Abstract
The existence of a hydrodynamically relevant endothelial glycocalyx has been established in capillaries, venules, and arterioles in vivo. The glycocalyx is thought to consist primarily of membrane-bound proteoglycans with glycosaminoglycan side-chains, membrane-bound glypicans, and adsorbed plasma proteins. The proteoglycans found on the luminal surface of endothelial cells are syndecans-1, -2, and -4, and glypican-1. The extent to which any of these proteins might serve to anchor the glycocalyx to the endothelium has not yet been determined. To test whether syndecan-1, in particular, is an essential anchoring protein, we performed experiments to determine the hydrodynamically relevant glycocalyx thickness in syndecan-1 deficient (Sdc1−/−) mice. Micro-particle image velocimetry data were collected using a previously described method. Microviscometric analysis of these data consistently revealed the existence of a hydrodynamically relevant endothelial glycocalyx in Sdc1−/− mice in vivo. The mean glycocalyx thickness found in Sdc1−/− mice was 0.45±0.10 μm (N=15), as compared with 0.54±0.12 μm (N=11) in wild-type (WT) mice (p=0.03). The slightly thinner glycocalyx observed in Sdc1−/− mice relative to WT mice may be due to the absence of syndecan-1. These findings show that healthy Sdc1−/− mice are able to synthesize and maintain a hydrodynamically relevant glycocalyx, which indicates that syndecan-1 is not an essential anchoring protein for the glycocalyx in Sdc1−/− mice. This may also be the case for WT mice; however, Sdc1−/− mice might adapt to the lack of syndecan-1 by increasing the expression of other proteoglycans. In any case, syndecan-1 does not appear to be a prerequisite for the existence of an endothelial glycocalyx.
Keywords: Glycocalyx, Endothelial surface layer, Endothelium, Syndecan-1, Syndecan, Proteoglycan, Intravital microscopy, Microvascular, Hemodynamics
Introduction
Much recent research has focused on the structure, composition, function, and clinical significance of the endothelial glycocalyx, a layer of membrane-bound macromolecules, ~0.5 μm in thickness, on the luminal surface of vascular endothelium. It has been implicated as a determinant in many physiological processes, including permeability, mechanotransduction, and inflammation (Henry and Duling, 1999; Mulivor and Lipowsky 2004; Smith et al., 2003; Potter et al., 2009). There is also evidence that the glycocalyx plays a significant role in various pathologies, such as atherosclerosis, vascular disease associated with diabetes, ischemia-reperfusion injury, and sepsis (van den Berg et al., 2006; Nieuwdorp et al., 2006; Platts et al., 2003; Chappell et al., 2009a; Hofmann-Kiefer et al., 2009). A wide variety of methods, in vivo, ex vivo, and in vitro, have been employed to investigate the structure, composition, and function of the glycocalyx (Vink and Duling, 1996; Smith et al., 2003; Damiano et al., 2004b; van den Berg et al., 2003, Jacob et al., 2009; Chappell et al., 2009b), but much remains unknown.
The basic structure that has been proposed for the glycocalyx by Pries et al. (2000) consists of a thin (<100 nm) layer of heparan sulfate proteoglycans (HSPGs), glycosaminoglycans (GAGs), and glycoproteins at the surface of the vascular endothelium. The remainder of the glycocalyx, ~400–500 nm in thickness, is made up of molecules adsorbed from the plasma; this portion of the layer is thought to be in dynamic equilibrium with the flowing plasma (Pries et al., 2000). Of the molecules found at the endothelial cell (EC) surface, HSPGs are thought to be the key components that anchor the glycocalyx to the cell (Pries et al., 2000; Weinbaum et al., 2007). HSPGs consist of a core protein with covalently attached GAG side-chains; those found on the apical surface of ECs are the transmembrane syndecans-1, -2, and -4, and membrane-bound glypican-1 (Rosenberg et al., 1997; Weinbaum et al., 2007). The extent to which any or all of these proteins might serve to anchor the glycocalyx to the endothelium has not yet been determined.
A variety of methods have been employed to investigate the glycocalyx, each of which has advantages and limitations. The first visualization of the glycocalyx was accomplished ex vivo, using electron microscopy (EM) (Luft, 1966). EM can provide information on glycocalyx charge, composition, and structure, but results vary greatly according to the fixation and staining methods employed. One of the main limitations of EM is the potential for damage to the glycocalyx, which can occur during preparation of the tissue. This damage may include the washing away of adsorbed plasma components or the removal of GAG side-chains from HSPGs, either of which could result in collapse of the glycocalyx structure (Pries et al., 2000; Weinbaum et al., 2007). There is also the possibility that these imaging studies can produce significant dehydration artifacts or crystallization artifacts resulting from the aqueous fixation techniques that are typically employed (Weinbaum et al., 2007). Until recently, EM images of the glycocalyx generally revealed a layer <0.1 μm in thickness, much thinner than the ~0.5-μm-thick layer observed in vivo. Perfusion fixation protocols that do not include post-fixation alcohol dehydration of the sample appear to preserve a larger portion of the glycocalyx (Van den Berg et al., 2003; Rehm et al., 2004), although the presence of fixation artifacts still cannot be entirely ruled out. Van den Berg et al. (2003) observed a glycocalyx that was ~0.2–0.5 μm in thickness in segments of capillaries from rat hearts that were perfusion fixed and stained with Alcian blue. Rehm et al. (2004) and Jacob et al. (2007) found a glycocalyx that was ~0.3 μm in thickness in capillaries of guinea pig hearts that were perfusion fixed and stained with Lanthanum.
Two methods have been developed to visualize the glycocalyx in vivo. The first was the dye-exclusion method developed by Vink and Duling (1996). This method, which can be applied in microvessels up to ~15 μm in diameter (Henry and Duling, 1999), was the first to show the full extent of the glycocalyx in vivo, revealing a ~0.5 μm layer in capillaries. The microviscometric method, developed by Damiano and coworkers to analyze intravital micro-particle image velocimetry (μ-PIV) data, has been used in vivo to interrogate the endothelial glycocalyx in venules and arterioles ~20–70 μm in diameter (Smith et al., 2003; Long et al., 2004; Damiano et al., 2004b; Savery and Damiano, 2008). It has also been employed in vitro in the study of ECs cultured in cylindrical collagen micro-channels (Potter and Damiano, 2008; Potter et al., 2009). This method uses intravital microscopy, combined with a detailed hemodynamic analysis, to estimate the hydrodynamically relevant thickness of the endothelial glycocalyx, where the hydrodynamically relevant thickness is defined as the thickness of the layer that significantly retards the flow of plasma (Smith et al., 2003; Long et al., 2004; Damiano et al., 2004b).
Many studies have attempted to elucidate the structure and composition of the glycocalyx using in vitro cell-culture models, which has led to some controversy regarding the status of the glycocalyx in vitro. While cultured ECs do produce what are thought to be glycocalyx components (Rosenberg et al., 1997; Pries et al., 2000), it has also been shown that, when cultured under standard conditions, HUVECs do not possess a glycocalyx that is structurally and functionally equivalent to that found in vivo (Potter and Damiano, 2008; Chappell et al., 2009; Jacob et al., 2007). In a study by Chappell et al. (2009), staining revealed the presence of heparan sulfate and syndecan-1 on the surface of both cultured HUVECs and on ex vivo human umbilical vein endothelium. Despite the presence of heparan sulfate and syndecan-1, EM revealed a glycocalyx thickness in vitro of only ~0.03 μm compared with a nearly 0.9-μm thick layer ex vivo. Thus, the mere presence of these molecules at the cell surface in vitro does not imply the existence of a glycocalyx structure comparable to that found in vivo. Potter and Damiano (2008) used μ-PIV to examine HUVECs cultured in collagen microchannels and also reported a mean hydrodynamically relevant glycocalyx thickness of ~0.03 μm, as compared with ~0.5 μm found in vivo in mouse cremaster-muscle venules (Damiano et al., 2004b; Long et al., 2004; Potter and Damiano, 2008).
Immunofluorescence staining and confocal microscopy have also been used to study the glycocalyx in vitro (Thi et al., 2004; Stevens et al., 2007). Thi et al. (2004) studied rat fat pad ECs and showed the presence of HSPGs extending from the cell surface. In a study of bovine lung microvascular ECs, Stevens et al. (2007) found heparan sulfate and hyaluronan extending up to 3 μm from the cell surface. A study by Barker et al. (2004) reported the glycocalyx on HUVECs to have a thickness of up to 2.5 μm, while Megens et al. (2007) reported a glycocalyx in isolated mouse arterial vessel preparations extending as far as 4.5 μm from the vessel wall. Chappell et al. (2009) attributed the large differences in glycocalyx dimension reported in the literature to the possibility that rarified fibrillar structures of the glycocalyx would be able to extend much further from the cell surface in quiescent media than it would under the shear rates that were present in their studies and in those of Potter and Damiano (2008). One notable difference between some of these studies (Thi et al., 2004; Stevens et al., 2007; Megens et al., 2007) and those of Chappell et al. and Potter and Damiano (2008) is the cell type used. While it is possible that glycocalyx composition and structure might vary between cell types, it is not difficult to conceive of an alternative explanation for how the discrepancy between the results of Potter and Damiano (2008) and those studies involving immunostaining might be resolved. The explanation lies in the fact that the μ-PIV method observes the effects of only the hydrodynamically relevant glycocalyx, while immunostaining methods reveal nothing about hydrodynamics. A result in which immunostaining shows the presence of glycocalyx components at the EC surface, while at the same time μ-PIV indicates the absence of a hydrodynamically relevant glycocalyx, could arise if the glycocalyx components had not assembled into a structure that is equivalent to that found in vivo. Therefore, whatever EC-surface molecules that might be present in culture would not change the fact that the absence of a hydrodynamically relevant layer in vitro cannot be assumed to exhibit many of the important functional roles that the glycocalyx is known to have under in vivo conditions (and many of which may be fundamentally dependent upon the hydrodynamical properties of the native glycocalyx in vivo). Until a cell-culture model is developed in which ECs in vitro are demonstrated to possess a glycocalyx that is equivalent to that found in vivo, the utility of these methods in investigating the structure, composition, and function of the glycocalyx is limited.
In order to gain insight into the biochemical composition and structure of the endothelial glycocalyx, we propose using the established in vivo methods to interrogate the glycocalyx in animals deficient in one or more macromolecules thought to comprise the glycocalyx. Syndecan-1 is the major syndecan found on vascular ECs, with syndecans-2 and -4 being expressed at lower levels (Alexopoulou et al., 2006). There is evidence that degradation of the glycocalyx, under certain conditions, may be associated with increased levels of syndecan-1 in the circulation (Bruegger et al., 2005; Chappell et al., 2007; Rehm et al., 2007), and several recent studies have used syndecan-1 as a marker for glycocalyx degradation in humans (Rehm et al., 2007; Steppan et al., 2011; Johansson et al., 2011). To test whether syndecan-1, in particular, is an essential anchoring protein for the endothelial glycocalyx, experiments were performed to estimate the hydrodynamically relevant glycocalyx thickness in syndecan-1 deficient (Sdc1−/−) mice before and after treatment with hyaluronidase to degrade the glycocalyx. These results were then compared with results from similar experiments performed in WT mice.
Methods
To investigate whether mice lacking syndecan-1 possess a hydrodynamically relevant glycocalyx, we collected and analyzed μ-PIV data in cremaster muscle venules of Sdc1−/− mice and compared these data with those obtained in WT mice. Data were collected before and after treatment with hyaluronidase, an enzyme that is known to degrade the glycocalyx in vivo (Henry et al., 1999; Potter et al., 2009). Microviscometric analysis was applied to each monotonically filtered subset of data to determine the velocity profile and corresponding estimated hydrodynamically relevant glycocalyx thickness that resulted in the lowest least squares error in the fit to the data (Damiano et al., 2004b; Long et al., 2004). This velocity profile was also used to calculate hemodynamic parameters, including the viscosity, shear-stress, and shear-rate distributions.
The use of hyaluronidase was not intended to specifically target syndecan-1 or to gain any additional information regarding glycocalyx composition, but rather to serve as a negative control by demonstrating that the microviscometric method does not detect a hydrodynically relevant glycocalyx when none is present. We have previously shown that no statistically significant difference was observed in the hydrodynamically relevant glycocalyx thickness after treatment with hyaluronidase, heparinase, or TNF-α, as all of these agents effectively abolish the hydrodynamically relevant layer (Potter et al., 2009). Other studies have shown that a variety of enzymes, including hyaluronidase, heparinase, chondroitinase, and neurminidase, are effective in degrading the glycocalyx in healthy WT mice (Desjardins and Duling, 1990; Vink and Duling, 1996; Pries et al., 1997; Henry et al., 1999; Smith et al., 2003; Damiano et al., 2004b). For consistency with our previous studies, we elected hyaluronidase as the degrading enzyme to be used here.
Animals
All animal experiments were conducted under a protocol approved by the Boston University Institutional Animal Care and Use Committee (protocol numbers 05-041 and 08-031). Experiments were performed in WT and Sdc1−/− male mice that were at least 8 weeks old and weighed 20–30 grams. WT C57BL/6 mice were obtained from Charles River labs (Wilmington, MA) or Jackson Labs (Bar Harbor, ME). Sdc1−/− mice were backcrossed 10 times onto the C57BL/6 background. The generation of the Sdc1−/− strain has been described by Alexander et al. (2000) and Park et al. (2001). Under normal laboratory conditions, Sdc1−/− mice develop normally and are fertile. They exhibit no decrease in viability and there are no apparent differences in routine serum chemistry, hematology, or tissue histology relative to WT mice under unchallenged conditions (Alexander et al., 2000; Park et al., 2001; Stepp et al., 2002; Hayashida et al., 2008)
Intravital Microscopy and μ-PIV
Mice were anesthetized with isofluorane and a jugular or carotid cannula was inserted for infusion of experimental reagents (Savery and Damiano, 2008; Potter et al., 2009). The cremaster muscle was prepared for intravital microscopy as described previously (Baez, 1973; Ley et al., 1995; Smith et al., 2003). The muscle was exteriorized, opened with a longitudinal incision, and spread over a stage, allowing for illumination from below. Throughout the experiment, the cremaster muscle was continuously superfused by a steady drip of thermo-controlled, sodium bicarbonate-buffered physiological salt solution. If necessary, 10μmol/l succinylcholine was added to the superfusion solution to reduce spontaneous skeletal muscle contractions; the superfusion of the cremaster muscle was local, and only the cremaster muscle preparation itself was affected by the paralytic agent.
Intravital microscopy and μ-PIV were performed using a variation on a previously described method (Smith et al., 2003; Damiano et al., 2004b; Long et al., 2004; Savery and Damiano, 2008). The electrocardiogram of the mouse was monitored throughout each experiment, and the experiment was terminated if the heart rate dropped below 250 beats per minute. Intravital microscopic observations were made on a Zeiss microscope (Axioskop II, Carl Zeiss, Inc.) with a 63× saline immersion objective (NA 1.0), focused on the midsaggital plane of the vessel. This was defined as the focal plane where the contrast of the edge of the intraluminal wall reversed (Gretz and Duling, 1995). Data was collected from segments of venules 20–65 μm in diameter that allowed for clear focus on the vessel wall, had consistent flow, and were located away from sources of flow disturbances or irregularities, such as sharp curves, bifurcations, or adherent leukocytes. Administration of a P-selectin antibody (20–40 μL, BD Biosciences, Inc.) helped systemically reduce leukocyte rolling and adhesion.
Neutrally buoyant fluoresbrite YG microspheres (0.538±0.01 μm, ρ=1.05 g/cm3) or Polychromatic red microspheres (0.513±0.015 μm, ρ = 1.05 g/cm3, Polysciences, Inc.,) were suspended in saline and a small volume (<0.05 ml) of this solution was slowly injected through the jugular or carotid cannula until 10-20 microspheres per second passed through the microvessel. A Dualview beam splitter (Optical Insights, LLC) separated infrared transillumination from the fluorescent microsphere images, allowing for simultaneous acquisition of the vessel wall and microspheres. Stroboscopic double-flash (4–9 ms apart, DPS-1 Video, Rapp OptoElectronic GmbH) epi-illumiation resulted in a dual image of each fluorescent microsphere, where the flash-time interval chosen such that the two exposures of a single microsphere were captured on the same digital image and appeared ~3–10 μm apart. Recordings were made with a CCD camera (Sensicam QE, Cooke Corp.) and a minimum of 300 images were captured from each vessel and processed with IP Lab/iVision-Mac software (BioVision Technologies Inc.).
Images were analyzed with the public domain software ImageJ (http://rsbweb.nih.gov/ij/), as previously described (Smith et al., 2003; Damiano et al., 2004b). The brightfield and fluorescent images were merged, and the brightness and contrast were adjusted so that both the vessel wall and the doubly exposed microspheres were clearly visible in the combined images. When the focal plane corresponds to the midsagittal plane, contrast at the intraluminal vessel wall reverses and the vessel wall is visible as dark band (Gretz and Duling, 1995). The endothelial surface was defined as the luminal edge of this band and the vessel diameter was taken to be the distance between the luminal edges of the dark bands on either side of the vessel (Smith et al., 2003). The radial location and velocity were measured for a least 50 microspheres in each microvessel. Since the data were collected in vessel segments away from flow disturbances, it can be shown that the velocity profile is axisymmetric, and each data set was folded onto to one-half of the vessel cross-section. Since not all of the recorded microspheres travelled in the midsaggital plane of the vessel, and only those that did were relevant to our analysis, a previously described monotonic filter (Smith et al., 2003; Damiano et al., 2004b) was then applied to each data set. A microsphere in the midsagittal plane travels faster than any other microsphere at that same measured radial location, therefore, only the fastest beads at a given radial location were retained for further analysis.
Microviscometric Analysis
Microviscometry provides a precise estimate of the hydrodynamic drag of sub-micron particle tracers that travel in the vessel lumen. This drag is greatest on particles near the vessel wall. A particle traveling at a fixed distance from the vessel wall in a vessel without a glycocalyx will experience less drag than it would at the same distance from that vessel wall if a hydrodynamically relevant glycocalyx were present. This is due to the enhanced hydrodynamic drag of plasma flowing within the glycocalyx that is exerting its influence on the fluid flow field within the vessel lumen outside of the glycocalyx. We emphasize that the particle tracers are deliberately chosen to be too large to invade the fiber matrix of the glycocalyx. Rather than attempt to directly visualize the flow field within the glycocalyx, we instead analyze the flow field within the vessel lumen, and use that information to interrogate the hydrodynamic properties of the glycocalyx. The analysis of μ-PIV data and the associated particle tracer motion, which we refer to collectively as microviscometric analysis, provides quantitative information about the hydrodynamically relevant glycocalyx thickness. The hydrodynamically relevant thickness that we have defined represents a functional dimension, corresponding to the distance from the endothelium where non-Newtonian hydrodynamic effects dominate. Borrowing upon the analogy made by Potter et al. (2009), “it is much like a viscous boundary layer region in fluid mechanics, and as such, represents a physically real and quantitative characterization of glycocalyx thickness in the literal sense.” As it also determines the physical extent of the non-circulating plasma layer bound up in the glycocalyx, it identifies the region over which the transport of small molecules is diffusion rather than convection limited. Furthermore, the hydrodynamically relevant thickness can be used as one metric for the overall hydrodynamic state of the glycocalyx, particularly in those instances where the absence of a hydrodynamically relevant layer is indicative of either damage, degradation, or some other structural deficiency (such as might arise in the absence of essential proteoglycan or glycoprotein structures).
Microviscometric analysis of the monotonically filtered μ-PIV data followed the methods of Damiano et al. (2004b). For each microsphere, the predicted velocity of a fluid particle at that radial location, in the absence of the microsphere, was calculated, using a three-dimensional analysis of fluid dynamics near the vessel wall (Damiano et al., 2004a). A fitting function for the velocity profile, consisting of a constant term, a quadratic term, and a growing and a decaying exponential term, was then used to perform a nonlinear regression analysis to determine the values of two constants that minimize the least-squares error in the fit to the fluid particle velocity data, for a given trial glycocalyx thickness. Further details regarding this regression analysis are given by Damiano et al. (2004b). Validation of microviscometric analysis in mouse cremaster muscle venules in vivo and in glass capillaries perfused with red cells suspended in plasma is provided by Long et al. (2004).
To estimate the hydrodynamically relevant glycocalyx thickness, the best-fit velocity profile to the data was calculated for a range of glycocalyx thicknesses. It is assumed that the microspheres do not invade the glycocalyx, and the maximum possible glycocalyx thickness is determined by the data point nearest to the vessel wall. The variation in the least-squares error, E, associated with the fit to the data was used to track the quality of the fit relative to a trial glycocalyx thickness. Defining a normalized least-squares error, E* = E/Emin, where Emin is the minimum value of E, our estimate of the velocity distribution and hydrodynamically relevant glycocalyx thickness for each vessel corresponded to the trial thickness associated with E*=1. The corresponding distributions in viscosity, μ(r), shear rate, , and shear stress, τ(r) were then calculated. In the analysis presented here, flow through the glycocalyx was assumed to be negligible (i.e. vanishingly small hydraulic permeability, which corresponds to a hydraulic resistivity, K → ∞). This results in a lower-bound estimate of the glycocalyx thickness, as a permeable layer would provide less resistance and therefore need to be thicker to provide the same observed hydrodynamic drag on the microspheres (Smith et al., 2003; Damiano et al., 2004b). In previous studies, Damiano and Stace (2002) estimated the hydraulic resistivity of the glycocalyx to be ~ 1010 – 1011 dyn-s/cm4, and Damiano et al. (2004b) found that, for K > 109 dyn-s/cm4, the glycocalyx thickness estimated by microviscometric analysis was insensitive to K.
The hydraulic resistivity, K, is dependent upon the hydrodynamic drag exerted by the macromolecular fiber matrix of the glycocalyx on the water being forced through the fiber matrix. The particle tracers that we use are intentionally chosen to be too large to penetrate the fiber matrix of the glycocalyx. However, the greater the hydrodynamic drag on the plasma being forced through the fiber matrix, the greater will be the drag on the plasma and passing microspheres in the lumen just outside the glycocalyx. In essence, the particle tracers themselves serve as hydrodynamic probes. Careful analysis of their motion just outside of the glycocalyx (Damiano et al., 2004a; Damiano et al., 2004b) reveals the extent to which fluid flow within the glycocalyx is retarded relative to what would be the case if the glycocalyx were absent. For a particular hydraulic permeability, then, an estimate of the hydrodynamically relevant glycocalyx thickness can be made (Damiano et al., 2004a; Damiano et al., 2004b; Long et al., 2004).
Leukocyte Adhesion
While collecting μ-PIV data, the number of adherent leukocytes in a venule was readily quantifiable. To quantify any difference that might exist between Sdc1−/− and WT mice, we counted the number of adherent leukocytes present in the mid-sagittal plane at the beginning of data collection for each vessel studied. A leukocyte was considered to be adherent if it remained firmly attached to the vessel wall, without rolling, for at least 1 minute after the start of data collection.
Statistical Analysis
Data are expressed as mean ± one standard deviation. All p-values were calculated using a two-tailed independent t-test.
Results
Estimated Glycocalyx Thickness
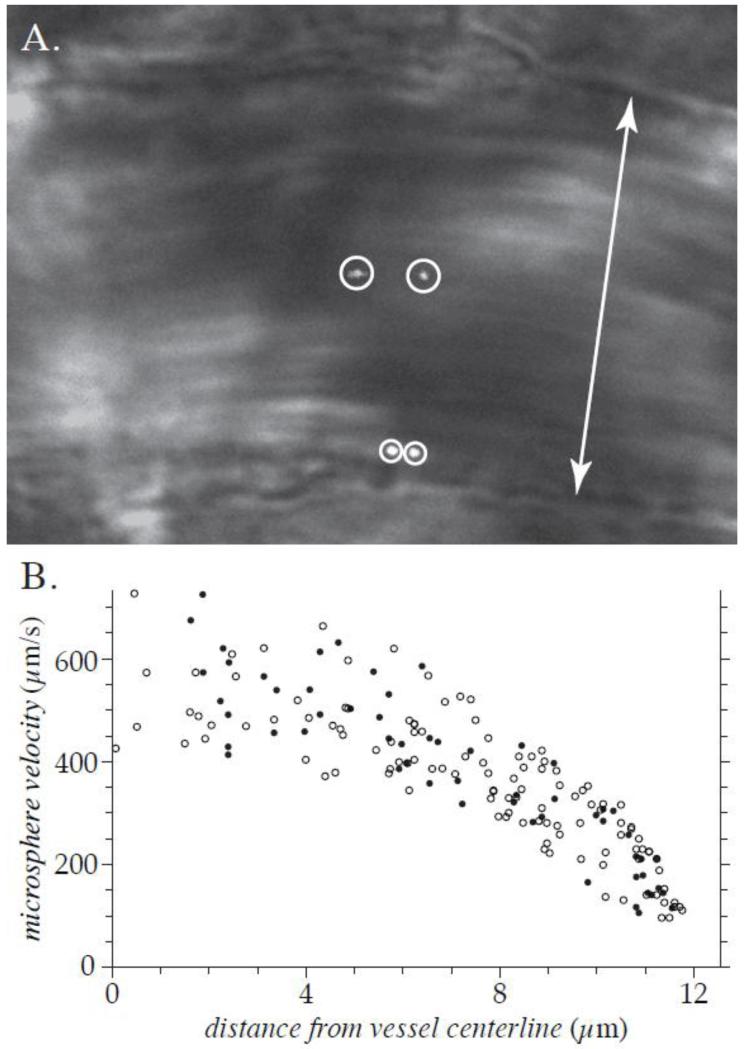
A typical image capture of a microvessel containing a particle tracer in the lumen is shown in Figure 1(A). The instantaneous bright-field image of the vessel has been merged with the corresponding simultaneously acquired fluorescent image of the microsphere so that both the vessel wall and the microsphere are visible. Dual exposures of two fluorescent microspheres can be seen in Figure 1(A). The dual images of each microsphere are separated in time by the double flash interval (9 ms for the data set shown). Note that the microsphere that is closer to the vessel wall has a lower velocity than the microsphere that is nearer to the vessel centerline. The full data set of all microsphere measurements made in this vessel is shown in Figure 1(B), where the velocity of each microsphere is plotted versus its position relative to the vessel centerline. Note that this data distribution is consistent with the assumption of an axisymmetric velocity profile. The monotonically filtered subset of data for this vessel is shown in Figure 2(A). All data sets were acquired and analyzed in this manner. Each data set analyzed contained measurements of at least 50 fluorescent microspheres distributed over the entire cross-section of the vessel.
Figure 1. Typical image and data obtained in vivo.
These results are from a 25.1-μm-diameter venule in the cremaster muscle of an Sdc1−/− mouse, prior to hyaluronidase treatment. Shown in (A) is the brightfield image of the vessel, merged with the simultaneously acquired dual images of two fluorescent microspheres (encircled). The double arrow indicates the diameter of the venule. The full μ-PIV data set for this venule, folded onto one-half of the vessel cross-section, is shown in (B). The closed circles indicate data points measured from the vessel wall that is nearer to the top of the image in (A) and open circles indicate data points measured from the opposite wall. The velocity and radial location were measured for 172 microspheres in this venule. The monotonically filtered subset of this data is shown in Figure 2(A).
Figure 2. Representative results of μ-PIV and microviscometric analysis in venules of Sdc1−/− mice in vivo.
Shown in the left column are results from a 25.1-μm-diameter venule before hyaluronidase treatment. In the right column are results from a 48.2-μm-diameter venule after hyaluronidase treatment to degrade the glycocalyx. Panels A and B show the monotonically filtered μ-PIV data (circles) and the fitted axisymmetric velocity profile (curve) corresponding to trial glycocalyx thickness that produced the smallest least-squares error of all admissible trial thicknesses. The shaded region near the vessel wall represents the glycocalyx. The corresponding relative blood viscosity (solid curve) and shear-rate (dashed curve) distributions are shown in panels C and D. Panels E and F show the variation in the normalized least-squares error associated with the fit to the μ-PIV data. The estimated glycocalyx thickness is 0.42 μm in the untreated venule and 0.0 μm in the hyaluronidase-treated venule.
Representative results from venules of Sdc1−/− mice, before and after hyaluronidase treatment, are shown in Figure 2. The monotonically filtered data and corresponding best-fit velocity profile are plotted, along with the resulting viscosity and shear-rate distributions. Also shown is the variation in the normalized least-squares error, E*, associated with the fit to the μ-PIV data plotted as a function of the trial glycocalyx thickness. The estimated glycocalyx thickness, R – a, corresponds to the trial glycocalyx thickness associated with the minimum in this curve. The estimated glycocalyx thickness is 0.42 μm in the venule not treated with hyaluronidase, whereas there is no detectable hydrodynamically relevant glycocalyx remaining in the hyaluronidase-treated venule. Representative results from WT mice are shown in Figure 3. In the WT venules shown, the hydrodynamically relevant glycocalyx thickness was found to be 0.50 μm in the control venule and 0.02 μm in the hyaluronidase treated venule.
Figure 3. Representative results of μ-PIV and microviscometric analysis in venules of WT mice in vivo, with the same interpretation as Figure 2.
Shown in the left column are results from a 43.7-μm-diameter venule before hyaluronidase treatment. In the right column are results from a 55.3-μm-diameter venule after hyaluronidase treatment to degrade the glycocalyx. The estimated hydrodynamically relevant glycocalyx thickness is 0.50 μm in the untreated venule and 0.02 μm in the hyaluronidase-treated venule.
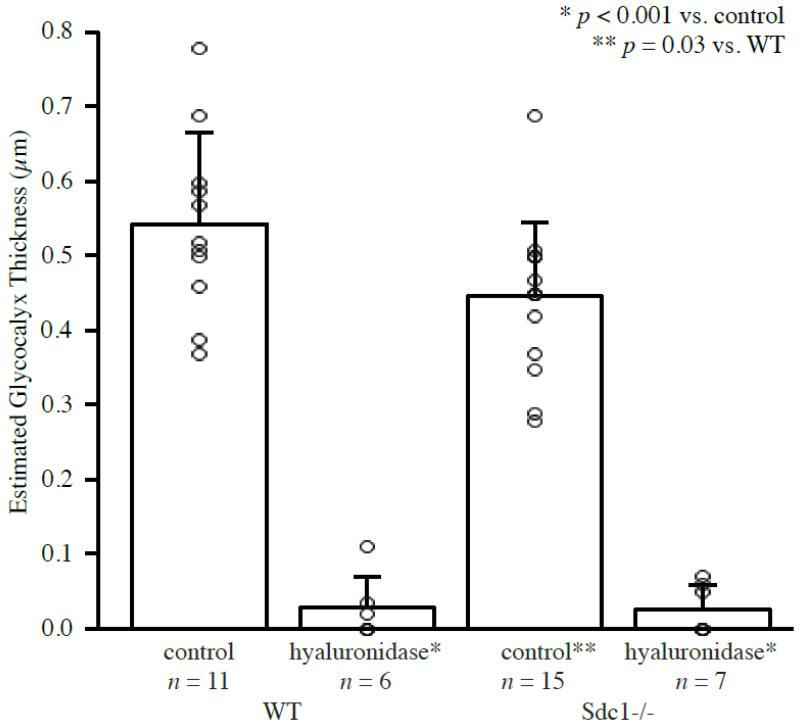
Microviscometric analysis of μ-PIV data collected from 15 venules in 6 Sdc1−/− mice revealed the existence of a hydrodynamically relevant glycocalyx with a mean thickness of 0.45±0.10 μm (0.28-0.69 μm). This is ~15% thinner than the mean glycocalyx thickness of 0.54±0.12 μm (0.37-0.78 μm) found in 11 venules of 7 WT mice (p=0.03). The mean glycocalyx thickness after hyaluronidase treatment was found to be 0.03±0.03 μm (0.00-0.07 μm) in 7 venules of 3 Sdc1−/− mice and 0.03±0.04 μm (0.00-0.11 μm) in 6 venules from 3 WT mice. These results are summarized in Figure 4. Mean vessel radius, glycocalyx thickness, and hemodynamic parameters for each group are summarized in Table 1. Note that while there is a large difference in the mean values of several hemodynamic parameters between the two Sdc1−/− groups, there is also a large standard deviation associated with each of these values and when they are compared p > 0.05.
Figure 4. Mean estimated glycocalyx thickness in venules of Sdc1−/− and WT mice before and after hyaluronidase treatment.
The bars show the mean and standard deviation for each group. The open circles indicate the estimated glycocalyx thickness for each individual vessel in the data set. In the untreated Sdc1−/− group, the mean glycocalyx thickness was found to be 0.45±0.10 μm (n=15 venules, N=6 mice). This is ~15% thinner than the 0.54±0.12 μm glycocalyx found in WT mice (n=11, N=7, p=0.03). After hyaluronidase treatment, the mean glycocalyx thickness was 0.03±0.03 μm in Sdc1−/− mice (n=7, N=3, p<0.001 vs. Sdc1−/− or WT mice before treatment) and 0.03±0.04 μm in WT mice (n=6, N=3, p<0.001 vs. Sdc1−/− or WT mice before treatment).
Table 1. Physical and hemodynamic parameters in Sdcl−/− and WT mice.
Data are presented as mean ± one SD, for Sdcl−/− and WT mice before and after hyaluronidase treatment. Tabulated are the vessel radius, R; estimated glycocalyx thickness, R-a; flow rate, Q; ratio of maximum to average flow velocity, Vmax/Vavg; shear-rate, , and shear stress, τ[a], magnitude at the blood-glycocalyx interface.
| WT | Sdc1−/− | |||
|---|---|---|---|---|
| before | after | before | after | |
| R (μm) | 20.2±7.4 | 19.3±7.4 | 16.6±4.1 | 16.4±4.3 |
| R-a (μm) | 0.54±0.12 | 0.03±0.04 | 0.45±0.10 | 0.03±0.03 |
| Vmax (103μm/s) | 2.67±2.06 | 2.53±2.06 | 3.08±1.97 | 1.52±0.66 |
| Q (106μm3/s) | 2.22±2.08 | 2.40±2.93 | 1.96±2.05 | 1.00±1.04 |
| Vmax/Vavg | 1.65±0.07 | 1.55±0.07 | 1.69±0.12 | 1.60±0.04 |
| 103s−1 | 1.64±1.63 | 1.25±0.73 | 1.44±1.15 | 0.54±0.20 |
| τ[a] (dyn/cm2) | 20.8±20.7 | 15.3±8.9 | 18.2±14.5 | 6.6±2.4 |
Leukocyte Adhesion
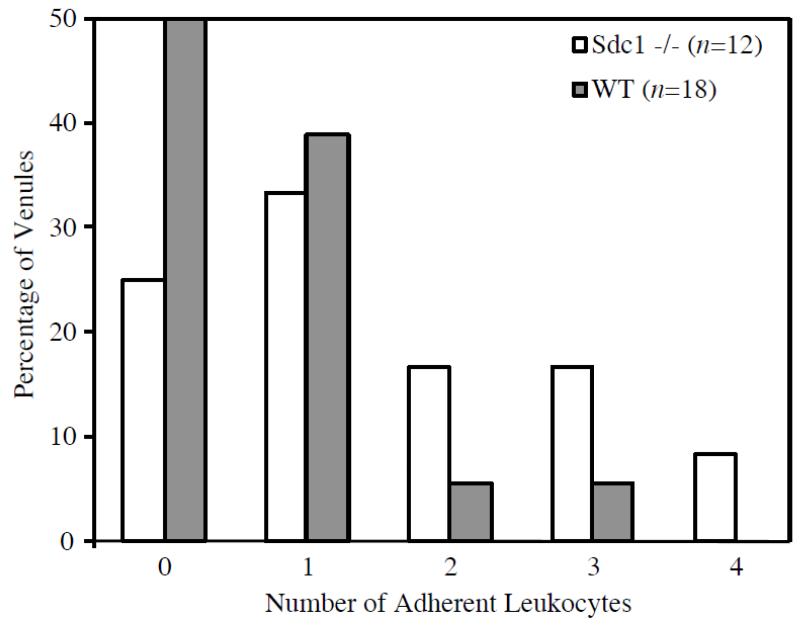
To assess whether the slightly thinner glycocalyx found in Sdc1−/− mice has functional significance, we compared the number of adherent leukocytes observed in vessel segments from Sdc1−/− and WT mice. The mean number of adherent leukocytes in WT mice was 0.8±1.0 (n=24, N=6), compared with 1.5±1.1 (n=25, N=13, p=0.02) found in Sdc1−/− mice. On average, data were collected from a greater number of venules per mouse in Sdc1−/− mice than in WT mice. Since the cremaster preparation tends to become more inflamed with increasing duration of the experiment, vessels observed later in the experiment would tend to exhibit more leukocyte adhesion. To account for this, we also analyzed a limited data set consisting of only the first 1-2 venules observed in each mouse (note that in some animals, data were only collected from one venule before enzyme treatment was performed). With this restricted data set, our findings of increased leukocyte adhesion in Sdc1−/− mice persisted. In the restricted data set, the mean number of adherent leukocytes in WT mice was 0.7±0.8 (n=18, N=13), compared with 1.5±1.3 (n=12, N=6, p=0.04) found in Sdc1−/− mice. Figure 5 shows the number of adherent leukocytes versus the percentage of vessels studied in Sdc1−/− and WT mice for the restricted data set. In WT mice, only 11% of vessels studied had 2 or more adherent leukocytes, whereas in Sdc1−/− mice this increased to 42%.
Figure 5. Leukocyte Adhesion in Sdc1−/− and WT mice. This chart shows the percentage of vessel segments in each group having 0, 1, 2, 3 or 4 adherent leukocytes.
None of the vessels observed had greater than 4 adherent leukocytes visible in the mid-sagittal plane. In Sdc1−/− mice, 42% of vessels studied had 2 or more adherent leukocytes, whereas in WT mice this number dropped to 11%. The mean number of adherent leukocytes was 1.5 ±1.3 (n=12) in Sdc1−/− mice and 0.7±0.8 (n=18; p=0.04) in WT mice.
Discussion
These results provide the first in vivo evidence that syndecan-1 is not an prerequisite for the existence of the endothelial glycocalyx. Despite the complete absence of syndecan-1, healthy Sdc1−/− mice are able to synthesize and maintain a hydrodynamically relevant glycocalyx. The glycocalyx found in Sdc1−/− mice appears to be ~0.1 μm thinner, on average, than that of WT mice, and this may be due to the absence of syndecan-1. The error associated with using μ-PIV and microviscometric analysis to estimate glycocalyx thickness has previously been estimated to be < 0.05 μm (Savery and Damiano, 2008). Therefore, a difference in glycocalyx thickness of 0.1 μm is large enough to be detectable by this method. Additionally, an approximately two-fold increase in leukocyte adhesion was observed in Sdc1−/− mice relative to WT mice. These differences suggest that the glycocalyx found in Sdc1−/− mice may be structurally and functionally different from that found in WT mice. As in WT mice, the hydrodynamically relevant glycocalyx in Sdc1−/− mice was almost completely degraded after enzymatic treatment with hyaluronidase.
Syndecan-1 has been linked to a wide variety of physiological processes and is expressed in many tissues during growth and development, including the vascular endothelium (Bernfield et al., 1992); however, mice lacking syndecan-1 develop normally and are fertile (Alexander et al., 2000; Park et al., 2001). Under normal laboratory conditions, they exhibit no decrease in viability and there are no apparent differences in routine serum chemistry, hematology, or tissue histology as compared with WT mice (Stepp et al., 2002). In this context, it is not surprising that Sdc1−/− mice are able to synthesize and maintain a hydrodynamically relevant endothelial glycocalyx. Even in some tissues that normally have high levels of syndecan-1 expression, no defects have been observed in these mice (Alexander et al., 2000). Therefore, these results do not indicate that syndecan-1 is not a component of the glycocalyx in animals with normal syndecan-1 expression, only that it is not a prerequisite for the existence of a hydrodynamically relevant endothelial glycocalyx.
There is evidence that only a subset of HS signaling pathways are affected in Sdc1−/−mice, while others, including those required for normal development, are not (Alexander et al., 2000). Additionally, there appears to be no difference in HS structure in Sdc1−/− mice, even in tissues with high syndecan-1 expression (Ledin et al., 2004). It has also been shown that the structure of HS chains is independent of the core protein, with HS chains on syndecan-1 and syndecan-4 being indistinguishable from each other (Zako et al., 2003). Thus, HS structures normally associated with syndecan-1 could bind to other core proteins instead, and any HS function that is independent of core protein structure could still occur (Zako et al., 2003; Ledin et al., 2004). It is possible that syndecan-1 is a major component of, or even an anchoring protein for, the glycocalyx in WT mice, but that in the absence of syndecan-1, other HSPGs are able to fill that role. On the other hand, previous studies have observed no differences in HS structure in tissues in Sdc1−/− mice compared to that of WT mice (Park et al., 2004). Furthermore, no evidence of compensation was observed in various models of disease where syndecan-1 plays a prominent role (Hayashida et al., 2008; 2009; Stanford et al., 2009). Together, these data suggest that loss of syndecan-1 is not compensated for by increased expression of other syndecans or HSPGs. These data further suggest that syndecan-1 functions prominently and specifically in vivo. Combining these data with the observations from the present study (revealing the existence of a somewhat less prominent hydrodynamically relevant endothelial glycocalyx in Sdc1−/− mice), suggests that other components (e.g. HSPGs, adsorbed plasma proteins from the blood, etc.) are not compensating for the deficiency in sydecan-1 and may, in fact, constitute much of the glycocalyx structure even in the presence of sydecan-1.
When challenged, as in cases of injuries or disease, Sdc1−/− mice exhibit a variety of phenotypes. It has been reported that they exhibit impaired wound healing and increased mortality, accompanied by defects in cell proliferation, and prolonged inflammation (Stepp et al., 2002; Floer et al., 2010). Similar observations have been made in Sdc1−/− mice exposed to certain toxins or diseases. Under such exposure, the animals experienced increased organ damage and mortality, which was accompanied by persistent systemic inflammation and exaggerated neutrophillic inflammation, relative to WT mice (Hayashida et al., 2008; Hayashida et al., 2009). In each of these studies, upregulation and/or dysregulation of certain cytokines, integrins, and adhesion molecules were also present (Stepp et al., 2002; Hayashida et al., 2008; Hayashida et al., 2009; Floer et al., 2010). In addition, while Sdc1−/− mice may exhibit a slight increase in leukocyte-endothelial interactions and adhesion under normal conditions, this difference is much more dramatic under inflammatory conditions, with 100-fold greater leukocyte adhesion occurring in Sdc1−/− versus WT mice after treatment with TNF-α (Götte et al., 2002; Götte et al., 2005). In these studies, increased leukocyte adhesion in Sdc1−/− was largely attributed to in the leukocytes resulting from increased activity of pro-adhesive molecules, however changes in the endothelium could not be excluded as a possible contributor (Götte et al., 2002; Götte et al., 2005). Taken together, these results demonstrate that syndecan-1 plays a critical role in inflammation, particularly in the resolution of inflammation, through the regulation of integrins, cytokines, and adhesion molecules and through the modulation of leukocyte activity and adhesion.
The slightly thinner glycocalyx and increased leukocyte adhesion observed in Sdc1−/− mice in the present study could be the result of mild inflammation that inevitably accompanies surgical preparation of the cremaster muscle. While much care is taken in handling the tissue, and a P-selectin antibody is used to minimize leukocyte rolling, the method is inherently invasive and mild inflammation is unavoidable. It has been established that the glycocalyx acts as a barrier to leukocyte adhesion and glycocalyx shedding is a normal part of the inflammatory response (Mulivor and Lipowsky, 2002). Given the evidence of a dysregulated inflammatory response in Sdc1−/− mice, it could be expected that even under mild inflammatory conditions, leukocyte adhesion and glycocalyx shedding would be greater than what is observed in WT mice. Thus, it is possible that under uninflamed conditions, the glycocalyx in Sdc1−/− mice is comparable in thickness to that found in WT mice. Whether or not the observed differences are the result of mild inflammation, they are suggestive of structural and functional differences between the glycocalyx in WT and Sdc1−/− mice. It has been proposed that one way in which the glycocalyx modulates leukocyte-EC interactions is by acting as a physical barrier to leukocyte attachment, and that primary capture occurs when the glycocalyx is crushed by leukocytes as they exit capillaries and enter inflamed post-capillary venules (Smith et al., 2003; Weinbaum et al., 2007). Therefore, in addition to increased leukocyte adhesion resulting from the dysregulation or upregulation of adhesion molecules in Sdc1−/− mice, leukocyte adhesion could be further facilitated by the decreased glycocalyx thickness.
This is the first investigation to assess the status of the endothelial glycocalyx in vivo in animals lacking what has been speculated to be a primary component of the glycocalyx. These results show that syndecan-1 is not an essential component of the glycocalyx, as Sdc1−/− mice are able to synthesize and maintain a hydrodynamically relevant layer; however, there is structural and functional evidence that the glycocalyx observed in these mice differs from what exists in WT mice. Now that the existence of a glycocalyx in Sdc1−/− mice has been established, investigations into the differences between this glycocalyx and that found in WT mice could be used to explore the role of syndecan-1 as a component of the endothelial glycocalyx and to elucidate mechanisms of glycocalyx function under inflammation.
Highlights.
We investigate the endothelial glycocalyx in syndecan-1 deficient mice in vivo.
Analysis of our data consistently revealed a glycocalyx in these mice.
This glycocalyx is slightly thinner that that observed in wild-type mice.
Syndecan-1 is not a prerequisite for the existence of a glycocalyx.
Lack of syndecan-1 may result in changes to glycocalyx structure and function.
Acknowledgements
Partial support for this work was provided by the National Institutes of Health, grant R01 HL076499 (E.R.D.) and by a graduate fellowship from the Clare Boothe Luce Foundation (M.D.S.).
Abbreviations
- EM
electron microscopy
- EC
endothelial cell
- GAG
glycosaminoglycan
- HS
heparan sulfate
- HSPG
heparan sulfate proteoglycan
- HUVEC
human umbilical vein endothelial cell
- μ-PIV
micro-particle image velocimetry
- Sdc1−/−
syndecan-1 null
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
The Sdc1−/− mice line was generated and bred in the laboratory of P.W. Park at Children’s Hospital, Boston, MA. All experiments were performed in the laboratory of E.R. Damiano in the Department of Biomedical Engineering at Boston University, Boston, MA. M.D. Savery contributed to the conception and design of the experiments and collection, analysis, and interpretation of data. J.X. Jiang contributed to the design of the experiments and collection of data. E.R. Damiano contributed to the conception and design of the experiments and interpretation of data. All authors contributed to drafting the article and/or revising it critically for important intellectual content. All authors approved the final version of the manuscript.
References
- Alexander CM, Reichsman F, Hinkes MT, Lincecum J, Becker KA, Cumberledge S, Bernfield M. Syndecan-1 is required for Wnt-1-induced mammary tumorigenesis in mice. Nat. Gen. 2000;25:329–332. doi: 10.1038/77108. [DOI] [PubMed] [Google Scholar]
- Alexopoulou AN, Multhaupt HAB, Couchman JR. Syndecans in wound healing, inflammation, and vascular biology. Int. J. Biochem. Cell Biol. 2007;39:505–528. doi: 10.1016/j.biocel.2006.10.014. [DOI] [PubMed] [Google Scholar]
- Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc. Res. 1973;5:384–394. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- Barker AL, Konopatskaya O, Neal CR, Macpherson JV, Whatmore JL, Winlove PC, Unwin PR, Shore AC. Observation and characterisation of the glycocalyx of viable human endothelial cells using confocal laser scanning microscopy. Int. Phys. Chem. Chem. Phys. 2004;6:1006–1011. [Google Scholar]
- Bernfield M, Kokenyesi R, Kato M, Hinkes MT, Spring J, Gallo RL, Lose EJ. Biology of the syndecans: a family of transmembrane heparan-sulfate proteoglycans. Annu. Rev. Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- Bruegger D, Jacob M, Rehm M, Loetsch M, Welsch U, Conzen P, Becker BF. Atrial natriuretic peptide induces shedding of the endothelial glycocalyx in coronary vascular bed of guinea pig hearts. Am J Physiol-Heart C. 2005;289:H1993–H1999. doi: 10.1152/ajpheart.00218.2005. [DOI] [PubMed] [Google Scholar]
- Chappell D, Jacob M, Hofmann-Kiefer K, Bruegger D, Rehm M, Conzen P, Welsch U, Becker BF. Hydrocortisone preserves the vascular barrier by protecting the endothelial glycocalyx. Anesthesiology. 2007;107:776–784. doi: 10.1097/01.anes.0000286984.39328.96. [DOI] [PubMed] [Google Scholar]
- Chappell D, Westphal M, Jacob M. The impact of glycocalyx on microcirculatory oxygen distribution in critical illness. Curr. Opin. Anaesthesiol. 2009a;22:155–162. doi: 10.1097/ACO.0b013e328328d1b6. [DOI] [PubMed] [Google Scholar]
- Chappell D, Jacob M, Paul O, Rehm M, Welsch U, Stoekelhuber M, Conzen P, Becker BF. The glycocalyx of the human umbilical vein endothelial cell: an impressive structure ex vivo but not in culture. Circ. Res. 2009b;104:1313–1317. doi: 10.1161/CIRCRESAHA.108.187831. [DOI] [PubMed] [Google Scholar]
- Damiano ER, Long DS, El-Khatib FH, Stace TM. On the motion of a sphere in a Stokes flow parallel to a Brinkman half-space. J. Fluid Mech. 2004a;500:75–101. [Google Scholar]
- Damiano ER, Long DS, Smith ML. Estimation of viscosity profiles using velocimetry data from parallel flows of linearly viscous fluids: application to microvascular haemodynamics. J. Fluid Mech. 2004b;512:1–19. [Google Scholar]
- Desjardins C, Duling BR. Heparinase treatment suggests a role for the endothelial cell glycocalyx in regulation of capillary hematocrit. Am. J. Physiol. 1990;258:H647–H654. doi: 10.1152/ajpheart.1990.258.3.H647. [DOI] [PubMed] [Google Scholar]
- Floer M, Götte M, Wild MK, Heidemann J, Gassar ES, Domschke W, Kiesel L, Luegering A, Kucharzik T. Enoxaparin improves the course of dextran sodium sulfate-induced colitis in syndecan-1-deficient mice. Am. J. Pathol. 2010;176:146–157. doi: 10.2353/ajpath.2010.080639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Götte M, Joussen AM, Klein C, Andre P, Wagner DD, Hinkes MT, Kirchhof B, Adamis AP, Bernfield M. Role of syndecan-1 in leukocyte-endothelial interactions in the ocular vasculature. Invest. Ophth. Vis. Sci. 2002;43:1135–1141. [PubMed] [Google Scholar]
- Götte M, Bernfield M, Joussen AM. Increased leukocyte-endothelial interactions in syndecan-1-deficient mice involve heparan sulfate-dependent and -independent steps. Curr. Eye Res. 2005;30:417–422. doi: 10.1080/02713680590956289. [DOI] [PubMed] [Google Scholar]
- Gretz JE, Duling BR. Measurement uncertainties associated with the use of bright-field and fluorescence microscopy in the microcirculation. Microvasc. Res. 1995;49:134–140. doi: 10.1006/mvre.1995.1011. [DOI] [PubMed] [Google Scholar]
- Hayashida K, Chen Y, Bartlett AH, Park PW. Syndecan-1 is an in vivo suppressor of Gram-positive toxic shock. J. Biol. Chem. 2008;283:19895–19903. doi: 10.1074/jbc.M801614200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashida K, Parks WC, Park PW. Syndecan-1 shedding facilitates the resolution of neutrophilic inflammation by removing sequestered CXC chemokines. Blood. 2009;114:3033–3043. doi: 10.1182/blood-2009-02-204966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CB, Duling BR. Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am. J. Physiol. 1999;277:H508–H514. doi: 10.1152/ajpheart.1999.277.2.H508. [DOI] [PubMed] [Google Scholar]
- Hofmann-Kiefer KF, Kemming GI, Chappell D, Flondor M, Kisch-Wedel H, Hauser A, Pallivathukal S, Conzen P, Rehm M. Serum heparan sulfate levels are elevated in endotoxemia. Eur. J. Med. Res. 2009;14:526–531. doi: 10.1186/2047-783X-14-12-526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacob M, Rehm M, Loetsch M, Paul JO, Breugger D, Welsch U, Conzen P, Becker BF. The endothelial glycocalyx prefers albumin for evoking shear stress-induced, nitric oxide-mediated coronary dilatation. J. Vasc. Res. 2007;44:435–443. doi: 10.1159/000104871. [DOI] [PubMed] [Google Scholar]
- Johansson PI, Stensballe J, Rasmussen LS, Ostrowski SR. A high admission syndecan-1 level, a marker of endothelial glycocalyx degradation, is associated with inflammation, protein C depletion, fibrinolysis, and increased mortality in trauma patients. Ann. Surg. 2011;254:194–200. doi: 10.1097/SLA.0b013e318226113d. [DOI] [PubMed] [Google Scholar]
- Ledin J, Staatz W, Li J, Götte M, Selleck S, Kjellén L, Spillmann D. Heparan sulfate structure in mice with genetically modified heparan sulfate production. J. Biol. Chem. 2004;279(41):42732–42741. doi: 10.1074/jbc.M405382200. [DOI] [PubMed] [Google Scholar]
- Ley K, Bullard DC, Arbonés ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long DS, Smith ML, Pries AR, Ley K, Damiano ER. Microviscometry reveals reduced blood viscosity and altered shear rate and shear stress profiles in microvessels after hemodilution. P. Natl. Acad. Sci. USA. 2004;101:10060–10065. doi: 10.1073/pnas.0402937101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft JH. Fine structures of capillary and endocapillary layer as revealed by ruthenium red. Fed. Proc. 1966;25:1773–1783. [PubMed] [Google Scholar]
- Megens RT, Reitsma S, Schiffers PH, Hilgers RH, De Mey JG, Slaaf DW, oude Egbrink MG, van Zandvoort MA. Two-photon microscopy of vital murine elastic and muscular arteries. Combined structural and functional imaging with subcellular resolution. J. Vasc. Res. 2007;44:87–98. doi: 10.1159/000098259. [DOI] [PubMed] [Google Scholar]
- Mulivor AW, Lipowsky HH. Role of glycocalyx in leukocyte-endothelial cell adhesion. Am. J. Physiol. 2002;283:H1282–H1291. doi: 10.1152/ajpheart.00117.2002. [DOI] [PubMed] [Google Scholar]
- Park PW, Pier GB, Hinkes TM, Bernfield M. Exploitation of syndecan-1 shedding by Pseudomonas aeruginosa enhances virulence. Nature. 2001;411:98–102. doi: 10.1038/35075100. [DOI] [PubMed] [Google Scholar]
- Park PW, Foster TJ, Nishi E, Duncan SJ, Klagsbrun M. Activation of syndecan-1 ectodomain shedding by staphylococcus aureus α-toxin and β-toxin. J. Biol. Chem. 2004;279:251–258. doi: 10.1074/jbc.M308537200. [DOI] [PubMed] [Google Scholar]
- Platts SH, Linden J, Duling BR. Rapid modification of the glycocalyx caused by ischemia-reperfusion is inhibited by adenosine A2A receptor activation. Am. J. Physiol. 2003;284:H2360–H2367. doi: 10.1152/ajpheart.00899.2002. [DOI] [PubMed] [Google Scholar]
- Potter DR, Damiano ER. The hydrodynamically relevant endothelial cell glycocalyx observed in vivo is absent in vitro. Circ. Res. 2008;102:770–776. doi: 10.1161/CIRCRESAHA.107.160226. [DOI] [PubMed] [Google Scholar]
- Potter DR, Jiang J, Damiano ER. The recovery time course of the endothelial glycocalyx in vivo and its implications in vitro. Circ. Res. 2009;104:1318–1325. doi: 10.1161/CIRCRESAHA.108.191585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Jacobs H, Sperandio M, Osterloh K, Gaehtgens P. Microvascular blood flow resistance: role of endothelial surface layer. Am. J. Physiol. 1997;273:H2272–H2279. doi: 10.1152/ajpheart.1997.273.5.H2272. [DOI] [PubMed] [Google Scholar]
- Pries AR, Secomb TW, Gaehtgens P. The endothelial surface layer. Pflugers Arch. 2000;440:653–666. doi: 10.1007/s004240000307. [DOI] [PubMed] [Google Scholar]
- Rehm M, Zahler S, Lötsch M, Welsch U, Conzen P, Jacob M, Becker BF. Endothelial glycocalyx as an additional barrier determining extravasation of 6% hydroxyethyl starch or 5% albumin solutions in the coronary vascular bed. Anesthesiology. 2004;100:1211–1223. doi: 10.1097/00000542-200405000-00025. [DOI] [PubMed] [Google Scholar]
- Rehm M, Bruegger D, Christ F, Conzen P, Thiel M, Jacob M, Chappell D, Stoeckelhuber M, Welsch U, Reichart B, Peter K, Becker BF. Shedding of the endothelial glycocalyx in patients undergoing major vascular surgery with global and regional ischemia. Circulation. 2007;116:1896–1906. doi: 10.1161/CIRCULATIONAHA.106.684852. [DOI] [PubMed] [Google Scholar]
- Rosenberg RD, Shworak NW, Liu J, Schwartz JJ, Zhang L. Heparan sulfate proteoglycans of the cardiovascular system. J. Clin. Invest. 1997;99:2062–2070. doi: 10.1172/JCI119377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savery MD, Damiano ER. The endothelial glycocalyx is hydrodynamically relevant in arterioles throughout the cardiac cycle. Biophys. J. 2008;95:1439–1447. doi: 10.1529/biophysj.108.128975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith ML, Long DS, Damiano ER, Ley K. Near-wall μ-PIV reveals a hydrodynamically relevant endothelial surface layer in venules in vivo. Biophys. J. 2003;85:637–645. doi: 10.1016/s0006-3495(03)74507-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanford KI, Bishop JR, Foley EM, Gonzales JC, Niesman IR, Witztum JL, Esko JD. Syndecan-1 is the primary heparan sulfate proteoglycan mediating hepatic clearance in triglyceride-rich lipoproteins in mice. J. Clin. Invest. 2009;119:3236–3245. doi: 10.1172/JCI38251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepp MA, Gibson HE, Gala PH, Iglesia DDS, Pajoohesh-Ganji A, Pal-Ghosh S, Brown M, Aquino C, Schwartz AM, Goldberger O, Hinkes MT, Bernfield M. Defects in keratinocyte activation during wound healing in the syndecan-1-deficient mouse. J. Cell Sci. 2002;115:4517–4531. doi: 10.1242/jcs.00128. [DOI] [PubMed] [Google Scholar]
- Steppan J, Hofer S, Funke B, Brenner T, Henrich M, Martin E, Weitz J, Hofmann U, Weigand MA. Sepsis and major abdominal surgery lead to flaking of the endothelial glycocalix. J. Surg. Res. 2011;165:136–141. doi: 10.1016/j.jss.2009.04.034. [DOI] [PubMed] [Google Scholar]
- Stevens AP, Hlady V, Dull RO. Fluorescence correlation spectroscopy can probe albumin dynamics inside lung endothelial glycocalyx. Am. J. Physiol. 2007;293:L328–L335. doi: 10.1152/ajplung.00390.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thi MM, Tarbell JM, Weinbaum S, Spray DC. The role of the glycocalyx in reorganization of the actin cytoskeleton under fluid shear stress: A “bumper car” model. P. Natl. Acad. Sci. USA. 2004;101:16483–16488. doi: 10.1073/pnas.0407474101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Berg BM, Vink H, Spaan JAE. The endothelial glycocalyx protects against myocardial edema. Circ. Res. 2003;92:592–594. doi: 10.1161/01.RES.0000065917.53950.75. [DOI] [PubMed] [Google Scholar]
- Vink H, Duling BR. Identification of distinct luminal domains for macromolecules, erythrocytes, and leukocytes within mammalian capillaries. Circ. Res. 1996;79:581–589. doi: 10.1161/01.res.79.3.581. [DOI] [PubMed] [Google Scholar]
- Weinbaum S, Tarbell JM, Damiano ER. The structure and function of the endothelial glycocalyx layer. Annu. Rev. Biomed. Eng. 2007;9:121–167. doi: 10.1146/annurev.bioeng.9.060906.151959. [DOI] [PubMed] [Google Scholar]
- Zako M, Dong J, Goldberger O, Bernfield M, Gallagher JT, Deakin JA. Syndecan-1 and -4 synthesized simultaneously by mouse mammary gland epithelial cells bear heparan sulfate chains that are apparently structurally indistinguishable. J. Biol. Chem. 2003;278:13561–13569. doi: 10.1074/jbc.M209658200. [DOI] [PubMed] [Google Scholar]