Abstract
Several contributions have reported an altered expression of pseudoneglect in psychiatric disorders, highlighting the existence of an anomalous brain lateralization in affected subjects. Surprisingly, no studies have yet investigated pseudoneglect in first-degree relatives (FdR) of psychiatric patients. We investigated performance on “paper and pencil” line bisection (LB) tasks in 68 schizophrenic patients (SCZ), 42 unaffected FdR, 41 unipolar depressive patients (UP), and 103 healthy subjects (HS). A subgroup of 20 SCZ and 16 HS underwent computerized LB and mental number line bisection (MNL) tasks requiring judgment of prebisected lines and numerical intervals. Moreover, we evaluated, in a subgroup of 15 SCZ, performance on LB and MNL before and after parietal transcranial direct current stimulation (tDCS). In comparison to HS and UP, SCZ showed a systematic rightward bias on LB, partially corrected by selective right posterior parietal tDCS. Interestingly, even FdR showed a lack of pseudoneglect on LB, expressing a mean error lying in the middle between those of HS and SCZ. On the other hand, our results showed no significant difference between the performance of SCZ and HS on MNL. Both groups showed a comparable leftward bias that could not be significantly altered after left or right parietal tDCS. These findings confirm the existence of reduced lateralization in SCZ, suggesting specific impaired functioning of the right parietal lobule. Notably, we report a lack of pseudoneglect not only in SCZ but also in FdR, raising the hypothesis that an inverted laterality pattern may be considered a concrete marker of schizotypal traits.
Keywords: pseudoneglect, schizophrenia, endophenotype, tDCS, posterior parietal cortex
Introduction
The human brain is characterized by a unique lateralization of cognitive functions between homologous areas of the 2 hemispheres. For instance, it is known that cortical networks of the right hemisphere (RH) involving the posterior parietal cortex (PPC) play a predominant role in visuospatial attention, so that RH lesions often induce visuospatial neglect, a severe neurological disorder characterized by failure to acknowledge or explore stimuli presented to the contralesional side of space.1–3 The most commonly used technique for detecting the presence of unilateral spatial neglect is the line bisection (LB) test: the patient is asked to place a pencil mark at the center of a series of horizontal lines. Displacement of the bisection mark toward the side of the brain lesion is interpreted as a symptom of neglect (referred to as perceptual neglect). On the other hand, the phenomenon known as “pseudoneglect”4 refers to the systematic leftward misbisection of horizontal lines made by neurologically intact observers. Recently, several studies have investigated this phenomenon in schizophrenia. In particular, many studies have shown that schizophrenia patients (SCZ) have an abnormal rightward bias in comparison to healthy subjects (HS) on the LB test, which has been hypothesized to be linked to right parietal dysfunction.5,6 On the other hand, one study has shown a leftward bias in affective patients.7,8 Surprisingly, till now no studies have investigated LB in patients’ unaffected first-degree relatives (FdR).
There is also evidence supporting the existence of representational pseudoneglect in addition to perceptual pseudoneglect. This can be detected through the mental number line bisection (MNL) test, in which numbers are conceived as falling along a mental number line spatially oriented from left to right.1,9,10 Recent studies have investigated representational pseudoneglect in schizophrenia: while Cavezian and colleagues11 found an exaggerated leftward bias in the MNL of SCZ in comparison to HS, a more recent study found no difference between the 2 groups.12
Therefore, in the current study, we aimed to compare the performance on the LB test and MNL test of SCZ, FdR of SCZ, unipolar patients (UP), and HS. Furthermore, in a following experiment, we sought to investigate whether anodal transcranial direct current stimulation (tDCS) over both the left and right PPC influenced LB and MNL performance in an SCZ subgroup, to better elucidate the putative role of the PPC in this abnormal behavior.
Methods
Subjects
Three hundred and five right-handed subjects were enrolled: 103 SCZ, 42 FdR, 41 UP, and 119 HS. All patients were inpatients and were recruited from the Psychiatric Clinic at Tor Vergata University in Rome. Diagnosis was determined by means of consultation with physicians and the Structured Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition. Both patients and controls were excluded from participating if they exhibited any neurological or ophthalmological disorders, a history of head trauma, or if they met criteria for substance dependence within the previous 6 months or substance abuse within the month preceding testing.
Before evaluation, we used the Positive and Negative Syndrome Scale and the Global Assessment of Functioning scale to index the severity of the psychopathology. The schizophrenia group exhibited primarily mixed (both negative and positive) symptomatology, while the degree of depression in the UP group was evaluated with the Hamilton rating scale for Depression. See table 1 for demographic and clinical data for the patient group.
Table 1.
Clinical and Demographic Data for all the Groups
| Age | Male | PANSS (+) | PANSS (−) | PANSS (tot) | HAM-D | GAF | |
| Experiment 1 | |||||||
| SCZ (n = 68) | 37.93 ± 11.61 | 40 | 20.87 ± 5.9 | 19.41 ± 7.34 | 74.56 ± 7.2 | — | 46.74 ± 11.3 |
| UP (n = 46) | 44.76 ± 10.69 | 17 | — | — | — | 13.24 ± 4.67 | 65.81 ± 14.3 |
| FdR (n = 42) | 46.10 ± 16.06 | 21 | — | — | — | — | — |
| HS (n = 103) | 34.49 ± 12.97 | 52 | — | — | — | — | — |
| Experiment 2 | |||||||
| SCZ (n = 20) | 39.33 ± 12.01 | 13 | 22.57 ± 6.7 | 18.39 ± 8.15 | 73.47 ± 9.1 | — | 43.53 ± 8.27 |
| HS (n = 16) | 37.86 ± 13.86 | 10 | — | — | — | — | — |
| Experiment 3 | |||||||
| SCZ (n = 15) | 34.33 ± 10.37 | 11 | 23.1 ± 5.3 | 20.75 ± 7.23 | 69.85 ± 8.1 | — | 47.47 ± 7.63 |
Note: PANSS (total) is the result of PANSS positive subscale (+) plus negative subscale (−) plus global psychopathological scale. GAF, Global Assessment of Functioning; HAM-D, Hamilton Rating Scale for Depression; PANSS, Positive and Negative Syndrome Scale; UP, unipolar patients; FdR, first-degree relatives; HS, healthy subjects; SCZ, schizophrenic patients.
All subjects were right-handed on the basis of the Edinburgh Handedness Inventory13 and they all gave written informed consent for the study. The experimental procedures used were approved by the local Ethics Committee and were carried out in accordance with the Declaration of Helsinki.
Experiment 1
Two hundred and fifty-four subjects were enrolled: 68 SCZ (28 women and 40 men; age, 37.93 ± 11.61), 42 FdR (21 women and 21 men; age, 46.10 ± 16.06), 41 UP (24 women and 17 men; age, 44.76 ± 10.69), and 103 HS (51 women and 52 men; age, 34.49 ± 12.97). Subjects were seated comfortably at a writing table and the pages (size A4) containing the tasks were placed in front of them. They were told to follow the instructions written in Italian at the top of each page. All tasks were to be performed using the dominant hand. The lines to be divided in the bisection task were centered on each page and located below the midline. The lines were 125-mm-long black lines on an otherwise blank white page. Every page instructed the subject to “divide the line in half as accurately as possible.” Participants were asked to bisect each line into 2 equal lengths using a pencil. In order to compute scores, each line was measured to the nearest millimeter. The deviation from the center of the line was calculated as the absolute error in millimeter. Negative values indicate leftward bias and positive values a rightward bias.14 The task was repeated 3 times on 3 different days, and the mean deviation was calculated.
Experiment 2
Thirty-six subjects were recruited: 20 SCZ (7 women and 13 men; age, 38.85 ± 12.01) and 16 HS (6 women and 10 men; age, 37.13 ± 11.74).
E-Prime (version 2.0, Psychological Software Tools, Inc.; http://www.pstnet.com) software was used to create computerized versions of the 2 pseudoneglect protocols (LB and MNL; their experimental designs are presented in detail in the following sections), to run experiments, and to record subjects’ responses. Stimulus presentation and data collection were performed on a 15.4-inch laptop computer.
Line Bisection.
Visual stimuli consisted of black 1 mm thick horizontal lines transected by a vertical bar 1 mm thick and 1 cm long, presented on a white background with the bisector exactly coincident with the center of the screen. A modified version of LB created by Fierro et al15 was performed to detect perceptual pseudoneglect in medicated psychiatric patients. Stimuli were presented for 750 msec. Three 15-cm-long lines were presented, differing in the position of the bisector (at midpoint, rightward, or leftward). Subjects were given 30 trials in random order, 10 with the bisector at the exact center (7.5 cm), 10 with a rightward bisector at 8.0 cm, and 10 with a leftward bisector at 7.00 cm. The interstimulus interval was 3750 msec.
Subjects were seated at a distance of 45 cm from the laptop monitor and were asked to fixate a central cross that disappeared as soon as the lines were presented.
Observers judged the position of the bisector in prebisected lines by pressing 1 of 3 buttons with the right index, middle, or ring finger for “left,” “equal,” or “right” responses. The performance of the subject on each trial was scored as follows: 0, correct response; 1, if subject judged the bisector to be rightward with respect to its real position; −1, if subject judged the bisector to be leftward with respect to its real position.
Mental Number Line.
Stimuli (integers from 1 to 99) consisted of 30 different 1 and 2-digit number triplets, made up of a middle number and 2 outer numbers defining a number interval on each side. The 3 number stimuli were spaced 25 mm apart. The numerical distance between the middle number and the outer numbers was equal (bisectable triplets: eg, 2_9_16), bigger on the right side (e.g., 9_15_6), or on the left side (e.g., 9_19_11) in an equal number of trials. Triplets that are part of a multiplication table were not included. Stimuli were presented for 750 msec, with the middle number coincident with the center of the 15.4-inch laptop display. The intertrial interval was 3750 msec. Subjects were seated at a distance of 45 cm from the monitor and were asked to fixate a central cross that disappeared as soon as the 3 numbers were presented.
Observers judged the magnitude of the middle number in relationship to the outer numbers by pressing 1 of 3 buttons with the right index, middle, or ring finger for “left,” “equal,” or “right” responses. The performance of the subject on each trial was scored as follows: 0, for a correct response; 1, if subjects judged the middle number nearer to the right number of the triplet with respect to the real position; −1, if subjects judged the middle number nearer to the left number of the triplet with respect to the real position.
Experiment 3
Fifteen SCZ were enrolled (4 women and 11 men; age, 34.33 ± 10.37). The tDCS was applied for 10 min through rectangular saline–soaked sponge electrodes (50 × 70 mm2) with a battery-driven stimulator (CX-6650, Rolf Schneider Electronics, Gleichen, Germany). Stimulus intensity was set at 1 mA.
We applied anodal stimulation over the right PPC and the left PPC in 2 different sessions at an interval of 7 days, while the reference cathode electrode was placed over the contralateral shoulder. The electrode position for right PPC tDCS was then defined relative to the P4 position of the 10–20 electroencephalography (EEG) system, while the electrode position for left PPC tDCS was then defined relative to the P3 position of the 10–20 EEG system. According to previous investigations adopting 3-dimensional magnetic resonance imaging (MRI) reconstruction, these sites are situated over the inferior parietal lobule (IPL) close to the posterior part of the adjoining intraparietal sulcus.16
The LB and MNL computerized protocols were the same as for Experiment 2 (see above for details). The protocol presentation order was randomized both before and after the tDCS sessions. The performance of the subjects on LB and MNL was evaluated immediately before and then 5 minutes after each session of tDCS.
Statistics
The distribution of the pseudoneglect indices was evaluated. In the case of a nonnormal distribution, a logarithmic transformation was performed prior to statistical analysis to achieve an appropriate equivalence to a normal distribution (Shapiro-Wilk test, P > .05 consistently).
A General Linear Model approach was used to analyze the 3 experiments. In addition to well-known indices of null hypothesis significance testing, we reported the effect size—a measure useful for recognizing the value of the degree of association among variables—partial Eta squared () in ANOVA models.
Post hoc tests were performed with Bonferroni’s CI adjustment for multiple comparisons to define which variables contributed to the major effects. Because we cannot have a P value greater than unity, if Bonferroni correction produced an adjusted P value greater than 1.0, the P value would be rounded down to 1.0. Statistical significance was set at P < .05.
Results
Experiment 1
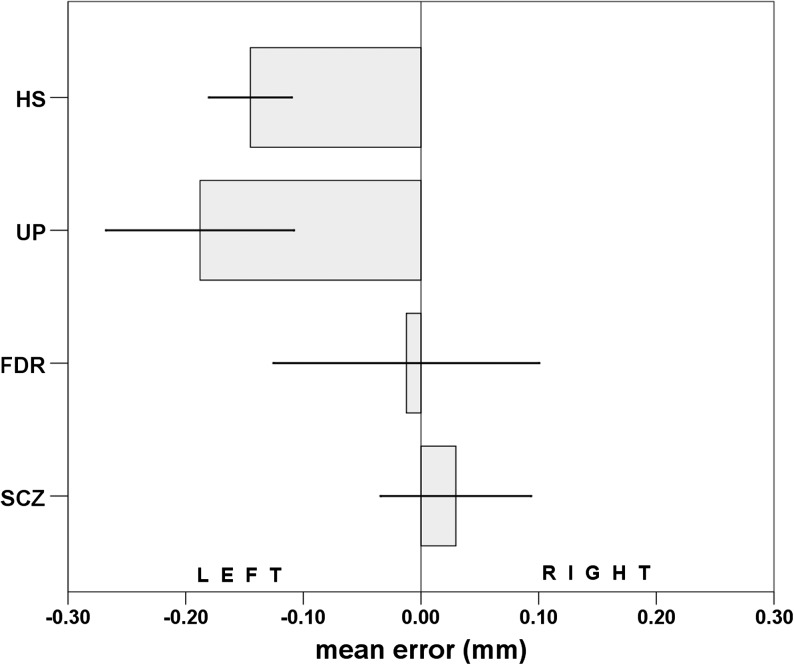
Mean error values were normally distributed (Shapiro-Wilk W = 0.99, P = .07). Age was statistically different among groups (F 3,250 = 11.395, P < .0001, = 0.12). A significant 2-way ANCOVA model (dependent variable: mean error; between-subject factors: “group,” SCZ vs FdR vs UP vs HS; “gender,” women vs men; covariate: age; F 8,250 = 4.176, P = .0001, adjusted R 2 = 0.09) showed a high difference in mean error among the 4 groups (F 3,245 = 10.755, P < .0001, = 0.12; figure 1); “age,” “gender,” and “gender × group” effects were not significant (respectively: F 1,245 = 1.629, P = 0.20, = 0.01; F 1,245 = 0.193, P = .66, = 0.00; F 3,245 = 0.398, P = .75, = 0.01). Post hoc analysis revealed SCZ had a significant rightward bias in comparison to UP (P = .0001) and HS (P < .0001). The same significant rightward bias emerged in the comparison between FdR and, respectively, UP (P = .02), and HS (P < .03). No difference was observed between SCZ and FdR (P = 1.00) or between UP and HS (P = 1.00).
Fig. 1.
LB paper and pencil performances of first-degree relatives (FdR), schizophrenic patients (SCZ), healthy subjects (HS), and unipolar patient (UP). The deviation from the center of the line was calculated as the absolute error in millimeter. Negative values indicate leftward bias and positive values a rightward bias.
Experiment 2
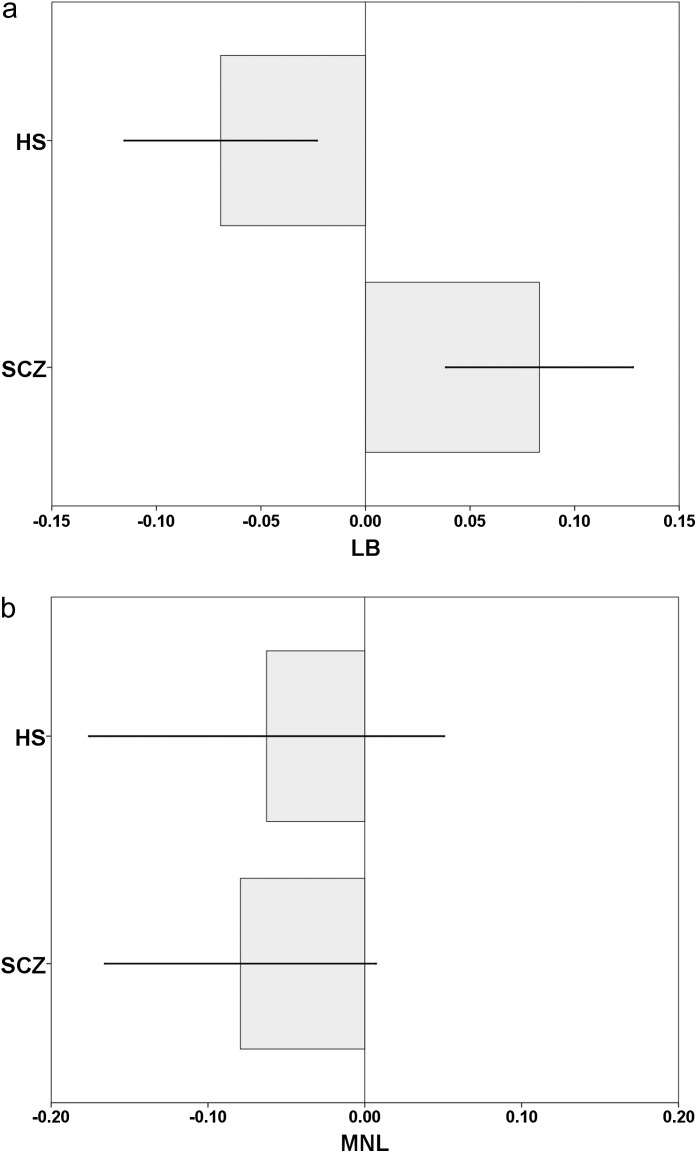
LB and MNL values were normally distributed (respectively, LB: Shapiro-Wilk W = 0.99, P = .09; MNL: Shapiro-Wilk W = 0.99, P = .08). Age was not statistically different between groups (t 34 = 0.433, P = .67). Two separate 1-way ANOVA analyses were carried out to test the difference respectively on LB and MNL between SCZ and HS. The first ANOVA model showed a significant rightward bias of LB measurements in SCZ with respect to HS (F 1,34 = 25.046, P < .0001, = 0.47; figure 2a). The second ANOVA model did not result in any difference of MNL measurements between SCZ and HS (F 1,34 = 0.066, P = .80, = 0.00; figure 2b).
Fig. 2.
(a) Schizophrenic patients (SCZ) and healthy subjects (HS) performances of line bisection (LB) on a PC screen with E-Prime programming software. The performance of the subject on each trial was scored as follows: 0.correct response; 1. if the subjects judged the transector to be rightward than its real position; −1. if the subjects judged the transector to be leftward than its real position. Negative values indicate leftward bias and positive values a rightward bias. (b) SCZ and HS performances of mental number line bisection (MNL) on a personal computer (PC) screen with E-Prime programming software. The performance of the subject on each trial was scored as follows: 0.correct response; 1. if the subjects judged the middle number nearer to the right number of the triplet; −1. if the subjects judged the middle number nearer to the left number of the triplet. Negative values indicate leftward bias and positive values a rightward bias.
Experiment 3
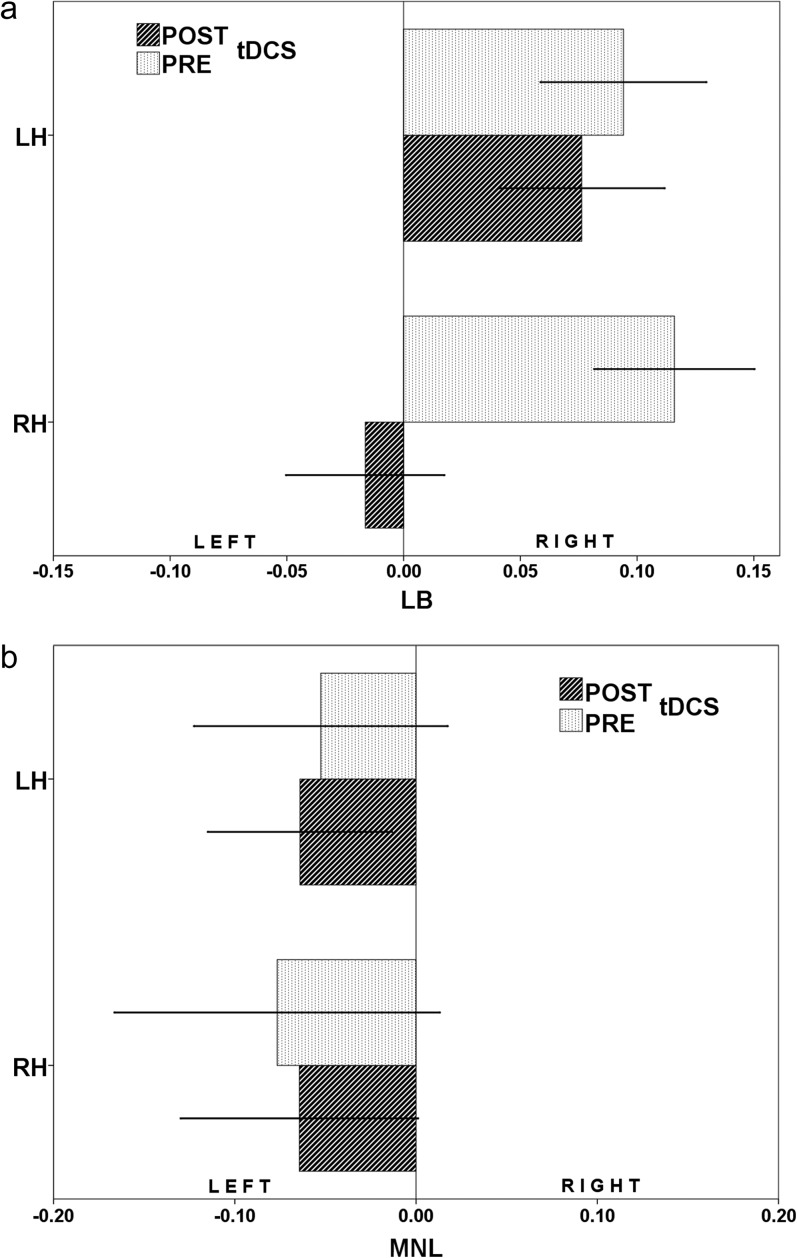
Two separate 2-way repeated measures ANOVA (rm-ANOVA) models were used to study the difference induced by left and right parietal tDCS (2 within-subjects factors: “condition,” pre-tDCS vs post-tDCS; “parietal stimulation,” left vs right) on LB and MNL. The first rm-ANOVA model showed a “condition” effect (F 1,14 = 46.166, P < .0001, = 0.77) and “condition” × “parietal stimulation” interaction effect (F 1,14 = 19.603, P = .0005, = 0.58; figure 3a) on LB measurement; the “parietal stimulation” effect was not significant (F 1,14 = 3.078, P = .10, = 0.18). Post hoc test-defined variables, which contributed to the major effects, were “pre-tDCS × left parietal stimulation” group vs “post-tDCS × left parietal stimulation” group (P < .0001). The second rm-ANOVA model did not show a significant “condition” (F 1,14 = 0.000, P = .99, = 0.00) and “parietal stimulation” effect (F 1,14 = 0.198, P = .66, = 0.02), and no significant “condition” × “parietal stimulation” interaction effect (F 1,14 = 1.131, P = .72, = 0.01; figure 3b) on MNL measurement.
Fig. 3.
(a) Observers judged the position of the transector in prebisected lines by pressing 1 of 3 buttons with the right index, middle, or ring finger for “left,” “equal,” or “right” responses. Negative values indicate leftward bias and positive values a rightward bias. This Figure shows LB scores before and after right hemisphere transcranial direct current stimulation (tDCS) (a) and after left hemisphere tDCS (b). LH stands for left hemisphere, while RH stands for right hemisphere.
Discussion
Schizophrenia is a complex disorder mainly characterized by thought disturbances, hallucinations, and decay of social and cognitive performance. From past attempts to identify the individual brain lesions responsible for specific domains of schizophrenia symptoms such as delusion and auditory hallucinations, recent data has pointed toward network alterations leading to abnormal brain asymmetry as important determinants of schizophrenia pathophysiology.17 Set in motion partly by Flor-Henry and Gur18–21 reports about the left hemisphere (LH) dysfunction in schizophrenia, a series of studies were undertaken to investigate hemisphere asymmetries of brain functions in schizophrenia. In the present study, we explored functional asymmetry, or lack thereof, by traditional means of LB and MNL tasks.
Experiment 1
In experiment 1, we found that SCZ did not exhibit the normal pattern of leftward bisection error, showing a systematic rightward bias on LB tasks in comparison to both HS and UP. This result is in line with several previous reports,5,6 confirming the hypothesis of schizophrenia as a loss of normal cerebral asymmetry. Moreover, no significant difference was found between UP and HS. Surprisingly, we found for the first time that even FdR of SCZ showed a lack of leftward bias on LB, with a mean error that was in the middle between those found in the HS and SCZ groups.
In short, we found that unaffected relatives of SCZ patients had a milder leftward bias on the LB task in comparison to HS. Because we found that lack of normal pseudoneglect is associated only with schizophrenia (not with unipolar depression), it manifests in patients both with positive and negative symptomatology and is found in a milder form in nonaffected family members, we may support the hypothesis that this phenomenon may be a potential endophenotype of schizophrenia according to the definition of Gottesman and Gould.22,23 Considering LB as a traditional means of studying functional asymmetry, this hypothesis is in line with previous brain imaging studies showing abnormal brain asymmetry in unaffected relatives.23,24
Experiment 2
In experiment 2, a subgroup of 20 SCZ and 16 HS underwent computerized LB and MNL tasks requiring judgment of prebisected lines and numerical intervals. Interestingly, SCZ patients expressed the same leftward bias in the visuospatial representation of numbers as the HS, showing dissociation in performance between visual line and number bisection in schizophrenia. Our data replicate the previous findings of Tian et al12 on 40 SCZ patients. Notably, Brugger et al25 have found that in HS, leftward bias is modulated by a mild form of schizotipy, suggesting that laterality in number representation may predispose to the development of fantastical ideas.
Complex phenomena are involved in the execution of MNL tasks. Till now, it has been commonly assumed that there is close interaction between the representations of number and space.9 The most compelling evidence in favor of number-space interactions derives from patients with RH damage who typically also show a bias toward larger numbers when asked to report the middle of a numerical interval (eg, when asked to specify the middle between 1 and 9, they might respond 7).26,27 Moreover, neuroimaging and Transcranial Magnetic Stimulation (TMS) studies in healthy humans28,29 have demonstrated that numerical tasks involve parietal areas, whose involvement in space perception and spatial attention is well known.30 On the other hand, recently, other studies have found dissociation between physical LB and MNL among different neglect patient groups.31 In the light of the above findings, several authors have reported that representational forms of neglect only occasionally coexist with neglect in physical space.32 Moreover, accurate neuropsychological examination revealed that the apparent left-sided neglect in the bisection of number intervals has a purely nonspatial origin and that verbal working memory may be crucial.33 Our hypothesis is that MNL in schizophrenia does not depend on spatial perception and may involve areas other than the parietal cortex, but a deeper evaluation is needed. In fact, in accordance with this hypothesis, neuroimaging studies have shown that while the visual LB task is related to the structure of the striate cortex, the extrastriate visual cortex, and the parietal lobe, the mental number bisection task is instead related to the right parietal lobe and prefrontal cortex.12
Experiment 3
Finally, in experiment 3, we tested whether LB and MNL performance may be influenced by tDCS over both the left and right PPC. To date, it has been demonstrated that tDCS cannot only modulate activity in the brain region directly underlying the stimulating electrode but also in a network of brain regions that are functionally related to the stimulated area.34 Furthermore, a magnetic resonance spectroscopy study has revealed that anodal stimulation over the PPC is able to increase glutamate and glutamine beneath the stimulating electrode.35
In our experiment, we found that only right parietal tDCS altered SCZ scores on LB with a partial correction of their lack of leftward bias. Our hypothesis is that the changes in the performance observed on LB tasks in SCZ could be due to an increase in the neural activity of the right PPC induced by tDCS. Several studies have reported the involvement of the right PPC36 and of the human IPL30 in the phenomenon of visual neglect. Interestingly, transient disruption of the right PPC, induced by focal repetitive TMS, determines contralateral visuospatial neglect in normal subjects.15 To this effect, several findings have reported dysfunction of the PPC in schizophrenia.37 To this regard, IPL cortical thickness in antipsychotic-naïve schizophrenia has been related to the presence of Schneiderian first rank symptoms.38 Furthermore, functional MRI data have revealed correlation of passivity symptoms with activation in the bilateral IPL, primary motor, and sensory cortices in the action monitoring condition.39
In the parallel experiment, we tested whether MNL performance may be influenced by tDCS over both the left and right PPC. In a previous study by Göbel et al,29 low frequency repetitive Transcranial Magnetic Stimulation (rTMS) over the right PPC simulated RH damage shifting the perceived midpoint of the numerical interval of HS significantly to the right while occipital rTMS had no effect on bisection performance. As we expect that the right PPC in HS is normal, we did not hypothesize any change after anodal tDCS and our results confirmed our expectations. We did not find any change in SCZ performance either after right PPC tDCS. This result is not surprising as we already found in experiment 2 that there was no difference in performance on MNL between SCZ and HS.
Conclusions
The major finding of this study is that a lack of a significant leftward bias in the LB test is presented both in SCZ and, in a milder way, in FdR. Furthermore, we hypothesize that this is due to a dysfunction of the right PPC because the lack of leftward bias reduces after selective stimulation of this area, supporting the hypothesis that this phenomenon may be a potential endophenotype of schizophrenia. There is general agreement that the RH is dominant in the distribution of attention for both hemifields, while the LH attentional vector is directed only to the right hemifield. Thus, lesions to the RH frequently render neglect patients pathologically inattentive to the left hemispace.2,40 In our model, we postulate that in the schizophrenic brain the RH loses its dominance, and this may be responsible for the well-observed lack of leftward bias in schizophrenia.
Acknowledgments
All authors report no biomedical financial interests or potential conflicts of interest.
References
- 1.Bisiach E, Luzzatti C. Unilateral neglect of representational space. Cortex. 1978;14:129–133. doi: 10.1016/s0010-9452(78)80016-1. [DOI] [PubMed] [Google Scholar]
- 2.Heilman KM, Valenstein E, Watson RT. Neglect and related disorders. Semin Neurol. 2000;20:463–470. doi: 10.1055/s-2000-13179. [DOI] [PubMed] [Google Scholar]
- 3.Vallar G, Bottini G, Paulesu E. Neglect syndromes: the role of the parietal cortex. Adv Neurol. 2003;93:293–319. [PubMed] [Google Scholar]
- 4.Bowers D, Heilman KM. Pseudoneglect: effects of hemispace on a tactile line bisection task. Neuropsychologia. 1980;18:491–498. doi: 10.1016/0028-3932(80)90151-7. [DOI] [PubMed] [Google Scholar]
- 5.Barnett KJ. Schizophrenia and rightward bias in line bisection. Laterality. 2006;11:36–42. doi: 10.1080/13576500500233628. [DOI] [PubMed] [Google Scholar]
- 6.McCourt ME, Shpaner M, Javitt DC, Foxe JJ. Hemispheric asymmetry and callosal integration of visuospatial attention in schizophrenia: a tachistoscopic line bisection study. Schizophr Res. 2008;102:189–196. doi: 10.1016/j.schres.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rao NP, Arasappa R, Reddy NN, Venkatasubramanian G, Gangadhar BN. Antithetical asymmetry in schizophrenia and bipolar affective disorder: a line bisection study. Bipolar Disord. 2010;12:221–229. doi: 10.1111/j.1399-5618.2010.00811.x. [DOI] [PubMed] [Google Scholar]
- 8.He W, Chai H, Zhang Y, Yu S, Chen W, Wang W. Line bisection performance in patients with generalized anxiety disorder and treatment-resistant depression. Int J Med Sci. 2010;7:224–231. doi: 10.7150/ijms.7.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dehaene S, Cohen L. Cerebral pathways for calculation: double dissociation between rote verbal and quantitative knowledge of arithmetic. Cortex. 1997;33:219–250. doi: 10.1016/s0010-9452(08)70002-9. [DOI] [PubMed] [Google Scholar]
- 10.McGeorge P, Beschin N, Colnaghi A, Rusconi ML, Della Sala S. A lateralized bias in mental imagery: evidence for representational pseudoneglect. Neurosci Lett. 2007;421:259–263. doi: 10.1016/j.neulet.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 11.Cavezian C, Rossetti Y, Danckert J, d'Amato T, Dalery J, Saoud M. Exaggerated leftward bias in the mental number line of patients with schizophrenia. Brain Cogn. 2007;63:85–90. doi: 10.1016/j.bandc.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 12.Tian Y, Wei L, Wang C, et al. Dissociation between visual line bisection and mental number line bisection in schizophrenia. Neurosci Lett. 2011;491:192–195. doi: 10.1016/j.neulet.2011.01.034. [DOI] [PubMed] [Google Scholar]
- 13.Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- 14.Zivotofsky AZ, Edelman S, Green T, Fostick L, Strous RD. Hemisphere asymmetry in schizophrenia as revealed through line bisection, line trisection, and letter cancellation. Brain Res. 2007;1142:70–79. doi: 10.1016/j.brainres.2007.01.046. [DOI] [PubMed] [Google Scholar]
- 15.Fierro B, Brighina F, Oliveri M, et al. Contralateral neglect induced by right posterior parietal rTMS in healthy subjects. Neuroreport. 2000;11:1519–1521. [PubMed] [Google Scholar]
- 16.Koch G, Fernandez Del Olmo M, Cheeran B, et al. Focal stimulation of the posterior parietal cortex increases the excitability of the ipsilateral motor cortex. J Neurosci. 2007;27:6815–6822. doi: 10.1523/JNEUROSCI.0598-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ribolsi M, Koch G, Magni V, et al. Abnormal brain lateralization and connectivity in schizophrenia. Rev Neurosci. 2009;20:61–70. doi: 10.1515/revneuro.2009.20.1.61. [DOI] [PubMed] [Google Scholar]
- 18.Flor-Henry P. Psychosis and temporal lobe epilepsy. A controlled investigation. Epilepsia. 1969;10:363–395. doi: 10.1111/j.1528-1157.1969.tb03853.x. [DOI] [PubMed] [Google Scholar]
- 19.Gur RE. Left hemisphere dysfunction and left hemisphere overactivation in schizophrenia. J Abnorm Physiol. 1978;87:226–238. doi: 10.1037//0021-843x.87.2.226. [DOI] [PubMed] [Google Scholar]
- 20.Gruzelier JH. Hemispheric imbalances in schizophrenia. Int J Psychophysiol. 1984;1:227–240. doi: 10.1016/0167-8760(84)90043-6. [DOI] [PubMed] [Google Scholar]
- 21.Crow TJ. A continuum of psychosis, one human gene, and not much else-the case for homogeneity. Schizophr Res. 1995;17:135–145. doi: 10.1016/0920-9964(95)00059-u. [DOI] [PubMed] [Google Scholar]
- 22.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 23.Sharma T, Lancaster E, Sigmundsson T, et al. Lack of normal pattern of cerebral asymmetry in familial schizophrenic patients and their relatives–The Maudsley Family Study. Schizophr Res. 1999;40:111–120. doi: 10.1016/s0920-9964(99)00143-7. [DOI] [PubMed] [Google Scholar]
- 24.Oertel V, Knochel C, Rotarska-Jagiela A, et al. Reduced laterality as a trait marker of schizophrenia–evidence from structural and functional neuroimaging. J Neurosci. 2010;30:2289–2299. doi: 10.1523/JNEUROSCI.4575-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brugger P, Schubiger M, Loetscher T. Leftward bias in number space is modulated by magical ideation. Cogn Behav Neurol. 2010;23:119–123. doi: 10.1097/WNN.0b013e3181d74901. [DOI] [PubMed] [Google Scholar]
- 26.Priftis K, Zorzi M, Meneghello F, Marenzi R, Umilta C. Explicit versus implicit processing of representational space in neglect: dissociations in accessing the mental number line. J Cogn Neurosci. 2006;18:680–688. doi: 10.1162/jocn.2006.18.4.680. [DOI] [PubMed] [Google Scholar]
- 27.Zorzi M, Priftis K, Umilta C. Brain damage: neglect disrupts the mental number line. Nature. 2002;417:138–139. doi: 10.1038/417138a. [DOI] [PubMed] [Google Scholar]
- 28.Dehaene S, Spelke E, Pinel P, Stanescu R, Tsivkin S. Sources of mathematical thinking: behavioral and brain-imaging evidence. Science. 1999;284:970–974. doi: 10.1126/science.284.5416.970. [DOI] [PubMed] [Google Scholar]
- 29.Gobel SM, Calabria M, Farne A, Rossetti Y. Parietal rTMS distorts the mental number line: simulating 'spatial' neglect in healthy subjects. Neuropsychologia. 2006;44:860–868. doi: 10.1016/j.neuropsychologia.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 30.Husain M, Nachev P. Space and the parietal cortex. Trends Cogn Sci. 2007;11:30–36. doi: 10.1016/j.tics.2006.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Doricchi F, Guariglia P, Gasparini M, Tomaiuolo F. Dissociation between physical and mental number line bisection in right hemisphere brain damage. Nat Neurosci. 2005;8:1663–1665. doi: 10.1038/nn1563. [DOI] [PubMed] [Google Scholar]
- 32.Loetscher T, Nicholls ME, Towse JN, Bradshaw JL, Brugger P. Lucky numbers: spatial neglect affects physical, but not representational, choices in a lotto task. Cortex. 2010;46:685–690. doi: 10.1016/j.cortex.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 33.van Dijck JP, Gevers W, Lafosse C, Doricchi F, Fias W. Non-spatial neglect for the mental number line. Neuropsychologia. 2011;49:2570–2583. doi: 10.1016/j.neuropsychologia.2011.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Zheng X, Alsop DC, Schlaug G. Effects of transcranial direct current stimulation (tDCS) on human regional cerebral blood flow. Neuroimage. 2011;58:26–33. doi: 10.1016/j.neuroimage.2011.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Clark VP, Coffman BA, Trumbo MC, Gasparovic C. Transcranial direct current stimulation (tDCS) produces localized and specific alterations in neurochemistry: a (1)H magnetic resonance spectroscopy study. Neurosci Lett. 2011;500:67–71. doi: 10.1016/j.neulet.2011.05.244. [DOI] [PubMed] [Google Scholar]
- 36.Malhotra P, Coulthard EJ, Husain M. Role of right posterior parietal cortex in maintaining attention to spatial locations over time. Brain. 2009;132:645–660. doi: 10.1093/brain/awn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Petty RG. Structural asymmetries of the human brain and their disturbance in schizophrenia. Schizophr Bull. 1999;25:121–139. doi: 10.1093/oxfordjournals.schbul.a033360. [DOI] [PubMed] [Google Scholar]
- 38.Venkatasubramanian G, Jayakumar PN, Keshavan MS, Gangadhar BN. Schneiderian first rank symptoms and inferior parietal lobule cortical thickness in antipsychotic-naive schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:40–46. doi: 10.1016/j.pnpbp.2010.07.023. [DOI] [PubMed] [Google Scholar]
- 39.Schnell K, Heekeren K, Daumann J, et al. Correlation of passivity symptoms and dysfunctional visuomotor action monitoring in psychosis. Brain. 2008;131:2783–2797. doi: 10.1093/brain/awn184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Weintraub S, Mesulam MM. Right cerebral dominance in spatial attention. Further evidence based on ipsilateral neglect. Arch Neurol. 1987;44:621–625. doi: 10.1001/archneur.1987.00520180043014. [DOI] [PubMed] [Google Scholar]