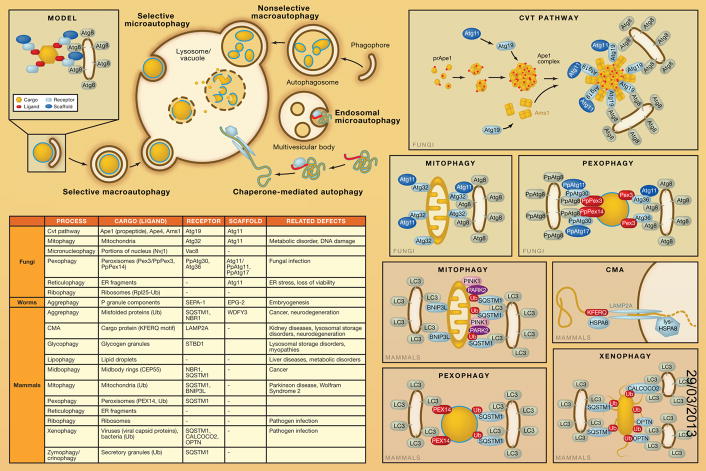
There are various types of autophagy, which can be categorized as nonselective or selective. Macroautophagy is an evolutionarily conserved process through which cells degrade and recycle cytoplasm. Nonselective macroautophagy randomly engulfs a portion of the cytoplasm into autophagosomes and then delivers them to the vacuole (in fungi or plants) or the lysosome (in other higher eukaryotes) for degradation. Selective macroautophagy, however, specifically recognizes and degrades a particular cargo, either a protein complex, an organelle, or an invading microbe. The morphological hallmark of macroautophagy is the formation of an initial sequestering compartment, the phagophore, which expands into the double-membrane autophagosome; the initial sequestration occurs in a compartment that is separate from the degradative organelle. Selective microautophagy utilizes the same cellular machinery, but in this case, the sequestration event takes place directly at the limiting membrane of the lysosome/vacuole. In higher eukaryotes, selective types of autophagy also include chaperone-mediated autophagy (CMA), and two similar processes, endosomal microautophagy (e-MI) and chaperone-assisted selective autophagy (CASA), each of which involves uptake at the limiting membrane of either the lysosome or endosome. In all cases, how a substrate is targeted for sequestration and segregated from other parts of the cell is one of the major questions in this research field. Nonselective autophagy is primarily a starvation response, whereas cells use selective autophagy for a variety of purposes, including remodeling to adapt to changing environmental/nutritional conditions and to eliminate damaged organelles. Accordingly, defects in selective autophagy are associated with a range of pathophysiologies in humans, including certain types of neurodegenerative diseases.
General Model
Selective macroautophagy utilizes the same core machinery used for nonselective macroautophagy. A small number of additional proteins suffice to make the process selective; a cargo-ligand-receptor-scaffold model is proposed to describe how cells achieve selectivity (see table). The ligand is the recognition component on the cargo that binds a receptor. In some cases, the receptor is a resident protein on the cargo (e.g., Atg32 or BNIP3L/NIX, which are located in the mitochondria outer membrane, in mitophagy). The interaction between the receptor and scaffold is vital for cargo recruitment to the phapophore assembly site (PAS), where an autophagosome forms. In yeast, Atg11 is the most commonly used scaffold protein, mediating several types of selective macroautophagy, including the cytoplasm-to-vacuole targeting (Cvt) pathway, mitophagy, pexophagy, and reticulophagy; however, in mammals a functional counterpart of Atg11 is yet to be discovered. In many cases, the receptor proteins subsequently bind Atg8, in yeast, or, in mammalian cells, one of the LC3 family proteins through a particular sequence referred to as AIM (Atg8-family-interacting motif) or LIR (LC3-interacting region). This interaction may connect the cargo directly with the macroautophagy machinery. Some aspects of this model can also be applied to CMA. In this case, the cargo is comprised of individual cytosolic proteins, which contain a consensus pentapeptide motif functioning as the ligand. The cytosolic chaperone HSPA8/HSC70 recognizes this sequence and delivers the substrate to LAMP2A in the lysosomal membrane, which serves as the receptor, and also acts as a translocation channel to move the unfolded substrate protein into the lumen of the lysosome.
Macroautophagy
The Cvt Pathway
The yeast Cvt pathway is the first characterized example of a biosynthetic process that utilizes the macroautophagy machinery. It is a transport route through which vacuolar resident enzymes are targeted from the cytosol to the vacuole, their final site of action. Enzymes that utilize the Cvt pathway include Ape1, Ape4, and Ams1. Atg19 is the primary receptor for these cargos, binding each through a different domain. The interaction of Atg11 with Atg19 mediates the transport of prApe1 oligomers in the form of a large complex to the PAS. Atg19 also interacts with Atg8 via a C-terminal WXXL motif that functions as an AIM to connect the cargo with the sequestration machinery.
Mitophagy
Selective degradation of surplus or dysfunctional organelles by autophagy has been observed both in yeast and mammals. Mitophagy, selective removal of mitochondria by autophagy (which can occur by either macro- or microautophagy), is one of the most studied types of “organelle autophagy” in part due to the connection between dysfunction of this process and certain diseases. In yeast, mitophagy is associated with the cellular remodeling that occurs upon the transition to a preferred carbon source. For example, when yeast cells are shifted from respiratory substrates such as lactate to glucose, excess mitochondria are degraded. The mitochondria outer membrane protein Atg32 functions as the receptor for mitophagy by interacting sequentially with Atg11 and Atg8. These interactions are critical for mitochondria delivery to, and subsequent degradation within, the vacuole.
In mammals, the functional counterpart of Atg32 is yet to be determined. Both BNIP3L and SQSTM1/p62 have been implied to function as receptors to link mitochondria with the autophagy machinery, depending on the cell type. During erythrocyte maturation, BNIP3L is essential for mitochondrial clearance where mitophagy plays a developmental role. Another such example is allophagy, a process observed in Caenorhabditis elegans embryos, in which sperm-derived paternal mitochondria and their mtDNA are degraded by autophagy. SQSTM1 may function as a receptor for depolarization-induced mitophagy. According to one model, PINK1 accumulates on the outer membrane of depolarized or damaged mitochondria where it recruits PARK2/Parkin, an E3 ubiquitin ligase. PARK2 mediates the ubiquitination of numerous outer mitochondrial membrane proteins, including MFN1 and MFN2. SQSTM1 contains a LIR motif, allowing it to bridge the ligand on the mitochondrial membrane with the autophagy machinery. However, SQSTM1 is not essential for mitophagy, suggesting that other factors may be able to function in its place.
Pexophagy
Pexophagy, the selective autophagic degradation of peroxisomes, shares certain features with mitophagy in that it can also occur by both macro- and microautophagic mechanisms and is primarily a response to changing nutrients. When fungi are grown on oleic acid or methanol, the peroxisomes proliferate because this organelle contains the enzymes necessary to utilize these carbon sources. When subsequently shifted to glucose or ethanol, the peroxisomes are rapidly and selectively degraded through autophagy. PpAtg30 and Atg36 function as receptors for pexophagy in Pichia pastoris and Saccharoymces cerevisiae, respectively.
Reticulophagy
The secretory pathway is an essential part of many cells, and massive numbers of newly synthesized proteins transit through all or part of this subcellular route. Due to the high level of protein synthesis associated with the endoplasmic reticulum (ER), there is the potential for problems with protein misfolding. Cells have specific mechanisms for handling routine protein misfolding, including lumenal chaperones, the unfolded protein response, and ER-associated degradation; however, these systems can be overwhelmed if the misfolding load is excessive. In this case, reticulophagy can be triggered. One of the key points with reticulophagy is that degradation of the sequestered cargo is not the key to its role in maintaining cellular homeostasis. Rather, sequestration of a portion of the ER within an autophagosome is sufficient to restore temporary homeostasis, maintaining viability and allowing the cell adequate time to recover from the acute stress.
Ribophagy
Ribosomes are nonselectively and selectively degraded by autophagy; ribophagy refers to the selective mode of degradation. Nonselective autophagic degradation of ribosomes may serve to keep a proper number of ribosomes in a cell under normal conditions. However, during starvation cells need amino acids and energy. Given the fact that ribosomes account for nearly half of the cellular proteins in a growing cell, cells are able to survive by selective autophagic degradation of ribosomes. This selective degradation is also suggested to rapidly downregulate translation, saving large amounts of amino acids and energy. The ubiquitin-specific protease Ubp3 and its cofactor Bre5 are required for ribophagy. A possible model for this regulation is that ubiquitination of ribosome subunits prevents degradation by selective autophagy; upon starvation, deubiquitination by Ubp3 causes the ribosome to be captured by autophagosomes, leading to its final degradation. In mammals, ribophagy may play a role in the immune response through the production of antimicrobial neopeptides.
Xenophagy
Xenophagy refers to the selective macroautophagic elimination of invasive microbes. Three receptors, SQSTM1, CALCOCO2/NDP52, and OPTN, have been implicated as receptors in the macroautophagic degradation of bacteria. The interactions of these receptors with both ubiquitinated proteins and LC3 are critical for effective xenophagy.
Viruses can also be selectively degraded by macroautophagy. For example, when the mouse CNS is infected with Sindbis (SIN) virus, SQSTM1 interacts with the SIN capsid proteins and mediates their degradation through macroautophagy, significantly reducing virally induced cell death.
Selective pressure has allowed the evolution of microbial strategies to circumvent the autophagic pathway. For example, the herpes simplex virus type 1 protein ICP34.5 binds BECN1, a component of the class III phosphatidylinositol 3-kinase complex, to block autophagy. Some bacteria induce macroautophagy but block fusion of the autophagosome with the lysosome, establishing a replicative niche within this compartment.
Other Macroautophagy Pathways
Formation of the phagophore during macroautophagy is referred to as a de novo process to distinguish it from the formation of vesicles throughout the secretory pathway. The critical point is that the phagophore expands to sequester various-sized cargo. The tremendous flexibility of the sequestration process means that almost any subcellular component can be a target for macroautophagy. In general, as with all types of selective macroautophagy, the cell utilizes the core machinery and adds on minimal components to target particular cargos. Aside from the cargos mentioned above, other examples include glycogen particles (glycophagy), lipid droplets (lipophagy), midbodies formed during cytokinesis (midbophagy), and zymogen granules (zymophagy), which are all targeted for degradation by macroautophagy. This process is not limited to mammals, as seen with aggrephagy in C. elegans, which reflects another developmental role for autophagy.
Chaperone-Mediated Autophagy
CMA is a selective type of autophagy that targets a specific subset of cytosolic proteins for lysosomal degradation. The distinctive feature of CMA is that the cargo proteins are directly translocated from the cytosol into the lysosome lumen for degradation independent of the core macroautophagy machinery. The cargo proteins of CMA have a consensus KFERQ-like motif that is recognized by a cytosolic chaperone protein, HSPA8. HSPA8 directs substrate proteins to the lysosomal-limiting membrane by interacting with the receptor protein LAMP2A, although several cochaperones have been identified that facilitate this process. In addition, a lumenal form of HSPA8 (Lys-HSPA8) is suggested to facilitate the translocation of the unfolded cargo proteins.
Chaperone-Assisted Selective Autophagy
CASA is a selective type of autophagy that targets polyubiquitinated proteins for degradation. BAG3, which is a cochaperone of HSPA8 and the small heat shock protein HSPB8, mediates CASA through interaction with the autophagy receptor SQSTM1. Despite its well-known role in proteasomal degradation, the ubiquitin ligase STUB1/CHIP is also involved in the CASA pathway. STUB1 mediates ubiquitination of cargo proteins and subsequent recruitment of SQSTM1. CASA has several physiological roles. First, CASA is a main route for the lysosomal degradation of cellular components under normal growth conditions, whereas CMA is induced by stress. CASA (like CMA) is also very important for protein quality control in aged cells, which can be demonstrated by elevated BAG3 levels and increased targeting of oxidized and ubiquitinated proteins to the lysosome in aged neurons. Moreover, CASA is essential for muscle maintenance.
Microautophagy
Endosomal Microautophagy
e-MI is a process in which some cytosolic proteins are selectively delivered to vesicles of late endosomes during multivesicular body biogenesis. Similar to CMA, e-MI relies on HSPA8 for cargo recruitment; however, the interaction between HSPA8 and cargo proteins is not through a KFERQ-like motif. Moreover, e-MI does not require LAMP2A but relies on the endosomal sorting complex required for transport (ESCRT)-I and -II components, such as VPS4 and TSG101, which are indispensible for multivesicular body formation. HSPA8 directs cargo proteins through electrostatic interaction of this chaperone with acidic phospholipids on the limiting membrane of late endosomes, but unfolding of the substrate is not needed for sequestration so that this process can also degrade protein complexes.
Micronucleophagy/Piecemeal Microautophagy of the Nucleus or PMN
Micronucleophagy occurs in S. cerevisiae at nucleus-vacuole junctions, which are mediated by Vac8 in the vacuole membrane and Nvj1 in the perinuclear ER. Upon starvation, portions of membrane containing nonessential nuclear materials protrude into the vacuole lumen, where they ultimately scission off and are degraded. The core Atg proteins are required for this process.
Figure 1.
References
- Cebollero E, Reggiori F, Kraft C. Reticulophagy and ribophagy: regulated degradation of protein production factories. Int J Cell Biol. 2012;2012:182834. doi: 10.1155/2012/182834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dice JF. Chaperone-mediated autophagy. Autophagy. 2007;3:295–299. doi: 10.4161/auto.4144. [DOI] [PubMed] [Google Scholar]
- Kettern N, Dreiseidler M, Tawo R, Höhfeld J. Chaperone-assisted degradation: multiple paths to destruction. Biol Chem. 2010;391:481–489. doi: 10.1515/BC.2010.058. [DOI] [PubMed] [Google Scholar]
- Lynch-Day MA, Klionsky DJ. The Cvt pathway as a model for selective autophagy. FEBS Lett. 2010;584:1359–1366. doi: 10.1016/j.febslet.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijaljica D, Nazarko TY, Brumell JH, Huang WP, Komatsu M, Prescott M, Simonsen A, Yamamoto A, Zhang H, Klionsky DJ, Devenish RJ. Receptor protein complexes are in control of autophagy. Autophagy. 2012;8:1701–1705. doi: 10.4161/auto.21332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orvedahl A, Levine B. Eating the enemy within: autophagy in infectious diseases. Cell Death Differ. 2009;16:57–69. doi: 10.1038/cdd.2008.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu R, Kaushik S, Clement CC, Cannizzo ES, Scharf B, Follenzi A, Potolicchio I, Nieves E, Cuervo AM, Santambrogio L. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20:131–139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani T, Huang WP, Stromhaug PE, Klionsky DJ. Mechanism of cargo selection in the cytoplasm to vacuole targeting pathway. Dev Cell. 2002;3:825–837. doi: 10.1016/s1534-5807(02)00373-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z, Klionsky DJ. Autophagosome formation: core machinery and adaptations. Nat Cell Biol. 2007;9:1102–1109. doi: 10.1038/ncb1007-1102. [DOI] [PubMed] [Google Scholar]
- Youle RJ, Narendra DP. Mechanisms of mitophagy. Nat Rev Mol Cell Biol. 2011;12:9–14. doi: 10.1038/nrm3028. [DOI] [PMC free article] [PubMed] [Google Scholar]