Abstract
Deoxynivalenol (DON, vomitoxin), a trichothecene mycotoxin produced by Fusarium sp. that frequently occurs in cereal grains, has been associated with human and animal food poisoning. Although a common hallmark of DON-induced toxicity is the rapid onset of emesis, the mechanisms for this adverse effect are not fully understood. Recently, our laboratory has demonstrated that the mink (Neovison vison) is a suitable small animal model for investigating trichothecene-induced emesis. The goal of this study was to use this model to determine the roles of two gut satiety hormones, peptide YY3–36 (PYY3–36) and cholecystokinin (CCK), and the neurotransmitter 5-hydroxytryptamine (5-HT) in DON-induced emesis. Following ip exposure to DON at 0.1 and 0.25mg/kg bw, emesis induction ensued within 15–30min and then persisted up to 120min. Plasma DON measurement revealed that this emesis period correlated with the rapid distribution and clearance of the toxin. Significant elevations in both plasma PYY3–36 (30–60min) and 5-HT (60min) but not CCK were observed during emesis. Pretreatment with the neuropeptide Y2 receptor antagonist JNJ-31020028 attenuated DON- and PYY-induced emesis, whereas the CCK1 receptor antagonist devezapide did not alter DON’s emetic effects. The 5-HT3 receptor antagonist granisetron completely suppressed induction of vomiting by DON and the 5-HT inducer cisplatin. Granisetron pretreatment also partially blocked PYY3–36-induced emesis, suggesting a potential upstream role for this gut satiety hormone in 5-HT release. Taken together, the results suggest that both PYY3–36 and 5-HT play contributory roles in DON-induced emesis.
Key Words: mycotoxin, trichothecene, deoxynivalenol, vomitoxin, 5-hydroxytryptamine, peptide YY3–36, emesis.
Deoxynivalenol (DON), a trichothecene mycotoxin produced by Fusarium sp. that frequently contaminates cereal staples, has been documented to cause human and animal food poisoning (Canady et al., 2001; Pestka, 2010a). DON remains stable during cereal storage and is relatively resistant to food processing techniques enabling it to persist in foods consumed by humans and domestic animals (Jackson and Bullerman, 1999). There is a growing concern that global changes in agricultural practice and climate have increased the incidence of Fusarium head blight with the end result being rising levels of DON contamination in grain-based foods (Pestka, 2010b).
DON was originally called “vomitoxin” by United States Department of Agriculture (USDA) researchers because of its potent emetic effects in pigs (Vesonder et al., 1973). Consistent with this colloquial name, investigations of foodborne illnesses associated with Fusarium and/or DON contamination identified vomiting as one of the primary symptoms (Luo, 1994; Ueno, 1987; Yoshizawa, 1983). For example, between 1961 and 1991, 53 gastroenteritis outbreaks in China were etiologically linked to consumption of cereals containing Fusarium and/or DON, with the largest outbreak affecting over 130,000 people (Luo, 1994). DON is not routinely measured in foods or clinical samples associated with U.S. food poisoning outbreaks by public health agencies as is commonly done for pathogens and bacterial toxins. However, in one notable exception, DON was sought and detected at < 1 ppm in the absence of other putative food poisoning agents in burritos associated with 16 large outbreaks of gastroenteritis with a characteristic rapid onset vomiting that affected over 1,900 schoolchildren in seven states (Anonymous, 1999; Steinberg et al., 2006).
Despite emesis being a likely consequence of DON-related food poisoning, relatively few studies have focused on the underlying mechanisms of this effect or potential human sensitivity. Emesis, a reflex that forcefully drives out contents of the upper gastrointestinal (GI) tract through the oral cavity (Andrews and Hawthorn, 1988), serves as a protective mechanism against food poisoning. Severe emesis can adversely affect human and animal health by causing nausea, disrupting normal nutrition, hydration, and electrolyte balance. The emetic response is a highly complex process integrating neurotransmitters, hormones, and visceral afferent neurons that are coordinated by a neuronal network known as the central pattern generator (CPG) (Hornby, 2001; Koga and Fukuda, 1992; Miller, 1999). The CPG is located in the medulla oblongata of the hindbrain where it coordinates the efferent autonomic and motor neurons.
Cytotoxic drugs used in the treatment of cancer trigger vomiting in at least two ways. One mechanism involves the action of peripheral blood- and cerebrospinal fluid (CSF)–borne emetic stimuli (e.g., hormones and neurotransmitters) at the area postrema (AP) of the medulla. The AP is a circumventricular organ that lies between the brain parenchyma and the CSF-containing ventricles, and it is believed to be the primary chemoreceptor trigger zone for humoral agent–mediated emesis (Carpenter, 1990). The AP lacks a specific blood-brain diffusion barrier allowing it to sense emetic stimuli in both the blood and CSF, leading to activation of the CPG and subsequent emesis (Borison, 1989; Carpenter, 1990; Hornby, 2001). In a second possible mechanism, emetic stimuli may act on the enterochromaffin (EC) cells to induce a local release of emetic mediators such as 5-HT. These mediators trigger emesis by binding to the corresponding receptors located on vagal afferent terminals that relay emetic stimuli to nucleus tractus solitarius (NTS) and ultimately activate the CPG (Andrews et al., 1990; Andrews and Horn, 2006; Hornby, 2001). The monoamine 5-hydroxytryptamine (5-HT, serotonin) is a well-known mediator of emesis, nausea, appetite, and GI functions (Endo et al., 2000; Kucharczyk and Harding, 1990; Stables et al., 1987). Although most 5-HT is synthesized and released from EC cells in the GI tract, it can also be produced in the neurons of the CNS (Kim and Camilleri, 2000). Receptors for this amine are the major targets of antiemetic drugs that combat the emetic actions of chemotherapeutic agents such as cisplatin (Percie du Sert et al., 2011). Although it could not be demonstrated in the pig that DON upregulates plasma concentrations of 5-HT or its metabolite 5-hydroxyindoleacetic acid (5-HIAA) (Prelusky, 1994), 5-HT3 receptor antagonists prevent DON-induced vomiting in this species (Prelusky and Trenholm, 1993). Thus, DON’s emetic effects likely involve serotonergic pathways.
The gut satiety hormones peptide YY (PYY) and cholecystokinin (CCK) are released in mice upon DON exposure (Flannery et al., 2012) and might also have the potential to mediate DON-induced emesis. PYY is a 36-amino-acid protein of the pancreatic polypeptide hormone family expressed in both endocrine cells and neurons (Ekblad and Sundler, 2002). PYY is released by L-endocrine cells of the ileum and colon in two endogenous forms, PYY1–36 and PYY3–36, with the latter exhibiting greater biological activity and abundance in the circulation (Pittner et al., 2004). PYY3–36 is an agonist of the neuropeptide Y2 receptor (Y2R) (Sloth et al., 2007) functioning to induce satiety and decrease gastric emptying (Ballantyne, 2006; Batterham et al., 2002; Halatchev and Cone, 2005; Koegler et al., 2005). PYY regulates food intake by acting on peripheral Y2Rs located on vagal afferent neurons (Abbott et al., 2005) and by acting centrally via the NTS and hypothalamus (Blevins et al., 2008). Importantly, PYY is highly emetogenic in dogs, a response that is mediated by the AP (Harding and McDonald, 1989; Perry et al., 1994). Furthermore, both nausea and vomiting are adverse side effects of PYY administration in humans (Gantz et al., 2007; Sloth et al., 2007).
In the mouse, ip and oralingual exposure to DON-induced rapid (15min) increases in plasma PYY (Flannery et al., 2012). It was further shown that pretreatment with an Y2R antagonist suppressed DON-induced anorexia, implicating a role for PYY. Although mice are useful for studying food intake, they are incapable of vomiting and therefore not an effective model for discerning linkages between DON-induced PYY and emesis. Nevertheless, the rapid and transient nature of PYY3–36-induced emesis (Harding and McDonald, 1989) is remarkably similar to that previously observed with DON in studies of the pig and other animal species (Forsyth et al., 1977; Hughes et al., 1999; Pestka et al., 1987; Wu et al., 2013), suggesting that this gut satiety hormone could be an unrecognized factor in DON-induced nausea and vomiting.
CCK is produced by I-cells in the duodenum (Liou et al., 2011) and acts primarily on peripheral CCK1 receptors (CCK1Rs) located on intestinal vagal afferents or located within the dorsal vagal complex (Baptista et al., 2007; Kopin et al., 1999; Sullivan et al., 2007). Previous research in monkeys has demonstrated nausea and vomiting upon iv treatment with CCK (Perera et al., 1993). Furthermore, DON markedly elevates plasma CCK concentrations in the mouse along with PYY (Flannery et al., 2012). It could thus be speculated that CCK might also contribute to nausea and vomiting following DON exposure.
Critical questions remain regarding the precise roles of 5-HT and the aforementioned gut satiety hormones in DON-induced emesis. Recently, our lab developed a mink model to study trichothecene-induced emesis and employed it to compare emetic effects between DON and its congeners (Wu et al., 2013). The sensitivity of this species to DON-induced emesis was found to be similar to that observed in larger animals. Here, we utilized the mink to test the hypothesis that 5-HT, PYY3–36, and CCK coordinate DON-induced emesis. The results indicated that plasma 5-HT and PYY3–36 but not CCK were elevated during DON-induced emesis, and furthermore, chemical antagonists for 5-HT and PYY receptors but not CCK receptor suppressed induction of vomiting by this mycotoxin.
MATERIALS AND METHODS
Laboratory animals.
Animal treatment followed National Institutes of Health guidelines and were approved by the Michigan State University Institutional Animal Care and Use Committee. Sixty standard dark, female mink (Neovison vison) of 1–2 years of age (average weight = 1.2 ± 0.2kg) were obtained from Michigan State University (MSU) Experimental Fur Farm. Animals were housed singly in wire cages (62cm long × 25cm wide × 38cm high) within an open-sided pole barn and were provided with a nest box (24cm long × 24cm wide × 29cm high) with aspen shavings and excelsior. Temperature, humidity, and photoperiod were dependent on ambient environment. Experiments were conducted in October, November, and December 2011 and January 2012, a period in which the mink were not in the estrus state, with average temperatures being 41–60, 32–47, 22–34, and 17–30°F for these months, respectively. These housing conditions met those specified in the Standard Guidelines for the Operation of Mink Farms in the United States (Fur Commission USA, 2010). Mink were acclimated for a minimum of 1 week prior to the initial experiment and fed the MSU Experimental Fur Farm ranch diet, which is formulated to meet the nutrient requirements of mink. To minimize variation in gastric contents among test animals, mink were fasted (no feed but water available ad libitum) for 24h (day 1) prior to all experiments and then given 50g feed just prior to the treatment. To minimize the number of animals used, mink employed for receptor antagonist studies were given a minimum 2 weeks of washout period between experiments, except cisplatin-treated mink (positive control to demonstrate that granisetron is an effective antiemetic in mink as in other species in response to a well-established emetic stimulus) that were not reused. We based this recovery time on (1) rapid clearance of DON in monogastric species (Pestka, 2010a), (2) rapid reversal of anorectic effects in DON-treated mice (Flannery et al., 2011), (3) rapid clearance of granisetron (Clarke et al., 1994) and JNJ-31020028 (Shoblock et al., 2010) in experimental animals, and (4) preliminary studies with mink showing the absence of anorectic, emetic, or weight effects after 24h.
Toxin and drugs.
Intraperitoneal and subcutaneous injections of the toxin, hormones, or pharmacologic agents were delivered in 1ml/kg bw using a sterile 20-G, 2.54-cm needle. Delivery volumes were adjusted in accordance with each animal’s body weight which was typically between 1.0 and 1.5kg. Although both ip and po DON exposure can induce emesis in mink, ip administration was employed for all studies to minimize stress and avoid variation in delivery amounts that might occur during oral gavage. DON was obtained from Dr. Tony Durst (University of Ottawa) and purity (> 98%) verified by elemental analysis. For exposure studies, DON was dissolved in filter-sterilized PBS (Sigma-Aldrich, St Louis, MO) and administered to mink at 0.1 and 0.25mg/kg bw. These doses were previously shown to effectively induce emesis in mink (Wu et al., 2013). Both PYY3–36 (Tocris Biosciences, Ellisville, MO) and CCK (23–33, sulfonated; Sigma-Aldrich) were prepared in PBS to provide ip injection at doses up to 0.01 and 0.025mg/kg bw, respectively. The Y2 receptor antagonist JNJ-31020028, a gift from Dr. P. Bonaventure (Janssen Research & Development, LLC, San Diego, CA), was dissolved in a vehicle composed of Pharmasolve (ISP Technologies, Wayne, NJ) plus 20% 2-hydroxypropyl-β-cyclodextrin (Sigma-Aldrich) and administered by sc injection at a dose of 15mg/kg bw (Shoblock et al., 2010). The CCK1R antagonist devazepide was dissolved in PBS containing 1% dimethylsulfoxide and administered by sc injection at 0.1mg/kg bw (Eberle-Wang and Simansky, 1992). Based on prior mink and ferret studies (Qian et al., 2009 , 2010a,b; Percie du Sert et al., 2011), the 5-HT3 receptor antagonist granisetron and cisplatin (Tocris) were prepared in PBS and administered by ip injection to mink at doses up to 2.5 and 7.5mg/kg bw, respectively.
Experimental design.
To relate dose-response and kinetics of DON-induced emesis to timing of PYY3–36, CCK, and 5-HT release, 48 fasted mink (n = 4 per group) were given 50g feed at 8:30h on the day of dosing and then allowed to eat for 30min (Fig. 1A). At 9:00h, 16 mink were dosed by ip injection with PBS, DON (0.1mg/kg bw), or DON (0.25mg/kg bw). Each individual retch or vomit was counted as described previously (Wu et al., 2013) and combined to yield total emetic events. Vomiting is characterized as rhythmic abdominal contraction with oral expulsion of either solid or liquid material, whereas retching is defined to responses that mimicked vomiting but without any material being expelled. The distribution of emetic events was calculated based on the total emetic events in 120min. After 15-, 30-, 60-, and 120-min intervals, mink groups (n = 4 per group) were anesthetized by intramuscular injection with ketamine (30mg/kg bw) and xylazine (1mg/kg bw). Blood was collected by heart puncture into vacutainers containing EDTA as anticoagulant, and mink were immediately euthanized by CO2 exposure. Blood was centrifuged at 1000 × g for 10min. Resultant plasma, which was platelet free, was frozen at −80°C until subsequent analyses for DON, PYY3-36, CCK, and 5-HT by ELISA.
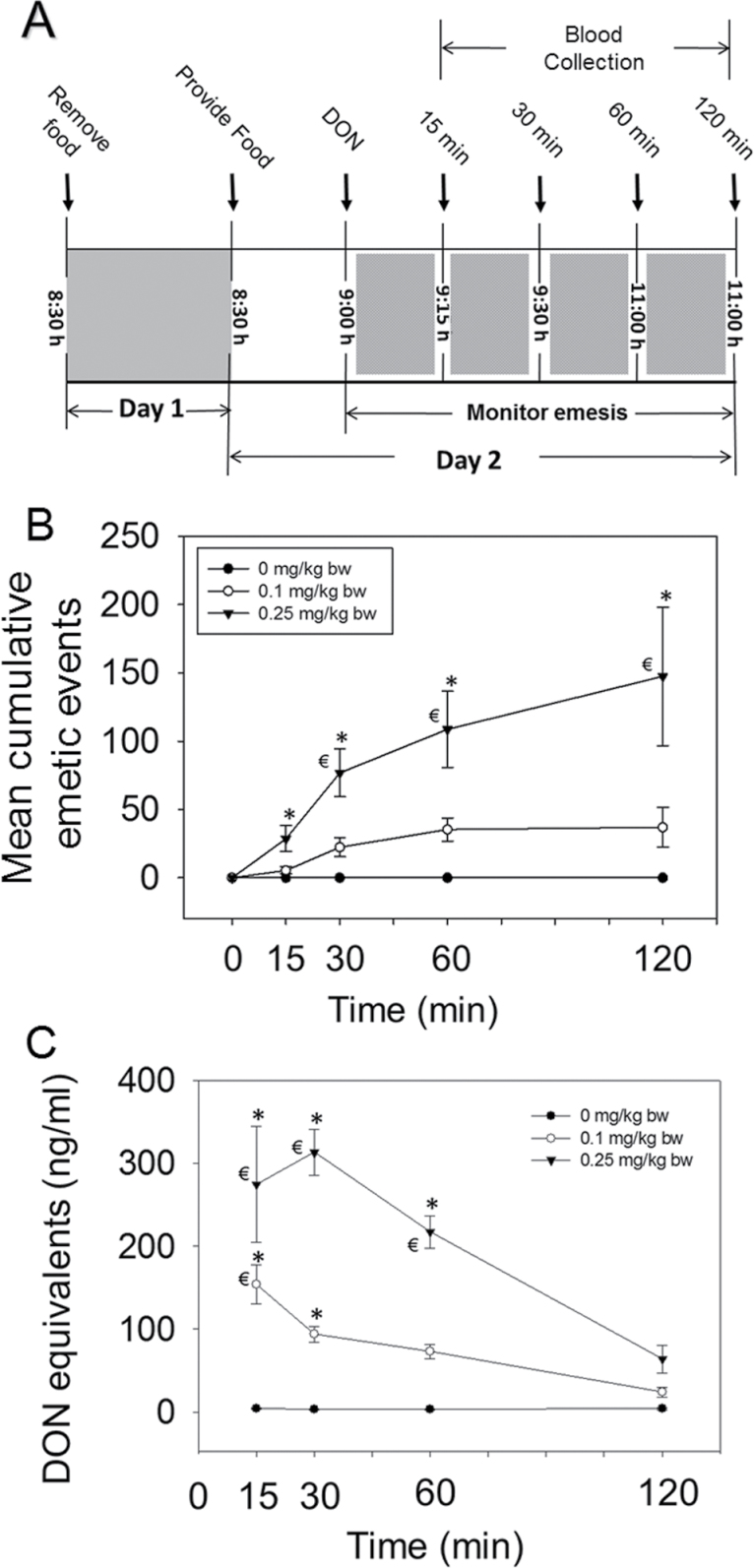
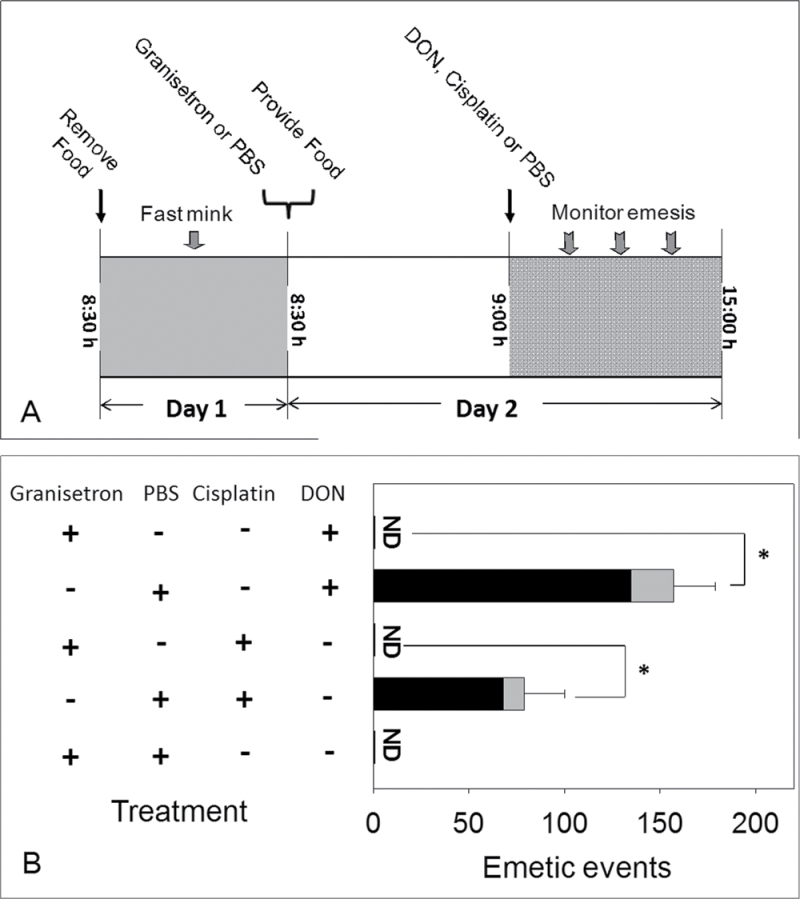
Fig. 1.
Emesis induction in mink corresponds to elevation in plasma DON. (A) Experimental design for DON-induced plasma PYY3–36 and 5-HT release; (B) mean cumulative emetic events (total retches and vomits) in mink following ip exposure to DON. Data are averages for both responders and nonresponders. The numbers of animals responding/tested at 15, 30, 60, and 120min were 6/16, 9/12, 8/8, and 4/4, respectively, for 0.1mg/kg bw DON group and 11/16, 12/12, 8/8, and 4/4, respectively, for 0.25mg/kg bw DON group. The mean latency time to onset of emesis for the 0.1 and 0.25mg/kg DON groups were 17±2 and 12±2min, respectively. (C) Kinetics of plasma DON concentration. Data represent mean ± SEM (n = 4 per group). A two-way ANOVA using Bonferroni t-test was used to assess significant differences in cumulative emetic events and kinetics of DON concentration in plasma. *p < 0.05 indicates statistically significant differences in emetic events or DON concentration compared with the control. ŧ p < 0.05 indicates a statistically significant difference in emetic events relative to the 0-min time point or significant difference in DON concentration relative to the 120-min time point within a given dose.
To assess the role of PYY on emesis induced by DON, fasted mink were first administered with the Y2R antagonist JNJ-31020028 (15mg/kg bw) (Cippitelli et al., 2011) or vehicle by sc injection at 8:45h and immediately provided 50g of feed. After 15min, DON (0.25mg/kg bw), PYY3–36 (0.01mg/kg bw, positive control), or PBS (negative control) was administered by ip injection. Mink were then returned to their cages and monitored for emesis over a 6-h period.
To determine if CCK contributed to DON-induced emesis, fasted mink were treated with the CCKR1 antagonist devazepide (0.1mg/kg bw) (Richards et al., 1996) or vehicle by sc injection at 8:30h and provided 50g of food. After 30min, DON (0.25mg/kg bw), CCK (0.0125 and 0.025mg/kg bw), or PBS (negative control) was administered by ip injection and emesis monitored.
To ascertain the role of 5-HT3 in DON-induced emesis, fasted mink were administered with the 5-HT3 receptor antagonists granisetron (2mg/kg bw) (Nakayama et al., 2005) or PBS by ip injection at 8:30h and provided 50g of feed immediately after. After 30min, DON (0.25mg/kg bw), cisplatin (7.5mg/kg bw, positive control), or PBS (negative control) was administered by ip injection, and mink were monitored for emesis. Cisplatin-treated mink were euthanized after the experiment because of the nephrotoxic effects of this drug.
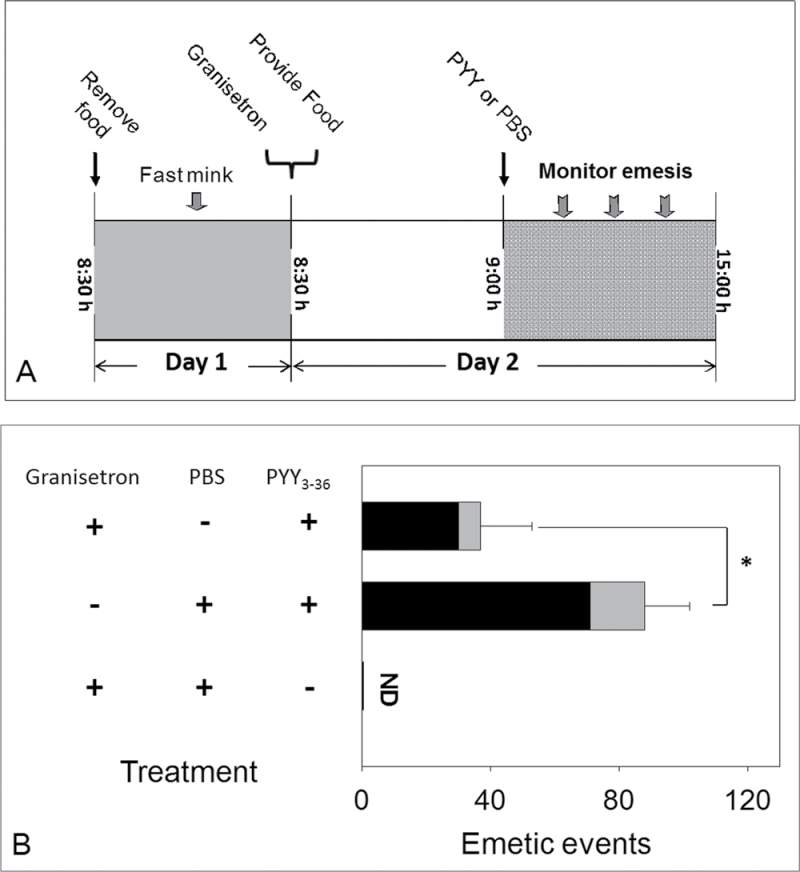
To evaluate the effect of PYY3–36 on 5-HT3-mediated emesis, mink were first administered with granisetron (2.5mg/kg bw) or PBS by ip exposure at 8:30h and provided 50g of feed immediately thereafter. After 30min, mink were administered with PYY3–36 (0.01mg/kg bw) or PBS via ip injection and mink were then monitored for emesis.
DON quantitation.
Plasma was diluted 1:6 (vol/vol) in PBS and then centrifuged at 15,000 × g for 10min. DON content in the supernatant fraction was determined using a Veratox High Sensitivity ELISA (Neogen, Lansing, MI) was performed as previously described (Pestka and Amuzie, 2008). Following addition of stop reagent (100 µl), plates were read on ELISA reader (Molecular Devices, Menlo Park, CA) at 690nm and DON concentrations determined with Softmax software (Molecular Devices).
Plasma hormone measurement.
Plasma hormones PYY and CCK were analyzed using enzyme immunoassay kits for PYY (PYY [3-36]; mouse, rat, porcine, and canine specific) and CCK (CCK [26-33], nonsulfated; human, rat, and mouse specific) (Phoenix Pharmaceuticals, Burlingame, CA). Plasma 5-HT was measured using a Serotonin EIA kit (Enzo Life Sciences, Plymouth Meeting, PA).
Data analysis.
Data were plotted and statistically analyzed using SigmaPlot 11 for Windows (Jandel Scientific; San Rafael, CA). Means were considered significantly different at p < 0.05. A two-way ANOVA using Bonferroni t-test was used to assess significant differences in cumulative emetic events, kinetics of DON, PYY, and 5-HT concentrations in plasma. A one-way ANOVA using Tukey’s test or t-test was used to analyze significant differences between treatments and the respective controls. If normality test failed, Kruskal-Wallis ANOVA on Ranks was used in conjunction with Student-Newman-Keuls test. The Spearman rank-order correlation coefficient was used for correlation between hormone levels and emetic events.
RESULTS
Mink Emetic Response Corresponds to DON Plasma Concentrations
Robust emesis was observed in mink exposed to 0.1 and 0.25mg/kg bw DON (Fig. 1B) At the 0.1mg/kg bw dose, most emesis occurred from 15 to 60min. At the 0.25mg/kg bw dose, 40, 41, 15, and 4% of emetic events were observed during 0- to 15-, 15- to 30-, 30- to 60-, and 60- to 120-min periods, respectively. Plasma DON concentrations in the 0.1mg/kg group were highest after 15min, whereas toxin concentrations peaked in the 0.25mg/kg bw group at 30min (Fig. 1C). The rate of emesis was greatest between 15 and 30min, which coincided with maximal DON plasma concentrations for both doses.
PYY3–36 Mediates DON-Induced Emesis
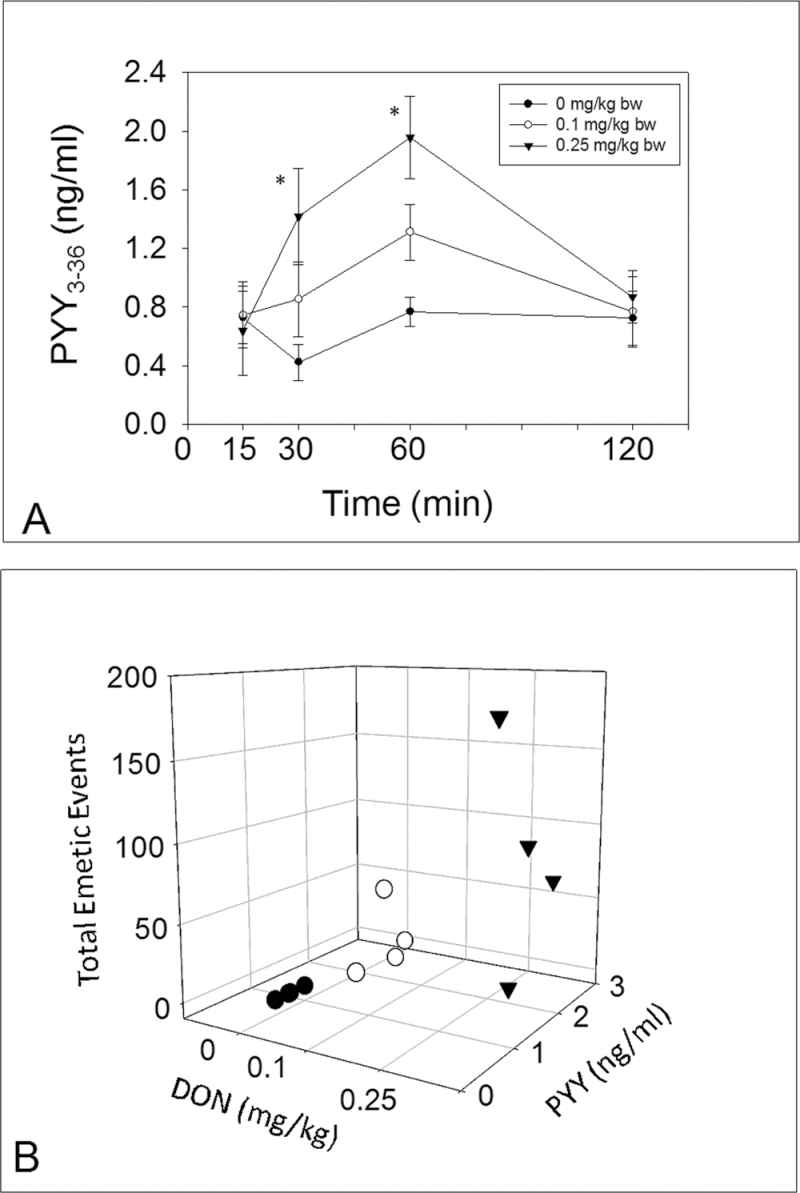
Exposure to DON at 0.25mg/kg bw resulted in PYY3–6 elevation at 30 and 60min with a similar trend being evident at 0.1mg/kg bw (Fig. 2A). Dose-dependent elevation of PYY3–36 at 60min correlated with cumulative emetic events (Fig. 2B). To further investigate the role of PYY in DON-induced emesis, mink were administered with either JNJ-31020028 or vehicle 15min prior to exposure to DON (0.25mg/kg bw), PYY, or PBS (Fig. 3A). Mink treated with vehicle and then DON had 179±17 total emetic events. Emetic events in mink administered with JNJ-31020028 prior to DON exposure were reduced by nearly 70%. JNJ-31020028 pretreatment completely suppressed PYY-induced emesis, confirming the efficacy of this drug. Animals pretreated with the drug and then vehicle did not exhibit emetic events. These results suggest that DON-induced emesis was mediated, in part, by PYY.
Fig. 2.
Emesis induction corresponds to elevation in plasma PYY3–36. (A) Kinetics of DON-induced plasma PYY3–36 concentration. Data are the mean ± SEM (n = 4 per group). A two-way ANOVA using Bonferroni t-test was used to assess significant differences in kinetics of PYY3–36 concentration in plasma. Asterisk indicates statistically significant differences in PYY3–36 concentration compared with the control at p < 0.05. (B) Relationship between total emetic events and PYY3–36 levels at 60min in DON-treated mink. Cumulative emetic events significantly correlated with PYY3–36 (Spearman rank-order correlation coefficient = 0.651, p < 0.05).
Fig. 3.
PYY3–36 mediates DON-induced emesis. Experimental design for Y2 receptor antagonist in DON-induced emesis; (B) suppression of DON- and PYY-induced emesis by Y2 receptor antagonists. Emetic events include vomiting (gray) and retching (black) episodes. ND = not detected. Data represent mean ± SEM (n = 4 per group). A one-way ANOVA using Tukey’s test or t-test was used to analyze significant differences between treatments and the respective controls. *p < 0.05 indicates statistically significant differences in emetic events between Y2 receptor antagonist treatment groups and only DON or PYY treatment group. These results and frequency, latency, and duration times are presented in tabular form in Supplementary data.
CCK Does Not Contribute to DON-Induced Emesis
Acute ip exposure to DON at 0.1 and 0.25mg/kg bw had no effect on plasma CCK concentrations over the course of 120min (data not shown). IP administration with CCK at doses of 0.0125mg/kg bw did not induce emesis, whereas 0.025mg/kg bw induced vomiting in one of three mink tested. CCK-induced emesis could be inhibited by pretreatment with the CCK1R antagonist devazepide; however, this drug had no effect on DON-induced emesis (Supplementary data). These observations indicate that CCK capacity to induce emesis was modest at best, and this hormone was unlikely to be a factor in emesis induction by this toxin.
5-HT Mediates DON-Induced Emesis
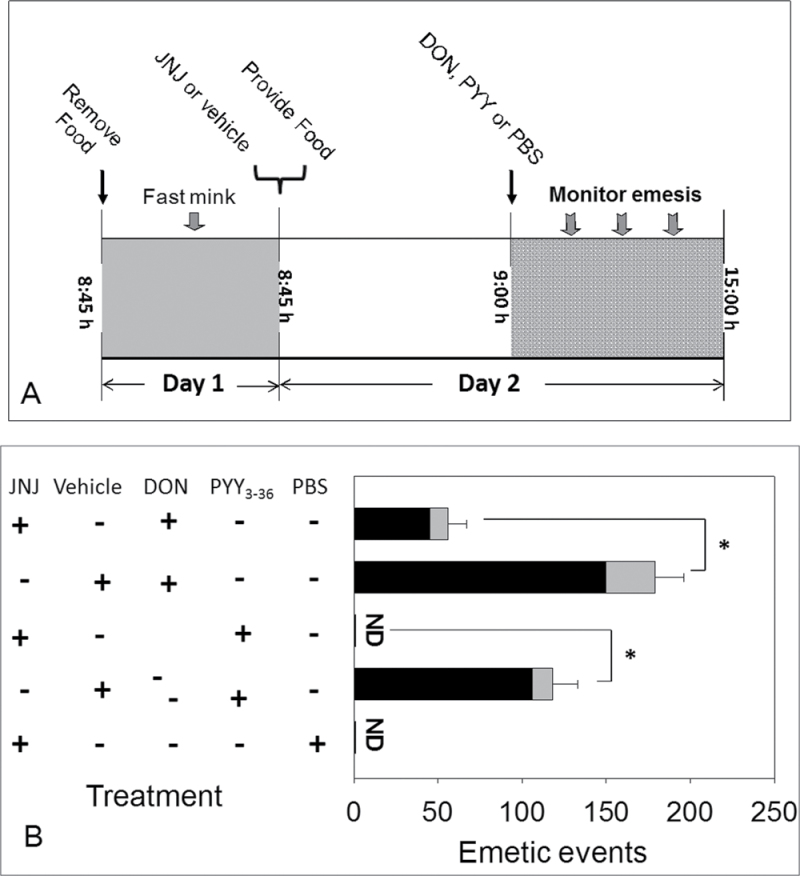
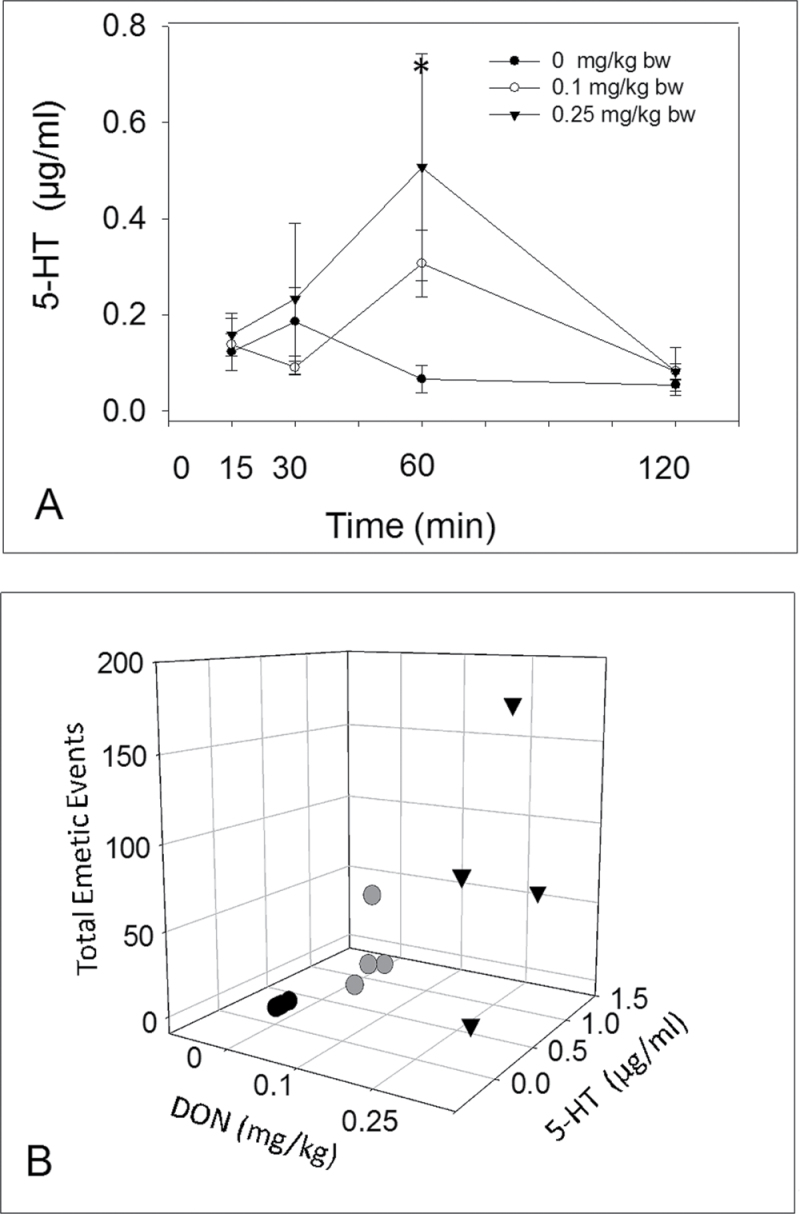
DON administered at 0.25mg/kg bw induced an increase in plasma 5-HT concentrations at 60min with a similar trend evident at 0.1mg/kg bw DON (Fig. 4A). Cumulative emetic events at 60min correlated with plasma 5-HT (Fig. 4B). The 5-HT3 receptor antagonist granisetron was used to verify the role of 5-HT3 receptor in DON-induced emesis (Fig. 5A). Pretreatment with PBS prior to DON dosing resulted in animals having 157±22 total emetic events (Fig. 5B). However, administration of granisetron 30min prior to DON exposure completely abolished this emetic response. Animals treated with PBS prior to cisplatin exhibited 79±21 total emetic events, whereas emesis was not observed in animals treated with granisetron followed by cisplatin. These data suggest that both 5-HT and 5-HT3 receptors are involved in DON-induced emesis.
Fig. 4.
Emesis induction corresponds to elevations in plasma 5-HT. (A) Kinetics of DON-induced plasma 5-HT. Data are the mean ± SEM (n = 4 per group). A two-way ANOVA using Bonferroni t-test was used to assess significant differences in kinetics of 5-HT concentration in plasma. Asterisk indicates statistically significant differences in 5-HT concentration compared with the control at p < 0.05. (B) Relationship between total emetic events and plasma 5-HT at 60min. Cumulative emetic events significantly correlated with plasma 5-HT (Spearman rank-order correlation coefficient = 0.623, p < 0.05).
Fig. 5.
5-HT mediates DON-induced emesis. (A) Experimental design for 5-HT3 receptor antagonist in DON- and cisplatin-induced emesis; (B) suppression of emesis by 5-HT3 receptor antagonist, granisetron. Emetic events include vomiting (gray) and retching (black) episodes. ND = not detected. Data represent mean ± SEM (n = 4 per group). A one-way ANOVA using Tukey’s test or t-test was used to analyze significant differences between treatments and the respective controls. Asterisk indicates statistically significant differences in emetic events between 5-HT3 receptor antagonist treatment groups and only DON or cisplatin treatment group at p < 0.05. These results and frequency, latency, and duration times are presented in tabular form in Supplementary data.
5-HT Contributes to PYY-Induced Emesis
A final study was conducted to determine the relationship between PYY and 5-HT pathways of emesis (Fig. 6A). Animals pretreated with PBS prior to treatment with PYY exhibited 88±14 total emetic events, whereas animals treated with granisetron prior to PYY had 37±16 total emetic events (Fig. 6B). No significant differences in latency or duration of PYY-induced emesis were evident between animals pretreated with granisetron and animals pretreated with PBS. These data suggest that PYY’s effects might be mediated, in part, by 5-HT.
Fig. 6.
PYY-induced emesis is mediated through 5-HT. (A) Experimental design; (B) suppression of PYY-induced emesis by 5-HT3 receptor antagonist granisetron. Emetic events include vomiting (gray) and retching (black) episodes. ND = not detected. Data represent mean ± SEM (n = 4 per group). A one-way ANOVA using Tukey’s test or t-test was used to analyze significant differences between treatments and the respective controls. Asterisk indicates statistically significant differences in emetic events between 5-HT3 receptor antagonist treatment group and only PYY treatment group at p < 0.05. These results and frequency, latency, and duration times are presented in tabular form in Supplementary data.
DISCUSSION
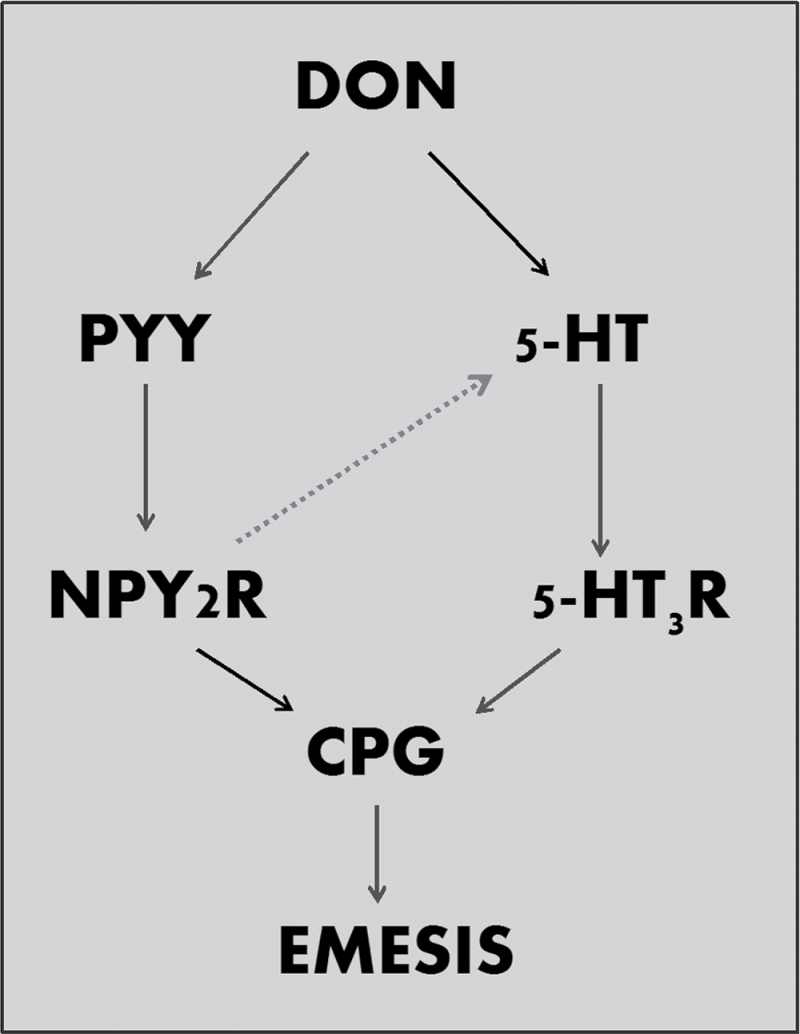
Given the frequent presence of DON in grain-based foods and animal feeds, it is important to understand the basic mechanisms by which it causes gastrointestinal illness in humans and animals. The findings presented herein suggested that DON elevated plasma concentrations of 5-HT and PYY3–36. Additionally, DON-induced emesis was inhibited by the 5-HT3 receptor antagonist granisetron and the Y2R antagonist JNJ-31020028, further confirming roles for these hormones in DON-induced emesis. Thus, both hormones might cocontribute to DON-induced emesis in the mink (Fig. 7).
Fig. 7.
Putative mechanisms for DON-induced emesis. The results presented here suggest that DON could act by inducing PYY release (e.g., L cells) and 5-HT release (e.g., EC cells). These might activate neuropeptide Y2 receptor (NPY2R) and 5-HT3 receptors (5-HT3R), respectively, in the peripheral and central nervous systems, ultimately inducing emesis via the CPG. The potential exists for significant crosstalk (dotted line)ss whereby PYY induces 5-HT release at peripheral and central sites.
The data presented herein were consistent with a prior study demonstrating that 5-HT3 receptors mediate DON-induced emesis in pigs (Prelusky and Trenholm, 1993). It is likely that DON induces exocytosis to EC cells, resulting in the release of 5-HT into the GI tract and subsequent stimulation of the 5-HT3 receptors located on the vagal afferents. These stimulated vagal afferents would then activate the CPG via signaling through the NTS and AP, thereby evoking vomiting. In support of this contention, DON exposure delays gastric emptying (a surrogate for emesis) in rodents via a pathway involving 5-HT3 receptors (Fioramonti et al., 1993). Further consistent with our finding, Andrews et al. (1990) demonstrated that the trichothecene diacetoxyscirpinol (anguidine) caused emesis in ferrets that could be attenuated by abdominal vagotomy or pretreatment with a 5-HT3 receptor antagonist.
Though less studied for its emetogenic properties, PYY is one of the most potent peptide inducers of vomiting known (Harding and McDonald, 1989). Here, DON administration rapidly increased plasma concentrations of PYY3–36, and furthermore, ip administration of PYY3–36 evoked emesis in mink, indicating that this peptide might indeed be a factor in the toxin’s emetic effects. PYY3–36 could act by binding to Y2R located either on the vagal afferents or within the AP (Dumont et al., 2007; Koda et al., 2005), ultimately activating signaling within the CPG. Studies in the dog found that selective ablation of the AP could completely block PYY-induced emesis (Harding and McDonald, 1989). Because JNJ-31020028 penetrates the blood-brain barrier (Shoblock et al., 2010; Swanson et al., 2011), it has the capacity to block Y2Rs both centrally and peripherally, making it impossible to discriminate central versus peripheral actions of PYY at this time.
Given DON’s capacity to robustly induce CCK release in mice (Flannery et al., 2012), the potential of this gut satiety peptide to affect vomiting was also considered. Plasma CCK was not affected in mink as was previously observed in the mouse. Additionally, the ability of ip CCK administration to induce vomiting was quite modest in the mink as was previously observed in monkeys (Perera et al., 1993) and ferrets (Percie du Sert et al., 2012). Relatedly, Billig et al. (2001) found that CCK induced plasma vasopressin (arginine vasopressin), a physiological correlate of nausea in emetic species but not vomiting in the ferret. Although it is feasible that the CCK could still exert emetogenic effects without detectable changes in plasma concentrations, we were unable to reduce DON-induced emesis with the CCKR1 antagonist devazapide. Accordingly, these data do not support the possibility that CCK mediates DON-induced vomiting.
Although emesis corresponded with the kinetics of DON distribution and clearance in plasma, it was notable that an elevation of 5-HT plasma concentration followed rather than preceded the onset and peak of emetic activity. Plasma 5-HT elevation was only detectable at 60min after DON administration at the highest dose, which differs with the prior findings showing no detectable changes in plasma 5-HT in pigs in response to DON (Prelusky, 1994). Although species differences, sampling times, and improvements in assay sensitivity could explain these differing results, other factors might be involved. Most (> 90%) of the total 5-HT is located in the GI tract within EC cells and enteric neurons (Feldberg and Toh, 1953; Kim and Camilleri, 2000; Resnick and Gray, 1961), with the remainder being found in the brain (Endo et al., 2000). Once 5-HT is released from EC cells, most 5-HT will be metabolized into 5-HIAA within the gut wall or liver; however, increased 5-HIAA in plasma or urine does not correlate with circulating 5-HT concentrations (Endo et al., 2000). Additionally, circulating 5-HT can be effectively up taken by platelets, leading to no increase in plasma concentrations (Thomas and Vane, 1967). Interestingly, in a chemotherapy-induced emesis study, circulating 5-HT was not associated with emesis induction by cisplatin, even though this adverse effect that could be countered with 5-HT3 receptor antagonists (Castejon et al., 1999).
It is particularly intriguing that plasma PYY was elevated (30min) prior to 5-HT (60min) and thus somewhat more in synchrony with emesis. Although it is possible that 5-HT release occurs earlier but is masked by rapid metabolism to 5-HIAA, this finding along with our observation that granisetron attenuated PYY-induced emesis suggests that this satiety peptide might indeed be capable of inducing 5-HT-mediated events. Two analogous prior studies support this contention. First, it has been established in the fistulated dog that iv administration of either PYY or 5-HT can reduce intestinal transit and, furthermore, PYY-induced suppression of intestinal transit can be blocked by the 5-HT3 receptor antagonist ordansetron (Lin et al., 2004). Second, studies using guinea pig colonic mucosal sections revealed that exogenously applied PYY induced sustained release of 5-HT that could be blocked with NPY receptor antagonists (Kojima et al., 2012). It was also shown that selective tachykinin NK2 receptor agonism evoke PYY and 5-HT release in the mucosal sections and that the latter can be suppressed with NPY receptor antagonists. Collectively, these findings suggest that PYY-containing L cells could control, in part, the release of 5-HT from intestinal EC cells (Fig. 7). Interestingly, Perry et al. (1994) have previously reported that plasma PYY and 5-HT are elevated in cisplatin-treated dogs. However, granisetron pretreatment impaired elevation of PYY but not 5-HT, suggesting different mechanisms are in play for this drug and model.
Although much of the present discussion focuses on gut endocrine cells as DON targets, it cannot be excluded that the toxin directly targets the AP or central nervous system to modulate emetic neurocircuitry by stimulating PYY and 5-HT release in the brain. Consistent with a possible direct action of DON on the central nervous system, we previously observed that the toxin is detectable in the brain of mice within 5min of oral exposure (Pestka et al., 2008). Furthermore, po administration of DON can induce c-Fos expression in the circumventricular organs of the brain (Girardet et al., 2011a,b). Recent immunohistochemical studies have shown the colocalization of PYY and 5-HT within neurons and that PYY-producing cells are present in the hindbrain (Gelegen et al., 2012) with 5-HT fibers in close apposition suggesting possible synaptic contacts. Therefore, the possibility that DON has direct effects on PYY and 5-HT in brain merits further investigation.
To summarize, the results provided herein suggest that PYY and 5-HT are prominent mediators of DON-induced emesis. Future studies should focus on discerning how DON and other trichothecenes act on L-cells and EC-cells within the gut and neurons within the brain. It will be of additional interest to understand how and where PYY affects serotonergic pathways. Strategies for such approaches could include (1) measurement of residual intestinal mucosal 5-HT and PYY following DON treatment, (2) determination of whether DON can cause direct release of 5-HT and PYY from intestine in vitro, (3) use of peripheral 5-HT hydroxylase inhibitors, and (4) ascertaining the effect of selective nerve lesions on DON-induced emesis. Ultimately, such studies will improve our understanding of how DON and other trichothecenes cause human food poisoning.
FUNDING
United States Department of Agriculture National Institute of Food and Agriculture Award (2011-0635); United States Department of Agriculture Wheat and Barley SCAB Initiative Award (59-0206-9-058); National Institutes of Health (Public Health Service Grant ES03553).
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to acknowledge Andrew Cohen-Barnhouse, Angelo Napolitano, and Mary Rosner for their assistance.
REFERENCES
- Abbott C. R., Monteiro M., Small C. J., Sajedi A., Smith K. L., Parkinson J. R., Ghatei M. A., Bloom S. R. (2005). The inhibitory effects of peripheral administration of peptide YY(3-36) and glucagon-like peptide-1 on food intake are attenuated by ablation of the vagal-brainstem-hypothalamic pathway. Brain Res. 1044, 127–131 [DOI] [PubMed] [Google Scholar]
- Andrews P. L., Davis C. J., Bingham S., Davidson H. I., Hawthorn J., Maskell L. (1990). The abdominal visceral innervation and the emetic reflex: Pathways, pharmacology, and plasticity. Can. J. Physiol. Pharmacol. 68, 325–345 [DOI] [PubMed] [Google Scholar]
- Andrews P. L., Hawthorn J. (1988). The neurophysiology of vomiting. Baillieres Clin. Gastroenterol. 2, 141–168 [DOI] [PubMed] [Google Scholar]
- Andrews P. L., Horn C. C. (2006). Signals for nausea and emesis: Implications for models of upper gastrointestinal diseases. Auton. Neurosci. 125, 100–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anonymous (1999). Outbreaks of gastrointestinal illness of unknown etiology associated with eating burritos—United States, October 1997-October 1998. MMWR Morb. Mortal. Wkly. Rep. 48, 210–213 [PubMed] [Google Scholar]
- Ballantyne G. H. (2006). Peptide YY(1-36) and peptide YY(3-36): Part I. Distribution, release and actions. Obes. Surg. 16, 651–658 [DOI] [PubMed] [Google Scholar]
- Baptista V., Browning K. N., Travagli R. A. (2007). Effects of cholecystokinin-8s in the nucleus tractus solitarius of vagally deafferented rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1092–R1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batterham R. L., Cowley M. A., Small C. J., Herzog H., Cohen M. A., Dakin C. L., Wren A. M., Brynes A. E., Low M. J., Ghatei M. A., et al. (2002). Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 418, 650–654 [DOI] [PubMed] [Google Scholar]
- Billig I., Yates B. J., Rinaman L. (2001). Plasma hormone levels and central c-Fos expression in ferrets after systemic administration of cholecystokinin. Am. J. Physiol. Regul. Integr. Comp. Physiol. 281, R1243–R1255 [DOI] [PubMed] [Google Scholar]
- Blevins J. E., Chelikani P. K., Haver A. C., Reidelberger R. D. (2008). PYY(3-36) induces Fos in the arcuate nucleus and in both catecholaminergic and non-catecholaminergic neurons in the nucleus tractus solitarius of rats. Peptides. 29, 112–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borison H. L. (1989). Area postrema: Chemoreceptor circumventricular organ of the medulla oblongata. Prog. Neurobiol. 32, 351–390 [DOI] [PubMed] [Google Scholar]
- Canady R., Coker R., Egan S., Krska R., Kuiper-Goodman T., Olsen M., Pestka J., Resnik S., Schlatter J. (2001). Deoxynivalenol. In Safety Evaluation of Certain Mycotoxins in Food, pp. 420–555 World Health Organization; Geneva: [Google Scholar]
- Carpenter D. O. (1990). Neural mechanisms of emesis. Can. J. Physiol. Pharmacol. 68, 230–236 [DOI] [PubMed] [Google Scholar]
- Castejon A. M., Paez X., Hernandez L., Cubeddu L. X. (1999). Use of intravenous microdialysis to monitor changes in serotonin release and metabolism induced by cisplatin in cancer patients: Comparative effects of granisetron and ondansetron. J. Pharmacol. Exp. Ther. 291, 960–966 [PubMed] [Google Scholar]
- Cippitelli A., Rezvani A. H., Robinson J. E., Eisenberg L., Levin E. D., Bonaventure P., Motley S. T., Lovenberg T. W., Heilig M., Thorsell A. (2011). The novel, selective, brain-penetrant neuropeptide Y Y2 receptor antagonist, JNJ-31020028, tested in animal models of alcohol consumption, relapse, and anxiety. Alcohol. 45, 567–576 [DOI] [PubMed] [Google Scholar]
- Clarke S. E., Austin N. E., Bloomer J. C., Haddock R. E., Higham F. C., Hollis F. J., Nash M., Shardlow P. C., Tasker T. C., Woods F. R. (1994). Metabolism and disposition of 14C-granisetron in rat, dog and man after intravenous and oral dosing. Xenobiotica. 24, 1119–1131 [DOI] [PubMed] [Google Scholar]
- Dumont Y., Moyse E., Fournier A., Quirion R. (2007). Distribution of peripherally injected peptide YY ([125I] PYY (3-36)) and pancreatic polypeptide ([125I] hPP) in the CNS: Enrichment in the area postrema. J. Mol. Neurosci. 33, 294–304 [DOI] [PubMed] [Google Scholar]
- Eberle-Wang K., Simansky K. J. (1992). The CCK-A receptor antagonist, devazepide, blocks the anorectic action of CCK but not peripheral serotonin in rats. Pharmacol. Biochem. Behav. 43, 943–947 [DOI] [PubMed] [Google Scholar]
- Ekblad E., Sundler F. (2002). Distribution of pancreatic polypeptide and peptide YY. Peptides. 23, 251–261 [DOI] [PubMed] [Google Scholar]
- Endo T., Minami M., Hirafuji M., Ogawa T., Akita K., Nemoto M., Saito H., Yoshioka M., Parvez S. H. (2000). Neurochemistry and neuropharmacology of emesis - the role of serotonin. Toxicology. 153, 189–201 [DOI] [PubMed] [Google Scholar]
- Feldberg W., Toh C. C. (1953). Distribution of 5-hydroxytryptamine (serotonin, enteramine) in the wall of the digestive tract. J. Physiol. (Lond.). 119, 352–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fioramonti J., Dupuy C., Dupuy J., Bueno L. (1993). The mycotoxin, deoxynivalenol, delays gastric emptying through serotonin-3 receptors in rodents. J. Pharmacol. Exp. Ther. 266, 1255–1260 [PubMed] [Google Scholar]
- Flannery B. M., Clark E. S., Pestka J. J. (2012). Anorexia induction by the trichothecene deoxynivalenol (vomitoxin) is mediated by the release of the gut satiety hormone peptide YY. Toxicol. Sci. 130, 289–297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery B. M., Wu W., Pestka J. J. (2011). Characterization of deoxynivalenol-induced anorexia using mouse bioassay. Food Chem. Toxicol. 49, 1863–1869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsyth D. M., Yoshizawa T., Morooka N., Tuite J. (1977). Emetic and refusal activity of deoxynivalenol to swine. Appl. Environ. Microbiol. 34, 547–552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fur Commission USA (2010). Standard Guidelines for the Operation of Mink Farms in the United States Available at: http://www.maninnature.com/FCUSA/Members/Resources/Minkguide.pdf Accessed October 9, 2012
- Gantz I., Erondu N., Mallick M., Musser B., Krishna R., Tanaka W. K., Snyder K., Stevens C., Stroh M. A., Zhu H., et al. (2007). Efficacy and safety of intranasal peptide YY3-36 for weight reduction in obese adults. J. Clin. Endocrinol. Metab. 92, 1754–1757 [DOI] [PubMed] [Google Scholar]
- Gelegen C., Chandarana K., Choudhury A. I., Al-Qassab H., Evans I. M., Irvine E. E., Hyde C. B., Claret M., Andreelli F., Sloan S. E., et al. (2012). Regulation of hindbrain Pyy expression by acute food deprivation, prolonged caloric restriction, and weight loss surgery in mice. Am. J. Physiol. Endocrinol. Metab. 303, E659–E668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardet C., Bonnet M. S., Jdir R., Sadoud M., Thirion S., Tardivel C., Roux J., Lebrun B., Mounien L., Trouslard J., et al. (2011a). Central inflammation and sickness-like behavior induced by the food contaminant deoxynivalenol: A PGE2-independent mechanism. Toxicol. Sci. 124, 179–191 [DOI] [PubMed] [Google Scholar]
- Girardet C., Bonnet M. S., Jdir R., Sadoud M., Thirion S., Tardivel C., Roux J., Lebrun B., Wanaverbecq N., Mounien L., et al. (2011b). The food-contaminant deoxynivalenol modifies eating by targeting anorexigenic neurocircuitry. PLoS ONE. 6, e26134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halatchev I. G., Cone R. D. (2005). Peripheral administration of PYY(3-36) produces conditioned taste aversion in mice. Cell Metab. 1, 159–168 [DOI] [PubMed] [Google Scholar]
- Harding R. K., McDonald T. J. (1989). Identification and characterization of the emetic effects of peptide YY. Peptides. 10, 21–24 [DOI] [PubMed] [Google Scholar]
- Hornby P. J. (2001). Central neurocircuitry associated with emesis. Am. J. Med. 111, 106S–112S [DOI] [PubMed] [Google Scholar]
- Hughes D. M., Gahl M. J., Graham C. H., Grieb S. L. (1999). Overt signs of toxicity to dogs and cats of dietary deoxynivalenol. J. Anim. Sci. 77, 693–700 [DOI] [PubMed] [Google Scholar]
- Jackson L. S., Bullerman L. B. (1999). Effect of processing on Fusarium mycotoxins. Adv. Exp. Med. Biol. 459, 243–261 [DOI] [PubMed] [Google Scholar]
- Kim D. Y., Camilleri M. (2000). Serotonin: A mediator of the brain-gut connection. Am. J. Gastroenterol. 95, 2698–2709 [DOI] [PubMed] [Google Scholar]
- Koda S., Date Y., Murakami N., Shimbara T., Hanada T., Toshinai K., Niijima A., Furuya M., Inomata N., Osuye K., et al. (2005). The role of the vagal nerve in peripheral PYY3-36-induced feeding reduction in rats. Endocrinology. 146, 2369–2375 [DOI] [PubMed] [Google Scholar]
- Koegler F. H., Enriori P. J., Billes S. K., Takahashi D. L., Martin M. S., Clark R. L., Evans A. E., Grove K. L., Cameron J. L., Cowley M. A. (2005). Peptide YY(3-36) inhibits morning, but not evening, food intake and decreases body weight in rhesus macaques. Diabetes. 54, 3198–3204 [DOI] [PubMed] [Google Scholar]
- Koga T., Fukuda H. (1992). Neurons in the nucleus of the solitary tract mediating inputs from emetic vagal afferents and the area postrema to the pattern generator for the emetic act in dogs. Neurosci. Res. 14, 166–179 [DOI] [PubMed] [Google Scholar]
- Kojima S., Tohei A., Anzai N. (2012). A role for endogenous peptide YY in tachykinin NK(2) receptor-triggered 5-HT release from guinea pig isolated colonic mucosa. Br. J. Pharmacol. 167, 1362–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopin A. S., Mathes W. F., McBride E. W., Nguyen M., Al-Haider W., Schmitz F., Bonner-Weir S., Kanarek R., Beinborn M. (1999). The cholecystokinin-A receptor mediates inhibition of food intake yet is not essential for the maintenance of body weight. J. Clin. Invest. 103 383–391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharczyk J., Harding R. K. (1990). Regulatory peptides and the onset of nausea and vomiting. Can. J. Physiol. Pharmacol. 68, 289–293 [DOI] [PubMed] [Google Scholar]
- Lin H. C., Neevel C., Chen J. H. (2004). Slowing intestinal transit by PYY depends on serotonergic and opioid pathways. Am. J. Physiol. Gastrointest. Liver Physiol. 286, G558–G563 [DOI] [PubMed] [Google Scholar]
- Liou A. P., Sei Y., Zhao X., Feng J., Lu X., Thomas C., Pechhold S., Raybould H. E., Wank S. A. (2011). The extracellular calcium-sensing receptor is required for cholecystokinin secretion in response to L-phenylalanine in acutely isolated intestinal I cells. Am. J. Physiol. Gastrointest. Liver Physiol. 300, G538–G546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X. (1994). Food poisoning caused by Fusarium toxins. In Proceedings of the Second Asian Conference on Food Safety. 18–23 September 1994, Bangkok, Thailand, pp. 129–136
- Miller A. D. (1999). Central mechanisms of vomiting. Dig. Dis. Sci. 44(8Suppl), 39S–43S [PubMed] [Google Scholar]
- Nakayama H., Yamakuni H., Higaki M., Ishikawa H., Imazumi K., Matsuo M., Mutoh S. (2005). Antiemetic activity of FK1052, a 5-HT3- and 5-HT4-receptor antagonist, in Suncus murinus and ferrets. J. Pharmacol. Sci. 98, 396–403 [DOI] [PubMed] [Google Scholar]
- Perry M. R., Rhee J., Smith W. L. (1994). Plasma levels of peptide YY correlate with cisplatin-induced emesis in dogs. J. Pharm. Pharmacol. 46, 553–557 [DOI] [PubMed] [Google Scholar]
- Percie du Sert N. P., Holmes A. M., Wallis R., Andrews P. L. R. (2012). Predicting the emetic liability of novel chemical entities: A comparative study. Brit. J. Pharmacol. 165, 1848–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Percie du Sert N. P, Rudd J. A., Apfel C. C., Andrews P. L. (2011). Cisplatin-induced emesis: Systematic review and meta-analysis of the ferret model and the effects of 5-HT receptor antagonists. Cancer Chemother. Pharmacol. 67, 667–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perera A. D., Verbalis J. G., Mikuma N., Majumdar S. S., Plant T. M. (1993). Cholecystokinin stimulates gonadotropin-releasing hormone release in the monkey (Macaca mulatta). Endocrinology. 132, 1723–1728 [DOI] [PubMed] [Google Scholar]
- Pestka J. J. (2010a). Deoxynivalenol: Mechanisms of action, human exposure, and toxicological relevance. Arch. Toxicol. 84, 663–679 [DOI] [PubMed] [Google Scholar]
- Pestka J. J. (2010b). Toxicological mechanisms and potential health effects of deoxynivalenol and nivalenol. World Mycotoxin J. 3, 323–347 [Google Scholar]
- Pestka J. J., Amuzie C. J. (2008). Tissue distribution and proinflammatory cytokine gene expression following acute oral exposure to deoxynivalenol: Comparison of weanling and adult mice. Food Chem. Toxicol. 46, 2826–2831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka J. J., Islam Z., Amuzie C. J. (2008). Immunochemical assessment of deoxynivalenol tissue distribution following oral exposure in the mouse. Toxicol. Lett. 178, 83–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka J. J., Lin W. S., Miller E. R. (1987). Emetic activity of the trichothecene 15-acetyldeoxynivalenol in swine. Food Chem. Toxicol. 25, 855–858 [DOI] [PubMed] [Google Scholar]
- Pittner R. A., Moore C. X., Bhavsar S. P., Gedulin B. R., Smith P. A., Jodka C. M., Parkes D. G., Paterniti J. R., Srivastava V. P., Young A. A. (2004). Effects of PYY[3-36] in rodent models of diabetes and obesity. Int. J. Obes. Relat. Metab. Disord. 28, 963–971 [DOI] [PubMed] [Google Scholar]
- Prelusky D. B. (1994). The effect of deoxynivalenol on serotoninergic neurotransmitter levels in pig blood. J. Environ. Sci. Health. B. 29, 1203–1218 [DOI] [PubMed] [Google Scholar]
- Prelusky D. B., Trenholm H. L. (1993). The efficacy of various classes of anti-emetics in preventing deoxynivalenol-induced vomiting in swine. Nat. Toxins. 1, 296–302 [DOI] [PubMed] [Google Scholar]
- Qian Q., Chen W., Yue W., Yang Z., Liu Z., Qian W. (2010a). Antiemetic effect of Xiao-Ban-Xia-Tang, a Chinese medicinal herb recipe, on cisplatin-induced acute and delayed emesis in minks. J. Ethnopharmacol. 128, 590–593 [DOI] [PubMed] [Google Scholar]
- Qian Q. H., Yue W., Chen W. H., Yang Z. H., Liu Z. T., Wang Y. X. (2010b). Effect of gingerol on substance P and NK1 receptor expression in a vomiting model of mink. Chin. Med. J. 123, 478–484 [PubMed] [Google Scholar]
- Qian Q. H., Yue W., Wang Y. X., Yang Z. H., Liu Z. T., Chen W. H. (2009). Gingerol inhibits cisplatin-induced vomiting by down regulating 5-hydroxytryptamine, dopamine and substance P expression in minks. Arch. Pharm. Res. 32, 565–573 [DOI] [PubMed] [Google Scholar]
- Resnick R. H., Gray S. J. (1961). Distribution of serotonin (5-hydroxytryptamine) in the human gastrointestinal tract. Gastroenterology. 41, 119–121 [PubMed] [Google Scholar]
- Richards W., Hillsley K., Eastwood C., Grundy D. (1996). Sensitivity of vagal mucosal afferents to cholecystokinin and its role in afferent signal transduction in the rat. J. Physiol. 497, 473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoblock J. R., Welty N., Nepomuceno D., Lord B., Aluisio L., Fraser I., Motley S. T., Sutton S. W., Morton K., Galici R., et al. (2010). In vitro and in vivo characterization of JNJ-31020028 (N-(4-{4-[2-(diethylamino)-2-oxo-1-phenylethyl]piperazin-1-yl}-3-fluorophenyl)-2-pyridin-3-ylbenzamide), a selective brain penetrant small molecule antagonist of the neuropeptide Y Y(2) receptor. Psychopharmacology (Berl.). 208, 265–277 [DOI] [PubMed] [Google Scholar]
- Sloth B., Holst J. J., Flint A., Gregersen N. T., Astrup A. (2007). Effects of PYY1-36 and PYY3-36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am. J. Physiol. Endocrinol. Metab. 292, E1062–E1068 [DOI] [PubMed] [Google Scholar]
- Stables R., Andrews P. L., Bailey H. E., Costall B., Gunning S. J., Hawthorn J., Naylor R. J., Tyers M. B. (1987). Antiemetic properties of the 5HT3-receptor antagonist, GR38032F. Cancer Treat. Rev. 14, 333–336 [DOI] [PubMed] [Google Scholar]
- Steinberg E. B., Henderson A., Karpati A., Hoekstra M., Marano N., Souza J. M., Simons M., Kruger K., Giroux J., Rogers H. S., et al. (2006). Mysterious outbreaks of gastrointestinal illness associated with burritos supplied through school lunch programs. J. Food Prot. 69, 1690–1698 [DOI] [PubMed] [Google Scholar]
- Sullivan C. N., Raboin S. J., Gulley S., Sinzobahamvya N. T., Green G. M., Reeve J. R., Jr, Sayegh A. I. (2007). Endogenous cholecystokinin reduces food intake and increases Fos-like immunoreactivity in the dorsal vagal complex but not in the myenteric plexus by CCK1 receptor in the adult rat. Am. J. Physiol. Regul. Integr. Comp. Physiol. 292, R1071–R1080 [DOI] [PubMed] [Google Scholar]
- Swanson D. M., Wong V. D., Jablonowski J. A., Shah C., Rudolph D. A., Dvorak C. A., Seierstad M., Dvorak L. K., Morton K., Nepomuceno D., et al. (2011). The discovery and synthesis of JNJ 31020028, a small molecule antagonist of the Neuropeptide Y Y(2) receptor. Bioorg. Med. Chem. Lett. 21, 5552–5556 [DOI] [PubMed] [Google Scholar]
- Thomas D. P., Vane J. R. (1967). 5-Hydroxytryptamine in the circulation of the dog. Nature. 216, 335–338 [DOI] [PubMed] [Google Scholar]
- Ueno Y. (1987). Trichothecenes in food. In Mycotoxins in Food (Krogh P, Ed.), pp. 123–147 Academic Press; London: [Google Scholar]
- Vesonder R. F., Ciegler A., Jensen A. H. (1973). Isolation of the emetic principle from Fusarium-infected corn. Appl. Microbiol. 26, 1008–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu W., Bates M. A., Bursian S. J., Link J. E., Flannery B. M., Sugita-Konishi Y., Watanabe M., Zhang H., Pestka J. J. (2013). Comparison of emetic potencies of the 8-ketotrichothecenes deoxynivalenol, 15-acetyldeoxynivalenol, 3-acetyldeoxynivalenol, fusarenon X, and nivalenol. Toxicol. Sci. 131, 279–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshizawa T. (1983). Red-mold diseases and natural occurrence in Japan. In Trichothecenes: Chemical, Biological, and Toxicological Aspects (Ueno Y, Ed.), pp. 195–209 Amsterdam, Elsevier: [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.