Abstract
Tumor necrosis factor (TNF) induces an antiviral state in various cell lines. This antiviral state is quite similar to that established by interferon (IFN), e.g., TNF treatment of HEp-2 cells induces 2',5'-oligoadenylate synthetase activity. Both antiviral activity and synthetase induction are greatly reduced when TNF treatment occurs in the presence of a beta interferon subtype 1 (IFN-beta 1)-neutralizing antiserum. However, no one has yet directly demonstrated IFN-beta 1 induction, either as an antiviral activity in supernatants from TNF-treated cells or as IFN-specific mRNA by Northern (RNA) blot analysis. We have adopted a recently described in vitro DNA amplification protocol for the detection of specific RNAs. By applying this method to RNA from HEp-2 cells, we could demonstrate increased levels of IFN-beta 1-specific transcripts after TNF treatment. Dose response and kinetics of IFN-beta 1 induction coincided with the TNF-induced antiviral state. Nuclear run-on analysis showed enhanced transcriptional activity of the IFN-beta 1 gene in TNF-treated cells. Our data substantiate a role of IFN-beta 1 as mediator of the biological activity of TNF in HEp-2 cells.
Full text
PDF

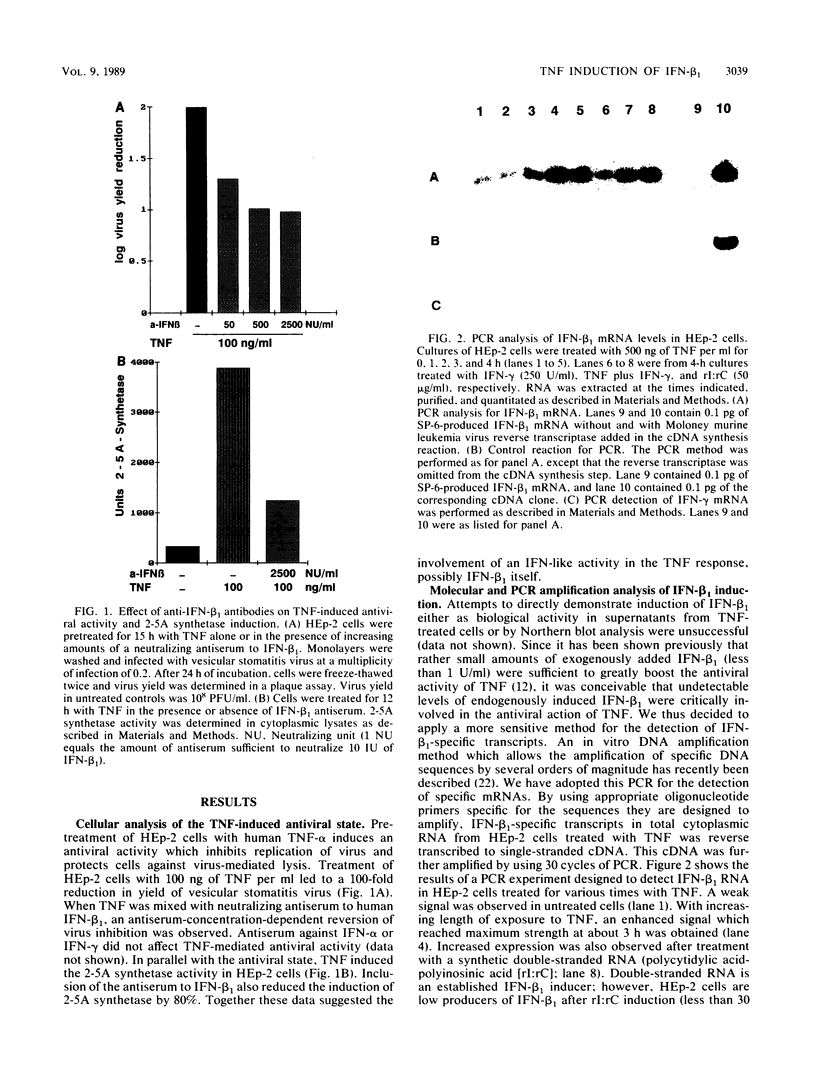
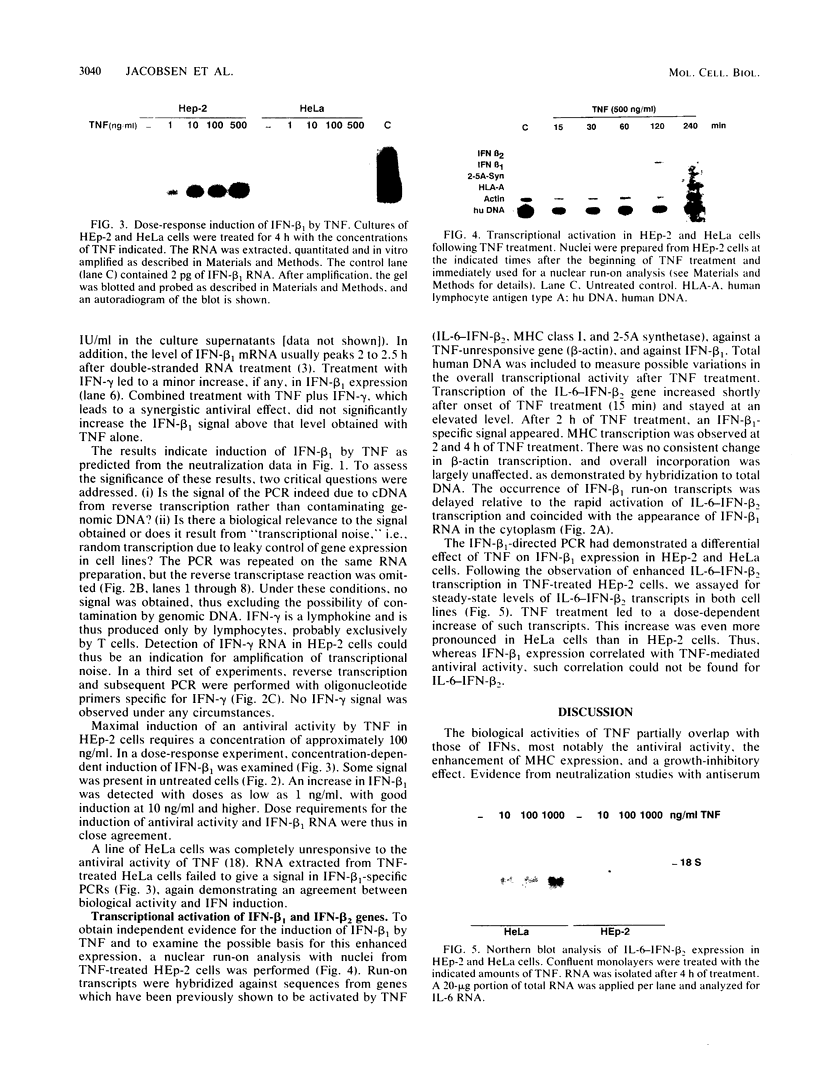


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aderka D., Holtmann H., Toker L., Hahn T., Wallach D. Tumor necrosis factor induction by Sendai virus. J Immunol. 1986 Apr 15;136(8):2938–2942. [PubMed] [Google Scholar]
- Carswell E. A., Old L. J., Kassel R. L., Green S., Fiore N., Williamson B. An endotoxin-induced serum factor that causes necrosis of tumors. Proc Natl Acad Sci U S A. 1975 Sep;72(9):3666–3670. doi: 10.1073/pnas.72.9.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalieri R. L., Havell E. A., Vilcek J., Pestka S. Induction and decay of human fibroblast interferon mRNA. Proc Natl Acad Sci U S A. 1977 Oct;74(10):4415–4419. doi: 10.1073/pnas.74.10.4415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelly J., Kaplan J. C., Maire P., Gautron S., Kahn A. Transcription of the dystrophin gene in human muscle and non-muscle tissue. Nature. 1988 Jun 30;333(6176):858–860. doi: 10.1038/333858a0. [DOI] [PubMed] [Google Scholar]
- Defilippi P., Poupart P., Tavernier J., Fiers W., Content J. Induction and regulation of mRNA encoding 26-kDa protein in human cell lines treated with recombinant human tumor necrosis factor. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4557–4561. doi: 10.1073/pnas.84.13.4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derynck R., Content J., DeClercq E., Volckaert G., Tavernier J., Devos R., Fiers W. Isolation and structure of a human fibroblast interferon gene. Nature. 1980 Jun 19;285(5766):542–547. doi: 10.1038/285542a0. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gray P. W., Goeddel D. V. Structure of the human immune interferon gene. Nature. 1982 Aug 26;298(5877):859–863. doi: 10.1038/298859a0. [DOI] [PubMed] [Google Scholar]
- Greenberg M. E., Ziff E. B. Stimulation of 3T3 cells induces transcription of the c-fos proto-oncogene. Nature. 1984 Oct 4;311(5985):433–438. doi: 10.1038/311433a0. [DOI] [PubMed] [Google Scholar]
- Groudine M., Peretz M., Weintraub H. Transcriptional regulation of hemoglobin switching in chicken embryos. Mol Cell Biol. 1981 Mar;1(3):281–288. doi: 10.1128/mcb.1.3.281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohase M., Henriksen-DeStefano D., May L. T., Vilcek J., Sehgal P. B. Induction of beta 2-interferon by tumor necrosis factor: a homeostatic mechanism in the control of cell proliferation. Cell. 1986 Jun 6;45(5):659–666. doi: 10.1016/0092-8674(86)90780-4. [DOI] [PubMed] [Google Scholar]
- Kohase M., May L. T., Tamm I., Vilcek J., Sehgal P. B. A cytokine network in human diploid fibroblasts: interactions of beta-interferons, tumor necrosis factor, platelet-derived growth factor, and interleukin-1. Mol Cell Biol. 1987 Jan;7(1):273–280. doi: 10.1128/mcb.7.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeuwenberg J. F., van Damme J., Jeunhomme G. M., Buurman W. A. Interferon beta 1, an intermediate in the tumor necrosis factor alpha-induced increased MHC class I expression and an autocrine regulator of the constitutive MHC class I expression. J Exp Med. 1987 Oct 1;166(4):1180–1185. doi: 10.1084/jem.166.4.1180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May L. T., Helfgott D. C., Sehgal P. B. Anti-beta-interferon antibodies inhibit the increased expression of HLA-B7 mRNA in tumor necrosis factor-treated human fibroblasts: structural studies of the beta 2 interferon involved. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8957–8961. doi: 10.1073/pnas.83.23.8957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinkoth J., Wahl G. Hybridization of nucleic acids immobilized on solid supports. Anal Biochem. 1984 May 1;138(2):267–284. doi: 10.1016/0003-2697(84)90808-x. [DOI] [PubMed] [Google Scholar]
- Mestan J., Brockhaus M., Kirchner H., Jacobsen H. Antiviral activity of tumour necrosis factor. Synergism with interferons and induction of oligo-2',5'-adenylate synthetase. J Gen Virol. 1988 Dec;69(Pt 12):3113–3120. doi: 10.1099/0022-1317-69-12-3113. [DOI] [PubMed] [Google Scholar]
- Mestan J., Digel W., Mittnacht S., Hillen H., Blohm D., Möller A., Jacobsen H., Kirchner H. Antiviral effects of recombinant tumour necrosis factor in vitro. 1986 Oct 30-Nov 5Nature. 323(6091):816–819. doi: 10.1038/323816a0. [DOI] [PubMed] [Google Scholar]
- Moos M., Gallwitz D. Structure of two human beta-actin-related processed genes one of which is located next to a simple repetitive sequence. EMBO J. 1983;2(5):757–761. doi: 10.1002/j.1460-2075.1983.tb01496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rappolee D. A., Mark D., Banda M. J., Werb Z. Wound macrophages express TGF-alpha and other growth factors in vivo: analysis by mRNA phenotyping. Science. 1988 Aug 5;241(4866):708–712. doi: 10.1126/science.3041594. [DOI] [PubMed] [Google Scholar]
- Reis L. F., Le J. M., Hirano T., Kishimoto T., Vilcek J. Antiviral action of tumor necrosis factor in human fibroblasts is not mediated by B cell stimulatory factor 2/IFN-beta 2, and is inhibited by specific antibodies to IFN-beta. J Immunol. 1988 Mar 1;140(5):1566–1570. [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Van Damme J., De Ley M., Van Snick J., Dinarello C. A., Billiau A. The role of interferon-beta 1 and the 26-kDa protein (interferon-beta 2) as mediators of the antiviral effect of interleukin 1 and tumor necrosis factor. J Immunol. 1987 Sep 15;139(6):1867–1872. [PubMed] [Google Scholar]
- Van Damme J., Opdenakker G., Simpson R. J., Rubira M. R., Cayphas S., Vink A., Billiau A., Van Snick J. Identification of the human 26-kD protein, interferon beta 2 (IFN-beta 2), as a B cell hybridoma/plasmacytoma growth factor induced by interleukin 1 and tumor necrosis factor. J Exp Med. 1987 Mar 1;165(3):914–919. doi: 10.1084/jem.165.3.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong G. H., Goeddel D. V. Tumour necrosis factors alpha and beta inhibit virus replication and synergize with interferons. 1986 Oct 30-Nov 5Nature. 323(6091):819–822. doi: 10.1038/323819a0. [DOI] [PubMed] [Google Scholar]
- Yasukawa K., Hirano T., Watanabe Y., Muratani K., Matsuda T., Nakai S., Kishimoto T. Structure and expression of human B cell stimulatory factor-2 (BSF-2/IL-6) gene. EMBO J. 1987 Oct;6(10):2939–2945. doi: 10.1002/j.1460-2075.1987.tb02598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberstein A., Ruggieri R., Korn J. H., Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J. 1986 Oct;5(10):2529–2537. doi: 10.1002/j.1460-2075.1986.tb04531.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zullo J. N., Cochran B. H., Huang A. S., Stiles C. D. Platelet-derived growth factor and double-stranded ribonucleic acids stimulate expression of the same genes in 3T3 cells. Cell. 1985 Dec;43(3 Pt 2):793–800. doi: 10.1016/0092-8674(85)90252-1. [DOI] [PubMed] [Google Scholar]