Abstract
Background
Using treated wastewater in agriculture irrigation could be a realistic solution for the shortage of fresh water in Iran, however, it is associated with environmental and health threats; therefore, effluent quality assessment is quite necessary before use. The present study aimed to evaluate the physicochemical and microbial quality of Shiraz wastewater treatment plant effluent for being used in agricultural irrigation. In this study, 20 physicochemical and 3 microbial parameters were measured during warm (April to September) and cold months (October to march). Using the measured parameters and the Canadian Water Quality Index, the quality of the effluent was determined in both warm and cold seasons and in all the seasons together.
Results
The calculated index for the physicochemical parameters in the effluent was equal (87) in warm and cold months and it was obtained as 85 for the seasons all together. When the microbial parameters were used in order to calculate the index, it declined to 67 in warm and cold seasons and 64 in all the seasons together. Also, it was found that three physicochemical parameters (TDS, EC, and NO3) and three microbial parameters (Fecal coliform, Helminthes egg, and Total coliform) had the most contribution to the reduction of the index value.
Conclusions
The results showed that the physicochemical quality of Shiraz Wastewater Treatment Plant Effluent was good for irrigation in the warm, cold, and total of the two kinds of seasons. However, by applying the microbial parameter, the index value declined dramatically and the quality of the effluent was marginal.
Keywords: Effluent, Water reuse, Irrigation, CWQI, Shiraz
Background
The Middle East and North Africa countries (MENA), with one percent of fresh water resources, are the most arid regions of the world [1]. Due to the scarcity of fresh water resources in these regions, wastewater reuse could be a realistic option to alleviate the shortage of fresh water resources in these communities and until now, the largest and most popular wastewater reuse has been in the agricultural irrigation field [2,3]. Wastewater reuse is not a new issue; for instance, indications of wastewater reuse for agricultural irrigation extends back about 3000 years to the Minoan Civilization in Greece [4]. Also, the history of wastewater reuse in Iran is related to the Safavieh era (1501-1722AD) [5]. The main advantages of using the municipal Wastewaters Treatment Plants Effluent (WWTPE) are availability, being inexpensive to irrigate farmland, and being a constant source of fresh water [6,7]. Other benefits of wastewater reuse are the possibility to recover the nutrients in the wastewater, reducing the use of fertilizers [6,8-11], resolving the problems associated with wastewater disposal [10-12], and groundwater recharge [10]. So today, there are plans for the wastewater reuse in many countries; for example, in Spain, using wastewater for irrigation is about 346 MCM/year and amount of wastewater reuse could be 1100 hm3 by 2012 [12,13]. In California, about 78% of the treated wastewater is used for agricultural irrigation in central Valley and coastal areas [8]. Moreover, it is estimated that the treated wastewater effluent could be the main (about 70%) source of water for irrigation in Israel by 2040 [2]. Nevertheless, since some materials remain in wastewater effluent, despite the above-mentioned benefits, wastewater reuse could be associated with some risks [14]. Thus, several studies have evaluated the probable health and environmental impacts of wastewater reuse in agricultural irrigation. For example, in separate studies, Surdyk et al. [15], Wang et al. [16], and Reboll et al. [17] concluded that irrigation with wastewater effluent had no negative impacts on various agricultural products. However, in some studies evaluating the long-term effects of the wastewater effluent on the soil, the heavy metal pollution and reduction of soil quality have been reported [11,18]. On the other hand, due to the presence of pathogens in wastewater effluent, the irrigation by this water resource could be associated with health hazards and increasing the risk of intestinal infections [19,20]. Therefore, it seems that quality assessment of wastewater effluent before reuse projects is essential in order to prevent adverse health and environmental impacts.
According to the indicators of UN and the International Water Management Institute (IWMI), Iran is in a severe water crisis situation [21]. Thus, using new fresh water resources is very important in this country, especially in Fars province (in the southwest of Iran) in which, drought is considered as the main climatic feature [22]. Overall, it seems that Shiraz Wastewater Treatment Plant Effluent (SWTPE) could potentially be considered as a good source of fresh water supply and Fars Regional Water Organization plans to reuse SWTPE (about 29.5 MCM/year); hence, the current study aims to evaluate SWTPE quality for agricultural irrigation.
Materials and Methods
Status of the Shiraz wastewater treatment plant
SWTP is located in the southeastern region of the city. It covers 409000 inhabitants right now and it is estimated that the final coverage of inhabitants in this WWTP will be about 548000 in future. The average inlet flow rate of this WWTP is about 930 LPS and it is expected to provide about 29.5MCM/year of fresh water for irrigation. Activated sludge is the biological wastewater treatment processes of this WWTP and it includes different units of screen bar unit, primary settling tank, selector, aerated tank, secondary settling tank, and chlorination unit.
Sampling and measured parameters
In order to determine the quality of the SWTP for being reused in the agricultural irrigation, 20 physicochemical and 3 microbial parameters were evaluated during warm (April to September) and cold months (October to march). Then, 11 samples in warm and 7 samples in cool seasons were taken and analyzed from effluent of WWTP (grab sampling was used). The measured physiochemical parameter were pH, EC, TSS, TDS, Res.Cl, HCO3, Cl, SO4, Ca, Mg, Na, Mn, Hg, Fe, As, Cd, DO, COD, BOD5, and NO3, while the 3 microbial parameters included Fecal coliform, Total coliform, and Helminthes egg. It is worth noting that due to some limitations, helminthes egg and SO4 were measured just 8 (4 times in warm seasons and 4 times in cold seasons) and 12 times (6 times in warm seasons and 6 times in cold seasons), respectively.
Apparatus
The EC and pH of the study samples were measured using EC meter Metrohm (model 856) and pH Meter metrohm (model 827). In addition, the amounts of Ca, Mg, and Na were measured by Flame photometer Jenway (model PFP7). In order to measure COD and SO4, Spectrophotometer HACH (model DR/2500) was used. Also, by an Atomic Absorption Spectrometer GBC Scientific Equipment (model savant AA AAS), the concentrations of Mn, Fe, Hg, As, and Cd were determined in the samples. The concentration of DO in SWTP effluent was measured by DO meter HACH (model 850045). Also, Spectrophotometer PG Instruments Ltd (model T80) and Manometric respirometer HACH (model BOD Trak II) were used in order to measure NO3 and BOD5, respectively. Finally, Nickon microscope (model E100) was used for counting the number of helminthes egg.
Determination of the effluent quality
In order to determine the quality of the SWTPE, Canadian Water Quality Index (CWQI) was used. In general, three factors (F1, F2, and F3) are used to determine the CWQI. F1 (scope) indicates the percentage of the variables which depart from their objectives (Eq. (1)), while F2 (Frequency) represents the percentage of the tests which do not meet the objectives (Eq. (2)) [23,24].
| (1) |
| (2) |
F3 (Amplitude) is calculated by an asymptotic capping function which scales the normalized sum of the excursions from the objectives (nse) in a range between 0 and 100 (Eq. (3)). F3 is obtained in a three-step process. At the first step, the "excursion" is calculated and the number of times an individual parameter is further than (when the objective is a minimum, less than) the objective is nominated as “excursion” and is calculated by Eq. (4) and Eq. (5). (In case the test value should not fall below the objective, Eq. (5) is used).
| (3) |
| (4) |
| (5) |
| (6) |
Then, the sum of the excursions from the objectives is calculated by Eq. (6) and, finally, the CWQI could be obtained from Eq. (7). It should be noted that 1.732, is a scaling factor and rearranges the index between 0 and 100 [25].
| (7) |
Different values obtained from the CWQI are classified in Table 1.
Table 1.
Classification of CWQI values[26]
| Rank | WQI value | Description |
|---|---|---|
| Excellent |
95-100 |
There is no threat to the water quality and these index values can only be obtained when all parameters are within objectives virtually all the time. |
| Very Good |
89-94 |
There is a slight presence of threat or impairment for the water quality |
| Good |
80-88 |
There is minor degree of threat for the water quality; conditions rarely depart from desirable levels. |
| Fair |
65-79 |
Water quality is usually protected but occasionally threatened; sometimes conditions depart from desirable conditions |
| Marginal |
45-64 |
Water quality is frequently threatened; conditions often depart from natural or desirable levels. |
| Poor | 0-44 | Water quality is almost always threatened andconditions usually depart from desirable levels. |
The objectives used in the present study were selected based on the Iranian Department of Environment (IDOE) standards for wastewater reuse in agricultural irrigation; however, due to the lack of IDOE standards in this field, WHO, USEPA, and Jordan standards were used (Table 2). Also, the 90% cumulative probability was calculated for all the parameters and compared with the standards. Furthermore, since the effect of sodium should be considered in association with calcium and magnesium, Sodium Adsorption Ratio was used (SAR) instead of Na for calculating the CWQI.
Table 2.
Minimum, maximum, mean, and cumulative probability of each measured parameter
| Parameter | Unit | Min | Max | Mean | Cumulative probability (less than90%) | Standard | Specific Multiplier | Contribution Value % | |
|---|---|---|---|---|---|---|---|---|---|
| pH (Warm) |
- |
7.46 |
8.25 |
7.861±0.281 |
7.57 |
6.5-8.5 (Iran) |
0.5 |
3.88 |
|
| pH (Cool) |
7.69 |
8.17 |
7.902±0.192 |
8.181 |
|||||
| pH (Overall) |
7.46 |
8.25 |
7.877±0.057 |
8.22 |
|||||
| EC (Warm) |
μmoh/cm |
1717 |
2351 |
1904±189.208* |
2100* |
700 (WHO) |
0.1 |
0.78 |
|
| EC (Cool) |
1722 |
2340 |
1928.14±221.977* |
2343* |
|||||
| EC (Overall) |
|
1717 |
2351 |
1913.39±46.306* |
2340* |
|
|
|
|
| TSS (Warm) |
mg/L |
18 |
115 |
61.18±33.722 |
80 |
100 (Iran) |
0.5 |
3.88 |
|
| TSS (Cool) |
|
15 |
163 |
69.57±49.027 |
165 |
|
|
|
|
| TSS (Overall) |
|
15 |
163 |
64.44±9.234 |
115 |
|
|
|
|
| TDS (Warm) |
mg/L |
1144 |
1518 |
1269.36±105.766* |
1365* |
450 (WHO) |
1 |
7.76 |
|
| TDS (Cool) |
1126 |
1530 |
1311.83±165.153* |
1533* |
|||||
| TDS (Overall) |
|
1126 |
1530 |
1284.35±30.633* |
1518* |
|
|
|
|
| Res. Cl (Warm) |
mg/L |
0 |
0 |
0±0 |
0 |
0.2 (Iran) |
0.5 |
3.88 |
|
| Res. Cl (Cool) |
0 |
0.25 |
0.057±0.101 |
0.26 |
|||||
| Res. Cl (Overall) |
|
0 |
0.25 |
0.022±0.015 |
0.15 |
|
|
|
|
| HCO3 (Warm) |
mg/L |
365.94 |
542.811 |
441.345±58.134 |
540.523 |
520 (Jordan) |
0.5 |
3.88 |
|
| HCO3 (Cool) |
378.138 |
518.415 |
424.316±54.987 |
523.315 |
|||||
| HCO3 (Overall) |
|
365.94 |
542.811 |
434.723±13.182 |
518.415 |
|
|
|
|
| Cl (Warm) |
mg/L |
248.171 |
372.256 |
283.624±33.596 |
369.564 |
600 (Iran) |
0.5 |
3.88 |
|
| Cl (Cool) |
219.808 |
301.35 |
271.721±27.418 |
308.593 |
|||||
| Cl (Overall) |
|
219.808 |
372.256 |
278.995±7.321 |
301.35 |
|
|
|
|
| SO4 (Warm) |
mg/L |
171.465 |
265.65 |
224.112±44.357 |
262.863 |
1000 (Jordan) |
0.5 |
3.88 |
|
| SO4 (Cool) |
182.091 |
444.36 |
256.473±100.383 |
500 |
|||||
| SO4 (Overall) |
|
171.465 |
444.36 |
240.292±21.909 |
444.36 |
|
|
|
|
| Ca (Warm) |
mg/L |
100.2 |
130.26 |
114.956±8.129 |
127.35 |
200 (EPA) |
0.5 |
3.88 |
|
| Ca (Cool) |
94.188 |
180.36 |
116.518±30.555 |
195 |
|||||
| Ca (Overall) |
|
94.188 |
180.36 |
115.564±4.527 |
130.26 |
|
|
|
|
| Mg (Warm) |
mg/L |
54.675 |
91.125 |
71.795±10.444 |
90.85 |
100 (Iran) |
0.5 |
3.88 |
|
| Mg (Cool) |
30.375 |
100.845 |
67.345±23.568 |
101.92 |
|||||
| Mg (Overall) |
|
30.375 |
100.845 |
70.065±3.838 |
91.125 |
|
|
|
|
| SAR (Warm) |
- |
2.677 |
5.156 |
3.405±0.830 |
4.7 |
9 (FAO) |
1 |
7.76 |
|
| SAR (Cool) |
2.897 |
5.087 |
3.63±0.840 |
5.3 |
|||||
| SAR (Overall) |
|
2.677 |
5.156 |
3.493±0.192 |
5.087 |
|
|
|
|
| Mn (Warm) |
mg/L |
0.0062 |
0.042 |
0.02±0.011 |
0.041 |
1 (Iran) |
1 |
7.76 |
|
| Mn (Cool) |
0.0025 |
0.044 |
0.023±0.016 |
0.045 |
|||||
| Mn (Overall) |
|
0.0025 |
0.044 |
0.021±0.003 |
0.042 |
|
|
|
|
| Fe (Warm) |
mg/L |
0.01 |
0.343 |
0.057±0.096 |
0.34 |
3 (Iran) |
0.5 |
3.88 |
|
| Fe (Cool) |
0.0207 |
0.288 |
0.116±0.103 |
0.3 |
|||||
| Fe (Overall) |
|
0.01 |
0.343 |
0.08±0.023 |
0.288 |
|
|
|
|
| Hg (Warm) |
mg/L |
0.0003 |
0.0035 |
0.00084±0.00091 |
0.003 |
0.01 (EPA) |
1 |
7.76 |
|
| Hg (Cool) |
0.0003 |
0.001 |
0.00075±0.00022 |
0.00104 |
|||||
| Hg (Overall) |
|
0.00026 |
0.0035 |
0.00081±0.00016 |
0.00098 |
|
|
|
|
| As (Warm) |
mg/L |
0.0006 |
0.0034 |
0.0021±0.0009 |
0.00342 |
0.1 (Iran) |
1 |
7.76 |
|
| As (Cool) |
0.0007 |
0.0021 |
0.0013±0.00041 |
0.0022 |
|||||
| As (Overall) |
|
0.0006 |
0.0034 |
0.0018±0.00020 |
0.00325 |
|
|
|
|
| Cd (Warm) |
mg/L |
0 |
0.003 |
0.00041±0.00091 |
0.00301 |
0.05 (Iran) |
1 |
7.76 |
|
| Cd (Cool) |
0 |
0.0038 |
0.00076±0.00134 |
0.0046 |
|||||
| Cd (Overall) |
|
0 |
0.0038 |
0.00055±0.00026 |
0.00381 |
|
|
|
|
| DO (Warm) |
mg/L |
2.87 |
7.4 |
5.766±1.318 |
7.3 |
2 (Iran) |
0.5 |
3.88 |
|
| DO (Cool) |
5.098 |
6.21 |
5.541143±0.401 |
6.3 |
|||||
| DO (Overall) |
|
2.87 |
7.4 |
5.678±0.246 |
6.81 |
|
|
|
|
| COD (Warm) |
mg/L |
14 |
203 |
103.82±63.653 |
200 |
200 (Iran) |
0.1 |
0.78 |
|
| COD (Cool) |
32 |
200 |
104.71±58.131 |
210 |
|||||
| COD (Overall) |
|
14 |
203 |
104.17±14.095 |
200 |
|
|
|
|
| BOD5 (Warm) |
mg/L |
8.1 |
107 |
52.518±32.198 |
104 |
100 (Iran) |
0.1 |
0.78 |
|
| BOD5 (Cool) |
16.8 |
88 |
49.429±27.869 |
88.5 |
|||||
| BOD5 (Overall) |
|
8.1 |
107 |
51.317±7.017 |
89.5 |
|
|
|
|
| NO3-N(Warm) |
mg/L |
3.79 |
46 |
24.067±13.444* |
41* |
5 (WHO) |
0.1 |
0.78 |
|
| NO3-N(Cool) |
11.015 |
149.9 |
57.712±50.442* |
165* |
|||||
| NO3-N(Overall) |
3.79 |
|
149.9 |
37.151±8.463* |
91.58* |
|
|
|
|
| TC (Warm) |
N/100ml |
20 |
2320 |
1014.82±1139.364* |
2313* |
1000 (Iran) |
0.5 |
3.88 |
|
| TC (Cool) |
24 |
2615 |
1725.28±1152.32* |
2618* |
|||||
| TC (Overall) |
20 |
2615 |
1291.11±1165.88* |
2437* |
|||||
| FC (Warm) |
N/100ml |
15 |
1985 |
864.45±1071.53* |
1980* |
400 (Iran) |
0.5 |
3.88 |
|
| FC (Cool) |
6 |
1220 |
377.86±893.088* |
1226* |
|||||
| FC (Overall) |
6 |
1985 |
675.22±1008.21* |
2341* |
|||||
| Helmith egg (Warm) |
N/L | 49 |
210 |
126.75±75.769* |
208* |
1 (Iran) | 0.5 | 3.88 | |
| Helmith egg (Cool) |
20 |
164 |
66.35*±65 |
168* |
|||||
| Helmith egg (Overall) | 20 | 210 | 73.61*±96.125 | 215* |
* Values which did not meet standards.
Moreover, in order to get a closer CWQI to the actual quality of SWTP effluent, the authors decided to give weight to each parameter based on its importance in the agricultural irrigation. Thus, as Table 2 shows, each parameter has its specific multiplier and contribution value to calculation of CWQI.
Results
After analyzing the samples collected from warm and cold seasons, the results shown in Table 2 were obtained. Besides, the meanvariations of the analyzed parameters are depicted in Figure 1.
Figure 1.
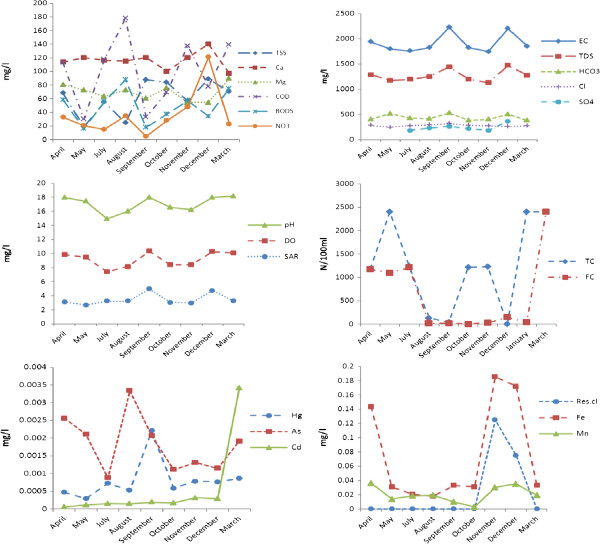
Mean variations of the measured parameters in SWWPTE.
As noted above, to determine the quality of the SWTPE, the CWQI was used. Therefore, the CWQI was calculated in the warm, cold, and overall seasons for the physiochemical parameters. In addition, F1, F2, and F3 were separately calculated and the results are depicted in Figure 2.
Figure 2.
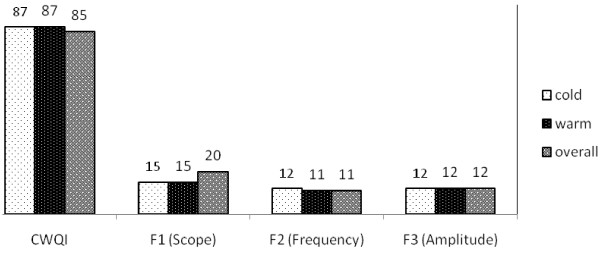
The value of calculated CWQI, F1, F2, and F3 for physicochemical parameters.
Analysis of SWTPE shows that in the cold seasons, 4 physicochemical parameters (EC, Res. Cl, TSS, TDS, Mg, and NO3) failed from the defined objectives (Scope) and among these; two parameters (EC and NO3) had the highest failure to meet the objectives (Frequency). Also,NO3 had the most deviation from the desired objective (Amplitude). In the warm seasons, NO3, BOD5, COD,HCO3,TDS, TSS, and EC departed from their objectives and EC and TDS had the most frequency of failure. Besides, similar to the cold seasons, NO3 had the most deviation from its objective in warm seasons, as well.
In all the cold and warm seasons, 9 parameters (EC, Res. Cl, TSS, TDS, Mg, NO3, BOD, COD, and HCO3) failed to meet their objectives over the sampling period. Among these parameters, similar to warm and cold seasons, the electrical conductivity had the most frequency of failure and NO3 had the most deviation from its objectives. Also, CWQI was calculated by applying the microbial parameters along with the physicochemical parameters (Figure 3). In this situation, the helminthes egg, instead of NO3, had the most deviation from its objective and fecal coliform as well as total coliform had failures to meet their objectives in warm, cold, and all the seasons together.
Figure 3.
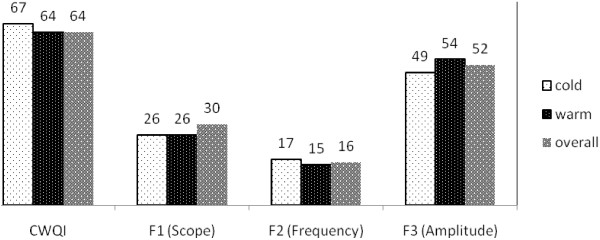
The value of calculated CWQI, F1, F2, and F3 for physicochemical and microbial parameters.
Discussion
There are two components for evaluating the quality of water resources: 1) measurement of water quality variables and 2) comparison of values to benchmarks, such as guidelines or objectives. However, assessment of the quality variable by variable and objective by objective is quite a difficult task [23]. Therefore, a method which combines all the variables and represents a final value as the quality index could be used as a management tool for decision makers [27,28]. The CWQI is a science-based communication tool which tests multivariable water quality data versus water quality objectives specified by the users [23]. This tool also simplifies the reporting of water quality data to both technical and non-technical individuals [26]. Thus, due to the advantages of CWQI, in order to assess SWTP effluent quality for agricultural irrigation, this was used in the present study. According to Figure 2 and Table 1, physicochemical quality of SWTPE in warm and cold seasons is in the good range and, consequently, the physicochemical quality of the SWTPE rarely falls from the desired quality. According to the obtained results and Table 2, it can be concluded that TDS, EC, and NO3 have the largest contribution to the decline of CWQI in cold, warm, and all the seasons together. EC and TDS are the most important parameters related to the water resources salinity [29]. Some studies have shown that using wastewater for irrigation can increase soil salinity [6,8,11]. In the current study, mean and 90% cumulative probability of EC and TDS, which were the main factors of decrease in CWQI, were exceeded from the standards; therefore, this effluent could increase the irrigated soil salinity in future. In general, when the total soluble salt reaches an excessive concentration in the irrigated soil, water uptake by plant is reduced due to osmotic effect and this situation leads to a phenomenon called "osmotic desiccation" which can reduce the harvest [12,29,30]. On the other hand, increasing salinity reduces organic complex for most metals, which induces the displacement of metal in the solid phase with the soil solution and this can pollute the aquifers [31]. Generally, the salinity of WWTPE is high and the conventional treatments cannot reduce the salinity to the desired values; thus, just the advanced treatments which increase the cost of water reuse are necessary [12]. Overall, there are some options for controlling SWTPE salinity. For instance, in order to prevent soil salinization by SWTPE irrigation, enough drainage and leaching could be applied [30,32]. Also, if the salinity of the effluent is higher than the cultivated plant tolerance threshold, salinity could be reduced to the desired level by mixing the effluent with fresh water [13]. In the present study, the mean and 90% cumulative probability of nitrogen in warm, cold, and all the seasons together were far from the WHO standard (5mg/l). Some studies have shown that using untreated wastewater can increase soil nitrogen [10]. Although using wastewater treatment plants effluent for irrigation can be as significant source of valuable nutrients like nitrogen [14], it should be considered that large quantities of nitrogen in the effluent could be unfavorable for plant growth [11]. On the other hand, nitrate is highly soluble and by leaching phenomena, the nitrate concentration could increase in groundwater and consuming this water by the infants could lead to methemoglobinemia [33]. Hence, it seems that advanced treatments are necessary in order to reduce the SWTPE nitrate to the guidelines value. As can be seen in Figure 3, when microbial parameters were applied for calculating CWQI, the value of this index fell dramatically (from 85 to 64 in all the seasons together) and, thus, the quality of the effluent was located in marginal situation. Figure 3 also shows that the quality of SWTPE in the cold seasons was better than warm seasons, which could be due to the lower levels of microbial indicators in the cold seasons. In fact, the mean of fecal coliform and helminthes egg in cold seasons were respectively 486 and 61 units less than the warm seasons. Just the mean of total coliform in cold seasons was greater than the warm seasons, which might result from more precipitation in the cold period, washing the pathways, and progression of the washed coliforms in to the SWTP. Many studies have shown that the microbial pollution in the recycled effluent could contaminate the soil as well as the crops and develop the risk of disease in both consumers and the farm workers. AL-Laham et al. showed that irrigating tomato by an effluent with high microbial index can cause contamination on fruit scar [7]. In another study, Forslund et al. showed that using effluents for irrigation of potatoes could increase the risk of gastroenteritis diseases for farm workers [34]. Palese et al. also conducted a study and concluded that the reuse of wastewater for irrigation could increase the soil microbial load, although after a day, the contamination of the soil had greatly reduced [2]. In separate studies, Habbari et al. and Ensink et al. showed that the prevalence of parasitic infections was quite high among the populations exposed to the areas irrigated with recycled wastewater [19,20]. Therefore, considering the high levels of microbial indicators (Fecal coliform, Total coliform, and Helminthes egg) in SWTPE, it seems that using this water resource for irrigation could cause health problems for both the crops consumers and the farm workers and in order to reduce the microbial load in this wastewater treatment plant, some additional treatment, such as sand filtering followed by UV disinfection, is recommended. Bakopoulou et al. evaluated four wastewater treatment effluents for agricultural irrigation and showed that the wastewater treatment plant which used the advance treatment (sand filtering and UV disinfection) not only had a better microbial situation, but its physicochemical parameters were also in a better status compared to the other WWTPs [35]. Furthermore, applying management measures can control the health risk to some extent; for example, subsurface irrigation can be used in order to reduce the exposure of workers and crops to the recycled water. Stopping the irrigation few days before harvesting the crops [2,36], planting the crops in depths of the soil, putting nets under the trees in order to prevent the crops from falling on the ground and contamination of the product [2], and cooking the harvested crops before consumption [7], are other management practices which can bring down the risk of recycled wastewater for irrigation. As Table 2 depicts, mean and 90% cumulative probability of BOD5, TSS, HCO3, Cl, SO4, Ca, Mg, SAR, DO, Mn, Hg, Fe, As, and Cd completely fulfilled the standards, which shows the desirable efficiency of the treatment of the physicochemical parameters in SWTPE which is confirmed by the obtained CWQI values (Figure 2). Therefore, it seems that if the problems related to the microbial load in SWTPE be resolved, even with the current situation of the physicochemical parameters which could not meet the objectives (NO3, TDS, and EC), the final quality of SWTPE for agricultural irrigation will be favorable.
Conclusion
The present study evaluated the SWTPE quality for agricultural irrigation by measuring the physicochemical and microbial parameters and then calculating the CWQI. The results showed that the effluent physicochemical quality was appropriate for irrigation; however, considering the microbial parameters, the quality of the effluent reduced dramatically which shows that the pathogens in this effluent can be a threat to the public health. Therefore, in order to protect the health of the consumers and the farm workers, advanced treatments, such as sand filtration and UV disinfection, are recommended.
Competing interest
This work was financially supported by vice chancellor for research affairs of Shiraz University of Medical Sciences, Shiraz, Iran (contract no. 6029).
Authors’ contributions
MAB participated in the data gathering, design of study, coordinated activities and revised manuscript. SN participated in the design of the study, final revised of manuscript and intellectual helping for analyzing of data. BD performed data collection, carried out statistical and technical analysis of data, participated in design of study and drafted manuscript. All authors read and approved the final manuscript.
Contributor Information
Mohammad A Baghapour, Email: baghapour@sums.ac.ir.
Simin Nasseri, Email: naserise@tums.ac.ir.
Babak Djahed, Email: Babak.Djahed@Gmail.com.
Acknowledgments
The information contained in this article was extracted from a master’s thesis by the author, Babak Djahed. Research Improvement Center of Shiraz University of Medical Sciences, Shiraz, Iran and Ms. A. Keivanshekouh are appreciated for their helping.
References
- Qadir M, Bahri A, Sato T, Al-Karadsheh E. Wastewater production, treatment, and irrigation in Middle East and North Africa. Irrigation and Drainage Systems. 2009;24:37–51. [Google Scholar]
- Palese A, Pasquale V, Celano G, Figliuolo G, Masi S, Xiloyannis C. Irrigation of olive groves in Southern Italy with treated municipal wastewater: Effects on microbiological quality of soil and fruits. Agric Ecosyst Environ. 2009;129:43–51. doi: 10.1016/j.agee.2008.07.003. [DOI] [Google Scholar]
- Alfarra A, Kemp-Benedict E, Hötzl H, Sader N, Sonneveld B. A Framework for Wastewater Reuse in Jordan: Utilizing a Modified Wastewater Reuse Index. Water Resources Management. 2011;25:1153–1167. doi: 10.1007/s11269-010-9768-8. [DOI] [Google Scholar]
- Asano T, Burton FL, Leverenz HL, Tsuchihashi R, Tchobanoglous G. water reuse: Issues, Technologies, and Applications. 1. New York: McGraw-Hill; 2006. [Google Scholar]
- Mohammadi P. A review on standards and experiences of using wastewaters effluents for irrigation 1edn. Tehran: Iranian national committee on irrigation and drainage; 2008. [in Persian] [Google Scholar]
- Heidarpour M, Mostafazadehfard B, Abedikoupai J, Malekian R. The effects of treated wastewater on soil chemical properties using subsurface and surface irrigation methods. Agricultural Water Management. 2007;90:87–94. doi: 10.1016/j.agwat.2007.02.009. [DOI] [Google Scholar]
- Al-Lahham O, El Assi NM, Fayyad M. Impact of treated wastewater irrigation on quality attributes and contamination of tomato fruit. Agricultural Water Management. 2003;61:51–62. doi: 10.1016/S0378-3774(02)00173-7. [DOI] [Google Scholar]
- Kalavrouziotis IK, Robolas P, Koukoulakis PH, Papadopoulos AH. Effects of municipal reclaimed wastewater on the macro- and micro-elements status of soil and of Brassica oleracea var. Italica, and B. oleracea var. Gemmifera. Agricultural Water Management. 2008;95:419–426. doi: 10.1016/j.agwat.2007.11.004. [DOI] [Google Scholar]
- Pedrero F, Alarcón JJ. Effects of treated wastewater irrigation on lemon trees. Desalination. 2009;246:631–639. doi: 10.1016/j.desal.2008.07.017. [DOI] [Google Scholar]
- Adrover M, Farrús E, Moyà G, Vadell J. Chemical properties and biological activity in soils of Mallorca following twenty years of treated wastewater irrigation. J Environ Manage. 2012;95:S188–S192. doi: 10.1016/j.jenvman.2010.08.017. [DOI] [PubMed] [Google Scholar]
- Xu J, Wu L, Chang AC, Zhang Y. Impact of long-term reclaimed wastewater irrigation on agricultural soils: A preliminary assessment. J Hazard Mater. 2010;183:780–786. doi: 10.1016/j.jhazmat.2010.07.094. [DOI] [PubMed] [Google Scholar]
- Candela L, Fabregat S, Josa A, Suriol J, Vigues N, Mas J. Assessment of soil and groundwater impacts by treated urban wastewater reuse. A case study: Application in a golf course (Girona, Spain) Sci Total Environ. 2007;374:26–35. doi: 10.1016/j.scitotenv.2006.12.028. [DOI] [PubMed] [Google Scholar]
- Pedrero F, Kalavrouziotis I, Alarcón JJ, Koukoulakis P, Asano T. Use of treated municipal wastewater in irrigated agriculture—Review of some practices in Spain and Greece. Agricultural Water Management. 2010;97:1233–1241. doi: 10.1016/j.agwat.2010.03.003. [DOI] [Google Scholar]
- Hamoda M. Water strategies and potentional of water reuse in south Mediterranean countries. Desalination. 2004;165:31–41. [Google Scholar]
- Surdyk N, Cary L, Blagojevic S, Jovanovic Z, Stikic R, Vucelic-Radovic B, Zarkovic B, Sandei L, Pettenati M, Kloppmann W. Impact of irrigation with treated low quality water on the heavy metal contents of a soil-crop system in Serbia. Agricultural Water Management. 2010;98:451–457. doi: 10.1016/j.agwat.2010.10.009. [DOI] [Google Scholar]
- Wang J, Wang G, Wanyan H. Treated wastewater irrigation effect on soil, crop and environment: Wastewater recycling in the loess area of China. J Environ Sci. 2007;19:1093–1099. doi: 10.1016/S1001-0742(07)60178-8. [DOI] [PubMed] [Google Scholar]
- Reboll V, Cerezo M, Roig A, Flors V. Influence of wastewater vs groundwater on young Citrus trees. J Sci Food Agric. 2000;80:1441–1446. doi: 10.1002/1097-0010(200008)80:10<1441::AID-JSFA664>3.0.CO;2-S. [DOI] [Google Scholar]
- Klay S, Charef A, Ayed L, Houman B, Rezgui F. Effect of irrigation with treated wastewater on geochemical properties (saltiness, C, N and heavy metals) of isohumic soils (Zaouit Sousse perimeter, Oriental Tunisia) Desalination. 2010;253:180–187. doi: 10.1016/j.desal.2009.10.019. [DOI] [Google Scholar]
- Ensink JHJ, Hoek W, Mukhtar M, Tahir Z, Amerasinghe FP. High risk of hookworm infection among wastewater farmers in Pakistan. Trans R Soc Trop Med Hyg. 2005;99:809–818. doi: 10.1016/j.trstmh.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Habbari K, Tifnouti A, Bitton G, Mandil A. Geohelminthic infections associated with raw wastewater reuse for agricultural purposes in Beni-Mellal, Morocco. Parasitol Int. 2000;48:249–254. doi: 10.1016/S1383-5769(99)00026-4. [DOI] [PubMed] [Google Scholar]
- Ehsani M. A Vision on Water Resources Situation, Irrigation and Agricultural Production in Iran. ICID 21st European Regional Conference. Frankfurt (Oder) and Slubice - Germany and Poland. 2005.
- Edalatgostar MD, Farzadian A, Amiri N. Presentation of a stochastic model for the drought prediction in shiraz city. The national conference on water crisis management Islamic Azad University, Marvdasht branch. 2009.
- Sd R, Duro DC. Dubé M: Comparative analysis of regional water quality in Canada using the Water Quality Index. Environ Monit Assess. 2008;156:223–240. doi: 10.1007/s10661-008-0480-6. [DOI] [PubMed] [Google Scholar]
- Hurley T, Sadiq R, Mazumder A. Adaptation and evaluation of the Canadian Council of Ministers of the Environment Water Quality Index (CCME WQI) for use as an effective tool to characterize drinking source water quality. Water Res. 2012;46:3544–3552. doi: 10.1016/j.watres.2012.03.061. [DOI] [PubMed] [Google Scholar]
- Lumb A, Halliwell D, Sharma T. Application of CCME Water Quality Index to Monitor Water Quality: A Case Study of the Mackenzie River Basin, Canada. Environ Monit Assess. 2006;113:411–429. doi: 10.1007/s10661-005-9092-6. [DOI] [PubMed] [Google Scholar]
- Khan AA, Paterson R, Khan H. Modification and Application of the Canadian Council of Ministers of the Environment Water Quality Index (CCME WQI) for the Communication of Drinking Water Quality Data in Newfoundland and Labrador. Water Qual Res J Canada. 2004;39:289–293. [Google Scholar]
- Said A, Stevens DK, Sehlke G. An Innovative Index for Evaluating Water Quality in Streams. Environ Manage. 2004;34:406–414. doi: 10.1007/s00267-004-0210-y. [DOI] [PubMed] [Google Scholar]
- Avvannavar SM, Shrihari S. Evaluation of water quality index for drinking purposes for river Netravathi, Mangalore, South India. Environ Monit Assess. 2007;143:279–290. doi: 10.1007/s10661-007-9977-7. [DOI] [PubMed] [Google Scholar]
- Duncan RR, Carrow RN, Huck MT. Turfgrass and Landscape Irrigation Water Quality "Assessment and Management". New York: CRC Press; 2009. [Google Scholar]
- Qadir M, Oster JD. Crop and irrigation management strategies for saline-sodic soils and waters aimed at environmentally sustainable agriculture. Sci Total Environ. 2004;323:1–19. doi: 10.1016/j.scitotenv.2003.10.012. [DOI] [PubMed] [Google Scholar]
- Tarchouna LG, Merdy P, Raynaud M, Pfeifer H-R, Lucas Y. Effects of long-term irrigation with treated wastewater. Part I: Evolution of soil physico-chemical properties. Appl Geochem. 2010;25:1703–1710. doi: 10.1016/j.apgeochem.2010.08.018. [DOI] [Google Scholar]
- Oster JD. Irrigation with poor quality water. Agricultural Water Management. 1994;25:271–297. doi: 10.1016/0378-3774(94)90064-7. [DOI] [Google Scholar]
- WHO. Guidelines for the safe use of wastewater, excreta and Greywater. Volume 2, wastewater use in agriculture. Geneva, Switzerland: World Health Organization; 2006. [Google Scholar]
- Forslund A, Ensink JHJ, Battilani A, Kljujev I, Gola S, Raicevic V, Jovanovic Z, Stikic R, Sandei L, Fletcher T. Faecal contamination and hygiene aspect associated with the use of treated wastewater and canal water for irrigation of potatoes (Solanum tuberosum) Agricultural Water Management. 2010;98:440–450. doi: 10.1016/j.agwat.2010.10.007. [DOI] [Google Scholar]
- Bakopoulou S, Emmanouil C, Kungolos A. Assessment of wastewater effluent quality in Thessaly region, Greece, for determining its irrigation reuse potential. Ecotoxicol Environ Saf. 2011;74:188–194. doi: 10.1016/j.ecoenv.2010.06.022. [DOI] [PubMed] [Google Scholar]
- Blumenthal UJ, Peasey A, Ruiz-Palacios G. Guidelines for wastewater reuse in agriculture and aquaculture: recommended revisions based on new research evidence. London: WELL Study; 2000. [Google Scholar]


