Abstract
Background
: Cognitive deficits form core features in schizophrenia. Several studies have shown improvements in prefrontal cognitive function by α 2 -agonists in schizophrenia. In the present study, it was investigated whether clonidine (an α 2 -adrenoceptor agonist) could normalize sensorimotor gating deficits in schizophrenia. Methods : In a double blind, placebo controlled, randomized, yet balanced, cross-over experiment, 20 male schizophrenia patients on stable medication were assessed in an auditory prepulse inhibition (PPI), sensitization, and habituation of the startle reflex paradigm on 5 occasions: once after oral administration of placebo and after a single dose of 25, 50, 75, and 150 µg of clonidine. Their results were compared with 20 age- and gender-matched healthy volunteers, who received no treatment. Results : In the placebo treatment, patients showed deficient PPI and sensitization, yet normal habituation compared with the controls. Except the highest dose, all dosages of clonidine significantly increased percentage PPI in the patients compared with placebo, to such levels that it no longer differed significantly from the healthy controls. However, none of the dosages increased sensitization or influenced habituation. Conclusions : This is the first study to show that even a single low dose of clonidine added to the medical treatment of patients with schizophrenia who are clinically stable on their antipsychotic medication not only significantly ameliorates their PPI deficits, but also normalizes them. The results have a potentially high clinical relevance for the medical treatment of schizophrenia.
Key words: chronic schizophrenia/PPI/habituation/sensitization/clonidine
Introduction
A large number of reports in literature provide evidence for disrupted central noradrenergic activity in schizophrenia. 1 – 3 Noradrenaline is one of the key neurotransmitters involved in cognition, 4 especially in those cognitive functions that involves the prefrontal cortex (PFC). 5 , 6 Unsurprisingly therefore, there is abundant evidence pointing toward a noradrenergic involvement in a large number of cognitive deficits that are usually found in patients with schizophrenia. 3 , 7 , 8 In the early years of research on noradrenergic involvement in schizophrenia, researchers have especially been focussing on the effects of clonidine, a specific α2-agonist, either administered in monotherapy or in combination with antipsychotics. These studies indicated that clonidine not only improved cognition, but also positive and even negative symptomatology. 9 – 11 For unknown reasons, these studies were not followed up, and from a more general perspective, investigators appeared to have lost interest in the noradrenergic system until recently. It is important to realize that the traditional concept that α2-receptors are only presynaptically located, and thus lower noradrenergic tone, is outdated. The current concept is that α2-receptors are also postsynaptically located, eg, in the PFC. 12 , 13 In other words, it is plausible that the above-described positive effects of clonidine in schizophrenic patients were not only simply due to a reduction of noradrenergic activity by stimulating presynaptic α2-receptors in the locus coeruleus (LC) but also might have been due to stimulating postsynaptic α2-receptors in the PFC.
In addition to disruptions in central noradrenergic activity in schizophrenia, patients often show reduced filtering of sensory information, which is widely believed to be reflected in a reduced prepulse inhibition (PPI) of the startle reflex. 14 Defects or flaws in these early sensory filter mechanisms have been proposed to lead to cognitive fragmentation and ultimately even to hallucinations and delusions. 15 Deficits in PPI may therefore underlie some of the cognitive deficits that are found in schizophrenia, although the mechanism behind this is currently still unclear. PPI deficits usually do not correlate directly with cognitive deficits in schizophrenia patients, 16 yet do, for instance, correlate with perceptional and reasoning disturbances. 15
Surprisingly, few studies report on the effects of noradrenergic activity on human PPI. In fact, to our knowledge, there are only 4 such studies. Quednow et al. 17 reported decreased PPI in patients with major depression following 14 days treatment with reboxetine. However, Phillips et al. 18 found no effects of a single dose of reboxetine in healthy volunteers. In contrast, in 2 separate studies from our laboratory, we found reduced sensorimotor gating following administration of imipramine (a combined noradrenergic [SNRI] and serotonergic [SSRI] reuptake inhibitor) and desipramine (specific SNRI) compared with healthy volunteers. 19 , 20 Also recent animal studies point toward an involvement of the noradrenergic system in PPI, especially in the direction of α1 and α2 receptor activity. 21 , 22 Taking all this together suggests a potential direct causal relation between aberrant noradrenergic activity and disrupted PPI, phenomena both commonly found in patients with schizophrenia.
The startle eliciting stimulus from the PPI paradigm can also be used to quantify an individual’s habituation and sensitization abilities, therefore, these processes were assessed simultaneously with PPI. Reports on startle habituation and sensitization in schizophrenia are inconsistent. Some studies have shown deficits, 23 while other studies have not. 24 Clonidine appears to have no influence on PPI in healthy controls—most likely because they already have optimal levels of PPI—but it does seem to reduce human startle responsivity. 25 It may therefore be expected that clonidine will influence habituation and sensitization.
In this study, the effects of clonidine were investigated on sensorimotor gating of patients with schizophrenia on stable medication. A single dose of clonidine was added to the patient’s current medication in 4 different dosages (25, 50, 75, and 150 µg), after which they were tested in a PPI, habituation, and sensitization of the startle reflex paradigm. Their results were compared with those from healthy age and gender-matched healthy volunteers, who were tested in the same paradigm but underwent no treatment at all. Based on the above, we expected clonidine to ameliorate PPI deficits in our patients with schizophrenia.
Methods
Subjects
The study was approved by the Ethics Committee of the Capital Region (H-KF-2006-6813), Copenhagen, with regards to the ethical principles for medical research involving human subjects as stated in the declaration of Helsinki (amendment of Washington, 2002). Written and oral information was given, after which written informed consent was obtained from all subjects. Chronic, yet clinically stable, medicated male patients with schizophrenia between 25 and 50 years of age were recruited from psychiatric hospitals and related outpatient clinics in the Copenhagen municipality and county. All included patients fulfilled the DSM-IV criteria for schizophrenia, ascertained by use of the Schedule for Clinical Assessment in Neuropsychiatry (SCAN), version 2.1. 26 The patients were matched to healthy controls based on age and gender. The healthy controls were recruited from the community and had no history of psychiatric illness (ascertained with the SCAN interview). Both patients and healthy controls passed a physical examination before inclusion to ascertain that they were all physically healthy. Exclusion criteria were coercive measures, a history of mental retardation, organic brain damage, or organic psychosis. Substance dependence (as defined by DSM-IV criteria) was an exclusion criterion. Healthy controls with a history of mental illness in first-degree relatives were also not included in the study. To screen for hearing deficits, subjects were tested at 500, 1000, and 6000 Hz (40 dB). Due to the naturalistic design, concomitant treatment with benzodiazepines and antidepressants was allowed. In total, 20 patients (mean age 37.5 years, SD 6.4) and 20 matched healthy control subjects (mean age 36.5 years, SD 6.5) completed the project. Two patients were treated with typical antipsychotics, 15 with atypical, and 3 with a combination of typical and atypical medication. Besides antipsychotics, the medical treatment of 5 patients included benzodiazepines and also 5 patients were simultaneously treated with antidepressants (only treatment with SSRIs with very low noradrenergic affinity [Ki < 1000 nM] were allowed) during their participation in the project [the medication of 3 patients included both antidepressants and benzodiazepines]. Importantly, none of the patients changed any of their medication during their participation in the project. Fourteen of the patients were smokers, whereas 4 of the controls were smokers. Three patients dropped out before the project was completed: 1 patient did not participate in the 25 µg clonidine session, 1 not in the 150 µg session, and 1 patient only participated in the 25 µg session. There were no nonresponders, ie, all subjects had mean startle amplitudes to pulse-alone trials larger than 20 µV in the placebo session. Table 1 shows the characteristics of all subjects.
Table 1.
Demographics and Clinical Characteristics
| Patients | ||||||
| Controls | Placebo | 25 µg Clonidine | 50 µg Clonidine | 75 µg Clonidine | 150 µg Clonidine | |
| Mean age (SEM) | 36.5 y (1.5) | 37.5 y (1.4) | ||||
| Average PANSS scores (SEM) | ||||||
| Positive | 13.0 (1.2) | 14.0 (1.6) | 12.7 (1.1) | 13.8 (1.6) | 12.5 (0.9) | |
| Negative | 14.3 (1.2) | 15.6 (1.0) | 15.4 (1.4) | 15.4 (1.2) | 14.3 (1.1) | |
| General | 25.4 (1.6) | 27.1 (1.9) | 25.1 (1.7) | 25.7 (1.6) | 24.4 (1.2) | |
| Total | 52.6 (3.4) | 56.1 (3.4) | 53.1 (3.4) | 54.8 (3.9) | 50.8 (2.4) |
Note: Characteristics of patients and matched healthy controls, showing no significant differences in age between patients and controls, nor significant differences in PANSS scores in the patients over the 5 different test sessions.
Experimental Design
In a double-blind, placebo-controlled, pseudorandomized [balanced] cross-over experiment all patients were tested in the Copenhagen Psychophysiological Testbattery [CPTB] on 5 occasions, separated by a minimum of 1 week: once after oral administration of placebo [little green soybeans, very similar in size and weight to the clonidine tablets] and once after a single dose of 25, 50, 75, and 150 µg of clonidine [Catapresan]. To avoid order effects as much as possible, we used a pseudorandomized design: randomly chosen, an equal number of patients per session started with each dose [ie, 4 patients started with placebo and 4 with 25, 50, 75, and 150 µg of clonidine] while the order of the subsequent doses was randomized. Both placebo and clonidine tablets were administered 4h before the psychophysiological assessments, in an opaque white capsule. On all 5 test days, before administration of the capsule, the patients’ symptomatology was rated with the Positive and Negative Syndrome Scale (PANSS). 27 It should be realized that the data of the controls were only used to assess whether patients showed significant deficits in our dependent variables (PPI, habituation, and sensitization) in spite of these patients being stable on their medication, and to evaluate the potential level of change in these variables as a result of clonidine administration. Therefore, the controls were assessed only once in the CPTB (including its PPI paradigm) without being administered to any compounds. Besides the currently investigated PPI paradigm, the CPTB includes P50 suppression, selective attention, and mismatch negativity (MMN) paradigms. To keep the study focused, only the results on PPI, habituation, and sensitization will be reported in the present article, the results on the other paradigms will be published elsewhere. At inclusion, neither the patients nor the healthy controls had ever participated in psychophysiological research before. To avoid acute and/or withdrawal effects of nicotine, 14 smoking was not allowed from 1h prior to testing. Similarly, all subjects were requested not to drink any caffeine-containing beverages on a test day, until all tests were completed. Because it is known that sedatives influence startle magnitude, although without affecting PPI, 14 patients were requested not to take any benzodiazepines from the evening before a test day (from 2300) until the completion of the tests. Furthermore, a urine sample of the subjects was screened on drug abuse (cannabis, cocaine, amphetamines).
Assessment of Habituation and PPI
The method has been described before. 23 In short, subjects were seated in a comfortable armchair in a room with a sound level below 40 dB and situated adjacent to the control room. They were instructed to sit still, to keep their eyes fixed on a spot on the wall directly in front of them, and were asked to stay awake. The assessment of PPI and habituation started with 5min of acclimation to the background noise (70 dBa white noise) after which 3 experimental blocks of stimuli were superimposed on the background noise. Blocks 1 and 3 were used to assess habituation of the acoustic startle reflex. The 2 blocks were identical and consisted of 8 pulse-alone trials (white noise with an intensity of 115 dBa, and a duration of 20ms, instant rise and fall) with randomized intertrial intervals between 10 and 20 s. Block 2 consisted of 50 trials presented in a pseudorandomized order, to assess PPI. Because it is known that prepulse intensity and interstimulus intervals can affect the levels of PPI,14 our paradigm contains 2 levels of each, ie, prepulse intensities of 6 and 15 dB (white noise, 20ms in duration) above background and stimulus onset asynchronies (SOA) of 60 and 120ms. The intertrial intervals were randomized between 10 and 20 s. Randomized across the session, 10 pulse alone and 10 of each prepulse-pulse combination (60ms/76 dBa, 60ms/85 dBa, 120ms/76 dBa, and 120ms/85 dBa) were presented. All auditory stimuli were presented by a computer running Presentation (Neurobehavioral Systems, Inc., Albany, NY) software (soundcard: Creative soundblaster 5.1, 2008 Creative Technology Ltd, Singapore, Singapore) and were presented binaurally through stereo insert earphones (Eartone ABR, 1996-2008 Interacoustics A/S, Assens, Denmark, C and H Distributors Inc, Milwaukee, WI). The soft- and hardware settings were calibrated by means of an artificial ear (Brüel and Kjær, type 2133, Odin Metrology Inc., Thousand Oaks, CA). PPI assessment took approximately 25min. Following offline filtering of the data between 25 and 250 Hz, startle amplitude was scored as the highest absolute amplitude in the time interval 20–100ms after the startle eliciting pulse, while PPI was expressed as [(1 − (PP/PA)) × 100%]; where PP: average startle amplitude to prepulse–pulse trials and PA: average startle amplitude to pulse-alone trials. Sensitization was defined as the percent increment in startle amplitude from the first to the third pulse-alone trial of block 1. To avoid influence of sensitization, habituation was defined as a subjects’ difference in response amplitude to pulse-alone stimuli of trials 5–8 of block 1 compared to trials 5–8 of block 3.
Signal Recording
The eye-blink component of the acoustic startle response was measured by recording electromyographic (EMG) activity from the right m. orbicularis oculi. Two electrodes were placed under the right eye for startle response measurement. The first of these was aligned with the pupil, the other positioned just laterally. The EMG recordings were assessed with BioSemi hardware (Biosemi, Amsterdam, Netherlands). Sampling started immediately before an experimental block started and lasted to the end of it. Auditory stimuli were presented binaurally through insert headphones. All signals were digitized online by computer at a rate of 4096 Hz, and a low-pass setting of 1/5 of the AD rate.
Statistical Analysis
All statistical analyses were performed with SPSS (version 11.0). All data were normally distributed (Kolmogorov-Smirnov test) and were therefore analyzed parametrically.
Group effects on PPI in the placebo session were analyzed by analysis of variance (ANOVA) with between factor “group” (schizophrenia patients or healthy controls) and within factor “prepulse intensity” (85 or 76 dBa) and “SOA” (60 or 120ms). Effect of clonidine on the raw startle amplitude of the 5 different trial types was analyzed with ANOVA with factors “dose” (placebo, 25, 50, 75, and 150 µg) and “trialtype” (pulse alone, 85/120, 85/60, 76/120, 76/60). Similar to PPI, also sensitization and habituation were analyzed with repeated measures ANOVA.
Psychopathology (positive, negative, general, and total PANSS score) was analyzed with ANOVA, to evaluate the clinical stability of the patients over the period that they participated in this project. The relationship between PPI, habituation, and symptomatology were investigated by means of Pearson’s correlation tests.
Age, use of benzodiazepines and antidepressants, and smoking were used as covariates in the analyses. However, because none of them reached statistical significance, they were removed from the analyses.
Results
Psychopathology
No significant differences were found in the patients PANSS scores in the 5 different sessions (assessed before administration of clonidine): PANSS positive: [F(4,12) 5 0.987, P 5 .45], PANSS negative: [F(4,12) 5 0.38, P 5 .82], PANSS general: [F(4,12) 5 0.33, P 5 .85], and PANSS total: [F(4,12) 5 0.81, P 5 .54]. The results indicate that the patients’ psychopathology was stable over time.
PPI
The only group effect that was found in the ANOVA on PPI in the placebo session was a highly significant interaction between group and intensity [F(1,37) 5 7.67, P 5 .009], indicating that the patients showed significantly less PPI than controls with the 85 dBa prepulses only, regardless of ISI. Patients and controls showed no differences on raw amplitude level in the placebo session, indicating that the differences in PPI were based on the patients’ response to pulse-alone trials being somewhat lower than controls, and their response to prepulse-pulse trials being somewhat higher than controls, but none of these differences reached statistical significance (the average amplitude of patients to the pulse-alone trials was 96 µV, where that of the controls was 116 µV; the average patients’ amplitude to the 85 dBa, 120ms ISI prepulse-pulse trials was 45 µV and to 85 dBa, 60ms ISI trials this was 49 µV, while that of the controls was 29 and 36 µV, respectively).
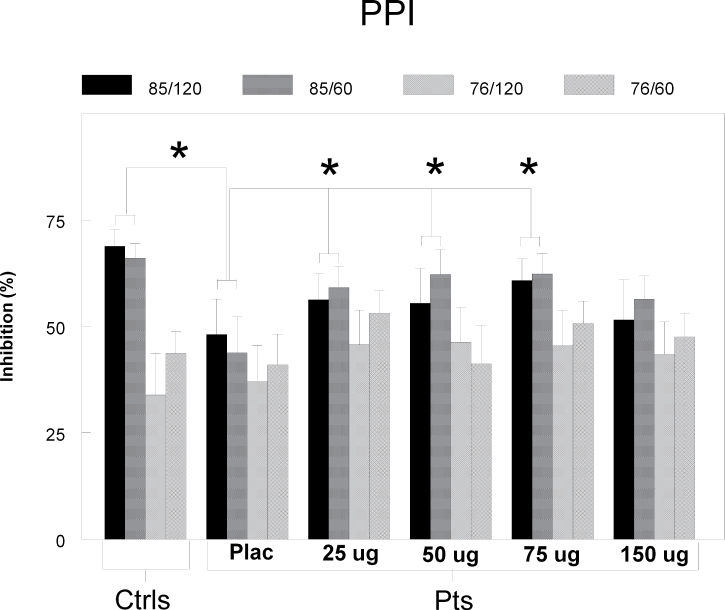
To test whether clonidine was able to increase the deficient levels of PPI of the patients in these trial types, we used an ANOVA with factor “dose” and “SOA,” which revealed a significant effect of dose [F(4,13) = 3.98, P = .025]. Further testing of this treatment effect revealed that all dosages of clonidine significantly increased the patients’ PPI in these 85 dBa trial types, except the highest dose: 25 µg: [F(1,17) = 9.70, P = .006], 50 µg: [F(1,18) = 4.41, P = .05], 75 µg: [F(1,18) = 9.57, P = .006], and 150 µg: [F(1,17) = 1.58, P = .23]. Comparison of these increased levels of PPI with those of the healthy volunteers showed no significant differences anymore (see figure 1). In spite of this increase in PPI, clonidine did not significantly influence the raw startle amplitudes of the trials [F(1,16) = 1.37, P = .59] (see online supplementary material for a color version of this figure).
Fig. 1.
PPI. Percentage PPI (±SEM) for all 4 different prepulse-pulse trials in patients (pts) and matched controls (ctrls) specified for all treatments. The patients showed significantly lower percentage PPI compared with the controls in the 85 dB/60ms and 85 dB/120ms trials in the placebo treatment. The 3 lower dosages of clonidine increased percentage PPI of these trial types significantly in the patients compared with placebo, to a level that was not significantly different from the controls. *P < .05.
Habituation and Sensitization
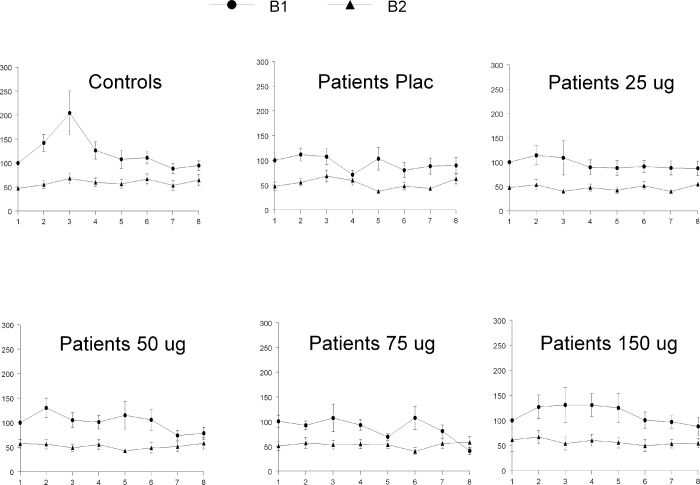
The ANOVA on sensitization in the placebo session revealed a significant main effect of sensitization [F(1,37) = 5.1, P = .03] and a trend level sensitization 3 group interaction effect [F(1,37) = 3.84, P = 0.058]. Further analyses indicated significant sensitization in controls t(19) = 2.28, P = .034) but not in patients t(18) = 0.46, P = .65). Clonidine treatment did not affect the patients’ sensitization [F(4,13) = 0.78, P = .56] (see figure 2).
Fig. 2.
Sensitization. Mean startle amplitudes in relation to the first trial for both patients and controls (percentage scores, 6SEM), specified for the 8 trials of block 1 (B1) and the 8 trials of block 3 (B3). Controls showed a highly significant increment from trial 1 to trial 3 of block 1 (5sensitization), where patients did not. Clonidine did not affect sensitization.
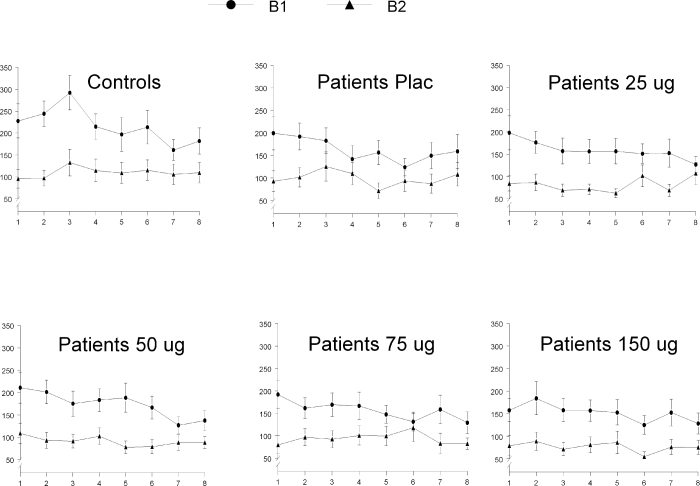
Both patients and controls showed highly significant habituation [F(1,37) = 30.83, P < .001] but did not significantly differ in their levels [F(1,37) = 0.02, P = .89]. Treatment with clonidine had no effect on the habituation level of the patients [F(4,13) = 0.13, P = .97] (see figure 3)
Fig. 3.
Habituation. Mean startle amplitudes for both patients and controls (in µV, 6SEM), specified for the 8 trials of block 1 (B1) and the 8 trials of block 3 (B3). Both patients and controls showed a highly significant decrement in startle amplitude of the last 4 trials of block 1 compared with the last 4 trials of block 3 (5habituation), without showing a significant group difference. Clonidine did not affect habituation.
Discussion
To our knowledge, this is the first study reporting the effects of clonidine on deficient sensorimotor gating in patients with schizophrenia. The main results indicate that, in agreement with our hypothesis, even low doses of clonidine added to the patients’ medical treatment are capable of significantly increasing their levels of PPI. In fact, their PPI increased to such levels that it was not significantly different from the controls anymore. However, although the highest dose of clonidine (150 µg) increased the patients’ level of PPI, it did not reach statistical significance.
The LC is responsible for approximately 90% of the noradrenergic innervation of the forebrain and 70% of the total amount of noradrenaline in the brain. 28 More specifically, the LC innervates, among others, several forebrain regions, such as the PFC, hippocampus, and amygdala, all areas known to be involved in sensorimotor gating, as indicated in preclinical studies. 29 From our current data, it is impossible to point toward a specific area in the brain where noradrenaline exerts its activity on sensorimotor gating. However, the PFC is a likely candidate. In a series of experiments, Kumari et al. 30 – 32 have investigated the neural correlates of PPI in both healthy controls as well as chronic and medicated patients with schizophrenia. Although the findings appeared not very consistent over the studies, involvement of the PFC in modulation of PPI was suggested in all. Very recent evidence from our own research group on antipsychotic naïve, first-episode patients with schizophrenia also pointed toward involvement of the PFC in PPI. 33
The finding that 3 different dosages of clonidine increased PPI in our schizophrenia patients, without significantly affecting their raw startle amplitude, is in agreement with our hypothesis that the deficient PPI in schizophrenia patients may, at least in part, be caused by aberrant noradrenergic activity. Our findings are supported by recent preclinical research, indicating that not only α2- but also α1-noradrenergic receptors are involved in PPI. Swerdlow et al. 34 found that cirazoline (an α1-adrenocepter agonist) disrupted PPI in male rats, which could be prevented by pretreatment with (systemically injected) clonidine. Similarly, in another recent preclinical study, stimulation of receptors in the LC was found to disrupt PPI in rats. This disruption could be prevented by pretreatment with either clonidine (infused into peri-LC) or prasozin (α1-adrenocepter antagonist, systemically injected) and also with quetiapine or olanzapine (both systemically injected). 21 In theory, treatment with clonidine would have reduced noradrenergic activity in both experiments by stimulating presynaptic α2-receptors in the LC, thereby effectively reducing the impact of cirazoline in the first study or reducing the activity of the LC in the second study. It cannot be excluded, however, that clonidine may have prevented disruption of PPI in the study of Swerdlow et al. 34 by direct stimulation of α2-receptors in the PFC. In this study, our patients were on stable medication before administration of clonidine. In spite of that, they still showed deficient levels of PPI in the placebo session compared with the healthy controls. Because a large number of the currently most prescribed antipsychotics—especially the atypical ones, which were also used by many of our patients—reduce noradrenergic activity, it means that reducing noradrenergic activity alone is not sufficient to reach normal levels of PPI. Clonidine’s ability to increase levels of PPI might therefore indicate that it acted by stimulation of α2noradrenergic activity, instead of by decreasing the already through antipsychotic treatment reduced noradrenergic activity even further. Taking all this together, it is indeed conceivable that both increased α1-noradrenergic activity, as well as decreased α2-noradrenergic activity reduces normal levels of PPI. This is in agreement with a preclinical study in which increased startle responses and more pronounced disruption of PPI were noted in D-amphetamine-treated α2A-receptor knock-out mice. 35
As mentioned above, several studies point toward an involvement of the PFC in PPI. 30–33 Therefore, and also because the PFC has both α1- and α2-noradrenergic receptors, it is a likely location for noradrenergic modulation of PPI. Indeed, infusion of a noradrenergic agonist into (among others) the medial PFC was recently found to disrupt PPI in rats. 22 Also interesting in this respect is the model described by Arnsten et al., 13 suggesting that stimulation of α1-receptors impair normal PFC functions, while stimulation of α2-receptors strengthen its function. Furthermore, they reason that α1-receptors are less sensitive to noradrenaline than α2-receptors, meaning that a modest increase in noradrenergic activity in the PFC would be beneficial for its functions, while a high increase would be disruptive. However, the fact that many other studies suggest involvement of dopaminergic activity in the PFC in PPI 19 , 36 , 37 as well, makes it more proper to assume that a well-functioning PFC in general is a prerequisite for normal levels of PPI. Nevertheless, obviously more research is necessary to reach firmer conclusions.
From a clinical perspective, deficits in filtering of sensory information are thought to lead to cognitive fragmentation and ultimately even to hallucinations and delusions. 14 , 15 Deficient PPI correlates for instance not only highly with measures of perceptual and reasoning disturbances, 15 but also with aspects of hallucinations. 38 Normalizing PPI in schizophrenia patients may therefore set the stage for improving their cognition and symptomatology, for which there is already some evidence found in earlier literature on the effects of α2 agonists in schizophrenia. 9 , 39 , 40 However, more research is necessary to evaluate the significance of our current findings.
There are strengths, but also limitations to this study. An obvious strength is that the essential part of this study had a within-subject design, where patients randomly received not only one but 4 different dosages of clonidine, and showing that 3 of these dosages not only significantly increased but also largely normalized the patients’ levels of PPI. A limitation of this study is that only the effects of single dosages of clonidine on PPI were studied. Therefore, investigation of long-term treatment effects of clonidine on PPI and cognition in schizophrenia is warranted. Another limitation is the fact that we tested our healthy subjects only once, whereas the patients were tested 5 times. However, from literature, we know that PPI and habituation have a high test-retest reliability not only in healthy volunteers, 41 but also in schizophrenia patients on stable medication. 42 This, in combination with the fact that the order of the 5 test sessions of the patients was balanced yet randomized, makes it highly unlikely that the multiple test exposures instead of the effects of clonidine, accounted for our current results.
Summarized, this study is the first to show that deficient sensorimotor gating in patients with schizophrenia on stable medication can be effectively normalized by adding low dosages of the α2-receptor agonist clonidine to their current treatment. Future research should focus on long-term treatment effects of clonidine on basic information processing deficits, cognition, and symptomatology in schizophrenia.
Supplementary Material
Funding
Danish Council for Independent Research (Medical Sciences, 271-06-0308); Lundbeck foundation (R25-A2701).
Supplementary material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Acknowledgments
This study was sponsored by The Danish Council for Independent Research (Medical Sciences, grant number: 271-06-0308) and the Lundbeck foundation (grant number: R25-A2701). The authors declare no conflict of interest.
References
- 1. Powchik P, Davidson M, Haroutunian V,, et al. Postmortem studies in schizophrenia Schizophr Bull 1998. ; 24 : 325–341 [DOI] [PubMed] [Google Scholar]
- 2. Farley IJ, Price KS, McCullough E, et al. Norepinephrine in chronic paranoid schizophrenia: above-normal levels in limbic forebrain Science 1978. ; 200 : 456–458 [DOI] [PubMed] [Google Scholar]
- 3. Yamamoto K, Hornykiewicz O. Proposal for a noradrenaline hypothesis of schizophrenia Prog Neuropsychopharmacol Biol Psychiatry 2004. ; 28 : 913–922 [DOI] [PubMed] [Google Scholar]
- 4. Clark CR, Geffen GM, Geffen LB. Catecholamines and attention: II. Pharmacological studies in normal humans Neurosci Biobehav Rev 1987. ; 11 : 353–364 [DOI] [PubMed] [Google Scholar]
- 5. Ramos BP, Arnsten AF. Adrenergic pharmacology and cognition: focus on the prefrontal cortex Pharmacol Ther 2007. ; 113 : 523–536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arnsten AF, Li BM. Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions Biol Psychiatry 2005. ; 57 : 1377–1384 [DOI] [PubMed] [Google Scholar]
- 7. Litman RE, Pickar D. Noradrenergic systems: a target for augmenting pharmacotherapy . In: Breier A , ed. The New Pharmacotherapy of Schizophrenia (Clinical Practice Series) Washington: American Psychiatric Press; ; 1996. : 133–152 [Google Scholar]
- 8. Friedman JI, Adler DN, Davis KL. The role of norepinephrine in the pathophysiology of cognitive disorders: potential applications to the treatment of cognitive dysfunction in schizophrenia and Alzheimer's disease Biol Psychiatry 1999. ; 46 : 1243–1252 [DOI] [PubMed] [Google Scholar]
- 9. Maas JW, Miller AL, Tekell JL,, et al. Clonidine plus haloperidol in the treatment of schizophrenia/psychosis J Clin Psychopharmacol 1995. ; 15 : 361–364 [DOI] [PubMed] [Google Scholar]
- 10. Fields RB, Van Kammen DP, Peters JL,, et al. Clonidine improves memory function in schizophrenia independently from change in psychosis. Preliminary findings Schizophr Res 1988. ; 1 : 417–423 [DOI] [PubMed] [Google Scholar]
- 11. Freedman R, Kirch D, Bell J,, et al. Clonidine treatment of schizophrenia. Double-blind comparison to placebo and neuroleptic drugs Acta Psychiatr Scand 1982. ; 65 : 35–45 [DOI] [PubMed] [Google Scholar]
- 12. Arnsten AF, Steere JC, Hunt RD. The contribution of alpha 2-noradrenergic mechanisms of prefrontal cortical cognitive function. Potential significance for attention-deficit hyperactivity disorder Arch Gen Psychiatry 1996; 53: 448–455 [DOI] [PubMed] [Google Scholar]
- 13. Arnsten AFT. Norepinephrine and cognitive disorders . In: Ordway GA, Schwartz MA, Frazer A , eds. Brain Norepinephrine, Neurobiology and Therapeutics Cambridge University Press; ; 2007. : 408–435 [Google Scholar]
- 14. Braff DL, Geyer MA, Swerdlow NR. Human studies of prepulse inhibition of startle: normal subjects, patient groups, and pharmacological studies Psychopharmacology (Berl) 2001. ; 156 : 234–258 [DOI] [PubMed] [Google Scholar]
- 15. Perry W, Geyer MA, Braff DL. Sensorimotor gating and thought disturbance measured in close temporal proximity in schizophrenic patients Arch Gen Psychiatry 1999. ; 56 : 277–281 [DOI] [PubMed] [Google Scholar]
- 16. Swerdlow NR, Light GA, Cadenhead KS, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: relationship to medications, symptoms, neurocognition, and level of function Arch Gen Psychiatry 2006. ; 63 : 1325–1335 [DOI] [PubMed] [Google Scholar]
- 17. Quednow BB, Kuhn KU, Stelzenmueller R, Hoenig K, Maier W, Wagner M. Effects of serotonergic and noradrenergic antidepressants on auditory startle response in patients with major depression Psychopharmacology (Berl) 2004. ; 175 : 399–406 [DOI] [PubMed] [Google Scholar]
- 18. Phillips MA, Langley RW, Bradshaw CM, Szabadi E. The effects of some antidepressant drugs on prepulse inhibition of the acoustic startle (eyeblink) response and the N1/P2 auditory evoked response in man J Psychopharmacol 2000. ; 14 : 40–45 [DOI] [PubMed] [Google Scholar]
- 19. Oranje B, Verbaten MN, Kemner C, Kahn RS. Modulating sensorimotor gating in healthy volunteers: the effects of desipramine and haloperidol Psychiatry Res 2004. ; 127 : 195–205 [DOI] [PubMed] [Google Scholar]
- 20. Hammer TB, Oranje B, Glenthoj BY. The effects of imipramine on P50 suppression, prepulse inhibition and habituation of the startle response in humans Int J Neuropsychopharmacol 2007. ; 10 : 787–795 [DOI] [PubMed] [Google Scholar]
- 21. Alsene KM, Bakshi VP. Pharmacological stimulation of locus coeruleus reveals a new antipsychotic-responsive pathway for deficient sensorimotor gating Neuropsychopharmacology 2011. ; 36 : 1656–1667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Alsene KM, Rajbhandari AK, Ramaker MJ, Bakshi VP. Discrete forebrain neuronal networks supporting noradrener gic regulation of sensorimotor gating Neuropsychopharmacology 2011. ; 36 : 1003–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Aggernaes B, Glenthoj BY, Ebdrup BH, Rasmussen H, Lublin H, Oranje B. Sensorimotor gating and habituation in antipsychotic-naïve, first-episode schizophrenia patients before and after six months treatment with quetiapine Int J Neuropsychopharmacol 2010. ; 13 : 1383–1395 [DOI] [PubMed] [Google Scholar]
- 24. Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naive schizophrenic patients Biol Psychiatry 2002. ; 52 : 863–873 [DOI] [PubMed] [Google Scholar]
- 25. Kumari V, Cotter P, Corr PJ, Gray JA, Checkley SA. Effect of clonidine on the human acoustic startle reflex Psychopharmacology (Berl) 1996. ; 123 : 353–360 [DOI] [PubMed] [Google Scholar]
- 26. Wing JK, Babor T, Brugha T,, et al. SCAN. Schedules for Clinical Assessment in Neuropsychiatry Arch Gen Psychiatry 1990. ; 47 : 589–593 [DOI] [PubMed] [Google Scholar]
- 27. Kay SR, Fiszbein A, Opler LA. The Positive and Negative Syndrome Scale (PANSS) for schizophrenia Schizophr Bull 1987. ; 13 : 261–276 [DOI] [PubMed] [Google Scholar]
- 28. Simpson KL, Lin RCS. Neuroanatomical and chemical organization of the locus coeruleus . In: Ordway GA, Schwartz MA, Frazer A , eds. Brain Norepinephrine, Neurobiology and Therapeutics Cambridge University Press; ; 2007. : 9–52 [Google Scholar]
- 29. Koch M. The neurobiology of startle Prog Neurobiol 1999. ; 59 : 107–128 [DOI] [PubMed] [Google Scholar]
- 30. Kumari V, Antonova E, Zachariah E, et al. Structural brain correlates of prepulse inhibition of the acoustic startle response in healthy humans Neuroimage 2005. ; 26 : 1052–1058 [DOI] [PubMed] [Google Scholar]
- 31. Kumari V, Gray JA, Geyer MA, et al. Neural correlates of tactile prepulse inhibition: a functional MRI study in normal and schizophrenic subjects Psychiatry Res 2003. ; 122 : 99–113 [DOI] [PubMed] [Google Scholar]
- 32. Kumari V, Fannon D, Geyer MA,, et al. Cortical grey matter volume and sensorimotor gating in schizophrenia Cortex 2008. ; 44 : 1206–1214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hammer TB, Oranje B, Skimminge A,, et al. Structural brain correlates of sensorimotor gating in antipsychotic-naïve, first-episode schizophrenia patients. J Psychiatry Neuroscience. 2012 doi: 10.1503/jpn.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Swerdlow NR, Bongiovanni MJ, Tochen L, Shoemaker JM. Separable noradrenergic and dopaminergic regulation of prepulse inhibition in rats: implications for predictive validity and Tourette Syndrome Psychopharmacology (Berl) 2006. ; 186 : 246–254 [DOI] [PubMed] [Google Scholar]
- 35. Lahdesmaki J, Sallinen J, MacDonald E, Scheinin M. Alpha2A-adrenoceptors are important modulators of the effects of D-amphetamine on startle reactivity and brain monoamines Neuropsychopharmacology 2004. ; 29 : 1282–1293 [DOI] [PubMed] [Google Scholar]
- 36. Kumari V, Mulligan OF, Cotter PA, et al. Effects of single oral administrations of haloperidol and d- amphetamine on prepulse inhibition of the acoustic startle reflex in healthy male volunteers Behav Pharmacol 1998. ; 9 : 567–576 [DOI] [PubMed] [Google Scholar]
- 37. Quednow BB, Schmechtig A, Ettinger U, et al. Sensorimotor gating depends on polymorphisms of the serotonin-2A receptor and catechol-O-methyltransferase, but not on neuregulin-1 Arg38Gln genotype: a replication study Biol Psychiatry 2009. ; 66 : 614–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Kumari V, Peters ER, Fannon D, et al. Uncontrollable voices and their relationship to gating deficits in schizophrenia Schizophr Res 2008. ; 101 : 185–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Friedman JI, Adler DN, Temporini HD,, et al. Guanfacine treatment of cognitive impairment in schizophrenia Neuropsychopharmacology 2001. ; 25 : 402–409 [DOI] [PubMed] [Google Scholar]
- 40. Van Kammen DP, Peters JL, Van Kammen WB,, et al. Clonidine treatment of schizophrenia: can we predict treatment response? Psychiatry Res 1989. ; 27 : 297–311 [DOI] [PubMed] [Google Scholar]
- 41. Abel K, Waikar M, Pedro B, Hemsley D, Geyer M. Repeated testing of prepulse inhibition and habituation of the startle reflex: a study in healthy human controls J Psychopharmacol 1998. ; 12 : 330–337 [DOI] [PubMed] [Google Scholar]
- 42. Ludewig K, Geyer MA, Etzensberger M, Vollenweider FX. Stability of the acoustic startle reflex, prepulse inhibition, and habituation in schizophrenia Schizophr Res 2002. ; 55 : 129–137 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.