Abstract
Maintaining attention for more than a few seconds is essential for mastering everyday life. Yet, our ability to stay focused on a particular task is limited, resulting in well-known performance decrements with increasing time on task. Intriguingly, such decrements are even more likely if the task is cognitively simple and repetitive. The attentional function that enables our prolonged engagement in intellectually unchallenging, uninteresting activities has been termed “vigilant attention.” Here we synthesized what we have learnt from functional neuroimaging about the mechanisms of this essential mental faculty. To this end, a quantitative meta-analysis of pertinent neuroimaging studies was performed, including supplementary analyses of moderating factors. Furthermore, we reviewed the available evidence on neural time-on-task effects, additionally considering information obtained from patients with focal brain damage. Integrating the results of both meta-analysis and review, a set of mainly right-lateralized brain regions was identified that may form the core network subserving vigilant attention in humans, including dorsomedial, mid- and ventrolateral prefrontal cortex, anterior insula, parietal areas (intraparietal sulcus, temporo-parietal junction), and subcortical structures (cerebellar vermis, thalamus, putamen, midbrain). We discuss the potential functional roles of different nodes of this network as well as implications of our findings for a theoretical account of vigilant attention. It is conjectured that sustaining attention is a multi-component, non-unitary mental faculty, involving a mixture of (i) sustained/recurrent processes subserving task-set/arousal maintenance and (ii) transient processes subserving the target-driven reorienting of attention. Finally, limitations of previous studies are considered and suggestions for future research are provided.
Keywords: sustained attention, alertness, vigilance, meta-analysis, ALE
Many everyday behaviors require continuous attention for more than a few seconds and, thus, rely on sustaining attention over time. Examples include scanning a supermarket shelf for a certain product, attending to a lecture, reading a book, or playing a demanding piece of music on an instrument. These examples may differ greatly in duration and cognitive complexity, but they share the requirement for maintaining attention over time. This ability to keep one’s mind continuously focused on a particular task is considered a fundamental dimension of attentional control, distinct from shifting or dividing the attentional focus or controlling its selectivity (Mirsky, Anthony, Duncan, Ahearn, & Kellam, 1991; Posner & Petersen, 1990; Raz & Buhle, 2006; Robertson, Ward, Ridgeway, & Nimmo-Smith, 1996; Sturm & Willmes, 2001; Stuss, Shallice, Alexander, & Picton, 1995; van Zomeren & Brouwer, 1994).
Anecdotal as well as experimental evidence shows that it is usually harder to maintain attention in intellectually unchallenging, monotonous situations than in cognitively demanding but interesting ones (Kahneman, 1973; Manly et al., 2003; Parasuraman, 1984; Poffenberger, 1927; Robinson & Bills, 1926; Wilkinson, 1964; see Robertson & O’Connell, 2010, for a recent review). This seemingly paradoxical, inverse relationship between cognitive challenge and effort required to sustain attention has long been known to industrial psychologists: simple, repetitive tasks requiring continuous attention were often found to be associated with increased stress responses and higher subjective effort expenditure, as compared with more complex, variable tasks (Frankenhaeuser & Gardell, 1976; Johansson, Aronsson, & Lindström, 1978; Thackray, 1981; Ulich, 1960; see also Warm, Parasuraman, & Matthews, 2008).
Vigilant Attention: Concept, Measurement, and Relevance
Concept
Acknowledging the empirical differentiation between cognitively simple and more complex tasks, Robertson and colleagues (Robertson & Garavan, 2004; see also Robertson & O’Connell, 2010) introduced the term “vigilant attention” (VA) for sustaining attention to monotonous, intellectually unchallenging tasks. Based on their definition, we use this term to denote the process of sustaining efficient conscious stimulus processing over periods longer than about 10 seconds up to many minutes. By definition, this “stimulus processing” refers to the simple detection or discrimination of stimuli, including a simple cognitive or motoric response but excluding “higher” attentional or executive functions such as spatial orienting, resolving interference, dividing attention, or selecting between several overt responses.
Our definition of VA deliberately includes instances of a rather short-term maintenance of attention, in line with models that assume VA to be implemented by “a short-cycle ‘refresh’ system … [that] operates in situations (usually dull or repetitive) where attention is not exogenously triggered by novelty or other similar processes” (Robertson, Ridgeway, Greenfield, & Parr, 1997, p. 291; see also Coull, 1998, p. 351). From experiments on temporal preparation processes, the cycle length of this endogenously controlled “refresh” system has been estimated to be much less than the minimum maintenance period (10 s) chosen here. In these experiments on preparatory attention it was shown that peak levels of preparedness cannot be maintained for more than a few seconds, requiring repreparation processes for sustaining readiness (Allegria, 1974; Gottsdanker, 1975). This makes our 10-s cut-off a rather conservative choice and roughly agrees with what others have previously considered a sustained (vs. transient) allocation of attention (cf. Cabeza et al., 2003; Robertson, Ridgeway et al., 1997; Thakral & Slotnick, 2009).
On a conceptual level, this endogenous “refresh” system was previously described (Shallice, Stuss, Alexander, Picton, & Derkzen, 2008; Stuss et al., 1995) as a set of control processes implemented by a supervisory attentional system (cf. Norman & Shallice, 1986; Shallice & Burgess, 1996). According to this framework, performing simple, repetitive and therefore easily over-learnt tasks such as simple stimulus detection or discrimination is based on activating and implementing a task schema (i.e. a memorized set of input–output rules). Theoretically, relevant schemata in routine tasks are triggered automatically by appropriate input, without the need for supervisory control. However, sustaining high performance levels over time is assumed to require top-down attentional control even in well-learnt tasks as simple as speeded stimulus detection (cf. Henderson & Dittrich, 1998). In particular, as proposed by Stuss et al. (1995): “If it is not continually used, a selected schema will gradually lose activation over several seconds, thereby decreasing its power to activate its lower-level component schemata. This can occur by intrinsic decay of activation or by the selection of some irrelevant competing schema, which then inhibits the task-schema” (pp. 195–196). On the other hand, fast-paced target presentation (i.e., continuous use of a given schema) is also no guarantee that automatic schema activation will persist over time, as over-rapid use may result in the task schema becoming increasingly refractory (Stuss et al., 1995, p. 199; see also van Breukelen et al., 1995).
Stuss et al. (1995) considered four supervisory-system processes as essential for preventing such situations and maintaining performance in VA tasks over time (see also Shallice et al., 2008): (i) monitoring the activation level of the task schema, (ii) (re)activating (“energizing”) the task schema, (iii) inhibiting conflicting schemata, and (iv) monitoring performance (i.e., checking the appropriateness of behavioral outputs against the task goal). Thus, according to this framework, VA is supported by a combination of stimulus-triggered (“bottom-up”) routine processing, in line with a fairly simple task schema, and supervisory-system (“top-down”) modulations thereof, which facilitate the recurrent implementation of the relevant schema.
Given this framework, the question remains as to what changes in these processes underlie the frequently observed difficulties in VA maintenance. First of all, laboratory research corroborates the aforementioned anecdotal evidence and observations in the field by showing that sustaining attention to simple, monotonous tasks (i.e., VA) is perceived as effortful and highly demanding, inducing subjective strain or even fatigue over time (Grier et al., 2003; Szalma et al., 2004; Warm et al., 2008). These findings led several researchers to propose that such subjective experiences as well as concurrent objective difficulties in maintaining high (initial) performance levels directly result from a depletion of attentional resources that occurs with the continuous allocation of attention (Grier et al., 2003; Helton & Warm, 2008; Smit, Eling, & Coenen, 2004). This “resource depletion” (or “mental fatigue”) account is strongly supported by several lines of research (Helton & Russell, 2011a,b; Helton et al., 2005; See, Howe, Warm, & Dember, 1995; Temple et al., 2000; see Warm et al., 2008, for a review). In the supervisory-attention framework, resource depletion would correspond to an insufficiently energized task schema.
Others, however, have argued that cognitively more challenging, interesting tasks (e.g., prolonged computer gaming vs. radar screen monitoring) can pose similar or even higher demands on attention but still fail to elicit any subjective experiences of strain and fatigue or objective performance deterioration over time; in fact, such tasks can even induce positive “flow” experiences (cf. Csikszentmihalyi, 1975). On that basis, negative subjective experiences in prolonged simple, repetitive tasks have been interpreted as reflecting the experience of boredom (Pattyn, Neyt, Henderickx, & Soetens, 2008; Scerbo, 1998), which has been found associated with increased absentmindedness and mind-wandering (Cheyne, Solman, Carriere, & Smilek, 2009; Smallwood et al., 2004; see Smallwood & Schooler, 2006, for a review). Here, mind-wandering is considered a state in which cognitive processing is driven by internally oriented goals, such as recalling previous experiences or simulating future actions. Thus, this mental “absenteeism” reflects a re-allocation of processing resources away from the VA task at hand toward some other goal and is, in turn, held responsible for the oft-observed performance decline over time (Manly, Robertson, Galloway, & Hawkins, 1999; Pattyn et al., 2008; Robertson, Manly, Andrade, Baddeley, & Yiend, 1997). In the supervisory-attention framework, this would correspond to an insufficient inhibition of competing schemata.
There is ample empirical evidence for both the fatigue/resource-depletion and the boredom/absentmindedness accounts, but neither is consistent with all the available data. We, therefore, would like to promote a recent proposal that synthesizes both views (Langner, Willmes, Chatterjee, Eickhoff, & Sturm, 2010). These authors argued that maintaining the attentional focus on an intrinsically non-rewarding (i.e. monotonous, “flow”-defying) but attentionally demanding task requires constant self-regulation (see Rueda, Posner, & Rothbart, 2011, for a detailed review on the relationship between attention and self-regulation). Thus, the account implies an imbalance between subjective costs (i.e. effort exertion) and benefits (i.e. intrinsic rewards) of maintaining VA over time. Self-regulatory power, in turn, is considered a limited resource that gets depleted with prolonged continuous use (Hagger, Wood, Stiff, & Chatzisarantis, 2010; Muraven & Baumeister, 2000). Accordingly, self-control strength should decline over time while trying to maintain vigilant attention. This decline, then, is thought to result in: (i) a diminished intensity of attention allocated to the task, leading to a weaker attentional modulation of task-relevant information processing (a state often labeled “mental fatigue” or “resource depletion”), and (ii) diminished goal maintenance, leading to task-irrelevant processing and task-unrelated thoughts (a state often labeled “absentmindedness” or “mind-wandering”). Thus, Langner, Willmes et al. (2010) consider sustained attentional demand a necessary condition (in line with resource theory) but not a sufficient one (in contrast to resource theory) for the typical time-related VA decrement to occur. In essence, Langner, Willmes et al.’s account introduced the mediator variable “self-regulatory power,” which is assumed to remain unaffected during attentionally demanding but intrinsically rewarding tasks and thus can potentially explain the specific difficulty in upholding attention during intellectually simple, monotonous tasks. It should be noted, though, that the empirical evidence directly supporting this account is sparse as yet.
Notably, all three above-mentioned accounts of time-related VA decline do not consider motivational issues, at least not explicitly. Other work, however, emphasized the relevance of motivational changes with time on task, which may lead to strategic shifts in effort investment during sustained cognitive performance (Boksem & Tops, 2008; Hockey, 1986, 1997). Broadly, these accounts assume an increasing imbalance between perceived costs and benefits of maintaining performance over time, resulting in a strategic reduction of effort invested into the VA task at hand. This switching of processing priorities can be incorporated into all three above accounts, leading either to diminished resource allocation to the VA task, interference from task-unrelated processing, or both.
Finally, we think it useful to touch briefly upon another concept that is relevant to VA: Although referring to non-identical (albeit related) constructs, the terms “arousal” and “sustained attention” (comprising the less inclusive concept “vigilant attention”) have sometimes been used interchangeably (see Parasuraman, 1984, for a related discussion). Since this indiscriminate use may have contributed to terminological confusion in research on attention, we would like to mention the conceptual differentiation: In our view, “arousal” is the net result from the joint action of several neuromodulatory brain systems; it drives the general excitability of cortical neurons and thus constitutes a basic precondition and modulator of (vigilant) attention (Fischer, Langner, Birbaumer, & Brocke, 2008; Pfaff, 2006; Sarter, Givens, & Bruno, 2001; see also Humphreys & Revelle, 1984).
This is by no means to say that we consider arousal a unitary concept — to the contrary, we take arousal to be multi-dimensional, having specialized and generalized subcomponents (cf. Pfaff, 2006; Pfaff, Ribeiro, Matthews, & Kow, 2008). As generalized arousal is thought to decrease with the predictability of the situation (Pfaff, 2006), one of the processes that contribute to the perceived high workload of simple, repetitive VA tasks might be the exertion of effort to compensate for the task-induced decrease in arousal (Fischer et al., 2008; O’Hanlon, 1981; Robertson & Garavan, 2004; Thackray, 1981). As recent research has shown, however, such attempts at compensation might be in vain: using self-report scales for measuring subjective arousal based on Thayer’s (1978) two-dimensional arousal model, it was found that prolonged VA maintenance was related to increases in avoidance-related “tense arousal” but decreases in approach-related “energetic arousal” (Helton & Warm, 2008; Szalma et al., 2004). And it is the latter arousal component that is thought to reflect the availability of attentional resources for performing the task at hand (Helton & Warm, 2008; Matthews & Westerman, 1994).
Measurement
The basic experimental paradigm for assessing VA has participants monitor their environment for a (more or less frequently occurring) pre-specified target. Mostly, one of the following paradigm subtypes has been used: (i) continuous stimulus detection (i.e., non-cued simple reaction-time tasks), (ii) continuous stimulus discrimination (i.e., non-cued go/no-go tasks), and (iii) sustained covert (i.e. silent) target counting. Stimulus-detection tasks require no stimulus identification, since all presented stimuli are targets. There is only one invariable response, and the only uncertain aspect is the exact moment of stimulus occurrence. This kind of task is typically used to assess an aspect of VA that has been described as readiness for speeded responding to unwarned stimulation, variably labeled “intrinsic alertness” (e.g., Langner, Kellermann, Eickhoff, et al., 2012; Sturm et al., 1999) or “psychomotor vigilance” (Lim & Dinges, 2008).
In the second type of paradigm (i.e., continuous stimulus discrimination), targets and non-targets are presented in an (usually unpredictably) intermixed fashion, with targets (“go” stimuli) requiring a response and non-targets (“no-go” stimuli) requiring withholding the response. Please note that forced-choice reaction-time tasks, which require selecting between two or more overt responses, are, by definition, not considered to assess VA but rather more complex attentional abilities such as concentration (cf. Alexander, Stuss, Shallice, Picton, & Gillingham, 2005; Steinborn, Flehmig, Westhoff, & Langner, 2009). Classically, go/no-go tasks used in VA research contain many more non-target (“no-go”) than target (“go”) events, including Mackworth’s well-known Clock Task (Mackworth, 1948) or the classic Continuous Performance Task (CPT; Rosvold, Mirsky, Sarason, Bransome, & Beck, 1956). Such tasks constitute the typical paradigm used in research on “vigilance” (cf. Davies & Parasuraman, 1982), which refers to maintaining VA over prolonged periods of time (i.e. at least several minutes). Some well-established variants of this paradigm type, such as the AX-CPT or the rapid visual information processing task (Wesnes & Warburton, 1983), additionally impose a modest working-memory demand, since their target definition includes sequential dependencies (i.e., target status depends on the preceding stimulus). Moreover, “reverse” vigilance paradigms with many more go than no-go events, such as Conners’ CPT (Conners, 1994) or the sustained-attention-to-response task (SART; Robertson, Manly, et al., 1997), have recently garnered much interest.
Relevance
Apart from the introductory examples regarding the real-life importance of our ability to sustain attention, we would like to point out a few more domains in which the relevance of VA maintenance becomes evident. For instance, Robertson (2003) related two examples of catastrophic traffic accidents, which arose from single lapses of attention during the prolonged performance of routine behaviours: one involved a train driver missing a critical stop signal and causing a devastating train crash; the other involved a sleep-deprived, tired car driver who accidentally drove his car off the motorway down onto a railroad track, causing the derailment of a train and killing several people. Indeed, such failures in maintaining VA are the most common cause of railway accidents (Edkins & Pollock, 1997). The second case implies that driver fatigue contributed to the fatal incident. A recent meta-analysis confirmed the special sensitivity of VA tasks to sleep deprivation (Lim & Dinges, 2010), and according to Wickens, Gordon, and Liu (1998, p. 397), fatigue is estimated to be a causal factor in about 200,000 car accidents per year.
Besides such time-critical man–machine interactions, in which remaining attentive can be vital, VA maintenance is important in all occupational settings that require sustained monitoring such as quality inspection at assembly lines, luggage screening at airports, or radar observation (the task that triggered human-factors research on vigilance in the late 1940s). Furthermore, VA is highly sensitive to psychiatric disorders and damage to the brain, substantially contributing to problems in everyday life in individuals suffering from, for example, schizophrenia (Green, 1996), attention-deficit/hyperactivity disorder (Bellgrove et al., 2005), traumatic brain injury (Robertson, Manly et al., 1997), or various neurodegenerative diseases (O’Keeffe et al., 2007).
Present Study
We think that a better understanding of the system that mediates VA (and its failures) in healthy human beings as well as in patients involves an understanding of its neural basis. Neuropsychology and, more recently, neuroergonomics are two subdisciplines, among others, that have spearheaded this view, applying knowledge about neural mechanisms to improve clinical treatments and the design of occupational or educational settings, respectively. Therefore, our study aimed at providing a synthesis of what is known about the brain circuitry involved in maintaining VA by means of a coordinate-based meta-analysis of findings from neuroimaging studies using perfusion positron emission tomography (PET) or functional magnetic resonance imaging (fMRI). Coordinate-based meta-analysis localizes the above-chance convergence of task-related brain activity across multiple neuroimaging experiments in a common 3-D reference space. In contrast to qualitative reviews, it weighs the concordance between neuroimaging results by relying on location probabilities rather than neuroanatomical nomenclature, which is often used inconsistently. Thus, coordinate-based meta-analysis is a powerful tool for synthesizing distributed neuroimaging findings in a quantitative and impartial fashion (cf. Eickhoff & Bzdok, in press).
Earlier qualitative reviews tended to converge on the conclusion that sustaining attention to simple detection or discrimination tasks was subserved by a predominantly right-lateralized network including various cortical and subcortical structures (Cabeza & Nyberg, 2000; Husain & Rorden, 2003; Parasuraman, Warm, & See, 1998; Sturm & Willmes, 2001). To our knowledge, however, our study is the first to examine quantitatively to what extent neuroimaging findings on VA converge into a core network. We also tested the neural effects of potentially important moderator variables that describe major differences between paradigms employed to assess VA. The first such variable was the duration VA needed to be maintained for without a break, as longer times of continuous attending increase the demand on the endogenous control of VA. Second, the task may or may not require overt motor responses to targets; if it does, it will involve sustained motor preparation (cf. Requin, Brener, & Ring, 1991). Third, the task may require either stimulus detection (i.e., all stimuli are targets) or stimulus identification (i.e., targets need to be discriminated from non-targets). Fourth, temporal unpredictability of event occurrence, resulting from variable interstimulus intervals, obviates short-term “resting” breaks in-between the sustained monitoring for targets and should therefore enhance the burden on the monitoring subsystem. Finally, a supplementary analysis tested for the effects of stimulus modality on VA-related brain activity across experiments.
Since the duration of uninterrupted VA maintenance is a major factor determining sustained performance (Davies & Parasuraman, 1982), the quest for neural correlates of time-related performance decrements is an important approach to discerning the relevance of particular brain areas for sustaining attention over time (cf. Parasuraman et al., 1998). For a quantitative integration, however, there have yet been too few neuroimaging studies testing such correlates. In addition to the meta-analysis, we therefore provide a qualitative review of the limited set of studies that investigated neural correlates of time on task in VA tasks. In this review, we also consider the few available studies on time-related VA changes in patients with focal brain damage. In our discussion, we integrate this evidence with the results of our meta-analysis toward a more comprehensive delineation of the neural mechanisms of VA. Finally, some implications of our synthesis for the concept of sustained attention are discussed and open research questions as well as possible approaches to answer them are pointed out.
Meta-Analysis of Neuroimaging Studies
Methods
Study Selection
We used a step-wise procedure to identify the relevant experimental studies. First, studies were selected through a standard search in the PubMed (http://www.pubmed.gov) and ISI Web of Science (http://apps.isiknowledge.com) databases using the terms ‘vigilant attention’, ‘sustained attention’, ‘continuous attention’, ‘vigilance’, ‘alertness’, or ‘continuous performance’) in combination with ‘fMRI’, ‘functional MRI’, ‘functional magnetic resonance’, ‘PET’, or ‘positron emission’. Other term combinations such as [‘attention’ AND (‘monitoring’ OR ‘tracking’)] were included to identify relevant studies that had been labeled more specifically by their authors.
To ameliorate the “file drawer” problem arising from a potential publication bias toward significant results (Rosenthal, 1979), we extended the search to studies which included VA tasks that were not the focus of the respective publication but served as a “high-level baseline” or control condition. For example, from working-memory studies that used the n-back paradigm and measured brain activity with PET or blocked fMRI designs, we included go/no-go 0-back control conditions. When the results of these control conditions were not reported in sufficient detail (but when further inclusion criteria were fulfilled; see below), corresponding authors were contacted and asked to provide the relevant data. Finally, further studies were found via the ‘related articles’ function of the PubMed database and by tracing the references from review articles and the papers identified before.
Experiments were considered relevant if they met the following inclusion criteria:
The task required participants to continuously direct their attention to external stimuli for more than 10 s.
The task posed only minimal cognitive demands, i.e. did not require more than stimulus discrimination associated with a simple overt (e.g. manual) or covert (e.g. counting) response.
The task put only minimal demands on the selectivity and “executive” aspects of attention; that is, it did not require shifting the attentional focus, suppressing distractors, resolving conflict, dividing attention or selecting between two or more overt responses.
In addition, the following selection criteria were applied:
-
-
Only studies reporting results of whole-brain group analyses as coordinates in a standard reference space (Talairach/Tournoux or Montreal Neurological Institute [MNI]) were included, while single-subject reports, results of region-of-interest analyses (e.g. Kinomura, Larsson, Gulyas, & Roland, 1996), and studies not reporting standardized stereotaxic coordinates (e.g. Lewin et al., 1996) were excluded.
-
-
Only data from healthy adults were included; results obtained from patients and children were excluded. When studies with patients included a healthy control group, the data of these healthy controls were included if separately reported or if the authors provided us with the necessary information upon being contacted.
-
-
Data from conditions with pharmacological or other “state” manipulations (e.g. sleep deprivation) were excluded, while results from normal control conditions without manipulation) were included if separately available.
-
-
Only activation data resulting from subtractions between target conditions and sensorimotor-control or resting-baseline conditions were included; thus, we did not consider deactivation data, correlations between brain activity and other predictors (such as performance or time on task; e.g. Coull, Frackowiak, & Frith, 1998), or results from connectivity analyses (e.g. Mottaghy et al., 2006).
Based on these criteria, 55 studies were identified as eligible for inclusion into the meta-analysis. Together, these studies reported 962 activation foci obtained from 1058 participants in 67 contrasts (Table A1 in the Appendix). Differences in coordinate spaces (MNI vs. Talairach space) were accounted for by transforming coordinates reported in Talairach space into MNI coordinates using a linear transformation (Lancaster et al., 2007). Convergence of reported activation coordinates was analysed for the main effect of VA-related activity as well as for the effects of potential moderator variables denoted above.
Activation Likelihood Estimation (ALE)
All meta-analyses were performed using the revised ALE algorithm for coordinate-based meta-analysis of neuroimaging results (Eickhoff et al., 2009; Turkeltaub, Eden, Jones, & Zeffiro, 2002). This algorithm seeks to identify brain areas whose activity converges across experiments more strongly than expected from a random spatial association. Reported coordinates are treated as centres of 3-D Gaussian probability distributions capturing the spatial uncertainty associated with each focus (Eickhoff et al., 2009). Hereby, the between-subject variance is weighted by the number of participants per study, since larger sample sizes are deemed to provide more reliable approximations of the “true” activation effect and should therefore be modeled by “narrower” Gaussian distributions.
Subsequently, the probabilities of all foci reported of a given experiment were combined for each voxel, yielding a modeled activation map (Turkeltaub et al., 2012). Voxel-wise ALE scores (i.e. the union across modeled activation maps) then quantified the convergence across experiments at each location in the brain. To distinguish “true” from random convergence, ALE scores were compared to an empirical null distribution reflecting a random spatial association among all modeled activation maps. The resulting random-effects inference focuses on the above-chance convergence across studies rather than the clustering within a particular study (Eickhoff et al., 2009). This null hypothesis was derived by computing the distribution that would be obtained when sampling a voxel at random from each of the modeled activation maps and taking the union of these values in the same manner as for the (spatially contingent) voxels in the original analysis (Eickhoff, Bzdok, Laird, Kurth, & Fox, 2012). The p-value of a “true” ALE score was then given by the proportion of equal or higher values obtained under the null distribution. The resulting non-parametric p-values were then cut off at a threshold of p < .05 (family-wise error–corrected at cluster level; cluster inclusion threshold at voxel level: p < .001) and transformed into Z-scores for display.
Testing for Differences Between Task Conditions
Differences between conditions were tested by first performing separate ALE meta-analyses for each condition and computing the voxel-wise difference between the ensuing ALE maps. The experiments contributing to either analysis were then pooled and randomly divided into two groups of the same size as the two sets of contrasted experiments (Eickhoff, Bzdok, et al., 2011). Voxel-wise ALE scores for these two randomly assembled groups were subtracted from each other. Repeating this process 10,000 times yielded an empirical null distribution of ALE-score differences between the two conditions. This was used for testing the significance of the observed difference in each voxel’s ALE scores by thresholding at a posterior probability of P > .95 for a true difference between the two samples. Surviving voxels were inclusively masked by the respective main effect, i.e. the significant effect of the ALE analysis for the minuend (Eickhoff, Bzdok, et al., 2011; Rottschy et al., 2012). In addition, a cluster extent threshold of k ≥ 25 voxels was applied.
Anatomical Labelling
Results were anatomically labeled by reference to probabilistic cytoarchitectonic maps of the human brain using the Maximum Probability Maps included in the Anatomy Toolbox (Eickhoff et al., 2007; Eickhoff et al., 2005) of the SPM5 software package (Wellcome Department of Imaging Neuroscience, London). Hereby, activations were assigned to the most probable histologically defined area at the respective location. This histology-based anatomical labeling is reported in each result table; references to details of the cytoarchitecture may be found in the respective table notes.
Results
Meta-Analysis Across All Included Experiments
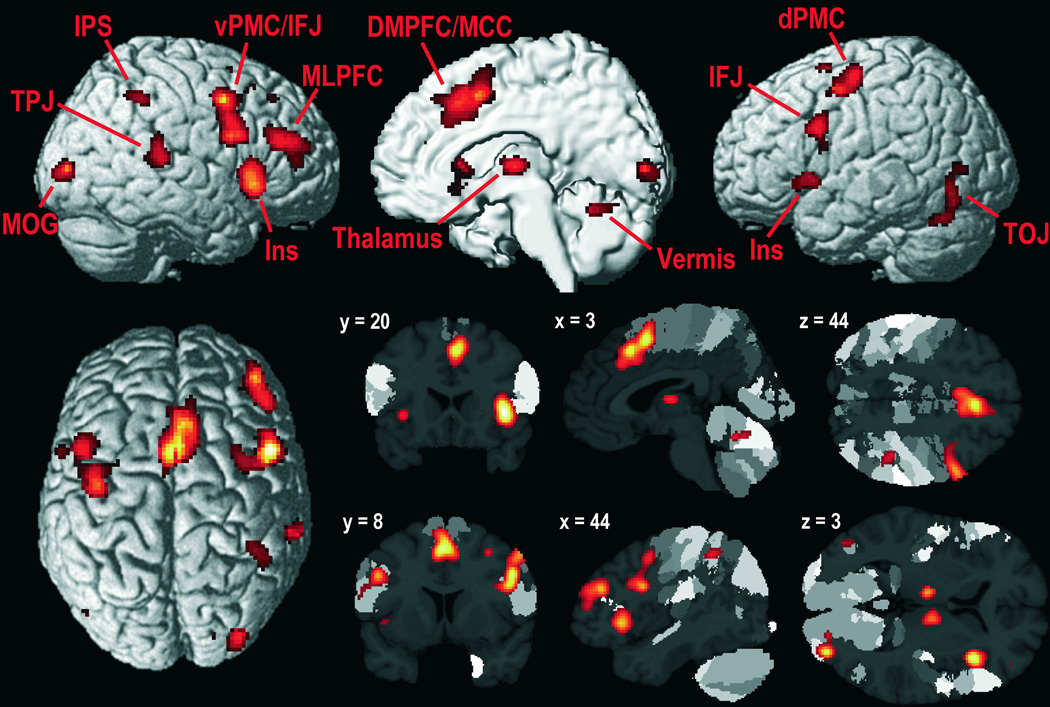
The meta-analysis of all 67 VA experiments revealed significant convergence in 14 distinct clusters (Table 1 and Fig. 1): bilateral (though right-dominant) pre-supplementary motor area (pre-SMA) and midcingulate cortex, extending to more anterior medial prefrontal cortex (PFC); bilateral inferior PFC (inferior frontal junction and dorsal area 44), extending to ventral premotor cortex (vPMC) in the right hemisphere; bilateral (though right-dominant) anterior insula and, in the right hemisphere, adjacent frontal operculum; bilateral thalamus; right midlateral PFC; right temporoparietal junction (TPJ); right inferior parietal lobule and intraparietal sulcus (IPS); right middle occipital gyrus, reaching medially to area 17; left dorsal PMC (dPMC); left temporo-occipital junction extending to fusiform gyrus; and cerebellar vermis.
Table 1.
Brain Regions of Significant Convergence of Activity Related to Vigilant Attention Across All Experiments
| Cluster/Macroanatomical Structure | x, y, z | Histological Assignment | t-score |
|---|---|---|---|
| Cluster 1 (k = 1201) | |||
| L/R anterior paracentral lobule (pre−SMA) | −2 8 50 | Area 6 | 6.4 |
| −2 6 60 | Area 6 | 5.4 | |
| R medial posterior SFG (BA 8) | 8 32 46 | - | 3.7 |
| L/R dorsal midcingulate cortex (BA 32) | 0 26 34 | - | 4.3 |
| Cluster 2 (k = 748) | |||
| R inferior frontal junction (BA 9) | 50 8 32 | - | 6.2 |
| R precentral sulcus (ventral PMC) | 50 4 42 | - | 5.2 |
| R posterior IFG (pars opercularis) | 48 6 22 | Area 44 | 5.0 |
| R posterior MFG (BA 9) | 54 8 46 | - | 4.4 |
| Cluster 3 (k = 529) | |||
| R anterior insula | 40 22 −4 | - | 6.9 |
| R inferior frontal gyrus (pars triangularis) | 42 24 4 | Area 45 | 4.4 |
| Cluster 4 (k = 431) | |||
| R inferior frontal sulcus (BA 46) | 46 36 20 | - | 6.3 |
| R MFG (BA 46) | 42 44 20 | - | 5.0 |
| Cluster 5 (k = 347) | |||
| L precentral gyrus (dorsal PMC) | −40 −12 60 | Area 6 | 6.0 |
| −40 −4 50 | Area 6 | 4.9 | |
| Cluster 6 (k = 249) | |||
| L inferior occipital gyrus | −46 −68 −6 | - | 4.4 |
| L fusiform gyrus | −40 −70 −16 | - | 3.7 |
| L middle occipital gyrus | −46 −76 4 | hOc5 (V5) | 3.5 |
| Cluster 7 (k = 231) | |||
| L inferior frontal junction | −48 8 30 | Area 44 | 5.1 |
| L posterior IFG | −58 6 20 | Area 44 | 3.4 |
| Cluster 8 (k = 217) | |||
| R temporoparietal junction | 62 −38 17 | - | 4.5 |
| 62 −36 20 | IPC (PF, PFcm) | 3.9 | |
| Cluster 9 (k = 192) | |||
| R anterior and middle thalamus | 8 −12 6 | - | 5.3 |
| Cluster 10 (k = 183) | |||
| R middle occipital gyrus | 32 −90 4 | - | 5.7 |
| R cuneus | 22 −88 6 | Area 17 | 3.8 |
| R middle occipital gyrus | 34 −96 2 | hOc3v (V3v) | 3.5 |
| Cluster 11 (k = 149) | |||
| L anterior insula | −42 12 −2 | - | 4.2 |
| −34 22 −4 | - | 4.1 | |
| Cluster 12 (k = 106) | |||
| L anterior and middle thalamus | −10 −14 6 | - | 5.3 |
| Cluster 13 (k = 104) | |||
| L/R cerebellum (vermis) | 6 −58 −18 | Lobules V, VI (Vermis) | 4.4 |
| Cluster 14 (k = 103) | |||
| R inferior parietal lobule | 44 −44 46 | IPC (PFm) | 3.9 |
| R intraparietal sulcus | 40 −42 44 | hIP2 | 3.8 |
| Additional clusters (40 < k < 80)* | |||
| R fusiform gyrus (k = 68) | 36 −60 −22 | - | 3.8 |
| R posterior inferior temporal gyrus (k = 61) | 46 −64 −12 | - | 3.9 |
| R posterior intraparietal sulcus (k = 55) | 36 −60 48 | hIP3 | 4.3 |
| L ponto−mesencephalic tegmentum (possibly PPTg) (k = 53) | −6 −22 −12 | - | 4.9 |
| R putamen (k = 53) | 26 6 0 | - | 4.1 |
| L putamen (k = 44) | −24 8 6 | - | 4.0 |
Note. Coordinates x, y, z of local maxima refer to Montreal Neurological Institute (MNI) space; k = number of voxels in cluster.
L = left; R = right; BA = Brodmann area; SFG/MFG/IFG = superior/middle/inferior frontal gyrus; pre-SMA = pre−supplementary motor area; PMC = premotor cortex; PPTg = pedunculopontine tegmental nucleus. References for histological assignments: Area 6: Geyer (2004); Area 17: Amunts et al. (2000); Areas 44, 45: Amunts et al. (1999); hIP2: Choi et al. (2006); hIP3: Scheperjans et al. (2008); hOc3v: Rottschy et al. (2007); hOc5: Malikovic et al. (2007); IPC (PF, PFm, PFcm): Caspers et al. (2006); Lobules V, VI (Vermis): Diedrichsen et al. (2009).
Significant at p < 0.05 (uncorrected at cluster-level; cluster-forming threshold at voxel level: p < 0.001).
Figure 1.
Foci of brain activity with significant convergence across all 67 experiments included in the meta-analysis (cluster-level p < .05, family-wise error–corrected for multiple comparisons; cluster-forming threshold p < .001 at voxel level). Brain sections show foci of significant convergence overlaid on the template brain with maps of cytoarchitectonically defined areas as included in the SPM Anatomy Toolbox 1.7 (Eickhoff et al., 2005). Coordinates refer to Montreal Neurological Institute (MNI) space and follow the neurological convention (left = left). DMPFC = dorsomedial prefrontal cortex (including pre-supplementary motor area); dPMC/vPMC = dorsal/ventral premotor cortex; IFJ = inferior frontal junction; Ins = anterior insula; IPS = intraparietal sulcus (including adjacent inferior parietal lobule); MLPFC = midlateral prefrontal cortex; MOG = middle occipital gyrus; TOJ/TPJ = temporo-occipital/temporoparietal junction.
Using a more lenient threshold (uncorrected cluster-level p < 0.05), additional loci of convergence were observed in right occipital areas (homotopic to the above-reported left-sided focus at the temporo-occipital junction), right posterior IPS, left medial midbrain–brainstem junction (in the vicinity of the left pedunculopontine tegmental nucleus [PPTg]), and bilateral anterior putamen (Table 1 and Supplementary Fig. S1).
Correlation of Brain Activity With Duration of VA Maintenance
The demand on the system subserving VA should increase with the time attention is to be maintained without a break. We, therefore, tested which nodes of the VA-related network identified in the main analysis (see above) were more likely to show activity with increasing time VA was maintained for without interruption. In the included experiments, these times ranged from 14 to 1800 s (M = 116.8, SD = 267.1 s; cf. Table A1). Significant positive correlations were found in a right-lateralized network comprising anterior insula, pre-SMA, midcingulate cortex, midlateral PFC, vPMC, posterior ventrolateral PFC, IPS and adjacent inferior parietal lobule, TPJ, thalamus, and cerebellar vermis (Table 2 and Fig. 2). We did not find any negative correlations; thus, no regions showed less consistent activation across studies with longer VA maintenance.
Table 2.
Brain Regions Showing Significantly Stronger Across-Experiment Convergence of VA-related Activity With Increasing Duration of Continuous Attending
| Cluster/Macroanatomical Structure | x, y, z | Histological Assignment | t-score |
|---|---|---|---|
| R anterior insula | 34 14 0 | - | 4.5 |
| R anterior paracentral lobule (pre-SMA), extending to dorsal midcingulate cortex | 2 20 52 | Area 6 | 5.9 |
| R inferior frontal sulcus, MFG (BA 46) | 38 42 18 | - | 4.5 |
| R anterior and middle thalamus | 10 −12 10 | - | 5.0 |
| R precentral sulcus (vPMC) | 46 0 38 | Area 6 | 4.6 |
| R IPS, inferior parietal lobule | 46 −44 46 | hIP1−3, IPC (PFm) | 4.5 |
| R posterior IFG (pars opercularis) | 50 8 20 | Area 44 | 4.5 |
| R/L cerebellum (vermis) | 2 −56 −22 | Lobule V | 5.7 |
| R temporoparietal junction (STG) | 60 −40 10 | - | 1.9 |
Note. Coordinates x, y, z of the cluster’s peak voxel refer to Montreal Neurological Institute (MNI) space; histological assignments refer to (parts of) the cluster (and not necessarily the peak voxel).
VA = vigilant attention; L = left; R = right; BA = Brodmann area; pre−SMA = pre−supplementary motor area; MFG/IFG = superior/middle/inferior frontal gyrus; vPMC = ventral premotor cortex; IPS: intraparietal sulcus; STG = superior temporal gyrus.
References for histological assignments: Area 6: Geyer (2004); Area 44: Amunts et al. (1999); hIP1, hIP2: Choi et al. (2006); hIP3: Scheperjans et al. (2008); IPC (PFm): Caspers et al. (2006); Lobule V: Diedrichsen et al. (2009).
Figure 2.
Foci of brain activity that show significantly stronger across-experiment convergence with increasing duration of uninterrupted maintenance of vigilant attention.
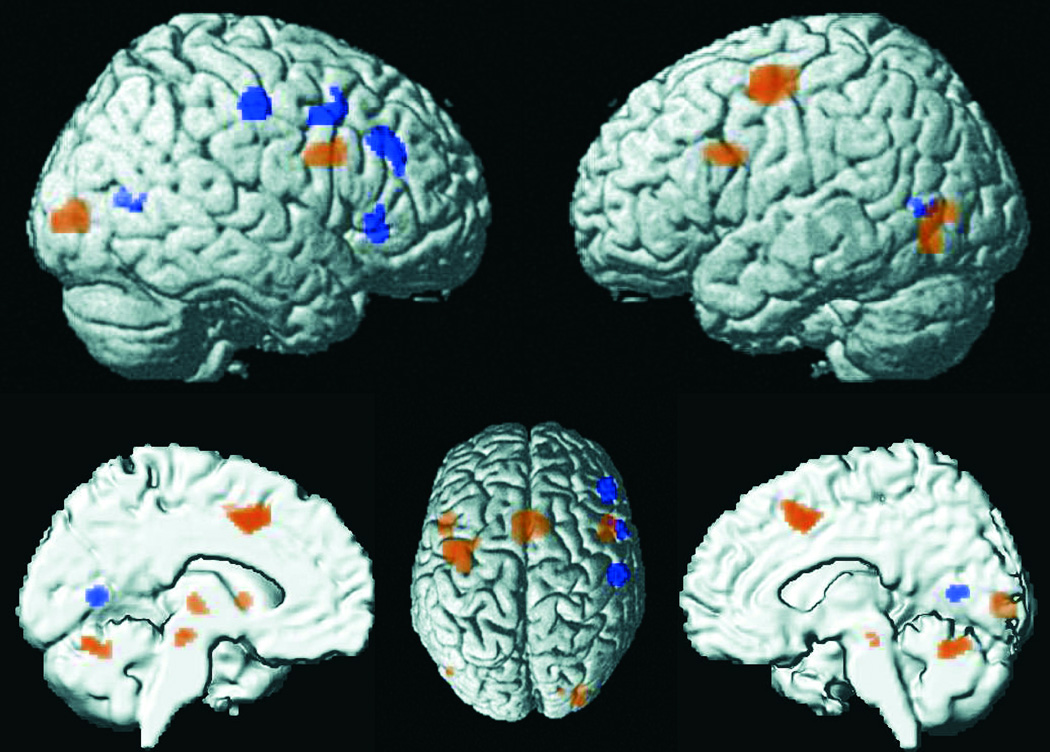
Meta-Analyses of Tasks With and Without Overt Responding
To assess how VA-related brain activity is influenced by the requirement for overt motor responses to targets, we contrasted tasks that involved motor responses (n = 55) and those that did not (n = 12). Motor tasks (vs. non-motor tasks) showed significantly stronger convergence of activity in bilateral pre-SMA, inferior frontal junction, occipital cortex, and cerebellar vermis as well as in left dPMC, putamen, ventrolateral thalamus, and brainstem (rostromedial pons) (Fig. 3 and Table S1). The absence of convergence in the primary motor cortex most likely results from the inconsistent use of the right versus left hand across experiments as well as from the fact that a substantial number of experiments (n = 11) contrasted VA-related activity against motor control conditions that involved regular button presses. Conversely, contrasting non-motor tasks with motor tasks yielded significantly stronger convergence of activity in a right-lateralized “triangle” consisting of vPMC, midlateral PFC, and ventrolateral PFC. Further foci were observed in bilateral anterior-medial occipital cortex, right postcentral gyrus, and left anterior insula (Fig. 3 and Table S2).
Figure 3.
Foci of brain activity with significantly stronger convergence in experiments involving an overt (yellow) or no overt (blue) motor response to target stimuli.
Meta-Analyses of Detection Versus Discrimination Tasks
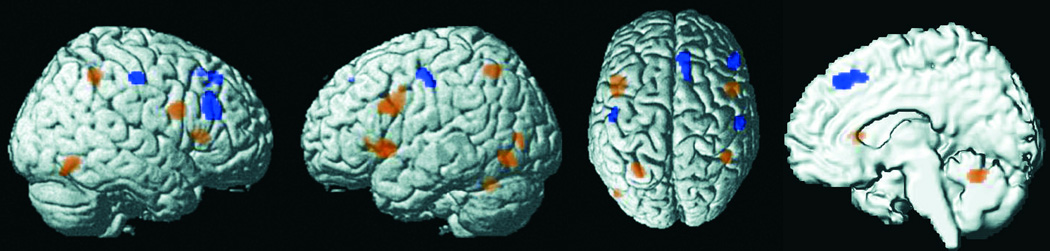
VA-related brain activity may also be affected by whether the task requires stimulus detection or discrimination. In discrimination tasks, as compared with simple detection ones, stimuli need be processed more “deeply,” as identification is required, and the stimulus–response (S-R) mapping is more complex, as each trial can either require the pre-defined response (to the “go” stimulus) or require withholding this response (to the “no-go” stimulus) (cf. Cavina-Pratesi et al., 2006). The cognitive simplicity of detection tasks, on the other hand, may render them even more repetitive and de-arousing, increasing the demand for endogenous control of VA maintenance.
To examine differences in brain activity related to the type of paradigm, we thus contrasted detection (n = 25) and discrimination (n = 42) tasks included in our sample. This comparison yielded clusters of significantly stronger convergence for detection compared with discrimination tasks in right dorsolateral and medial PFC, right postcentral gyrus as well as left posterior ventrolateral PFC (Fig. 4 and Table S3). Conversely, significantly stronger convergence for discrimination compared with detection tasks was observed in a bilateral pattern of foci comprising inferior frontal junction, intraparietal sulcus, and temporo-occipital junction as well as left anterior insula and left putamen (Fig. 4 and Table S4).
Figure 4.
Foci of brain activity with significantly stronger convergence in experiments involving detection (yellow) or discrimination (blue) tasks.
Analysis of the Effect of Time Uncertainty
A fixed temporal structure of stimulus occurrence can improve performance by directing attention to relevant moments in time according to implicit temporal expectations (Coull & Nobre, 2008). This might lead to regular fluctuations in VA level without performance loss, since VA peaks can be well synchronized with stimulus occurrence. Conversely, temporal variation of stimulus presentations hampers implicit preparation for (early) moments of potential stimulus occurrence (Niemi & Näätänen, 1981; Steinborn & Langner, 2011; 2012), and therefore, VA needs to be maintained at a more stable level over the entire time. Indeed, temporally irregular signal occurrence has been shown to negatively affect performance in VA tasks (Richter, Senter, & Warm, 1981; Scerbo, Warm, & Fisk, 1986; Shaw, Finomore, Warm, & Matthews, 2012).
To examine neural effects of such temporal uncertainty in tasks tapping VA, we contrasted the respective subsamples of experiments (tasks with temporally predictable stimulus occurrence: n = 37; tasks with temporally unpredictable stimulus occurrence: n = 30). Temporal predictability was associated with significantly stronger convergence in a bilateral network comprising inferior frontal junction, IPS, and temporo-occipital junction. Unilaterally increased convergence related to temporal predictability was observed in left anterior insula, lateral occipital cortex, putamen, and cerebellum as well as right ventrolateral PFC and cerebellar vermis (Fig. 5 and Table S5). Conversely, when contrasting tasks with temporally unpredictable against predictable stimulus occurrence, we observed significantly stronger convergence in right superior medial and midlateral PFC as well as bilateral postcentral gyrus (Fig. 5 and Table S6).
Figure 5.
Foci of brain activity with significantly stronger convergence in experiments with temporally predictable (yellow) or unpredictable (blue) stimulus occurrence.
Supplementary Analysis of Modality-Specific Effects
We also tested for the effects of using auditory (n = 13) versus visual (n = 45) stimuli Experiments using (only) tactile stimuli were too few (n = 4) for separate analysis. Contrasting auditory with visual tasks revealed significantly stronger convergence in bilateral auditory-belt regions (superior temporal and adjacent inferior parietal cortex), Broca’s region, and right ventrolateral PFC (Fig. S2 and Table S7). Conversely, comparing visual against auditory tasks yielded a network comprising bilateral visual areas (lateral occipital cortex, temporo-occipital junction) and posterior parietal cortex as well as left inferior frontal junction, left putamen, and bilateral pre-SMA extending to more anterior medial PFC and midcingulate cortex (Fig. S2 and Table S8).
Review of Studies on Neural Correlates of Time-Related Effects in VA Tasks
For the review of studies on neural correlates of time-on-task effects in VA tasks, the search was extended to studies in patients with circumscribed grey-matter lesions (excluding studies in populations with diffuse brain damage, such as traumatic brain injury, or interventions targeting white-matter tracts, such as commissurotomy). Crucially, we included only those studies in the review that reported statistical associations between time-related performance decline and lesion location, or between time on task and regional changes in hemodynamic brain activity.
Studies in Patients With Focal Brain Damage
A study by Rueckert and Grafman (1996) reported that patients with right frontal lesions, as compared to patients with left frontal lesions and healthy controls, not only showed slower overall responses and missed more targets in a detection as well as a discrimination task but also showed a steeper performance decline over 10 min in the discrimination task. In a follow-up study (Rueckert & Grafman, 1998), patients with lesions in posterior (parietal) cortex performed worse overall and showed a stronger time-related performance decline in the discrimination task. This time, however, no significant difference between right- and left-hemisphere lesions was found, although the authors themselves speculated that this null-result may have been due to a lack of statistical power.
A recent study (Malhotra, Coulthard, & Husain, 2009) in patients with right posterior parietal lesions replicated the overall deficit of patients in sustained detection as well as discrimination tasks. A more severe performance decline over 8 min, however, was only found in those right-hemisphere patients that had developed a neglect syndrome, and it was restricted to discrimination tasks that required maintaining attention to spatial locations. Another patient study (Koski & Petrides, 2001) compared the effects of right or left frontal or temporal lesions on performance in a 30-min simple-RT task with spatially cued lateralized targets. It was found that patients with right frontal damage showed a stronger time-on-task decrement than all other patient groups or healthy controls. Finally, comparing healthy participants and patients with focal PFC lesions (left or right lateral, inferior medial or dorsomedial PFC), Shallice et al. (2008) reported that only lesions in dorsomedial PFC (including anterior midcingulate cortex) were associated with stronger performance decline over time in a task requiring the silent counting of tones presented serially about every 3 s.
Studies Using Functional Neuroimaging
As with patient studies, only a handful of imaging studies have so far investigated intraindividual changes in VA-related brain activity with time on task. A landmark PET study by Paus and colleagues (Paus et al., 1997) employed a 60-min auditory discrimination task and found time-related decreases in a right-lateralized cortical network including ventro- and dorsolateral PFC, parietal and temporal cortex, as well as in mainly left-sided subcortical structures (thalamus, putamen). Regression analyses revealed that RT slowing over time was selectively associated with decreasing activity in a subnetwork comprising thalamus, striatum, midcingulum, and ponto-mesencephalic tegmentum. This time-dependent decrease was interpreted as reflecting a decline in arousal level during monotonous, highly repetitive tasks. At the same time, activity in several areas involved in processing visual information increased over time. The authors argued, however, that these increases only constituted a return of activity to baseline levels, reflecting a decrement in the cross-modal suppression of processing input in a task-irrelevant (i.e., visual) modality.
Another PET study (Coull, Frackowiak, & Frith, 1998) examined time-related changes in brain activity during a discrimination and a detection task, lasting 18 min each. RT increased significantly over time, and brain activity decreased across both tasks in right dorso- and ventrolateral PFC, right bilateral inferior parietal cortex, left anterior middle frontal gyrus, and left thalamus. Time-related increases in activity were observed in the right caudate nucleus and posterior cingulate cortex. Interestingly, the decrease in right prefrontal and inferior parietal areas was exclusively driven by the detection task. This specificity argues against a general role of these areas in regulating arousal (see also Paus et al., 1997). Rather, since a discrimination task poses stronger selectivity demands, it indicates an interaction of maintenance and selectivity aspects of attention in right frontal and parietal cortices, consistent with the above-mentioned findings on right-hemisphere lesions. The authors suggested that increased selection demands during the discrimination task counteracted the typical time-related right fronto-parietal deactivation.
Using arterial spin labeling, Lim et al. (2010) measured time-related changes in brain perfusion during a 20-min detection task. Response speed decreased significantly with time on task, and significant brain activity decreases were observed in right midlateral PFC and bilateral posterior cingulum; no time-related activity increases were reported. Further, no significant associations between neural and performance changes over time were found. These results are more circumscribed than those of previous PET studies, which may be related to methodological differences, but they again demonstrate an involvement of the right midlateral PFC in sustained speeded detection. This is in line with our results, which revealed significantly stronger convergence of activity in this area for detection compared with discrimination tasks. Finally, a recent fMRI study (Breckel, Giessing, & Thiel, 2011) investigated time-on-task effects during a 32-min simple detection task with lateralized motion distractors using standard fMRI. Over time, RT significantly increased, and hemodynamic activity decreased in a large detection-related network including bilateral ventrolateral PFC, IFJ, TPJ, anterior insula, and somatosensory cortices. No significant time-related increases were found; performance-related neural changes were not reported.
Toward a Brain Network Model for Vigilant Attention
Our study delineated the neural network involved in VA (i.e., sustaining attention to repetitive, cognitively unchallenging tasks) by means of an ALE meta-analysis of 67 neuroimaging experiments. We found that VA engages an extended cortico-subcortical network with a predominant right-lateralization. This lateralization confirms previous qualitative reviews of neuroimaging findings (e.g. Cabeza & Nyberg, 2000; Husain & Rorden, 2003) and agrees with studies using functional transcranial Doppler sonography or near-infrared spectroscopy (Helton et al., 2007; Helton et al., 2010; Hitchcock et al., 2003; Shaw et al., 2012; Shaw et al., 2009). The right-hemisphere dominance for controlling VA is also corroborated by studies using VA tasks with lateralized stimuli (Heilman & Van Den Abell, 1979; Warm, Richter, Sprague, Porter, & Schumsky, 1980; Whitehead, 1991) and by studies in patients with focal right-lateralized brain damage, who showed stronger VA impairments (i.e., worse average performance) than left-hemisphere patients (Coslett, Bowers, & Heilman, 1987; Howes & Boller, 1975; Ladavas, 1987; Posner, Inhoff, Friedrich, & Cohen, 1987). Finally, segregating brain networks based on their functional connectivity patterns during both unconstrained and task-related cognition (i.e., extracting independent components from resting-state fMRI activity and across-study co-activation patterns, respectively) yielded, among others, a right-dominant fronto-parietal brain network similar to ours (Smith et al., 2009; cf. network no. 920). This overlap contributes further evidence for the functional distinctiveness of the right-lateralized brain network observed in our analysis.
Helton et al. (2010) suggested that this VA-related right-lateralization may be related to the attentional simplicity of typical VA tasks: When the salience of targets was reduced to increase discrimination difficulty, the right-hemisphere dominance observed with easy-to-discriminate targets vanished and turned into a more symmetrical activity pattern (see also Demeter, Hernandez-Garcia, Sarter, & Lustig, 2011; Nebel et al., 2005). Similar effects were observed when comparing phasic alerting (i.e., performance in forewarned simple-RT tasks) with intrinsic alerting (i.e., performance in continuous unwarned simple-RT tasks), with the former imposing higher discrimination demands than the latter (Sturm & Willmes, 2001). The current findings support this view: compared with simple detection tasks, discrimination tasks showed increased activity in a bilateral fronto-parieto-occipital network (cf. Fig. 4 and Table S4) as well as in left anterior insula and putamen. These findings accord with cooperative-interaction models of hemispheric specialization in which cognitive processes are assumed not to be completely lateralized but to be (asymmetrically) subserved by both hemispheres, with lateralization decreasing with increasing task difficulty (Allen, 1983; Hoptman & Davidson, 1994). Findings of positive correlations between interhemispheric communication efficiency and sustained attentional performance corroborate the relevance of hemispheric interactions in VA (Rueckert, Baboorian, Stavropoulos, & Yasutake, 1999; Rueckert, Sorensen, & Levy, 1994). We conclude that the right hemisphere plays the dominant role in endogenously maintaining the attentional focus, whereas the left hemisphere is additionally recruited by cognitive challenges such as increased selection demand.
Time-Related Effects of VA Maintenance
Evidence From Patients With Focal Brain Damage
The available evidence for relationships between lesion site and time-related performance decline in VA tasks indicates that intact right frontal, bilateral dorsomedial frontal as well as posterior temporal and parietal areas are critical for VA maintenance. This is consistent with our findings of the (predominantly right-lateralized) convergence of VA-related activity in midlateral and dorsomedial PFC, IPS, and TPJ with an increasing duration of uninterrupted VA maintenance. The fact that lesion effects tended to be stronger in discrimination than detection tasks suggests that maintenance and selectivity aspects of attention share some neural substrates localized in the damaged frontal and temporoparietal areas. This view is supported by studies demonstrating that phasic alerting can transiently compensate not only for impairments in sustaining VA but also for deficits in spatial attention observed in right-hemisphere patients (Robertson, Mattingley, Rorden, & Driver, 1998; Robertson, Tegnér, Tham, Lo, & Nimmo-Smith, 1995; see also Posner, 1993). The right-hemisphere preponderance of this interaction also provides further evidence that the additional left frontal activity in response to increased selectivity demands (see above) reflects “higher” attentional processes on top of maintaining a stable attentional focus, which cannot compensate for right-hemisphere lesions.
Functional Neuroimaging Data: Positive Correlations With Time on Task
To test the notion that VA-related right-hemisphere activity is specifically related to the maintenance aspect of VA, rather than, for example, selectivity demands, we examined the positive across-study correlation between the duration VA was sustained for and the probability of brain activity to converge in a particular location. This analysis rested on the assumption that brain regions critical for sustaining VA should show more robust activity with longer durations of uninterrupted maintenance demands requiring enhanced attentional effort (Kahneman, 1973; Sarter, Gehring, & Kozak, 2006). Our analysis revealed significant associations in the right anterior insula, pre-SMA and adjacent midcingulate cortex, midlateral PFC, vPMC, posterior ventrolateral PFC (IFJ), IPS and adjacent inferior parietal lobule, TPJ, and thalamus as well as bilateral cerebellar vermis. This clear right-lateralization corroborates the view of a specific role of the right hemisphere in continuously maintaining the attentional focus.
The few neuroimaging studies that directly examined positive intraindividual associations between VA maintenance duration and brain activity yielded heterogeneous results, potentially due to methodological differences, which range from task parameters to measurement duration to imaging modality. Most likely, these factors also contribute to the small overlap with the outcome of our analysis, while this comparison may be additionally affected by differences in the level of analysis: individual studies examined the consistency of intraindividual time-related changes in brain activity across participants, whereas our meta-analytic approach examined the time-related consistency of brain activity across experiments. In spite of these discrepancies, we found an overlap between our and Breckel et al.’s (2011) findings, which corroborates the involvement of the overlapping regions (pre-SMA and mid-cingulum as well as right midlateral PFC, vPMC, and thalamus) in maintaining VA.
Functional Neuroimaging Data: Inverse Correlations With Time on Task
Not a single brain region became less consistently activated with increasing VA maintenance duration. Moreover, given the sparse reporting of deactivation patterns, we could not perform a quantitative analysis of brain regions that may consistently deactivate with time on task. However, functional neuroimaging studies on intraindividual changes with time on task repeatedly revealed activity decreases in mainly right-hemisphere regions and subcortical structures including dorso- and ventrolateral PFC, inferior parietal cortex, anterior insula, and thalamus. These findings converge with results obtained with transcranial Doppler sonography that demonstrated stronger time-on-task decrease in hemodynamic activity during sustained discrimination tasks in the right than left hemisphere (Hitchcock et al., 2003; Shaw et al., 2009; Warm, Matthews, & Parasuraman, 2009). At first surprising, the regions showing an intraindividual activity decrease with increasing time on task correspond well to the foci identified in our analysis of positive associations between across-study convergence probability and VA maintenance duration. Put differently, the brain regions that, over time, become activated with increasing consistency are often those that suffer most from prolonged VA maintenance. On a functional level, this pattern may be interpreted in terms of mental fatigue: prolonged exertion of attentional effort leads to resource depletion with associated reductions in effort and endogenous attentional control.
Although most studies reported time-related performance decrements, only few examined or observed direct associations between this decline and changes in brain activity (see Paus et al., 1997, for an exception). Therefore, the relevance of specific changes in neural activity for observed behavioral changes (and vice versa) remains an open question. Also, self-report data on subjective-state dimensions such as perceived fatigue, arousal, or motivation have hardly been collected and associated with regional changes in brain activity (see Lim et al., 2010, for an exception). Functional interpretations of neural activity changes in terms of fatigue- or arousal-related decline, therefore, rest more on indirect evidence from related research. This points to open questions regarding the relationships between brain activity, behavior and cognitive-energetic processes in tasks that tax VA.
Are Short- and Long-Term VA Qualitatively Different?
Posner and colleagues (Posner, 1978; Posner & Boies, 1971; see also Parasuraman et al., 1998) suggested that short-term VA (in the range of seconds) and long-term VA (in the range of minutes or even hours) are basically equivalent, arguing that the “foreperiod of a reaction time task may be considered as a miniature vigilance situation where alertness must be developed rapidly and maintained over a relatively brief interval” (Posner & Boies, 1971, p. 391). Indeed, our data show time-related changes in the across-study convergence probability within the network presumably subserving VA. This activation pattern is more consistent with Posner’s view, since assuming a qualitative difference between short- and long-term VA would have predicted the involvement of distinct brain regions rather than mere quantitative differences within the same network. It should be noted, though, that our interpretation only applies to maintaining VA for more than 10 s, whereas Posner’s original suggestion appears to include even shorter maintenance periods as found in typical foreperiod RT paradigms (cf. Niemi & Näätänen, 1981).
In contradiction to Posner’s hypothesis, Breckel et al. (2011) recently dissociated two brain networks related to short-term (i.e., average inter-trial interval: 20 s) versus long-term (i.e., time on task: 32 min) VA maintenance and assumed them to reflect two distinct mechanisms. Yet, the parametric-modulation approach employed decreases the probability to find spatial overlap a priori, precluding strong inferences. In particular, hierarchical orthogonalization of the parametric regressors may have resulted in the first modulator (reflecting time on task) explaining variance potentially shared between short- and long-term VA (cf. Wood, Nuerk, Sturm, & Willmes, 2008). This, in turn, might have biased the analysis of brain activity related to short-term VA duration toward subprocesses that are sensitive to energetic short-term but not long-term modulation (e.g., implicit temporal preparation; cf. Langner, Steinborn, Chatterjee, Sturm, & Willmes, 2010). From this, however, it may not necessarily be inferred that short-term VA maintenance does not involve any subprocesses that are also sensitive to long-term modulation (e.g. by fatigue) and vice versa. We, therefore, suggest that the two distinct association patterns observed by Breckel et al. rather reflect two classes of subprocesses involved in maintaining VA. Based on previous evidence, we assume that the first class of processes, being sensitive to energetic short-term modulations (within seconds), is related to the expectancy-driven establishing of a preparatory set, which is modulated by sequential trial-to-trial arousal changes (Näätänen, 1970; Steinborn & Langner, 2012; Vallesi & Shallice, 2007). In contrast, the second class of processes, being sensitive to longer-term changes (within minutes to hours), would be related to top-down monitoring and energizing of the task schema (Shallice et al., 2008; Stuss et al., 1995), which are modulated by decreasing task engagement over time (Langner, Willmes, et al., 2010; Lorist et al., 2000; Pattyn et al., 2008; Robertson, Manly, et al., 1997; Warm et al., 2008).
In conclusion, the available data on time-related brain activity changes during VA task performance agree with the notion of a right-sided network of frontal, cingulate, insular, parietal, and subcortical regions involved in maintaining VA. This appears to apply to both short- and longer-term maintenance, albeit to different degrees. We, therefore, argue that it is these regions that constitute the core brain network subserving VA.
Functional Significance of Core Network Nodes
Midlateral PFC
Significant convergence in the right midlateral PFC is corroborated by VA-related effects in this region in early neuroimaging studies not meeting our inclusion criteria (Buchsbaum et al., 1990; Cohen et al., 1988; Cohen, Semple, Gross, King, & Nordahl, 1992; Lewin et al., 1996; see also Cabeza & Nyberg, 2000) as well as by lesion data (Godefroy, Lhullier-Lamy, & Rousseaux, 2002; Rueckert & Grafman, 1996; Wilkins, Shallice, & McCarthy, 1987). Our analyses revealed that the right midlateral PFC showed stronger convergence (i) with longer VA maintenance, (ii) in tasks with covert versus overt responses, (iii) in simple detection versus discrimination tasks, and (iv) in tasks with a variable versus fixed temporal structure of event occurrence. At the same time, convergence in this region was independent of stimulus modality. This pattern argues against a specific role of this area in mediating speeded motor responses but is consistent with implementing a continuous monitoring for relevant external events. Within the framework proposed by Stuss and co-workers (Shallice et al., 2008; Stuss et al., 1995), this process is viewed as monitoring the input-induced activation level of the task schema. Monitoring is required independently of stimulus modality and may be even more demanding in the absence of overt motor action that produces sensory feedback potentially acting as an external “reminder” to reactivate the monitoring process; the same may hold for the distinction between detection versus discrimination. Further, constant monitoring is especially crucial with longer VA maintenance and in temporally variable event sequences, in which the moments of event occurrence cannot be predicted with certainty.
A role of the midlateral PFC in monitoring agrees with conclusions from patient studies. In patients with right lateral PFC lesions, Shallice et al. (2008) observed a specific impairment in a fast version of a sustained covert target counting task, which imposed more demands on event monitoring than the slow version. Furthermore, in tasks with unpredictable event onset, behavioral advantages arise from monitoring the changing conditional probability of stimulus occurrence with elapsing time since the last stimulus (Nobre, Correa, & Coull, 2007) for conversion into enhanced expectancy and preparation (Niemi & Näätänen, 1981). Since conditional probability monitoring per se seems not related to PFC activity (Cui, Stetson, Montague, & Eagleman, 2009; Janssen & Shadlen, 2005), the PFC might be more involved in this second process: the conversion of subjective probabilities into expectations or predictions. Supporting an essential role of the right PFC in conditional probability–based temporal preparation, Stuss et al. (2005) observed a specific deficit for patients with right lateral PFC lesions in gaining a response time advantage from a decrease in the conditional probability of target occurrence with increasing foreperiod length (see also Vallesi, Shallice, & Walsh, 2007). These findings may constitute instances of the more general filter function ascribed to the midlateral PFC (Corbetta, Patel, & Shulman, 2008), by which expectations are implemented across time via a selective modulation (“biasing”) of sensorimotor processing (cf. Beck & Kastner, 2009).
Inferior Frontal Junction
Our analyses yielded stronger convergence in bilateral IFJ – a region in the vicinity of the junction of the inferior frontal sulcus and the inferior precentral sulcus – (i) with longer VA maintenance (here restricted to right IFJ), (ii) in tasks with overt versus covert responses, (iii) in discrimination versus detection tasks, and (iv) in tasks with temporally fixed versus variable event occurrence. This pattern favors a role of the IFJ in mediating the mapping between target stimuli and instructed motor response, since this mapping is (i) exclusively required in VA tasks involving motor output, and (ii) it is more demanding in discrimination tasks, which require continuous decisions about the response alternative (i.e., go vs. no-go) that the stimulus is mapped onto.
IFJ involvement in setting up stimulus–response (S-R) connections is supported by Hartstra et al. (2011) reporting that left IFJ activity was associated with implementing new S-R rules. This left dominance agrees with a selective deficit in patients with left (vs. medial or right) frontal lesions (including IFJ) in acquiring S-R mappings in a choice-RT task (Alexander et al., 2005). A recent study (Verbruggen, Aron, Stevens, & Chambers, 2010) using transcranial magnetic stimulation (TMS) further delineated the role of the right IFJ by showing a specific involvement in detecting infrequent but action-relevant signals (cf. Chikazoe et al., 2009). Right IFJ may thus link non-dominant responses and stimulus features that trigger them, possibly by sending control signals to inferior parietal cortex implementing a stimulus-driven (re)orienting of attention. Finally, Ruge and Wolfensteller (2010) observed a rapid practice-related decline in bilateral IFJ activity associated with mapping instructed responses to stimuli, which reached an asymptotic level above baseline. This argues for a reduced but continuous IFJ involvement in S-R mapping over time.
Our data suggest that despite over-learning such mappings in simple VA tasks, sustaining efficiency in sensorimotor responding may depend on continuous (right) IFJ engagement. On a cognitive level, this might correspond to holding the representation of the S-R link active for continued top-down facilitation of the over-learnt mapping (see also Brass, Derrfuss, Forstmann, & von Cramon, 2005). Of note, sustained hemodynamic activity in the IFJ across a block of trials could also reflect recurrent (vs. genuinely sustained) IFJ engagement associated with repeated reactivation processes rather than continuous maintenance; mixed blocked/event-related fMRI designs might clarify this issue. The stronger involvement of the left IFJ in discrimination versus detection tasks is in line with the difficulty-related hemispheric asymmetry reduction alluded to above; however, it might also reflect (covert or even overt) verbal rehearsal of the more complex S-R mapping in discrimination tasks (cf. Friederici, 2002). Finally, the stronger convergence in tasks with a fixed (vs. unpredictable) temporal event sequence indicates that the representation of mappings may be stronger when the moment of their application can be prepared.
Dorsomedial PFC, Midcingulate Cortex, and Anterior Insula
The main analysis across all experiments yielded significant convergence in a large cluster in medial PFC, which included pre-SMA and more anterior regions of the dorsomedial PFC as well as anterior midcingulate cortex [aMCC; previously often (mis-)labeled dorsal anterior cingulate cortex based on Brodmann’s coarser segmentation (cf. Vogt, 2005)]. Our differential analyses revealed stronger convergence in central parts of this cluster (i.e., pre-SMA and aMCC) with longer VA maintenance. Further, the mid-dorsal part of this cluster (i.e., pre-SMA) showed stronger convergence in tasks with overt motor (vs. non-motor) responses, while the most anterior part of this cluster (i.e., the medial superior frontal gyrus, mSFG) did so in detection (vs. discrimination) tasks as well as in tasks with a variable (vs. fixed) temporal structure.
Pre-SMA has been associated with the cognitive control of motor output (Cieslik, Zilles, Grefkes, & Eickhoff, 2011; for review, see Nachev, Kennard, & Husain, 2008), ranging from motor preparation (Cunnington, Windischberger, & Moser, 2005; Hülsmann, Erb, & Grodd, 2003) and facilitation (Mars et al., 2009) to motor inhibition (Chen, Muggleton, Tzeng, Hung, & Juan, 2009; Picton et al., 2007). As VA tasks with motor output benefit from maintaining a preparatory (motor) set, which includes managing the balance between motor preparation and inhibition (Burle, Tandonnet, & Hasbroucq, 2010; J. R. Jennings & van der Molen, 2005), pre-SMA may subserve the sustained representation of relevant motor plans and the sustained prevention of their premature release by putting constraints on activity in non-primary motor cortical areas.
The most anterior part of the medial cluster (i.e., mSFG) appears to be selectively involved in withholding pre-planned responses (Brass & Haggard, 2007). Such “veto” decisions may play an important role for maintaining efficient responding over time in that they allow the preparation and simulation of action without execution. Indeed, activity in anterior medial PFC has been shown to code action intentions across delays (Haynes et al., 2007), and dorsomedial PFC might subserve a “brake” function that enables postponing execution until target occurrence by down-regulation of motor activity (Danielmeier, Eichele, Forstmann, Tittgemeyer, & Ullsperger, 2011). In sustained performance, this might become necessary when the distance between fluctuating motor cortex baseline activity and motor action limit becomes too small. Evidence for this assumption comes from a study (Eichele et al., 2008) that revealed a decline in dorsomedial PFC activity before erroneous responses, which may reflect a gradual “release of the brake,” shifting neural baseline motor activity too close to the motor action limit. This interpretation fits with the reported co-occurring decline in precuneus activity, a region associated with task-free cognition and mind-wandering (Christoff, Gordon, Smallwood, Smith, & Schooler, 2009), suggesting that pre-error drifts toward task disengagement mediated the “brake release.”
Simple detection tasks only involve a single response, which can be fully pre-planned and easily simulated, and typically evoke more premature responses than discrimination tasks, whose irregularly interspersed no-go trials discourage continuous motor simulation. Therefore, continuous detection tasks should more often invoke veto decisions in-between imperative stimuli, consistent with the observed stronger convergence in mSFG in these tasks. The view of mSFG activity as mediating the prevention of acting out task-related intentions is also supported by our finding stronger convergence in this region when event onsets were unpredictable. Here, the “urge to act” should arise more irregularly, potentially evoking more veto decisions to prevent premature responses.
The midcingulate cortex has been conceptualized as a region where motor intentions and motivational signals interface (Paus, 2001; see also Shackman et al., 2011). Several studies demonstrated a role of this region in response to cues that announce an impending need for sensorimotor processing (Langner, Kellermann, Boers, et al., 2012; Luks, Simpson, Feiwell, & Miller, 2002; Murtha, Chertkow, Beauregard, Dixon, & Evans, 1996; Weissman, Gopalakrishnan, Hazlett, & Woldorff, 2005). Patient studies (Alexander et al., 2005; Shallice et al., 2008; Stuss et al., 2005; Stuss, Binns, Murphy, & Alexander, 2002) indicated that the aMCC region is required for sustaining the intention and preparation to respond (overtly or covertly) in non-routine tasks or when responses must occur at a particular moment in time. Apart from such proactive processing, the aMCC has also been implicated in performance monitoring and signaling the need for attentional adjustments after committing an error (see Danielmeier & Ullsperger, 2011, for review). Furthermore, besides adapting task-specific cognitive-control parameters, such attentional adjustments should also entail the top-down regulation of midbrain and brainstem arousal systems (Aston-Jones & Cohen, 2005; Fischer et al., 2008; Mottaghy et al., 2006; Sarter et al., 2001).
Such an arousal regulation system will likely include the anterior insula, an area that was also revealed by our meta-analysis. The anterior insula is involved in representing emotional and bodily states (Craig, 2002; Critchley, Wiens, Rotshtein, Öhman, & Dolan, 2004; Kurth, Zilles, Fox, Laird, & Eickhoff, 2010) and has been associated with self-reported arousal (Knutson & Greer, 2008), interoception (Pollatos, Schandry, Auer, & Kaufmann, 2007), and sympathetic autonomic activity (Critchley, Corfield, Chandler, Mathias, & Dolan, 2000). Anatomically and functionally, it is tightly connected with the anterior and middle cingulate cortex (Augustine, 1996; Eckert et al., 2009; Medford & Critchley, 2010; Taylor, Seminowicz, & Davis, 2009).
An fMRI study (Sridharan, Levitin, & Menon, 2008) demonstrated the causal involvement of this insular-midcingulate network in switching between central-executive and default-mode networks, which presumably mediate states of task engagement versus disengagement, respectively. In fact, it has been argued that recurring intrusions of task-unrelated (“default-mode”) brain activity associated with mind-wandering challenge the integrity of task-related functional networks and lie at the heart of attentional fluctuations during sustained task performance (Sonuga-Barke & Castellanos, 2007). Thus, such periodic intrusions, which compete with VA-related activity, need be suppressed or, at least, immediately undone to protect the maintenance of VA and prevent attentional drifts away from the task at hand. Initiating this switching back to VA-related processing might be subserved by the anterior insula (cf. Sridharan et al., 2008). In accord with this claim, it was recently shown via source localization of electrocortical activity that the cue-induced switching from default activity during the inter-trial interval into a mind-set for speeded responding is led by activity in aMCC and anterior insula/inferior frontal cortex (Fischer, Langner, Diers, Brocke, & Birbaumer, 2010). Together, these findings suggest that the consistently observed anterior insula/MCC activity during VA tasks might not result from genuinely sustained but rather from frequently recurrent activity related to bringing the mind back “on track” (i.e., reactivating the VA task schema) while it is about to (i) reduce the intensity of its task engagement or (ii) even “wander off” completely. The positive association between consistency of activity in these regions and duration of VA maintenance supports this view, as drifts toward task disengagement should become more frequent over time (cf. Smallwood, O’Connor, Sudbery, & Obonsawin, 2007). In summary, the available data suggest that during VA tasks, the right anterior insula may be involved in signaling the need for attentional effort investment to facilitate target detection (i.e., to stay on, or get back to, the job at hand), particularly under “energetically” challenging conditions such as increasing time on task.
Collectively, the processes assumed to be subserved by the medial PFC and anterior insular clusters can be subsumed under what Stuss, Shallice and colleagues (Shallice et al., 2008; Stuss et al., 2005; Stuss et al., 1995) referred to as “energizing” [i.e., the (re-)activation of the currently relevant task schema without initiating motor actions] and performance monitoring. Converging evidence for this interpretation was provided by Dosenbach et al. (2006), who observed sustained activity in these frontomedial regions and the anterior insula across different tasks, suggesting that it reflected the initial implementation and stable maintenance (or recurrent reactivation; cf. Sridharan et al., 2008) of task sets (see also Dosenbach et al., 2007). Dorsomedial PFC/aMCC and anterior insula may thus monitor performance and energetic state and, if needed, provide reactive control signals to (re)engage in task-relevant processing with optimal intensity. Target regions for such control signals may include the midlateral PFC and parietal cortex for adjusting input expectations, as well as subcortical arousal systems for adjusting ascending modulatory inputs.
Midbrain Tegmentum and Thalamus
We observed a small cluster of convergence in the ponto-mesencephalic tegmentum, which was significantly more active in VA-related tasks that required an overt motor (vs. non-motor) response. The cluster was in the vicinity of the pedunculopontine tegmental nucleus, which is a major source of cholinergic innervation of the cerebral cortex by way of its projections to the thalamus and the basal cholinergic forebrain (for review, see Jones, 2003; Kobayashi & Isa, 2002). While this localization must be considered with caution given the spatial resolution of neuroimaging, there is abundant evidence for VA improvement through cholinergic modulation (for review, see Koelega, 1993; Sarter, Hasselmo, Bruno, & Givens, 2005). Furthermore, PET studies reported increased hemodynamic activity in this midbrain region during VA maintenance (Kinomura et al., 1996) and a time-related decline of its activity along with a performance decrement (Paus et al., 1997).
Tonic increases in cholinergic neurotransmission might lead to widespread enhancements of cortical arousal (Hasselmo & Sarter, 2011; Sarter et al., 2001; Steriade, Datta, Pare, Oakson, & Curro Dossi, 1990), acting in concert with noradrenergic modulation originating in the locus coeruleus (Briand, Gritton, Howe, Young, & Sarter, 2007). There also is, however, accumulating evidence for phasic, more specifically localized acetylcholine release, which enhances signal-to-noise ratios in task-relevant cortical modules and thus facilitates cue detection. For sustaining attention, such phasic acetylcholine release may be recurrently evoked, as a neural correlate of investing attentional effort, to stabilize task-relevant attentional circuits and performance over time (Hasselmo & Sarter, 2011; Kozak, Bruno, & Sarter, 2006; Sarter et al., 2006; Sarter et al., 2005). Importantly, such transient acetylcholine release is most likely under top-down control from PFC, which in turn is partially driven by glutamatergic input from mediodorsal thalamus (Hasselmo & Sarter, 2011), which likewise showed significant convergence in our data.
Thalamic activity during VA, however, may also be related to mediating cortical arousal via relaying the inputs of noradrenergic and other brainstem arousal systems to the cortex (McCormick, 1992; Paus, 2000; Sarter et al., 2001). Especially the noradrenergic system has been considered essential for maintaining alertness (Posner & Petersen, 1990; Smith & Nutt, 1996). Later research confirmed that the tonic firing mode of the diffuse noradrenergic projections to thalamus and cortex is more related to regulating general arousal than specific attentional functions (Aston-Jones & Cohen, 2005; see also Sarter et al., 2001). Concurrent evidence was provided by a genetic study, in which noradrenergic genotype was found to predict lapses in VA, possibly mediated via effects on the physiological efficiency of the VA-related brain network (Greene, Bellgrove, Gill, & Robertson, 2009). One reason for not observing significant convergence in the vicinity of the noradrenergic locus coeruleus in our analysis (despite some reports in individual studies) might be the difficulty to reliably detect activity in small brainstem structures with standard neuroimaging approaches.
Regarding thalamic activity during VA tasks, neuroimaging studies revealed that it not only decreases over time (Coull et al., 1998; Paus et al., 1997) but also varies as a function of arousal changes induced by sleep deprivation (Thomas et al., 2000, 2003; Wu et al., 1991), falling asleep (Hofle et al., 1997), or pharmacological challenges (Coull, Frith, Dolan, Frackowiak, & Grasby, 1997; Fiset et al., 1999). However, decreased thalamic activity under low-arousal conditions was also found to bounce back when new demands on attention were imposed by the experimenter (Coull et al., 1997; Portas et al., 1998). This is consistent with the view that maintaining arousal (or compensating its time-related decline) is an integral part of maintaining VA (cf. Introduction). Thus, the consistent thalamic activity we observed may be taken to indicate that arousal did not drop substantially in the majority of tasks under study. On the other hand, this activity might in part reflect the exertion of compensatory attentional effort. The positive correlation between thalamic activity and duration of VA maintenance supports this reasoning, since increasing maintenance duration poses a growing challenge for the VA system (cf. Sarter et al., 2006).
Temporoparietal Junction
Our analyses revealed significant convergence of right TPJ activity across all experiments but in particular for longer VA maintenance. Increased activity in this supramodal association area was previously observed when task-relevant sensory changes in the environment were detected (Downar, Crawley, Mikulis, & Davis, 2000) or expectations about stimuli were violated (Corbetta & Shulman, 2002). Recent theorizing (Jakobs et al., 2012) conceptualized the right TPJ as a crucial cortical node for the integration of stimulus input with task context (i.e., instructions and expectations), including the comparison of prepared motor programs with current requirements and/or updating of action-related expectations (cf. Eickhoff, Pomjanski, Jakobs, Zilles, & Langner, 2011). We would thus suggest that the right TPJ contributes to optimal VA task performance by comparing expectations with incoming sensory information, providing an interface between top-down and bottom-up processing and facilitating the stimulus-driven (re)orienting to those inputs that are task-relevant (see also Corbetta et al., 2008; Downar, Crawley, Mikulis, & Davis, 2001).
This reasoning implies that the right TPJ should also become active when attention has drifted away from the task and needs to be re-focused once a new task-relevant event occurs. Indeed, an fMRI study (Weissman, Roberts, Visscher, & Woldorff, 2006) corroborated this implication by showing that right TPJ activity on the current trial was correlated with RT on the subsequent, but not current, trial, presumably reflecting post-lapse reorienting of attention. In this case, reorienting may be triggered by internally generated signals of the performance monitoring system (cf. Smallwood, Riby, Heim, & Davies, 2006), most likely subserved by the dorsal anterior/middle cingulate cortex (Ridderinkhof, Ullsperger, Crone, & Nieuwenhuis, 2004). This assumption is supported by increased cingulate activity on trials with slow responses (Weissman et al., 2006). Finding increased TPJ convergence with longer VA maintenance is concordant with assuming that lapse- or error-triggered reorienting occurs increasingly often over time, along with the typical time-related increase in the number of attentional drifts away from the task (as expressed, e.g., by the number of very slow responses that are immediately followed by much faster responses; cf. Bertelson & Joffe, 1963).
Intraparietal Sulcus
Beyond the main effect, our analyses yielded stronger convergence in right anterior IPS and adjacent inferior parietal cortex (i) with longer VA maintenance, (ii) in discrimination versus simple detection tasks, and (iii) in tasks with fixed versus variable temporal structures of event occurrence. An essential role of this region in maintaining VA is also supported by deficits in patients with parietal lesions (Malhotra et al., 2009; Rueckert & Grafman, 1998).
The IPS is associated with guiding the focus of attention in space (for review, see Corbetta & Shulman, 2002; Posner & Petersen, 1990; or Raz & Buhle, 2006) but appears to be also involved in non-spatial attentional orienting (see Husain & Rorden, 2003), such as directing attention to stimulus modality (Langner, Kellermann, Boers, et al., 2012) or moments in time (Coull & Nobre, 1998). Synthesizing several lines of research, Ptak (2012) argued for a central role of this region in computing a feature- and modality-independent priority map of the environment. This priority map is thought to integrate multidimensional feature information provided by sensory cortex and representations of behavioral goals and expectations originating in frontal cortex (see also Bisley and Goldberg, 2010). Since perceptually more demanding tasks should benefit more from attentional facilitation, the increased IPS activity observed in response to discrimination (vs. simple detection) demands supports this reasoning. The greater IPS activity in VA tasks with predictable event onsets also concurs with this notion, since attention will be voluntarily directed to the relevant moments in time under such conditions.
Given a balance between attentional drifts away from the task and reorienting toward it during sustained performance (cf. above; see also Smallwood & Schooler, 2006), we assume that IPS activity does not reflect a steady “holding” of the attentional focus (and its intensity; cf. Kahneman, 1973; Spitzer, Desimone, & Moran, 1988) but rather recurrent readjustments thereof. Since attentional readjustments can only occur after the preceding disengagement of attention, the reorienting process should engage both IPS and TPJ (Corbetta et al., 2008). Indeed, both regions are frequently co-activated during target detection and stimulus-driven reorienting (Corbetta & Shulman, 2002; Giessing, Thiel, Rösler, & Fink, 2006; Jakobs et al., 2012; Kincade, Abrams, Astafiev, Shulman, & Corbetta, 2005; Marois, Leung, & Gore, 2000).
Cerebellar Vermis
Our meta-analyses yielded stronger convergence in the cerebellar vermis (i) with longer VA maintenance, (ii) in tasks with overt versus covert responses, and (iii) in tasks with fixed versus variable temporal structures of event occurrence. This suggests a specific role for the cerebellar vermis in the anticipatory timing of motor output, in line with a study (Diedrichsen, Verstynen, Lehman, & Ivry, 2005) that found impaired anticipatory timing of postural adjustments in patients with cerebellar lesions (see also Trillenberg, Verleger, Teetzmann, Wascher, & Wessel, 2004) as well as a functional-connectivity analysis (Pollok, Gross, Kamp, & Schnitzler, 2008) that revealed cerebellar involvement in both anticipatory motor control and mismatch-contingent updating of internal sensorimotor models. Together, these findings suggest that the medial cerebellum supports efficient performance in VA tasks with motor output by synchronizing motor preparation with the predicted temporal structure of target events. This conjecture is further corroborated by a recent study (Michael, Garcia, Bussy, Lion-Francois, & Guibaud, 2009), which demonstrated that the absence of the cerebellar vermis because of a congenital dysplasia is related to an impairment in endogenously maintaining responsiveness to visual signals.
Theoretical Implications
In this section, we summarize some implications our results have for the question of how top-down and bottom-up processes may interact in the control of VA. Here, “top-down” refers to the goal-directed facilitation of input and response processing by attentional biasing, while “bottom-up” refers to processes driven by stimulus input and this input’s ability to attract attention and activate appropriate responses. In the aforementioned conceptual framework proposed by Stuss, Shallice and co-workers (Shallice et al., 2008; Stuss et al., 2005; Stuss et al., 1995), bottom-up control of VA corresponds to the automatic, input-driven activation and selection of the relevant task schema (“contention scheduling”; cf. Norman & Shallice, 1986; Shallice & Burgess, 1996). In contrast, top-down control of VA is thought to comprise several supervisory-system processes (i.e., energizing the relevant task schema and suppressing irrelevant schemata as well as monitoring schema activation level and implementation success) that together mediate the goal-driven facilitation of task-relevant processing.
Our meta-analysis revealed a network that substantially overlaps with the right-lateralized ventral attention network (Corbetta & Shulman, 2002), which has consistently been associated with the stimulus-driven (bottom-up) reorienting of attention (Corbetta et al., 2008). In an event-related fMRI study (Shulman et al., 2003), the ventral attention network was activated during a continuous visual search task when a target was detected but deactivated between targets, whereas the right IPS and frontal eye fields (i.e., parts of the dorsal “top-down” attention network) showed sustained activity. Similarly, there is extensive overlap between the VA-related network and regions activated by the sudden recognition of a slowly revealed target (Ploran et al., 2007). These and related (e.g., Shulman, Astafiev, McAvoy, d’Avossa, & Corbetta, 2007; Todd, Fougnie, & Marois, 2005) findings suggest that top-down filter signals, possibly from midlateral PFC, convey information on the task relevance of given sensory inputs and enable the selective, stimulus-driven activation of the ventral system for reorienting attention to task-relevant inputs and preventing reorienting to irrelevant events.
Although inferences from brain activity patterns on cognitive processes are necessarily limited (cf. Poldrack, 2006), the substantial overlap between the ventral attention network and the core VA network suggests that sustaining VA may entail maintaining a state in which target stimuli can optimally elicit a reorientation of attention to themselves. Put differently, VA may constitute a task set that enables (recurrent) efficient task-contingent attentional capture (cf. Folk, Remington, & Johnston, 1992; see also Kiefer & Martens, 2010; Serences et al., 2005). According to the attentional-control framework proposed by Stuss and colleagues (Shallice et al., 2008; Stuss et al., 2005; Stuss et al., 1995), such a state is maintained via monitoring the degree of the task schema’s activation and implementation and, if needed, reactivating the relevant schema. In Figure 6, we present a putative and simplified hierarchical model of how these processes might map onto the cortical core network nodes revealed in our meta-analysis.
Figure 6.
Simplified hierarchical model of the putative functions and interrelations of cortical core nodes of the brain network involved in vigilant attention (see text for details). Solid lines denote top-down signaling; broken lines denote bottom-up signaling; dotted lines denote within-level signaling.
Abbreviations: aIns = anterior insula; aMCC/dmPFC = anterior midcingulate cortex; IFJ = inferior frontal junction; I-O = Input–Output; IPS = intraparietal sulcus; MLPFC = midlateral prefrontal cortex; TPJ = temporoparietal junction.
Our conclusions contradict a view of VA as simply constituting a continuous form of focused, goal-directed attention, being controlled in a purely top-down manner. Rather, they agree with viewing sustained attention as a product of top-down and bottom-up modes of control (Sarter et al., 2001; see also Egeth & Yantis, 1997). Behavioral evidence for an interaction of both processes in VA is, for instance, provided by greater time-on-task performance decrements with less conspicuous targets (Helton & Warm, 2008; Langner, Willmes, et al., 2010), which typically arise from a stronger decline in top-down attentionally mediated observer sensitivity (Langner, Eickhoff, & Steinborn, 2011; see See et al., 1995, for review). Within this framework, time-on-task performance decrements would reflect either increasingly less efficient contingent attentional capture (i.e., reductions in top-down modulatory intensity) or gradual shifts from predominantly contingent to more frequent non-contingent capture (i.e., reductions in top-down modulatory selectivity). It remains to be shown which of the presumed stimulus-driven and/or supervisory-system processes are affected by prolonged time on task under what circumstances. So far, most accounts have focused on changes in top-down regulation, resulting from a depletion of some (often underspecified) mental resource(s) (Grier et al., 2003; Langner, Willmes, et al., 2010; Smit et al., 2004; Warm et al., 2008; cf. Introduction) or strategic-motivational shifts in attentional effort investment (Boksem & Tops, 2008; Hockey, 1986, 1997).
At any rate, the existence and predictability of time-related VA decrements provides a powerful example for the pervasive influence of “energetic” factors on human cognition (Hockey, Coles, & Gaillard, 1986). Such factors have been considered noise in many psychological models of human information processing, which has often limited these models’ predictive or explanatory value in applied settings. Observing VA-related brain activity and its change occurring with increasing time on task is a reminder of the biological grounding of human cognition, which emerges from a “wet” (i.e. physiologically based) mind (Kosslyn & Koenig, 1992) and is subject to modulations by fatigue, effort, circadian rhythms, etc.
Finally, our analysis of VA-related brain activity corroborates the long-standing view that maintaining VA is no mean task, as it involves the intricate interplay of a substantial number of brain regions. The differential sensitivity of these regions to the duration of VA maintenance suggests, along with other evidence, that maintaining VA is not a unitary process. This provides further support for multi-process models of VA regulation, as, for example, proposed by Stuss et al. (1995). Recognizing this, one might not consider the seemingly paradoxical dissociation between (low) intellectual demand and (high) subjective effort expenditure all that paradoxical anymore.
Limitations and Future Directions
Limitations of the Meta-Analysis
As meta-analyses are based on the available empirical data, their results may be affected by a publication bias in the literature that disfavors null results (R. G. Jennings & Van Horn, 2012; Rosenthal, 1979). We were able to mitigate this bias by including a set of results from VA tasks that had served as control conditions and, therefore, had not been published as stand-alone findings. Moreover, as detailed elsewhere (Eickhoff & Bzdok, in press; Rottschy et al., 2012), coordinate-based meta-analyses of neuroimaging data are less susceptible to publication bias than standard meta-analytic approaches that examine effect sizes, as the assessment of spatial convergence across experiments would not be affected by additionally including (observed but unpublished) null results. We, therefore, are confident that the validity of our results was not substantially undermined by such bias.
Second, we were not able to include brain activity correlates of performance parameters or time on task in our quantitative analysis, since such results are yet too sparse. However, we conducted a qualitative review of the few pertinent data from functional neuroimaging and patient studies and integrated the results in our discussion to strengthen our conclusions on the functional significance of the brain areas involved in maintaining VA.
Future Directions
Knowing that there are so many brain network nodes involved in VA regulation, it should be little surprising that there are so many disorders of mind and brain associated with VA impairments. These impairments are by no means restricted to pathological hypofunction — they also include dysfunctional up-regulation, as exemplified by the hypervigilance syndrome in post-traumatic stress disorder. The challenge for studies to come is to delineate the neurocomputational operations of each network node during VA maintenance and their contribution to the behavioral outcome. Understanding the functional significance of the different parts of the VA system should also help to understand its failures. This, in turn, has direct relevance for the prediction, diagnosis, and treatment of deficient VA regulation in various neurological and psychiatric patient groups, since understanding the neural basis of specific cognitive subprocesses opens the window to more specifically targeted diagnostic approaches and therapeutic interventions, holding the promise of substantially improved outcomes.
For instance, one avenue for improving the therapy of VA deficits may entail the use of real-time fMRI neurofeedback to support the training of self-alerting strategies, which have proven successful in alleviating attentional deficits after brain damage (Robertson et al., 1995). In fact, it has already been demonstrated that such self-alert training benefits from being combined with an autonomic arousal biofeedback protocol (O’Connell et al., 2008). Another possibility may lie in the treatment of attention-deficit/hyperactivity disorder, where the classic electroencephalography-based neurofeedback methods could be combined with fMRI. This way, learning to self-regulate one’s brain activity might not only be focused on relevant electrocortical frequency bands but also on relevant brain structures. Finally, examining the neural correlates of VA dysfunction in different mental disorders may also reveal surprising commonalities across pathophysiological features that in turn suggest the application of established therapeutic approaches for a given condition to other ones. Recently, for example, it has been proposed, in part on physiological grounds, that psychostimulants, being an established treatment for attention-deficit/hyperactivity disorder, could also be useful in the treatment of mania (cf. Hegerl, Himmerich, Engmann, & Hensch, 2010).
A deeper understanding of the (neural) mechanisms involved in VA regulation should benefit from simultaneously manipulating several of the key factors that determine performance in VA tasks, such as target salience, task duration, task variability, or incentives. Incorporating these dimensions into the designs of neuroimaging or patient studies has only just begun. On the dependent-variables side, self-report measures have already been developed (e.g., Matthews et al., 2002) to capture multiple dimensions of subjective state such as perceived fatigue, effort, mind-wandering, and hedonic tone. Future studies may benefit from analyzing both brain–behavior as well as brain–subjective-state relationships across and within participants. This should also include self-report data collected during neuroimaging sessions, as has been successfully demonstrated for thought probes in studies on mind-wandering (Christoff et al., 2009; Stawarczyk, Majerus, Maquet, & D’Argembeau, 2011). Integrating multi-dimensional data on neurobiology, performance, and subjective state should lead to more differentiated and firmer conclusions about the specific functional roles of the brain regions involved in VA (cf. Smallwood, Beach, Schooler, & Handy, 2008).
Furthermore, apart from inter individual differences, analyzing neural correlates of intra individual differences in behavior (e.g., the comparison of trials with fast versus slow responses; cf. Drummond et al., 2005) might be revealing. Since increasing time on task is presumably correlated with changes in several variables (e.g., facets of arousal, fatigue, task engagement, or effort investment), the functional interpretation of time-related changes in brain activity might benefit from a moderation analysis approach, which examines how within-subject associations between brain and performance or subjective state are moderated by time on task, including non-linear relationships (cf. Giambra & Quilter, 1987).
Another issue concerns the unit of measurement in functional neuroimaging. Assessing both sustained and transient processes simultaneously (e.g., by applying mixed blocked/event-related designs; cf. Donaldson, Petersen, Ollinger, & Buckner, 2001) may help to differentiate the neural correlates of genuinely tonic versus recurrent phasic processes involved in maintaining VA. To this end, it might also be useful to employ paradigms with event and target rates at intermediate levels: high event rates, as in rapid visual information processing tasks, obviate the separate analysis of brain activity associated with each event and may provide undesirably strong bottom-up stimulation or may produce neural adaptation. Conversely, very low target rates, as in many vigilance tasks, may not yield enough relevant data points for the reliable modeling of target-related brain activity. Furthermore, understanding the neural mechanisms of VA should also profit from moving beyond regional brain activity toward integrating distributed activation patterns by examining functional and effective connectivity between network nodes.
Finally, more studies are needed on time-related performance changes in patients with focal brain damage, since the lesion approach allows inference on regions and their connections that are essential to VA maintenance. These studies, however, need to carefully examine the effects of time since lesion occurrence, since the brain’s plasticity might lead to compensatory adaptations that mask the “essentiality” of the damaged area (Rehme, Eickhoff, Wang, Fink, & Grefkes, 2011). To facilitate connecting results of patient and neuroimaging studies, sophisticated analysis methods such as voxel-based lesion–symptom mapping (Bates et al., 2003) have been developed. Finally, TMS should be used as a complementary approach to study virtual lesion effects in healthy individuals (Chouinard & Paus, 2010).
Conclusion
We synthesized current knowledge about the neural mechanisms of human VA, a major attentional function that enables us to stay focused on intellectually simple, monotonous yet attention-demanding tasks. To this end, we combined a coordinate-based meta-analysis of pertinent neuroimaging data with a review of neuroimaging and patient studies of time-on-task effects on VA. The meta-analysis quantitatively tested VA-related regional brain activity for across-study consistency, while the review qualitatively summarized individual studies that tested for time-related changes in regional brain activity or associations between time-related performance decrease and brain lesion site in VA tasks.
Taken together, our analyses and review provided evidence for a mainly right-lateralized cortico-subcortical network subserving VA maintenance. The putative core network comprises dorsomedial, mid- and ventrolateral PFC, anterior insula, and parietal areas (IPS, TPJ) as well as cerebellar vermis, thalamus, basal ganglia (putamen) and midbrain. Based on these and previous findings we conjecture that instead of simply maintaining a steady attentional focus, sustaining VA might rather be conceptualized as a mixture of (i) sustained and/or recurrent top-down processes related to task-set/arousal maintenance and (ii) transient bottom-up processes related to the target-driven reorienting of attention. Even though this notion needs further elaboration and empirical testing, the current neurobiological evidence clearly disfavors views that consider VA (or, more generally, sustained attention) a unitary attentional function.
Supplementary Material
Acknowledgments
This research was supported by National Institute of Mental Health grant 1R01MH074457-01A1 and the Initiative and Networking Fund of the Helmholtz Association within the Helmholtz Alliance on Systems Biology (Human Brain Model). We thank all contacted authors who contributed results of relevant contrasts not explicitly reported in their original publications, and we apologize to all authors whose eligible papers we might have missed. We are grateful to Ian Robertson, Jonathan Smallwood, Deak Helton, Merim Bilalić, Danilo Bzdok, and Susanne Leiberg for providing valuable comments on an earlier version of this paper. We are especially indebted to Thomas Fischer and Michael Steinborn for helpful discussions surrounding many issues touched upon in this work.
Appendix
Table A1.
Description of All Contrasts Included in the Meta-Analysis
| Study | n | Contrast | Task | Modality | Motor Response |
Time (s) | Event Rate |
WM | TP |
|---|---|---|---|---|---|---|---|---|---|
| Adler et al. 2001 | 9 | task > sensory control | discrim | visual | yes | 30 | high | yes | yes |
| Adler et al. 2001 | 9 | task > sensory control | discrim | visual | yes | 30 | high | no | yes |
| *Ayalon et al. 20091 | 14 | task > rest | discrim | visual | yes | 21 | high | no | yes |
| Belin et al. 1998 | 7 | task > sensory control | discrim | auditory | no | 120 | high | no | yes |
| Belin et al. 2002 | 7 | task > sensory control | discrim | auditory | no | 120 | high | no | yes |
| Benedict et al. 1998 | 7 | task > rest | discrim | auditory | yes | 60 | high | no | yes |
| Benedict et al. 2002 | 12 | task > sensory control | discrim | auditory | no | 60 | high | no | yes |
| *Cabeza et al. 2003 | 20 | task > rest | detect | visual | no | 15 | low | no | no |
| Coull et al. 1996 | 8 | task > rest | discrim | visual | yes | 150 | high | yes | yes |
| Coull et al. 1997 | 13 | task > rest | discrim | visual | yes | 90 | high | yes | yes |
| Coull & Frith 1998 | 6 | task > rest | discrim | visual | yes | 90 | high | no | yes |
| Coull & Frith 1998 | 4 | task > rest | discrim | visual | yes | 90 | high | no | yes |
| Eyler et al. 2004 | 10 | task > rest | discrim | visual | yes | 21 | high | no | yes |
| Fassbender et al. 2004 | 21 | task > rest | discrim | visual | yes | 90 | high | no | yes |
| Gamma et al. 2001 | 11 | task > sensory control | discrim | visual | yes | 60 | high | yes | yes |
| *Gilbert et al. 2006 | 14 | task > motor control | detect | visual | yes | 24 | high | no | no |
| Gitelman et al. 1996 | 8 | task > sensorimotor control | detect | auditory | yes | 60 | low | no | no |
| Goldstein et al. 2007 | 12 | task > rest | discrim | visual | yes | 63 | high | no | yes |
| *Habel et al. 20072 | 47 | task > rest | discrim | visual | yes | 30 | high | no | yes |
| Herath et al. 2001 | 10 | task > sensory control | detect | tactile | yes | 30 | high | no | no |
| Herath et al. 2001 | 10 | task > sensory control | detect | visual | yes | 30 | high | no | no |
| Holcomb et al. 1998 | 12 | task > rest | detect | auditory | yes | 60 | high | no | yes |
| *Honey et al. 2005 | 12 | task > sensory control | discrim | visual | yes | 30 | high | no | yes |
| *Hong et al. 2011 | 24 | task > sensory control | discrim | visual | yes | 90 | high | yes | yes |
| *Hong et al. 2011 | 24 | task > rest | discrim | visual | yes | 90 | high | no | yes |
| Horn et al. 2003 | 12 | task > sensory control | discrim | auditory | no | 32 | high | no | yes |
| Johannsen et al. 1997 | 16 | task > sensory control | discrim | visual | no | 40 | high | no | no |
| Johannsen et al. 1997 | 16 | task > sensory control | discrim | tactile | no | 40 | high | no | no |
| Kansaku et al. 2004 | 10 | task > rest | detect | mixed | no | 27 | high | no | no |
| Kansaku et al. 2004 | 10 | task > rest | detect | mixed | yes | 27 | high | no | no |
| Kawashima et al. 1996 | 9 | task > sensory control | discrim | visual | yes | 60 | high | no | no |
| Kim et al. 2006 | 12 | task > rest | discrim | visual | yes | 360 | low | no | no |
| *Krug et al. 2008 | 85 | task > sensory control | discrim | visual | yes | 30 | high | no | yes |
| Langner et al. 2012 | 20 | task > sensorimotor control | detect | mixed | yes | 20 | high | no | no |
| Langner et al. 2012 | 20 | task > sensorimotor control | detect | mixed 3 | yes | 20 | high | no | no |
| *Lawrence et al. 20024 | 15 | task > rest | discrim | visual | yes | 90 | high | no | yes |
| *Lawrence et al. 20024 | 15 | task > sensorimotor control | discrim | visual | yes | 90 | high | yes | yes |
| *Lawrence et al. 2003 | 25 | task > rest | discrim | visual | yes | 90 | high | no | yes |
| Lawrence et al. 2003 | 25 | task > sensorimotor control | discrim | visual | yes | 90 | high | yes | yes |
| Lim et al. 2010 | 14 | task > rest | detect | visual | yes | 1200 | low | no | no |
| Lockwood et al. 2008 | 12 | task > rest | discrim | auditory | yes | 60 | high | no | no |
| Marklund et al. 2007 | 13 | task > rest | detect | visual | yes | 90 | low | no | no |
| Naito et al. 2000 | 9 | task > rest | detect | mixed | yes | 200 | high | no | no |
| Ogg et al. 2008 | 30 | task > rest | discrim | visual | yes | 21 | high | no | yes |
| *Okamura et al. 2000 | 12 | task > rest | discrim | visual | yes | 135 | high | no | yes |
| Ortuno et al. 2002 | 10 | task > sensory control | detect | auditory | no | 60 | high | yes | yes |
| Pardo et al. 1991 | 10 | task > rest | detect | tactile | no | 40 | low | no | no |
| Pardo et al. 1991 | 9 | task > rest | detect | tactile | no | 40 | low | no | no |
| Pardo et al. 1991 | 19 | task > rest | detect | visual | no | 40 | low | no | no |
| Paus et al. 1997 | 8 | task > sensory control | discrim | auditory | yes | 60 | high | no | yes |
| Perin et al. 2010 | 16 | task > sensorimotor control | detect | visual | yes | 60 | high | no | no |
| Pfefferbaum et al. 2001 | 10 | task > rest | discrim | visual | yes | 36 | high | no | yes |
| *Salgado-Pineda et al. 2004 | 14 | task > sensorimotor control | discrim | visual | yes | 220 | high | yes | yes |
| *Schlagenhauf et al. 2008 | 10 | task > rest | discrim | visual | yes | 31 | high | no | yes |
| Schmidt et al. 20095 | 31 | task > rest | detect | visual | yes | 690 | low | no | no |
| *Schneider et al. 2007 | 81 | task > sensory control | discrim | visual | yes | 30 | high | no | yes |
| Schnell et al. 2007 | 15 | task > sensorimotor control | detect | visual | yes | 30 | low | no | no |
| *Smits et al. 2009 | 11 | task > rest | discrim | auditory | yes | 30 | high | no | yes |
| Sturm et al. 1999 | 15 | task > sensorimotor control | detect | visual | yes | 40 | high | no | no |
| Sturm et al. 2004 | 10 | task > sensorimotor control | detect | auditory | yes | 40 | high | no | no |
| Sturm et al. 2006 | 10 | task > rest | detect | visual | yes | 60 | high | no | no |
| Tana et al. 2010 | 8 | task > sensory control | discrim | visual | yes | 120 | high | no | no |
| Thakral et al. 2009 | 8 | task > sensory control | detect | visual | yes | 14 | low | no | no |
| Vandenberghe et al. 2001 | 6 | task > rest | detect | visual | yes | 27 | low | no | no |
| Welander-Vatn et al. 2009 | 28 | task > rest | discrim | visual | yes | 20 | high | no | yes |
| Wingen et al. 2008 | 10 | task > rest | discrim | visual | yes | 1800 | high | no | yes |
| Zatorre et al. 1999 | 8 | task > rest | discrim | auditory | yes | 60 | high | no | no |
Note. An asterisk (*) indicates that the data included here had not been explicitly reported in the publication but were provided by the authors upon request.
n = number of participants; Modality: sensory modality of response signals (“mixed” refers to activity averaged across different modalities); Time: duration of continuous maintenance of vigilant attention (with concurrent measurement of brain activity); WM = working memory: task put (modest) demands on WM; TP = temporal predictability: fixed timing of task events enabled prediction of the moment of stimulus occurrence; detect = stimulus detection task; discrim = stimulus discrimination task
Task data reflect activity averaged across all trials (go and no-go trials) in healthy controls only.
Data are based on a joint reanalysis of the data used by Habel et al. (2007) and Koch et al. (2007), which both employed the same paradigm in two partially overlapping samples; the reanalysis joined the data from the non-overlapping subgroups of each sample.
Data are based on a condition in which stimulus modality (visual, auditory, or tactile) randomly varied between trials (as opposed to a between-block variation that all other “mixed modality” contrasts are based on).
Data are only based on the smoker subsample (under placebo conditions); all non-smoking controls were part of the sample used in Lawrence et al. (2003).
Data reflect global alertness effect (based on trials with intermediate reaction time during the evening session) across the entire sample.
References
References marked with an asterisk indicate studies included in the meta-analysis.
- *.Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg L, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42:266–272. doi: 10.1002/syn.1112. [DOI] [PubMed] [Google Scholar]
- Alegria J. The time course of preparation after a first peak: Some constraints of reacting mechanisms. Quarterly Journal of Experimental Psychology. 1974;26:622–632. [Google Scholar]
- Alexander MP, Stuss DT, Shallice T, Picton TW, Gillingham S. Impaired concentration due to frontal lobe damage from two distinct lesion sites. Neurology. 2005;65:572–579. doi: 10.1212/01.wnl.0000172912.07640.92. [DOI] [PubMed] [Google Scholar]
- Allen M. Models of hemispheric specialization. Psychological Bulletin. 1983;93:73–104. [PubMed] [Google Scholar]
- Amunts K, Malikovic A, Mohlberg H, Schormann T, Zilles K. Brodmann's areas 17 and 18 brought into stereotaxic space-where and how variable? NeuroImage. 2000;11:66–84. doi: 10.1006/nimg.1999.0516. [DOI] [PubMed] [Google Scholar]
- Amunts K, Schleicher A, Bürgel U, Mohlberg H, Uylings HB, Zilles K. Broca's region revisited: cytoarchitecture and intersubject variability. Journal of Comparative Neurology. 1999;412:319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annual Review of Neuroscience. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Augustine JR. Circuitry and functional aspects of the insular lobe in primates including humans. Brain Research. Brain Research Reviews. 1996;22:229–244. doi: 10.1016/s0165-0173(96)00011-2. [DOI] [PubMed] [Google Scholar]
- *.Ayalon L, Ancoli-Israel S, Aka AA, McKenna BS, Drummond SP. Relationship between obstructive sleep apnea severity and brain activation during a sustained attention task. Sleep. 2009;32:373–381. doi: 10.1093/sleep/32.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature Neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Beck DM, Kastner S. Top-down and bottom-up mechanisms in biasing competition in the human brain. Vision Research. 2009;49:1154–1165. doi: 10.1016/j.visres.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Belin P, McAdams S, Smith B, Savel S, Thivard L, Samson S, Samson Y. The functional anatomy of sound intensity discrimination. Journal of Neuroscience. 1998;18:6388–6394. doi: 10.1523/JNEUROSCI.18-16-06388.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Belin P, McAdams S, Thivard L, Smith B, Savel S, Zilbovicius M, Samson Y. The neuroanatomical substrate of sound duration discrimination. Neuropsychologia. 2002;40:1956–1964. doi: 10.1016/s0028-3932(02)00062-3. [DOI] [PubMed] [Google Scholar]
- Bellgrove MA, Hawi Z, Kirley A, Gill M, Robertson IH. Dissecting the attention deficit hyperactivity disorder (ADHD) phenotype: sustained attention, response variability and spatial attentional asymmetries in relation to dopamine transporter (DAT1) genotype. Neuropsychologia. 2005;43:1847–1857. doi: 10.1016/j.neuropsychologia.2005.03.011. [DOI] [PubMed] [Google Scholar]
- *.Benedict RH, Lockwood AH, Shucard JL, Shucard DW, Wack D, Murphy BW. Functional neuroimaging of attention in the auditory modality. Neuroreport. 1998;9:121–126. doi: 10.1097/00001756-199801050-00024. [DOI] [PubMed] [Google Scholar]
- *.Benedict RH, Shucard DW, Santa Maria MP, Shucard JL, Abara JP, Coad ML, Lockwood A. Covert auditory attention generates activation in the rostral/dorsal anterior cingulate cortex. Journal of Cognitive Neuroscience. 2002;14:637–645. doi: 10.1162/08989290260045765. [DOI] [PubMed] [Google Scholar]
- Bertelson P, Joffe R. Blockings in prolonged serial responding. Ergonomics. 1963;6:109–116. [Google Scholar]
- Bisley JW, Goldberg ME. Attention, intention, and priority in the parietal lobe. Annual Review of Neuroscience. 2010;33:1–21. doi: 10.1146/annurev-neuro-060909-152823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boksem MA, Tops M. Mental fatigue: costs and benefits. Brain Research. Brain Research Reviews. 2008;59:125–139. doi: 10.1016/j.brainresrev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Brass M, Derrfuss J, Forstmann B, von Cramon DY. The role of the inferior frontal junction area in cognitive control. Trends in Cognitive Sciences. 2005;9:314–316. doi: 10.1016/j.tics.2005.05.001. [DOI] [PubMed] [Google Scholar]
- Brass M, Haggard P. To do or not to do: The neural signature of self-control. Journal of Neuroscience. 2007;27:9141–9145. doi: 10.1523/JNEUROSCI.0924-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breckel TP, Giessing C, Thiel CM. Impact of brain networks involved in vigilance on processing irrelevant visual motion. NeuroImage. 2011;55:1754–1762. doi: 10.1016/j.neuroimage.2011.01.025. [DOI] [PubMed] [Google Scholar]
- Briand LA, Gritton H, Howe WM, Young DA, Sarter M. Modulators in concert for cognition: modulator interactions in the prefrontal cortex. Progress in Neurobiology. 2007;83:69–91. doi: 10.1016/j.pneurobio.2007.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchsbaum MS, Nuechterlein KH, Haier RJ, Wu J, Sicotte N, Hazlett E, Guich S. Glucose metabolic rate in normals and schizophrenics during the Continuous Performance Test assessed by positron emission tomography. British Journal of Psychiatry. 1990;156:216–227. doi: 10.1192/bjp.156.2.216. [DOI] [PubMed] [Google Scholar]
- Burle B, Tandonnet C, Hasbroucq T. Excitatory and inhibitory motor mechanisms of temporal preparation. In: Nobre AC, Coull JT, editors. Attention and time. Oxford, UK: Oxford University Press; 2010. pp. 243–255. [Google Scholar]
- *.Cabeza R, Dolcos F, Prince SE, Rice HJ, Weissman DH, Nyberg L. Attention-related activity during episodic memory retrieval: a cross-function fMRI study. Neuropsychologia. 2003;41:390–399. doi: 10.1016/s0028-3932(02)00170-7. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Nyberg L. Imaging cognition II: An empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Caspers S, Geyer S, Schleicher A, Mohlberg H, Amunts K, Zilles K. The human inferior parietal cortex: cytoarchitectonic parcellation and interindividual variability. NeuroImage. 2006;33:430–448. doi: 10.1016/j.neuroimage.2006.06.054. [DOI] [PubMed] [Google Scholar]
- Cavina-Pratesi C, Valyear KF, Culham JC, Kohler S, Obhi SS, Marzi CA, Goodale MA. Dissociating arbitrary stimulus-response mapping from movement planning during preparatory period: evidence from event-related functional magnetic resonance imaging. Journal of Neuroscience. 2006;26:2704–2713. doi: 10.1523/JNEUROSCI.3176-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen CY, Muggleton NG, Tzeng OJ, Hung DL, Juan CH. Control of prepotent responses by the superior medial frontal cortex. NeuroImage. 2009;44:537–545. doi: 10.1016/j.neuroimage.2008.09.005. [DOI] [PubMed] [Google Scholar]
- Cheyne JA, Solman GJ, Carriere JS, Smilek D. Anatomy of an error: a bidirectional state model of task engagement/disengagement and attention-related errors. Cognition. 2009;111:98–113. doi: 10.1016/j.cognition.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Chikazoe J, Jimura K, Asari T, Yamashita K, Morimoto H, Hirose S, Konishi S. Functional dissociation in right inferior frontal cortex during performance of go/no-go task. Cerebral Cortex. 2009;19:146–152. doi: 10.1093/cercor/bhn065. [DOI] [PubMed] [Google Scholar]
- Choi HJ, Zilles K, Mohlberg H, Schleicher A, Fink GR, Armstrong E, Amunts K. Cytoarchitectonic identification and probabilistic mapping of two distinct areas within the anterior ventral bank of the human intraparietal sulcus. Journal of Comparative Neurology. 2006;495:53–69. doi: 10.1002/cne.20849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouinard PA, Paus T. What have we learned from"perturbing" the human cortical motor system with transcranial magnetic stimulation? Frontiers in Human Neuroscience. 2010;4:173. doi: 10.3389/fnhum.2010.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gordon AM, Smallwood J, Smith R, Schooler JW. Experience sampling during fMRI reveals default network and executive system contributions to mind wandering. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:8719–8724. doi: 10.1073/pnas.0900234106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cieslik EC, Zilles K, Grefkes C, Eickhoff SB. Dynamic interactions in the fronto-parietal network during a manual stimulus–response compatibility task. NeuroImage. 2011;58:860–869. doi: 10.1016/j.neuroimage.2011.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen RM, Semple WE, Gross M, Holcomb HH, Dowling MS, Nordahl TE. Functional localization of sustained attention: Comparison to sensory stimulation in the absence of instruction. Neuropsychiatry, Neuropsychology, and Behavioral Neurology. 1988;1:3–20. [Google Scholar]
- Cohen RM, Semple WE, Gross M, King AC, Nordahl TE. Metabolic brain pattern of sustained auditory discrimination. Experimental Brain Research. 1992;92:165–172. doi: 10.1007/BF00230392. [DOI] [PubMed] [Google Scholar]
- Conners CK. The Conners continuous performance test. Toronto, Canada: Multi-Health Systems; 1994. [Google Scholar]
- Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature Reviews. Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Coslett HB, Bowers D, Heilman KM. Reduction in cerebral activation after right hemisphere stroke. Neurology. 1987;37:957–962. doi: 10.1212/wnl.37.6.957. [DOI] [PubMed] [Google Scholar]
- Coull JT. Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Progress in Neurobiology. 1998;55:343–361. doi: 10.1016/s0301-0082(98)00011-2. [DOI] [PubMed] [Google Scholar]
- Coull JT, Frackowiak RSJ, Frith CD. Monitoring for target objects: Activation of right frontal and parietal cortices with increasing time on task. Neuropsychologia. 1998;36:1325–1334. doi: 10.1016/s0028-3932(98)00035-9. [DOI] [PubMed] [Google Scholar]
- *.Coull JT, Frith CD. Differential activation of right superior parietal cortex and intraparietal sulcus by spatial and nonspatial attention. NeuroImage. 1998;8:176–187. doi: 10.1006/nimg.1998.0354. [DOI] [PubMed] [Google Scholar]
- *.Coull JT, Frith CD, Dolan RJ, Frackowiak RS, Grasby PM. The neural correlates of the noradrenergic modulation of human attention, arousal and learning. European Journal of Neuroscience. 1997;9:589–598. doi: 10.1111/j.1460-9568.1997.tb01635.x. [DOI] [PubMed] [Google Scholar]
- *.Coull JT, Frith CD, Frackowiak RS, Grasby PM. A fronto-parietal network for rapid visual information processing: a PET study of sustained attention and working memory. Neuropsychologia. 1996;34:1085–1095. doi: 10.1016/0028-3932(96)00029-2. [DOI] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Where and when to pay attention: The neural systems for directing attention to spatial locations and to time intervals as revealed by both PET and fMRI. Journal of Neuroscience. 1998;18:7426–7435. doi: 10.1523/JNEUROSCI.18-18-07426.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT, Nobre AC. Dissociating explicit timing from temporal expectation with fMRI. Current Opinion in Neurobiology. 2008;18:137–144. doi: 10.1016/j.conb.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Reviews. Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Corfield DR, Chandler MP, Mathias CJ, Dolan RJ. Cerebral correlates of autonomic cardiovascular arousal: a functional neuroimaging investigation in humans. Journal of Physiology. 2000;523(Pt 1):259–270. doi: 10.1111/j.1469-7793.2000.t01-1-00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Rotshtein P, Öhman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Csikszentmihalyi M. Beyond boredom and anxiety: Experiencing flow in work and play. San Francisco, CA: Jossey-Bass; 1975. [Google Scholar]
- Cui X, Stetson C, Montague PR, Eagleman DM. Ready...go: Amplitude of the fMRI signal encodes expectation of cue arrival time. PLoS Biol. 2009;7:e1000167. doi: 10.1371/journal.pbio.1000167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunnington R, Windischberger C, Moser E. Premovement activity of the pre-supplementary motor area and the readiness for action: studies of time-resolved event-related functional MRI. Human Movement Science. 2005;24:644–656. doi: 10.1016/j.humov.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Danielmeier C, Eichele T, Forstmann BU, Tittgemeyer M, Ullsperger M. Posterior medial frontal cortex activity predicts post-error adaptations in task-related visual and motor areas. Journal of Neuroscience. 2011;31:1780–1789. doi: 10.1523/JNEUROSCI.4299-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielmeier C, Ullsperger M. Post-error adjustments. Frontiers in Psychology. 2011;2:233. doi: 10.3389/fpsyg.2011.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies DR, Parasuraman R. The psychology of vigilance. San Diego, CA: Academic Press; 1982. [Google Scholar]
- Demeter E, Hernandez-Garcia L, Sarter M, Lustig C. Challenges to attention: A continuous arterial spin labeling (ASL) study of the effects of distraction on sustained attention. NeuroImage. 2011;54:1518–1529. doi: 10.1016/j.neuroimage.2010.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diedrichsen J, Balsters JH, Flavell J, Cussans E, Ramnani N. A probabilistic MR atlas of the human cerebellum. NeuroImage. 2009;46:39–46. doi: 10.1016/j.neuroimage.2009.01.045. [DOI] [PubMed] [Google Scholar]
- Diedrichsen J, Verstynen T, Lehman SL, Ivry RB. Cerebellar involvement in anticipating the consequences of self-produced actions during bimanual movements. Journal of Neurophysiology. 2005;93:801–812. doi: 10.1152/jn.00662.2004. [DOI] [PubMed] [Google Scholar]
- Donaldson DI, Petersen SE, Ollinger JM, Buckner RL. Dissociating state and item components of recognition memory using fMRI. NeuroImage. 2001;13:129–142. doi: 10.1006/nimg.2000.0664. [DOI] [PubMed] [Google Scholar]
- Dosenbach NU, Fair DA, Miezin FM, Cohen AL, Wenger KK, Dosenbach RA, Petersen SE. Distinct brain networks for adaptive and stable task control in humans. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:11073–11078. doi: 10.1073/pnas.0704320104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosenbach NU, Visscher KM, Palmer ED, Miezin FM, Wenger KK, Kang HC, Petersen SE. A core system for the implementation of task sets. Neuron. 2006;50:799–812. doi: 10.1016/j.neuron.2006.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nature Neuroscience. 2000;3:277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. The effect of task relevance on the cortical response to changes in visual and auditory stimuli: an event-related fMRI study. NeuroImage. 2001;14:1256–1267. doi: 10.1006/nimg.2001.0946. [DOI] [PubMed] [Google Scholar]
- Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–1068. [PubMed] [Google Scholar]
- Eckert MA, Menon V, Walczak A, Ahlstrom J, Denslow S, Horwitz A, Dubno JR. At the heart of the ventral attention system: the right anterior insula. Human Brain Mapping. 2009;30:2530–2541. doi: 10.1002/hbm.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edkins G, Pollock C .The influence of sustained attention on railway accidents. Accident Analysis and Prevention. 1997;29:533–539. doi: 10.1016/s0001-4575(97)00033-x. [DOI] [PubMed] [Google Scholar]
- Egeth HE, Yantis S. Visual attention: control, representation, and time course. Annual Review of Psychology. 1997;48:269–297. doi: 10.1146/annurev.psych.48.1.269. [DOI] [PubMed] [Google Scholar]
- Eichele T, Debener S, Calhoun VD, Specht K, Engel AK, Hugdahl K, Ullsperger M. Prediction of human errors by maladaptive changes in event-related brain networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:6173–6178. doi: 10.1073/pnas.0708965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D. Meta-analyses in basic and clinical neuroscience: State of the art and perspective. In: Ulmer S, Jansen O, editors. fMRI: Basics and clinical applications. 2nd ed. Berlin, Germany: Springer; (in press) [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Kurth F, Fox PT. Activation likelihood estimation meta-analysis revisited. NeuroImage. 2012;59:2349–2361. doi: 10.1016/j.neuroimage.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Bzdok D, Laird AR, Roski C, Caspers S, Zilles K, Fox PT. Co-activation patterns distinguish cortical modules, their connectivity and functional differentiation. NeuroImage. 2011;57:938–949. doi: 10.1016/j.neuroimage.2011.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Laird AR, Grefkes C, Wang LE, Zilles K, Fox PT. Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Human Brain Mapping. 2009;30:2907–2926. doi: 10.1002/hbm.20718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eickhoff SB, Paus T, Caspers S, Grosbras MH, Evans AC, Zilles K, Amunts K. Assignment of functional activations to probabilistic cytoarchitectonic areas revisited. NeuroImage. 2007;36:511–521. doi: 10.1016/j.neuroimage.2007.03.060. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Pomjanski W, Jakobs O, Zilles K, Langner R. Neural correlates of developing and adapting behavioural biases in speeded choice reactions: An fMRI study on predictive motor coding. Cerebral Cortex. 2011;21:1178–1191. doi: 10.1093/cercor/bhq188. [DOI] [PubMed] [Google Scholar]
- Eickhoff SB, Stephan KE, Mohlberg H, Grefkes C, Fink GR, Amunts K, Zilles K. A new SPM toolbox for combining probabilistic cytoarchitectonic maps and functional imaging data. NeuroImage. 2005;25:1325–1335. doi: 10.1016/j.neuroimage.2004.12.034. [DOI] [PubMed] [Google Scholar]
- *.Eyler LT, Olsen RK, Jeste DV, Brown GG. Abnormal brain response of chronic schizophrenia patients despite normal performance during a visual vigilance task. Psychiatry Research. 2004;130:245–257. doi: 10.1016/j.pscychresns.2004.01.003. [DOI] [PubMed] [Google Scholar]
- *.Fassbender C, Murphy K, Foxe JJ, Wylie GR, Javitt DC, Robertson IH, Garavan H. A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Research. Cognitive Brain Research. 2004;20:132–143. doi: 10.1016/j.cogbrainres.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Fischer T, Langner R, Birbaumer N, Brocke B. Arousal and attention: self-chosen stimulation optimizes cortical excitability and minimizes compensatory effort. Journal of Cognitive Neuroscience. 2008;20:1443–1453. doi: 10.1162/jocn.2008.20101. [DOI] [PubMed] [Google Scholar]
- Fischer T, Langner R, Diers K, Brocke B, Birbaumer N. Temporo-spatial dynamics of event-related EEG beta activity during the initial contingent negative variation. PLoS One. 2010;5 doi: 10.1371/journal.pone.0012514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiset P, Paus T, Daloze T, Plourde G, Meuret P, Bonhomme V, Evans AC. Brain mechanisms of propofol-induced loss of consciousness in humans: a positron emission tomographic study. Journal of Neuroscience. 1999;19:5506–5513. doi: 10.1523/JNEUROSCI.19-13-05506.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk CL, Remington RW, Johnston JC. Involuntary covert orienting is contingent on attentional control settings. Journal of Experimental Psychology: Human Perception and Performance. 1992;18:1030–1044. [PubMed] [Google Scholar]
- Frankenhaeuser M, Gardell B. Underload and overload in working life: Outline of a multidisciplinary approach. Journal of Human Stress. 1976;2:35–46. doi: 10.1080/0097840X.1976.9936068. [DOI] [PubMed] [Google Scholar]
- Friederici AD. Towards a neural basis of auditory sentence processing. Trends in Cognitive Sciences. 2002;6:78–84. doi: 10.1016/s1364-6613(00)01839-8. [DOI] [PubMed] [Google Scholar]
- *.Gamma A, Buck A, Berthold T, Vollenweider FX. No difference in brain activation during cognitive performance between ecstasy (3,4-methylenedioxy�methamphetamine) users and control subjects: A [(H2O)-O-15]-positron emission tomography study. Journal of Clinical Psychopharmacology. 2001;21:66–71. doi: 10.1097/00004714-200102000-00012. [DOI] [PubMed] [Google Scholar]
- Geyer S. The microstructural border between the motor and the cognitive domain in the human cerebral cortex. Advances in Anatomy, Embryology and Cell Biology. 2004;174:1–89. doi: 10.1007/978-3-642-18910-4. [DOI] [PubMed] [Google Scholar]
- Giambra LM, Quilter RE. A two-term exponential functional description of the time course of sustained attention. Human Factors. 1987;29:635–643. doi: 10.1177/001872088702900603. [DOI] [PubMed] [Google Scholar]
- Giessing C, Thiel CM, Rösler F, Fink GR. The modulatory effects of nicotine on parietal cortex activity in a cued target detection task depend on cue reliability. Neuroscience. 2006;137:853–864. doi: 10.1016/j.neuroscience.2005.10.005. [DOI] [PubMed] [Google Scholar]
- *.Gilbert SJ, Simons JS, Frith CD, Burgess PW. Performance-related activity in medial rostral prefrontal cortex (area 10) during low-demand tasks. Journal of Experimental Psychology: Human Perception and Performance. 2006;32:45–58. doi: 10.1037/0096-1523.32.1.45. [DOI] [PubMed] [Google Scholar]
- *.Gitelman DR, Alpert NM, Kosslyn S, Daffner K, Scinto L, Thompson W, Mesulam MM. Functional imaging of human right hemispheric activation for exploratory movements. Annals of Neurology. 1996;39:174–179. doi: 10.1002/ana.410390206. [DOI] [PubMed] [Google Scholar]
- Godefroy O, Lhullier-Lamy C, Rousseaux M. SRT lengthening: role of an alertness deficit in frontal damaged patients. Neuropsychologia. 2002;40:2234–2241. doi: 10.1016/s0028-3932(02)00109-4. [DOI] [PubMed] [Google Scholar]
- *.Goldstein RZ, Tomasi D, Alia-Klein N, Zhang L, Telang F, Volkow ND. The effect of practice on a sustained attention task in cocaine abusers. NeuroImage. 2007;35:194–206. doi: 10.1016/j.neuroimage.2006.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottsdanker R. The attaining and maintaining of preparation. In: Rabbitt PMA, Dornic S, editors. Attention and performance V. London: Academic Press; 1975. pp. 33–42. [Google Scholar]
- Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? American Journal of Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- Greene CM, Bellgrove MA, Gill M, Robertson IH. Noradrenergic genotype predicts lapses in sustained attention. Neuropsychologia. 2009;47:591–594. doi: 10.1016/j.neuropsychologia.2008.10.003. [DOI] [PubMed] [Google Scholar]
- Grier RA, Warm JS, Dember WN, Matthews G, Galinsky TL, Szalma JL, Parasuraman R. The vigilance decrement reflects limitations in effortful attention, not mindlessness. Human Factors. 2003;45:349–359. doi: 10.1518/hfes.45.3.349.27253. [DOI] [PubMed] [Google Scholar]
- *.Habel U, Koch K, Pauly K, Kellermann T, Reske M, Backes V, Schneider F. The influence of olfactory-induced negative emotion on verbal working memory: individual differences in neurobehavioral findings. Brain Research. 2007;1152:158–170. doi: 10.1016/j.brainres.2007.03.048. [DOI] [PubMed] [Google Scholar]
- Hagger MS, Wood C, Stiff C, Chatzisarantis NL. Ego depletion and the strength model of self-control: a meta-analysis. Psychological Bulletin. 2010;136:495–525. doi: 10.1037/a0019486. [DOI] [PubMed] [Google Scholar]
- Hartstra E, Kühn S, Verguts T, Brass M. The implementation of verbal instructions: an fMRI study. Human Brain Mapping. 2011;32:1811–1824. doi: 10.1002/hbm.21152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Sarter M. Modes and models of forebrain cholinergic neuromodulation of cognition. Neuropsychopharmacology. 2011;36:52–73. doi: 10.1038/npp.2010.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes JD, Sakai K, Rees G, Gilbert S, Frith C, Passingham RE. Reading hidden intentions in the human brain. Current Biology. 2007;17:323–328. doi: 10.1016/j.cub.2006.11.072. [DOI] [PubMed] [Google Scholar]
- Hegerl U, Himmerich H, Engmann B, Hensch T. Mania and attention-deficit/hyperactivity disorder: common symptomatology, common pathophysiology and common treatment? Current Opinion in Psychiatry. 2010;23:1–7. doi: 10.1097/YCO.0b013e328331f694. [DOI] [PubMed] [Google Scholar]
- Heilman KM, Van Den Abell T. Right hemispheric dominance for mediating cerebral activation. Neuropsychologia. 1979;17:315–321. doi: 10.1016/0028-3932(79)90077-0. [DOI] [PubMed] [Google Scholar]
- Helton WS, Hollander TD, Warm JS, Matthews G, Dember WN, Hancock PA. Signal regularity and the mindlessness model of vigilance. British Journal of Psychology. 2005;96:249–261. doi: 10.1348/000712605X38369. [DOI] [PubMed] [Google Scholar]
- Helton WS, Hollander TD, Warm JS, Tripp LD, Parsons K, Matthews G, Hancock PA. The abbreviated vigilance task and cerebral hemodynamics. Journal of Clinical and Experimental Neuropsychology. 2007;29:545–552. doi: 10.1080/13803390600814757. [DOI] [PubMed] [Google Scholar]
- Helton WS, Russell PN. Feature absence–presence and two theories of lapses of sustained attention. Psychological Research. 2011a;75:384–392. doi: 10.1007/s00426-010-0316-1. [DOI] [PubMed] [Google Scholar]
- Helton WS, Russell PN. Working memory load and the vigilance decrement. Experimental Brain Research. 2011b;212:429–437. doi: 10.1007/s00221-011-2749-1. [DOI] [PubMed] [Google Scholar]
- Helton WS, Warm JS. Signal salience and the mindlessness theory of vigilance. Acta Psychologica. 2008;129:18–25. doi: 10.1016/j.actpsy.2008.04.002. [DOI] [PubMed] [Google Scholar]
- Helton WS, Warm JS, Tripp LD, Matthews G, Parasuraman R, Hancock PA. Cerebral lateralization of vigilance: A function of task difficulty. Neuropsychologia. 2010;48:1683–1688. doi: 10.1016/j.neuropsychologia.2010.02.014. [DOI] [PubMed] [Google Scholar]
- Henderson L, Dittrich WH. Preparing to react in the absence of uncertainty: I. New perspectives on simple reaction time. British Journal of Psychology. 1998;89(Pt 4):531–554. doi: 10.1111/j.2044-8295.1998.tb02702.x. [DOI] [PubMed] [Google Scholar]
- *.Herath P, Klingberg T, Young J, Amunts K, Roland P. Neural correlates of dual task interference can be dissociated from those of divided attention: an fMRI study. Cerebral Cortex. 2001;11:796–805. doi: 10.1093/cercor/11.9.796. [DOI] [PubMed] [Google Scholar]
- Hitchcock EM, Warm JS, Matthews G, Dember WN, Shear PK, Tripp LD, Parasuraman R. Automation cueing modulates cerebral blood flow and vigilance in a simulated air traffic control task. Theoretical Issues in Ergonomics Science. 2003;4:89–112. [Google Scholar]
- Hockey GRJ. A state control theory of adaptation to stress and individual differences in stress management. In: Hockey GRJ, Gaillard AWK, Coles MGH, editors. Energetics and human information processing. Dordrecht, The Netherlands: Martinus Nijhoff; 1986. pp. 285–298. [Google Scholar]
- Hockey GRJ. Compensatory control in the regulation of human performance under stress and high workload: A cognitive-energetical framework. Biological Psychology. 1997;45:73–93. doi: 10.1016/s0301-0511(96)05223-4. doi: 123. [DOI] [PubMed] [Google Scholar]
- Hockey GRJ, Coles MGH, Gaillard AWK. Energetical issues in research on human information processing. In: Hockey GRJ, Gaillard AWK, Coles MGH, editors. Energetics and human information processing. Dordrecht: Martinus Nijhoff; 1986. pp. 3–21. [Google Scholar]
- Hofle N, Paus T, Reutens D, Fiset P, Gotman J, Evans AC, Jones BE. Regional cerebral blood flow changes as a function of delta and spindle activity during slow wave sleep in humans. Journal of Neuroscience. 1997;17:4800–4808. doi: 10.1523/JNEUROSCI.17-12-04800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Holcomb HH, Medoff DR, Caudill PJ, Zhao Z, Lahti AC, Dannals RF, Tamminga CA. Cerebral blood flow relationships associated with a difficult tone recognition task in trained normal volunteers. Cerebral Cortex. 1998;8:534–542. doi: 10.1093/cercor/8.6.534. [DOI] [PubMed] [Google Scholar]
- *.Honey GD, Pomarol-Clotet E, Corlett PR, Honey RA, McKenna PJ, Bullmore ET, Fletcher PC. Functional dysconnectivity in schizophrenia associated with attentional modulation of motor function. Brain. 2005;128:2597–2611. doi: 10.1093/brain/awh632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Hong LE, Schroeder M, Ross TJ, Buchholz B, Salmeron BJ, Wonodi I, Stein EA. Nicotine enhances but does not normalize visual sustained attention and the associated brain network in schizophrenia. Schizophrenia Bulletin. 2011;37:416–425. doi: 10.1093/schbul/sbp089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptman MJ, Davidson RJ. How and why do the two cerebral hemispheres interact? Psychological Bulletin. 1994;116:195–219. doi: 10.1037/0033-2909.116.2.195. [DOI] [PubMed] [Google Scholar]
- *.Horn H, Syed N, Lanfermann H, Maurer K, Dierks T. Cerebral networks linked to the event-related potential P300. European Archives of Psychiatry and Clinical Neuroscience. 2003;253:154–159. doi: 10.1007/s00406-003-0419-4. [DOI] [PubMed] [Google Scholar]
- Howes D, Boller F. Simple reaction time: Evidence for focal impairment from lesions of the right hemisphere. Brain. 1975;98:317–332. doi: 10.1093/brain/98.2.317. [DOI] [PubMed] [Google Scholar]
- Hülsmann E, Erb M, Grodd W. From will to action: sequential cerebellar contributions to voluntary movement. NeuroImage. 2003;20:1485–1492. doi: 10.1016/s1053-8119(03)00307-0. [DOI] [PubMed] [Google Scholar]
- Humphreys MS, Revelle W. Personality, motivation, and performance: A theory of the relationship between individual differences and information processing. Psychological Review. 1984;91:153–184. [PubMed] [Google Scholar]
- Husain M, Rorden C. Non-spatially lateralized mechanisms in hemispatial neglect. Nature Reviews. Neuroscience. 2003;4:26–36. doi: 10.1038/nrn1005. [DOI] [PubMed] [Google Scholar]
- Jakobs O, Langner R, Caspers S, Roski C, Cieslik EC, Zilles K, Eickhoff SB. Across-study and within-subject functional connectivity of a right temporo-parietal junction subregion involved in stimulus–context integration. NeuroImage. 2012;60:2389–2398. doi: 10.1016/j.neuroimage.2012.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janssen P, Shadlen MN. A representation of the hazard rate of elapsed time in macaque area LIP. Nature Neuroscience. 2005;8:234–241. doi: 10.1038/nn1386. [DOI] [PubMed] [Google Scholar]
- Jennings JR, van der Molen MW. Preparation for speeded action as a psychophysiological concept. Psychological Bulletin. 2005;131:434–459. doi: 10.1037/0033-2909.131.3.434. [DOI] [PubMed] [Google Scholar]
- Jennings RG, Van Horn JD. Publication bias in neuroimaging research: implications for meta-analyses. Neuroinformatics. 2012;10:67–80. doi: 10.1007/s12021-011-9125-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Johannsen P, Jakobsen J, Bruhn P, Hansen SB, Gee A, Stodkilde-Jorgensen H, Gjedde A. Cortical sites of sustained and divided attention in normal elderly humans. NeuroImage. 1997;6:145–155. doi: 10.1006/nimg.1997.0292. [DOI] [PubMed] [Google Scholar]
- Johansson G, Aronsson G, Lindström BO. Social psychological and neuroendocrine stress reactions in highly mechanised work. Ergonomics. 1978;21:583–599. doi: 10.1080/00140137808931761. [DOI] [PubMed] [Google Scholar]
- Jones BE. Arousal systems. Frontiers in Bioscience. 2003;8:s438–s451. doi: 10.2741/1074. [DOI] [PubMed] [Google Scholar]
- Kahneman D. Attention and effort. Englewood Cliffs, NJ: Prentice-Hall; 1973. [Google Scholar]
- *.Kansaku K, Hanakawa T, Wu T, Hallett M. A shared neural network for simple reaction time. NeuroImage. 2004;22:904–911. doi: 10.1016/j.neuroimage.2004.02.006. [DOI] [PubMed] [Google Scholar]
- *.Kawashima R, Satoh K, Itoh H, Ono S, Furumoto S, Gotoh R, Fukuda H. Functional anatomy of GO/NO-GO discrimination and response selection: A PET study in man. Brain Research. 1996;728:79–89. [PubMed] [Google Scholar]
- Kiefer M, Martens U. Attentional sensitization of unconscious cognition: Task sets modulate subsequent masked semantic priming. Journal of Experimental Psychology: General. 2010;139:464–489. doi: 10.1037/a0019561. [DOI] [PubMed] [Google Scholar]
- *.Kim J, Whyte J, Wang J, Rao H, Tang KZ, Detre JA. Continuous ASL perfusion fMRI investigation of higher cognition: quantification of tonic CBF changes during sustained attention and working memory tasks. NeuroImage. 2006;31:376–385. doi: 10.1016/j.neuroimage.2005.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kincade JM, Abrams RA, Astafiev SV, Shulman GL, Corbetta M. An event-related functional magnetic resonance imaging study of voluntary and stimulus-driven orienting of attention. Journal of Neuroscience. 2005;25:4593–4604. doi: 10.1523/JNEUROSCI.0236-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- Knutson B, Greer SM. Anticipatory affect: neural correlates and consequences for choice. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 2008;363:3771–3786. doi: 10.1098/rstb.2008.0155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi Y, Isa T. Sensory-motor gating and cognitive control by the brainstem cholinergic system. Neural Networks. 2002;15:731–741. doi: 10.1016/s0893-6080(02)00059-x. [DOI] [PubMed] [Google Scholar]
- *.Koch K, Pauly K, Kellermann T, Seiferth NY, Reske M, Backes V, Habel U. Gender differences in the cognitive control of emotion: An fMRI study. Neuropsychologia. 2007;45:2744–2754. doi: 10.1016/j.neuropsychologia.2007.04.012. [DOI] [PubMed] [Google Scholar]
- Koelega HS. Stimulant drugs and vigilance performance: a review. Psychopharmacology. 1993;111:1–16. doi: 10.1007/BF02257400. [DOI] [PubMed] [Google Scholar]
- Koski L, Petrides M. Time-related changes in task performance after lesions restricted to the frontal cortex. Neuropsychologia. 2001;39:268–281. doi: 10.1016/s0028-3932(00)00110-x. [DOI] [PubMed] [Google Scholar]
- Kosslyn SM, Koenig O. Wet mind: The new cognitive neuroscience. New York, NY: The Free Press; 1992. [Google Scholar]
- Kozak R, Bruno JP, Sarter M. Augmented prefrontal acetylcholine release during challenged attentional performance. Cerebral Cortex. 2006;16:9–17. doi: 10.1093/cercor/bhi079. [DOI] [PubMed] [Google Scholar]
- *.Krug A, Markov V, Eggermann T, Krach S, Zerres K, Stocker T, Kircher T. Genetic variation in the schizophrenia-risk gene neuregulin1 correlates with differences in frontal brain activation in a working memory task in healthy individuals. NeuroImage. 2008;42:1569–1576. doi: 10.1016/j.neuroimage.2008.05.058. [DOI] [PubMed] [Google Scholar]
- Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Structure and Function. 2010;214:519–534. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladavas E. Is the hemispatial deficit produced by right parietal lobe damage associated with retinal or gravitational coordinates? Brain. 1987;110(Pt 1):167–180. doi: 10.1093/brain/110.1.167. [DOI] [PubMed] [Google Scholar]
- Lancaster JL, Tordesillas-Gutierrez D, Martinez M, Salinas F, Evans A, Zilles K, Fox PT. Bias between MNI and Talairach coordinates analyzed using the ICBM-152 brain template. Human Brain Mapping. 2007;28:1194–1205. doi: 10.1002/hbm.20345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Eickhoff SB, Steinborn MB. Mental fatigue modulates dynamic adaptation to perceptual demand in speeded detection. PLoS One. 2011;6:e28399. doi: 10.1371/journal.pone.0028399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Kellermann T, Boers F, Sturm W, Willmes K, Eickhoff SB. Modality-specific perceptual expectations selectively modulate baseline activity in auditory, somatosensory, and visual cortices. Cerebral Cortex. 2012;21:2850–2862. doi: 10.1093/cercor/bhr083. [DOI] [PubMed] [Google Scholar]
- *.Langner R, Kellermann T, Eickhoff SB, Boers F, Chatterjee A, Willmes K, Sturm W. Staying responsive to the world: Modality-specific and -nonspecific contributions to speeded auditory, tactile, and visual stimulus detection. Human Brain Mapping. 2012;33:398–418. doi: 10.1002/hbm.21220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langner R, Steinborn MB, Chatterjee A, Sturm W, Willmes K. Mental fatigue and temporal preparation in simple reaction-time performance. Acta Psychologica. 2010;133:64–72. doi: 10.1016/j.actpsy.2009.10.001. [DOI] [PubMed] [Google Scholar]
- Langner R, Willmes K, Chatterjee A, Eickhoff SB, Sturm W. Energetic effects of stimulus intensity on prolonged simple reaction-time performance. Psychological Research. 2010;74:499–512. doi: 10.1007/s00426-010-0275-6. [DOI] [PubMed] [Google Scholar]
- *.Lawrence NS, Ross TJ, Hoffmann R, Garavan H, Stein EA. Multiple neuronal networks mediate sustained attention. Journal of Cognitive Neuroscience. 2003;15:1028–1038. doi: 10.1162/089892903770007416. [DOI] [PubMed] [Google Scholar]
- *.Lawrence NS, Ross TJ, Stein EA. Cognitive mechanisms of nicotine on visual attention. Neuron. 2002;36:539–548. doi: 10.1016/s0896-6273(02)01004-8. [DOI] [PubMed] [Google Scholar]
- Lewin JS, Friedman L, Wu D, Miller DA, Thompson LA, Klein SK, Duerk JL. Cortical localization of human sustained attention: Detection with functional MR using a visual vigilance paradigm. Journal of Computer Assisted Tomography. 1996;20:695–701. doi: 10.1097/00004728-199609000-00002. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. Sleep deprivation and vigilant attention. Annals of the New York Academy of Sciences. 2008;1129:305–322. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychological Bulletin. 2010;136:375–389. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lim J, Wu WC, Wang J, Detre JA, Dinges DF, Rao H. Imaging brain fatigue from sustained mental workload: an ASL perfusion study of the time-on-task effect. NeuroImage. 2010;49:3426–3435. doi: 10.1016/j.neuroimage.2009.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Lockwood AH, Wack DS, Benedict RH, Coad ML, Sussman JE, Burkard RF. Multi-site phasic neural activity mediates the execution of an auditory continuous performance task: a PET and electrophysiological study. Journal of Neuroimaging. 2008;18:364–374. doi: 10.1111/j.1552-6569.2007.00196_1.x. [DOI] [PubMed] [Google Scholar]
- Lorist MM, Klein M, Nieuwenhuis S, De Jong R, Mulder G, Meijman TF. Mental fatigue and task control: Planning and preparation. Psychophysiology. 2000;37:614–625. [PubMed] [Google Scholar]
- Luks TL, Simpson GV, Feiwell RJ, Miller WL. Evidence for anterior cingulate cortex involvement in monitoring preparatory attentional set. NeuroImage. 2002;17:792–802. [PubMed] [Google Scholar]
- Mackworth NH. The breakdown of vigilance during prolonged visual search. Quarterly Journal of Experimental Psychology. 1948;1:6–21. [Google Scholar]
- Malhotra P, Coulthard EJ, Husain M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain. 2009;132:645–660. doi: 10.1093/brain/awn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malikovic A, Amunts K, Schleicher A, Mohlberg H, Eickhoff SB, Wilms M, Zilles K. Cytoarchitectonic analysis of the human extrastriate cortex in the region of V5/MT+: a probabilistic, stereotaxic map of area hOc5. Cerebral Cortex. 2007;17:562–574. doi: 10.1093/cercor/bhj181. [DOI] [PubMed] [Google Scholar]
- Manly T, Owen AM, McAvinue L, Datta A, Lewis GH, Scott SK, Robertson IH. Enhancing the sensitivity of a sustained attention task to frontal damage: convergent clinical and functional imaging evidence. Neurocase. 2003;9:340–349. doi: 10.1076/neur.9.4.340.15553. [DOI] [PubMed] [Google Scholar]
- Manly T, Robertson IH, Galloway M, Hawkins K. The absent mind: further investigations of sustained attention to response. Neuropsychologia. 1999;37:661–670. doi: 10.1016/s0028-3932(98)00127-4. [DOI] [PubMed] [Google Scholar]
- *.Marklund P, Fransson P, Cabeza R, Petersson KM, Ingvar M, Nyberg L. Sustained and transient neural modulations in prefrontal cortex related to declarative long-term memory, working memory, and attention. Cortex. 2007;43:22–37. doi: 10.1016/s0010-9452(08)70443-x. [DOI] [PubMed] [Google Scholar]
- Marois R, Leung HC, Gore JC. A stimulus-driven approach to object identity and location processing in the human brain. Neuron. 2000;25:717–728. doi: 10.1016/s0896-6273(00)81073-9. [DOI] [PubMed] [Google Scholar]
- Mars RB, Klein MC, Neubert FX, Olivier E, Buch ER, Boorman ED, Rushworth MF. Short-latency influence of medial frontal cortex on primary motor cortex during action selection under conflict. Journal of Neuroscience. 2009;29:6926–6931. doi: 10.1523/JNEUROSCI.1396-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews G, Campbell SE, Falconer S, Joyner LA, Huggins J, Gilliland K, Warm JS. Fundamental dimensions of subjective state in performance settings: task engagement, distress, and worry. Emotion. 2002;2:315–340. doi: 10.1037/1528-3542.2.4.315. [DOI] [PubMed] [Google Scholar]
- Matthews G, Westerman SJ. Energy and tension as predictors of controlled visual and memory search. Personality and Individual Differences. 1994;17:617–626. [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex and their role in neuromodulation of thalamocortical activity. Progress in Neurobiology. 1992;39:337–388. doi: 10.1016/0301-0082(92)90012-4. [DOI] [PubMed] [Google Scholar]
- Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Structure and Function. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michael GA, Garcia S, Bussy G, Lion-Francois L, Guibaud L. Reactivity to visual signals and the cerebellar vermis: Evidence from a rare case with rhombencephalosynapsis. Behavioral Neuroscience. 2009;123:86–96. doi: 10.1037/a0013726. [DOI] [PubMed] [Google Scholar]
- Mirsky AF, Anthony BJ, Duncan CC, Ahearn MB, Kellam SG. Analysis of the elements of attention: a neuropsychological approach. Neuropsychology Review. 1991;2:109–145. doi: 10.1007/BF01109051. [DOI] [PubMed] [Google Scholar]
- Mottaghy FM, Willmes K, Horwitz B, Müller HW, Krause BJ, Sturm W. Systems level modeling of a neuronal network subserving intrinsic alertness. NeuroImage. 2006;29:225–233. doi: 10.1016/j.neuroimage.2005.07.034. [DOI] [PubMed] [Google Scholar]
- Muraven M, Baumeister RF. Self-regulation and depletion of limited resources: Does self-control resemble a muscle? Psychological Bulletin. 2000;126:247–259. doi: 10.1037/0033-2909.126.2.247. [DOI] [PubMed] [Google Scholar]
- Murtha S, Chertkow H, Beauregard M, Dixon R, Evans A. Anticipation causes increased blood flow to the anterior cingulate cortex. Human Brain Mapping. 1996;4:103–112. doi: 10.1002/(SICI)1097-0193(1996)4:2<103::AID-HBM2>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Näätänen R. The diminishing time-uncertainty with the lapse of time after the warning signal in reaction-time experiments with varying fore-periods. Acta Psychologica. 1970;34:399–419. doi: 10.1016/0001-6918(70)90035-1. [DOI] [PubMed] [Google Scholar]
- Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nature Reviews. Neuroscience. 2008;9:856–869. doi: 10.1038/nrn2478. [DOI] [PubMed] [Google Scholar]
- *.Naito E, Kinomura S, Geyer S, Kawashima R, Roland PE, Zilles K. Fast reaction to different sensory modalities activates common fields in the motor areas, but the anterior cingulate cortex is involved in the speed of reaction. Journal of Neurophysiology. 2000;83:1701–1709. doi: 10.1152/jn.2000.83.3.1701. [DOI] [PubMed] [Google Scholar]
- Nebel K, Wiese H, Stude P, de Greiff A, Diener HC, Keidel M. On the neural basis of focused and divided attention. Brain Research. Cognitive Brain Research. 2005;25:760–776. doi: 10.1016/j.cogbrainres.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Niemi P, Näätänen R. Foreperiod and simple reaction time. Psychological Bulletin. 1981;89:133–162. [Google Scholar]
- Nobre AC, Correa A, Coull JT. The hazards of time. Current Opinion in Neurobiology. 2007;17:465–470. doi: 10.1016/j.conb.2007.07.006. [DOI] [PubMed] [Google Scholar]
- Norman D, Shallice T. Attention to action: Willed and automatic control of behavior. In: Davidson RJ, Schwartz GE, Shapiro D, editors. Consciousness and self regulation. Vol. 4. New York, NY: Plenum Press; 1986. pp. 1–18. [Google Scholar]
- O’Connell RG, Bellgrove MA, Dockree PM, Lau A, Fitzgerald M, Robertson IH. Self-Alert Training: volitional modulation of autonomic arousal improves sustained attention. Neuropsychologia. 2008;46:1379–1390. doi: 10.1016/j.neuropsychologia.2007.12.018. [DOI] [PubMed] [Google Scholar]
- O’Hanlon JF. Boredom: Practical consequences and a theory. Acta Psychologica. 1981;49:53–82. doi: 10.1016/0001-6918(81)90033-0. [DOI] [PubMed] [Google Scholar]
- *.Ogg RJ, Zou P, Allen DN, Hutchins SB, Dutkiewicz RM, Mulhern RK. Neural correlates of a clinical continuous performance test. Magnetic Resonance Imaging. 2008;26:504–512. doi: 10.1016/j.mri.2007.09.004. [DOI] [PubMed] [Google Scholar]
- *.Okamura N, Yanai K, Higuchi M, Sakai J, Iwata R, Ido T, Itoh M. Functional neuroimaging of cognition impaired by a classical antihistamine, d-chlorpheniramine. British Journal of Pharmacology. 2000;129:115–123. doi: 10.1038/sj.bjp.0702994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Keeffe FM, Murray B, Coen RF, Dockree PM, Bellgrove MA, Garavan H, Robertson IH. Loss of insight in frontotemporal dementia, corticobasal degeneration and progressive supranuclear palsy. Brain. 2007;130:753–764. doi: 10.1093/brain/awl367. [DOI] [PubMed] [Google Scholar]
- *.Ortuno F, Ojeda N, Arbizu J, Lopez P, Marti-Climent JM, Penuelas I, Cervera S. Sustained attention in a counting task: normal performance and functional neuroanatomy. NeuroImage. 2002;17:411–420. doi: 10.1006/nimg.2002.1168. [DOI] [PubMed] [Google Scholar]
- Parasuraman R. Sustained attention in detection and discrimination. In: Parasuraman R, Davies DR, editors. Varieties of attention. Orlando, FL: Academic Press; 1984. pp. 243–271. [Google Scholar]
- Parasuraman R, Warm JS, See JE. Brain systems of vigilance. In: Parasuraman R, editor. The attentive brain. Cambridge, MA: MIT Press; 1998. pp. 221–256. [Google Scholar]
- *.Pardo JV, Fox PT, Raichle ME. Localization of a human system for sustained attention by positron emission tomography. Nature. 1991;349:61–64. doi: 10.1038/349061a0. [DOI] [PubMed] [Google Scholar]
- Pattyn N, Neyt X, Henderickx D, Soetens E. Psychophysiological investigation of vigilance decrement: Boredom or cognitive fatigue? Physiology and Behavior. 2008;93:369–378. doi: 10.1016/j.physbeh.2007.09.016. [DOI] [PubMed] [Google Scholar]
- Paus T. Functional anatomy of arousal and attention systems in the human brain. Progress in Brain Research. 2000;126:65–77. doi: 10.1016/S0079-6123(00)26007-X. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews. Neuroscience. 2001;2:417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- *.Paus T, Zatorre RJ, Hofle N, Caramanos Z, Gotman J, Petrides M, Evans AC. Time-related changes in neural systems underlying attention and arousal during the performance of an auditory vigilance task. Journal of Cognitive Neuroscience. 1997;9:392–408. doi: 10.1162/jocn.1997.9.3.392. [DOI] [PubMed] [Google Scholar]
- *.Perin B, Godefroy O, Fall S, de Marco G. Alertness in young healthy subjects: an fMRI study of brain region interactivity enhanced by a warning signal. Brain and Cognition. 2010;72:271–281. doi: 10.1016/j.bandc.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Pfaff DW. Brain arousal and information theory: Neural and genetic mechanisms. Cambridge, MA: Harvard University Press; 2006. [Google Scholar]
- Pfaff DW, Ribeiro A, Matthews J, Kow LM. Concepts and mechanisms of generalized central nervous system arousal. Annals of the New York Academy of Sciences. 2008;1129:11–25. doi: 10.1196/annals.1417.019. [DOI] [PubMed] [Google Scholar]
- *.Pfefferbaum A, Desmond JE, Galloway C, Menon V, Glover GH, Sullivan EV. Reorganization of frontal systems used by alcoholics for spatial working memory: an fMRI study. NeuroImage. 2001;14:7–20. doi: 10.1006/nimg.2001.0785. [DOI] [PubMed] [Google Scholar]
- Picton TW, Stuss DT, Alexander MP, Shallice T, Binns MA, Gillingham S. Effects of focal frontal lesions on response inhibition. Cerebral Cortex. 2007;17:826–838. doi: 10.1093/cercor/bhk031. [DOI] [PubMed] [Google Scholar]
- Ploran EJ, Nelson SM, Velanova K, Donaldson DI, Petersen SE, Wheeler ME. Evidence accumulation and the moment of recognition: dissociating perceptual recognition processes using fMRI. Journal of Neuroscience. 2007;27:11912–11924. doi: 10.1523/JNEUROSCI.3522-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poffenberger AT. The effects of continuous mental work. The American Journal of Psychology. 1927;39:283–296. [Google Scholar]
- Poldrack RA. Can cognitive processes be inferred from neuroimaging data? Trends in Cognitive Sciences. 2006;10:59–63. doi: 10.1016/j.tics.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Pollatos O, Schandry R, Auer DP, Kaufmann C. Brain structures mediating cardiovascular arousal and interoceptive awareness. Brain Research. 2007;1141:178–187. doi: 10.1016/j.brainres.2007.01.026. [DOI] [PubMed] [Google Scholar]
- Pollok B, Gross J, Kamp D, Schnitzler A. Evidence for anticipatory motor control within a cerebello-diencephalic-parietal network. Journal of Cognitive Neuroscience. 2008;20:828–840. doi: 10.1162/jocn.2008.20506. [DOI] [PubMed] [Google Scholar]
- Portas CM, Rees G, Howseman AM, Josephs O, Turner R, Frith CD. A specific role for the thalamus in mediating the interaction of attention and arousal in humans. The Journal of Neuroscience. 1998;18:8979–8989. doi: 10.1523/JNEUROSCI.18-21-08979.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Posner MI. Chronometric explorations of mind. Hillsdale, NJ: Lawrence Erlbaum; 1978. [Google Scholar]
- Posner MI. Interaction of arousal and selection in the posterior attention network. In: Baddeley AD, Weiskrantz L, editors. Attention: Selection, awareness, and control. A tribute to Donald Broadbent. New York, NY: Clarendon Press; 1993. pp. 390–405. [Google Scholar]
- Posner MI, Boies SJ. Components of attention. Psychological Review. 1971;78:391–408. [Google Scholar]
- Posner MI, Inhoff AW, Friedrich FJ, Cohen A. Isolating attentional systems: A cognitive-anatomical analysis. Psychobiology. 1987;15:107–121. [Google Scholar]
- Posner MI, Petersen SE. The attention system of the human brain. Annual Review of Neuroscience. 1990;13:25–42. doi: 10.1146/annurev.ne.13.030190.000325. [DOI] [PubMed] [Google Scholar]
- Ptak R. The frontoparietal attention network of the human brain: action, saliency, and a priority map of the environment. Neuroscientist. 2012;18:502–515. doi: 10.1177/1073858411409051. [DOI] [PubMed] [Google Scholar]
- Raz A, Buhle J. Typologies of attentional networks. Nature Reviews. Neuroscience. 2006;7:367–379. doi: 10.1038/nrn1903. [DOI] [PubMed] [Google Scholar]
- Rehme AK, Eickhoff SB, Wang LE, Fink GR, Grefkes C. Dynamic causal modeling of cortical activity from the acute to the chronic stage after stroke. NeuroImage. 2011;55:1147–1158. doi: 10.1016/j.neuroimage.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Requin J, Brener J, Ring C. Preparation for action. In: Jennings JR, Coles MGH, editors. Handbook of cognitive psychophysiology: Central and autonomic nervous system approaches. Oxford, UK: John Wiley & Sons; 1991. pp. 357–448. [Google Scholar]
- Richter DO, Senter RJ, Warm JS. Effects of the rate and regularity of background events on sustained attention. Bulletin of the Psychonomic Society. 1981;18:207–210. [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Robertson IH. The absent mind: Attention and error. The Psychologist. 2003;16:476–479. [Google Scholar]
- Robertson IH, Garavan H. Vigilant attention. In: Gazzaniga MS, editor. The cognitive neurosciences. 3rd ed. Cambridge, MA: MIT Press; 2004. pp. 631–640. [Google Scholar]
- Robertson IH, Manly T, Andrade J, Baddeley BT, Yiend J. ‘Oops!’: performance correlates of everyday attentional failures in traumatic brain injured and normal subjects. Neuropsychologia. 1997;35:747–758. doi: 10.1016/s0028-3932(97)00015-8. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Mattingley JB, Rorden C, Driver J. Phasic alerting of neglect patients overcomes their spatial deficit in visual awareness. Nature. 1998;395:169–172. doi: 10.1038/25993. [DOI] [PubMed] [Google Scholar]
- Robertson IH, O’Connell RG. Vigilant attention. In: Nobre AC, Coull JT, editors. Attention and time. Oxford, UK: Oxford University Press; 2010. pp. 79–88. [Google Scholar]
- Robertson IH, Ridgeway V, Greenfield E, Parr A. Motor recovery after stroke depends on intact sustained attention: a 2-year follow-up study. Neuropsychology. 1997;11:290–295. doi: 10.1037//0894-4105.11.2.290. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Tegnér R, Tham K, Lo A, Nimmo-Smith I. Sustained attention training for unilateral neglect: Theoretical and rehabilitation implications. Journal of Clinical and Experimental Neuropsychology. 1995;17:416–430. doi: 10.1080/01688639508405133. [DOI] [PubMed] [Google Scholar]
- Robertson IH, Ward T, Ridgeway V, Nimmo-Smith I. The structure of normal human attention: The Test of Everyday Attention. Journal of the International Neuropsychological Society. 1996;2:525–534. doi: 10.1017/s1355617700001697. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Bills AG. Two factors in the work decrement. Journal of Experimental Psychology. 1926;9:415–443. [Google Scholar]
- Rosenthal R. The"file drawer problem� and tolerance for null results. Psychological Bulletin. 1979;86:638–641. [Google Scholar]
- Rosvold H, Mirsky AF, Sarason I, Bransome ED, Jr, Beck LH. A continuous performance test of brain damage. Journal of Consulting Psychology. 1956;20:343–350. doi: 10.1037/h0043220. [DOI] [PubMed] [Google Scholar]
- Rottschy C, Eickhoff SB, Schleicher A, Mohlberg H, Kujovic M, Zilles K, Amunts K. Ventral visual cortex in humans: cytoarchitectonic mapping of two extrastriate areas. Human Brain Mapping. 2007;28:1045–1059. doi: 10.1002/hbm.20348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottschy C, Langner R, Dogan I, Reetz K, Laird AR, Schulz JB, Eickhoff SB. Modelling neural correlates of working memory: A coordinate-based meta-analysis. NeuroImage. 2012;60:830–846. doi: 10.1016/j.neuroimage.2011.11.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert L, Baboorian D, Stavropoulos K, Yasutake C. Individual differences in callosal efficiency: correlation with attention. Brain and Cognition. 1999;41:390–410. doi: 10.1006/brcg.1999.1142. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Grafman J. Sustained attention deficits in patients with right frontal lesions. Neuropsychologia. 1996;34:953–963. doi: 10.1016/0028-3932(96)00016-4. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Grafman J. Sustained attention deficits in patients with lesions of posterior cortex. Neuropsychologia. 1998;36:653–660. doi: 10.1016/s0028-3932(97)00150-4. [DOI] [PubMed] [Google Scholar]
- Rueckert L, Sorensen L, Levy J. Callosal efficiency is related to sustained attention. Neuropsychologia. 1994;32:159–173. doi: 10.1016/0028-3932(94)90003-5. [DOI] [PubMed] [Google Scholar]
- Rueda MR, Posner MI, Rothbart MK. Attentional control and self-regulation. In: Vohs KD, Baumeister RF, editors. Handbook of self-regulation: Research, theory, and applications. 2nd ed. New York, NY: Guilford Press; 2011. pp. 284–299. [Google Scholar]
- Ruge H, Wolfensteller U. Rapid formation of pragmatic rule representations in the human brain during instruction-based learning. Cerebral Cortex. 2010;20:1656–1667. doi: 10.1093/cercor/bhp228. [DOI] [PubMed] [Google Scholar]
- *.Salgado-Pineda P, Junque C, Vendrell P, Baeza I, Bargallo N, Falcon C, Bernardo M. Decreased cerebral activation during CPT performance: structural and functional deficits in schizophrenic patients. NeuroImage. 2004;21:840–847. doi: 10.1016/j.neuroimage.2003.10.027. [DOI] [PubMed] [Google Scholar]
- Sarter M, Gehring WJ, Kozak R. More attention must be paid: The neurobiology of attentional effort. Brain Research Reviews. 2006;51:145–160. doi: 10.1016/j.brainresrev.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Sarter M, Givens B, Bruno JP. The cognitive neuroscience of sustained attention: Where top-down meets bottom-up. Brain Research Reviews. 2001;35:146–160. doi: 10.1016/s0165-0173(01)00044-3. [DOI] [PubMed] [Google Scholar]
- Sarter M, Hasselmo ME, Bruno JP, Givens B. Unraveling the attentional functions of cortical cholinergic inputs: interactions between signal-driven and cognitive modulation of signal detection. Brain Research Reviews. 2005;48:98–111. doi: 10.1016/j.brainresrev.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Scerbo MW. What’s so boring about vigilance? In: Hoffman RB, Sherrick MF, Warm JS, editors. Viewing psychology as a whole: The integrative science of William N. Dember. Washington, DC: APA; 1998. pp. 145–166. [Google Scholar]
- Scerbo MW, Warm JS, Fisk AD. Event asynchrony and signal regularity in sustained attention. Current Psychological Research & Reviews. 1986;5:335–343. [Google Scholar]
- Scheperjans F, Hermann K, Eickhoff SB, Amunts K, Schleicher A, Zilles K. Observer-independent cytoarchitectonic mapping of the human superior parietal cortex. Cerebral Cortex. 2008;18:846–867. doi: 10.1093/cercor/bhm116. [DOI] [PubMed] [Google Scholar]
- *.Schlagenhauf F, Wustenberg T, Schmack K, Dinges M, Wrase J, Koslowski M, Heinz A. Switching schizophrenia patients from typical neuroleptics to olanzapine: effects on BOLD response during attention and working memory. European Neuropsychopharmacology. 2008;18:589–599. doi: 10.1016/j.euroneuro.2008.04.013. [DOI] [PubMed] [Google Scholar]
- *.Schmidt C, Collette F, Leclercq Y, Sterpenich V, Vandewalle G, Berthomier P, Peigneux P. Homeostatic sleep pressure and responses to sustained attention in the suprachiasmatic area. Science. 2009;324:516–519. doi: 10.1126/science.1167337. [DOI] [PubMed] [Google Scholar]
- *.Schneider F, Habel U, Reske M, Kellermann T, Stocker T, Shah NJ, Gaebel W. Neural correlates of working memory dysfunction in first-episode schizophrenia patients: an fMRI multi-center study. Schizophrenia Research. 2007;89:198–210. doi: 10.1016/j.schres.2006.07.021. [DOI] [PubMed] [Google Scholar]
- *.Schnell K, Heekeren K, Schnitker R, Daumann J, Weber J, Hesselmann V, Gouzoulis-Mayfrank E. An fMRI approach to particularize the frontoparietal network for visuomotor action monitoring: Detection of incongruence between test subjects’ actions and resulting perceptions. NeuroImage. 2007;34:332–341. doi: 10.1016/j.neuroimage.2006.08.027. [DOI] [PubMed] [Google Scholar]
- See JE, Howe SR, Warm JS, Dember WN. Meta-analysis of the sensitivity decrement in vigilance. Psychological Bulletin. 1995;117:230–249. [Google Scholar]
- Serences JT, Shomstein S, Leber AB, Golay X, Egeth HE, Yantis S. Coordination of voluntary and stimulus-driven attentional control in human cortex. Psychological Science. 2005;16:114–122. doi: 10.1111/j.0956-7976.2005.00791.x. [DOI] [PubMed] [Google Scholar]
- Shackman AJ, Salomons TV, Slagter HA, Fox AS, Winter JJ, Davidson RJ. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews. Neuroscience. 2011;12:154–167. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shallice T, Burgess P. The domain of supervisory processes and temporal organization of behaviour. Philosophical Transactions of the Royal Society of London. Series B: Biological Sciences. 1996;351:1405–1411. doi: 10.1098/rstb.1996.0124. [DOI] [PubMed] [Google Scholar]
- Shallice T, Stuss DT, Alexander MP, Picton TW, Derkzen D. The multiple dimensions of sustained attention. Cortex. 2008;44:794–805. doi: 10.1016/j.cortex.2007.04.002. [DOI] [PubMed] [Google Scholar]
- Shaw TH, Finomore V, Warm J, Matthews G. Effects of regular or irregular event schedules on cerebral hemovelocity during a sustained attention task. Journal of Clinical and Experimental Neuropsychology. 2012;34:57–66. doi: 10.1080/13803395.2011.621890. [DOI] [PubMed] [Google Scholar]
- Shaw TH, Warm JS, Finomore V, Tripp L, Matthews G, Weiler E, Parasuraman R. Effects of sensory modality on cerebral blood flow velocity during vigilance. Neuroscience Letters. 2009;461:207–211. doi: 10.1016/j.neulet.2009.06.008. [DOI] [PubMed] [Google Scholar]
- Shulman GL, Astafiev SV, McAvoy MP, d’Avossa G, Corbetta M. Right TPJ deactivation during visual search: functional significance and support for a filter hypothesis. Cerebral Cortex. 2007;17:2625–2633. doi: 10.1093/cercor/bhl170. [DOI] [PubMed] [Google Scholar]
- Shulman GL, McAvoy MP, Cowan MC, Astafiev SV, Tansy AP, d’Avossa G, Corbetta M. Quantitative analysis of attention and detection signals during visual search. Journal of Neurophysiology. 2003;90:3384–3397. doi: 10.1152/jn.00343.2003. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Beach E, Schooler JW, Handy TC. Going AWOL in the brain: Mind wandering reduces cortical analysis of external events. Journal of Cognitive Neuroscience. 2008;20:458–469. doi: 10.1162/jocn.2008.20037. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Davies JB, Heim D, Finnigan F, Sudberry M, O'Connor R, Obonsawin M. Subjective experience and the attentional lapse: Task engagement and disengagement during sustained attention. Consciousness and Cognition. 2004;13:657–690. doi: 10.1016/j.concog.2004.06.003. [DOI] [PubMed] [Google Scholar]
- Smallwood J, O’Connor RC, Sudbery MV, Obonsawin M. Mind-wandering and dysphoria. Cognition & Emotion. 2007;21:816–842. [Google Scholar]
- Smallwood J, Riby L, Heim D, Davies JB. Encoding during the attentional lapse: Accuracy of encoding during the semantic sustained attention to response task. Consciousness and Cognition. 2006;15:218–231. doi: 10.1016/j.concog.2005.03.003. [DOI] [PubMed] [Google Scholar]
- Smallwood J, Schooler JW. The restless mind. Psychological Bulletin. 2006;132:946–958. doi: 10.1037/0033-2909.132.6.946. [DOI] [PubMed] [Google Scholar]
- Smit AS, Eling PATM, Coenen AML. Mental effort causes vigilance decrease due to resource depletion. Acta Psychologica. 2004;115:35–42. doi: 10.1016/j.actpsy.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Smith A, Nutt D. Noradrenaline and attention lapses. Nature. 1996;380:291. doi: 10.1038/380291a0. [DOI] [PubMed] [Google Scholar]
- Smith SM, Fox PT, Miller KL, Glahn DC, Fox PM, Mackay CE, Beckmann CF. Correspondence of the brain’s functional architecture during activation and rest. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:13040–13045. doi: 10.1073/pnas.0905267106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Smits M, Dippel DW, Houston GC, Wielopolski PA, Koudstaal PJ, Hunink MG, van der Lugt A. Postconcussion syndrome after minor head injury: brain activation of working memory and attention. Human Brain Mapping. 2009;30:2789–2803. doi: 10.1002/hbm.20709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonuga-Barke EJ, Castellanos FX. Spontaneous attentional fluctuations in impaired states and pathological conditions: a neurobiological hypothesis. Neuroscience and Biobehavioral Reviews. 2007;31:977–986. doi: 10.1016/j.neubiorev.2007.02.005. [DOI] [PubMed] [Google Scholar]
- Spitzer H, Desimone R, Moran J. Increased attention enhances both behavioral and neuronal performance. Science. 1988;240:338–340. doi: 10.1126/science.3353728. [DOI] [PubMed] [Google Scholar]
- Sridharan D, Levitin DJ, Menon V. A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proceedings of the National Academy of Sciences of the United States of America. 2008;105:12569–12574. doi: 10.1073/pnas.0800005105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stawarczyk D, Majerus S, Maquet P, D’Argembeau A. Neural correlates of ongoing conscious experience: both task-unrelatedness and stimulus-independence are related to default network activity. PLoS One. 2011;6:e16997. doi: 10.1371/journal.pone.0016997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinborn MB, Flehmig HC, Westhoff K, Langner R. Differential effects of prolonged work on performance measures in self-paced speed tests. Advances in Cognitive Psychology. 2009;5:105–113. doi: 10.2478/v10053-008-0070-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinborn MB, Langner R. Distraction by irrelevant sound during foreperiods selectively impairs temporal preparation. Acta Psychologica. 2011;136:405–418. doi: 10.1016/j.actpsy.2011.01.008. [DOI] [PubMed] [Google Scholar]
- Steinborn MB, Langner R. Arousal modulates temporal preparation under increased time uncertainty: Evidence from higher-order sequential foreperiod effects. Acta Psychologica. 2012;139:65–76. doi: 10.1016/j.actpsy.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Steriade M, Datta S, Pare D, Oakson G, Curro Dossi RC. Neuronal activities in brain-stem cholinergic nuclei related to tonic activation processes in thalamocortical systems. Journal of Neuroscience. 1990;10:2541–2559. doi: 10.1523/JNEUROSCI.10-08-02541.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *.Sturm W, de Simone A, Krause BJ, Specht K, Hesselmann V, Radermacher I, Willmes K. Functional anatomy of intrinsic alertness: evidence for a fronto-parietal-thalamic-brainstem network in the right hemisphere. Neuropsychologia. 1999;37:797–805. doi: 10.1016/s0028-3932(98)00141-9. [DOI] [PubMed] [Google Scholar]
- *.Sturm W, Longoni F, Fimm B, Dietrich T, Weis S, Kemna S, Willmes K. Network for auditory intrinsic alertness: a PET study. Neuropsychologia. 2004;42:563–568. doi: 10.1016/j.neuropsychologia.2003.11.004. [DOI] [PubMed] [Google Scholar]
- *.Sturm W, Schmenk B, Fimm B, Specht K, Weis S, Thron A, Willmes K. Spatial attention: more than intrinsic alerting? Experimental Brain Research. 2006;171:16–25. doi: 10.1007/s00221-005-0253-1. [DOI] [PubMed] [Google Scholar]
- Sturm W, Willmes K. On the functional neuroanatomy of intrinsic and phasic alertness. NeuroImage. 2001;14:S76–S84. doi: 10.1006/nimg.2001.0839. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Alexander MP, Shallice T, Picton TW, Binns MA, Macdonald R, Katz DI. Multiple frontal systems controlling response speed. Neuropsychologia. 2005;43:396–417. doi: 10.1016/j.neuropsychologia.2004.06.010. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Binns MA, Murphy KJ, Alexander MP. Dissociations within the anterior attentional system: effects of task complexity and irrelevant information on reaction time speed and accuracy. Neuropsychology. 2002;16:500–513. doi: 10.1037//0894-4105.16.4.500. [DOI] [PubMed] [Google Scholar]
- Stuss DT, Shallice T, Alexander MP, Picton TW. A multidisciplinary approach to anterior attentional functions. Annals of the New York Academy of Sciences. 1995;769:191–211. doi: 10.1111/j.1749-6632.1995.tb38140.x. [DOI] [PubMed] [Google Scholar]
- Szalma JL, Warm JS, Matthews G, Dember WN, Weiler EM, Meier A, Eggemeier FT. Effects of sensory modality and task duration on performance, workload, and stress in sustained attention. Human Factors. 2004;46:219–233. doi: 10.1518/hfes.46.2.219.37334. [DOI] [PubMed] [Google Scholar]
- *.Tana MG, Montin E, Cerutti S, Bianchi AM. Exploring cortical attentional system by using fMRI during a Continuous Perfomance Test. Computational Intelligence and Neuroscience. 2010;10:329213. doi: 10.1155/2010/329213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor KS, Seminowicz DA, Davis KD. Two systems of resting state connectivity between the insula and cingulate cortex. Human Brain Mapping. 2009;30:2731–2745. doi: 10.1002/hbm.20705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple JG, Warm JS, Dember WN, Jones KTS, LaGrange CM, Matthews G. The effects of signal salience and caffeine on performance, workload, and stress in an abbreviated vigilance task. Human Factors. 2000;42:183–194. doi: 10.1518/001872000779656480. [DOI] [PubMed] [Google Scholar]
- Thackray RI. The stress of boredom and monotony: a consideration of the evidence. Psychosomatic Medicine. 1981;43:165–176. doi: 10.1097/00006842-198104000-00008. [DOI] [PubMed] [Google Scholar]
- *.Thakral PP, Slotnick SD. The role of parietal cortex during sustained visual spatial attention. Brain Research. 2009;1302:157–166. doi: 10.1016/j.brainres.2009.09.031. [DOI] [PubMed] [Google Scholar]
- Thayer RE. Toward a psychological theory of multidimensional activation (arousal) Motivation and Emotion. 1978;2:1–34. [Google Scholar]
- Thomas ML, Sing HC, Belenky G, Holcomb HH, Mayberg HS, Dannals RF, Redmond D. Neural basis of alertness and cognitive performance impairments during sleepinessIEffects of 24 h of sleep deprivation on waking human regional brain activity. Journal of Sleep Research. 2000;9:335–352. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Sing HC, Belenky G, Holcomb HH, Mayberg HS, Dannals RF, Popp KA. Neural basis of alertness and cognitive performance impairments during sleepiness: II. Effects of 48 and 72 h of sleep deprivation on waking human regional brain activity. Thalamus & Related Systems. 2003;2:199–229. doi: 10.1046/j.1365-2869.2000.00225.x. [DOI] [PubMed] [Google Scholar]
- Todd JJ, Fougnie D, Marois R. Visual short-term memory load suppresses temporo-parietal junction activity and induces inattentional blindness. Psychological Science. 2005;16:965–972. doi: 10.1111/j.1467-9280.2005.01645.x. [DOI] [PubMed] [Google Scholar]
- Trillenberg P, Verleger R, Teetzmann A, Wascher E, Wessel K. On the role of the cerebellum in exploiting temporal contingencies: evidence from response times and preparatory EEG potentials in patients with cerebellar atrophy. Neuropsychologia. 2004;42:754–763. doi: 10.1016/j.neuropsychologia.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eden GF, Jones KM, Zeffiro TA. Meta-analysis of the functional neuroanatomy of single-word reading: method and validation. NeuroImage. 2002;16:765–780. doi: 10.1006/nimg.2002.1131. [DOI] [PubMed] [Google Scholar]
- Turkeltaub PE, Eickhoff SB, Laird AR, Fox M, Wiener M, Fox PT. Minimizing within-experiment and within-group effects in activation likelihood estimation meta-analyses. Human Brain Mapping. 2012;33:1–13. doi: 10.1002/hbm.21186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ulich E. Unterforderung als arbeitspsychologisches Problem. [Underload as a problem of work psychology] Psychologie und Praxis. 1960;4:156–161. [Google Scholar]
- Vallesi A, Shallice T. Developmental dissociations of preparation over time: Deconstructing the variable foreperiod phenomena. Journal of Experimental Psychology: Human Perception and Performance. 2007;33:1377–1388. doi: 10.1037/0096-1523.33.6.1377. [DOI] [PubMed] [Google Scholar]
- Vallesi A, Shallice T, Walsh V. Role of the prefrontal cortex in the foreperiod effect: TMS evidence for dual mechanisms in temporal preparation. Cerebral Cortex. 2007;17:466–474. doi: 10.1093/cercor/bhj163. [DOI] [PubMed] [Google Scholar]
- van Breukelen GJ, Roskam EE, Eling PA, Jansen RW, Souren DA, Ickenroth JG. A model and diagnostic measures for response time series on tests of concentration: historical background, conceptual framework, and some applications. Brain and Cognition. 1995;27:147–179. doi: 10.1006/brcg.1995.1015. [DOI] [PubMed] [Google Scholar]
- van Zomeren AH, Brouwer WH. Clinical neurospychology of attention. New York: Oxford Press; 1994. [Google Scholar]
- Vandenberghe R, Gitelman DR, Parrish TB, Mesulam MM. Functional specificity of superior parietal mediation of spatial shifting. NeuroImage. 2001;14:661–673. doi: 10.1006/nimg.2001.0860. [DOI] [PubMed] [Google Scholar]
- Verbruggen F, Aron AR, Stevens MA, Chambers CD. Theta burst stimulation dissociates attention and action updating in human inferior frontal cortex. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:13966–13971. doi: 10.1073/pnas.1001957107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt BA. Pain and emotion interactions in subregions of the cingulate gyrus. Nature Reviews. Neuroscience. 2005;6:533–544. doi: 10.1038/nrn1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warm JS, Matthews G, Parasuraman R. Cerebral hemodynamics and vigilance performance. Military Psychology. 2009;21(Suppl 1):S75–S100. [Google Scholar]
- Warm JS, Parasuraman R, Matthews G. Vigilance requires hard mental work and is stressful. Human Factors. 2008;50:433–441. doi: 10.1518/001872008X312152. [DOI] [PubMed] [Google Scholar]
- Warm JS, Richter DO, Sprague RL, Porter PK, Schumsky DA. Listening with a dual brain: Hemispheric asymmetry in sustained attention. Bulletin of the Psychonomic Society. 1980;15:229–232. [Google Scholar]
- Weissman DH, Gopalakrishnan A, Hazlett CJ, Woldorff MG. Dorsal anterior cingulate cortex resolves conflict from distracting stimuli by boosting attention toward relevant events. Cerebral Cortex. 2005;15:229–237. doi: 10.1093/cercor/bhh125. [DOI] [PubMed] [Google Scholar]
- Weissman DH, Roberts KC, Visscher KM, Woldorff MG. The neural bases of momentary lapses in attention. Nature Neuroscience. 2006;9:971–978. doi: 10.1038/nn1727. [DOI] [PubMed] [Google Scholar]
- *.Welander-Vatn AS, Jensen J, Lycke C, Agartz I, Server A, Gadmar OB, Andreassen OA. No altered dorsal anterior cingulate activation in bipolar II disorder patients during a Go/No-go task: an fMRI study. Bipolar Disorders. 2009;11:270–279. doi: 10.1111/j.1399-5618.2009.00680.x. [DOI] [PubMed] [Google Scholar]
- Wesnes K, Warburton DM. Effects of smoking on rapid information processing performance. Neuropsychobiology. 1983;9:223–229. doi: 10.1159/000117969. [DOI] [PubMed] [Google Scholar]
- Whitehead R. Right hemisphere processing superiority during sustained visual attention. Journal of Cognitive Neuroscience. 1991;3:329–334. doi: 10.1162/jocn.1991.3.4.329. [DOI] [PubMed] [Google Scholar]
- Wickens CD, Gordon SE, Liu Y. An introduction to human factors engineering. New York, NY: Addison Wesley Longman; 1998. [Google Scholar]
- Wilkins AJ, Shallice T, McCarthy R. Frontal lesions and sustained attention. Neuropsychologia. 1987;25:359–365. doi: 10.1016/0028-3932(87)90024-8. [DOI] [PubMed] [Google Scholar]
- Wilkinson RT. Effects of up to 60 hours’ sleep deprivation on different types of work. Ergonomics. 1964;7:175–186. [Google Scholar]
- *.Wingen M, Kuypers KP, van de Ven V, Formisano E, Ramaekers JG. Sustained attention and serotonin: a pharmaco-fMRI study. Human Psychopharmacology. 2008;23:221–230. doi: 10.1002/hup.923. [DOI] [PubMed] [Google Scholar]
- Wood G, Nuerk HC, Sturm D, Willmes K. Using parametric regressors to disentangle properties of multi-feature processes. Behavioral and Brain Functions. 2008;4:38. doi: 10.1186/1744-9081-4-38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Gillin JC, Buchsbaum MS, Hershey T, Hazlett E, Sicotte N, Bunney WE., Jr The effect of sleep deprivation on cerebral glucose metabolic rate in normal humans assessed with positron emission tomography. Sleep. 1991;14:155–162. [PubMed] [Google Scholar]
- *.Zatorre RJ, Mondor TA, Evans AC. Auditory attention to space and frequency activates similar cerebral systems. NeuroImage. 1999;10:544–554. doi: 10.1006/nimg.1999.0491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.