Abstract
Functional vascularization is a key requirement for the development and function of most tissues, and most critically cardiac muscle. Rapid and irreversible loss of cardiomyocytes during cardiac infarction directly results from the lack of blood supply. Contractile cardiac grafts, engineered using cardiovascular cells in conjunction with biomaterial scaffolds, are an actively studied method for cardiac repair. In this article, we focus on biomaterial scaffolds designed to mediate the development and maturation of vascular networks, by immobilized growth factors. The interactive effects of multiple vasculogenic factors are discussed in the context of cardiac tissue engineering.
Introduction
Native myocardium consists of cardiomyocytes, cardiac fibroblasts and endothelial cells (ECs). Cardiomyocytes which comprise about 20-40% of the total cells by number, and about 80-90% by volume, are multinucleated elongated cells aligned in parallel to the heart wall. These aligned cardiomyocytes are interspaced by dense vascular network, with inter-capillary distances being only 20 μm [1]. Essentially, every cardiomyocyte in adult heart is flanked between two capillaries. The extensive vascular network supports the high metabolic activity of the heart by providing rapid exchange of nutrients, oxygen and metabolites, over very short distances. The vascular network is essential for the survival and function of all cells in the heart, through its role in mass transport and cell signaling.
The best illustration of the importance of blood flow for cardiac function is the sequence of events that results from the interruption of blood flow, which triggers myocardial infarction. The interruption of blood flow results in hypoxia - lack of oxygen supply to the cells, causing the release of apoptotic factors and cell death. Upon myocardial infarction, a patient can lose as much as 50 grams of cardiac muscle mass, which is significantly beyond the regenerative capacity of adult heart. Following myocardial infarction, the heart undergoes a three-step healing process characterized by the inflammatory, proliferative and maturation phase. Within seconds of myocardial ischemia, hypoxia sets in the myocardium, and within minutes, adenosine triphosphate (ATP) depletion of cardiomyocytes leads to an inability to contract, and eventually necrosis of the myocytes. Dying cardiovascular cells release pro-inflammatory signals, leading to rapid infiltration of neutrophils that begin to clear the cell debris within the infarct. Within 24 hours, the myocardium is invaded by monocytes, which phagocytose neutrophils, resulting in the release of cytokines (e.g., TGF-ß) that initiate tissue remodeling [2]. In the next phase, the inflammatory response leads to the formation of granulation tissue and eventually to the remodeling of myocardium into a fibrous scar.
Engineering of functional patches of cardiac tissue, using cardiovascular cells or their progenitors in conjunction with biomaterial scaffolds and bioreactors, has been actively explored for more than a decade, as a possible method for cardiac repair. Engineered heart muscle must meet multiple functional criteria: (i) support physiologic levels of diastolic loads, (ii) produce systolic forces sufficient to contract muscle and create the pressure for pumping blood, (iii) have the ability to integrate both electrically and mechanically with the host muscle, and (iv) contain functional vasculature that can connect to the vasculature of the host. Vascularization is in a way the prerequisite for meeting all other requirements, as it determines the survival and function of the grafted cells. An effective approach to achieving vascularization is through the use of biomaterial scaffolds that are functionalized by incorporation of multiple growth factors, which act in concert to first induce angiogenesis and then stabilize and mature the new blood vessels.
In this Feature Article, we discuss biomaterial scaffolds for cardiac tissue engineering that are specifically designed to mediate the development and maturation of vascular networks by immobilized growth factors. Our focus is on interactive effects of immobilized vascular endothelial growth factor (VEGF) and angiopoietin-1 (Ang-1) which are known to respectively induce and stabilize vascular networks. We use examples from our recent study [3] to document the use of these scaffolds to induce blood vessel formation in vitro and in vivo.
Cardiac tissue engineering and the need for vascularization
Myocardial infarction causes irreversible damage to the cardiac muscle, which has only a minimal ability to regenerate, as the terminally differentiated cardiomyocytes are arrested in a post-mitotic state. A recent landmark paper [4], reported that cardiomyocytes do in fact renew themselves at a rate of 1% per year during the first 25 years of life, and that this rate decreases to only 0.45% per year by the age of 75. Remarkably, less than a half of the heart cells we are born with is being replaced over the entire life span. Over the last two decades, the field of cardiac tissue engineering has been striving to provide functional cardiac constructs that can repair injured myocardium. Tissue engineering offers the possibility of controlling cell differentiation and tissue assembly, by a coordinated use of three principle components: (1) cells, (2) biomaterial scaffold, and (3) bioreactor. Responding to the signalling imparted by the scaffold and bioreactor, cardiogenic cells engineer new tissue, by changing their phenotypes, forming intracellular junctions, interacting with the extracellular matrix, and assembling tissue structure at all hierarchical levels.
Within the tissue engineering system, biomaterial scaffold provides a structural template for the cells to adhere to and interact with each other, and an informational template by virtue of incorporated regulatory factors and specifically tailored structural and mechanical properties. Scaffolds can be engineered to interact with the cells, contain specific surface ligands, release bioactive factors at pre-determined or cell-regulated rates, and to have mechanical properties and degradation rates tailored to support the formation of a specific tissue. For cardiac tissue engineering, scaffolds are intended to provide instructional and temporary support to cells – inducing alignment, providing stiffness appropriate for generating physiological forces, and degrading as the cells replace the scaffold with new extracellular matrix proteins. Bioreactors are designed to provide environmental control and regulatory factors (molecular, hydrodynamic, mechanical and electrical). Overall, the cultivation of cells on scaffolds in bioreactors can provide the culture conditions conducive to cardiac tissue formation in vitro.
Ideally, a scaffold for cardiac tissue engineering should mimic the native composition, architecture and mechanical properties of the native heart matrix at certain developmental stages. General design requirements include surface chemistry and morphology suitable for cell attachment, high porosity (typically >95%) with a network of interconnected pores of an appropriate size (~100 μm for most efficient cell seeding), structural properties (including channels for vascularization, and structural anisotropy), and the biomechanical properties in compression and tension. In recent years, passive scaffolding materials (permissive and conducive to exogenous signals, but without specific bioactive roles) are now being replaced with “cell-instructive” materials designed to mimic the native matrix and actively interact with the cells at multiple levels, from molecular to cellular and tissue levels.
One “designer scaffold” has been designed using methods for “on the go” modifications of hydrogel properties by laser light, after the cells have been encapsulated [5]. This method enables geometrically precise degradation of hydrogel, and form channels for cell migration or vascular conduits. Another “designer scaffold” was engineered to mimic the anisotropic structure and biomechanics of cardiac muscle [6]. The scaffold material was a highly porous degradable elastomer, with the tensile stiffness matching that of native rat myocardium. The material was processed into an accordion-like honeycomb structure with geometric properties designed to mimic the structural and biomechanical anisotropy of native heart muscle. This scaffold induced the alignment and coupling of neonatal heart myocytes, and resulted in direction-dependent contractile behavior, a situation much closer to native heart tissue properties than it can be achieved with isotropic scaffolds. It will be interesting to see if these scaffolds will also support the development of vascular networks, and be compatible with the use of perfusion bioreactors, necessary for creating thick and compact tissue grafts [7].
In terms of mechanical properties, it has been confirmed that cells exhibit their differentiated phenotype on substrates that match the stiffness of their native ECM [8, 9]. Thus, it is also thought that the function and morphological properties of the engineered heart tissue would be physiological on substrates with stiffness comparable to that of the native heart. The stiffness of the adult rat myocardium was reported to be ~70 kPa [10], while the stiffness of the adult rat right ventricle was reported to be 54 ± 8 kPa in the circumferential direction and 20 ± 4 kPa in the longitudinal direction [11]. Adult rat left ventricle was measured to have the stiffness of 18 ± 2 kPa by atomic force microscopy [12]. We recently found the stiffness of the neonatal (4.0-11.4 kPa) and adult rat myocardium (11.9-46.2 kPa) to be in a reasonable agreement with the previously reported values [13]. Interestingly, when neonatal rat heart cells were cultivated as monolayers of on substrates of the stiffness from 3-144 kPa, the group combining reasonable levels of electrical excitability and high contraction force was the 50 kPa gel of stiffness consistent with the native heart. Substrate stiffness, also influence the functional maturation of neonatal rat ventricular cardiomyocytes [14]. Jacot et al demonstrated that the optimal sarcomere structure was obtained for cells on 10 kPa substrates [14].
The main role of vascular supply is mass transport of nutrients, metabolites and regulatory molecules to and from the cells, by a combination of convective flow (via large blood vessels, over large distances) and molecular diffusion (between capillaries and the surrounding tissue, over very short distances, down concentration gradients). The most critical molecule is oxygen, due to its extremely low solubility in plasma (only ~ 7 mg/liter). Reversible binding of oxygen to hemoglobin enables high rates of oxygen transport between blood and tissues. To achieve sufficient mass transport through engineered tissues, researchers have used perfusion-based tissue culture, channeled scaffolds, and oxygen carriers [15-18]. Early studies characterized the diffusion limits of cardiac tissue, and demonstrated that diffusional supply of oxygen (for example, into the tissue constructs that are bathed in culture medium) can maintain cell viability only within an 100 μm outer layer of the tissue, a thickness that is several hundred times smaller than the thickness of adult human heart muscle.
Scaffold architectures can be adapted to enable the application of convective regimes of oxygen transport. While medium flow through porous scaffolds enables higher cell survival, direct perfusion resulted in non-physiologic shear stress on the cells. In native heart muscle, blood flows through the vasculature lined with endothelial cells that are exposed to the shear forces, while the muscle is shielded from these forces. Exposure of cardiomyocytes to shear may affect their phenotypic stability and ability to form the necessary cell-matrix interactions.
To provide a native-like oxygen supply, without exposing cells to hydrodynamic shear, channeled scaffolds have been used as a means of mimicking vasculature [17]. Highly porous elastomers were laser pierced to enable medium perfusion through the channels, rather than through the cell-seeded bulk phase of the scaffold. While the channels made thus far have been on the order of 200 μm in diameter, and spaced several hundred micrometers apart, in vivo capillaries are on the order of 10 μm with intercapillary distances of 20 μm, approximately one capillary between every two cardiomyocytes [1].
Patterning of high resolution features to create vascular structures at physiological density and size within engineered heart muscle may be the next step forward in scaffold development. The use of perfusion-based culture systems combined with other biophysical signaling paradigms may further enhance engineering of thick, functional cardiac tissues for large animal studies. One condition for the effective use of these systems is the development of biomaterial scaffolds with high porosity and interconnected pores (to allow cell attachment, and protect the cells from flow-induced hydrodynamic shear), and an array of parallel channels (to allow organized flow of culture medium, and lumens for the attachment of endothelial cells).
Thick and compact cardiac grafts can thus be engineered using channeled scaffolds and perfusion bioreactors. While this approach improves the viability of the cardiac patches in vitro, it does not sustain patch viability once grafted into the patient. The vascularization of the patch requires the infiltration and growth of blood vessels into the patch, which is a very slow process. An alternative solution is to design a physiologically interactive replacement consisting of functional blood vessels for the injured vascular tissue. By inducing vascularization within the engineered tissue in vitro prior to implantation, limited transport capacity of oxygen and nutrients into the tissue could be overcome, thus improving its survival both in vitro and in vivo.
Several approaches have been previously investigated to engineer vascularized cardiac tissues [19-21]. In one approach [19], functional myocardial tissues of up to 1mm in thickness were engineered by a polysurgery approach, in which cardiomyocyte sheets were transplanted layer by layer at 1- or 2-day intervals (after adequate neovascularization occurred for the layer) to recreate cell-dense cardiac tissues. However, this approach is limited by the high risk of complications from each surgery. In the second approach [20], a three-dimensional vascularized human cardiac tissue was constructed by using multiple cell types, including cardiomyocytes, endothelial cells and embryonic fibroblasts. Endothelial cells were found to play an important role in prevascularization of the tissue, while fibroblasts decreased the death of endothelial cells and stabilized the formed vessels [20]. In the third approach [21], vascularized cardiac tissues were created by placing an arterio-venous blood vessel loop inside a cell-seeded polycarbonate chamber, which was then implanted into a rat groin. Other meritorious approaches include the inclusion of vascular cells in the construct to facilitate functional vessel formation [22, 23], use of channeled scaffolds seeded with endothelial cells to mimic native vasculature [24], and use of pro-angiogenic factors to attract native vasculature to infiltrate into the scaffold [3].
At this time, our inability to vascularize tissue grafts remains the main factor limiting cell survival and function upon in vivo implantation. The critical challenge associated with the graft implantation is that once an engineered tissue is removed from its in vitro culture system where medium perfusion enables nutrient transport, it needs to immediately connect with the host vasculature. The overall success of tissue engineering basically depends on functional vascularization of the tissue graft, in a way that enables rapid establishment of blood supply after implantation.
Angiogenesis and angiogenic factors
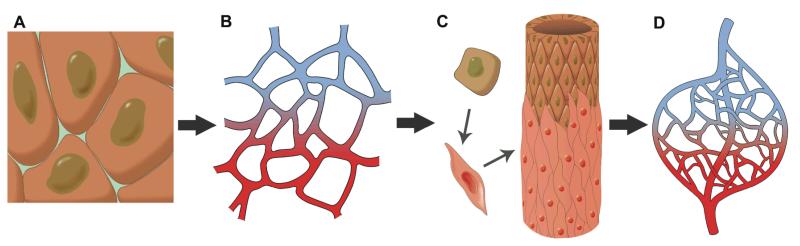
An understanding of the processes underlying the formation of new blood vessels is critical for the treatment of a broad spectrum of clinical conditions, from ischemic disease to wound healing and cancer [25]. The formation of new blood vessels by endothelial cells or their progenitors, a process described as vasculogenesis, can occur in several different ways, that are briefly summarized here (for detailed review, please see [26]) (Figure 1).
Figure 1. Formation of vascular networks.
Endothelial progenitors (A) differentiate into arterial and venous endothelial cells that form a capillary plexus (B). The resulting vessels are sprouting and being stabilized by the recruitment of smooth muscle cells (C), to result in mature vasculature (D).
Angiogenesis, sprouting of endothelial cells or their progenitors, leads to the formation of capillaries consisting of only endothelial cells. These endothelial cell sprouts can be further stabilized and matured by mural cells, either pericytes surrounding the sprouts (to form medium size blood vessels), or smooth muscle cells (forming the walls of large blood vessels), in a process described as arteriogenesis. In addition, collateral growth of already existing blood vessels can be a source of new blood vessels and connections between the vascular networks.
Angiogenesis and the subsequent maturation of the newly formed blood vessels progress through a complex sequence of events that involves multiple cell types and is regulated by multiple growth factors. Initial steps include disintegration of the basement membrane, proliferation and migration of endothelial cells, and their sprouting into vascular tubes. The end of cell migration and proliferation coincides with the recruitment of perivascular support cells, formation of a new basement membrane, and the formation of mature and durable vessels by incorporation of smooth muscle cells into the vessel walls.
Recent work has shown that endothelial progenitors, thought for a long time to contribute to the vascularization only during the development, are also major contributors to vascularization of ischemic or inflamed adult tissues, and could thus be used for therapeutic vasculogenesis [26] and prevascularization of engineered tissue grafts. Early steps in vasculogenesis (including the basement membrane dissolution, endothelial cell migration, endothelial cell proliferation and sprouting) are VEGF-mediated, whereas the later steps (pericyte recruitment, and vessel stabilization and maturation) are mediated by Ang1.
Identification of the key factors mediating vasculogenesis opens the possibility for the use of these factors to mediate vascularization. Fibroblast growth factor (FGF), platelet-derived endothelial cell growth factor (PDGF) and vascular endothelial growth factor (VEGF) have been identified long ago as stimulatory factors of angiogenesis in vivo [27]. Studies showed that the presence of vascular endothelial growth factor and acidic or basic fibroblast growth factor (aFGF or bFGF) promoted the formation of vascular structures [25]. In vivo rodent studies also showed that local delivery of insulin-like growth factor-I (IGF) and platelet-derived growth factor-BB (PDGF-BB) to the myocardium led to improved contractility and decreased apoptosis in cardiomyocytes [28].
Among these angiogenic growth factors, vascular endothelial growth factor and angiopoietins are the most important regulators of blood vessel formation [29]. VEGF receptors, known as fms-like tyrosine kinase (Flt-1 or VEGFR-1) and kinase insert domain-containing receptor (KDR or Flk-1 or VEGFR-2), and Ang1 receptor, known as tyrosine kinase with immunoglobulin-like and EGF-like domains 2 (Tie2), are found in endothelial cells [30]. VEGF promotes the formation of new capillary vessels, while Ang1 induces the maturation and stabilization of new vessel networks, suggesting a complementary relationship between these two angiogenic factors [31]. This raises a possibility that VEGF and Ang1, incorporated into scaffolds for tissue engineering, could theoretically work together to first form the new vessels, and then stabilize and mature these vessels to grow a functional vascular network.
Vascular Endothelial Growth Factor (VEGF)
Vascular endothelial growth factor is a family of homodimeric glycoproteins consisting of VEGF-A to VEGF-D. They are critically required for normal vascular development, as demonstrated on mice with deletion of VEGF [32]. VEGF receptors on endothelial cells are Flk-1 and Flt-1 [33]. Specifically, process such as endothelial cell proliferation, migration, survival and vascular permeability that are critical for angiogenesis and vascularization of engineered tissues, are mediated by binding of VEGF-A to VEGFR-2, resulting in receptor dimerization, autophosphorylation and activation of downstream signaling pathways [34]. For example, the Raf-Mek-Erk pathway is responsible for cell proliferation, the phosphatidylinositol-3 kinase (PI3K)-Akt pathway [35] is responsible for cell survival, and Mitogen-Activated Protein Kinase (MAPK) pathway is responsible for cell migration. VEGF also stimulates glucose uptake, which may be related to the improved cell survival and proliferation.
Signaling through VEGFR-2 can also increase vascular permeability, resulting in the movement of fluids between vascular and extravascular areas [36]. This is advantageous in terms of improving the ability to form new vessels by causing dissolution of existing basement membrane, but disadvantageous in terms of causing instability in the walls of forming vessels. Therefore, VEGF-induced vessel formation alone could not effectively increase tissue perfusion in vivo [37]. Despite the increase in the number of vessels present when adeno-associated virus (AAV) vectors were used to prolong the expression of VEGF, perfusion decreased due to the formation of leaky vascular lacunae [37]. VEGF causes vascular permeability by affecting endothelial cell-cell junctions regulated by adhesion molecules that make up tight, gap and adherens junctions [36].
Angiopoietin-1 (Ang1)
The interaction of Ang1 with the Tie2 receptor is known to be necessary for regulating vascular stabilization and remodeling [38]. While VEGF acts at early stages of angiogenesis by promoting proliferation and vascular permeability, Ang1 acts at later stages by controlling the survival and migration of endothelial cells and by recruiting pericytes to the vessel walls [38]. In vitro studies showed that Ang1 was weakly mitogenic for endothelial cells, unlike VEGF [29]. Ang1 activates Tie2 in a concentration-dependent manner, with maximum levels of Tie2 activation when cells were stimulated with 800ng/mL Ang1 (Bogdanovic et al., 2006), and can bind to the Tie1 receptor [39, 40].
Ang1 binds to the extracellular domain of Tie2 in endothelial cells, leading to the autophosphorylation of receptor and the activation of intracellular signaling pathways. Phosphorylation on tyrosine residues 1102 and 1108 of Tie2 is important for the initiation of downstream signaling pathways [41] that in turn cause migration and survival of endothelial cells [42]. For example, phosphatidylinositol-3 kinase (PI3K) activation [43] prevents serum induced apoptosis and induces tube formation in vivo [44]. Binding of Ang1 induces rapid internalization and degradation of Tie2, while Ang1 itself is released from the cell surface [42]. Tie2 receptor internalization is necessary for regulating the magnitude and duration of Ang1 signal transduction, and maintaining the cellular homeostasis [42].
In adult microvasculature, Ang1 binds to Tie2 receptor to stabilize endothelial cell interactions with the extracellular matrix and junctional proteins, enhancing the endothelial barrier function [45]. Transgenic mice overexpressing Ang1 in ear dermal microvessels were resistant to vascular leakage induced by VEGF and other inflammatory agents [46]. Recombinant Ang1 inhibited hyperpermeability induced by VEGF and thrombin in human umbilical vein endothelial cell (HUVEC) monolayers (Gamble et al. 2000). This effect is mediated by blocking the VEGF-mediated activation of protein kinase C (PKCβ) [47] and activation of mDia through RhoA and association of mDia with Src [48]. Ang1 can also block the VEGF-induced Ca2+ influx into endothelial cells that is required for formation of interendothelial junctional gaps [45].
Need for interaction of VEGF and Ang1
The interaction between VEGF and Ang1 may be required for successful vascularization of engineered tissues, as neither Ang1 nor VEGF alone induced angiogenesis in a Matrigel plug assay [49]. Ang1 and VEGF, acting in concert, stimulated the infiltration of cluster of differentiation 31 (CD31) negative and vimentin positive cells that expressed VEGF and Ang1 receptors [49]. VEGF was found necessary for upregulation of Tie2 in mural cells, aiding the Ang1-mediated phosphorylation of Tie2, and recruiting the mural cells to new blood vessels [49]. This supports the need for combined growth factors, as VEGF and Ang1 have complementary and interdependent effects. Signaling through Akt contributed to endothelial cell survival [50], where Akt was required and sufficient to mediate Ang1-induced cell survival, and sufficient but not required for VEGF-induced cell survival. Akt also plays an important role in endothelial cell sprouting induced by Ang1 and VEGF [50]. Promoting the survival and sprouting of endothelial cells aids to vascularization, and helps to overcome diffusion limitations for oxygen and nutrients and improve survival of cardiac cells in the myocardium
Biomaterials for controlled delivery of angiogenic factors
Vascularization of engineered tissues involves the need for formation of the whole blood vessel network, ranging from capillaries (which are involved in the direct exchange of nutrients, oxygen and metabolites with the tissue cells) to larger blood vessels (which provide bulk flow of blood to the tissue). The requirement for vascularization is one of the main drivers of biomaterial design for tissue engineering scaffolds, with the need to provide both the initial cell-instructive signals for angiogenesis and the support of the formation and maturation of functional blood vessels.
In native tissues, the extracellular matrix (containing collagen, laminin, elastin and other components) prevents the blood vessels from collapsing, by providing the necessary contacts between the endothelial cells and the surrounding tissue. Also, the collagen and laminin provide connections between the vascular cells and the integrity and elastic nature of the vessel wall. The extracellular matrix further supports and modulates the formation and function of blood vessels through multiple regulatory factors that are incorporated in its structure. When vascular cells undergo sprouting, the extracellular matrix is broken down by cell-secreted enzymes, its structure changes and the new epitopes are being presented to the cells, in coordination with the formation of a new provisional matrix guiding vessel formation [26]. These multiple and highly interrelated functions of the extracellular matrix need to be provided in some way by any scaffolding material designed for engineering of vascularized tissues.
Collagen scaffolds
Simple collagen scaffolds have been previously shown to induce angiogenesis and arteriogenesis when on healthy and cryoinjured left ventricles of rat hearts, by evaluation of blood vessel density and extravascular cell infiltration at day 15 and day 60 after implantation [51]. The main advantage of the scaffolds is that their shape and size can be tailored easily for the specific application. Their highly porous structure enables he exchange of nutrients with the surrounding medium and the pore walls provide anchorage sites for the cells. However, they may interfere with the development of active force [52]. Collagen cardiac patches were integrated with the surface of the rat heart, and were populated with new capillaries (less than 50μm in diameter) and arterioles (more than 50μm in diameter). The implantation of collagen increased vessel density by 2.7-fold for arterioles and 4-fold for capillaries, with higher overall vessel density in cryoinjured than healthy hearts [51]. Interestingly, the cells in collagen patches showed markers of endothelial and smooth muscle cells, but not cardiomyocytes [51].
In a separate study, VEGF-soaked collagen sponges (10μg/mL VEGF) were surgically inserted into the anterior cricoid cartilage of rabbits. The larynx was harvested at Day 10 to analyze the degree of closure and the presence of inflammatory cells [53]. It was found that both control and VEGF-soaked sponges had complete epithelial and soft tissue closure, and the VEGF-soaked sponges led to lower (although not significantly lower) acute inflammatory response [53].
Theoretically, local delivery of angiogenic growth factors using controlled-release scaffolds can help in tissue regeneration following injury. After the tissue is damaged, the implantation (e.g., by injection) of a growth factor delivery device (i.e. biodegradable polymeric matrix with growth factors) causes a release of growth factor, which induces angiogenesis to increase blood vessels and re-epithelialization, while the polymer degrades and the wound heals [25]. Controlled-release scaffolds include hydrogel systems, heparin-binding growth factor delivery, encapsulation, and supercritical carbon dioxide processing.
Hydrogels
Microspheres and hydrogels have been used to deliver growth factors in a controlled manner to induce neovascularization in vitro and in vivo, to provide local and sustained release over the cultivation period [25]. The main advantage of hydrogels for use in cardiac tissue engineering, is that they enable the development of high active force by the encapsulated cardiomyocytes [52] connected into the syncytium. However, it is significantly more difficult to control the shape and size of the hydrogel based cardiac constructs in comparison to the porous or fibrous scaffold based constructs. The cell alignment which is critical for cardiac function, is achieved in hydrogels by hydrogel remodeling in response to the tractional forces by the cells. In general, the anchorage points are provided in or around the hydrogel that prevent collapse of the structure into a sphere and as a result guide the gel compaction process such that the alignment of cells is achieved [54, 55]. Hydrogels are fabricated by physical or chemical crosslinking to create networks of water-soluble polymers [56]. Bioactive molecules can be introduced to the biomaterial in a liquid state. The temperature is then increased to entrap the molecules within the gel network and the molecules are subsequently released by a combination of hindered diffusion through the hydrogel pores and hydrogel degradation. Hydrogels with preloaded VEGF, Ang1, keratinocyte growth factor (KGF) and PDGF were implanted into mouse ear pinna to elicit vascular maturity [57].
In addition, in situ forming hydrogels have been extensively investigated for myocardial cell therapy in recent years, due to their injectability and ability to control crosslinking chemistry. Early studies relied on cell injection using natural hydrogels such as Matrigel [58, 59] or fibrin [60-62] reporting structural stabilization, reduced infarct size and improved vascularization upon injection of undifferentiated ESC [58, 59] or bone marrow cells [60-62]. Alginate alone was demonstrated to reduce pathological remodeling and improve function [63], initiating commercialization efforts of this hydrogel. A synthetic material, self-assembling peptide hydrogel (AcN-RARADADARARADADA-CNH) was also used, forming a nano-fibrous structure upon injection into the myocardium that promoted recruitment of endogenous ECs and supported survival of injected CMs [64].
Insulin-like growth factor-1 (IGF) bound to the self-assembling peptide was demonstrated to improve grafting and survival of CMs injected into MI [65]. Laflamme and Murry demonstrated that targeting of multiple pathways related to cell survival by encapsulating a number of biomolecules in Matrigel, significantly increased the survival and grafting of the human ESC-derived CM injected into infracted rat hearts [66]. We have modified chitosan with the peptide QHREDGS derived from angiopoietin-1, to create a hydrogel that reduces cardiomyocyte apoptosis under adverse conditions such as taxol treatment[67].
Recent studies collectively indicate that an injection of hydrogel alone, without the reparative cells, may also attenuate pathological remodeling upon myocardial infarction [63, 68-70]. It is thought that in those cases hydrogels act by changing the ventricular geometry and mechanics, thus reducing elevated local wall stresses that have been implicated in pathological remodeling[71]. Finite element modeling of wall stresses indicated that upon injection of the material of elastic modulus 10-20kPa in the infarct, injection improved ejection fraction and the stroke volume/end-diastolic volume relationship. In addition, injections of the material in the border zone decreased endsystolic fiber stress proportionally to the volume and the stiffness of the injected material.
Heparin-binding growth factor delivery
For heparin-binding growth factor delivery [56], bi-domain peptides are covalently crosslinked to a biomaterial, like fibrin. The peptides contain Factor XIIIa and heparin binding sequences, allowing heparin to electrostatically associate with the heparin binding domain and subsequently causing heparin-binding growth factors like bFGF to attach. The controlled release occurs via cell-mediated cleavage of susceptible peptide sequences. Heparin is a highly sulfated glycosaminoglycan that has been used to bind and stabilize many growth factors to allow local delivery from hydrogels and microcapsules [31]. It protects growth factors from inactivation, increases affinity of the growth factors to receptors, and sustains their release over a period of time. Heparin modified materials also decreases initial burst effects [25]. Heparized hydrogels (i.e. alginate and chitosan-alginate) were used to provide long-term supply of angiogenic factors, such as VEGF and bFGF, and induce neovascularization in vivo [72].
The release of growth factors from hydrogels depends on the diffusion from the hydrogel matrix, while their release from heparinized hydrogels depends on both the diffusion and the binding affinity to surface heparin [72]. In this way, heparinized hydrogels allow loaded growth factors to be released in a more sustained manner. Heparin was also covalently incorporated onto collagen matrices using 1-ethyl-3-(3-dimethylaminopropyl)-carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS), and the VEGF was physically immobilized to the heparin [73]. It was found that heparinized collagen matrices increased endothelial cell proliferation and angiogenic potential, with further improvements in angiogenic potential when VEGF was incorporated [73].
Microspheres
To encapsulate growth factors in microspheres, a water-oil emulsion is first created, with the growth factor dissolved in the water phase and the polymer dissolved in the organic phase. The two solutions are mixed, and then a second emulsion is formed by dispersing the mixture in an aqueous phase using homogenization or sonication. The second emulsion is stirred to evaporate the solvent to form porous microspheres. Epidermal growth factor (EGF), PDGF and VEGF have been incorporated into degradable microspheres using this method [56]. Sustained VEGF delivery using alginate microparticles [74] or poly(lactide-co-glycolide) microspheres [75] improved survival of transplanted endothelial cells and increased the capillary density.
Staged delivery of multiple growth factors
Multiple growth factors can be delivered in a controlled manner using dual delivery [25, 76, 77]. For example, PDGF was encapsulated into microspheres and mixed with lyophilized VEGF [25, 76]. These microspheres were processed into porous scaffolds with poly(lactide-co-glycolide) polymer [25, 76]. In this way, VEGF was released due to surface erosion and PDGF was released due to degradation [25]. Alginate hydrogels used as an injectable delivery system for controlled co-release of VEGF and PDGF induced growth of mature vessels, thus improving cardiac function after myocardial infarction [77].
Scaffolds with Covalently Immobilized Growth Factors
The covalent immobilization of growth factors onto biomaterials is becoming an increasingly promising method of growth factor delivery [78-84]. The purpose of immobilization is to protect growth factors against cellular inactivation and digestion, and to allow highly localized activity. Immobilization inhibits down-regulation, which occurs when the cells decompose signaling molecules to reduce their stimulation [85]. As a result, stimulation by growth factors is prolonged. Since the activity is localized to mimic the local in vivo microenvironment, the ligand-receptor complexes are also aggregated to increase receptor-mediated functions. Immobilization can help overcome the diffusional limitation of soluble growth factor delivery into the centre of the scaffold [84]. As the immobilized growth factors are not released to the environment, covalent immobilization is preferred over controlled delivery if the effect of growth factors is to be seen locally within the biomaterial (i.e. vascularization of the biomaterial) rather than in the surrounding tissue. Immobilized molecules also have improved stability [85].
In one study, the collagen-binding domain polypeptide of fibronectin was fused to hepatocyte growth factor (HGF), an angiogenic factor, to help immobilize HGF on collagen and stabilize the molecule for prolonged activity [83]. In another study, insulin and epidermal growth factor immobilized on synthetic polymeric substrates increased cell growth compared to soluble or adsorbed insulin [78]. These stimulations mimic juxtacrine stimulation of membrane-anchored growth factors [78]. Co-immobilization of different growth factors enhanced the stimulation by growth factors [78] and induced a cross talk of receptors [85].
Covalently immobilized VEGF was shown to promote the growth of endothelial cells and angiogenesis [84]. When VEGF was immobilized on substrates to control the adhesion and growth of endothelial cells, it was found that cell growth was significantly increased [80]. Growth factors can also be patterned by immobilization in order to achieve formation of blood vessel networks in vitro [80], or to induce micropatterning of different cell types for co-cultures [85].
The reported methods for covalent immobilization of VEGF includes: (i) the use of carbodiimide chemistry to immobilize VEGF on poly(acrylic acid) surfaces [79], (ii) crosslinking of VEGF to fibronectin coated surface by a cysteine tag that was incorporated to VEGF molecule [81], (3) the use of a homobifunctional crosslinker to immobilize VEGF in collagen matrices [82], and (iv) the photoimmobilization of VEGF on gelatin substrate in a micropatterning manner [80].
Case study: Collagen scaffolds with covalently immobilized VEGF and Ang1
We hypothesized that immobilization of additional growth factors such as Ang1 may be required to form stable capillary-like structures in collagen scaffolds. Thus, we studied the effect of immobilized single growth factors (VEGF or Ang1) and co-immobilized VEGF and Ang1 on the various stages of angiogenesis, such as EC proliferation, tube formation and vessel formation. Our results indicate that co-immobilized growth factors were superior in promoting H5V [3] and primary rat aortic endothelial cell[86] proliferation, tube formation, as well as angiogenesis in the Chicken Chorioallantoic Membrane (CAM) assay in comparison to single growth factors and the soluble controls. The commercially available Ultrafoam collagen sponge chosen for the experiment is commonly used in tissue engineering due to its ability to support cell attachment and growth [87],[88]. Ultrafoam collagen scaffold is also FDA approved as a hemostat [51],[89] (Figure 2).
Figure 2. Scaffold characterization.
SEM images of collagen sponges for (A) PBS only treated scaffold (PBS), (B) Scaffold treated with EDC crosslinker in PBS without growth factors (PBS+EDC), (C) Scaffold with immobilized VEGF (VEGF), (D) Scaffold with immobilized Ang1 (Ang1), (E) Scaffold with lower dose of co-immobilized VEGF and Ang1 (1/2VEGF+1/2Ang1), and (F) Scaffold with higher dose of co-immobilized VEGF and Ang1 (VEGF+Ang1). Images at 100X with 500X insets. (G) Tensile modulus of scaffolds treated as above. # denotes statistically significant difference compared to PBS control. (P < 0.05; one-way ANOVA). Figure reproduced from [14] with permission. Copyright Elsevier.
EDC chemistry was used to couple carboxyl groups to the amine groups. EDC is a water-soluble carbodiimide and a zero-length crosslinking agent that forms amide bonds between proteins [90]. EDC reacts with carboxyl groups of collagen to form an amine-reactive O-acylisourea derivative, which is converted into amine-reactive sulfo-NHS esters in the presence of sulfo-NHS [90]. The reactive esters then react with amine groups on the second molecule (i.e. VEGF and/or Ang1) to form stable amide bonds [90].
The concentrations of VEGF and Ang1 in the basal H5V medium were 13.77 ± 4.46ng/mL and 0.66 ± 0.04ng/mL respectively, as measured by enzyme-linked immunosorbent assay (ELISA). This is equivalent to 13.77 ± 4.46ng VEGF and 0.66 ± 0.04ng Ang1 since 1mL culture medium was used for each sample. As expected, increased amounts of immobilized VEGF or Ang1 were found in scaffolds when a higher concentration of VEGF or Ang1 was used in the immobilization solution (Table 1).
Table 1.
Quantification of immobilized growth factors within collagen scaffolds by ELISA. The calculations were done for a cylindrical scaffold 7 mm in diameter and 2mm in thickness.
| Name of Experimental Group |
Concentration of Growth Factors in Immobilization Solution |
Amount of Growth Factors Immobilized in Collagen Scaffolds |
||||
|---|---|---|---|---|---|---|
|
| ||||||
| VEGF (μg/mL) |
Angl (μg/mL) |
VEGF (ng) | VEGF (ng/mg scaffold) |
Angl (ng) | Angl (ng/mg scaffold) |
|
| PBS | -- | -- | -- | -- | -- | -- |
| PBS+EDC | -- | -- | -- | -- | -- | -- |
| VEGF | 1 | -- | 57.94±1.65 | 13.12±0.79 (19.2±0.5nM) |
-- | -- |
| Ang1 | -- | 1 | -- | -- | 42.02±3.51 | 10.55±0.48 (7.8±0.6nM) |
| 1/2VEGF+1/2Ang1 | 0.5 | 0.5 | 19.37±3.25a | 4.30±0.73a (6.5±1.0nM) |
23.81±2.44c | 5.29±0.55c (4.4±0.4nM) |
| VEGF+Ang1 | 1 | 1 | 38.37±4.66b | 8.78±1.09b (12.7±1.6nM) |
51.41±8.21 | 11.83±2.24 (9.5±1.6nM) |
significantly lower amount compared to VEGF and VEGF+Ang1 groups.
significantly lower amount compared to VEGF group.
significantly lower amount compared to Ang1 and VEGF+Ang1 groups.
(P < 0.05; one-way ANOVA with post-hoc Tukey test)
There were no apparent differences in the pore structure and porosity of scaffolds with or without immobilized growth factors (Figure 2). The tensile moduli (Figure 2G) of scaffolds with immobilized growth factors, as well as PBS+EDC control scaffolds, were all statistically significantly higher than that of the PBS control sponges (one-way ANOVA, P = 0.0353). This is likely due to the crosslinking of the collagen sponge in which collagen is bound to itself through EDC chemistry. However, there were no statistically significant differences in the tensile moduli amongst the groups treated with EDC (i.e. the growth factor groups and PBS+EDC group).
Immobilized VEGF and Ang1 on collagen scaffolds promoted cell proliferation in vitro[3]. However, it was unclear whether or not soluble growth factors can achieve the same effect, and whether the results were in fact due to soluble growth factors when collagen scaffolds begin to degrade over time. In addition, it was required to determine whether mechanical properties of the collagen scaffolds played a role in cell proliferation. To answer these questions, we focused on our best group from the cell proliferation studies, i.e. VEGF+Ang1 group (Figure 3). Since 1mL culture medium was used, the amounts of soluble VEGF and Ang1 (50ng VEGF and 50ng Ang1) were comparable to those immobilized on the scaffolds for VEGF+Ang1 group (Table 1).
Figure 3. Comparison of soluble and immobilized growth factors in a 3-day cultivation of H5V endothelial cells.
Soluble VEGF (50ng/mL) and Ang1 (50ng/mL) were applied to cell-seeded collagen scaffolds treated with PBS alone (S-(VEGF+Ang1)), or scaffolds treated with EDC crosslinker in PBS (S-(VEGF+Ang1)+EDC). These scaffolds were compared to scaffolds with co-immobilized VEGF and Ang1 (VEGF+Ang1). 50,000 cells were seeded on the freshly made scaffolds. (A) XTT assay indicating final cell numbers in collagen scaffolds. (B) Lactate production rate. (C) Glucose consumption rate. * denotes statistically significant difference (P < 0.05; one-way ANOVA with post-hoc Tukey test). Figure reproduced from [14] with permission. Copyright Elsevier.
The soluble growth factors applied to the crosslinked scaffold did not significantly increase the final cell number (Figure 3A) as compared to PBS and PBS+EDC controls. However, the scaffolds with immobilized VEGF and Ang1 had higher cell number as compared to both PBS and PBS+EDC controls. The immobilized VEGF+Ang1 group also had higher cell number lactate production and glucose consumption rates compared to its corresponding soluble S-(VEGF+Ang1) group (Figure 3). Glucose consumption rate was also higher for immobilized VEGF+Ang1 group when compared to PBS and PBS+EDC groups.
Although crosslinked scaffolds from the S-(VEGF+Ang1)+EDC group showed higher final cell number compared to scaffolds from the S-(VEGF+Ang1) group, the lactate production and glucose consumption rates were not elevated (Figure 3). Also, the final cell number in the S-(VEGF+Ang1)+EDC group was not increased compared to PBS and PBS+EDC groups, in contrast to the immobilized VEGF+Ang1 group (Figure 3A). Thus, the effects could be attributed to the immobilized growth factors, rather than the soluble growth factors applied to the scaffolds of higher stiffness.
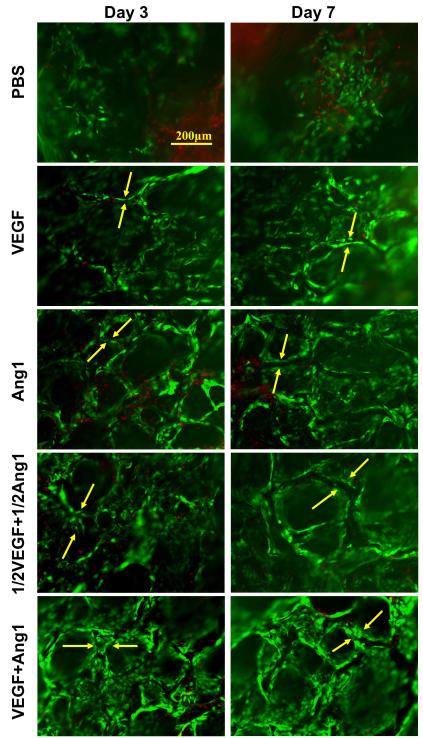
After 3 and 7 days of in vitro cultivation, live/dead images revealed the formation of tube structures by H5V endothelial cells in modified collagen scaffolds with immobilized VEGF and/or Ang1, but not in PBS controls (Figure 4) and not in the PBS+EDC group. Double staining of collagen scaffold red and the cells green (Figure 5), indicated that the cells were not merely aggregating and lining the pore walls of the scaffolds. Tube formation was evident away from the pore wall as indicated by the lack of overlapping green/red staining in the VEGF+Ang1 group (Figure 5).
Figure 4. Tube formation by H5V cells on collagen scaffolds with independently immobilized and co-immobilized growth factors in 3-day and 7-day cultivation periods.
Representative live/dead images under fluorescence microscopy (173,333 cells initially seeded on the freshly made scaffolds; green represents CFDA staining of live cells and red represents propidium iodide (PI) staining of dead cells; tube is indicated between two yellow arrows). Figure reproduced from [14] with permission. Copyright Elsevier.
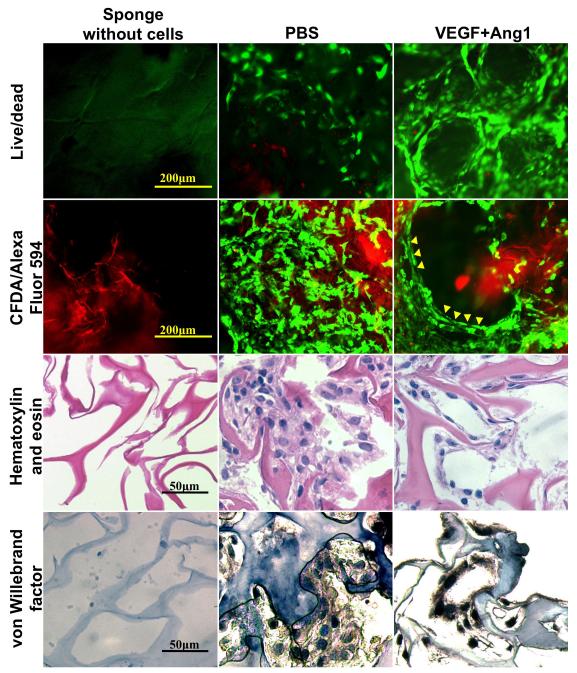
Figure 5. Tube formation after 7-day cultivation.
Images of PBS control and VEGF+Ang1 groups with live/dead staining (CFDA stains live cells green, PI stains dead cells red), cells (stained with CFDA) on collagen scaffolds labelled with Alexa Fluor 594 (red), hematoxylin and eosin staining, and von Willebrand factor staining. Sponges without cells are also shown. Figure reproduced from [59] with permission. Copyright Wiley.
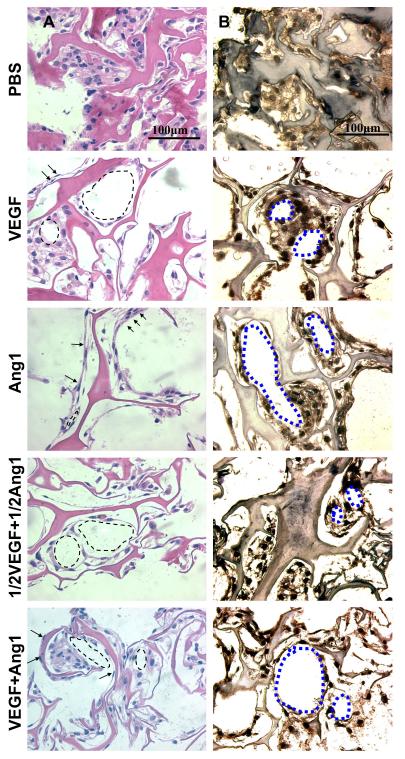
Histological evaluation further confirmed the formation of tube-like structures. Hematoxylin and eosin stained images demonstrated elongated thin cells and nuclei in peripheral position, which are typical structural appearances of endothelial cells in capillaries (Figure 6A). Von Willebrand (Factor VIII) staining (Figure 6B) revealed circular structures formed by endothelial cells for the groups with immobilized VEGF and/or Ang1. In contrast, PBS group showed less elongated cells that were clustered randomly within the pores of the collagen scaffold (Figure 6).
Figure 6. Cell morphology and organization after 7-day in vitro cultivation.
(A) Hematoxylin and eosin stained images (173,333 cells initially seeded on the freshly made scaffolds; arrows indicate elongated cells; arrowheads indicate circular structures). Note that darker pink in hematoxylin and eosin staining indicates the collagen scaffold, while the lighter pink is part of the cells. (B) Von Willebrand factor stained images (173,333 cells initially seeded; brown represents positive von Willebrand factor staining, blue represents counterstain; circular structures indicated by blue dotted outlines). Figure reproduced from [14] with permission. Copyright Elsevier.
The chorioallantoic membrane (CAM) of a developing chicken embryo serves as a surface for gas and nutrient exchanges. The allantois of the chicken embryo appears at Day 3 of incubation and grows until Day 10. It is fused with the adjacent mesodermal layers of the chorion to form the CAM, which is characterized by a dense capillary network. Due to a dense capillary network, the CAM is commonly used for studying angiogenic response to bioactive molecules [91]. The advantages of using the CAM assay include low cost, speed of results and low level of technical skills required, as compared to other animal models of angiogenesis [91] (Figure 7).
Figure 7. Chicken CAM assay using Fresh scaffolds.

Photographs of chicken eggs for (A) PBS and (B) VEGF+Ang1 groups. Arrow indicates location of collagen sponge. Figure reproduced from [59] with permission. Copyright Wiley.
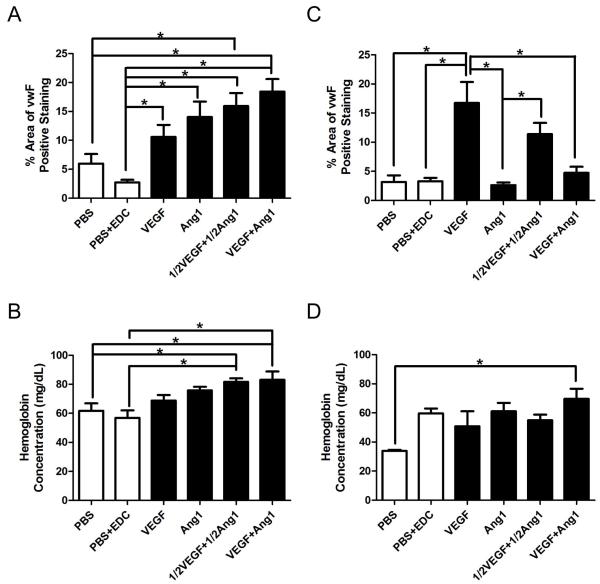
We evaluated vascularization of freshly prepared scaffolds and those aged in PBS for 28 days in the CAM assay. The area positive for Factor VIII, a marker of endothelial cells, in the initially cell-free scaffolds was indicative of the infiltrating ECs, and strikingly higher for VEGF+Ang1 group compared to all other groups in Fresh scaffolds (Figure 8A). Hemoglobin concentration is often measured to evaluate neovascularization, since an increase in hemoglobin content indicates an increase in the number of blood vessels that are connected to the host circulation [92]. Co-immobilization of VEGF and Ang1 showed increased hemoglobin concentration compared to PBS and PBS+EDC groups for Fresh scaffolds (Figure 8B). A dose response of immobilized VEGF and Ang1 was seen in the CAM assay with Aged scaffolds (Figure 8D), where only VEGF+Ang1 group (and not 1/2VEGF+1/2Ang1) showed higher hemoglobin concentration within the biomaterial compared to PBS controls. This indicates some loss of bioactivity for the growth factors over time, and suggests that the higher initial amounts of immobilized VEGF and Ang1 are necessary for eliciting an angiogenic response. However, it also indicates the maintenance of bioactivity of immobilized growth factors even after 28 days in PBS, a significant advantage over the use of control release systems and soluble growth factors.
Figure 8. Image analysis and hemoglobin content analysis of chicken CAM assay.
(A) Percentage of area with positive Factor VIII (FVIII) staining for Fresh scaffolds. (B) Hemoglobin concentration within Fresh scaffolds. (C) Percentage of area with positive FVIII staining for Aged scaffolds. (D) Hemoglobin concentration within Aged scaffolds. * denotes statistically significant difference (P < 0.05; one-way ANOVA with post-hoc Tukey test). Figure reproduced from [14] with permission. Copyright Elsevier.
Conclusions and Future Directions
In summary, functional vascularization, a key requirement for the development and function of most tissues, remains one of the key challenges of tissue engineering. In this article, we discussed biomaterial scaffolds designed to mediate the development and maturation of vascular networks, by immobilized growth factors. A case study was presented for co-immobilization of two important vasculogenic growth factors: VEGF and Ang-1. Our results indicate that covalently co-immobilized VEGF and Ang1 in scaffolds for tissue engineering enable scaffold vascularization both in vitro and in vivo. In addition, we demonstrated recently that covalently immobilized VEGF promotes endothelial differentiation of Flk1+ cardiovascular progenitors derived from mouse embryonic stem cells [93]. Thus, it may be possible to pre-vascularize scaffolds for cardiac tissue engineering by seeding of vascular progenitors into growth factor modified scaffolds. Future studies should involve pre-seeding of scaffolds with endothelial cells, characterization of pericyte recruitment to the scaffolds and studying how substrate stiffness modulates cellular response to immobilized growth factors. Also, the next great advances in tissue engineering may require the development of novel scaffolds that are adaptable to their environment and can facilitate communication between the cells, matrix and exogenous signals.
Biomaterial scaffolds provide a structural and logistic template for tissue formation, with vascularization being a key determinant of cell survival and function. In this Feature Article, we focus on biomaterial scaffolds for cardiac tissue engineering designed to mediate vascular development and maturation by immobilized growth factors.
Acknowledgments
The authors gratefully acknowledge funding support by a Canadian Institutes of Health Research (CARE project to MR), the Heart and Stroke Foundation Grant-in-Aid (NA6077 to MR), NSERC Alexander Graham Bell Canada Graduate Scholarship (to L.L.Y.C.), and NIH (HL076485, EB002520 and HL088913 to GVN). We also thank Dr Nebo Mirkovic for his expert help with the preparation of figures.
Contributor Information
Loraine Chiu, University of Toronto, Department of Chemical Engineering and Applied Chemistry, 164 College Street, Room 407, Toronto, Ontario, Canada M5S 3G9.
Milica Radisic, University of Toronto, Department of Chemical Engineering and Applied Chemistry, 164 College Street, Room 407, Toronto, Ontario, Canada M5S 3G9.
Gordana Vunjak-Novakovic, Columbia University, Department of Biomedical Engineering, 622 west 168th Street, VC12=234, New York NY 10032, U.S.A..
References
- [1].Rakusan K, Korecky B. Growth. 1982;46:275–81. [PubMed] [Google Scholar]
- [2].Frangogiannis NG. Pharmacol Res. 2008;58:88–111. doi: 10.1016/j.phrs.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Chiu LL, Radisic M. Biomaterials. 2010;31:226–41. doi: 10.1016/j.biomaterials.2009.09.039. [DOI] [PubMed] [Google Scholar]
- [4].Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnab√©-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, Fris√©n J. Science. 2009;324:98–102. doi: 10.1126/science.1164680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Engelmayr GC, Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Nature materials. 2008;7:1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Freytes DO, Wan LQ, Vunjak-Novakovic G. J Cell Biochem. 2009;108:1047–58. doi: 10.1002/jcb.22355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Engler AJ, Sen S, Sweeney HL, Discher DE. Cell. 2006;126:677–89. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- [9].Engler AJ, Griffin MA, Sen S, Bonnemann CG, Sweeney HL, Discher DE. J Cell Biol. 2004;166:877–87. doi: 10.1083/jcb.200405004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Boublik J, Park H, Radisic M, Tognana E, Chen F, Pei M, Vunjak-Novakovic G, Freed LE. Tissue Eng. 2005;11:1122–32. doi: 10.1089/ten.2005.11.1122. [DOI] [PubMed] [Google Scholar]
- [11].Engelmayr GC, Jr., Cheng M, Bettinger CJ, Borenstein JT, Langer R, Freed LE. Nat Mater. 2008;7:1003–10. doi: 10.1038/nmat2316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Berry MF, Engler AJ, Woo YJ, Pirolli TJ, Bish LT, Jayasankar V, Morine KJ, Gardner TJ, Discher DE, Sweeney HL. Am J Physiol Heart Circ Physiol. 2006;290:H2196–203. doi: 10.1152/ajpheart.01017.2005. [DOI] [PubMed] [Google Scholar]
- [13].Bhana B, Iyer RK, Chen WL, Zhao R, Sider KL, Likhitpanichkul M, Simmons CA, Radisic M. Biotechnol Bioeng. 105:1148–60. doi: 10.1002/bit.22647. [DOI] [PubMed] [Google Scholar]
- [14].Jacot JG, McCulloch AD, Omens JH. Biophys J. 2008;95:3479–87. doi: 10.1529/biophysj.107.124545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Radisic M, Deen W, Langer R, Vunjak-Novakovic G. American journal of physiology Heart and circulatory physiology. 2005;288:H1278–89. doi: 10.1152/ajpheart.00787.2004. [DOI] [PubMed] [Google Scholar]
- [16].Radisic M, Malda J, Epping E, Geng W, Langer R, Vunjak-Novakovic G. Biotechnology and Bioengineering. 2006;93:332–343. doi: 10.1002/bit.20722. [DOI] [PubMed] [Google Scholar]
- [17].Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. Nature protocols. 2008;3:719–38. doi: 10.1038/nprot.2008.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Tissue Engineering. 2006;12:2077–2091. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- [19].Shimizu T, Sekine H, Yang J, Isoi Y, Yamato M, Kikuchi A, Kobayashi E, Okano T. FASEB J. 2006;20:708–10. doi: 10.1096/fj.05-4715fje. [DOI] [PubMed] [Google Scholar]
- [20].Caspi O, Lesman A, Basevitch Y, Gepstein A, Arbel G, Habib IH, Gepstein L, Levenberg S. Circ Res. 2007;100:263–72. doi: 10.1161/01.RES.0000257776.05673.ff. [DOI] [PubMed] [Google Scholar]
- [21].Morritt AN, Bortolotto SK, Dilley RJ, Han X, Kompa AR, McCombe D, Wright CE, Itescu S, Angus JA, Morrison WA. Circulation. 2007;115:353–60. doi: 10.1161/CIRCULATIONAHA.106.657379. [DOI] [PubMed] [Google Scholar]
- [22].Stevens KR, Kreutziger KL, Dupras SK, Korte FS, Regnier M, Muskheli V, Nourse MB, Bendixen K, Reinecke H, Murry CE. Proc Natl Acad Sci U S A. 2009;106:16568–73. doi: 10.1073/pnas.0908381106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N, Holbova R, Feinberg MS, Dror S, Etzion Y, Leor J, Cohen S. Proc Natl Acad Sci U S A. 2009;106:14990–5. doi: 10.1073/pnas.0812242106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Radisic M, Park H, Chen F, Salazar-Lazzaro JE, Wang Y, Dennis R, Langer R, Freed LE, Vunjak-Novakovic G. Tissue Eng. 2006;12:2077–91. doi: 10.1089/ten.2006.12.2077. [DOI] [PubMed] [Google Scholar]
- [25].Lee H, Chung HJ, Park TG. Journal of Bioactive and Compatible Polymers. 2007;22:89–114. [Google Scholar]
- [26].Carmeliet P. Nat Med. 2003;9:653–60. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- [27].Risau W. Pharmacol Ther. 1991;51:371–6. doi: 10.1016/0163-7258(91)90066-u. [DOI] [PubMed] [Google Scholar]
- [28].Cheng M, Park H, Engelmayr GC, Moretti M, Freed LE. Tissue Eng. 2007;13:2709–19. doi: 10.1089/ten.2006.0414. [DOI] [PubMed] [Google Scholar]
- [29].Koblizek TI, Weiss C, Yancopoulos GD, Deutsch U, Risau W. Curr Biol. 1998;8:529–32. doi: 10.1016/s0960-9822(98)70205-2. [DOI] [PubMed] [Google Scholar]
- [30].Zhou L, Ma W, Yang Z, Zhang F, Lu L, Ding Z, Ding B, Ha T, Gao X, Li C. Gene Ther. 2005;12:196–202. doi: 10.1038/sj.gt.3302416. [DOI] [PubMed] [Google Scholar]
- [31].Kim SH, Kiick KL. Peptides. 2007;28:2125–36. doi: 10.1016/j.peptides.2007.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Nature. 1996;380:435–9. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- [33].Wang D, Lehman RE, Donner DB, Matli MR, Warren RS, Welton ML. Am J Physiol Gastrointest Liver Physiol. 2002;282:G1088–96. doi: 10.1152/ajpgi.00250.2001. [DOI] [PubMed] [Google Scholar]
- [34].Cross MJ, Dixelius J, Matsumoto T, Claesson-Welsh L. Trends Biochem Sci. 2003;28:488–94. doi: 10.1016/S0968-0004(03)00193-2. [DOI] [PubMed] [Google Scholar]
- [35].Ferrara N, Gerber HP, LeCouter J. Nat Med. 2003;9:669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- [36].Weis SM, Cheresh DA. Nature. 2005;437:497–504. doi: 10.1038/nature03987. [DOI] [PubMed] [Google Scholar]
- [37].Zacchigna S, Tasciotti E, Kusmic C, Arsic N, Sorace O, Marini C, Marzullo P, Pardini S, Petroni D, Pattarini L, Moimas S, Giacca M, Sambuceti G. Hum Gene Ther. 2007;18:515–24. doi: 10.1089/hum.2006.162. [DOI] [PubMed] [Google Scholar]
- [38].Morisada T, Kubota Y, Urano T, Suda T, Oike Y. Endothelium. 2006;13:71–9. doi: 10.1080/10623320600697989. [DOI] [PubMed] [Google Scholar]
- [39].Carlson TR, Feng Y, Maisonpierre PC, Mrksich M, Morla AO. J Biol Chem. 2001;276:26516–25. doi: 10.1074/jbc.M100282200. [DOI] [PubMed] [Google Scholar]
- [40].Saharinen P, Kerkela K, Ekman N, Marron M, Brindle N, Lee GM, Augustin H, Koh GY, Alitalo K. J Cell Biol. 2005;169:239–43. doi: 10.1083/jcb.200411105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Jones N, Chen SH, Sturk C, Master Z, Tran J, Kerbel RS, Dumont DJ. Mol Cell Biol. 2003;23:2658–68. doi: 10.1128/MCB.23.8.2658-2668.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Bogdanovic E, Nguyen VP, Dumont DJ. J Cell Sci. 2006;119:3551–60. doi: 10.1242/jcs.03077. [DOI] [PubMed] [Google Scholar]
- [43].Kwak HJ, Lee SJ, Lee YH, Ryu CH, Koh KN, Choi HY, Koh GY. Circulation. 2000;101:2317–24. doi: 10.1161/01.cir.101.19.2317. [DOI] [PubMed] [Google Scholar]
- [44].Saito M, Hamasaki M, Shibuya M. Cancer Sci. 2003;94:782–90. doi: 10.1111/j.1349-7006.2003.tb01519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Jho D, Mehta D, Ahmmed G, Gao XP, Tiruppathi C, Broman M, Malik AB. Circ Res. 2005;96:1282–90. doi: 10.1161/01.RES.0000171894.03801.03. [DOI] [PubMed] [Google Scholar]
- [46].Thurston G, Suri C, Smith K, McClain J, Sato TN, Yancopoulos GD, McDonald DM. Science. 1999;286:2511–4. doi: 10.1126/science.286.5449.2511. [DOI] [PubMed] [Google Scholar]
- [47].Wang Y, Pampou S, Fujikawa K, Varticovski L. J Cell Physiol. 2004;198:53–61. doi: 10.1002/jcp.10386. [DOI] [PubMed] [Google Scholar]
- [48].Gavard J, Patel V, Gutkind JS. Dev Cell. 2008;14:25–36. doi: 10.1016/j.devcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- [49].Metheny-Barlow LJ, Tian S, Hayes AJ, Li LY. Microvasc Res. 2004;68:221–30. doi: 10.1016/j.mvr.2004.08.005. [DOI] [PubMed] [Google Scholar]
- [50].DeBusk LM, Hallahan DE, Lin PC. Experimental Cell Research. 2004;298:167–77. doi: 10.1016/j.yexcr.2004.04.013. [DOI] [PubMed] [Google Scholar]
- [51].Callegari A, Bollini S, Iop L, Chiavegato A, Torregrossa G, Pozzobon M, Gerosa G, De Coppi P, Elvassore N, Sartore S. Biomaterials. 2007;28:5449–61. doi: 10.1016/j.biomaterials.2007.07.022. [DOI] [PubMed] [Google Scholar]
- [52].Zimmermann WH, Didie M, Doker S, Melnychenko I, Naito H, Rogge C, Tiburcy M, Eschenhagen T. Cardiovasc Res. 2006 doi: 10.1016/j.cardiores.2006.03.023. [DOI] [PubMed] [Google Scholar]
- [53].Schroeder JW, Jr., Rastatter JC, Walner DL. Otolaryngol Head Neck Surg. 2007;137:465–70. doi: 10.1016/j.otohns.2007.04.027. [DOI] [PubMed] [Google Scholar]
- [54].Zimmermann WH, Schneiderbanger K, Schubert P, Didie M, Munzel F, Heubach JF, Kostin S, Nehuber WL, Eschenhagen T. Circulation Research. 2002;90:223–230. doi: 10.1161/hh0202.103644. [DOI] [PubMed] [Google Scholar]
- [55].Zimmermann WH, Melnychenko I, Wasmeier G, Didie M, Naito H, Nixdorff U, Hess A, Budinsky L, Brune K, Michaelis B, Dhein S, Schwoerer A, Ehmke H, Eschenhagen T. Nat Med. 2006;12:452–458. doi: 10.1038/nm1394. [DOI] [PubMed] [Google Scholar]
- [56].Whitaker MJ, Quirk RA, Howdle SM, Shakesheff KM. J Pharm Pharmacol. 2001;53:1427–37. doi: 10.1211/0022357011777963. [DOI] [PubMed] [Google Scholar]
- [57].Hosack LW, Firpo MA, Scott JA, Prestwich GD, Peattie RA. Biomaterials. 2008;29:2336–47. doi: 10.1016/j.biomaterials.2008.01.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Balsam LB, Wagers AJ, Christensen JL, Kofidis T, Weissman IL, Robbins RC. Nature. 2004;428:668–73. doi: 10.1038/nature02460. [DOI] [PubMed] [Google Scholar]
- [59].Kofidis T, Lebl DR, Martinez EC, Hoyt G, Tanaka M, Robbins RC. Circulation. 2005;112:I173–7. doi: 10.1161/CIRCULATIONAHA.104.526178. [DOI] [PubMed] [Google Scholar]
- [60].Christman KL, Fok HH, Sievers RE, Fang Q, Lee RJ. Tissue Eng. 2004;10:403–9. doi: 10.1089/107632704323061762. [DOI] [PubMed] [Google Scholar]
- [61].Christman KL, Vardanian AJ, Fang Q, Sievers RE, Fok HH, Lee RJ. J Am Coll Cardiol. 2004;44:654–60. doi: 10.1016/j.jacc.2004.04.040. [DOI] [PubMed] [Google Scholar]
- [62].Ryu JH, Kim IK, Cho SW, Cho MC, Hwang KK, Piao H, Piao S, Lim SH, Hong YS, Choi CY, Yoo KJ, Kim BS. Biomaterials. 2005;26:319–26. doi: 10.1016/j.biomaterials.2004.02.058. [DOI] [PubMed] [Google Scholar]
- [63].Landa N, Miller L, Feinberg MS, Holbova R, Shachar M, Freeman I, Cohen S, Leor J. Circulation. 2008;117:1388–96. doi: 10.1161/CIRCULATIONAHA.107.727420. [DOI] [PubMed] [Google Scholar]
- [64].Davis ME, Motion JP, Narmoneva DA, Takahashi T, Hakuno D, Kamm RD, Zhang S, Lee RT. Circulation. 2005;111:442–50. doi: 10.1161/01.CIR.0000153847.47301.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Davis ME, Hsieh PC, Takahashi T, Song Q, Zhang S, Kamm RD, Grodzinsky AJ, Anversa P, Lee RT. Proc Natl Acad Sci U S A. 2006;103:8155–60. doi: 10.1073/pnas.0602877103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Laflamme MA, Chen KY, Naumova AV, Muskheli V, Fugate JA, Dupras SK, Reinecke H, Xu C, Hassanipour M, Police S, O’Sullivan C, Collins L, Chen Y, Minami E, Gill EA, Ueno S, Yuan C, Gold J, Murry CE. Nat Biotechnol. 2007;25:1015–24. doi: 10.1038/nbt1327. [DOI] [PubMed] [Google Scholar]
- [67].Rask F, Dallabrida SM, Ismail NS, Amoozgar Z, Yeo Y, Rupnick MA, Radisic M. J Biomed Mater Res A. 2010 doi: 10.1002/jbm.a.32808. [DOI] [PubMed] [Google Scholar]
- [68].Fujimoto KL, Ma Z, Nelson DM, Hashizume R, Guan J, Tobita K, Wagner WR. Biomaterials. 2009;30:4357–68. doi: 10.1016/j.biomaterials.2009.04.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Dobner S, Bezuidenhout D, Govender P, Zilla P, Davies N. J Card Fail. 2009;15:629–36. doi: 10.1016/j.cardfail.2009.03.003. [DOI] [PubMed] [Google Scholar]
- [70].Leor J, Tuvia S, Guetta V, Manczur F, Castel D, Willenz U, Petnehazy O, Landa N, Feinberg MS, Konen E, Goitein O, Tsur-Gang O, Shaul M, Klapper L, Cohen S. J Am Coll Cardiol. 2009;54:1014–23. doi: 10.1016/j.jacc.2009.06.010. [DOI] [PubMed] [Google Scholar]
- [71].Wall ST, Walker JC, Healy KE, Ratcliffe MB, Guccione JM. Circulation. 2006;114:2627–35. doi: 10.1161/CIRCULATIONAHA.106.657270. [DOI] [PubMed] [Google Scholar]
- [72].Lee KW, Yoon JJ, Lee JH, Kim SY, Jung HJ, Kim SJ, Joh JW, Lee HH, Lee DS, Lee SK. Transplant Proc. 2004;36:2464–5. doi: 10.1016/j.transproceed.2004.08.078. [DOI] [PubMed] [Google Scholar]
- [73].Steffens GC, Yao C, Prevel P, Markowicz M, Schenck P, Noah EM, Pallua N. Tissue Eng. 2004;10:1502–9. doi: 10.1089/ten.2004.10.1502. [DOI] [PubMed] [Google Scholar]
- [74].Jay SM, Saltzman WM. J Control Release. 2009;134:26–34. doi: 10.1016/j.jconrel.2008.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Rocha FG, Sundback CA, Krebs NJ, Leach JK, Mooney DJ, Ashley SW, Vacanti JP, Whang EE. Biomaterials. 2008;29:2884–90. doi: 10.1016/j.biomaterials.2008.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Richardson TP, Peters MC, Ennett AB, Mooney DJ. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- [77].Hao X, Silva EA, Mansson-Broberg A, Grinnemo KH, Siddiqui AJ, Dellgren G, Wardell E, Brodin LA, Mooney DJ, Sylven C. Cardiovasc Res. 2007;75:178–85. doi: 10.1016/j.cardiores.2007.03.028. [DOI] [PubMed] [Google Scholar]
- [78].Ito Y, Chen G, Imanishi Y. Bioconjug Chem. 1998;9:277–82. doi: 10.1021/bc970190b. [DOI] [PubMed] [Google Scholar]
- [79].Taguchi T, Kishida A, Akashi M, Maruyama I. Journal of Bioactive andCompatible Polymers. 2000;15:309–320. [Google Scholar]
- [80].Ito Y, Hasuda H, Terai H, Kitajima T. J Biomed Mater Res A. 2005;74:659–65. doi: 10.1002/jbm.a.30360. [DOI] [PubMed] [Google Scholar]
- [81].Backer MV, Patel V, Jehning BT, Claffey KP, Backer JM. Biomaterials. 2006;27:5452–8. doi: 10.1016/j.biomaterials.2006.06.025. [DOI] [PubMed] [Google Scholar]
- [82].Koch S, Yao C, Grieb G, Prevel P, Noah EM, Steffens GC. J Mater Sci Mater Med. 2006;17:735–41. doi: 10.1007/s10856-006-9684-x. [DOI] [PubMed] [Google Scholar]
- [83].Takashi H, Katsumi M, Toshihiro A. Biochem Biophys Res Commun. 2007;359:151–6. doi: 10.1016/j.bbrc.2007.05.079. [DOI] [PubMed] [Google Scholar]
- [84].Shen YH, Shoichet MS, Radisic M. Acta Biomater. 2008;4:477–89. doi: 10.1016/j.actbio.2007.12.011. [DOI] [PubMed] [Google Scholar]
- [85].Ito Y. Soft Matter. 2008;4:46–56. doi: 10.1039/b708359a. [DOI] [PubMed] [Google Scholar]
- [86].Chiu LLY, Weisel RD, Li R-K, Radisic M. Journal of Tissue Engineering and Regenerative Medicine. doi: 10.1002/term.292. in press. [DOI] [PubMed] [Google Scholar]
- [87].Radisic M, Yang L, Boublik J, Cohen RJ, Langer R, Freed LE, Vunjak-Novakovic G. Am J Physiol Heart Circ Physiol. 2004;286:H507–16. doi: 10.1152/ajpheart.00171.2003. [DOI] [PubMed] [Google Scholar]
- [88].Park H, Radisic M, Lim JO, Chang BH, Vunjak-Novakovic G. In Vitro Cell Dev Biol Anim. 2005;41:188–96. doi: 10.1290/0411071.1. [DOI] [PubMed] [Google Scholar]
- [89].Radisic M, Fast VG, Sharifov OF, Iyer RK, Park H, Vunjak-Novakovic G. Tissue Eng Part A. 2009;15:851–60. doi: 10.1089/ten.tea.2008.0223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Grabarek Z, Gergely J. Anal Biochem. 1990;185:131–5. doi: 10.1016/0003-2697(90)90267-d. [DOI] [PubMed] [Google Scholar]
- [91].Oates M, Chen R, Duncan M, Hunt JA. Biomaterials. 2007;28:3679–86. doi: 10.1016/j.biomaterials.2007.04.042. [DOI] [PubMed] [Google Scholar]
- [92].Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, Yura H, Morimoto Y, Takase B, Matsui T, Kikuchi M, Maehara T. Biomaterials. 2004;25:699–706. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- [93].Chiang CK, Chowdhury MF, Iyer RK, Stanford WL, Radisic M. Acta Biomater. 6:1904–16. doi: 10.1016/j.actbio.2009.12.005. [DOI] [PubMed] [Google Scholar]