Abstract
NR4A2 (nuclear receptor subfamily 4 group A member 2) or Nurr1 is a transcription factor implied in the differentiation, maturation, and survival of dopaminergic neurons. It also has a role in the expression of several proteins that are necessary for the synthesis and regulation of dopamine (DA), such as tyrosine hidroxilase, dopamine transporter, vesicular monoamine transporter 2, and cRET. DA is an important neurotransmitter in attentional pathways. Our aim was to evaluate the influence of NR4A2 gene in the performance of schizophrenia (SZ) patients and healthy subjects on a sustained attention task. For this study, we collected 188 SZ subjects (Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition) and 100 control individuals. We genotyped 5 tag SNPs in NR4A2 gene: rs1150143 (C/G), rs1150144 (A/G), rs834830 (A/G), rs1466408 (T/A), and rs707132 (A/G). We also analyzed the influence of its haplotypes (frequency >5%). To examine sustained attention, all the individuals completed the Degraded Stimulus Continuous Performance Test. We evaluated “hits,” “reaction time,” “sensibility a,” and “false alarms.” In the schizophrenic group, recessive genotypes of rs1150143, rs1150144, rs834830, and rs707132 were associated with a worse performance. SZ subjects who carried GGGTG haplotype showed less hits (P < .004), lower sensibility a scores (P < .009), and a higher reaction time (P = .013). We observed a sex effect of the gene: genotype and haplotype associations were only present in the male group. We conclude that NR4A2 gene is involved in attentional deficits of SZ patients, modifying hits, sensibility a, and reaction time.
Keywords: neuroscience, genetics, haplotype, dopamine, cognition, neuropsychology
Introduction
Schizophrenia (SZ) is a chronic, severe, and disabling brain disorder that affects approximately 1 of 100 people worldwide (∼26.3 millions of people). According to the World Health Organization, schizophrenia is the fifth leading cause of years lost due to disability in men and the sixth in women. Schizophrenic patients exhibit pervasive neuropsychological disturbances, in which impairment in attentional processing is considered one of the core deficits.1 Attention deficits may be manifested by easy distractibility, poor concentration, perseveration, impulsivity, disinhibition, or difficulty in the completion of planned activities. Prefrontal cortex (PFC), which has been critically involved in sustained attention,2 has an important role in regulating brain circuitries, involving connections between the orbitofrontal cortex, other limbic structures, the nucleus accumbens, mediodorsal thalamus, and ventral pallidum.3 Attentional deficits that have been described in SZ have been commonly linked to dysfunction of these “loops.”4–6 Mesocortical neurochemical modulation of attentional processes depends on several neurotransmitters, among which dopamine stands out.3
The nuclear receptor subfamily 4, group A, member 2 (NR4A2, also called Nurr1/NOT/TINUR/RNR-1/HZF-3) is a transcription factor that has been implicated in the specification/differentiation, maturation, and survival of dopamine neurons in animal studies.7,8 NR4A2-deficient mice fail to express early dopaminergic markers such as tyrosine hidroxilase, a key enzyme in the catecholamine biosynthetic pathway.9,10 This transcription factor regulates the expression of the former, and other proteins that are required for dopamine synthesis and regulation, such as vesicular monoamine transporter 2, dopamine transporter, and receptor tyrosine kinase (cRET).11,12 Moreover, the combination of this and other transcription factors such as Pitx3 is necessary for other developmental events in midbrain dopaminergic neurons.13 This nuclear receptor is also involved in the expression of other target genes involved in important neuronal processes such as neuronal patterning, axon outgrowth, and terminal differentiation.14,15 At early stages in development, expression of NR4A2 is restricted to the midbrain but subsequently expression extends to other central nervous system regions including the cortex and the hippocampus.16,17
NR4A2 gene, located in chromosome 2q22-23, has been previously associated with susceptibility to schizophrenia by other authors18,19 and by our own group (manuscript in preparation); although there is some controversy about the results.20,21 Association between this chromosomic region and schizophrenia has also been detected in genome-wide studies.22,23 Besides, expression of this transcription factor is reduced in the PFC of subjects with SZ.24 In addition, NR4A2 heterozygous mutant mice have been proposed as animal models for SZ.16,25 Finally, some studies have postulated the importance of NR4A2 expression for learning and memory in mice.26,27
This study sought to verify if NR4A2 gene, is associated, either genotypically or haplotypically, with the performance of schizophrenic patients and/or healthy subjects in a sustained attention task. Due to the results of a previous case-control report carried out in this population (in preparation), we also hypothesized a sexually dimorphic effect of the gene on this trait.
Methodology
Samples
We enrolled in the study 188 unrelated patients diagnosed with schizophrenia (134 men and 54 women) and 100 unrelated control subjects (48 men and 52 women), aged 18–60 years. Schizophrenic diagnoses were based on Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (DSM-IV) criteria. Patients were evaluated with the Structured Clinical Interview for DSM-IV (SCID-I:P. http://www.scid4.org/), and control subjects were screened using the SCID-I nonpatient version to exclude subjects with prior psychiatric history. Consensus DSM-IV diagnoses were assigned by board-certified psychiatrists. Patients with DSM-IV criteria for either schizoaffective or schizophreniform disorder were excluded from this study. Patients were in a nonacute phase of the illness. They were evaluated monthly and were assumed to be stabilized when the total Positive and Negative Syndrome Scale score did not change by more than 3 points, and there had not been changes in pharmacological treatment during at least 6 months preceding neuropsychological evaluation. Subjects were recruited from the Virgen de la Luz Hospital (Cuenca, Spain) and the Mental Health Area of the Clínico San Carlos Hospital (Madrid, Spain). All control individuals and schizophrenic patients were Caucasians. Exclusion criteria were severe medical or neurological disease, illiteracy or low education levels that hindered cognitive assessment, mental retardation, a history of electroconvulsive therapy in the last 2 years, substance dependence or abuse at the moment of the study (except for tobacco consumption), and head injury with loss of consciousness. Control subjects had no first-degree relatives with BD, psychosis, or other psychiatric disorders. Approval was obtained from Ethics Committees in both hospitals. Written informed consent was obtained from all participants after the study procedures had been explained in full.
Genotyping Assays
NR4A2 gene is located in chromosome 2 and spans 8.25 kb (positions 156,889,197–156,897,446 according to HapMap Data release 24/phase II, November 2008. www.hapmap.org). The haplotype block definition was made according to Gabriel et al28 CIs. The single nucleotide polymorphism (SNP) markers were selected across the whole gene to cover the complete linkage disequilibrium (LD) structure of the gene. This gene is contained in a single LD block (36 kb) that comprises the tag SNPs rs1150143, rs1150144, rs834830, rs1466408, and rs707132 (equivalent to rs12803) (see online supplementary figure 1). All these polymorphisms were genotyped and included in the analysis.
Genomic DNA was extracted from 10 ml of EDTA anticoagulated whole blood using standard methods. Briefly, lysis with a high sucrose solution (0.3 M sucrose, 0.01 M Tris-HCl, ph 7.5, 0.005 M MgCl2, and 1% Triton ×100) and subsequent centrifugation were performed. DNA was then isolated from these cells with DNAzol—Genomic DNA Isolation Reagent (Molecular Research Center, Inc.).29
Genotyping was performed using the MassARRAY iPLEX Gold technology (Sequenom Inc.), following the protocol provided by Sequenom (http://www.sequenom.com). The polymerase chain reaction and extension primers were designed using Assay Design 3.0 (Sequenom Inc). Analysis and scoring were completed using Typer 3.3 (Sequenom Inc).
Cognitive Assessment
Sustained attention was assessed throughout a computerized version of the Degraded Stimulus Continuous Performance Test (DS-CPT), version 8.12, from Nuechterlein and Asarnow.30 The entire test takes approximately 8 minutes to complete, and 6 blocks of 80 trials are given in total. Afterward, these 6 blocks are directly combined into 3 blocks of 160 trials, allowing the study of early, medium, and late attention stages (blocks 1, 2, and 3). Performance on the test was analyzed by different measures: hits, false alarms, reaction time, and sensibility a. Sensibility a is the ability to discriminate between the signal target and noise; it combines both hits and false alarms. All scores were obtained automatically from the program.
Besides, participants completed the vocabulary section of the Wechsler Adult Intelligence Scale , to estimate the premorbid IQ.
Data Analysis
After a case-control study and according to Akaike’s Criterion Information, we assumed the recessive genetic model for the analysis (see online supplementary table S1; analysis performed using SNPStats Software). Statistical analyses were carried out using SPSS, version 15.0, for Windows. According to the fit of the variables, linear (β) or logistic (odds ratio) regressions were used to determine associations between candidate polymorphisms and DS-CPT performance; including in the analysis age, gender, years of education, and IQ as potential confounders due to their possible influence on sustained attention performance.31 Cohen’s d value was calculated to estimate the effect size (d = M 1−M 2/S pooled). These effects were we defined as trivial (d < 0.2), small (0.2 ≤ d < 0.5), medium (0.5 ≤ d < 0.8), and large (d ≥ 0.8). To evaluate results from haplotypes, due to the unknown linkage phase of the polymorphisms, we computed statistics through Haplo.Stats 1.3.8 program, (http://mayoresearch.mayo.edu/mayo/research/schaid_lab/software.cfm) in the free software environment R.32 The general linear model was used to examine associations with cognitive traits and scores were calculated controlling for previously mentioned covariates, both in cases and controls separately. All haplotypes with an estimated overall frequency of ≥5% were considered in the analyses. Minority haplotypes were combined into a single group defined as “rare haplotypes.” Correction for multiple comparisons was performed via False Discovery Rate (FDR). A P value ≤ .05 was considered significant.
Results
Clinical and Sociodemographical Data
Schizophrenic patients were younger, had received 3.5 years of education less, and evidenced a lower premorbid IQ than healthy controls. Besides, the percentage of men was higher in the former group and only one-third of patients had a job vs almost 90% of control subjects. These and other sociodemographical and clinical data are collected in table 1.
Table 1.
Sociodemographical and Clinical Data of the Sample
| Controls (N = 100) | SCH (N = 188) | P Value | ||
| Sociodemographic | ||||
| Age ( ± SD) | 42.6 ± 12.6 | 38.4 ± 8.7 | .003 | |
| Sex (% men) | 48 | 71.2 | <.001 | |
| Years of education ( ± SD) | 15.5 ± 5.3 | 12 ±3.2 | <.001 | |
| IQ ( ± SD) | 42 ± 9.1 | 35.6 ± 9.5 | <.001 | |
| Number of cigarettes ( ± SD) | 6.4 ± 11.7 | 16.3 ± 14.5 | <.001 | |
| Tobacco consumption (% SM/% FS/% NS) | 28.3/13.1/58.6 | 66.8/5.2/28 | <.001 | |
| Labor status (% active) | 89.7 | 32.8 | <.001 | |
| Clinical | ||||
| % Deficit syndrome | 34.7 | |||
| Age at onset ( ± SD) | 23.4 ± 6.2 | |||
| Duration of illness ( ± SD) | 14.6 ± 8.4 | |||
| Age at first hospitalization ( ± SD) | 26.5 ± 7.6 | |||
| Number of hospitalizations ( ± SD) | 3.5 ± 3.7 | |||
| Stabilization (months) ( ± SD)a | 45.6 ± 45.4 | |||
| Suicide attempts (%) | 13.2 | |||
| PANSS ( ± SD) | Total | 54.9 ± 15.7 | ||
| Positive | 11.5 ± 4.4 | |||
| Negative | 17.4 ± 7.7 | |||
| GS | 26.3 ± 6.3 | |||
| Pharmacological | ||||
| Number of psychopharmacs ( ± SD) | 1.6 ± 0.8 | |||
| Antipsychotics (% No/% FGA/% SGA) | 2.1/17.6/80.3 | |||
| Antidepressive (%) | 10.4 | |||
| BZD (%) | 25.9 | |||
Note: , media; SD, standard deviation; SCH, schizophrenia; SM, smokers; FS, former smokers; NS, nonsmokers; DF, deficitary; GS, general syndrome; FGA, first-generation antipsychotic; SGA, second generation antipsychotic; BZD, benzodiazepine; In bold, significant associations.
Minimum 3 months.
Association of NR4A2 With Sustained Attention Performance
Genotypic Associations.
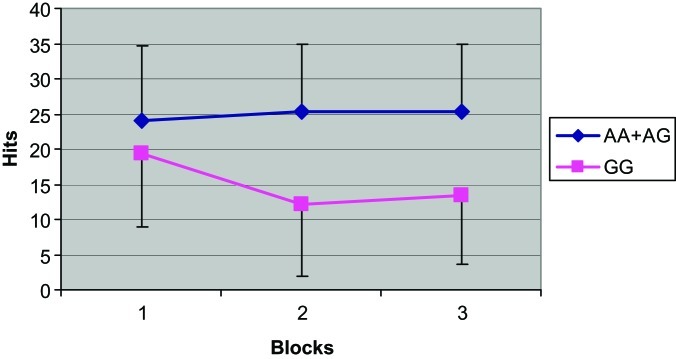
We assayed the candidate polymorphisms in the case and the control groups independently. Frequencies of genotypes are shown in online supplementary table S2. rs1150143, rs1150144, rs834830, and rs707132 showed significant associations with attentional performance in the schizophrenic group (tables 2 and 3). In the 4 loci, recessive homozygotes carriers evidenced impairment in several measures of the test. Those subjects showed a decrement in the number of the hits (P values reached values between 4 × 10−5 and .05), an increment in reaction time (.003 ≤ P ≤ .05), and lower sensibility a scores (8.2 × 10−5 ≤ P ≤ .017). Moreover, these disturbances emerged with the progression of the task. Gender-specific analyses were conducted at NR4A2 polymorphisms and evidenced that the whole group results were only maintained in the male group (significant P values in the 3 variables were comprised between 4.4 × 10−6 ≤ P ≤ .05) (table 3). Effect sizes of these genotypic differences, according to Cohen’s d, were medium and large except for rs707132, which only showed small effects. Individual values are reflected in online supplementary table S4. Not a single association was found with false alarms rate. An example of these associations is shown in figure 1. This figure evidences a lower number of hits of those schizophrenic patients who carry the recessive homozygote in rs834830.
Table 2.
Rawa Scores Obtained in DS-CPT by Carriers of the Different Genotypes of Candidate Polymorphisms of NR4A2b
| Whole Schizophrenic Group (n = 188)c | Only Men Schizophrenic Group (n = 134)d | |||||
| Hits | Reaction Time | Sensibility a | Hits | Reaction Time | Sensibility a | |
| Block 1 | ||||||
| rs1150143 | CC+CG: 0.85 ± 0.12; GG: 0.78 ± 0.23 | CC+CG: 0.85 ± 0.11; GG: 0.72 ± 0.28 | ||||
| rs1150144 | AA+AG: 24.5 ± 10.4; GG: 16.5 ± 9.3 | AA+AG: 0.85 ± 0.12; GG: 0.73 ± 0.26 | AA+AG: 24 ± 10.6; GG: 16.3 ± 8.6 | AA+AG: 0.85 ± 0.11; GG: 0.69 ± 0.30 | ||
| rs834830 | AA: 0.85 ± 0.11; GG: 0.66 ± 0.3 | AA+AG: 0.85 ± 0.12; GG: 0.6 ± 0.31 | ||||
| rs1466408 | ||||||
| rs707132 | ||||||
| Block 2 | ||||||
| rs1150143 | CC+CG: 25.8 ± 9.3; GG: 15.9 ± 11.3 | CC+CG: 0.86 ± 0.13; GG: 0.76 ± 0.16 | CC+CG: 25.3 ± 9.1; GG: 13.2 ± 9.6 | CC+CG: 0.86 ± 0.11; GG: 0.70 ± 0.17 | ||
| rs1150144 | AA+AG: 25.8 ± 9.3; GG: 13.3 ± 10.9 | AA+AG: 0.55 ± 0.09; GG: 0.62 ± 0.15 | AA+AG: 0.86 ± 0.12; GG: 0.72 ± 0.17 | AA+AG: 25.3 ± 9.2; GG: 12.8 ± 8.3 | AA+AG: 0.86 ± 0.12; GG: 0.69 ± 0.17 | |
| rs834830 | AA+AG: 25.4 ± 9.6; GG: 12.2 ± 10.4 | AA+AG: 0.85 ± 0.12; GG: 0.7 ± 0.2 | AA+AG: 24.9 ± 9.4; GG: 13.5 ± 11.5 | AA+AG: 0.85 ± 0.12; GG: 0.7 ± 0.23 | ||
| rs1466408 | ||||||
| rs707132 | AA+AG: 25.5 ± 9.7; GG: 23.2 ± 9.9 | AA+AG: 25 ± 9.5; GG: 22.1 ± 9.7 | AA+AG: 0.85 ± 0.1; GG: 0.81 ± 0.14 | |||
| Block 3 | ||||||
| rs1150143 | CC+CG: 25.5 ± 9.5; GG: 19.4 ± 12 | CC+CG: 0.54 ± 0.09; GG: 0.61 ± 0.1 | CC+CG: 0.86 ± 0.1; GG: 0.78 ± 0.2 | CC+CG: 25 ± 9.5; GG: 16.1 ± 11.8 | CC+CG: 0.55 ± 0.09; GG: 0.62 ± 0.10 | CC+CG: 0.86 ± 0.10; GG: 0.70 ± 0.23 |
| rs1150144 | AA+AG: 25.7± 9.4; GG: 14.2 ± 9.7 | AA+AG: 0.55 ± 0.09; GG: 0.63 ± 0.13 | AA+AG: 0.86 ± 0.12; GG: 0.72 ± 0.2 | AA+AG: 25.2 ± 9.4; GG: 12.6 ± 9.2 | AA+AG: 0.55 ± 0.09; GG: 0.63 ± 0.11 | AA+AG: 0.86 ± 0.11; GG: 0.67 ± 0.20 |
| rs834830 | AA+AG: 25.3 ± 9.6; GG: 13.4 ± 9.8 | AA+AG: 0.55 ± 0.09; GG: 0.64 ± 0.11 | AA+AG: 0.86 ± 0.11; GG: 0.64 ± 0.3 | AA+AG: 24.8 ± 9.6; GG: 10.5 ± 8.5 | AA+AG: 0.55 ± 0.09; GG: 0.65 ± 0.13 | AA+AG: 0.86 ± 0.11; GG: 0.58 ± 0.3 |
| rs1466408 | ||||||
| rs707132 | AA+AG: 25.6 ± 9.4; GG: 22.4 ± 10.9 | AA+AG: 0.86 ± 0.11; GG: 0.81 ± 0.16 | AA+AG: 25.1 ± 9.3; GG: 20.7 ± 11.6 | AA+AG: 0.86 ± 0.1; GG: 0.8 ± 0.17 | ||
Note: CPT-DS, Continuous Performance Test—Degraded Stimulus version.
Scores obtained directly from the program, before adjustment for age, sex, years of education, and IQ.
Only significative comparisons shown.
Number of genotype carriers: rs1150143: CC+CG: 173, GG: 15; rs1150144: AA+AG: 177, GG: 11; rs834830: AA+AG: 183, GG: 5; rs1466408: AA+AT: 1, TT: 187; rs707132: AA+AG: 140, GG: 48.
Number of genotype carriers: rs1150143: CC+CG: 125, GG: 9; rs1150144: AA+AG: 126, GG: 8; rs834830: AA+AG: 130, GG: 4; rs1466408: AA+AT: 1, TT: 133; rs707132: AA+AG: 104, GG: 30.
Table 3.
Main Effect Model of the NR4A2 Gene After Adjustment for Conventional DS-CPT Confounder Factorsa , b (Regression Analysis: Recessive Homozygotes vs Dominant Homozygotes + Heterozygotes)
| Whole Schizophrenic Group (n = 188)c | Only Men Schizophrenic Group (n = 134)d | |||||
| Hits | RT | S.a | Hits | RT | S.a | |
| Block 1 | ||||||
| rs1150143 | β = −.08 P = .017 | β= −.135P = .003 | ||||
| rs1150144 | β= −8.6 P = .009 | β = −.135P = .001 | β= −8.1 P = .037 | β= −.168P =.0003 | ||
| rs834830 | β= −.207 P = .0003 | β= −.266 P = 5.7 × 10−5 | ||||
| rs1466408 | ||||||
| rs707132 | ||||||
| Block 2 | ||||||
| rs1150143 | β= −10.4 P = 6.6 × 10−5 | β= −.1 P = .002 | β= −12.4 P = .0001 | β= −.162 P = .0001 | ||
| rs1150144 | β= −12.4P = 4 × 10−5 | β= .060 P = .036 | β= −.134P = .0005 | β= −12.4 P = .0004 | β= −.163 P = .0003 | |
| rs834830 | β= −12.9P = .004 | β= −.137 P = .015 | β= −11.5 P = .02 | β= −.140 P = .013 | ||
| rs1466408 | ||||||
| rs707132 | β= −2.9 P = .05 | |||||
| Block 3 | ||||||
| rs1150143 | β= −6.6 P = .012 | β= .063 P = .008 | β= −.086 P = .009 | β= −9.1 P = .007 | β= .068 P = .025 | β= −.154P =.0002 |
| rs1150144 | β= −11.5P = .0001 | β= .083P = .003 | β= −.147P = .0001 | β= −12.4P = .0004 | β= .075 P = .02 | β= −.184P= 1.5 × 10−5 |
| rs834830 | β= −11.8 P = .008 | β=.08 P = .05 | β= −.219P = 8.2 × 10−5 | β= −14.7P = .003 | β= .087 P = .05 | β= −.278P = 4.4 × 10−6 |
| rs1466408 | ||||||
| rs707132 | β= −4.1 P = .013 | β= −.06P = .005 | β= −5.2 P = .012 | β= −.07 P = .007 | ||
Note: CPT-DS, Continuous Performance Test—Degraded Stimulus version; RT, Reaction Time; S.a, Sensibility a; WAIS, Wechsler Adult Intelligence Scale; FDR, False Discovery Rate. P values before correction for multiple comparisons. In bold, significant associations that were maintained after FDR correction for multiple testing (q values not shown).
Age, sex, years of education, and WAIS-IQ.
Only significant associations shown.
Number of genotype carriers: rs1150143: CC+CG: 173, GG: 15; rs1150144: AA+AG: 177, GG: 11; rs834830: AA+AG: 183, GG: 5; rs1466408: AA+AT: 1, TT: 187; rs707132: AA+AG: 140, GG: 48.
Number of genotype carriers: rs1150143: CC+CG: 125, GG: 9; rs1150144: AA+AG: 126, GG: 8; rs834830: AA+AG: 130, GG: 4; rs1466408: AA+AT: 1, TT: 133; rs707132: AA+AG: 104, GG: 30.
Fig. 1.
Number of hits achieved by schizophrenic patients in the Continuous Performance Test (Degraded stimulus version) blocks, according to their genotype in rs834830. Error bars refer to SD values. Genotypes are grouped assuming a recessive genetic model.
In the control group, there was not any significant association; neither in the group as a whole or in the sex-specific analysis.
Haplotypic Associations.
Haplotype CAATA was chosen as the baseline because it was the most frequent combination in the control (47.5%) and in the schizophrenic group (49.7%) and there was no difference among frequencies in both groups (P value = .63). Frequencies of haplotypes (freq ≥ 5%) are shown in online supplementary table S3. Rare haplotypes group included several combinations: CAGTG, CGATA, CGATG, CGGTG, GAGTG, GGATA and GGATG.
Schizophrenic subjects carrying GGGTG haplotype (20.8%) performed worse than those carrying the reference haplotype. This effect was evidenced in the whole group and in the men group. No associations were found in the women group. In the first block, subjects with this haplotype showed an estimated decrement in sensibility a scores of 0.25 (β = −.25; P = .0001; FDR q value = 0.005). In the men group, the decrement in the estimated average score was of 0.34 (β =−.34; P = 7.8 × 10−6; FDR q value = 0.0005). In the second block, this haplotype was associated with a reduction in the number of hits of 14.3 (β = −14.3; P = .0036; nonsignificant after FDR) and a reduction in the estimated average score of 0.17 in sensibility a (β = −.17; P = .0089; nonsignificant after FDR). The same effect was evidenced in men, with a reduction of 13 hits over the estimated average score (β = −13; P = .019; nonsignificant after FDR) and a reduction in sensibility a scores (β = −.18; P = .016; nonsignificant after FDR). Finally, in the third block, subjects who carried this combination in the whole group got less hits (β = −14.6; P = .002; FDR q value = 0.05), a decrement in the estimated average score of 0.29 in sensibility a (β = −.29; P = 6 × 10−7; FDR q value = 5.8 × 10−5) and an increment in reaction time of 0.11 (β = .11; P = .013; nonsignificant after FDR). In the sex-specific analysis, GGGTG men performed worse than those who carried CAATA combination with a decrement in the estimated average hits of 18.7 (β = −18.7; P = .0004; FDR q value = 0.015), a reduction in sensibility a score of 0.37 (β = −18.7; P = 3.1 × 10−9; FDR q value =5.7 × 10−7) and an increment in the estimated average reaction time of 0.14 (β = .14; P = .009; nonsignificant after FDR).
On the other hand, subjects who carried the haplotype GGAAG evidenced an increment in the estimated average reaction time of 0.2 in the second block (β = .2; P = .032; nonsignificant after FDR). In men, this effect was observed in the first (β = .18; P = .05; nonsignificant after FDR) and in the second block (β = .2; P = .027; nonsignificant after FDR)
No significant associations were found in the control group, neither in the group as a whole or in the sex-specific analysis.
Discussion
The present study tested the relationship between NR4A2 gene and performance on the DS-CPT in both, schizophrenic and control populations. According to our results, significant genotype and haplotype effects were found in the schizophrenic group. Moreover, we sought to clarify if there was a gender-specific effect of the gene.
NR4A2 is associated with synaptic plasticity and is also implicated in the regulation of several dopaminergic genes, acting as a transcription factor. Since DA is an important neurotransmitter for cognition, this gene could be modulating neuropsychological performance. Our genotypic analysis yielded several SNP associations. rs1150143, rs1150144, rs834830, and rs707132 were associated with attentional performance in the schizophrenic group. Subjects who carry recessive homozygote genotypes in these loci show disturbances in DS-CPT task. Marker rs1466408 did not raise any effect. We consider that this lack of findings could be due to the low frequency of the A allele (1/188). Since the 5 polymorphisms analyzed are in the same LD block, we performed a 5-marker haplotype analysis. Haplotype analysis reduces false-positive associations and is more specific than single SNP analysis. Subjects carrying GGGTG combination got poorer results in the attentional task, with P values as low as 6 × 10−7. It is an expected result since the first, second, third, and fifth alleles of the haplotypic block had been related to attentional disturbances in the genotypic analysis.
In spite of the evidenced results, a direct explanation is difficult because none of the detected variants were functional. Probably, the observed effects are due to the existence of LD between our candidate SNPs and the real risk variants. Some of these plausible causal variants are contained in the same LD block: eg, rs13428968 is located in the promoter region, and rs834835 is an intronic enhancer (FASTSNP programme attributes to these variants a risk of 1–3 and 1–2, respectively; [http://fastsnp.ibms.sinica.edu.tw]), while rs12803 is a exonic splicing enchancer (www.genecards.org). On the other hand, it could happen that these variants and their haplotypes were identifying an accumulation of mutations in the exonic sequence of the gene. Xing et al24 described a deficient prefrontal NR4A2 expression in schizophrenia that could be associated with some of the mentioned markers. Moreover, changes in performance could be due to the formation of different splicing variants with different transcriptional activity and therefore dopaminergic phenotype. Multiple isoforms of NR4A2 have been found in human brain.33 To clarify these hypotheses, functional analyses are required both in control and pathological populations.
The second finding emerging in our study is a gender effect for NR4A2 involvement in attentional function in schizophrenic patients. When our patients were divided by gender, the positive association of the gene with the schizophrenic group was only maintained, and was even stronger, in the male subgroup. Recent studies have evidenced that sex may play an important role in the etiology of schizophrenia, with different clinical profiles between genders in age of onset, symptomatology, cognition, neuroanatomy, response to treatment, clinical outcome, and prognosis.34,35 Some authors have suggested an association of these effects with the different levels of sex hormones, such as estrogens and androgens, reached in male and female individuals. Specifically, estrogen modulates the dopaminergic activity in the brain.36 Other sex-specific associations with schizophrenia have been reported for several dopaminergic genes.37,38 Moreover, Rojas et al25 showed that NR4A2 heterozygous (+/−) mice, a candidate animal model for schizophrenia, displayed different patterns of dopaminergic transmission according to the gender of the rodents. Our finding is consistent with this converging evidence.
We would also like to highlight that the division of the task in 3 blocks allows us to analyze the evolution of performance and to focus on changes in sustained vigilance during the CPT task. Interestingly, the evidenced associations emerged and increased over the course of the test (first block < second block < third block). At the beginning of the task, the use of other cognitive components such as selective attention, flexibility, or inhibition could be necessary. These findings indicate that NR4A2 polymorphisms affect specific aspects of attention.
Finally, we would like to point out the absence of effects on the control group. The described results could be suggesting an “inverted U” model.39 In healthy individuals, in which basal DA levels are in a balanced position, DA changes associated with this gene might not be influencing brain functioning stability. However, in schizophrenic patients and probably shaped by the complex synergism of multiple risk gene variants of the dopaminergic pathway, these subtle modifications could drive to suboptimal levels of DA and to decrements in cognitive performance; be it for an excess or for a deficit in dopaminergic signaling. Another possibility for the lack of association in the control group is sample size. Although there is not any significant association, effects of rs1150143, rs1150144, and rs834830 genotypes are in the opposite direction as in the schizophrenic sample (data not shown): recessive homozygotes performed worse than the other genotypic groups. These data might support the mentioned idea of different basal and optimal levels of dopamine in cases and controls.
The main limitation of our study is that sample size was limited and led to a small number of individuals in some of the genotype classification groups. For this reason, these data should be confirmed in further studies involving a larger number of subjects.
Conclusions
NR4A2 is a perfect candidate that is suitable for the most common hypotheses of schizophrenia: the neurodevelopmental theory and the one that leans toward disturbances in dopamine neurotransmission.40,41 Besides, both of these models could be implicated in the neuropsychological deficits in cognitive domains displayed by these patients. According to our results, this gene is influencing sustained attention performance in schizophrenic patients, mainly in the male group. In these subjects, NR4A2 modulates variables of accuracy (hits and sensibility a) and of speed of response (reaction time). On the contrary, this gene does not contribute to impulsivity (false alarms rate).
Funding
This work was supported by the National Institute of Health Carlos III (grant numbers FIS Exp. PI030544, FIS N 060628); the Mutua Madrile ñ a Foundation; and the Committee of Castilla la Mancha (Exp: 0316-02). I.A. has received a research grant from the Foundation for Biomedical Research of the Cl í nico San Carlos Hospital, Madrid (Spain). This research was supported the Fund for Scientific Research Flanders (Belgium; FWO-F), M.A. holds an FWO PhD scholarship.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors would like to thank all patients for their participation in the study and the personnel of the VIB Genetic Service Facility (www.vibgeneticservicefacility.be). We are also grateful to Dr Nuechterlein and Dr Asarnow for their computerized version of the DS-CPT (Degraded Stimulus Continuous Performance Test (DS-CPT) Program for IBM-Compatible Microcomputers. Version 8.12). Finally, we thank Dra. Cristina Fernández, from the Department of Epidemiology of the Clínico San Carlos Hospital (Madrid, Spain) for providing statistical advice. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Hagh-Shenas H, Toobai S, Makaremi A. Selective, sustained, and shift in attention in patients with diagnoses of schizophrenia. Percept Mot Skills. 2002;95:1087–1095. doi: 10.2466/pms.2002.95.3f.1087. [DOI] [PubMed] [Google Scholar]
- 2.Arnsten AF. The emerging neurobiology of attention deficit hyperactivity disorder: the key role of the prefrontal association cortex. J Pediatr. 2009;154:I-S43. doi: 10.1016/j.jpeds.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- 4.Silver H, Feldman P. Evidence for sustained attention and working memory in schizophrenia sharing a common mechanism. J Neuropsychiatry Clin Neurosci. 2005;17:391–398. doi: 10.1176/jnp.17.3.391. [DOI] [PubMed] [Google Scholar]
- 5.Ojeda N, Ortuno F, Arbizu J, et al. Functional neuroanatomy of sustained attention in schizophrenia: contribution of parietal cortices. Hum Brain Mapp. 2002;17:116–130. doi: 10.1002/hbm.10055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cohen RM, Semple WE, Gross M, et al. Dysfunction in a prefrontal substrate of sustained attention in schizophrenia. Life Sci. 1987;40:2031–2039. doi: 10.1016/0024-3205(87)90295-5. [DOI] [PubMed] [Google Scholar]
- 7.Park CH, Kang JS, Shin YH, et al. Acquisition of in vitro and in vivo functionality of Nurr1-induced dopamine neurons. FASEB J. 2006;20:2553–2555. doi: 10.1096/fj.06-6159fje. [DOI] [PubMed] [Google Scholar]
- 8.Perlmann T, Wallen-Mackenzie A. Nurr1, an orphan nuclear receptor with essential functions in developing dopamine cells. Cell Tissue Res. 2004;318:45–52. doi: 10.1007/s00441-004-0974-7. [DOI] [PubMed] [Google Scholar]
- 9.Eells JB, Misler JA, Nikodem VM. Reduced tyrosine hydroxylase and GTP cyclohydrolase mRNA expression, tyrosine hydroxylase activity, and associated neurochemical alterations in Nurr1-null heterozygous mice. Brain Res Bull. 2006;70:186–195. doi: 10.1016/j.brainresbull.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 10.Kim KS, Kim CH, Hwang DY, et al. Orphan nuclear receptor Nurr1 directly transactivates the promoter activity of the tyrosine hydroxylase gene in a cell-specific manner. J Neurochem. 2003;85:622–634. doi: 10.1046/j.1471-4159.2003.01671.x. [DOI] [PubMed] [Google Scholar]
- 11.Smits SM, Ponnio T, Conneely OM, Burbach JP, Smidt MP. Involvement of Nurr1 in specifying the neurotransmitter identity of ventral midbrain dopaminergic neurons. Eur J Neurosci. 2003;18:1731–1738. doi: 10.1046/j.1460-9568.2003.02885.x. [DOI] [PubMed] [Google Scholar]
- 12.Wallen AA, Castro DS, Zetterstrom RH, et al. Orphan nuclear receptor Nurr1 is essential for Ret expression in midbrain dopamine neurons and in the brain stem. Mol Cell Neurosci. 2001;18:649–663. doi: 10.1006/mcne.2001.1057. [DOI] [PubMed] [Google Scholar]
- 13.Martinat C, Bacci JJ, Leete T, et al. Cooperative transcription activation by Nurr1 and Pitx3 induces embryonic stem cell maturation to the midbrain dopamine neuron phenotype. Proc Natl Acad Sci U S A. 2006;103:2874–2879. doi: 10.1073/pnas.0511153103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shintani A, Ono Y, Kaisho Y, Igarashi K. Characterization of the 5'-flanking region of the human brain-derived neurotrophic factor gene. Biochem Biophys Res Commun. 1992;182:325–332. doi: 10.1016/s0006-291x(05)80148-2. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs FM, van der Linden AJ, Wang Y, et al. Identification of Dlk1, Ptpru and Klhl1 as novel Nurr1 target genes in meso-diencephalic dopamine neurons. Development. 2009;136:2363–2373. doi: 10.1242/dev.037556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eells JB, Lipska BK, Yeung SK, Misler JA, Nikodem VM. Nurr1-null heterozygous mice have reduced mesolimbic and mesocortical dopamine levels and increased stress-induced locomotor activity. Behav Brain Res. 2002;136:267–275. doi: 10.1016/s0166-4328(02)00185-7. [DOI] [PubMed] [Google Scholar]
- 17.Rojas P, Joodmardi E, Perlmann T, Ogren SO. Rapid increase of Nurr1 mRNA expression in limbic and cortical brain structures related to coping with depression-like behavior in mice. J Neurosci Res. 2010;88:2284–2293. doi: 10.1002/jnr.22377. [DOI] [PubMed] [Google Scholar]
- 18.Chen YH, Tsai MT, Shaw CK, Chen CH. Mutation analysis of the human NR4A2 gene, an essential gene for midbrain dopaminergic neurogenesis, in schizophrenic patients. Am J Med Genet. 2001;105:753–757. doi: 10.1002/ajmg.10036. [DOI] [PubMed] [Google Scholar]
- 19.Buervenich S, Carmine A, Arvidsson M, et al. NURR1 mutations in cases of schizophrenia and manic-depressive disorder. Am J Med Genet. 2000;96:808–813. doi: 10.1002/1096-8628(20001204)96:6<808::aid-ajmg23>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 20.Ruano D, Macedo A, Dourado A, et al. NR4A2 and schizophrenia: lack of association in a Portuguese/Brazilian study. Am J Med Genet B Neuropsychiatr Genet. 2004;128B:41–45. doi: 10.1002/ajmg.b.30031. [DOI] [PubMed] [Google Scholar]
- 21.Carmine A, Buervenich S, Galter D, et al. NURR1 promoter polymorphisms: Parkinson's disease, schizophrenia, and personality traits. Am J Med Genet B Neuropsychiatr Genet. 2003;120B:51–57. doi: 10.1002/ajmg.b.20033. [DOI] [PubMed] [Google Scholar]
- 22.Lewis CM, Levinson DF, Wise LH, et al. Genome scan meta-analysis of schizophrenia and bipolar disorder, part II: Schizophrenia. Am J Hum Genet. 2003;73:34–48. doi: 10.1086/376549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ng MY, Levinson DF, Faraone SV, et al. Meta-analysis of 32 genome-wide linkage studies of schizophrenia. Mol Psychiatry. 2009;14:774–785. doi: 10.1038/mp.2008.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xing G, Zhang L, Russell S, Post R. Reduction of dopamine-related transcription factors Nurr1 and NGFI-B in the prefrontal cortex in schizophrenia and bipolar disorders. Schizophr Res. 2006;84:36–56. doi: 10.1016/j.schres.2005.11.006. [DOI] [PubMed] [Google Scholar]
- 25.Rojas P, Joodmardi E, Hong Y, Perlmann T, Ogren SO. Adult mice with reduced Nurr1 expression: an animal model for schizophrenia. Mol Psychiatry. 2007;12:756–766. doi: 10.1038/sj.mp.4001993. [DOI] [PubMed] [Google Scholar]
- 26.Ibi D, Takuma K, Koike H, et al. Social isolation rearing-induced impairment of the hippocampal neurogenesis is associated with deficits in spatial memory and emotion-related behaviors in juvenile mice. J Neurochem. 2008;105:921–932. doi: 10.1111/j.1471-4159.2007.05207.x. [DOI] [PubMed] [Google Scholar]
- 27.Colon-Cesario WI, Martinez-Montemayor MM, Morales S, et al. Knockdown of Nurr1 in the rat hippocampus: implications to spatial discrimination learning and memory. Learn Mem. 2006;13:734–744. doi: 10.1101/lm.407706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gabriel SB, Schaffner SF, Nguyen H, et al. The structure of haplotype blocks in the human genome. Science. 2002;296:2225–2229. doi: 10.1126/science.1069424. [DOI] [PubMed] [Google Scholar]
- 29.Mackey K, Williams P, Seim S, Chomczynski P. The use of DNAzol for the rapid isolation of genomic DNA from whole blood. Biomed Prod. 1996;(suppl):13–15. [Google Scholar]
- 30.Nuechterlein KH, Parasuraman R, Jiang Q. Visual sustained attention: image degradation produces rapid sensitivity decrement over time. Science. 1983;220:327–329. doi: 10.1126/science.6836276. [DOI] [PubMed] [Google Scholar]
- 31.Hsieh PC, Chu CL, Yang YK, et al. Norms of performance of sustained attention among a community sample: Continuous Performance Test study. Psychiatry Clin Neurosci. 2005;59:170–176. doi: 10.1111/j.1440-1819.2005.01353.x. [DOI] [PubMed] [Google Scholar]
- 32.Sinnwell JP, Schaid DJ. haplo.stats: Statistical methods for haplotypes when linkage phase is ambiguous. R package version 1.2.2. 2005 http://mayoresearch.mayo.edu/mayo/research/schaid_lab/upload/manualHaploStats.pdf. Accessed November 22, 2011. Ref Type: Computer Program [Google Scholar]
- 33.Michelhaugh SK, Vaitkevicius H, Wang J, et al. Dopamine neurons express multiple isoforms of the nuclear receptor nurr1 with diminished transcriptional activity. J Neurochem. 2005;95:1342–1350. doi: 10.1111/j.1471-4159.2005.03458.x. [DOI] [PubMed] [Google Scholar]
- 34.Aleman A, Kahn RS, Selten JP. Sex differences in the risk of schizophrenia: evidence from meta-analysis. Arch Gen Psychiatry. 2003;60:565–571. doi: 10.1001/archpsyc.60.6.565. [DOI] [PubMed] [Google Scholar]
- 35.Goldstein JM, Seidman LJ, O'Brien LM, et al. Impact of normal sexual dimorphisms on sex differences in structural brain abnormalities in schizophrenia assessed by magnetic resonance imaging. Arch Gen Psychiatry. 2002;59:154–164. doi: 10.1001/archpsyc.59.2.154. [DOI] [PubMed] [Google Scholar]
- 36.Cyr M, Calon F, Morissette M, Di PT. Estrogenic modulation of brain activity: implications for schizophrenia and Parkinson's disease. J Psychiatry Neurosci. 2002;27:12–27. [PMC free article] [PubMed] [Google Scholar]
- 37.Hoenicka J, Garrido E, Ponce G, et al. Sexually dimorphic interaction between the DRD1 and COMT genes in schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2010;153B:948–954. doi: 10.1002/ajmg.b.31065. [DOI] [PubMed] [Google Scholar]
- 38.Rybakowski JK, Borkowska A, Czerski PM, Kapelski P, Dmitrzak-Weglarz M, Hauser J. An association study of dopamine receptors polymorphisms and the Wisconsin Card Sorting Test in schizophrenia. J Neural Transm. 2005;112:1575–1582. doi: 10.1007/s00702-005-0292-6. [DOI] [PubMed] [Google Scholar]
- 39.Tunbridge EM, Harrison PJ, Weinberger DR. Catechol-o-methyltransferase, cognition, and psychosis: Val158Met and beyond. Biol Psychiatry. 2006;60:141–151. doi: 10.1016/j.biopsych.2005.10.024. [DOI] [PubMed] [Google Scholar]
- 40.Fatemi SH, Folsom TD. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr Bull. 2009;35:528–548. doi: 10.1093/schbul/sbn187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Howes OD, Kapur S. The dopamine hypothesis of schizophrenia: version III–the final common pathway. Schizophr Bull. 2009;35:549–562. doi: 10.1093/schbul/sbp006. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.