et al., 2002). However, MeHg does not target the transporter to induce release (Gassó et al., 2000) and may decrease transporter reuptake activity (Bonnet et al., 1994; Dreiem et al., 2009).
Roles for extracellular Ca2+ and vesicular exocytosis in MeHg-induced catecholamine release have also been documented (Gassó et al., 2000); however, nonvesicular and extracellular Ca2+-independent DA release has also been described following MeHg exposure (Faro et al., 2002; Kalisch and Racz, 1996). Despite the coupling between release and synthesis, no reports to date have investigated a potential role for DA synthesis in MeHg-mediated release. Therefore, the goals of this study were to (1) identify pathways by which MeHg induces spontaneous DA release in a well-characterized cell model and differentiate between transporter-mediated and nontransporter-mediated release, (2) determine the dependence of MeHg-induced DA release on extracellular Ca2+, and (3) examine the coupling between DA synthesis and MeHg-induced DA release.
Given the multiplicity of targets affected by MeHg and the complex and dynamic process of transmitter release, an in vitro model allows the separate steps to be examined in isolation, obviating potentially confounding effects of multiple pathways present in vivo. Undifferentiated rat pheochromocytoma (PC12) cells synthesize and store large amounts of DA, contain high concentrations of the enzymes necessary for DA synthesis and metabolism, and release DA via Ca2+-dependent vesicular exocytosis (Greene and Rein, 1977; Kishimoto et al., 2005). The effects of MeHg have been well described in this system (Shafer and Atchison, 1991).
MATERIALS AND METHODS
Chemicals and Solutions
Cell culture supplies, including RPMI-1640 medium, horse serum, trypsin, and penicillin-streptomycin, were purchased from GIBCO BRL (Grand Island, NY). Hyclone fetal bovine serum was purchased from Thermo Scientific (Logan, UT). Hoechst 33342 and propidium iodide were purchased from Invitrogen (Grand Island, NY). α-Methyl-DL-tyrosine methyl ester hydrochloride (AMT), 3-hydroxybenzylhydrazine (NSD-1015), reserpine, desipramine (DMI), poly-D-lysine hydrobromide, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid (HEPES), and bicinchoninic acid protein assay buffer were all purchased from Sigma-Aldrich (St Louis, MO). Methyl mercuric chloride (MeHg) was purchased from ICN Biochemicals Inc. (Aurora, OH).
The standard physiological saline used for extracellular solution was HEPES-buffered saline (HBS), which contained the following (mM): 150 NaCl, 5 KCl, 2.4 CaCl2, 1.6 MgSO4, 20 HEPES, and 20 d-glucose (pH 7.3). The Ca2+-free buffer had the same composition as the HBS with the following exceptions (mM): 0 CaCl2 and 0.02 EDTA. MeHg, AMT, and DMI were prepared as 10mM stock solutions and NSD-1015 as a 1mM stock solution in distilled water. Reserpine was dissolved in 100% dimethyl sulfoxide (DMSO) as a 10mM stock solution and then diluted so the final concentration of DMSO was less than 0.05% (vol/vol). MeHg and pharmacological inhibitors were diluted to working concentrations in HBS or culture medium on the day of each experiment.
Culture of PC12 Cells
PC12 cells (Gift of Dr M. L. Contreras) were grown in RPMI-1640 medium supplemented with 10% (vol/vol) horse serum, 2.5% (vol/vol) fetal bovine serum, and 1% (vol/vol) penicillin-streptomycin (pH 7.3). Cultures were maintained in either 25-cm2 or 75-cm2 T-flasks in a humidified environment containing 5% CO2 at 37°C. Culture medium was changed every 2–3 days. Every 4–5 days PC12 cultures were detached from the flasks with 0.25% (vol/vol) trypsin and subcultured at a density of 3×105cells/ml for a 5-day culture or 4×105cells/ml for a 4-day culture. All cultures were maintained at 80–90% confluence at the time of subculture. To maintain consistency from experiment to experiment, cells were used between passages 16 and 19 from our receipt. Experimental conditions were repeated in biological triplicate, and experiments were replicated at least thrice from separate cultures to minimize the risk of culture-specific confound.
Cell Viability Analysis
PC12 cells were seeded in 96-well plates coated with poly-D-lysine at 4×105 cells/ml 48h prior to treatment with HBS, 1, 2, or 5µM MeHg for 15, 30, 60, or 120min. Hoechst 33342 (10mg/ml) and propidium iodide (1mg/ml) were diluted 1:10,000 in existing treatment medium. Cells were incubated in fluorophores at 37°C for 15min prior to visualization on a Nikon Ellipse TE2000-U inverted microscope with diascopic illumination pillar (Nikon Instruments, Inc., Melville, NY) equipped with a T-FL EPO fluorescence attachment and X-Cite metal halide illumination system. 4′,6-Diamidino-2-phenylindole (358nm/461nm) and rhodamine filters (610nm) were used for visualization. Images were acquired using MetaMorph image acquisition and analysis software (Molecular Devices, Sunnyvale, CA). One image was acquired per well, and each treatment was replicated in triplicate wells in three separate experiments. Viable cells had a blue-stained (Hoechst) nucleus, whereas nonviable cells had a low blue, high red (propidium iodide) fluorescence (Yuan et al., 2009).
Measurements of Extracellular and Intracellular Neurochemistry
PC12 cells were seeded in 6-well plates coated with poly-D-lysine at a density of 6×105 cells/ml 48h prior to the following treatments:
Concentration response and time course.
Culture medium was aspirated and replaced with HBS or HBS containing 1, 2, or 5µM MeHg. DA concentration was sampled 15, 30, 60, and 120min following continuous exposure to only one MeHg concentration.
Ca2+-free treatment.
Culture medium was aspirated and replaced with HBS or Ca2+-free HBS containing vehicle or 2µM MeHg for 60min.
Pharmacological inhibition.
Several pharmacological treatments were used to analyze distinct components of the dopaminergic biosynthetic pathway or vesicular release. These included (1) reserpine (50nM), which inhibits the vesicular monoamine transporter and at a concentration of 50nM depletes DA stores in PC12 cells by 50% within 1h (Drukarch et al., 1996); (2) DMI (1μM), which inhibits the norepinephrine (NE) transporter (Brüss et al., 1997), the primary catecholamine transport mechanism in PC12 cells (Lorang et al., 1994); (3) AMT (300µM), which inhibits TH, the rate-limiting enzyme in DA synthesis (Spector et al., 1965); and (4) NSD-1015 (10µM), a 3,4-dihydroxyphenylalanine (DOPA) decarboxylase inhibitor (Carlsson et al., 1972) used to estimate TH activity by assessment of DOPA accumulation (Demarest and Moore, 1979).
Pharmacological interventions consisted of two distinct approaches. In the first approach, cells were pretreated in culture medium with reserpine for 60min, DMI for 15min, or AMT for either 24h or 90min. Following pretreatment, culture medium was aspirated and replaced with HBS or HBS containing 2µM MeHg and/or the pharmacological inhibitor used in pretreatment for the 60min MeHg exposure. In the second approach, cells were exposed to HBS or HBS containing 2µM MeHg and/or NSD-1015 for 60min. For both approaches, cell plates were centrifuged at 500 × g for 3min at 4°C to terminate the experiment. Treatment medium was reserved and acidified (1:1) with ice-cold tissue buffer (0.1M phosphate-citrate buffer containing 15% methanol (vol/vol), pH 2.5). Cells were rinsed once with 1ml ice-cold PBS, harvested, and pelleted by centrifugation at 12,000 × g for 5min at 4°C. After centrifugation, the supernatant was removed and replaced with 100 μl of ice-cold tissue buffer.
DA content in the supernatant was determined by means of high-pressure liquid chromatography coupled with electrochemical detection using a Water 515 HPLC pump (Waters Corp., Milford, MA) and an ESA Coulochem 5100A electrochemical detector with an oxidation potential of +0.4V. DA content was quantified by comparing peak height of each sample to peak heights of standards. It was then normalized to milliliter per sample for extracellular measurements or milligram protein for intracellular measurements as determined by the bicinchoninic acid protein assay.
Calculation of the Rate Constant
Releasable stores of transmitter in catecholamine secreting cells are maintained by the feedback regulation of the balance between vesicular release and de novo synthesis-dependent replenishment. The slope of decline (or rate constant) of intracellular DA following inhibition of synthesis represents a reliable, indirect measurement of release or DA storage utilization (Brodie et al., 1966). In the present study, the rate constant was calculated from intracellular DA concentrations after 90-min AMT treatment (Brodie et al., 1966). The slope of the lines representing the difference between cells treated with 2µM MeHg alone or combined with 300µM AMT was calculated and then compared with that of HBS-treated cells in the absence or presence of AMT.
Statistical Analysis
SigmaPlot software version 12.0 (SysStat Software, Inc., Point Richmond, CA) was used to make statistical comparisons among groups using unpaired t-test, one- and two-way ANOVA, or nonparametric alternatives as appropriate. If a significant difference was detected, post hoc between-group comparisons were performed using Tukey’s test. Statistical significance was set at p < 0.05.
RESULTS
Spontaneous DA Release Is Increased by MeHg in a Concentration- and Time-Dependent Manner
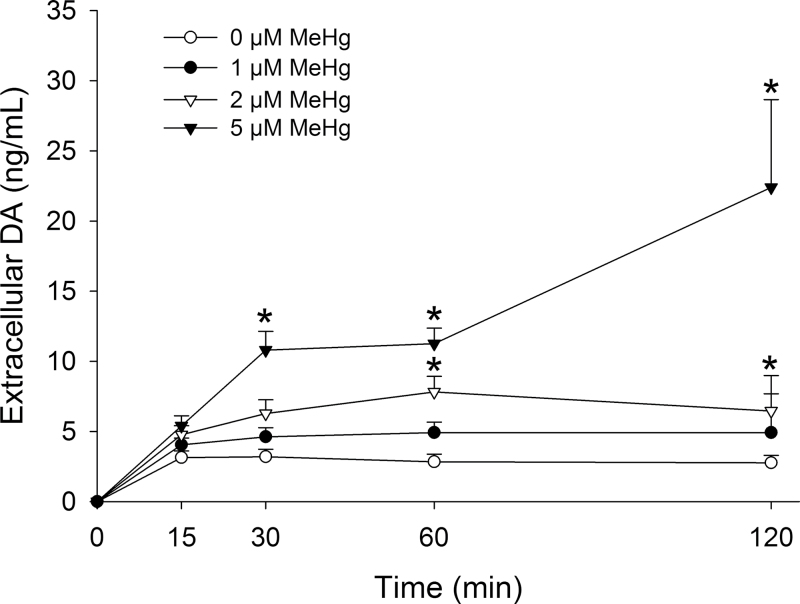
Measurements of DA in the medium reflect the balance between changes in DA release and transporter-mediated reuptake. DA was not detected in treatment medium in the absence of cells, and any subsequent treatment-induced change in medium DA was, therefore, due to cellular DA release. In the absence of MeHg, the concentration of extracellular DA stabilized within the first 15min and remained at a steady state throughout the 120-min sampling period (Fig. 1). MeHg caused both a concentration- and time-dependent increase in medium DA. At 1µM, MeHg did not significantly alter extracellular DA accumulation, whereas 2 and 5µM MeHg significantly increased the concentration of extracellular DA by 60 and 30min, respectively. These elevated levels were maintained for the duration of the experiment. The significant increase in extracellular DA concentrations induced by 5µM MeHg at 60 and 120min was associated with a significant incidence of cytotoxicity in a parallel set of cultures (Table 1). Because 2µM MeHg induced a significant increase in DA release by 60min without inducing significant levels of cytotoxicity, this concentration and time point were selected for further analysis of release mechanisms.
Fig. 1.
Concentration-response and time-course effects of MeHg on extracellular DA concentration. PC12 cells were treated with 0µM (white circles), 1µM (black circles), 2µM (white triangles), or 5µM (black triangles) MeHg in HBS for 15, 30, 60, or 120min. * indicates a significant difference compared with 0µM MeHg for a given time point (p ≤ 0.05). Values are means ± SEM (n = 3–4, three replicates per n).
Table 1 .
Effects of MeHg Exposure on Cell Viability in Undifferentiated Pheochromocytoma (PC12) Cells
| MeHg (µM)a | Cell death (% of population) | |||
|---|---|---|---|---|
| Time (min) | ||||
| 15 | 30 | 60 | 120 | |
| 0 | 0.8±0.2b | 1.0±0.3 | 0.8±0.2 | 4.1±0.9 |
| 1 | 0.8±0.3 | 1.4±0.6 | 1.8±0.4 | 5.8±3.4 |
| 2 | 0.7±0.2 | 2.2±0.2 | 2.8±0.9 | 4.6±2.1 |
| 5 | 2.0±0.4 | 10.3±4.9 | 48.3±17.8* | 71.6±21.0* |
Notes. aPC12 cells were exposed to 0, 1, 2, or 5µM MeHg in HBS for 15, 30, 60, or 120min. Following toxicant treatment, cells were incubated with Hoechst 33342 and propidium iodide for 15min at 37°C prior to visualization and image acquisition. Viable cells had a blue-stained (Hoechst) nucleus, whereas nonviable cells had a low blue, high red (propidium iodide) fluorescence.
bValues are means ± SEM.
*The asterisk indicates a value significantly different from 0µM MeHg within time point (p < 0.05).
Spontaneous MeHg-Mediated DA Release Is Partially Dependent upon the Presence of Extracellular Ca2+
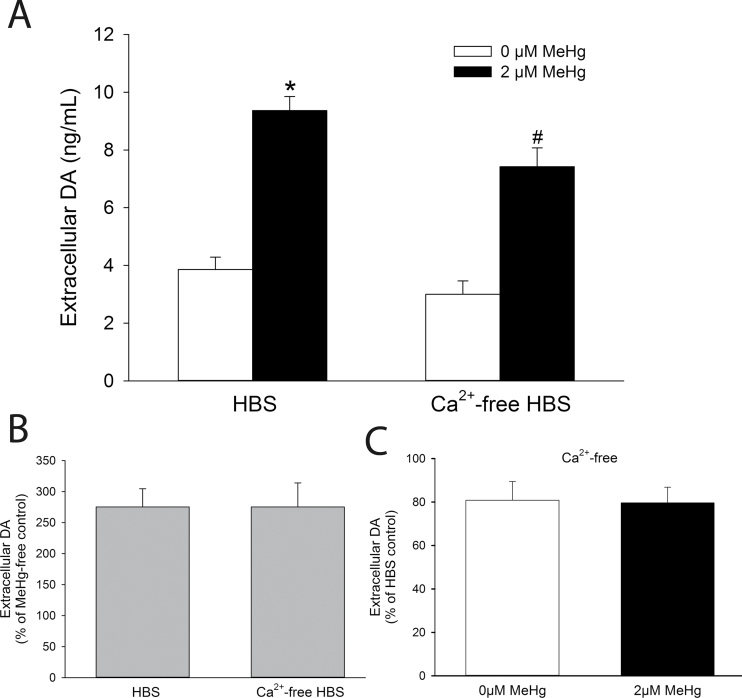
DA is released from PC12 cells through Ca2+-dependent exocytosis (Kishimoto et al., 2005). Because MeHg induces extracellular Ca2+ influx (Marty and Atchison, 1997), a role for extracellular Ca2+ in MeHg-mediated DA release from PC12 cells was evaluated by measuring extracellular DA concentrations after exposure to MeHg in a Ca2+-free solution. There was a slight nonsignificant decrease in spontaneous DA release in cells incubated in Ca2+-free HBS (Fig. 2A). In the absence of extracellular Ca2+, MeHg-induced DA release from PC12 cells was significantly attenuated compared with that from HBS-treated cells. However, there was still a dramatic increase in DA released by MeHg in the absence of extracellular Ca2+. It was not significantly different from DA release in the presence of extracellular Ca2+ (Fig. 2B). Removal of Ca2+ from the medium did not attenuate DA release in either the absence or the presence of MeHg (Fig. 2C).
Fig. 2.
Role of extracellular Ca2+ in MeHg-induced DA release. (A) The concentration of extracellular DA was measured from PC12 cells treated for 60min with 0µM (white bars) or 2µM (black bars) MeHg in the presence (HBS) or absence (Ca2+-free HBS) of extracellular Ca2+. * indicates a significant difference from HBS-Control (p ≤ 0.05). # indicates a significant difference from both HBS-Control and HBS-MeHg (p ≤ 0.05). (B) The percentage change of extracellular DA after treatment with MeHg was calculated in cells treated with either HBS or Ca2+-free HBS. (C) The percentage reduction of extracellular DA by treatment with Ca2+-free HBS in cells treated with either 0 or 2µM MeHg. Values are means ± SEM (n = 4, three replicates per n).
Spontaneous MeHg-Mediated DA Release Requires a Functional Vesicular Transporter, but Not a Functional Membrane Transporter
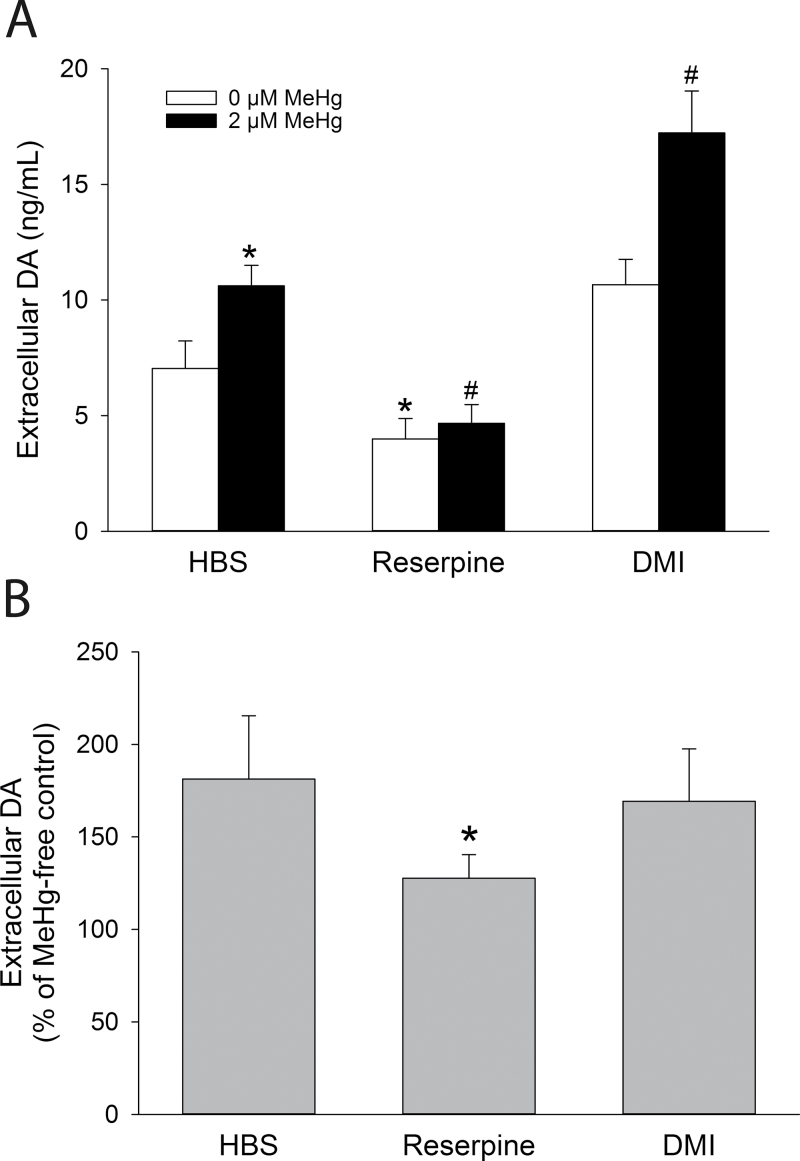
In PC12 cells, DA is primarily released from large dense-core vesicles concentrated near the plasma membrane (Bauerfeind et al., 1993; Fornai et al., 2007) and recaptured by the NE transporter (Lorang et al., 1994). To investigate the contributions of vesicular release and reuptake transporter activity in spontaneous MeHg-mediated DA release from PC12 cells, reserpine was used to inhibit the vesicular monoamine transporter (Schuldiner et al., 1993), and DMI was used to block the NE transporter (Brüss et al., 1997). Reserpine (50nM) decreased spontaneous basal release and blocked the significant increase in extracellular DA induced by MeHg. In contrast, DMI had no significant effect on extracellular DA concentration (p > 0.05) but significantly augmented MeHg-induced DA release (Fig. 3A). However, although reserpine significantly attenuated the percentage change in extracellular DA induced by MeHg, DMI did not (p > 0.05; Fig. 3B).
Fig. 3.
Effects of DA storage depletion and reuptake transporter inhibition on MeHg-induced DA release. (A) Concentrations of extracellular DA from PC12 cells preincubated with 50nM reserpine for 60min, 1µM DMI for 15min, or vehicle in culture medium prior to cotreatment with 0µM (white bars) or 2µM (black bars) MeHg in HBS for 60min. * indicates a significant difference from 0µM MeHg-HBS (control) (p ≤ 0.05). # indicates a significant difference from 2µM MeHg-HBS (control) (p ≤ 0.05). (B) The percentage change of extracellular DA after treatment with MeHg in cells treated with HBS, 50nM reserpine, or 1µM DMI. * indicates a significant difference from HBS (p ≤ 0.05). Values are means ± SEM (n = 3, three replicates per n).
MeHg-Mediated DA Release Is Associated with Increased Intracellular DA Concentrations and Utilization of DA Stores
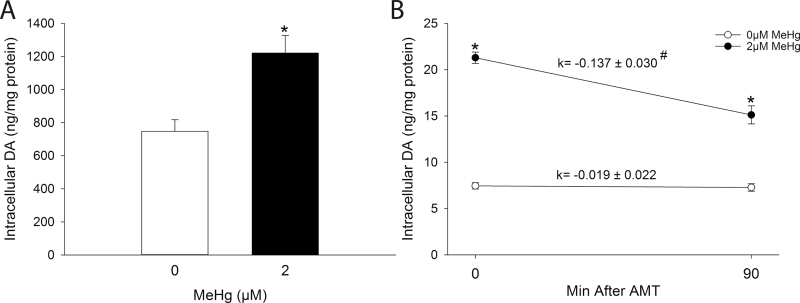
Spontaneous DA release induced by MeHg was associated with a significant increase in the concentration of intracellular DA (Fig. 4A). To determine whether increased DA stores correlated with increased release, DA storage utilization was assessed using AMT (Brodie et al., 1966). The rate constant of decline of intracellular DA was significantly greater in MeHg-treated cells compared with HBS-treated cells, demonstrating that utilization of DA stores was significantly increased following MeHg exposure (Fig. 4B).
Fig. 4.
(A) Effect of MeHg on intracellular DA concentration. PC12 cells were treated with either 0µM (white bars) or 2µM (black bars) MeHg in HBS for 60min. * indicates a significant difference from 0µM MeHg (p ≤ 0.05). (B) Effect of MeHg on DA storage utilization. PC12 cells were preincubated with 300µM AMT or vehicle in culture medium for 30min prior to treatment with either 0µM (white bars) or 2µM (black bars) MeHg in HBS in the presence or absence of 300µM AMT for 60min. The rate constant (k) was calculated as the slope of the line between 0 and 90min after AMT. * indicates a significant difference from 0µM MeHg within time point (p ≤ 0.05). # indicates a significant difference between rate constants (p ≤ 0.05). Values are means ± SEM (n = 3, three replicates per n).
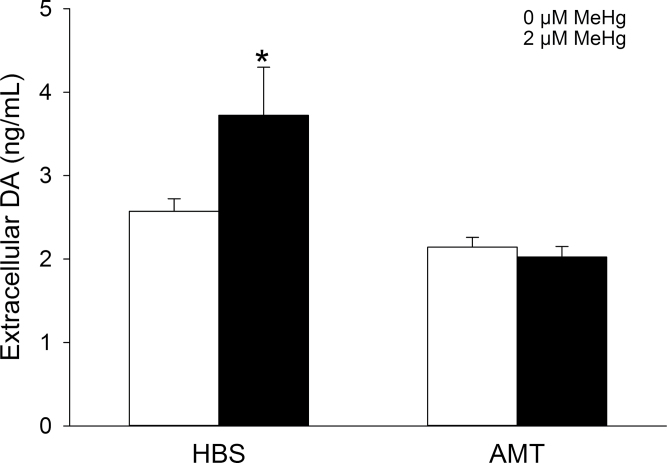
MeHg-Mediated DA Release Is Associated with De Novo DA Synthesis and Increased TH Enzymatic Activity
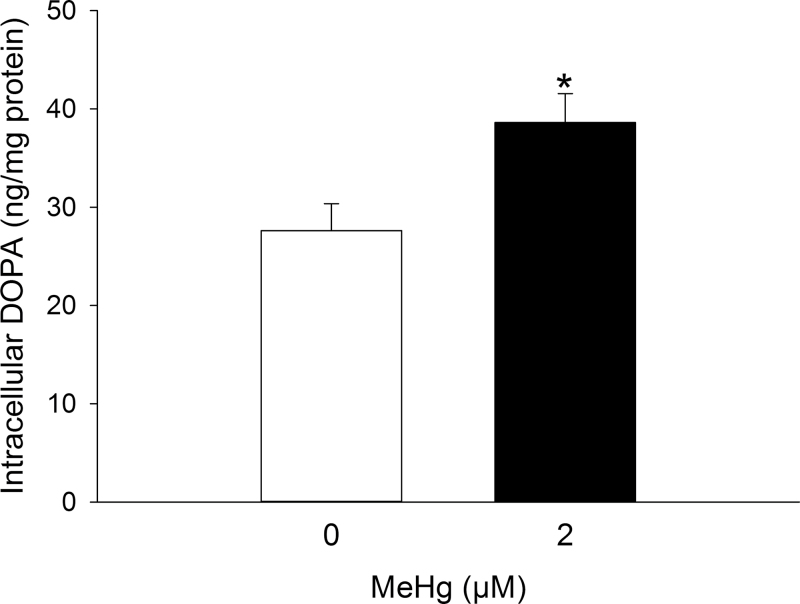
To examine the role of de novo DA synthesis in MeHg-mediated DA release, PC12 cells were treated with either the competitive TH inhibitor AMT (300μM) for 24h or the DOPA decarboxylase inhibitor NSD-1015 (10μM) for 60min. Complete inhibition of DA synthesis with AMT abolished the MeHg-mediated increase in extracellular DA concentrations but did not alter basal DA release (Fig. 5). The DA precursor DOPA was not detectable except in the presence of NSD-1015, after which its concentration was proportional to the rate of TH enzymatic activity. MeHg significantly increased the concentration of intracellular DOPA compared with HBS-treated cells (Fig. 6).
Fig. 5.
Effect of TH inhibition on MeHg-induced DA release. PC12 cells were preincubated with 300µM AMT or vehicle in culture medium for 24h prior to cotreatment with either 0µM (white bars) or 2µM (black bars) MeHg in HBS for 60min. * indicates a significant difference from 0µM MeHg-HBS (p ≤ 0.05). Values are means ± SEM (n = 3, three replicates per n).
Fig. 6.
Effect of DOPA decarboxylase inhibition on TH enzymatic activity. PC12 cells were treated with 0µM (white bars) or 2µM (black bars) MeHg in HBS for 60min in the presence of 10µM NSD-1015. DOPA accumulation was quantified using high-pressure liquid chromatography coupled with electrochemical detection as an index of TH enzymatic activity. * indicates a significant difference between groups (p ≤ 0.05). Values are means ± SEM (n = 4, three replicates per n).
DISCUSSION
This study contributes significantly to our understanding of mechanisms by which MeHg induces spontaneous DA release, in particular its interaction with the coupling between synthesis and vesicular exocytosis. Our data are consistent with the following conclusions: (1) MeHg causes a concentration- and time-dependent increase in DA release from PC12 cells; (2) MeHg-induced DA release does not depend upon the presence of extracellular Ca2+; (3) vesicular exocytosis, but not reuptake transporter activity, is responsible for both basal and MeHg-induced DA release; and (4) MeHg-induced DA release is dependent upon de novo DA synthesis and accelerated TH activity.
MeHg-Induced DA Release
Under basal conditions, DA release from PC12 cells is maintained at a steady state. During the first 15min, DA concentrations increased from 0 to ~3ng/ml and then did not fluctuate significantly for the duration of the experiment. The consistent level of extracellular DA in the medium likely reflects coupling between release and reuptake under basal conditions (Schmitz et al., 2003).
Increased DA release after exposure to MeHg has been well documented in other systems, including 3H-DA release from rat striatum (Minnema et al., 1989) and endogenous DA release from striatal synaptosomes (Dreiem et al., 2009), mouse striatal slices (Kalisch and Racz, 1996), and the striatum of conscious, free-moving rats (Faro et al., 2002). Results from the present study replicate these observations and demonstrate that MeHg-induced DA release is both concentration- and time-dependent. Low concentrations of MeHg (1μM) did not alter DA release; however, higher concentrations (2–5μM) increased DA release. Furthermore, 5μM MeHg increased DA release more rapidly than did 2μM.
The increase in extracellular DA after exposure to 5μM MeHg at 60 and 120min was substantially higher than that observed at lower concentrations but was more variable and associated with an increased incidence of cell death. Exposure to 5μM has previously been associated with rapid impairment of mitochondrial activity and membrane lysis consistent with necrotic cell death (Castoldi et al., 2000). As cells lyse, DA would be purged from the cell and artificially increase its extracellular concentration. DA released upon cell lysis would account for the sizable increase in extracellular DA and associated high variability. On the other hand, 2μM MeHg increased DA release by 60min, an effect not associated with cell death. The increase in DA release in the absence of cell death demonstrates that MeHg-induced DA release is associated with intracellular mechanisms unrelated to cell lysis. Ca2+ plays an essential role in neurotransmitter release, and Ca2+-dependent neurotransmitter release is a known target of MeHg toxicity (Atchison, 1986; Atchison and Narahashi, 1982). Therefore, MeHg-induced Ca2+ signaling could be a primary site of action linking MeHg exposure to increased DA release.
Role of Extracellular Ca2+ in Spontaneous and MeHg-Mediated DA Release
Spontaneous basal DA release from PC12 cells was independent of extracellular Ca2+ because removal of Ca2+ from the extracellular medium did not reduce the concentration of extracellular DA. Although DA release from PC12 cells is classically described as Ca2+ dependent (Greene and Rein, 1977), the source of Ca2+ can be intracellular (Kishimoto et al., 2005).
Therefore, spontaneous DA release from PC12 cells is most likely modulated by changes in cytosolic Ca2+ independent of extracellular Ca2+ influx.
MeHg induces a biphasic rise in intracellular Ca2+. Initially, Ca2+ is released from intracellular stores followed by Ca2+ entry from the extracellular space (Marty and Atchison, 1997). Removal of Ca2+ from the medium attenuated the rise in extracellular DA following exposure to MeHg, demonstrating a partial dependence on extracellular Ca2+. This observation suggests that the second-phase Ca2+ influx following exposure to the toxicant contributes to DA release. However, further analysis revealed that the contribution of extracellular Ca2+ is minimal; MeHg induced comparable increases in extracellular DA in the absence and presence of extracellular Ca2+.
These data are consistent with previous reports in the literature, demonstrating that spontaneous DA release is diminished, but not abolished, in a Ca2+-free superfusate (Kalisch and Racz, 1996). A large contribution of extracellular Ca2+ to the observed MeHg-induced DA release was not expected, considering that release was not evoked by depolarization in the present study. Rather, the phenomenon is more likely to due to release of Ca2+ from intracellular stores that results during first-phase Ca2+ influx following exposure to MeHg (Marty and Atchison, 1997). Alternatively, inhibition of the Na+/K+ ATPase by MeHg could also produce an increase in intracellular Ca2+ that contribute to release (Berg and Miles, 1979).
Vesicular Transport, but Not Membrane Transport, Contributes to MeHg-Induced DA Release
Vesicular exocytosis and transporter-mediated reuptake are essential components of catecholamine release and recycling in PC12 cells (Fornai et al., 2007; Kishimoto et al., 2005; Lorang et al., 1994). In the present study, depletion of storage vesicles decreased basal release, confirming a role for vesicular stores in DA release from PC12 cells. NE transporter inhibition caused a small, although nonsignificant, increase in extracellular DA likely due to inhibition of reuptake of released DA.
The observations that MeHg-induced DA release is partially dependent upon extracellular Ca2+ and intracellular Ca2+ concentrations are increased by MeHg (Marty and Atchison, 1997), suggest that mobilization of vesicular DA stores contributes to the stimulatory effect of MeHg on DA release. Indeed, depletion of DA stores abolishes MeHg-induced DA release. A role for vesicular exocytosis in MeHg-induced catecholamine release is consistent with reports in the literature, demonstrating that reserpine pretreatment blocks 3H-NE release from hippocampal slices (Gassó et al., 2000).
Inhibition of the NE transporter increased DA release above that induced by MeHg alone. However, the percent change in extracellular DA after MeHg was comparable in control and DMI groups. This suggests that the NE transporter is functional but not contributing to the rise in extracellular DA following exposure to MeHg. In contrast, transporter activity is reportedly decreased following exposure to MeHg (Bonnet et al., 1994; Dreiem et al., 2009) due to either a direct interaction between MeHg and the thiol group on the DA transporter (Bonnet et al., 1994) or indirectly through inhibition of the Na+/K+ ATPase (Berg and Miles, 1979) or reduction of ATP needed to maintain the ion gradient used by the transporter (Gatti et al., 2004; Torres et al., 2003). In the present study, it is possible that reuptake by the NE transporter was partially inhibited because MeHg and DMI treatment together produced a larger increase in extracellular DA that exceeded MeHg or DMI treatment alone.
Evidence for a Role of De Novo DA Synthesis in MeHg-Induced DA Release
MeHg-induced DA release was accompanied by an increase in intracellular DA stores and acceleration of DA storage utilization. Increased DA stores could result from either stimulated DA synthesis and/or impaired DA metabolism. MeHg impairs DA metabolism; concentrations of the DA metabolites 3,4-dihydroxyphenylacetic acid and homovanillic acid are significantly decreased following exposure to MeHg (Dreiem et al., 2009). However, the increased rate of storage utilization following MeHg exposure also suggests a role for DA synthesis. Newly synthesized DA is preferentially released (Kopin et al., 1968); thus, MeHg could also accelerate DA synthesis to increase DA release.
To elucidate the contribution of the DA biosynthetic pathway to the effects of MeHg, two pharmacological manipulations were used. Inhibition of TH with AMT completely abolished MeHg-induced DA release. This confirms that MeHg alters DA synthesis by targeting the rate-limiting enzyme in DA synthesis, TH. Furthermore, indirect assessment of TH activity using NSD-1015 suggests that MeHg stimulated TH activity; i.e., DOPA accumulation was increased following toxicant treatment. TH activity is regulated via phosphorylation of critical serine residues located on its N-terminus (Haycock et al., 1992). Because MeHg increases intracellular Ca2+, TH phosphorylation mediated by Ca2+-dependent protein kinases (Albert et al., 1984) may be responsible for the observed increase in TH activity following exposure to MeHg. Increased cytosolic Ca2+ could also inhibit the activity of protein phosphastase 2A or 2C (Bevilaqua et al., 2003), preventing TH dephosphorylation.
This is the first report demonstrating a stimulatory effect of acute MeHg exposure on TH activity that culminates in an increase in DA synthesis and consequently DA release. A previous report documented elevated TH activity in brain homogenates isolated from rats treated with MeHg for 7 days (Omata et al., 1982); however, the downstream consequences of MeHg-activated TH activity on catecholamine release were not studied. Results presented herein describe how MeHg targeting of the DA biosynthetic pathway contributes to MeHg-mediated DA release. This could be one mechanism by which MeHg induces cell-specific neurotoxicity. Decoupling of the regulation between biosynthesis and release, leading to an abnormally high level of extracellular DA, could disrupt the signal-to-noise ratio associated with normal neuronal activity-evoked release at dopaminergic synapses.
FUNDING
National Institutes of Health (R01ES03299, R25NS006577).
ACKNOWLEDGMENTS
Authors gratefully acknowledge the advice and technical assistance of Dr Ravindra Hajela, Dr Seung-Hoon Baek, and Sara Fox.
REFERENCES
- Albert K. A., Helmer-Matyjek E., Nairn A. C., Müller T. H., Haycock J. W., Greene L. A., Goldstein M., Greengard P. (1984). Calcium/phospholipid-dependent protein kinase (protein kinase C) phosphorylates and activates tyrosine hydroxylase. Proc. Natl. Acad. Sci. U.S.A. 81, 7713–7717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atchison W. D. (1986). Extracellular calcium-dependent and -independent effects of methylmercury on spontaneous and potassium-evoked release of acetylcholine at the neuromuscular junction. J. Pharmacol. Exp. Ther. 237, 672–680 [PubMed] [Google Scholar]
- Atchison W. D., Clark A. W., Narahashi T. (1984). Presynaptic effects of methylmercury at the mammalian neuromuscular junction. In Cellular and Molecular Neurotoxicity. (Narahashi T., Ed.), pp. 23–43 Raven Press, New Jersey: [Google Scholar]
- Atchison W. D., Narahashi T. (1982). Methylmercury-induced depression of neuromuscular transmission in the rat. Neurotoxicology. 3, 37–50 [PubMed] [Google Scholar]
- Bauerfeind R., Régnier-Vigouroux A., Flatmark T., Huttner W. B. (1993). Selective storage of acetylcholine, but not catecholamines, in neuroendocrine synaptic-like microvesicles of early endosomal origin. Neuron. 11, 105–121 [DOI] [PubMed] [Google Scholar]
- Berg G. G., Miles E. F. (1979). Mechanisms of inhibition of active transport ATPases by mercurials. Chem. Biol. Interact. 27, 199–219 [DOI] [PubMed] [Google Scholar]
- Bevilaqua L., Cammorota M., Dickson P. W., Sim A. T. R., Dunkley P. R. (2003). Role of protein phosphatase 2C from bovine adrenal chromaffin cells in the dephosphorylation of phospho-serine 40 tyrosine hydroxylase. J. Neurochem. 85, 1368–1373 [DOI] [PubMed] [Google Scholar]
- Bonnet J. J., Benmansour S., Amejdki-Chab N., Costentin J. (1994). Effect of CH3HgCl and several transition metals on the dopamine neuronal carrier; peculiar behaviour of Zn2+ . Eur. J. Pharmacol. 266, 87–97 [DOI] [PubMed] [Google Scholar]
- Brodie B. B., Costa E., Dlabac A., Neff N. H., Smookler H. H. (1966). Application of steady state kinetics to the estimation of synthesis rate and turnover time of tissue catecholamines. J. Pharmacol. Exp. Ther. 154, 493–498 [PubMed] [Google Scholar]
- Brüss M., Pörzgen P., Bryan-Lluka L. J., Bönisch H. (1997). The rat norepinephrine transporter: Molecular cloning from PC12 cells and functional expression. Brain Res. Mol. Brain Res. 52, 257–262 [DOI] [PubMed] [Google Scholar]
- Carlsson A., Davis J. N., Kehr W., Lindqvist M., Atack C. V. (1972). Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Naunyn Schmiedebergs Arch. Pharmacol. 275, 153–168 [DOI] [PubMed] [Google Scholar]
- Castoldi A. F., Barni S., Turin I., Gandini C., Manzo L. (2000). Early acute necrosis, delayed apoptosis and cytoskeletal breakdown in cultured cerebellar granule neurons exposed to methylmercury. J. Neurosci. Res. 59, 775–787 [DOI] [PubMed] [Google Scholar]
- Ceccatelli S., Daré E., Moors M. (2010). Methylmercury-induced neurotoxicity and apoptosis. Chem. Biol. Interact. 188, 301–308 [DOI] [PubMed] [Google Scholar]
- Demarest K. T., Moore K. E. (1979). Comparison of dopamine synthesis regulation in the terminals of nigrostriatal, mesolimbic, tuberoinfundibular and tuberohypophyseal neurons. J. Neural Transm. 46, 263–277 [DOI] [PubMed] [Google Scholar]
- Dreiem A., Shan M., Okoniewski R. J., Sanchez-Morrissey S., Seegal R. F. (2009). Methylmercury inhibits dopaminergic function in rat pup synaptosomes in an age-dependent manner. Neurotoxicol. Teratol. 31, 312–317 [DOI] [PubMed] [Google Scholar]
- Drukarch B., Jongenelen C. A., Schepens E., Langeveld C. H., Stoof J. C. (1996). Glutathione is involved in the granular storage of dopamine in rat PC12 pheochromocytoma cells: Implications for the pathogenesis of Parkinson’s disease. J. Neurosci. 16, 6038–6045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunkley P. R., Bobrovskaya L., Graham M. E., von Nagy-Felsobuki E. I., Dickson P. W. (2004). Tyrosine hydroxylase phosphorylation: Regulation and consequences. J. Neurochem. 91, 1025–1043 [DOI] [PubMed] [Google Scholar]
- Faro L. R., do Nascimento J. L., Alfonso M., Durán R. (2002). Mechanism of action of methylmercury on in vivo striatal dopamine release. Possible involvement of dopamine transporter. Neurochem. Int. 40, 455–465 [DOI] [PubMed] [Google Scholar]
- Fornai F., Lenzi P., Lazzeri G., Ferrucci M., Fulceri F., Giorgi F. S., Falleni A., Ruggieri S., Paparelli A. (2007). Fine ultrastructure and biochemistry of PC12 cells: A comparative approach to understand neurotoxicity. Brain Res. 1129, 174–190 [DOI] [PubMed] [Google Scholar]
- Gassó S., Suñol C., Sanfeliu C., Rodríguez-Farré E., Cristòfol R. M. (2000). Pharmacological characterization of the effects of methylmercury and mercuric chloride on spontaneous noradrenaline release from rat hippocampal slices. Life Sci. 67, 1219–1231 [DOI] [PubMed] [Google Scholar]
- Gatti R., Belletti S., Uggeri J., Vettori M. V., Mutti A., Scandroglio R., Orlandini G. (2004). Methylmercury cytotoxicity in PC12 cells is mediated by primary glutathione depletion independent of excess reactive oxygen species generation. Toxicology. 204, 175–185 [DOI] [PubMed] [Google Scholar]
- Greene L. A., Rein G. (1977). Release, storage and uptake of catecholamines by a clonal cell line of nerve growth factor (NGF) responsive pheo-chromocytoma cells. Brain Res. 129, 247–263 [DOI] [PubMed] [Google Scholar]
- Haycock J. W., Ahn N. G., Cobb M. H., Krebs E. G. (1992). ERK1 and ERK2, two microtubule-associated protein 2 kinases, mediate the phosphorylation of tyrosine hydroxylase at serine-31 in situ. Proc. Natl. Acad. Sci. U.S.A. 89, 2365–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalisch B. E., Racz W. J. (1996). The effects of methylmercury on endogenous dopamine efflux from mouse striatal slices. Toxicol. Lett. 89, 43–49 [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Liu T. T., Hatakeyama H., Nemoto T., Takahashi N., Kasai H. (2005). Sequential compound exocytosis of large dense-core vesicles in PC12 cells studied with TEPIQ (two-photon extracellular polar-tracer imaging-based quantification) analysis. J. Physiol. (Lond.). 568 (Pt 3), 905–915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin I. J., Breese G. R., Krauss K. R., Weise V. K. (1968). Selective release of newly synthesized norepinephrine from the cat spleen during sympathetic nerve stimulation. J. Pharmacol. Exp. Ther. 161, 271–278 [PubMed] [Google Scholar]
- Leviel V. (2011). Dopamine release mediated by the dopamine transporter, facts and consequences. J. Neurochem. 118, 475–489 [DOI] [PubMed] [Google Scholar]
- Limke, T. L., Heidemann, S. R. and Atchison, W. D. (2004). Disruption of intraneuronal divalent cation regulation by methylmercury: are specific targets involved in altered neuronal development and cytotoxicity in methylmercury poisoning? Neurotoxicology 25, 741–760. [DOI] [PubMed]
- Lorang D., Amara S. G., Simerly R. B. (1994). Cell-type-specific expression of catecholamine transporters in the rat brain. J. Neurosci. 14, 4903–4914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty M. S., Atchison W. D. (1997). Pathways mediating Ca2+ entry in rat cerebellar granule cells following in vitro exposure to methyl mercury. Toxicol. Appl. Pharmacol. 147, 319–330 [DOI] [PubMed] [Google Scholar]
- Minnema D. J., Cooper G. P., Greenland R. D. (1989). Effects of methylmercury on neurotransmitter release from rat brain synaptosomes. Toxicol. Appl. Pharmacol. 99, 510–521 [DOI] [PubMed] [Google Scholar]
- Møller-Madsen B. (1994). Localization of mercury in CNS of the rat. An autometallographic study. Pharmacol. Toxicol. 75(Suppl. 1 ) 1–41 [DOI] [PubMed] [Google Scholar]
- Ngim C. H., Devathasan G. (1989). Epidemiologic study on the association between body burden mercury level and idiopathic Parkinson’s disease. Neuroepidemiology. 8, 128–141 [DOI] [PubMed] [Google Scholar]
- Omata S., Momose Y., Ueki H., Sugano H. (1982). In vivo effect of methylmercury on protein synthesis in peripheral nervous tissues of the rat. Arch. Toxicol. 49, 203–214 [DOI] [PubMed] [Google Scholar]
- Schmitz Y., Benoit-Marand M., Gonon F., Sulzer D. (2003). Presynaptic regulation of dopaminergic neurotransmission. J. Neurochem. 87, 273–289 [DOI] [PubMed] [Google Scholar]
- Schuldiner S., Liu Y., Edwards R. H. (1993). Reserpine binding to a vesicular amine transporter expressed in Chinese hamster ovary fibroblasts. J. Biol. Chem. 268, 29–34 [PubMed] [Google Scholar]
- Shafer T. J., Atchison W. D. (1991). Transmitter, ion channel and receptor properties of pheochromocytoma (PC12) cells: A model for neurotoxicological studies. Neurotoxicology. 12, 473–492 [PubMed] [Google Scholar]
- Spector S., Sjoerdsma A., Udenfriend S. (1965). Blockade of endogenous norepinephrine synthesis by α-methyl-tyrosine, an inhibitor of tyrosine hydroxylase. J. Pharmacol. Exp. Ther. 147, 86–95 [PubMed] [Google Scholar]
- Takeuchi T., Morikawa N., Matsumoto H., Shiraishi Y. (1962). A pathological study of Minamata disease in Japan. Acta Neuropathol. 2, 40–57 [Google Scholar]
- Torres G. E., Gainetdinov R. R., Caron M. G. (2003). Plasma membrane monoamine transporters: Structure, regulation and function. Nat. Rev. Neurosci. 4, 13–25 [DOI] [PubMed] [Google Scholar]
- Wermuth L., von Weitzel-Mudersbach P., Jeune B. (2000). A two-fold difference in the age-adjusted prevalences of Parkinson’s disease between the island of Als and the Faroe Islands. Eur. J. Neurol. 7, 655–660 [DOI] [PubMed] [Google Scholar]
- Yuan W., Guo J., Li X., Zou Z., Chen G., Sun J., Wang T., Lu D. (2009). Hydrogen peroxide induces the activation of the phospholipase C-γ1 survival pathway in PC12 cells: Protective role in apoptosis. Acta Biochim. Biophys. Sin. (Shanghai). 41, 625–630 [DOI] [PubMed] [Google Scholar]