Abstract
In mice, in utero exposure to 2,3,7,8-tetrachlorodibenzo-p- dioxin (TCDD) reduces the number of dorsolateral prostatic buds resulting in a smaller dorsolateral prostate and prevents formation of ventral buds culminating in ventral prostate agenesis. The genes and signaling pathways affected by TCDD that are responsible for disrupting prostate development are largely unknown. Here we show that treatment of urogenital sinus (UGS) organ cultures with known inhibitors of canonical Wnt signaling also inhibits prostatic bud formation. In support of the hypothesis that TCDD decreases canonical Wnt signaling, we identify inhibitory effects of TCDD on multiple components of the canonical Wnt signaling pathway in the UGS that temporally coincide with the inhibitory effect of TCDD on prostatic bud formation: (1) expression of R-spondins (Rspo2 and Rspo3) that promote canonical Wnt signaling is reduced; (2) expression of Lef1, Tcf1, and Wif1, established canonical Wnt target genes, is decreased; (3) expression of Lgr5, a RSPO receptor that activates canonical Wnt signaling, is reduced; and (4) expression of Dickkopfs (Dkks), inhibitors of canonical Wnt signaling, is not increased by TCDD. Thus, the TCDD-induced reduction in canonical Wnt signaling is associated with a decrease in activators (Rspo2 and Rspo3) rather than an increase in inhibitors (Dkk1 and Dkk2) of the pathway. This study focuses on determining whether treatment of TCDD-exposed UGS organ cultures with RSPO2 and/or RSPO3 is capable of rescuing the inhibitory effects of TCDD on canonical Wnt signaling and prostatic bud formation. We discovered that each RSPO alone or in combination partially rescues TCDD inhibition of both canonical Wnt signaling and prostatic bud formation.
Key Words: prostate, urogenital sinus, TCDD, R-spondin, canonical Wnt, mouse, development, bud.
Mouse prostate derives from the urogenital sinus (UGS), which is located between the bladder and pelvic urethra, and arises on embryonic day (E) 13.5. The UGS is composed of an epithelium surrounded by a dense mesenchymal layer. Prostate development is androgen dependent, requiring 5α-dihydrotestosterone (DHT) that binds to androgen receptors in the urogenital mesenchyme (UGM) and activates downstream signaling factors that interact with the urogenital epithelium (UGE) and initiate subsequent signaling events in the UGE, resulting in the formation of prostatic buds and later development of the mature prostate (Cunha and Chung, 1981).
Prostatic bud development is orchestrated through a series of timely events beginning with specification, where developmental signals determine the exact positioning of buds, followed by initiation, where the buds begin to arise from the basal epithelium (BE), and elongation, where the buds continue to grow out into the surrounding UGM. These events take place between E13.5 and E18.5, and by E18.5, all prostatic buds have formed. By puberty, the prostatic buds will give rise to ductal networks that constitute the four separate and distinct lobes of the mature prostate: anterior, dorsal, lateral, and ventral (Lin et al., 2003; Timms et al., 1994).
Treating pregnant C57BL/6J mice with 2,3,7,8- tetrachlorodibenzo-p-dioxin (TCDD; 5 µg/kg, po) on E13.5 results in severe prostatic budding defects in male fetuses on E18.5 including mispositioning and reduction in the number of dorsal and lateral buds and complete ablation of ventral prostatic buds (Lin et al., 2003). A time-course study determined that TCDD acts during the bud specification stage with the critical window for ventral bud inhibition occurring between E15.5 and E16.5 (Vezina et al., 2008b). Impairment of budding by TCDD is not a consequence of androgen insufficiency or a reduction in androgen receptor activity (Ko et al., 2004b). Rather it is most likely caused by misregulation of downstream signaling pathways triggered by TCDD activation of the aryl hydrocarbon receptor (AHR) in the UGM (Ko et al., 2004a). Likely candidates include paracrine factors that are secreted from UGM and stimulate prostatic bud formation by interacting with signaling pathways found in the basal UGE from which prostatic buds arise.
Growing evidence supports an essential role for canonical Wnt signaling during prostatic bud formation (Francis et al., 2013; Mehta et al., 2013). Recent findings showed canonical Wnt signaling to be critical for lineage commitment and bud outgrowth during early prostate development and have shown LEF1, a canonical Wnt target gene, to be a marker of prostatic buds (Simons et al., 2012; Wu et al., 2011). Other groups have shown canonical Wnt signaling to be important during later phases of prostate development such as in the regulation of branching morphogenesis, which is also disrupted by TCDD (Ko et al., 2002; Kudryavtseva et al., 2011). Wnt signaling is required for budding in multiple organs including breast, tooth, and hair follicle (Faraldo et al., 2006; Gat et al., 1998; Liu et al., 2008; Lo Celso et al., 2004) and WNT5A, a noncanonical Wnt agonist, inhibits prostatic bud formation in vitro. Importantly, treatment with a WNT5A antibody rescued TCDD inhibition of prostatic budding (Allgeier et al., 2008).
TCDD effects on canonical Wnt signaling during prostatic bud formation have not been extensively explored. R-spondins 1–4 (RSPOs) are activators of the canonical Wnt signaling pathway. The exact mechanisms by which RSPOs activate canonical Wnt signaling remain inconclusive. RSPOs appear to initiate Wnt signaling by several mechanisms including inhibition of Dickkofs (DKKs) that are extracellular antagonists of canonical Wnt signaling, synergism with canonical Wnts, activation of noncanonical Wnt signaling through heparin sulfate proteoglycan binding, and recently by activation of LGR receptors to enhance Wnt signaling (Carmon et al., 2011; de Lau et al., 2011; Glinka et al., 2011; Kim et al., 2008).
This study focuses on determining the effects of TCDD on canonical Wnt signaling in the UGS during the same time that it inhibits prostatic bud formation. We show that TCDD decreases Rspo2 and Rspo3 mRNA levels as well as Wnt target genes, Lef1, Tcf1, and Wif1, during prostatic bud development. We demonstrate that RSPOs enhance prostatic bud formation and partially rescue the inhibition of budding caused by TCDD. We demonstrate that stimulatory effects of RSPOs on budding are mediated through canonical Wnt signaling and explore mechanisms for TCDD inhibition of RSPOs by examining the expression of Dkks and Lgrs. We conclude that inhibition of canonical Wnt signaling significantly contributes to the reduction in prostatic bud formation in UGS organ cultures exposed to TCDD.
MATERIALS AND METHODS
Animals and treatments.
C57BL/6J mice (Jackson Laboratory, Bar Harbor, ME) were maintained as previously described (Vezina et al., 2008b). All procedures were approved by the University of Wisconsin Animal Care and Use Committee. Timed-pregnant females were set up by pairing males with females overnight. The following day was considered E0.5. Pregnant females were administered a single dose of TCDD (5 µg/kg) or vehicle (corn oil, 5ml/kg), po, on E15.5. Dams were euthanized via CO2 asphyxiation when fetuses were at developmental stages E14.5, E16.5, E17.5, or E18.5.
UGS organ culture and prostatic bud counting.
Male UGSs were harvested on E14.5 and grown in serum-free culture media containing 10nM DHT on a 0.4-µm Millicell-CM filter for 3 or 4 days. Media and supplements were replenished every 2 days. The following supplements were added, alone or in combination, to UGS organ culture media: 0.1% dimethyl sulfoxide (DMSO; vehicle control); 1nM TCDD (Cambridge Isotope Laboratories, Woburn, MA); recombinant mouse RSPO2 and RSPO3; DKK1 and DKK2 proteins (R&D Systems, Minneapolis, MN); and XAV-939 (Selleck Chemicals, Houston, TX). Some UGS tissues were harvested after 3 days and frozen in liquid nitrogen for real-time (RT) PCR analysis. Other UGS tissues were harvested following 4 days in culture and fixed in 4% paraformaldehyde (PF) overnight followed by graded dehydration into 100% MeOH in which the tissues were stored at −20°C until further analysis.
Whole-mount immunohistochemistry (IHC) was performed as described previously (Keil et al., 2012). Primary antibody against e-cadherin (1:500; Cell Signaling, Danvers, MA) was added overnight at 4°C. Following washes, samples were treated with anti-rabbit-AlexaFluor488 (Jackson ImmunoResearch, West Grove, PA [1:500]), washed, cleared with a series of glycerol dilutions, and mounted in antifade media (PBS containing 80% glycerol and 0.2% n-propyl gallate). Images were taken on an Olympus Fluoview FV1000 confocal microscope, and total bud number was assessed using a z series to visualize the entire UGS epithelium. Every third z series section was counted to obtain total bud number (see Supplementary fig. 1 for illustration of bud counting method). We counted all prostatic buds on the UGS and excluded the small, nonprostatic buds present on the pelvic urethra. Prostatic buds projected out from the UGE surface and were readily identified as buds. Whole UGS images in Figs. 1, 4, and 5 represent a compilation of the z series for a “representative” single UGS in each treatment group. Each representative image shows only a fraction of the prostatic buds that were counted in that particular UGS and is intended only as a visual aid for making gross comparisons between groups at the level of the whole UGS. Analyses were performed on at least four litter-independent samples.
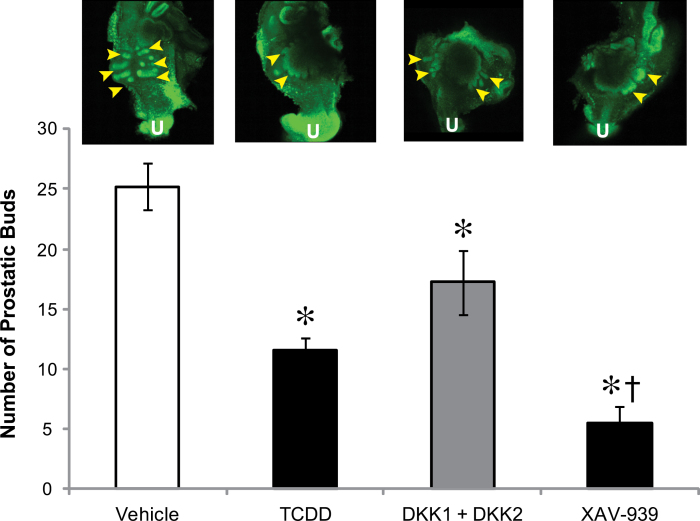
Fig. 1.
Inhibitors of canonical Wnt signaling inhibit prostatic bud number similar to TCDD. E14.5 male UGSs were cultured for 4 days in media containing 10nM DHT with vehicle, 1nM TCDD, recombinant DKK1 + DKK2 (500ng/ml each), or 10µM XAV-939 to inhibit canonical Wnt signaling. Buds were visualized by performing IHC specific for e-cadherin, an epithelium marker. The number of prostatic buds was determined by confocal microscopy. Arrowheads indicate areas where buds are present. U indicates urethra. Results are mean ± SE for at least four litter-independent samples per treatment. Asterisk indicates a significant decrease compared with control p < 0.05.
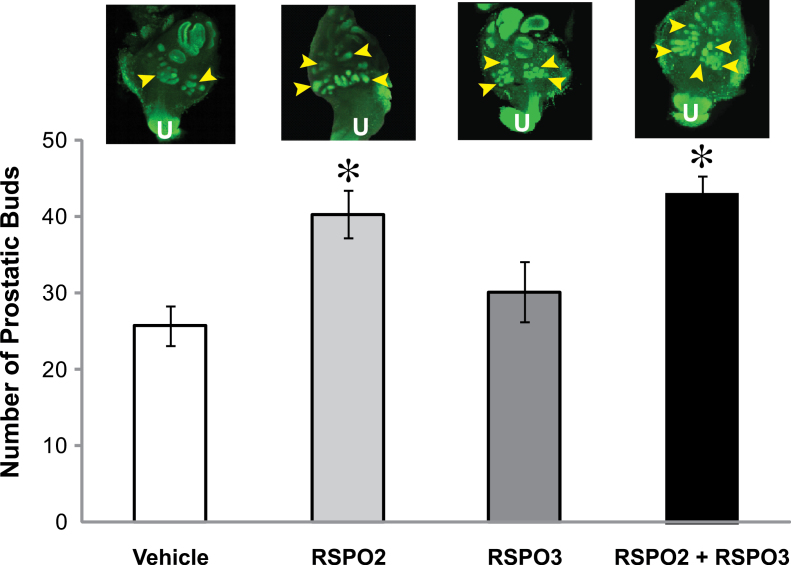
Fig. 4.
Exogenous RSPO2 and RSPO3 increase prostatic bud number in control UGS in vitro. E14.5 male UGSs were cultured for 4 days in media containing DHT (10nM, control) with or without RSPO2 (100ng/ml), RSPO3 (100ng/ml), or RSPO2 + RSPO3 (100ng/ml each). Prostatic buds were counted as described in the Figure 1 legend. Arrowheads indicate regions where buds are present. U indicates urethra. Results are mean ± SE of at least four litter-independent samples per treatment. Asterisk indicates a significant difference compared with vehicle (p < 0.05).
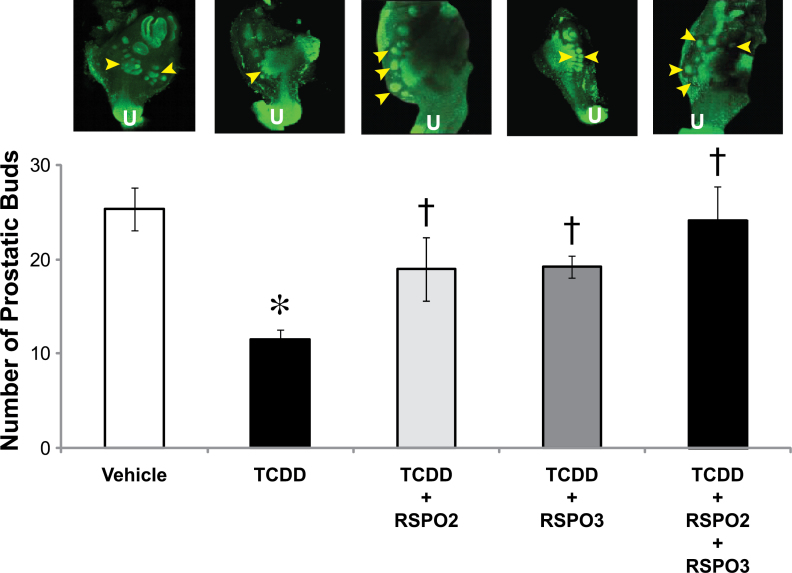
Fig. 5.
Treatment with exogenous RSPO2 and RSPO3 partially protects against TCDD inhibition of prostatic bud development. E14.5 male UGSs were cultured for 4 days in DHT (10nM) containing media. Supplements to the media were either vehicle (0.1% DMSO) or TCDD (1nM) and RSPO2 and/or RSPO3 (100ng/ml each). Treatment groups were vehicle, TCDD, RSPO2 + TCDD, RSPO3 + TCDD, and RSPO2 + RSPO3 + TCDD. Buds were visualized and quantified as described in the Figure 1 legend. Arrowheads represent areas where prostatic buds are present. U indicates urethra. Results are the mean ± SE of at least four litter-independent samples per treatment. Asterisk denotes a significant difference compared with vehicle, whereas a cross denotes a significant difference compared with TCDD (p < 0.05).
Sectional and whole-mount in situ hybridization.
Sectional in situ hybridization (ISH) and whole-mount ISH were performed according to protocols found at www.gudmap.org and described previously (Abler et al., 2011). Primer sequences for riboprobes are as follows: Lgr5F′ GCCTTAGAGCAGGAGAGCAT; Lgr5R′ CGATGTTAATACGACTCACTATAGGGTGTATAGTCCTGGCTGTCCT; Lgr4F′ GCTCATACCTTGAGCTGTCT; Lgr4R′ CGATGTTAATACGACTCAC TATAGGGACACTGAGAGGGGAATCACT. The reverse primers contained a synthetic T7 RNA polymerase recognition sequence. Riboprobes for Rspo2 and Rspo3 have been described previously (Mehta et al., 2011). The expression for each riboprobe was analyzed in at least three litter-independent UGSs per time and treatment. Vehicle- and TCDD-treated tissues were processed together to allow for comparisons to be made between biological replicates and treatment groups.
RNA isolation and RT PCR.
UGS homogenization, RNA isolation, and reverse transcriptase PCR were previously described in Vezina et al. (2008a). RT PCR was performed as described by Lin et al. (2002, 2003) using the Roche LightCycler 1.5 (Roche Applied Science, Indianapolis, IN). Results are shown relative to cyclophilin mRNA abundance to normalize expression on a per cell basis. RT PCR primer sequences can be found in Supplementary table 1.
Sectional IHC.
UGS tissues were fixed overnight in 4% PF, dehydrated into 100% MeOH, embedded in paraffin, and cut into 5-µm sagittal sections. Samples were rehydrated and boiled in 10mM sodium citrate for 20min to unmask epitopes. Samples were washed with PBST (1× PBS, 0.1% Tween-20) and blocked in PBST containing 5% normal goat serum and 1% bovine serum albumin for 1h. Primary antibody against LEF1 or TCF1 (1:100; Cell Signaling) was added in combination with antibody against cytokeratin 14 (KRT14) (1:100; Millipore, Billerica, MA) overnight at 4°C. Samples were washed and treated with anti-rabbit-AlexaFluor488 to detect LEF1 or TCF1 expression and anti-mouse-AlexaFluor546 (Sigma-Aldrich, St Louis, MO) for 1h to detect KRT14 expression. Samples were washed and counterstained with DAPI and mounted in antifade media. Images were acquired on a confocal microscope. Percent of LEF1 or TCF1 positive BE cells in the UGS was determined by counting total number of BE cells positive for LEF1 or TCF1 staining in the entire BE, excluding the bladder, Wolffian and Mullerian ducts, and pelvic urethra and dividing this number by the total number of BE cells identified by positive KRT14 staining.
Litter independence and statistical analysis.
In vitro UGS organ culture was performed on UGSs from four or more independent litters per group. Whole-mount e-cadherin staining and sectional IHC were performed on UGSs from three or more litters per treatment. RT PCR analysis was performed on at least four litter-independent UGSs from each treatment group. Error bars represent SE. ANOVA and Student’s t-test were conducted on data that passed Levene’s test for homogeneity of variance and appeared to be normally distributed.
RESULTS
Inhibitors of Canonical Wnt Signaling Reduce Prostatic Bud Formation
To determine whether inhibition of canonical Wnt signaling plays a role in TCDD inhibition of prostatic budding, UGS organ culture was performed in the presence of vehicle and TCDD. Prostatic bud formation with these treatments was compared with cultures treated with known inhibitors of canonical Wnt signaling, the DKKs and XAV-939, that antagonize by different mechanisms. DKK1 and DKK2 are extracellular antagonists of canonical Wnt signaling that inhibit by promoting internalization of LRP Wnt coreceptors, thereby decreasing canonical Wnt signaling (Niehrs, 2006). DKKs inhibit canonical Wnt signaling in vitro at concentrations above 100ng/ml (Im and Quan, 2010). In contrast to the DKKs, the chemical canonical Wnt inhibitor, XAV-939, is less well studied. XAV-939 acts by preventing AXIN degradation, the rate-limiting step in the destruction of β-catenin.
Our goal was to determine whether treatment of UGS organ cultures with DKKs or XAV-939 would inhibit prostatic bud formation, similar to TCDD. However, because XAV-939 is less well established as a canonical Wnt inhibitor than the DKKs, we first sought to determine whether the concentration of XAV-939 used in our UGS organ culture experiments actually inhibited canonical Wnt signaling. UGSs were grown for 3 days in DHT-containing media. RT PCR was performed to determine mRNA levels of Wnt target genes, Lef1 and Lgr5 (Barker et al., 2007; Filali et al., 2002), in vehicle (control)- versus XAV-939-treated UGSs. We found that XAV-939 downregulated the expression of both target genes when added at a 10µM concentration (64% reduction of Lef1 and 25% reduction of Lgr5 compared with vehicle), showing that this XAV-939 concentration inhibited canonical Wnt pathway target gene expression as predicted (data not shown).
Next, we examined effects of TCDD and these two types of canonical Wnt inhibitors on prostatic budding (Fig. 1). Total bud numbers were compared among vehicle- (control), TCDD- (1nM), DKK1 + DKK2- (500ng/ml each), and XAV-939- (10µM) treated UGSs by staining the UGE for e-cadherin and counting prostatic buds. All treatments decreased the total number of prostatic buds compared with control UGSs (p < 0.05, Fig. 1, bottom). A confocal image that is representative of the UGS for each treatment is also shown (Fig. 1, top). The arrowheads in each image point to prostatic buds, which are more plentiful in the vehicle (control) UGS than in the TCDD-, DKK1 + DKK2-, or XAV-939-treated UGS. Thus, both TCDD and the canonical Wnt inhibitors reduced prostatic bud formation (vehicle-25 buds, TCDD-12 buds, DKK1 + DKK2-17 buds, and XAV-939-7 buds). These results provide a rationale for investigating inhibition of canonical Wnt signaling as a potential mechanism for disruption of prostatic bud formation by TCDD.
TCDD Decreases Canonical Wnt Signaling Target Gene Expression in the UGS
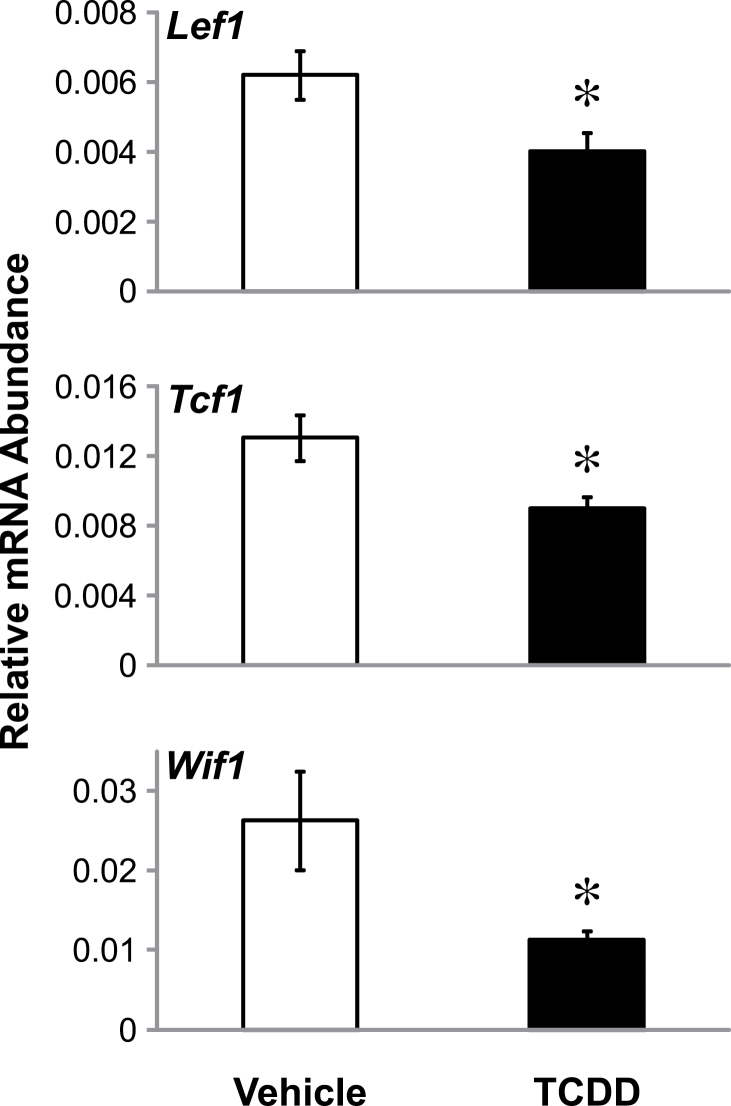
To explore whether TCDD is having an inhibitory effect on canonical Wnt signaling, we performed RT PCR using primers specific to confirmed Wnt signaling targets, including Lef1, Tcf1, and Wif1 (Filali et al., 2002; Roose and Clevers, 1999; Yan et al., 2001). UGSs treated with either vehicle or TCDD at a concentration known to reduce prostatic bud formation were examined after 3 days in culture to determine whether TCDD altered the mRNA expression of Wnt target genes. Consistent with our hypothesis of TCDD downregulating canonical Wnt signaling, we found that Lef1, Tcf1, and Wif1 mRNA levels were all significantly decreased following treatment with TCDD (Fig. 2). This finding supports a role for misregulation of the canonical Wnt signaling pathway in the reduction of prostatic bud number by TCDD.
Fig. 2.
TCDD downregulates canonical Wnt target genes in the mouse UGS. E14.5 male UGSs were cultured for 3 days in media containing 10nM DHT and either vehicle (0.1% dimethyl sulfoxide [DMSO]) or TCDD (1nM). RT PCR was performed to distinguish differences in mRNA expression of the canonical Wnt target genes, Lef1, Tcf1, and Wif1, between vehicle and TCDD-treated UGS samples. Results are mean ± SE for at least four litter-independent samples per treatment. Asterisk indicates a significant decrease compared with control p < 0.05.
TCDD Decreases Rspo2 and Rspo3 mRNA Expression in the UGS
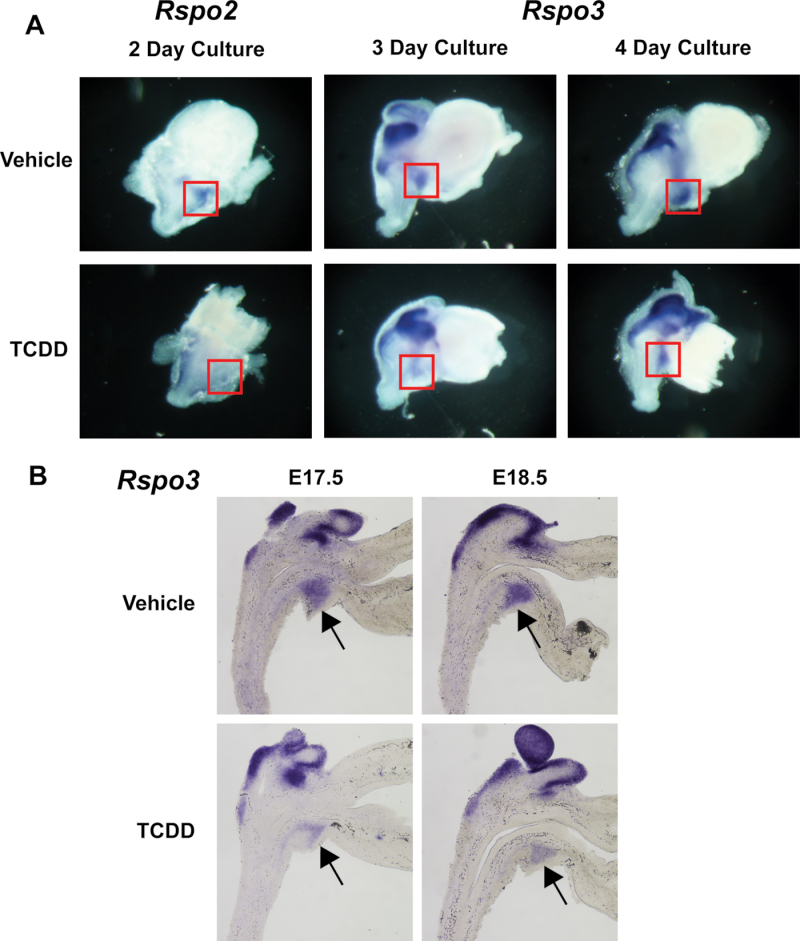
Previous ISH results from our laboratory revealed at E16.5 that Rspo2 and Rspo3 mRNA levels were decreased in UGSs exposed to TCDD on E15.5 (5 µg/kg) compared with UGSs exposed to vehicle (5ml/kg corn oil, po)(Moore et al., 2011). To determine whether these in vivo effects can be reproduced in vitro, a time-course study of Rspo2 and Rspo3 expression was performed in UGS organ culture. Male UGSs were harvested on E14.5 and treated in culture for 2, 3, or 4 days in DHT-containing media that contained either vehicle or TCDD (1nM).
Rspo2 mRNA expression in the UGS appears strongest after the 2-day culture and is prominently expressed in the ventral mesenchymal pad (VMP), a cell population known to mediate mesenchymal/epithelial interactions during prostatic bud development (Timms et al., 1995). Rspo2 expression in TCDD-treated cultures was reduced in the VMP after 2 days (Fig. 3A, compare staining inside boxed area) consistent with our previous in vivo results (Moore et al., 2011). However, we did not detect a significant TCDD-induced change in Rspo2 expression in 3- or 4-day cultures or in UGSs from E17.5 or E18.5 fetuses exposed in utero to vehicle or TCDD on E15.5 (results not shown).
Fig. 3.
Rspo2 and Rspo3 mRNA expression is inhibited by TCDD in the UGS. E14.5 male UGSs were cultured for 2, 3, or 4 days in the presence of 10nM DHT and either vehicle (0.1% DMSO) or TCDD (1nM). They were then subjected to ISH to visualize mRNA expression of Rspo2 and Rspo3 (A). Boxes indicate regions that differ in expression between vehicle- and TCDD-treated UGSs. Near mid-sagittal, 50µM sections of male UGS were processed by ISH to visualize mRNA expression of Rspo3 on E17.5 and E18.5. Dams from which fetal UGSs were obtained were treated with either vehicle (5ml/kg corn oil) or TCDD (5 µg/kg) po on E15.5 (B). Arrows indicate regions where expression differences exist between vehicle- and TCDD-treated UGSs. Results are representative of six litter-independent UGSs at each time.
Rspo3 expression overlaps with Rspo2 expression in the VMP, and it is strongly expressed in Wolffian structures. The expression of Rspo3 transcript remains robust throughout the entire time course, and no inhibition of Rspo3 expression was observed after 2 days of exposure to TCDD (results not shown). However, there was a decrease in Rspo3 levels in the VMP region of the UGS organ cultures after 3 and 4 days of exposure to TCDD compared with vehicle (Fig. 3A, compare staining inside boxed area). To assess expression of Rspo3 in the UGS of mouse fetuses exposed in vivo to TCDD, a time-course study was performed. E17.5 and E18.5 UGSs exposed to TCDD on E15.5 had less expression of Rspo3 in the VMP than respective vehicle control UGSs (Fig. 3B, arrow). Thus, the Rspo findings demonstrate that TCDD causes a time- and region-specific reduction in Rspo2 and Rspo3 expression in the ventral UGM. This is the UGS tissue compartment where TCDD activation of AHR is required to ablate ventral prostatic bud formation (Ko et al., 2004a).
RSPOs Promote Prostatic Bud Formation
RSPOs induce canonical Wnt signaling in other systems (Kim et al., 2008; Nam et al., 2006). We hypothesized that RSPO2 and RSPO3 would enhance prostatic bud formation in the developing UGS by enhancing canonical Wnt signaling. UGSs were treated for 4 days with the following: vehicle; recombinant RSPO2 protein (100ng/ml); recombinant RSPO3 protein (100ng/ml); or the combination of RSPO2 and RSPO3 (100ng/ml each). These concentrations have been shown to activate canonical Wnt signaling by other research groups (Kim et al., 2008). Whole-mount IHC was performed using an antibody specific to e-cadherin, and total prostatic bud number was assessed between treatment groups. RSPO2 and the combination of RSPO2 and RSPO3 significantly increased mean total prostatic bud number compared with vehicle control (mean of 40 buds for RSPO2 and 43 buds for RSPO2 + RSPO3 compared with 25 buds for vehicle). The RSPO3 results were not statistically significant, but they did show a trend toward an increase in bud formation (Fig. 4). These results support the hypothesis that RSPOs can increase total bud number in UGSs by activating canonical Wnt signaling.
RSPOs Protect Against TCDD Inhibition of Prostatic Bud Number
Because TCDD reduces transcript abundance of both Rspo2 and Rspo3, we sought to determine whether RSPO2 and/or RSPO3 could protect against the reduction in bud number caused by TCDD, by treating UGS organ cultures with these components in combination with TCDD. E14.5 male UGSs were grown for 4 days with the following supplements: vehicle, TCDD (1nM), RSPO2 (100ng/ml), RSPO3 (100ng/ml), RSPO2 + RSPO3 (100ng/ml each), and all RSPO treatments combined with TCDD. TCDD treatment decreased the number of prostatic buds by over 50% compared with UGSs treated with vehicle, from a mean of 25 buds to 12 buds. Addition of RSPO2 and RSPO3 to TCDD-exposed UGSs partially rescued the total number of buds to 19 buds when added to the TCDD-containing culture media. The combination of RSPO2 and RSPO3 led to even greater rescue of bud number, from an average of 12 buds with TCDD alone to 24 buds, which is comparable with vehicle-treated UGSs (mean of 25 buds). Although RSPOs were able to increase prostatic bud number in TCDD-treated UGSs to the vehicle level, they were not able to increase it to the number caused by RSPOs alone (19 buds and 24 buds in RSPO2 + TCDD and RSPO2 + RSPO3 + TCDD treatments compared with 40 and 43 buds in RSPO2 and RSPO2 + RSPO3 treatments) (Fig. 5). This could be due to RSPOs not being the sole mechanism responsible for TCDD inhibitory effects on budding.
TCDD Inhibits Canonical Wnt Signaling and RSPOs Counteract the Effect
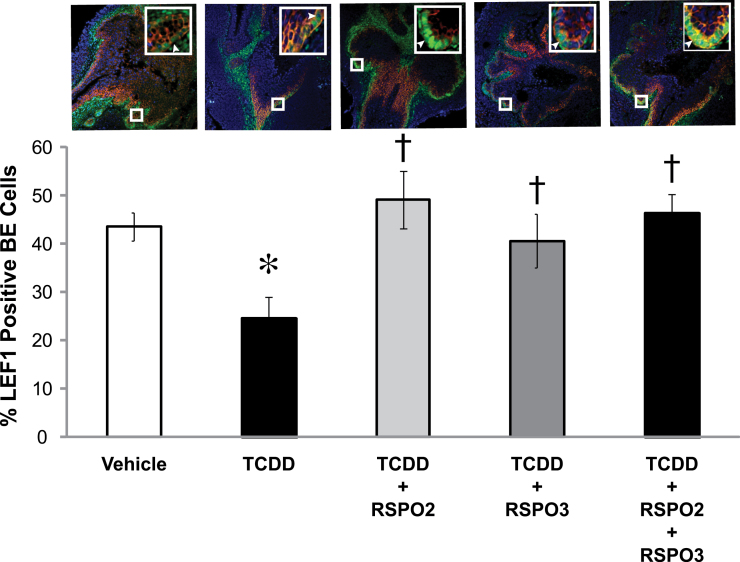
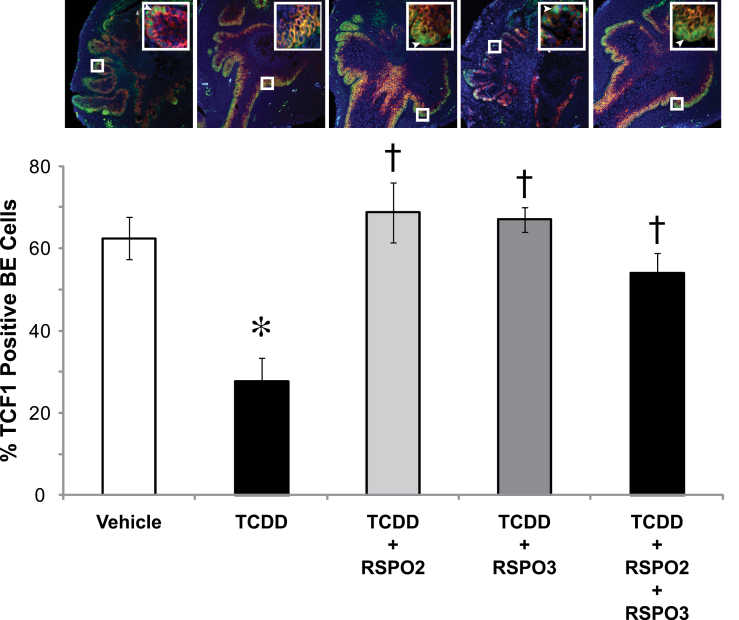
To further examine whether prostatic budding defects from TCDD exposure are due to disruption of the canonical Wnt signaling pathway, we examined how RSPOs and TCDD affected expression of downstream canonical WNT target proteins, LEF1 and TCF1, in cultured UGSs. In vitro UGS organ culture was performed for 4 days with media containing RSPO2 or RSPO3 alone or in combination with TCDD. Sectional IHC was performed using antibodies specific for LEF1 and TCF1. Sections were counterstained with KRT14 to mark BE and DAPI to mark nuclei. Percent of LEF1 or TCF1 positive BE cells in the UGS was determined by counting total number of BE cells positive for LEF1 or TCF1 staining in the entire UGS BE and dividing this number by the total number of BE cells identified by positive KRT14 staining. Our results show that both LEF1 and TCF1 are significantly reduced upon exposure to TCDD from 45 to 25% for LEF1 positive BE cells and from 60 to 25% for TCF1 positive BE cells (Figs. 6 and 7, compare high magnification insets). It should be noted that in the LEF1/KRT14 image, there is far less positive staining compared with vehicle control, and in the TCF1/KRT14 image, the inset shows no positive staining of TCF1, illustrating the inhibition of LEF1/TCF1 expression caused by addition of TCDD.
Fig. 6.
TCDD inhibits the downstream canonical WNT target LEF1, and RSPOs fully restore LEF1. E14.5 male UGSs were cultured for 4 days in DHT (10nM) containing media. Supplements to the media were either vehicle (0.1% DMSO) or TCDD (1nM) and RSPO2 and/or RSPO3 (100ng/ml each). Treatment groups were vehicle, TCDD, RSPO2 + TCDD, RSPO3 + TCDD, and RSPO2 + RSPO3 + TCDD. Near mid-sagittal sections (5 µm) were immunostained for LEF1 and counterstained for KRT14 to mark BE cells, which are the site of prostatic bud formation. Nuclei were stained with DAPI. Large boxes indicate enlarged images (200×) of small box areas in each sample to demonstrate colocalization of LEF1 and KRT14 in BE cells. Arrows indicate cells positive for LEF1/KRT14. Percent of BE cells positive for LEF1 was determined by counting the number of BE cells positive for LEF1 in the entire UGS and dividing that number by the total number of BE cells. Results are mean ± SE of at least four litter-independent UGSs per treatment. Asterisk denotes a significant difference compared with vehicle, whereas a cross indicates a significant difference compared with TCDD (p < 0.05).
Fig. 7.
TCDD exposure inhibits the downstream canonical WNT target, TCF1, and RSPOs fully restore TCF1 levels. E14.5 male UGSs were cultured for 4 days in DHT (10nM) containing media. Supplements to the media were either vehicle (0.1% DMSO) or TCDD (1nM) and RSPO2 and/or RSPO3 (100ng/ml each). Treatment groups were vehicle, TCDD, RSPO2 + TCDD, RSPO3 + TCDD, and RSPO2 + RSPO3 + TCDD. Near mid-sagittal sections (5 µm) were immunostained for TCF1 and counterstained for KRT14 to mark BE cells, which are the site of prostatic bud formation. Nuclei were stained with DAPI. Large boxes indicate enlarged images (200×) of small box areas in each sample to demonstrate colocalization of TCF1 and KRT14 in BE cells. Arrows point to cells positively stained for TCF1/KRT14. There is no arrow present in the TCDD inset as no cells are positive for TCF1. Percent of BE cells positive for TCF1 was determined by counting the number of BE cells positive for TCF1 in the entire UGS and dividing that number by the total number of BE cells. Results are mean ± SE of at least three litter-independent UGSs per treatment. Asterisk denotes a significant difference compared with vehicle, whereas a cross indicates a significant difference compared with TCDD (p < 0.05).
To determine whether RSPOs are able to protect against the inhibitory effects of TCDD on LEF1 and TCF1 expression, we performed sectional IHC on 4-day UGS cultures treated with RSPO2 (100ng/ml), RSPO3 (100ng/ml), or RSPO2 + RSPO3 (100ng/ml each). RSPO2, RSPO3, and the combination of RSPOs were able to fully rescue the expression of LEF1 and TCF1 in the BE, demonstrating that RSPOs exert their effects through the canonical WNT signaling pathway and can overcome TCDD effects on Wnt signaling (Figs. 6 and 7). However, in vehicle-exposed UGS organ cultures, additions of RSPO2 and/or RSPO3 were not able to significantly induce LEF1 or TCF1 expression. Nevertheless, there was a nearly significant trend for induction of LEF1 in both the RSPO2 treatment and RSPO2 + RSPO3 treatment groups (p = 0.07 and p = 0.08, respectively; results not shown). These findings confirm that both transcript and protein levels of LEF1 and TCF1 are inhibited by TCDD in 3- and 4-day UGS cultures, respectively, and that RSPO2 and RSPO3 are able to reverse the inhibition.
Assessing Other Canonical Wnt Signaling Regulatory Genes for Misexpression by TCDD
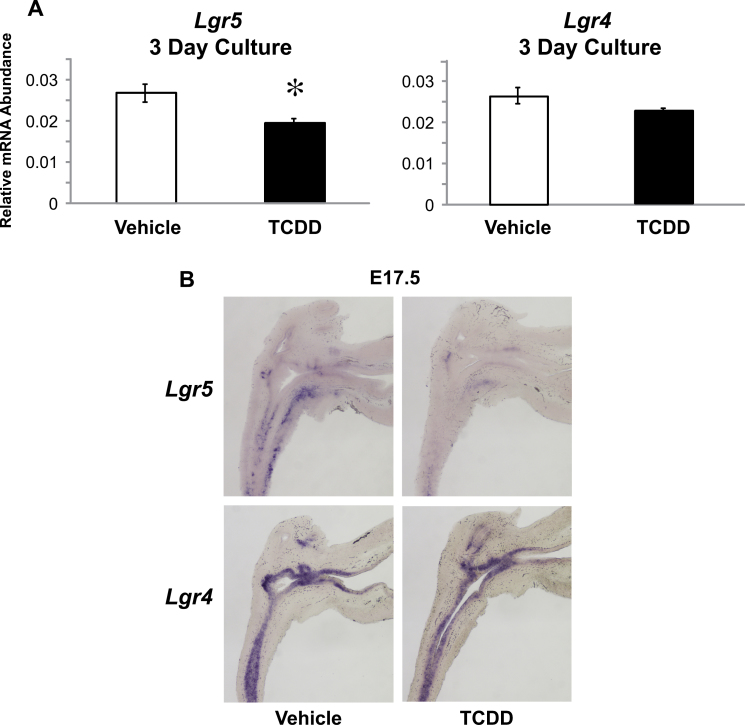
Reduction in Rspo2 and Rspo3 by TCDD leads to a decrease in canonical Wnt signaling. There has been growing evidence that RSPOs activate canonical Wnt signaling by binding to LGRs (Carmon et al., 2011; de Lau et al., 2011; Glinka et al., 2011). We found that TCDD significantly inhibits the mRNA expression of Lgr5 but not Lgr4 in UGSs from 3-day organ culture (Fig. 8A). To determine the expression of Lgr5 and Lgr4 in the developing UGS, we performed an in vivo time course using sectional ISH. Lgr5 is not highly expressed at E16.5 (not shown), but at E17.5, Lgr5 is expressed in both the UGE and the ventral UGM. The expression is depleted following TCDD exposure. Lgr4 is strongly expressed in the UGE at both E16.5 (not shown) and E17.5, but its expression does not seem to be affected by TCDD (Fig. 8B). Thus, TCDD inhibits canonical Wnt signaling by at least two mechanisms: it reduces Rspo2/3 abundance in UGM and reduces the abundance of their cognate receptor (Lgr5) in UGE.
Fig. 8.
TCDD downregulates Lgr5. E14.5 male UGSs were cultured for 3 days in DHT (10nM) containing media. RT PCR was performed to distinguish mRNA differences for Lgr5 and Lgr4 in vehicle- versus TCDD-treated samples (A). Results are mean ± SE of at least four litter-independent samples per treatment p < 0.05. Near mid-sagittal sections were cut from E17.5 fetal UGSs obtained from dams treated with either vehicle (5 ml/kg) or TCDD (5 µg/kg), po on E15.5 (B). Results represent at least three litter-independent samples per treatment.
Another way that TCDD could decrease canonical Wnt signaling in the UGE is by increasing DKK activity. We showed in Fig. 1 that DKKs, recognized inhibitors of canonical Wnt signaling, were capable of decreasing prostatic bud formation in mouse UGS organ cultures. This raised the possibility that TCDD might be decreasing canonical Wnt signaling not only by reducing the expression of activators of the pathway, Rspo2 and Rspo3, but also by increasing the expression of the inhibitors, Dkk1 and Dkk2. This would lead to a decrease in canonical Wnt signaling and prostatic bud formation. Accordingly, UGSs were treated in culture for 3 days with either vehicle or TCDD followed by RT PCR analysis. We did not see either Dkk1 or Dkk2 transcript levels increased in the presence of TCDD (Supplementary fig. 2A). We also performed sectional ISH for Dkk1 and Dkk2 on the UGS of E17.5 fetuses exposed to vehicle (control) or TCDD on E15.5. Both Dkks were highly expressed in the UGM, but the expression of neither Dkk was affected by TCDD (Supplementary fig. 2B). We conclude that the TCDD-induced decrease in canonical Wnt signaling associated with a reduction in prostatic bud formation is not mediated by an increase in Dkk1/2 expression.
DISCUSSION
Canonical Wnt Signaling Is Important for Prostatic Bud Formation and Is Inhibited by TCDD
We hypothesize that TCDD disrupts the balance between canonical and noncanonical Wnt signaling, and recent evidence from our laboratory and others supports this hypothesis. Previous data from our lab focused on the noncanonical pathway and showed that TCDD enhanced noncanonical Wnt signaling as evidenced by addition of WNT5A, leading to inhibition of prostate budding, and by an antibody against WNT5A, fully rescuing the inhibitory budding effects of TCDD (Allgeier et al., 2008). Noncanonical Wnt signaling also was shown to be upregulated following TCDD treatment in LNCaP prostate adenocarcinoma cells (Hrubá et al., 2011). Evidence for canonical Wnt β-catenin signaling being downregulated by TCDD has been demonstrated both in liver progenitor cells and in the prevention of spheroid attachment to endometrial cells (Procházková et al., 2011; Tsang et al., 2012). In zebrafish, TCDD treatment inhibits fin regeneration secondary to upregulation of Rspo1 and a morpholino against Rspo1 protected against this effect of TCDD (Mathew et al., 2008). Thus, there is a precedent for TCDD exposure adversely affecting development by causing misregulation of Rspos, activators of canonical Wnt signaling. Here we examined TCDD effects on canonical Wnt signaling and found the pathway to be inhibited and the expression of Rspo2 and Rspo3, canonical Wnt agonists, to be downregulated.
Canonical Wnt β-catenin signaling is crucial for normal development. Signaling is tightly regulated and either too much or too little results in early developmental arrest (Kharaishvili et al., 2011). Canonical Wnt signaling is critical in organs originating from a budding program including breast, tooth, and hair follicle (Faraldo et al., 2006; Gat et al., 1998; Liu et al., 2008; Lo Celso et al., 2004). Importantly, these organs are also all targets of TCDD toxicity (Keller et al., 2007; La Merrill et al., 2010; Panteleyev et al., 1997). We provide evidence that canonical Wnt signaling is important for the formation of prostatic buds and that TCDD inhibits this pathway. Using the canonical Wnt inhibitor, XAV-939, and the extracellular antagonists of canonical Wnt signaling, DKK1 and DKK2, we showed that inhibition of canonical Wnt signaling reduced the formation of prostatic buds in vitro as did treatment with TCDD. Additionally, we were able to demonstrate downregulation of the canonical Wnt target genes Lef1, Tcf1, and Wif1 in UGSs following in utero TCDD treatment, and reduced protein expression of LEF1 and TCF1 in UGSs following in vitro TCDD treatment. These genes are not only downstream targets of canonical Wnt β-catenin signaling but also major components of the pathway. Lef1 and Tcf1 are DNA binding partners of β-catenin and regulate transcription of β-catenin target genes. Wif1 is an extracellular antagonist of Wnt signaling. When these components are collectively downregulated, this could lead to drastic consequences in UGS development.
Rspo2 and Rspo3 Are Downregulated by TCDD
Preliminary data from our lab showed that Rspo2 and Rspo3 were reduced in the VMP of E16.5 UGSs exposed to TCDD (Moore et al., 2011). The UGM is the site of TCDD action in the UGS (Ko et al., 2004a), and the VMP is a region of the UGS that secretes paracrine factors required for development of prostatic buds (Donjacour et al., 2003). We continued the investigation of TCDD effects on Rspo2 and Rspo3 by analyzing their expression in vitro using UGS organ culture analysis and also in vivo to explore later stages of prostatic bud development at E17.5 and E18.5 to look for differences between vehicle and TCDD-treated samples. We found that in vitro, Rspo2 levels are decreased following 2-day culture, consistent with previous in vivo results at E16.5, whereas at later stages of development Rspo2 expression does not appear to be affected by TCDD. Rspo3 expression does appear to be reduced in the VMP at both the E17.5 and E18.5 time points. This was also true in whole-mount in vitro samples after 3- and 4-day culture.
It is particularly significant that the TCDD-induced decrease in expression of the Rspo2 and Rspo3 was specific to the VMP region of the UGS. This is the same region of the UGS where TCDD exerts its inhibitory effect on ventral prostatic bud formation, resulting in ventral prostate agenesis. Like Rspo2 and Rspo3, another inducer of prostatic bud formation in the mouse UGS, Fgf10, is expressed in the VMP. However, expression of Fgf10 in the VMP is not affected by TCDD nor is activity of the FGF10 pathway in the UGS (Vezina et al., 2009). Prior to the discovery that TCDD downregulates Rspo2 and Rspo3 expression in a VMP region–specific manner, the identity of a gene or signaling pathway with expression localized to the ventral UGM that could potentially explain the TCDD impairment in ventral prostate development was unknown. Exactly how TCDD produces this ventral UGM region-specific effect on Rspo expression is not understood. It is not caused by the expression of AhR or ARNT in the UGS being confined to the VMP nor is it associated with a ventral UGS region-specific activation of AhR signaling by TCDD (Vezina et al., 2008b). AhR and ARNT are expressed ubiquitously throughout the UGS, not just in the VMP. We believe that reduction in both Rspo2 and Rspo3 contributes to impairment of prostatic bud development and downregulation of canonical Wnt signaling by TCDD.
RSPOs Promote Prostatic Bud Formation and Partially Protect Against Budding Inhibition by TCDD
Rspo2 and Rspo3 are present in the VMP, and we predicted that they would promote prostatic bud formation by activating canonical Wnt signaling in the UGE. Supportive of this hypothesis, we found that addition of exogenous RSPO2 and RSPO3 in UGS organ culture media increases total prostatic buds formed. We also found that TCDD reduces Rspo2 and Rspo3 mRNA abundance, and we wanted to see whether restoring RSPO2 and RSPO3 activities could rescue the inhibitory budding effect of TCDD. We found that the addition of exogenous RSPO2 and RSPO3, alone or in combination, to TCDD-containing media restores prostatic bud numbers back to the level observed in UGSs grown in the absence of TCDD. We also found that RSPOs rescue the inhibition of LEF1 and TCF1 protein expression by TCDD, revealing that RSPOs are acting through the canonical Wnt signaling pathway to promote bud formation.
Differential Effects of TCDD on Expression of Other Canonical Wnt Signaling Regulatory Genes
Another possible mechanism of TCDD inhibition of Rspo2 and Rspo3 is through inhibition of LGR-induced canonical Wnt signaling. LGR4 and LGR5 enhance canonical Wnt signaling through binding to RSPOs (Carmon et al., 2011; de Lau et al., 2011; Glinka et al., 2011). RSPO binding to LGRs results in a synergistic response of canonical Wnt signaling, greater than achieved with Wnt ligands alone (Carmon et al., 2011). Consistent with this mechanism, we found that TCDD significantly reduced Lgr5 transcript levels in 3-day UGS organ culture and inhibited Lgr5 expression in ventral UGM and BE in E17.5 UGSs in vivo. We were not able to detect any expression differences in Lgr4 between vehicle (control) and TCDD treatment groups; however, it was highly expressed in the entire UGE. We predict that RSPOs are secreted from the VMP and bind to LGRs in the BE to initiate prostatic bud formation. The fact that Lgr5 is reduced, compiled with evidence for both Rspo2 and Rspo3 transcript reduction in the VMP by TCDD, could inhibit canonical Wnt signaling and bud formation. In contrast to Rspos and Lgrs contributing to the TCDD-induced decrease in canonical Wnt signaling, we found no evidence that a TCDD-induced increase in the expression of Dkks in the UGS occurs.
CONCLUSION
TCDD disrupts prostatic bud formation by altering the balance between noncanonical and canonical Wnt signaling. Previously, we showed that noncanonical Wnt signaling is increased by TCDD in the UGS, and we show in this study that canonical Wnt signaling is decreased. The decrease is principally caused by a profound reduction in the expression of Rspo2 and Rspo3, activators of canonical Wnt signaling. This results in downregulation of canonical Wnt target genes (Lef1, Tcf1, Wif1, and Lgr5) and a reduction in prostatic bud formation. Significantly, the TCDD-induced impairment in budding can be protected against by recombinant RSPO2 and RSPO3 proteins, which restore the balance in Wnt signaling.
SUPPLEMENTARY DATA
Supplementary data are available online at http://toxsci.oxfordjournals.org/.
FUNDING
National Institutes of Health (ES01332 to R.E.P.); National Institute of Environmental Health Sciences (T32 ES007015 to A.M.B.).
Supplementary Material
REFERENCES
- Abler L. L., Keil K. P., Mehta V., Joshi P. S., Schmitz C. T., Vezina C. M. (2011). A high-resolution molecular atlas of the fetal mouse lower urogenital tract. Dev. Dyn. 240, 2364–2377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allgeier S. H., Lin T. M., Vezina C. M., Moore R. W., Fritz W. A., Chiu S. Y., Zhang C., Peterson R. E. (2008). WNT5A selectively inhibits mouse ventral prostate development. Dev. Biol. 324, 10–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker N., van Es J. H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P. J., et al. (2007). Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 449, 1003–1007 [DOI] [PubMed] [Google Scholar]
- Carmon K. S., Lin Q., Gong X., Thomas A., Liu Q. (2011). LGR5 interacts and cointernalizes with Wnt receptors to modulate Wnt/ß-catenin signaling. Mol. Cell. Biol. 32, 2054–2064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha G. R., Chung L. W. (1981). Stromal-epithelial interactions–I. Induction of prostatic phenotype in urothelium of testicular feminized (Tfm/y) mice. J. Steroid Biochem. 14, 1317–1324 [DOI] [PubMed] [Google Scholar]
- de Lau W., Barker N., Low T. Y., Koo B. K., Li V. S., Teunissen H., Kujala P., Haegebarth A., Peters P. J., van de Wetering M., et al. (2011). Lgr5 homologues associate with Wnt receptors and mediate R-spondin signalling. Nature 476, 293–297 [DOI] [PubMed] [Google Scholar]
- Donjacour A. A., Thomson A. A., Cunha G. R. (2003). FGF-10 plays an essential role in the growth of the fetal prostate. Dev. Biol. 261, 39–54 [DOI] [PubMed] [Google Scholar]
- Faraldo M. M., Taddei-De La Hosseraye I., Teulière J., Deugnier M. A., Moumen M., Thiery J. P., Glukhova M. A. (2006). [Mammary gland development: Role of basal myoepithelial cells]. J. Soc. Biol. 200, 193–198 [DOI] [PubMed] [Google Scholar]
- Filali M., Cheng N., Abbott D., Leontiev V., Engelhardt J. F. (2002). Wnt-3A/beta-catenin signaling induces transcription from the LEF-1 promoter. J. Biol. Chem. 277, 33398–33410 [DOI] [PubMed] [Google Scholar]
- Francis J. C., Thomsen M. K., Taketo M. M., Swain A. (2013). ß-Catenin is required for prostate development and cooperates with pten loss to drive invasive carcinoma. PLoS Genet. 9, e1003180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gat U., DasGupta R., Degenstein L., Fuchs E. (1998). De novo hair follicle morphogenesis and hair tumors in mice expressing a truncated beta-catenin in skin. Cell 95, 605–614 [DOI] [PubMed] [Google Scholar]
- Glinka A., Dolde C., Kirsch N., Huang Y. L., Kazanskaya O., Ingelfinger D., Boutros M., Cruciat C. M., Niehrs C. (2011). LGR4 and LGR5 are R-spondin receptors mediating Wnt/ß-catenin and Wnt/PCP signalling. EMBO Rep. 12, 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hrubá E., Vondrácek J., Líbalová H., Topinka J., Bryja V., Soucek K., Machala M. (2011). Gene expression changes in human prostate carcinoma cells exposed to genotoxic and nongenotoxic aryl hydrocarbon receptor ligands. Toxicol. Lett. 206, 178–188 [DOI] [PubMed] [Google Scholar]
- Im G. I., Quan Z. (2010). The effects of Wnt inhibitors on the chondrogenesis of human mesenchymal stem cells. Tissue Eng. Part A. 16, 2405–2413 [DOI] [PubMed] [Google Scholar]
- Keil K. P., Mehta V., Branam A. M., Abler L. L., Buresh-Stiemke R. A., Joshi P. S., Schmitz C. T., Marker P. C., Vezina C. M. (2012). Wnt inhibitory factor 1 (Wif1) is regulated by androgens and enhances androgen-dependent prostate development. Endocrinology 153, 6091–6103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller J. M., Huet-Hudson Y. M., Leamy L. J. (2007). Qualitative effects of dioxin on molars vary among inbred mouse strains. Arch. Oral Biol. 52, 450–454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kharaishvili G., Simkova D., Makharoblidze E., Trtkova K., Kolar Z., Bouchal J. (2011). Wnt signaling in prostate development and carcinogenesis. Biomed. Pap. Med. Fac. Univ. Palacky. Olomouc. Czech. Repub. 155, 11–18 [DOI] [PubMed] [Google Scholar]
- Kim K. A., Wagle M., Tran K., Zhan X., Dixon M. A., Liu S., Gros D., Korver W., Yonkovich S., Tomasevic N., et al. (2008). R-Spondin family members regulate the Wnt pathway by a common mechanism. Mol. Biol. Cell. 19, 2588–2596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko K., Moore R. W., Peterson R. E. (2004a). Aryl hydrocarbon receptors in urogenital sinus mesenchyme mediate the inhibition of prostatic epithelial bud formation by 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Appl. Pharmacol. 196, 149–155 [DOI] [PubMed] [Google Scholar]
- Ko K., Theobald H. M., Moore R. W., Peterson R. E. (2004b). Evidence that inhibited prostatic epithelial bud formation in 2,3,7,8-tetrachlorodibenzo-p-dioxin-exposed C57BL/6J fetal mice is not due to interruption of androgen signaling in the urogenital sinus. Toxicol. Sci. 79, 360–369 [DOI] [PubMed] [Google Scholar]
- Ko K., Theobald H. M., Peterson R. E. (2002). In utero and lactational exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin in the C57BL/6J mouse prostate: Lobe-specific effects on branching morphogenesis. Toxicol. Sci. 70, 227–237 [DOI] [PubMed] [Google Scholar]
- Kudryavtseva E., Forde T. S., Pucker A. D., Adarichev V. A. (2011). Wnt signaling genes of murine chromosome 15 are involved in sex-affected pathways of inflammatory arthritis. Arthritis Rheum. 64, 1057–1068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Merrill M., Harper R., Birnbaum L. S., Cardiff R. D., Threadgill D. W. (2010). Maternal dioxin exposure combined with a diet high in fat increases mammary cancer incidence in mice. Environ. Health Perspect. 118, 596–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin T. M., Rasmussen N. T., Moore R. W., Albrecht R. M., Peterson R. E. (2003). Region-specific inhibition of prostatic epithelial bud formation in the urogenital sinus of C57BL/6 mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Toxicol. Sci. 76, 171–181 [DOI] [PubMed] [Google Scholar]
- Lin T. M., Simanainen U., Moore R. W., Peterson R. E. (2002). Critical windows of vulnerability for effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin on prostate and seminal vesicle development in C57BL/6 mice. Toxicol. Sci. 69, 202–209 [DOI] [PubMed] [Google Scholar]
- Liu F., Chu E. Y., Watt B., Zhang Y., Gallant N. M., Andl T., Yang S. H., Lu M. M., Piccolo S., Schmidt-Ullrich R., et al. (2008). Wnt/beta-catenin signaling directs multiple stages of tooth morphogenesis. Dev. Biol. 313, 210–224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo Celso C., Prowse D. M., Watt F. M. (2004). Transient activation of beta-catenin signalling in adult mouse epidermis is sufficient to induce new hair follicles but continuous activation is required to maintain hair follicle tumours. Development 131, 1787–1799 [DOI] [PubMed] [Google Scholar]
- Mathew L. K., Sengupta S. S., Ladu J., Andreasen E. A., Tanguay R. L. (2008). Crosstalk between AHR and Wnt signaling through R-Spondin1 impairs tissue regeneration in zebrafish. FASEB J. 22, 3087–3096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V., Abler L. L., Keil K. P., Schmitz C. T., Joshi P. S., Vezina C. M. (2011). Atlas of Wnt and R-spondin gene expression in the developing male mouse lower urogenital tract. Dev. Dyn. 240, 2548–2560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta V., Schmitz C. T., Keil K. P., Joshi P. S., Abler L. L., Lin T.-M., Taketo M. M., Sun X., Vezina C. M. (2013). Beta-catenin (CTNNB1) induces Bmp expression in urogenital sinus epithelium and participates in prostatic bud initiation and patterning. Dev. Biol. 376, 125–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore R. W., Abler L. L., Mehta V., Vezina C. M., Peterson R. E. (2011). Identification of Wnt and Rspo genes whose expression patterns in fetal mouse urogential sinus (UGS) are altered by 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Toxicol. Sci. 120(suppl), 350 [Google Scholar]
- Nam J. S., Turcotte T. J., Smith P. F., Choi S., Yoon J. K. (2006). Mouse cristin/R-spondin family proteins are novel ligands for the Frizzled 8 and LRP6 receptors and activate beta-catenin-dependent gene expression. J. Biol. Chem. 281, 13247–13257 [DOI] [PubMed] [Google Scholar]
- Niehrs C. (2006). Function and biological roles of the Dickkopf family of Wnt modulators. Oncogene 25, 7469–7481 [DOI] [PubMed] [Google Scholar]
- Panteleyev A. A., Thiel R., Wanner R., Zhang J., Roumak V. S., Paus R., Neubert D., Henz B. M., Rosenbach T. (1997). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCCD) affects keratin 1 and keratin 17 gene expression and differentially induces keratinization in hairless mouse skin. J. Invest. Dermatol. 108, 330–335 [DOI] [PubMed] [Google Scholar]
- Procházková J., Kabátková M., Bryja V., Umannová L., Bernatík O., Kozubík A., Machala M., Vondrácek J. (2011). The interplay of the aryl hydrocarbon receptor and β-catenin alters both AhR-dependent transcription and Wnt/β-catenin signaling in liver progenitors. Toxicol. Sci. 122, 349–360 [DOI] [PubMed] [Google Scholar]
- Roose J., Clevers H. (1999). TCF transcription factors: Molecular switches in carcinogenesis. Biochim. Biophys. Acta. 1424, M23–M37 [DOI] [PubMed] [Google Scholar]
- Simons B. W., Hurley P. J., Huang Z., Ross A. E., Miller R., Marchionni L., Berman D. M., Schaeffer E. M. (2012). Wnt signaling through beta-catenin is required for prostate lineage specification. Dev. Biol. 371, 246–255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timms B. G., Lee C. W., Aumüller G., Seitz J. (1995). Instructive induction of prostate growth and differentiation by a defined urogenital sinus mesenchyme. Microsc. Res. Tech. 30, 319–332 [DOI] [PubMed] [Google Scholar]
- Timms B. G., Mohs T. J., Didio L. J. (1994). Ductal budding and branching patterns in the developing prostate. J. Urol. 151, 1427–1432 [DOI] [PubMed] [Google Scholar]
- Tsang H., Cheung T. Y., Kodithuwakku S. P., Chai J., Yeung W. S., Wong C. K., Lee K. F. (2012). 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) suppresses spheroids attachment on endometrial epithelial cells through the down-regulation of the Wnt-signaling pathway. Reprod. Toxicol. 33, 60–66 [DOI] [PubMed] [Google Scholar]
- Vezina C. M., Allgeier S. H., Fritz W. A., Moore R. W., Strerath M., Bushman W., Peterson R. E. (2008a). Retinoic acid induces prostatic bud formation. Dev. Dyn. 237, 1321–1333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina C. M., Allgeier S. H., Moore R. W., Lin T. M., Bemis J. C., Hardin H. A., Gasiewicz T. A., Peterson R. E. (2008b). Dioxin causes ventral prostate agenesis by disrupting dorsoventral patterning in developing mouse prostate. Toxicol. Sci. 106, 488–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vezina C. M., Hardin H. A., Moore R. W., Allgeier S. H., Peterson R. E. (2009). 2,3,7,8-Tetrachlorodibenzo-p-dioxin inhibits fibroblast growth factor 10-induced prostatic bud formation in mouse urogenital sinus. Toxicol. Sci. 113, 198–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Daniels G., Shapiro E., Xu K., Huang H., Li Y., Logan S., Greco M. A., Peng Y., Monaco M. E., et al. (2011). LEF1 identifies androgen-independent epithelium in the developing prostate. Mol. Endocrinol. 25, 1018–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan D., Wiesmann M., Rohan M., Chan V., Jefferson A. B., Guo L., Sakamoto D., Caothien R. H., Fuller J. H., Reinhard C., et al. (2001). Elevated expression of axin2 and hnkd mRNA provides evidence that Wnt/beta -catenin signaling is activated in human colon tumors. Proc. Natl. Acad. Sci. U.S.A. 98, 14973–14978 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.