Abstract
Tumor-associated macrophages (TAMs) have been linked to promoting tumor progression by stimulating angiogenesis, cell growth and inflammation. NFKB activity in TAMs may mediate inflammation-associated tumor formation. However, most isolated TAMs from established tumors express a M2 phenotype with less NFKB activation and show a strong immunosuppressive phenomenon. How tumors affect the dynamic of NFKB activity in TAMs, and hence maintain their pro-tumor M2 phenotype is still poorly understood. We recently found that hepatoma-derived toll-like receptor 2 (TLR2)-related ligands are capable of stimulating M2 macrophage differentiation via controlling NFKB RELA/p65 protein homeostasis by selective autophagy. TLR2 signal induces NFKB RELA cytosolic ubiquitination and leads to its degradation by SQSTM1/p62-mediated autophagy. Inhibition of autophagy will rescue NFKB activity and shape the phenotype of hepatoma-polarized M2 macrophages. This suggests that autophagy might play a role in manipulating TAM functions and tumor-associated immune responses. Our study also demonstrates that autophagy can directly control a transcriptional factor in addition to its regulatory molecules. This finding uncovers a new role of autophagy in controlling cellular functions.
Keywords: tumor-associated macrophages, selective autophagy, NFKB, TLR2, SQSTM1/p62
A prominent population of infiltrating leukocytes in tumors is composed of tumor-associated macrophages. However, most TAMs have been considered to promote tumor progression instead of inhibiting it. In fact, in many human cancers, including hepatocellular carcinoma, a high density of infiltrating TAMs is associated with poor prognosis. In established tumors, TAMs generally express a M2 phenotype, which present IL10high IL12 low cells with a high level of scavenger and mannose receptors and ARG1/arginase I, but poor antigen presentation capacity. It has become increasingly clear that M2-type TAMs suppress antitumor immune responses. TAMs produce various immunosuppressive mediators into tumor environments and hence inhibit T cell activity. Additionally, TAMs also contribute to induce T regulatory cell differentiation, whereas those cells have been shown to carry strong immune suppressive activities. The immunosuppressive tumor environments modulated by TAMs favor tumor growth and progression. Although TAMs have been considered as having pro-tumor functions, fully activated classical macrophages (or M1) have the potential to elicit tumors. Microbial products (e.g., LPS) or IFNG/interferon gamma can activate macrophages as classical or M1 macrophages, which are characterized as having increased antigen presentation capacity, high IL12, and pro-inflammatory cytokine production. These M1 macrophages are able to polarize Th1 responses to/against intracellular microorganisms and tumor cells as well. In genetic animal models, IKBKB−/− or NFKB1/p50−/− TAMs represent an IL12high M1-like phenotype and show antitumor activity in vivo. This suggests that reprograming of M2 type TAM to M1 in the tumor area can be a potential strategy against tumors.
NFKB is an important transcription factor to regulate inflammation and tumor development. Activation of NFKB in macrophages has been suggested to mediate inflammation-associated tumor formation. Many stimulators can trigger NFKB activation, including TLR ligands. Upon stimulation of TLRs, a signaling cascade will lead to degradation of NFKB inhibitor, NFKBIA/IκBα, and cause NFKB nuclear translocation. Activated-NFKB can be terminated by two established mechanisms. One is to export the activated-NFKB from the nucleus with newly synthesized NFKBIA by NFKB itself, and the other is through proteasome-dependent degradation of ubiquitinated-NFKB in the nucleus. On the one hand, NFKB activity is usually upregulated in classical M1 macrophages to produce a high level of IL12 and other pro-inflammatory cytokines. On the other hand, M2 macrophages are considered to have less NFKB activity. Several studies indicated that activated NFKB in TAMs promotes their pro-tumor capability, but many isolated TAMs from human or mice established tumors present less NFKB activity. The reduced activity of NFKB in TAMs is correlated with impaired expression of the antitumor cytokine IL12. Apparently, it is a conspicuous contrast with TAMs as a pro-inflammatory cell in the early steps of tumor formation. The plasticity change of TAMs from M1 to M2 might correlate with the dynamics of NFKB activity. It has been suggested that tumor microenvironments may induce such TAMs phenotype switching, however, the regulatory mechanisms remain unclear.
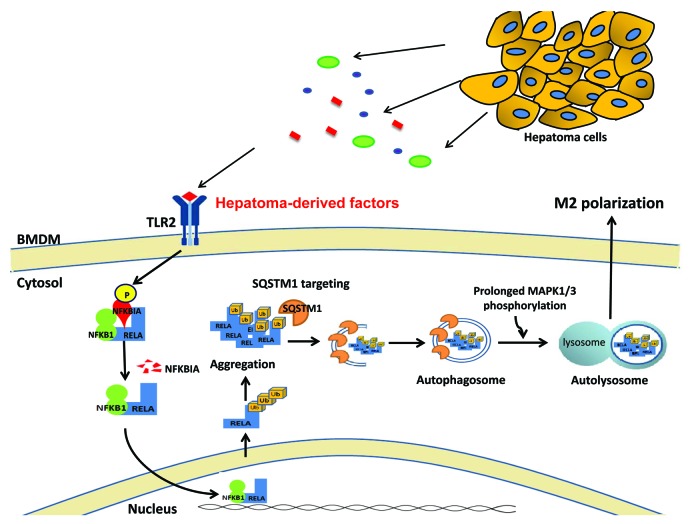
Autophagy is an evolutionarily conserved lysosome-dependent system to control cell homeostasis by breaking down cellular components. Cellular proteins that are covalently targeted with ubiquitin are generally degraded by 26S proteasomes. However, when ubiquitinated proteins are aggregated or too large to be degraded by proteasomes, those protein complexes will be targeted selectively by autophagy. Degradation of the ubiquitinated proteins by selective autophagy not only maintains the equilibrium of proteins, but also regulates signaling transduction within cells. Whether autophagy is able to directly target transcriptional factors to control cell functions has not been defined before. We recently reported that autophagy can directly regulate transcriptional factor NFKB to control hepatoma-associated macrophage M2 differentiation. A TLR2-dependent signal from hepatoma conditioned medium or agonists stimulates accumulation of ubiquitinated NFKB RELA in the cytoplasm, and recruits the ubiquitin binding protein SQSTM1 to form aggresome-like structures (ALS) in bone-marrow derived macrophages. The NFKB RELA-containing ALS is subsequently recognized by autophagosomes, and then degraded through lysosomes. The sustained phosphorylation of MAPK1/3 (extracellular signal-regulated kinase 1/2, ERK1/2) is required for this TLR2-induced NFKB RELA degradation by promoting autophagosome maturation. Inhibition of autophagy will prevent hepatoma-induced NFKB RELA degradation and force polarized M2 macrophages to produce M1-like cytokines, including a high level of IL12 (Fig. 1). This is the first demonstration that autophagy can directly target a transcription factor to control cell biological functions. Our findings indicate not only a novel pathway of NFKB regulation by selective autophagy, but also a therapeutic target for cancer therapy.
Figure 1. TLR2-dependent selective autophagy targets NFKB RELA in hepatoma-induced M2 macrophage polarization. In hepatoma microenvironments, tumor cells can produce various types of factors to manipulate TAM functions. Some of them are TLR2-related ligands, which are capable of triggering NFKBIA degradation and activating NFKB RELA nuclear translocation in TAMs. After turning on related gene expression, those nuclear NFKB RELA are exported into the cytoplasm, where they can become ubiquitinated to form aggresome-like structures. The aggregated NFKB RELA are further recognized by SQSTM1 and delivered to autophagosomes and lysosomes, which requires sustained phosphorylation of MAPK1/3. This TLR2-induced autophagosomal degradation of NFKB RELA limits NFKB activity and drives TAM to M2 macrophage polarization. BMDM, bone marrow-derived macrophage.
Acknowledgments
This work was supported by grants NSC 101-2320-B-006-044 and NSC101-2120-M-007-014 from the National Science Council, Taiwan, ROC.
Glossary
Abbreviations:
- TLR
toll-like receptor
- SQSTM1/p62
sequestosome-1/p62
- TAM
tumor-associated macrophage
- ERK1/2
extracellular signal-regulated kinase 1/2
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/autophagy/article/23546