Abstract
Chronic hepatitis B can currently be medically managed with either pegylated interferon-alpha (pegIFN-α) or one of the five nucleos(t)ide analogue Direct Acting Antivirals (DAAs) that inhibit the hepatitis B virus (HBV) DNA polymerase. While pegIFN-α is effective in approximately one-third of the treated patients, the polymerase inhibitors significantly reduce viral load in the vast majority of those treated. However, neither pegIFN-α nor nucleosid(t)e analogues are capable of reliably eliminating the virus and achieving a cure. Moreover, the interferons and polymerase inhibitors are recommended by US, European and Asian professional society practice guidelines for use in only a subset of those infected with HBV. This subset is the population with the greatest levels of circulating viral DNA and abnormal liver function. Although this is the population at the highest risk for cirrhosis and liver cancer, those who fall outside the treatment guidelines, with low levels of viral replication and normal serum ALTs, may also benefit from antiviral therapy. The questions are thus: are new classes of drugs needed to manage chronic hepatitis B? Is a cure possible? Is a cure even necessary? It is therefore important to define the meaning of a cure and determine what the goals of new therapies should be. In this article, we address those questions and propose two operational definitions of medically attainable cures. The first is a “functional cure” based on the clinical outcome, in which the patient’s life expectancy becomes the same as that of an individual who has resolved his HBV infection without therapy. Because such an outcome cannot be measured over the short term, we also define an “apparent virological cure,” based on the stable off-drug suppression of HBV viremia and antigenemia and the normalization of ALTs and other laboratory tests. We suggest that such a virological cure should be the goal of future therapeutics in all patients with chronic hepatitis B. The extent to which a virological cure predicts a functional cure will only be determined by long-term follow-up.
1. Introduction
Recent reports of new curative therapies for patients with chronic hepatitis C have created an expectation in the public advocacy community that hepatitis B can also be cured (Fox and Jacobson 2012; Lee, Tong et al. 2012; Seeff 2012). In the case of hepatitis C, cures have been associated with a sustained virological response (SVR) to therapy, as defined by the absence of detectable hepatitis C virus (HCV) RNA in the serum six months after the cessation of treatment. However, virologists are uncomfortable with unqualified analogies between hepatitis B virus (HBV) and HCV infections. HCV is an RNA virus that completes its entire replication cycle within the cytoplasm. In contrast, HBV is a DNA virus with a stable intranuclear form of its genome, covalently closed-circular DNA (cccDNA), which is not directly affected by nucleoside/nucleotide analogue therapy. However, in view of recent progress in treating hepatitis C, it may be time to consider the meaning of a “cure” for chronic hepatitis B, and how far new direct-acting antiviral (DAA) therapies can and should go.
Infection with HBV results in either acute or chronic infection of the liver (Lok and McMahon 2009). Acute hepatitis B is characterized by viremia, with or without apparent clinical symptoms. In adults, the infection usually resolves within six months, but about 5% of patients remain chronically infected, while about 90% of cases of perinatal transmission result in lifelong infection (Ganem and Prince 2004). Chronic hepatitis B is characterized by the continuous presence of the viral envelope protein, or surface antigen (HBsAg), in the circulation and a 15-40% lifetime risk of death from liver disease (Block, Guo et al. 2007; Dienstag 2008; Lok and McMahon 2009). HBV genotypes vary in virulence (Lin and Kao 2011), but most chronically infected individuals experience decades of viremia and antigenemia, in the absence of any apparent compromise of liver function. However, even in this “immune tolerant” phase, chronic infection with any HBV genotype is a significant risk factor for fatal liver disease.
More than 2 billion people worldwide are infected with HBV, as defined by the presence of antibodies to viral core proteins, but some 80% have resolved the infection (El-Serag and Mason 2000; Lavanchy 2004; Dienstag 2008). Resolution of acute hepatitis B, and rare instances of the natural resolution of chronic hepatitis B, are characterized by the disappearance of HBV DNA and HBsAg from the circulation and the development of robust humoral and cellular immune responses, involving CD4+ and CD8+ T cells and B cells (Thimme, Wieland et al. 2003; Guidotti and Chisari 2006). Resolution of infection is accompanied by a return to normal liver function, as indicated by the normalization of serum alanine and aspartate aminotransferase (ALT and AST) levels, presumably reflecting the abatement of the necro-inflammatory disease that resulted, in part, from chronic immunological attacks on infected hepatocytes (Guidotti and Chisari 2006).
2. Defining a “cure” for chronic hepatitis B
To evaluate medical interventions for chronic hepatitis B, it is useful to define what it means to be “cured.” According to a standard dictionary, a cure is “the restoration of health following disease.” Though admirable, this definition is obviously inadequate for determining standards and endpoints in antiviral drug development. We therefore suggest that the term “cure,” when used alone, should mean an “absolute” cure, in which an individual with chronic hepatitis B completely resolves the infection, and is then at the same risk of death from liver disease as someone the same age who has never been infected (Table 1). This is presumably what most patients believe it means to be “cured” of hepatitis B. However, it is now well established that even persons with naturally resolved acute HBV infection have a risk of death from hepatocellular carcinoma (HCC) three times greater than those who have never been infected, indicating that even a natural cure is not “absolute” (Beasley 1988; Amin, Law et al. 2006; Chen, Lin et al. 2006; Papatheodoridis, Lampertico et al. 2010; Evans, London et al. 2012).
Table 1.
Proposed definitions of absolute, functional and virological cures for new DAA therapies for chronic hepatitis B, as discussed in the text. Cures are based on the degree to which the patient’s age- and gender-adjusted risk of death from liver disease returns to that of an individual who has never been infected with HBV, or who has naturally resolved the infection, and can be predicted by virological markers. The correlation of an “Apparent virological cure” with reduced risk of death from liver disease will only be determined once long-term survival data have been obtained. Viral load is the DNA level detected in the circulation by standard commercial assays (Lok & McMahon, 2009). “Off-drug” indicates that therapeutic benefits continue after discontinuation of medication.
| Parameter measured |
Absolute cure | Functional cure | Apparent virological cure |
|---|---|---|---|
|
Risk of death from
liver disease |
Same as person who was never infected. |
Same as person with naturally resolved infection. |
To be determined* |
| Viral load | Undetectable | Undetectable | Undetectable |
| HBsAg | Undetectable | Undetectable | Undetectable |
| cccDNA | Undetectable | Undetectable | Undetectable or repressed |
| HBsAb | Present | Variable | Present or Absent |
| Treatment status | Off-drug | Off-drug | Off-drug |
It is therefore unrealistic to expect DAA therapy to return a patient with chronic hepatitis B to his pre-infection risk of death from liver disease. However, a treatment that reduces this risk to that of a person who has spontaneously resolved the infection would still provide a tremendous health benefit. By analogy to the current management of human immunodeficiency virus (HIV) infection (Richman, Margolis et al. 2009; Cohen 2011), such an outcome could be designated a “functional cure,” and it is clearly the minimum goal for future therapies (Table 1). However, it will take many years to quantify a reduction in risk of death from liver disease resulting from a new DAA therapy for chronic hepatitis B. For practical reasons, it is therefore necessary to identify surrogate markers that can be measured over a period of months to a few years, which correlate with the clinical outcome of a functional cure. We therefore propose the therapeutic goal of an “apparent virological cure,” defined as the sustained off-drug suppression of virological markers (serum HBsAg, HBeAg and viral DNA) and the normalization of liver function (serum ALT and AST levels) (Table 1.)
The term “sustained virological response” (SVR) has been used to describe the continuous suppression of viral load following the cessation of therapy. A “virological” cure of chronic hepatitis B therefore includes a SVR, but adds the loss of all circulating virological markers and suppression of cccDNA. However, because people who have naturally resolved their chronic hepatitis B can experience a reactivation of the disease as a consequence of immunosuppression or the use of anti-inflammatory medications (Hoofnagle 2009), we have chosen to use the term “apparent” in our definition.
Because a cure of HBV infection should mean the complete elimination of both intracellular and circulating viral genes and gene products, our definition of a virological cure is necessarily a compromise. However, it still requires the elimination or suppression both of circulating markers and of viral cccDNA, and assumes that such complete suppression will eventually be proved to be a “functional” cure. This is based, in part, on the assumption that a “functional” cure would require the overwhelming, if not complete and enduring, suppression of viremia and antigenemia, and a substantial immune restoration, mimicking the natural resolution of infection. However, although the achievement of a “virological cure” might lead to a beneficial activation or unmasking of humoral and cell-mediated recognition of HBV epitopes and relief from “immune exhaustion” (Dienstag 2008; Tzeng, Tsai et al. 2012), our definition does not require the development of anti-HBs antibodies, because we propose that immunological and therapeutic clearance of HBV markers are two parallel means to the same ends. In terms of reducing the patient’s long-term risk of severe liver disease, we do not think that one clearance mechanism can currently be known to be superior to the other. Of course, in the absence of a beneficial immune response to HBV, the patient will remain vulnerable to a reactivated or new infection. An “apparent virological cure” of HBV infection will therefore be defined as complete, sustained off-drug virological suppression, with measurable endpoints to determine when the goal has been achieved.
3. Uses and limitations of current therapies
As shown in Table 2, current therapeutic guidelines call for the treatment of chronic hepatitis B patients who have elevated circulating levels of viral DNA, ALT and AST. People with a family history of HCC who are viremic, but have normal liver enzyme levels, may also be recommended for treatment (Chien, Liaw et al. 1999; Heathcote, Marcellin et al. 2011; Yuen and Lai 2011). Although there are no firm numbers, it is generally believed that one-third to one-half of the chronically infected population fall within these guidelines (Cohen, Holmberg et al. 2011; Evans, London et al. 2012; Zoulim and Mason 2012). Although they are the population at greatest risk of developing HCC, persons who do not meet the guidelines might also benefit from treatment, since they are also at significantly elevated risk of liver disease, compared to the non-infected population.
Table. 2.
Recommended guidelines of three major professional organizations for considering the initiation of antiviral therapy for chronic hepatitis B. Levels of circulating viral DNA and ALT are those recommended in the cited reference as thresholds for beginning treatment with pegIFN-α or nucleoside/nucleotide analogues. The viral load is the circulating level of HBV DNA, measured in IU/mL or genomes/mL. ALT levels are expressed as multiples of the upper limit of normal (ULN). AASLD: American Association for the Study of Liver Disease. EASL: European Association for the Study of Liver Disease. APASL: Asia-Pacific Association for the Study of Liver Disease.
| Professional organization |
Serum HBeAg+ | Serum HBeAg− | Reference | ||
|---|---|---|---|---|---|
| HBV viral load | ALT (x ULN) |
HBV viral load | ALT (x ULN) |
||
| AASLD | > 20,000 (105) | >2 | > 2000 (104) | >1 | (Lok and McMahon 2009) |
| EASL | > 2000 (104) | >1 | > 2000 (104) | >1 | EASL (2009) |
| APASL | > 20,000 (105) | >2 | > 2000 (104) | >2 | (Liaw, Leung et al. 2008) |
In HBeAg+ patients, the loss of serum HBeAg, seroconversion to an anti-HBe status and loss of circulating HBV DNA are considered major therapeutic milestones (Lok and McMahon 2009). Current therapies lead to HBeAg seroconversion in 20-30% of such patients, and a roughly 50% reduction in the risk of death from liver disease over a ten-year period (Liaw, Sung et al. 2004; Liaw, Leung et al. 2008; Sung, Tsoi et al. 2008; Lok and McMahon 2009; Lok 2011; Evans, London et al. 2012). There is clearly a need for new medical interventions to increase the percentage of HBeAg+ patients who seroconvert and that reduce the numbers of people who still, despite current therapy, succumb to liver disease. The sustained off-drug suppression of viremia in HBeAg− patients offers another surrogate marker of successful treatment. Studies also indicate that, even with therapy, approximately half of patients who HBeAg seroconvert go on to develop serious liver disease -- a significantly increased risk over that of an age-matched, uninfected control population or persons with naturally resolved acute hepatitis B. This adverse outcome probably reflects the fact that these HBeAg− patients initiated antiviral therapy too late in the course of their disease.
4. Can current DAAs be curative?
Given enough time, treatment with DAAs might produce a “virological” cure of chronic hepatitis B. Indeed, despite the limitations of current therapies, up to 10% of patients, and in some studies up to 17%, appear to achieve stable off-drug suppression of viremia, and even the loss of HBsAg or anti-HBs seroconversion (Perrillo, Gish et al. 2006; Lok and McMahon 2009). It is therefore possible for DAAs, alone to eliminate all virological markers and achieve the clinical and biological benefits that, borrowing from HIV nomenclature, we have defined as a “functional” cure (Cohen 2011), in at least a sub-set of patients.
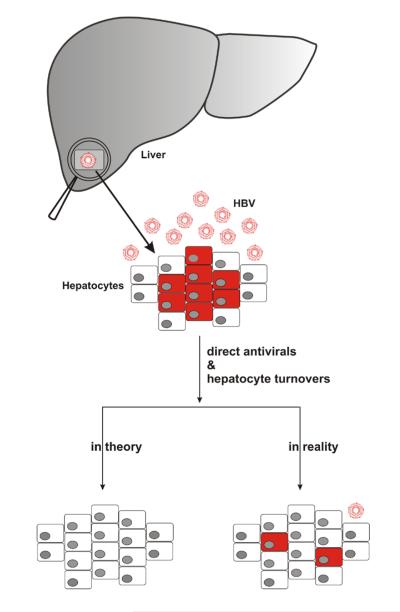
Hepatocytes are self-renewing, with a half-life in the healthy liver of approximately 6 months (Mason, Jilbert et al. 2005; Mason, Low et al. 2009; Mason, Liu et al. 2010). In HBV-infected individuals, in contrast, the half-life is dramatically shortened to only 3-30 days (Nowak 1996; Ciupe, Ribeiro et al. 2007). Therefore, as illustrated in Figure 1, if DAA therapy alone could absolutely block HBV replication, then inhibition at any step of the life cycle should eventually eliminate all viral replicative intermediates, resulting in a stable SVR once the treatment period has exceeded 10 hepatocyte half-lives (Fig. 2). Such an outcome should therefore be achieved by the currently used DAAs, even though they target the viral polymerase and have no direct effect on cccDNA. However, the reality appears to be more complicated (Fig. 2). Substantial HBsAg antigenemia may persist even after 5-10 years of DAA therapy, indicating the persistence of significant numbers of productively infected cells or virus reservoirs, making it impossible to discontinue treatment without a risk of rebound viremia (Lok 2011). This has certainly been the experience with monotherapy.
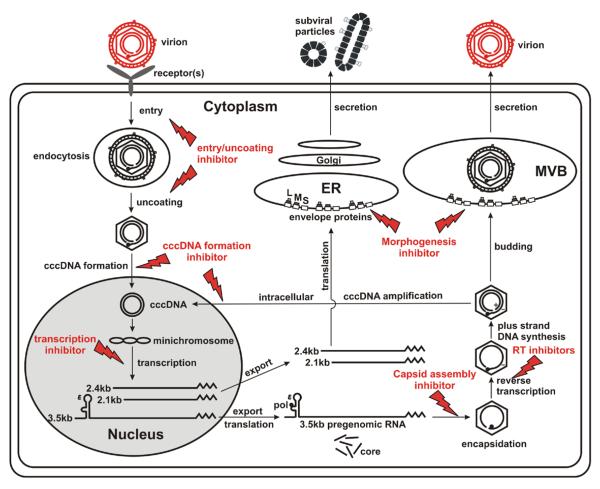
Figure 1. Inhibition of the HBV replication cycle.
Major steps in the life cycle of the hepatitis B virus in an infected cell. The steps targeted by currently used antiviral drugs are indicated, as well as possible drug targets. The figure indicates how inhibition of any step in the life cycle, if complete, prevents all downstream virological events, the production of new progeny virus and new rounds of infection.
Figure 2. Reduction of the number of infected hepatocytes in treated HBV infection.
A. An infected liver, with infected cells colored in red, and virions indicated by red circles. B. In theory, preventing the production of progeny virus should, after 10-20 hepatocyte half-lives, result in the death of all infected cells, so that no detectable virus-infected cells remain. In reality, however, infected nests of cells persist even after 5 years, or more than 20-50 half lives of polymerase therapy.
The reason for the inability of current DAAs to reliably achieve a stable SVR, and thus “virologically” cure chronic hepatitis B (Table 1), may be due to their failure to completely suppress viral replication in vivo. Consistent with this notion is the observation that, while antiviral therapy often lowers the serum viral load by more than 6 logs, it only reduces intracellular HBV cccDNA and core DNA by 1 or 2 logs, respectively (Werle-Lapostolle, Bowden et al. 2004; Wursthorn, Lutgehetmann et al. 2006; Sung, Tsoi et al. 2008; Takkenberg, Terpstra et al. 2011). The reason for this disconnection between the reduction in circulating and intracellular forms of HBV DNA is unclear. One possibility is that the maturation of viral genomes to produce secreted virions is much more sensitive to the current polymerase inhibitor DAA therapy than the intracellular generation of immature virions. Alternatively, there might be drug-refractory viral “reservoirs,” with two possible scenarios. In the first, a subpopulation of infected cells is “off limits” to the current DAAs, creating sanctuaries from drugs. In the other, a percentage of HBV-infected cells are long-lived, and their nuclear cccDNA is quiescent and stable, serving as a persistent source of virus reactivation.
Understanding why current polymerase inhibitor DAA therapy fails to resolve chronic HBV infection holds the key to achieving a “virological cure” For example, if the incomplete suppression of HBV replication results from the failure of metabolic activation of nucleoside/nucleotide analogues in a small percentage of infected hepatocytes, then combination therapy with drugs that target both the viral DNA polymerase and other steps in DNA synthesis might be beneficial.
5. How great a decline in viral load is needed for clinical benefit?
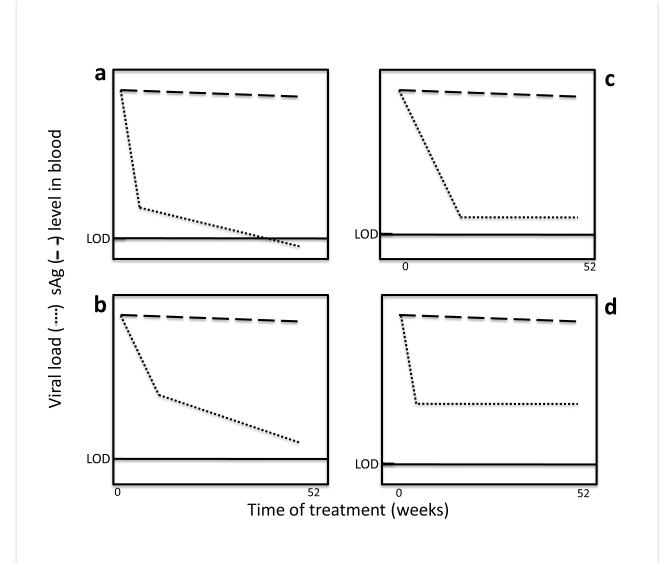
Kinetic studies have shown that HBV viremia declines in a multistep, at least biphasic manner in most patients treated with DAAs (Nowak 1996; Ciupe, Ribeiro et al. 2007). Based on such reports (Tsiang, Rooney et al. 1999; Lewin, Ribeiro et al. 2001; Wang, Holte et al. 2004), we have graphed some ”hypothetical” effects of the current polymerase inhibitor DAAs on viral and HBsAg load (Fig. 3). The responses can be divided into four groups. Unless an individual is infected with a drug-resistant virus, all four response groups show an initial, rapid drop in viral load (circulating viral DNA), usually within the first 4 weeks, followed by a slower decline which varies among patients. In some, the second period of decline is gradual but steady, ending up below the level of detection (Fig. 3a). In others, although the viral load declines, it either does not go below (Fig. 3b) or stabilizes just above (Fig. 3c) or significantly above (Fig. 3d) the level of detection.
Figure 3. Hypothetical kinetics of HBV viremia and HBs antigenemia during DAA therapy.
The graphs indicate four different responses to treatment of chronic hepatitis B patients with the currently available DAAs (lamivudine, tenofovir or barraclude), as reflected in circulating levels of HBV viral DNA and HBsAg (Lok and McMahon 2009). The time of treatment 0 to 52 weeks. The values of viral load and HBsAg are relative and arbitrary, although the scale for viral load is in logs (usually covering at least 8 logs) and antigen levels as percentage of baseline (0-100%, or 2 logs). The solid bar stemming from the y axis (LOD) indicates the limit of detection of viral DNA, and shows that in many patients it stabilizes at or above the LOD.
In marked contrast to the HBV DNA level, which may drop more than 5 logs during the first year of nucleoside/nucleotide analogue therapy, the level of HBsAg rarely falls more than 1 log (Fig. 3). (An apparent exception is telbivudine, which can produce a >1 log decline in HBsAg after 4 years of treatment (Wursthorn, Jung et al. 2010; Chan, Thompson et al. 2011)). Even though DNA polymerase inhibitors do not directly suppress viral transcription or HBsAg synthesis, a decline in the number of infected cells should cause a decrease in HBsAg. It thus appears that, even after virus production has been inhibited for a year by current DAA therapy, a significant number of infected cells remain. This observation is consistent with previously cited reports of the continued presence of intracellular replicative forms of viral DNA and cccDNA, long after the viral load has fallen near the level of detection (Werle-Lapostolle, Bowden et al. 2004; Sung, Wong et al. 2005; Wursthorn, Lutgehetmann et al. 2006; Pan, Hu et al. 2012). In this regard, it should be noted, as illustrated in the graphs of hypothetical outcomes in Fig. 4, that pegIFN-α therapy may cause a more rapid decline in circulating HBsAg, associated with the loss of HBeAg and the eventual loss of detectable HBsAg, or even anti-HBs seroconversion, producing a stable off-drug benefit (Moucari, Mackiewicz et al. 2009; Ma, Yang et al. 2010; Thompson, Nguyen et al. 2010; Liaw 2011; Chen, Jeng et al. 2012). These data suggest that long-term efficacy is related to the kinetics of the drug-induced reduction in viral gene products. In chronic hepatitis C, a rapid reduction in viral load is an excellent predictor of a beneficial outcome (Poordad, Reddy et al. 2008). Perhaps the same is true for HBV infection.
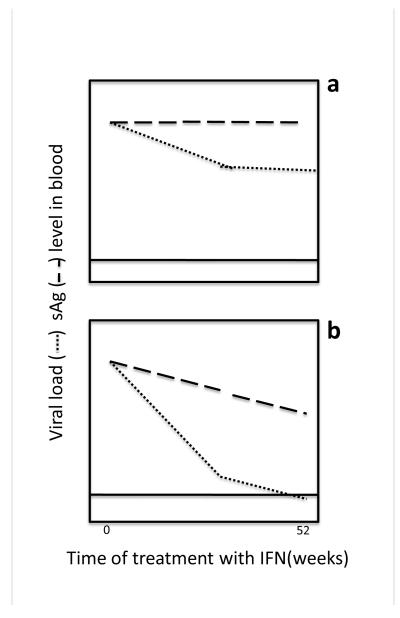
Figure 4. Hypothetical kinetics of HBV viremia and HBs antigenemia during therapy with pegIFN-α.
As in Fig. 3, the plots are summaries, sub-categorizing data from original papers such as (Moucari, Mackiewicz et al. 2009; Ma, Yang et al. 2010; Thompson, Nguyen et al. 2010; Chen, Jeng et al. 2012). The information is based on only HBeAg+ patients. Circulating viral DNA and HBsAg levels are depicted in arbitrary units on the y axis (where, as in Fig. 3, the scale for viral load values is 0-8 logs and for antigen, 2 logs) as a function of time of IF treatment (indicated by the bar), which was usually 28-42 weeks. Hypothetical profile for individuals treated with pegIFN-α who (a) did not experience a rapid reduction of circulating HBsAg and have the least likelihood of eliminating circulating HBV viral DNA load and losing HBeAg and (b) those who do experience a “rapid” decline of HBsAg and thus have the greatest likelihood of eliminating circulating detectable viral DNA load and HBeAg sero-conversion. Solid horizontal line: limit of detection of viral DNA. Dotted line: HBV DNA level in the blood. Hashed line: HBsAg level in the blood.
6. Can combination therapy achieve what monotherapy cannot?
Experience with the treatment of HIV and HCV infection has shown that combination therapy with drugs targeting multiple steps in the replication cycle suppresses viral load more rapidly and completely than monotherapy. If the same proves to be true for HBV infection, combination therapy might eventually achieve complete suppression, and therefore a type of viral “sterilization.” However, one must remember that, in the absence of effective immune control, even a single cell containing a competent, reactivatable viral genome could be the source of a re-established infection.
This raises the question of whether a rapid reduction in viral antigenemia, which current antiviral interventions do not achieve, would lead to the “unmasking” of neutralizing antibodies to HBsAg, and perhaps to other desirable immunologic effects. Chronically infected individuals with high levels of circulating HBsAg have actually been shown to possess anti-HBs, but it is presumably involved in immune complexes, and therefore not detectable (Michalak, Hodgson et al. 2000). Perhaps a rapid reduction in HBsAg would produce an excess of free anti-HBs antibodies, which might then “gain the upper hand” and contribute to humoral control of infection. Along these lines, excessive HBsAg antigenemia appears to repress T- and B-cell function (Menne, Roneker et al. 2002; Menne, Roneker et al. 2002) and to interfere with dendritic cell function and the MHC presentation of HBV epitopes (Oberhaus and Newbold 1995; Op den Brouw, Binda et al. 2009).
There is empirical support for the notion that a rapid reduction in circulating HBV gene products produces greater benefits than a slow decline. A significant and rapid fall in the HBsAg level has been associated with interferon efficacy and immune recovery in chronic hepatitis B patients (Liaw 2011; Chen, 2012). Drugs that directly target HBsAg, causing the circulating level to drop rapidly, would likely provide beneficial immunostimulatory activities, in addition to their antiviral effects. Since such drugs are now under development, it may soon be possible to test this hypothesis (Dougherty, Guo et al. 2007; Noordeen, Vaillant et al. 2007; Norton, Menne et al. 2010).
Because all HBV transcripts, antigens and viral progeny are derived from nuclear cccDNA, interventions that eliminate or durably silence cccDNA would, in principle, achieve all of the virological goals of therapy. In a complementary approach, treatments that restore immune recognition of HBV antigens should also eliminate cccDNA, by controlling or killing HBV-infected hepatocytes. However, caution is evidently needed, since the abrupt and large-scale destruction of infected cells could harm the patient. Despite these concerns, there has recently been great interest in activators of innate host defense pathways, via pattern recognition receptors, and in drugs that disrupt natural immune cell tolerance or putative “immune exhaustion” mediated by PD1/PD1–ligand interaction (Boni, Fisicaro et al. 2007; Guo, Jiang et al. 2009; Hsu, Yang et al. 2010; Tzeng, Tsai et al. 2012). In particular, there have been encouraging reports of the ability of a synthetic TLR7 agonist to suppress HBsAg and viral load in woodchucks (Menne, Tennant et al. 2011) and in chimpanzees (Lanford, Guerra et al. 2011). The compound has reached Phase I clinical trials (Lopatin, Wolfgang et al. 2011).
Can small-molecule drugs target cccDNA? High-throughput screening has recently identified two compounds that inhibit the formation of cccDNA, providing proof of concept that drugs can block its de novo synthesis (Cai, Mills et al. 2012). It will clearly be more challenging to target established cccDNA, given its long (if not indefinite) nuclear half-life and its dependence on host factors for its function. However, there is evidence that established cccDNA can be repressed and/or destabilized. For example, it has been shown that inflammatory cytokines produced by activated lymphocytes and/or other cell types can induce the clearance of established cccDNA through a noncytolytic mechanism (Guidotti, Rochford et al. 1999; Wieland, Thimme et al. 2004), suggesting that the same mechanisms could be activated by a biological stimulator or a small molecule (Lanford, Guerra et al. 2011). Recent success in repressing cccDNA in vitro with IFN-α (Belloni, Allweiss et al. 2012) (Liu, Guo et al., submitted) also raises hope that established cccDNA is not invulnerable to therapy. Indeed, we have used an LMH cell-based duck hepatitis B virus (DHBV) replication system to identify drugs that target cccDNA transcription and to demonstrate that treatment with IFN-α accelerates the decay of established cccDNA and efficiently inhibits its transcription (Liu, Guo et al., submitted).
cccDNA is also eliminated when the host immune response destroys virus-infected cells (Locarnini and Mason 2006; Mason, Low et al. 2009). Although HBV is not cytopathic, it imposes physiological stresses (metabolic and cell cycle disruption, endoplasmic reticulum (ER) stress, etc.) on infected cells. It should therefore be possible to identify agents that potentiate those stresses, are thus selectively cytotoxic. In that case, the pharmacological agent would act as a “chemical T-cell,” selectively eliminating remaining infected cells once their total numbers have been reduced by conventional DAA treatment. Although this idea may seem far-fetched, our work in progress suggests that drugs that potentiate ER stress and apoptosis pathways selectively kill HBV replicating cells, at least in tissue culture (Cuconati, unpublished observations).
Finally, we note that current nucleoside/nucleotide analogues may have therapeutic effects that cannot be accounted for entirely by their inhibition of viral DNA synthesis. For example, patients treated with HBV polymerase inhibitors often show normalization of serum ALT levels within months or even weeks after the initiation of therapy (Chien, Liaw et al. 1999; Thompson, Muir et al. 2010; Takkenberg, Terpstra et al. 2011). This suggests a more rapid abatement of immunological attack on infected cells than would be expected if the drug is only preventing HBV DNA synthesis, since the number of infected cells would not be expected to have declined by these early time points. Similarly, inhibitors of capsid formation may affect cccDNA function, and viral resistance to innate host defenses, since capsid protein in the nucleus and cytoplasm may have regulatory activity, and inhibitors of HBsAg folding can alter viral antigen presentation, enhancing innate and adaptive immune responses (Norton, Menne et al. 2010). These approaches, in combination with other strategies, may have in vivo benefits that cannot be predicted from their in vitro activity.
7. Conclusions
What should be the goal of new therapies for chronic hepatitis B? Will the introduction of new DAAs make it possible to cure the disease? What is the meaning of a “cure”? In this paper, we have defined three possible types of cures:
an “absolute” cure, in which the patient is returned to his state of health prior to illness, including his age- and gender-matched likelihood of developing cirrhosis or HCC;
a “functional” cure, in which the patient is returned to a state of health equivalent to that of a person who has recovered spontaneously from HBV infection, and has a similar likelihood of developing cirrhosis or HCC; and
an “apparent virological” cure, defined as the sustained off-drug suppression of virological markers and the normalization of liver function.
In offering these three definitions, we have used the risk of death from liver disease as the major criterion for sub-categorization, believing it to be most relevant to the ultimate goal of therapy. For practical reasons, however, it is clear that quantifiable endpoints are needed to measure the efficacy of a new drug in an individual patient (Tables 3 and 4).
Table 3.
Efficacy of current medical interventions in the management of chronic hepatitis B. The data are derived from summaries in AASLD practice guidelines (Lok et al., 2009) and in Heathcote & Marcellin (2010) and Perillo & Gish (2005), consolidating results obtained with three polymerase inhibitors (tenofovir, lamivudine and entecavir). Results are reported separately for chronically infected individuals who were HBeAg positive or negative at the time of treatment initiation. HBeAg seroconversion and HBsAg loss at 48-52 weeks of treatment. Histologic improvement is based on post treatment biopsies *As detected by PCR branched chain hybridization. For pegIFN-α, the lower limit was 105 copies/mL, for other studies 250 copies/mL.
| HBeAg+ | Milestone | Polymerase inhibitors |
PegIFN-α | PegIFNα + lamivudine |
|---|---|---|---|---|
| HBeAg sero- conversion |
19-26% | 27-30% | 24-27% | |
| HBV DNA PCR negative* |
44-76% | 25% | 69% | |
| ALT normal and/or histologic Improvement |
41-77% | ~39% | ~46% | |
| HBsAg loss | 0-3% | 3% | 3% | |
| HBeAg− | HBV DNA PCR negative* |
60-93% | 63% | 87% |
| ALT normal and/or histologic Improvement |
60-79% | 48% | 38% |
Table 4.
Suggested virological end points for new DAAs, as compared to currently available therapies. End points are based on readily measured blood levels of HBV DNA and gene products. HBeAg+ and HBeAg− patients are those with or without detectable HBeAg in the blood at the start of therapy. Viral DNA is detected by PCR, and HBeAg and HBsAg are detected by standard commercial ELISA. As indicated in Table 3, following at least 48 weeks of current therapies, up to 77% of HBeAg+ patients experience reductions of viremia to undetectable levels, 33% show HBeAg seroconversion and 1-5% have HBsAg loss (Gish, Marrero et al. 2010). Under the same regimens, up to 95% of HBeAg− patients experience reductions of viremia to undetectable levels, none have stable sustained reductions in viremia off-drug and 1-5% show loss of HBsAg.
| Circulating marker |
HBeAg+ patients | HBeAg− patients |
|---|---|---|
| viral DNA | Reductions to undetectable levels in all treated patients |
Reductions to undetectable levels in all treated patients |
| More rapid kinetics of reduction | More rapid kinetics of reduction | |
| Sustained suppression of viremia off drug |
Sustained suppression of viremia off drug |
|
| HBeAg | Seroconversion to HBeAg negativity in 100% of those treated |
Not applicable |
| HBsAg | Undetectable in all treated patients |
Undetectable in all treated patients |
In the submitted revised manuscript, footnote 9 in Table 4 refers to “HBeAg positive patients”, even though the column is headed “HBeAg negative patients.”
New therapies for chronic hepatitis B should therefore aim to achieve a “virological” cure, in which viral load, HBsAg and cccDNA are durably repressed, which should be associated with the clinical outcome of a “functional” cure. Short-term strategic advances, such as increasing the percentage of HBeAg+ patients who seroconvert, and enabling HBeAg− negative patients to come off therapy without rebound viremia, would be admirable, but they should be regarded only as tactical milestones. The true goal should be a “functional” cure in 100% of patients. Can new drugs be expected to achieve this goal? The recent growth in interest in anti-HBV therapeutics makes us optimistic. Stay tuned.
ACKNOWLEDGEMENTS
Preparation of this manuscript was supported in part by The US National Institutes of Health (The National Cancer Institute and The National Institute of Allergy and Infectious Diseases), The Hepatitis B Foundation and The Commonwealth of Pennsylvania. Dr. Mike Bray (NIH) is thanked for his helpful comments and Ms. Erica Litschi is thanked for help in manuscript preparation.
REFERENCES
- EASL Clinical Practice Guidelines: management of chronic hepatitis B. J Hepatol. 2009;50(2):227–42. doi: 10.1016/j.jhep.2008.10.001. [DOI] [PubMed] [Google Scholar]
- Amin J, Law MG, et al. Causes of death after diagnosis of hepatitis B or hepatitis C infection: a large community-based linkage study. Lancet. 2006;368(9539):938–45. doi: 10.1016/S0140-6736(06)69374-4. [DOI] [PubMed] [Google Scholar]
- Beasley RP. Hepatitis B virus. The major etiology of hepatocellular carcinoma. Cancer. 1988;61(10):1942–56. doi: 10.1002/1097-0142(19880515)61:10<1942::aid-cncr2820611003>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Belloni L, Allweiss L, et al. IFN-alpha inhibits HBV transcription and replication in cell culture and in humanized mice by targeting the epigenetic regulation of the nuclear cccDNA minichromosome. J Clin Invest. 2012;122(2):529–37. doi: 10.1172/JCI58847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block TM, Guo H, et al. Molecular virology of hepatitis B virus for clinicians. Clin Liver Dis. 2007;11(4):685–706. vii. doi: 10.1016/j.cld.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boni C, Fisicaro P, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai D, Mills C, et al. Identification of the Disubstituted Sulfonamide Compounds as Specific Inhibitors of Hepatitis B Virus Covalently Closed Circular DNA Formation. Antimicrobial Agents and Chemotherapy. 2012 doi: 10.1128/AAC.00473-12. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan HL, Thompson A, et al. Hepatitis B surface antigen quantification: why and how to use it in 2011 - a core group report. J Hepatol. 2011;55(5):1121–31. doi: 10.1016/j.jhep.2011.06.006. [DOI] [PubMed] [Google Scholar]
- Chen G, Lin W, et al. Past HBV viral load as predictor of mortality and morbidity from HCC and chronic liver disease in a prospective study. American Journal of Gastroenterology. 2006;101(8):1797–803. doi: 10.1111/j.1572-0241.2006.00647.x. [DOI] [PubMed] [Google Scholar]
- Chen YC, Jeng WJ, et al. Decreasing levels of HBsAg predict HBsAg seroclearance in patients with inactive chronic hepatitis B virus infection. Clin Gastroenterol Hepatol. 2012;10(3):297–302. doi: 10.1016/j.cgh.2011.08.029. [DOI] [PubMed] [Google Scholar]
- Chien RN, Liaw YF, et al. Pretherapy alanine transaminase level as a determinant for hepatitis B e antigen seroconversion during lamivudine therapy in patients with chronic hepatitis B. Asian Hepatitis Lamivudine Trial Group. Hepatology. 1999;30(3):770–4. doi: 10.1002/hep.510300313. [DOI] [PubMed] [Google Scholar]
- Ciupe SM, Ribeiro RM, et al. The role of cells refractory to productive infection in acute hepatitis B viral dynamics. Proceedings of the National Academy of Sciences. 2007;104(12):5050–5. doi: 10.1073/pnas.0603626104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen C, Holmberg SD, et al. Is chronic hepatitis B being undertreated in the United States? J Viral Hepat. 2011;18(6):377–83. doi: 10.1111/j.1365-2893.2010.01401.x. [DOI] [PubMed] [Google Scholar]
- Cohen J. The emerging race to cure HIV infections. Science. 2011;332(6031):784–5. 787–9. doi: 10.1126/science.332.6031.784. [DOI] [PubMed] [Google Scholar]
- Dienstag JL. Hepatitis B virus infection. N Engl J Med. 2008;359(14):1486–500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- Dougherty AM, Guo H, et al. A substituted tetrahydro-tetrazolo-pyrimidine is a specific and novel inhibitor of hepatitis B virus surface antigen secretion. Antimicrob Agents Chemother. 2007;51(12):4427–37. doi: 10.1128/AAC.00541-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Serag HB, Mason AC. Risk factors for the rising rates of primary liver cancer in the United States. Arch Intern Med. 2000;160(21):3227–30. doi: 10.1001/archinte.160.21.3227. [DOI] [PubMed] [Google Scholar]
- Evans AE, London WT, et al. Chronic HBV infection outside treatment guidelines: is treatment needed? Antiviral Therapy. 2012 doi: 10.3851/IMP2325. In press. [DOI] [PubMed] [Google Scholar]
- Fox AN, Jacobson IM. Recent successes and noteworthy future prospects in the treatment of chronic hepatitis C. Clin Infect Dis. 2012;55(Suppl 1):S16–24. doi: 10.1093/cid/cis391. [DOI] [PubMed] [Google Scholar]
- Ganem D, Prince AM. Hepatitis B infection: natural history and clinical consequences. New England Journal of Medicine. 2004;350(11):1118–29. doi: 10.1056/NEJMra031087. [DOI] [PubMed] [Google Scholar]
- Gish RG, Marrero JA, et al. A multidisciplinary approach to the management of hepatocellular carcinoma. Gastroenterol Hepatol (N Y) 2010;6(3 Suppl 6):1–16. [PMC free article] [PubMed] [Google Scholar]
- Guidotti LG, Chisari FV. IMMUNOBIOLOGY AND PATHOGENESIS OF VIRAL HEPATITIS. Annual Review of Pathology: Mechanisms of Disease. 2006;1(1):23–61. doi: 10.1146/annurev.pathol.1.110304.100230. [DOI] [PubMed] [Google Scholar]
- Guidotti LG, Rochford R, et al. Viral clearance without destruction of infected cells during acute HBV infection. Science. 1999;284(5418):825–9. doi: 10.1126/science.284.5415.825. [DOI] [PubMed] [Google Scholar]
- Guo H, Jiang D, et al. Activation of pattern recognition receptor-mediated innate immunity inhibits the replication of hepatitis B virus in human hepatocyte-derived cells. J Virol. 2009;83(2):847–58. doi: 10.1128/JVI.02008-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heathcote EJ, Marcellin P, et al. Three-year efficacy and safety of tenofovir disoproxil fumarate treatment for chronic hepatitis B. Gastroenterology. 2011;140(1):132–43. doi: 10.1053/j.gastro.2010.10.011. [DOI] [PubMed] [Google Scholar]
- Hoofnagle JH. Reactivation of hepatitis B. Hepatology. 2009;49(5 Suppl):S156–65. doi: 10.1002/hep.22945. [DOI] [PubMed] [Google Scholar]
- Hsu PN, Yang TC, et al. Increased PD-1 and decreased CD28 expression in chronic hepatitis B patients with advanced hepatocellular carcinoma. Liver Int. 2010;30(9):1379–86. doi: 10.1111/j.1478-3231.2010.02323.x. [DOI] [PubMed] [Google Scholar]
- Lanford RE, Guerra B, et al. 100 THERAPEUTIC EFFICACY OF THE TLR7 AGONIST GS-9620 FOR HBV CHRONIC INFECTION IN CHIMPANZEES. Journal of Hepatology. 2011;54(Supplement 1(0)):S45. [Google Scholar]
- Lavanchy D. Hepatitis B virus epidemiology, disease burden, treatment, and current and emerging prevention and control measures. J Viral Hepat. 2004;11(2):97–107. doi: 10.1046/j.1365-2893.2003.00487.x. [DOI] [PubMed] [Google Scholar]
- Lee LY, Tong CY, et al. New therapies for chronic hepatitis C infection: a systematic review of evidence from clinical trials. Int J Clin Pract. 2012;66(4):342–55. doi: 10.1111/j.1742-1241.2012.02895.x. [DOI] [PubMed] [Google Scholar]
- Lewin SR, Ribeiro RM, et al. Analysis of hepatitis B viral load decline under potent therapy: complex decay profiles observed. Hepatology. 2001;34(5):1012–20. doi: 10.1053/jhep.2001.28509. [DOI] [PubMed] [Google Scholar]
- Liaw YF. Clinical utility of hepatitis B surface antigen quantitation in patients with chronic hepatitis B: a review. Hepatology. 2011;54(2):E1–9. doi: 10.1002/hep.24473. [DOI] [PubMed] [Google Scholar]
- Liaw YF, Leung N, et al. Asian-Pacific consensus statement on the management of chronic hepatitis B: a 2008 update. Hepatol Int. 2008;2(3):263–283. doi: 10.1007/s12072-008-9080-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw YF, Sung JJ, et al. Lamivudine for patients with chronic hepatitis B and advanced liver disease. N Engl J Med. 2004;351(15):1521–31. doi: 10.1056/NEJMoa033364. [DOI] [PubMed] [Google Scholar]
- Lin CL, Kao JH. The clinical implications of hepatitis B virus genotype: Recent advances. J Gastroenterol Hepatol. 2011;26(Suppl 1):123–30. doi: 10.1111/j.1440-1746.2010.06541.x. [DOI] [PubMed] [Google Scholar]
- Locarnini S, Mason WS. Cellular and virological mechanisms of HBV drug resistance. J Hepatol. 2006;44(2):422–31. doi: 10.1016/j.jhep.2005.11.036. [DOI] [PubMed] [Google Scholar]
- Lok AS. Does antiviral therapy for hepatitis B and C prevent hepatocellular carcinoma? J Gastroenterol Hepatol. 2011;26(2):221–7. doi: 10.1111/j.1440-1746.2010.06576.x. [DOI] [PubMed] [Google Scholar]
- Lok AS, McMahon BJ. Chronic hepatitis B: update 2009. Hepatology. 2009;50(3):661–2. doi: 10.1002/hep.23190. [DOI] [PubMed] [Google Scholar]
- Lopatin U, Wolfgang G, et al. 737 A PHASE-I, RANDOMIZED, DOUBLE-BLIND, PLACEBO-CONTROLLED STUDY TO EVALUATE THE SAFETY, TOLERABILITY, PHARMACOKINETICS AND PHARMACODYNAMICS OF SINGLE ESCALATING ORAL DOSES OF GS-9620 IN HEALTHY SUBJECTS. Journal of Hepatology. 2011;54(Supplement 1(0)):S296. [Google Scholar]
- Ma H, Yang RF, et al. Quantitative serum HBsAg and HBeAg are strong predictors of sustained HBeAg seroconversion to pegylated interferon alfa-2b in HBeAg-positive patients. J Gastroenterol Hepatol. 2010;25(9):1498–506. doi: 10.1111/j.1440-1746.2010.06282.x. [DOI] [PubMed] [Google Scholar]
- Mason WS, Jilbert AR, et al. Clonal expansion of hepatocytes during chronic woodchuck hepatitis virus infection. Proc Natl Acad Sci U S A. 2005;102(4):1139–44. doi: 10.1073/pnas.0409332102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WS, Liu C, et al. Clonal expansion of normal-appearing human hepatocytes during chronic hepatitis B virus infection. J Virol. 2010;84(16):8308–15. doi: 10.1128/JVI.00833-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason WS, Low HC, et al. Detection of clonally expanded hepatocytes in chimpanzees with chronic hepatitis B virus infection. J Virol. 2009;83(17):8396–408. doi: 10.1128/JVI.00700-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne S, Roneker CA, et al. Immunization with surface antigen vaccine alone and after treatment with 1-(2-fluoro-5-methyl-beta-L-arabinofuranosyl)-uracil (L-FMAU) breaks humoral and cell-mediated immune tolerance in chronic woodchuck hepatitis virus infection. J Virol. 2002;76(11):5305–14. doi: 10.1128/JVI.76.11.5305-5314.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menne S, Roneker CA, et al. Immunogenic effects of woodchuck hepatitis virus surface antigen vaccine in combination with antiviral therapy: breaking of humoral and cellular immune tolerance in chronic woodchuck hepatitis virus infection. Intervirology. 2002;45(4-6):237–50. doi: 10.1159/000067914. [DOI] [PubMed] [Google Scholar]
- Menne S, Tennant BC, et al. 1114 ANTI-VIRAL EFFICACY AND INDUCTION OF AN ANTIBODY RESPONSE AGAINST SURFACE ANTIGEN WITH THE TLR7 AGONIST GS-9620 IN THE WOODCHUCK MODEL OF CHRONIC HBV INFECTION. Journal of Hepatology. 2011;54(Supplement 1(0)):S441. [Google Scholar]
- Michalak TI, Hodgson PD, et al. Posttranscriptional inhibition of class I major histocompatibility complex presentation on hepatocytes and lymphoid cells in chronic woodchuck hepatitis virus infection. Journal of Virology. 2000;74(10):4483–4494. doi: 10.1128/jvi.74.10.4483-4494.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moucari R, Mackiewicz V, et al. Early serum HBsAg drop: a strong predictor of sustained virological response to pegylated interferon alfa-2a in HBeAg-negative patients. Hepatology. 2009;49(4):1151–7. doi: 10.1002/hep.22744. [DOI] [PubMed] [Google Scholar]
- Noordeen F, Vaillant A, et al. Preclinical development of the amphipathic DNA polymer rep 9AC for the treatment of HBV infection. HepDart 2007 - Frontiers in Drug Development for Viral Hepatitis. R. F. Schinazi. Lahaina, Hawaii, Global Antiviral Journal. 2007;3:26–27. [Google Scholar]
- Norton PA, Menne S, et al. Glucosidase inhibition enhances presentation of de-N-glycosylated hepatitis B virus epitopes by major histocompatibility complex class I in vitro and in woodchucks. Hepatology. 2010;52(4):1242–50. doi: 10.1002/hep.23806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nowak MA, Bonhoeffer S, Hill AM, Boehme R, Thomas HC, McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci U S A. 1996;93(9):4398–402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberhaus SM, Newbold JE. Detection of an RNase H activity associated with hepadnaviruses. J Virol. 1995;69(9):5697–704. doi: 10.1128/jvi.69.9.5697-5704.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Op den Brouw ML, Binda RS, et al. The mannose receptor acts as hepatitis B virus surface antigen receptor mediating interaction with intrahepatic dendritic cells. Virology. 2009;393(1):84–90. doi: 10.1016/j.virol.2009.07.015. [DOI] [PubMed] [Google Scholar]
- Pan CQ, Hu KQ, et al. Response to tenofovir monotherapy in chronic hepatitis B patients with prior suboptimal response to entecavir. J Viral Hepat. 2012;19(3):213–9. doi: 10.1111/j.1365-2893.2011.01533.x. [DOI] [PubMed] [Google Scholar]
- Papatheodoridis GV, Lampertico P, et al. Incidence of hepatocellular carcinoma in chronic hepatitis B patients receiving nucleos(t)ide therapy: a systematic review. J Hepatol. 2010;53(2):348–56. doi: 10.1016/j.jhep.2010.02.035. [DOI] [PubMed] [Google Scholar]
- Perrillo RP, Gish RG, et al. Chronic hepatitis B: a critical appraisal of current approaches to therapy. Clin Gastroenterol Hepatol. 2006;4(2):233–48. doi: 10.1016/s1542-3565(05)00983-3. [DOI] [PubMed] [Google Scholar]
- Poordad F, Reddy KR, et al. Rapid virologic response: a new milestone in the management of chronic hepatitis C. Clin Infect Dis. 2008;46(1):78–84. doi: 10.1086/523585. [DOI] [PubMed] [Google Scholar]
- Richman DD, Margolis DM, et al. The challenge of finding a cure for HIV infection. Science. 2009;323(5919):1304–7. doi: 10.1126/science.1165706. [DOI] [PubMed] [Google Scholar]
- Seeff LB. Sustained virologic response: Is this equivalent to cure of chronic hepatitis C? Hepatology. 2012 doi: 10.1002/hep.25964. [DOI] [PubMed] [Google Scholar]
- Sung JJ, Tsoi KK, et al. Meta-analysis: Treatment of hepatitis B infection reduces risk of hepatocellular carcinoma. Aliment Pharmacol Ther. 2008;28(9):1067–77. doi: 10.1111/j.1365-2036.2008.03816.x. [DOI] [PubMed] [Google Scholar]
- Sung JJ, Wong ML, et al. Intrahepatic hepatitis B virus covalently closed circular DNA can be a predictor of sustained response to therapy. Gastroenterology. 2005;128(7):1890–7. doi: 10.1053/j.gastro.2005.03.009. [DOI] [PubMed] [Google Scholar]
- Takkenberg B, Terpstra V, et al. Intrahepatic response markers in chronic hepatitis B patients treated with peginterferon alpha-2a and adefovir. J Gastroenterol Hepatol. 2011;26(10):1527–35. doi: 10.1111/j.1440-1746.2011.06766.x. [DOI] [PubMed] [Google Scholar]
- Thimme R, Wieland S, et al. CD8(+) T cells mediate viral clearance and disease pathogenesis during acute hepatitis B virus infection. J Virol. 2003;77(1):68–76. doi: 10.1128/JVI.77.1.68-76.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson AJ, Muir AJ, et al. Interleukin-28B polymorphism improves viral kinetics and is the strongest pretreatment predictor of sustained virologic response in genotype 1 hepatitis C virus. Gastroenterology. 2010;139(1):120–9. e18. doi: 10.1053/j.gastro.2010.04.013. [DOI] [PubMed] [Google Scholar]
- Thompson AJ, Nguyen T, et al. Serum hepatitis B surface antigen and hepatitis B e antigen titers: disease phase influences correlation with viral load and intrahepatic hepatitis B virus markers. Hepatology. 2010;51(6):1933–44. doi: 10.1002/hep.23571. [DOI] [PubMed] [Google Scholar]
- Tsiang M, Rooney JF, et al. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology. 1999;29(6):1863–9. doi: 10.1002/hep.510290626. [DOI] [PubMed] [Google Scholar]
- Tzeng HT, Tsai HF, et al. PD-1 Blockage Reverses Immune Dysfunction and Hepatitis B Viral Persistence in a Mouse Animal Model. PLoS One. 2012;7(6):e39179. doi: 10.1371/journal.pone.0039179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CC, Holte S, et al. Kinetics of hepatitis B viral load during 48 weeks of treatment with 600 mg vs 100 mg of lamivudine daily. J Viral Hepat. 2004;11(5):443–7. doi: 10.1111/j.1365-2893.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- Werle-Lapostolle B, Bowden S, et al. Persistence of cccDNA during the natural history of chronic hepatitis B and decline during adefovir dipivoxil therapy. Gastroenterology. 2004;126(7):1750–8. doi: 10.1053/j.gastro.2004.03.018. [DOI] [PubMed] [Google Scholar]
- Wieland S, Thimme R, et al. Genomic analysis of the host response to hepatitis B virus infection. Proc Natl Acad Sci U S A. 2004;101(17):6669–74. doi: 10.1073/pnas.0401771101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wursthorn K, Jung M, et al. Kinetics of hepatitis B surface antigen decline during 3 years of telbivudine treatment in hepatitis B e antigen-positive patients. Hepatology. 2010;52(5):1611–20. doi: 10.1002/hep.23905. [DOI] [PubMed] [Google Scholar]
- Wursthorn K, Lutgehetmann M, et al. Peginterferon alpha-2b plus adefovir induce strong cccDNA decline and HBsAg reduction in patients with chronic hepatitis B. Hepatology. 2006;44(3):675–84. doi: 10.1002/hep.21282. [DOI] [PubMed] [Google Scholar]
- Yuen MF, Lai CL. Treatment of chronic hepatitis B: Evolution over two decades. J Gastroenterol Hepatol. 2011;26(Suppl 1):138–43. doi: 10.1111/j.1440-1746.2010.06545.x. [DOI] [PubMed] [Google Scholar]
- Zoulim F, Mason WS. Reasons to consider earlier treatment of chronic HBV infections. Gut. 2012;61(3):333–6. doi: 10.1136/gutjnl-2011-300937. [DOI] [PubMed] [Google Scholar]