Abstract
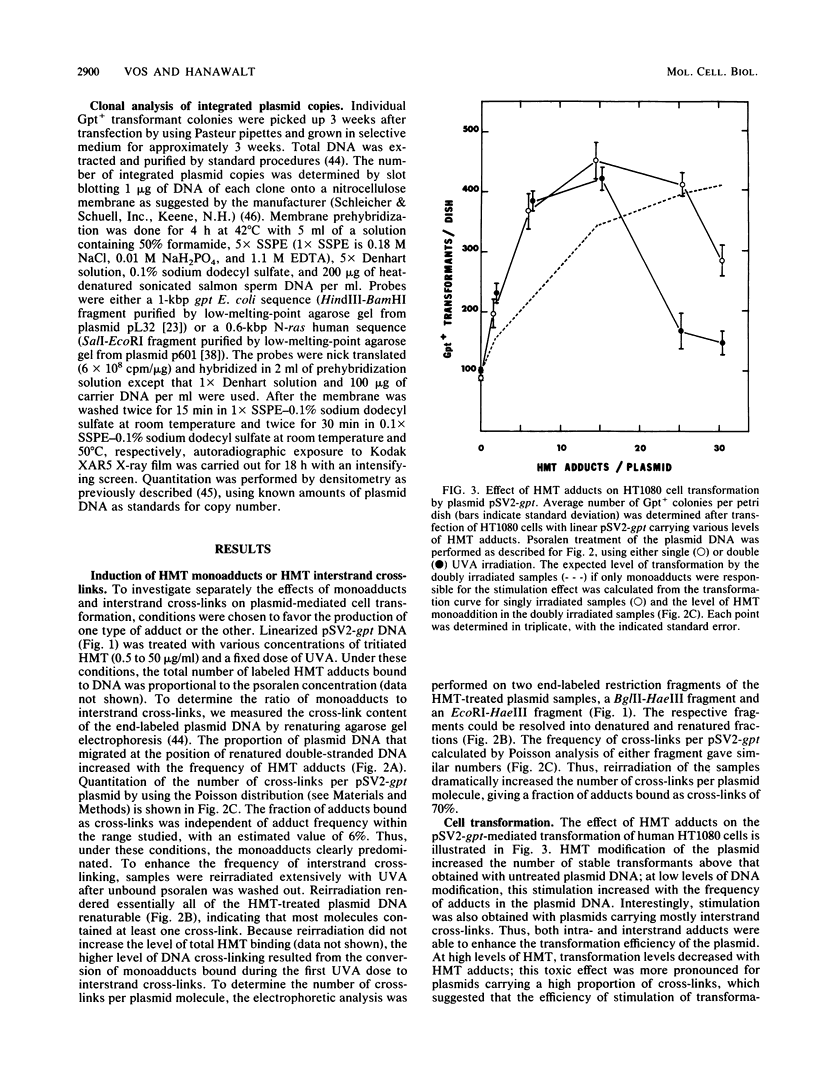
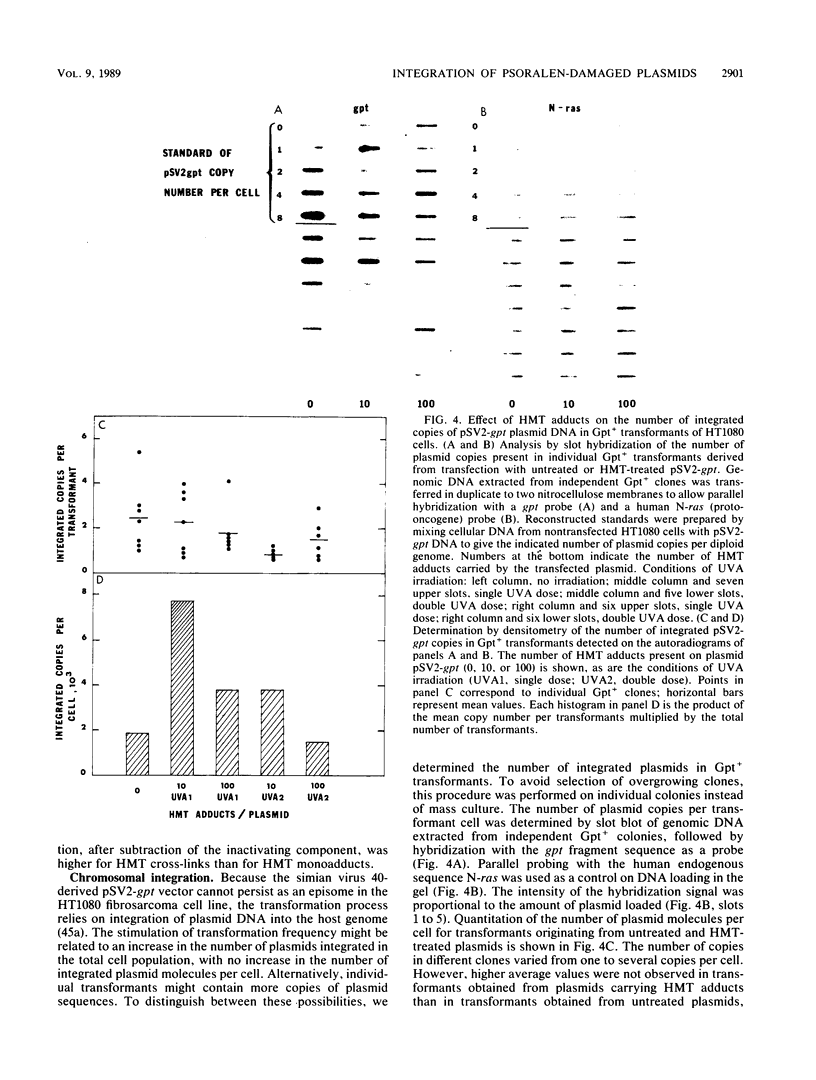
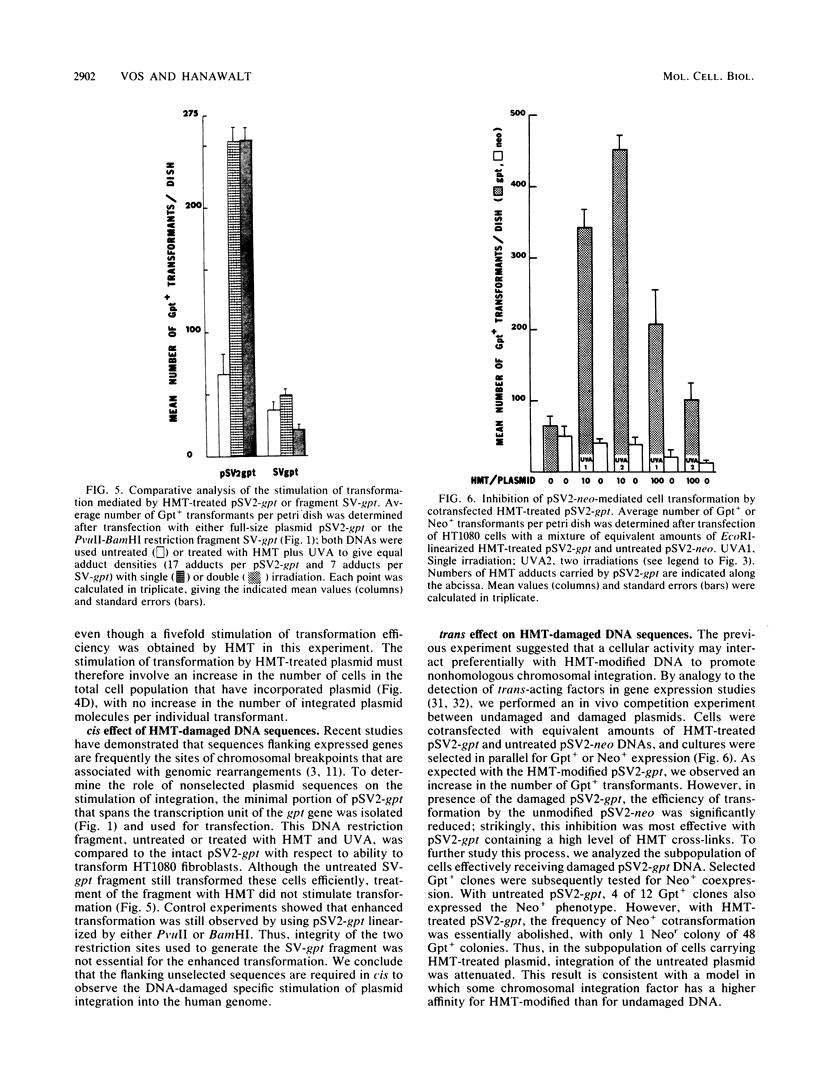
We have used integrative pSV2 plasmids to learn how DNA lesions affect nonhomologous recombination with human chromosomes. Enhanced stable transformation of fibrosarcoma cells with a selectable gene was observed after chemical modification of the plasmid DNA; thus, cells transfected with plasmid pSV2-gpt carrying photoadducts of the cross-linking agent 4'-hydroxymethyl-4,5',8-trimethylpsoralen (HMT) yielded four- to sevenfold-higher levels of Gpt+ transformants than were obtained with untreated plasmid. The enhancement due to HMT interstrand cross-links was at least as great as that due to the monoadducts. DNA hybridization analysis indicated that the enhanced transformation frequency resulted from an increased number of cells carrying integrated plasmid sequences rather than from a higher copy number per transformant. The enhancement was not seen with a plasmid missing the sequences flanking the minimal simian virus 40 gpt transcription unit. Cotransfection with untreated and HMT-treated plasmids suggested that the HMT-containing DNA interacted preferentially with some cellular factor that promoted chromosomal integration of the plasmid DNA. It is concluded that (i) interstrand cross-linking as well as intrastrand DNA adducts promote nonhomologous recombination in human chromatin and (ii) DNA sequences flanking the selectable genes are the targets for such recombinational events.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barklis E., Mulligan R. C., Jaenisch R. Chromosomal position or virus mutation permits retrovirus expression in embryonal carcinoma cells. Cell. 1986 Nov 7;47(3):391–399. doi: 10.1016/0092-8674(86)90596-9. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. The molecular genetics of cancer. Science. 1987 Jan 16;235(4786):305–311. doi: 10.1126/science.3541204. [DOI] [PubMed] [Google Scholar]
- Bodrug S. E., Ray P. N., Gonzalez I. L., Schmickel R. D., Sylvester J. E., Worton R. G. Molecular analysis of a constitutional X-autosome translocation in a female with muscular dystrophy. Science. 1987 Sep 25;237(4822):1620–1624. doi: 10.1126/science.3629260. [DOI] [PubMed] [Google Scholar]
- Bullock P., Champoux J. J., Botchan M. Association of crossover points with topoisomerase I cleavage sites: a model for nonhomologous recombination. Science. 1985 Nov 22;230(4728):954–958. doi: 10.1126/science.2997924. [DOI] [PubMed] [Google Scholar]
- Canaani D., Berg P. Regulated expression of human interferon beta 1 gene after transduction into cultured mouse and rabbit cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5166–5170. doi: 10.1073/pnas.79.17.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereghini S., Yaniv M. Assembly of transfected DNA into chromatin: structural changes in the origin-promoter-enhancer region upon replication. EMBO J. 1984 Jun;3(6):1243–1253. doi: 10.1002/j.1460-2075.1984.tb01959.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino G. D., Gamper H. B., Isaacs S. T., Hearst J. E. Psoralens as photoactive probes of nucleic acid structure and function: organic chemistry, photochemistry, and biochemistry. Annu Rev Biochem. 1985;54:1151–1193. doi: 10.1146/annurev.bi.54.070185.005443. [DOI] [PubMed] [Google Scholar]
- Conklin K. F., Groudine M. Varied interactions between proviruses and adjacent host chromatin. Mol Cell Biol. 1986 Nov;6(11):3999–4007. doi: 10.1128/mcb.6.11.3999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M. Loss of mouse chromosomes in somatic cell hybrids between HT-1080 human fibrosarcoma cells and mouse peritioneal macrophages. Proc Natl Acad Sci U S A. 1976 Sep;73(9):3248–3252. doi: 10.1073/pnas.73.9.3248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croce C. M. Role of chromosome translocations in human neoplasia. Cell. 1987 Apr 24;49(2):155–156. doi: 10.1016/0092-8674(87)90552-6. [DOI] [PubMed] [Google Scholar]
- Dinsart C., Cornelis J. J., Klein B., van der Eb A. J., Rommelaere J. Transfection with extracellularly UV-damaged DNA induces human and rat cells to express a mutator phenotype towards parvovirus H-1. Mol Cell Biol. 1984 Feb;4(2):324–328. doi: 10.1128/mcb.4.2.324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Thomas K., Capecchi M. R. Nonreciprocal exchanges of information between DNA duplexes coinjected into mammalian cell nuclei. Mol Cell Biol. 1985 Jan;5(1):59–69. doi: 10.1128/mcb.5.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Knox R. J., Lydall D. A., Friedlos F., Basham C., Roberts J. J. The effect of monofunctional or difunctional platinum adducts and of various other associated DNA damage on the expression of transfected DNA in mammalian cell lines sensitive or resistant to difunctional agents. Biochim Biophys Acta. 1987 Apr 29;908(3):214–223. doi: 10.1016/0167-4781(87)90101-1. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchange formation. Annu Rev Genet. 1981;15:11–55. doi: 10.1146/annurev.ge.15.120181.000303. [DOI] [PubMed] [Google Scholar]
- Leadon S. A., Ganesan A. K., Hanawalt P. C. Enhanced transforming activity of ultraviolet-irradiated pSV2-gpt is due to damage outside the gpt transcription unit. Plasmid. 1987 Sep;18(2):135–141. doi: 10.1016/0147-619x(87)90041-2. [DOI] [PubMed] [Google Scholar]
- Lin F. L., Sperle K., Sternberg N. Recombination in mouse L cells between DNA introduced into cells and homologous chromosomal sequences. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1391–1395. doi: 10.1073/pnas.82.5.1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu-Lee V. W., Heddle J. A., Arlett C. F., Broughton B. Genetic effects of specific DNA lesions in mammalian cells. Mutat Res. 1984 Jul;127(2):139–147. doi: 10.1016/0027-5107(84)90015-0. [DOI] [PubMed] [Google Scholar]
- McMaster G. K., Beard P., Engers H. D., Hirt B. Characterization of an immunosuppressive parvovirus related to the minute virus of mice. J Virol. 1981 Apr;38(1):317–326. doi: 10.1128/jvi.38.1.317-326.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Expression of a bacterial gene in mammalian cells. Science. 1980 Sep 19;209(4463):1422–1427. doi: 10.1126/science.6251549. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker B. A., Stark G. R. Regulation of simian virus 40 transcription: sensitive analysis of the RNA species present early in infections by virus or viral DNA. J Virol. 1979 Aug;31(2):360–369. doi: 10.1128/jvi.31.2.360-369.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearlman D. A., Holbrook S. R., Pirkle D. H., Kim S. H. Molecular models for DNA damaged by photoreaction. Science. 1985 Mar 15;227(4692):1304–1308. doi: 10.1126/science.3975615. [DOI] [PubMed] [Google Scholar]
- Reeves R., Gorman C. M., Howard B. Minichromosome assembly of non-integrated plasmid DNA transfected into mammalian cells. Nucleic Acids Res. 1985 May 24;13(10):3599–3615. doi: 10.1093/nar/13.10.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth D. B., Wilson J. H. Nonhomologous recombination in mammalian cells: role for short sequence homologies in the joining reaction. Mol Cell Biol. 1986 Dec;6(12):4295–4304. doi: 10.1128/mcb.6.12.4295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahar E., Kittrel C., Fulghum S., Feld M., Latt S. A. Sister-chromatid exchange induction in Chinese hamster ovary cells by 8-methoxypsoralen and brief pulses of laser light: assessment of the relative importance of 8-methoxypsoralen--DNA monoadducts and crosslinks. Mutat Res. 1981 Aug;83(1):91–105. doi: 10.1016/0027-5107(81)90074-9. [DOI] [PubMed] [Google Scholar]
- Schöler H. R., Gruss P. Specific interaction between enhancer-containing molecules and cellular components. Cell. 1984 Feb;36(2):403–411. doi: 10.1016/0092-8674(84)90233-2. [DOI] [PubMed] [Google Scholar]
- Scrable H. J., Witte D. P., Lampkin B. C., Cavenee W. K. Chromosomal localization of the human rhabdomyosarcoma locus by mitotic recombination mapping. Nature. 1987 Oct 15;329(6140):645–647. doi: 10.1038/329645a0. [DOI] [PubMed] [Google Scholar]
- Smithies O., Gregg R. G., Boggs S. S., Koralewski M. A., Kucherlapati R. S. Insertion of DNA sequences into the human chromosomal beta-globin locus by homologous recombination. Nature. 1985 Sep 19;317(6034):230–234. doi: 10.1038/317230a0. [DOI] [PubMed] [Google Scholar]
- Spivak G., Ganesan A. K., Hanawalt P. C. Enhanced transformation of human cells by UV-irradiated pSV2 plasmids. Mol Cell Biol. 1984 Jun;4(6):1169–1171. doi: 10.1128/mcb.4.6.1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivak G., Leadon S. A., Vos J. M., Meade S., Hanawalt P. C., Ganesan A. K. Enhanced transforming activity of pSV2 plasmids in human cells depends upon the type of damage introduced into the plasmid. Mutat Res. 1988 Mar;193(2):97–108. doi: 10.1016/0167-8817(88)90040-5. [DOI] [PubMed] [Google Scholar]
- Steele R. E., Bakken A. H., Reeder R. H. Plasmids containing mouse rDNA do not recombine with cellular ribosomal genes when introduced into cultured mouse cells. Mol Cell Biol. 1984 Apr;4(4):576–582. doi: 10.1128/mcb.4.4.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taparowsky E., Shimizu K., Goldfarb M., Wigler M. Structure and activation of the human N-ras gene. Cell. 1983 Sep;34(2):581–586. doi: 10.1016/0092-8674(83)90390-2. [DOI] [PubMed] [Google Scholar]
- Thomas K. R., Folger K. R., Capecchi M. R. High frequency targeting of genes to specific sites in the mammalian genome. Cell. 1986 Feb 14;44(3):419–428. doi: 10.1016/0092-8674(86)90463-0. [DOI] [PubMed] [Google Scholar]
- Tomic M. T., Wemmer D. E., Kim S. H. Structure of a psoralen cross-linked DNA in solution by nuclear magnetic resonance. Science. 1987 Dec 18;238(4834):1722–1725. doi: 10.1126/science.3686011. [DOI] [PubMed] [Google Scholar]
- Vanin E. F. Processed pseudogenes. Characteristics and evolution. Biochim Biophys Acta. 1984 Jul 18;782(3):231–241. doi: 10.1016/0167-4781(84)90057-5. [DOI] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Effect of DNA damage on stable transformation of mammalian cells with integrative and episomal plasmids. Mutat Res. 1989 Mar-May;220(2-3):205–220. doi: 10.1016/0165-1110(89)90025-0. [DOI] [PubMed] [Google Scholar]
- Vos J. M., Hanawalt P. C. Processing of psoralen adducts in an active human gene: repair and replication of DNA containing monoadducts and interstrand cross-links. Cell. 1987 Aug 28;50(5):789–799. doi: 10.1016/0092-8674(87)90337-0. [DOI] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. The path of DNA in the nucleosome. Cell. 1982 Jul;29(3):724–726. doi: 10.1016/0092-8674(82)90433-0. [DOI] [PubMed] [Google Scholar]
- Winocour E., Keshet I. Indiscriminate recombination in simian virus 40-infected monkey cells. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4861–4865. doi: 10.1073/pnas.77.8.4861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worton R. G., Duff C., Sylvester J. E., Schmickel R. D., Willard H. F. Duchenne muscular dystrophy involving translocation of the dmd gene next to ribosomal RNA genes. Science. 1984 Jun 29;224(4656):1447–1449. doi: 10.1126/science.6729462. [DOI] [PubMed] [Google Scholar]
- Yunis J. J., Soreng A. L., Bowe A. E. Fragile sites are targets of diverse mutagens and carcinogens. Oncogene. 1987 Mar;1(1):59–69. [PubMed] [Google Scholar]
- Yunis J. J. The chromosomal basis of human neoplasia. Science. 1983 Jul 15;221(4607):227–236. doi: 10.1126/science.6336310. [DOI] [PubMed] [Google Scholar]
- van Duin M., Westerveld A., Hoeijmakers J. H. UV stimulation of DNA-mediated transformation of human cells. Mol Cell Biol. 1985 Apr;5(4):734–741. doi: 10.1128/mcb.5.4.734. [DOI] [PMC free article] [PubMed] [Google Scholar]