Abstract
Background:
Social cognition is significantly impaired in schizophrenia and contributes to poor community functioning. This study examined whether cognitive remediation (CR; COGPACK), shown to improve neurocognition, improves an integral component of social cognition, emotion perception, compared with CR combined with a computerized Emotion Perception intervention (Mind Reading: Interactive Guide to Emotions [MRIGE]).
Methods:
59 stable schizophrenia or schizoaffective predominantly inpatients were randomized to either CR (N = 27) alone or CR + MRIGE (N = 32) for 12 weeks. Assessments included the Facial Emotion Identification Task (FEIT), Facial Emotion Discrimination Task (FEDT), MCCB-MATRICS, Personal and Social Performance Scale, and the Positive and Negative Syndrome Scale.
Results:
There was a significant group-by-time effect on FEIT (F = 11.509, P = .004); CR + MRIGE demonstrated significantly greater improvement than CR alone (CR + MRIGE, Z = 1.89, P = .05; CR alone Z = 0.57, P = .13). There was significant group-by-time effect on FEDT (F = 5.663, P = .022); CR + MRIGE demonstrated significantly greater improvement than CR alone (CR + MRIGE, Z = 1.90, P = .05; CR alone Z = 0.67, P = .21). There was also a significant group by time effect for social cognition, measured by the Mayer-Salovey-Caruso Emotional Intelligence Test (F = 5.473, P = .050): CR + MRIGE demonstrated significantly greater improvement than CR alone (CR + MRIGE, Z = 1.98, P = .02; CR alone, Z = 1.00, P = .05).
Conclusions:
Combined CR with emotion perception remediation produced greater improvements in emotion recognition, emotion discrimination, social functioning, and neurocognition compared with CR alone in chronic schizophrenia.
Key words: schizophrenia, social cognition, cognitive remediation
Introduction
Social cognition, which captures affect perception, social cue perception, empathy, attributional style, and theory of mind,1 is significantly impaired in schizophrenia. With increasing evidence of the importance of social cognition to community functioning in schizophrenia,2 efforts to ameliorate these deficits have intensified.3–6 Social cognition interventions include programs that address the broad range of social cognitive domains,3,6–8 as well as targeted intervention approaches targeting specified components of social cognition, and most commonly, emotion recognition.8,9
Social cognition is a unique area of functioning in schizophrenia, which is supported, in part, by basic neurocognitive functions, as well as having direct pathways to broader aspects of community functioning, such as social and independent living skills.5,10–16 Because of the importance of neurocognition to social cognition, cognitive remediation (CR) has the potential to improve social cognition, although this has not been well studied. For example, a recent meta-analysis involving 40 controlled trials of CR training17 indicated that only 7 studies assessed social cognition, with a minority assessing emotion perception. Another recent meta-analysis, examining the efficacy and specificity of computer-assisted CR in schizophrenia on cognitive functions, found a small effect size of 0.38 (CI 0.20–0.55) on general cognition and a medium effect size of 0.64 (CI 0.29–0.99) for computerized CR on social cognition.18 However, many of these studies combined CR with group therapies specifically targeting social cognition domains. Two well-articulated interventions, Integrated Psychological Therapy and Cognitive Enhancement Therapy also combine CR with manualized social group therapy and have demonstrated in multiple trials benefits on neurocognition, social cognition, and functional outcomes.19,20 One CR intervention, Neurocognitive Enhancement Therapy (NET), has demonstrated improve- ments in emotion recognition measures without specific remediation of social cognition.21 Therefore, conclusions regarding the separate contribution of CR alone to improvements in social cognition are still limited. Given the putative role of neurocognitive functioning in supporting social cognitive functioning, it is plausible that gains in neurocognitive functioning occurring during CR would contribute to improved social cognition. Thus, it is of interest to evaluate potential benefits of CR on social cognition and to investigate its potential contribution to social cognition interventions, such as “boosting” the effects of the social cognition intervention similar to findings observed in the augmentation of work therapy programs by CR.22
Social cognition programs developed for individuals with autism spectrum disorders (ASD) have become of interest for application to schizophrenia populations because autism and schizophrenia share many similarities with respect to social cognitive dysfunctions. Similarities in social cognitive deficits between schizophrenia patients and patients with ASD are intriguing.23 The program “Mind Reading: An Interactive Guide to Emotions” (MRIGE)24 was developed to improve facial and emotion recognition in patients with ASD. Golan and Baron-Cohen (2006)25 found that the MRIGE intervention significantly increased the ability of persons with Asperger’s disorder and high functioning autistic participants to recognize emotional states via facial and voice cues. Additional features of MRIGE that contribute to its feasibility for use in schizophrenia is that it provides individualized practice that is adjusted to patient performance levels and allows a high degree of control and autonomy to users, features that have contributed to the feasibility of computerized CR in schizophrenia.26
The aim of the present study was to compare the effects of a 12-week CR program (CR alone), having demonstrated efficacy on neurocognition in the patient population included in the present study, with CR combined with MRIGE, a novel training program of emotion perception (CR + MRIGE), on measures of emotion perception, social functioning, and neurocogniton. Our hypothesis was that the combined CR + MRIGE intervention would result in greater improvements in social cognition and measures of social functioning compared with CR alone.
Methods
Fifty-nine in- and outpatients were randomly assigned to CR (N = 27; 25 inpatients, 2 outpatients), or to CR + MRIGE (N = 32; 30 inpatients; 2 outpatients). All patients were clinically stable on antipsychotic medications for 1 month prior to study inclusion. Emotion recognition, social functions, neurocognitive functions, and psychopathology were assessed at baseline and posttreatment. During screening, patients were evaluated for study eligibility by review of past history and diagnostic eligibility. Retrospective stability was defined by absence of significant changes in medication and medication doses for 4 weeks prior to screening. Inclusion criteria: (1) age 18–65 years, (2) DSM-IV diagnosis of schizophrenia (all subtypes) or schizoaffective disorder with illness duration >5 years to establish greater homogeneity of the sample; (3) auditory and visual acuity adequate to complete cognitive tests; (4) stable dose of oral atypical antipsychotic for at least 4 weeks; (5) good physical health determined by physical examination from medical chart review; (6) capacity and willingness to give written informed consent; and (7) MMSE > 24. Exclusion criteria: (1) inability to read or speak English; (2) documented disease of the central nervous system; (3) intellectual disability; (4) clinically significant or unstable cardiovascular, renal, hepatic, gastrointestinal, pulmonary, or hematological conditions; (5) HIV+; and (6) diagnosis of substance dependence.
Following randomization, patients entered the 12-week treatment period. Patients who were randomized to receive CR + MRIGE received 2h of CR and 1h of MRIGE/week, while patients assigned to CR alone received 3h of CR/week, with both interventions delivered over 12 weeks. Both MRIGE and CR took place in a computer laboratory. In the inpatient setting, the interventions took place within the “treatment mall”, which is a 20-h/week comprehensive psychiatric rehabilitation program. Outpatients received an identical program, with MRIGE and CR conducted in a computer laboratory at the outpatient clinic within a comprehensive psychiatric rehabilitation program. There was no attempt at a formal integration of the Treatment Mall program with the study intervention beyond the customary interdisciplinary staff communications. The MRIGE and CR treatment programs were supervised by rehabilitation staff with extensive experience with the CR.
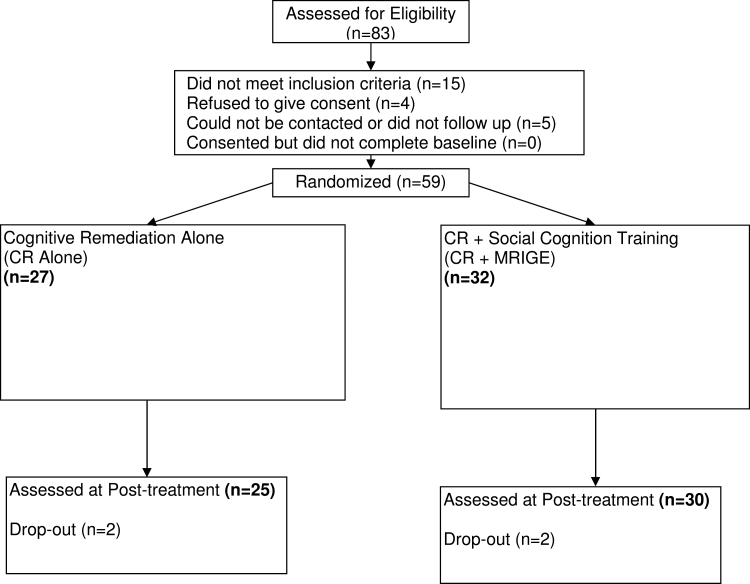
See figure 1 for the CONSORT diagram and study flow. All patients provided informed consent.
Fig. 1.
Patient disposition and study flow.
Social Cognition Intervention
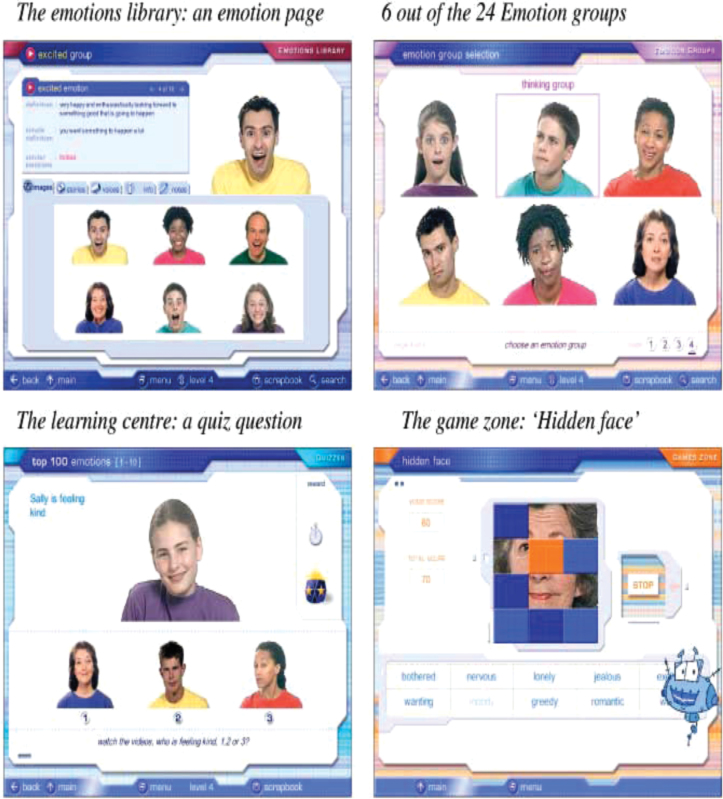
We used an innovative computerized program, MRIGE,24 which is an interactive computerized program practicing the recognition of emotions and mental states developed for patients with Autism. It is based on a taxonomic system of 412 emotions and mental states, grouped into 24 emotion groups and 6 developmental levels (from age 4 to adulthood). Each emotion is defined and demonstrated in 6 silent films of faces, 6 voice recordings, and 6 written examples of situations that evoke this emotion. The resulting library of emotional “assets” (video clips, audio clips, or brief stories) comprises 7416 units of emotion information used for learning to recognize or to understand emotions. The MRIGE emotion database is accessed using 3 applications: (1) The emotion library allows users to browse freely through the different emotions and emotion groups, play the faces, voices and scenarios giving examples of the emotions, read stories, add their own notes, and compare different emotional expressions in the face and the voice. (2) The learning center uses lessons, quizzes, and several reward practices to teach about emotions in a more structured and directive way. (3) The game zone comprises 5 educational games, allowing users to enjoy a game while studying about emotions. Patients were lead through a standardized curriculum of practice, with levels of challenge automatically adjusted by the computer program to offer a consistent ratio of challenge and positive reinforcement. Its use in patients with schizophrenia is new, with only 1 reported case study available to date indicating positive results,27 figure 2 shows screen shots from the software (www.jkp.com/mindreading). Prior to randomization, all patients were assigned their own computer workstation and received training on handling the mouse.
Fig. 2.
Screenshots from Mind Reading: The Interactive Guide to EmotionsSource: S. Baron-Cohen et al., 2004, London: Jessica Kingsley Limited. [Copyright 2003 by the University of Cambridge.]
Neurocognitive Intervention
We used COGPACK (Version 8.1; Marker Software, Ladenburg, Germany) for the CR intervention. COGPACK is a computerized, commercially available CR program developed for persons with severe mental illness, with demonstrated efficacy in schizophrenia.26,28,29 The curriculum of COGPACK exercises used in the current study is similar to that used in past controlled trials.22,26,30 The curriculum consists of a standardized set of computerized exercises that provides practice of the broad range of cognitive functions and overlaps with cognitive domains assessed by the MCCB-MATRICS.31
Both groups received 36h of intervention as follows: Patients randomized to the CR + MRIGE group received 12h of training (once per week) with MRIGE and 24h of training with COGPACK (twice/week; Version 8.1; Marker Software, Ladenburg, Germany), over 12 weeks, for a total of 36h of training. Patients randomized to the CR alone group received 36h of training (3 times/week) with COGPACK over 12 weeks.
Assessment of Social Cognition
Emotion Recognition. The Facial Emotion Identification Test (FEIT)32 consists of black and white photographs of facial emotions that are presented on a Digital Video Disk (DVD) of 19 faces each depicting 1 of 6 different emotions (happiness, sadness, anger, surprise, disgust, and shame), shown 1 at a time for 15 s, with 10 s of blank screen between each stimulus presentation. After each stimulus, the participant makes a forced choice by selecting which of the 6 emotions is depicted. The score is the sum of the number of correct emotion identifications (0–19). We also used the Facial Emotion Discrimination Test (FEDT).32 The task uses black and white photographs of facial emotions that are presented on a DVD. The FEDT consists of 30 pairs of photographs, each pair showing 2 different people displaying 1 or 2 of the 6 emotions depicted in the FEIT. The pairs are presented simultaneously for 15 s, with 15 s of blank screen between each presentation. The task is to judge whether the 2 faces have the same or different emotions.
The Managing Emotions subtest of the Mayer-Salovey-Caruso Emotional Intelligence Test (MSCEIT), which assesses the ability to be open to feelings and to modulate them in self and others, was administered in the MATRICS battery. The MSCEIT is an ability-based test designed to measure the 4 branches of the emotional intelligence model of Mayer and Salovey (2002).33 The MSCEIT is part of the MATRICS assessment battery for patients with schizophrenia.
Assessment of Neurocognitive Functions
The MATRICS Consensus Cognitive Battery (MCCB)31 was used to assess general cognitive performance.34,35 For the current analyses, the following MCCB domains were examined: speed of processing, attention/vigilance, working memory, verbal learning, visual learning, reasoning and problem solving, and social cognition. Additionally, an overall neurocognitive composite score based on the average T-scores for the 7 domains was computed.
Assessment of Psychopathology and Social Functions
The Positive and Negative Syndrome Scale (PANSS)36 was administered by trained raters to assess severity of psychopathology symptoms of schizophrenia at baseline and at endpoint. For the PANSS, all raters were required to have a graduate degree (MA, MS, PhD, or MD) with at least 1-year experience in psychiatry or psychology in order to administer the PANSS and raters were required to score Intraclass correlation within the range of ≥0.82 to ≤0.89 with the Gold Score rating after viewing a series of 5 videotaped Gold Scored PANSS interviews. In order to measure patient’s level of functioning, the Personal and Social Performance Scale (PSP)37,38 was administered at baseline and at week 12. The PSP is a validated scale to assess the social functioning of a patient with schizophrenia, and ratings are based on the outcome of a structured clinical interview, divided into 4 categories: (1) socially useful activities, (2) personal and social relationships, (3) self-care, and (4) disturbing and aggressive behaviors. The PSP was administered using a structured clinical interview for the scale; however, interrater reliability of the PSP measures was not performed for this study.
Raters were not blind to the group assignment of patients, but none were involved with the implementation of the remediation interventions and raters were not aware of the hypothesis of the study. The scoring of the primary outcome measures, the FEIT and the FEDT, is based on categorical “yes” or “no” answers by the subject during the stimulus presentation. There is no room for rater interpretation in scoring the subjects’ answers.
Statistical Analysis
Patient demographic characteristics were summarized using descriptive statistics. For continuous variables, descriptive statistics were provided; for categorical variables, patient counts and percentages were provided. The FEDT and FEIT scores were the primary efficacy variables. Actual values and changes from baseline to final visit (12 week) were summarized using descriptive statistics. To assess medication effects, chlorpromazine (CPZ) equivalent doses were calculated at baseline according to standard methodology based on published equivalencies for oral conventional and atypical antipsychotics.39,40 For depot formulations of haloperidol, fluphenazine, and risperidone, manufacturers’ recommended equivalent dose for the depot to oral conversion for the same drug converted to oral CPZ equivalents were used.41 In case of significant differences at baseline, CPZ equivalents would be controlled for in the primary efficacy analysis.
Group differences over time (0 weeks, 12 weeks) were examined using an intent-to-treat approach with all 59 patients who were randomized and had baseline data. The primary test employed was a linear mixed-effects model to examine group differences at endpoint (12 weeks) on the primary measures, FEDT, FEIT, and PSP. All mixed-effects models used random intercept and slope parameters, were estimated using restricted maximum likelihood, employed autoregressive error structures where appropriate. Missing data were estimated using the maximum-likelihood expectation maximization approach controlling for baseline scores.42 If there were significant baseline to endpoint group effects, a pairwise comparison of slopes between each group using z tests (based on the maximum-likelihood parameter estimates of the slopes and their standard errors) was performed. Similar analyses were performed for T-scores from baseline to endpoint for the cognitive domains of the MCCB-MATRICS (speed of processing, attention vigilance, working memory, verbal memory, reasoning and problem solving and social cognition measured by the MSCEIT) and the MCCB- MATRICS composite score. We also examined the percent of correct responses of recognition of positive emotions (happy, and surprised) vs negative emotions (ashamed, sad, angry, and afraid) using Chi Square tests, both at baseline and at endpoint for both groups and compared changes from baseline to endpoint for both groups. Effect sizes for the primary efficacy measure were provided for the treatment groups: CR alone and CR + MRIGE group. Cohen’s d effect size was used to compute effect size using the final mean (±SD) in the CR Alone group and the CR + Mind Reader group as follows [(Mean CR + Mind Reader − Mean CR)/pooled SD]. This procedure to compute Cohen’s d is presented further in McGough and Faraone (2009).43
We examined whether changes in emotion perception are associated with change in neurocognitive functioning. To take baseline differences into account when measuring change in treatment response, we used the residual change score analysis to assess changes in MCCB neurocognitive domains scores for each group (CR alone, CR + MRIGE). Multiple linear regression predicted a follow-up score for each subject on the basis of their neurocognitive domain scores and the degree of change in the whole sample. The difference between the predicted and the actual follow-up neurocognitive domain score for each subject constituted the residual change score. Pearsons correlations between the residual change scores were computed. All statistical tests were 2 tailed, using a significance level of P < .05.
Results
Fifty-nine patients (82% males; mean age 43.8; average education 8.9 years) were randomly assigned to CR alone or CR plus Social Cognition Training (CR + MRIGE) between August 2009 and September 2011. Of the 59 patients enrolled, 55 patients completed the 12-week course of treatment. Three patients were discharged from the inpatient facility without receiving the endpoint assessment, and 1 withdrew consent after 1 week of study entry and did not receive endpoint assessment (See figure 1 for the CONSORT diagram). There were no significant differences between the 2 groups on baseline variables, including background characteristics (see table 1), the FEIT and FEDT measure, the MCCB domain scores, the composite cognitive index, PSP scores, and psychopathology measures (see table 2). All patients who completed CR (n = 55) in the CR alone group received 36h of training, and all patients who completed CR = MRIGE group received 24h of training with COGPACK and 12h of training with MRIGE. For the intent-to-treat sample, the distribution of sessions is as follows: CR group (Mean number of sessions = 33.93, SD = 7.46; Range = 6–36), CR + MRIGE (Mean number of sessions = 34.23, SD = 6.90; Range = 6–36).
Table 1.
Demographic Characteristics of Sample at Baseline
| CR Alone (n = 27) | CR + Mind Reader (n = 32) | Effect Sizes Cohen’s d a | ||
|---|---|---|---|---|
| Gender | N (%) | N (%) | Difference between groups | |
| Male | 22 (81.48) | 26 (81.25) | Chi Square = 0.059P = .920 | N/A |
| Female | 5 (18.52) | 6 (18.75) | N/A | |
| Race | ||||
| Caucasian | 4 (14.81) | 5 (15.63) | Chi Square = 1.176P = .089 | |
| African American | 15 (55.56) | 17 (53.13) | ||
| Hispanic | 7 (25.93) | 9 (28.13) | ||
| Asian | 1 (3.70) | 1 (3.13) | ||
| Continuous variables | Mean (SD) | Mean (SD) | ||
| Age at time of testing | 42.48 (9.09) | 43.95 (11.12) | F = 0.208, P = .83 | 0.15 |
| Level of education (y) | 8.86 (4.33) | 9.12 (3.10) | F = 0.012, P = .99 | 0.07 |
| Age at first hospitalization | 18.67 (10.19) | 18.48 (11.49) | F = 0.011, P = .099 | 0.02 |
Note: Effect size that cannot be computed as variables are categorical. N/A, not applicable.
aCohen’s d was used to compute effect size using the mean at baseline (±SD) in the CR Alone group and the CR + MRIGE group as follows ((Mean CR + MRIGE – Mean CR)/pooled SD)).
*P ≤ .05, **P ≤ .001.
Table 2.
Change in Emotion Perception, MCCB-MATRICS Cognitive Scores and Social Performance
| Emotion Perception (Mean Scores) | CR Alone (n = 27) | CR + Mind Reader (n = 32) | Treatment, df (1,57) | Time, df (1,54) | Treatment × Time, df (1,54) | Estimated Linear Slopesa | Effect Sizes Cohen’s d b | ||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | Endpoint | Baseline | Endpoint | ||||||
| FEIT | 10.75 ± 2.79 | 11.12 ± 2.77 | 11.12 ± 3.11 | 14.23 ± 2.11 | F = 10.123, P = .005* | F = 9.234, P = .010* | F = 11.509, P = .004* | CR: Z = 0.57, P = .13CR + MRIGE: Z = 1.89, P = .05 | 1.27 |
| FEDT | 21.12 ± 3.24 | 22.05 ± 3.30 | 22.59 ± 3.52 | 26.49 ± 3.87 | F = 4.657, P = .023* | F = 4.615, P = .030* | F = 5.663, P = .022* | CR: Z = .67, P = .21CR + MRIGE: Z = 1.90, P = .05 | 1.24 |
| MCCB-MATRICS (T Scores) | |||||||||
| Speed of processing | 31.64 (6.33) | 33.87 (6.22) | 31.44 (8.76) | 35.99 (7.11) | F = 5.136, P = .042* | F = 5.834, P = .034* | F = 5.643, P = .039* | CR: Z = 1.55, P = .03CR + MRIGE: Z = 1.91, P = 0.02 | 0.32 |
| Attention/vigilance | 32.11 (5.09) | 35.21 (5.99) | 31.33 (7.89) | 36.94 (7.25) | F = 6.003, P = .038* | F = 5.412, P = .038* | F = 5.787, P = .030* | CR: Z = 1.51, P = .03CR + MRIGE: Z = 1.89, P = .02 | 0.26 |
| Working memory | 30.13 (6.02) | 33.31 (6.03) | 29.94 (6.19) | 35.37 (6.89) | F = 5.713, P = .034* | F = 5.597, P = .041* | F = 5.670, P = .039* | CR: Z = 1.11, P = .06CR + MRIGE: Z = 2.01, P = .02 | 0.32 |
| Verbal learning | 30.04 (5.16) | 33.30 (6.14) | 30.14 (5.91) | 33.44 (5.93) | F = 1.113, P = .612 | F = 2.032, P = .519 | F = 1.198, P = .595 | N/A | 0.02 |
| Visual learning | 30.67 (7.28) | 34.58 (6.35) | 31.31 (7.24) | 35.93 (7.05) | F = 1.698, P = .620 | F = 2.001, P = .637 | F = 1.746, P = .613 | N/A | 0.20 |
| Reasoning and problem solving | 34.28 (5.29) | 38.41 (6.00) | 32.61 (5.98) | 38.79 (6.06) | F = 3.694, P = .070 | F = 3.006, P = .099 | F = 3.946, P = .065 | N/A | 0.06 |
| Social cognition (MSCEIT) | 32.02 (5.15) | 34.49 (5.96) | 31.96 (9.12) | 39.16 (6.92) | F = 5.139, P = .057 | F = 5.119, P = .059 | F = 5.473, P = .050* | CR:Z = 1.00, P = .05CR + MRIGE: Z = 1.98, P = .02 | 0.73 |
| MCCB composite | 35.31 (6.79) | 39.93 (6.03) | 34.01 (6.74) | 41.51 (4.39) | F = 5.956, P = .048* | F = 4.687, P = .064 | F = 5.511, P = .050* | CR: Z = 0.90, P = .08CR + MRIGE: Z = 2.51, P = .01 | 0.30 |
| Social functioning | |||||||||
| PSP total score | 43.41 (11.16) | 45.46 (10.55) | 44.10 (9.97) | 50.56 (11.23) | F = 5.269, P = .029* | F = 3.947, P = .048* | F = 4.237, P = .037* | CR: Z = 0.60, P = .16CR + MRIGE: Z = 1.91, P = .05 | 0.47 |
| Psychopathology | |||||||||
| PANSS positive subscale | 24.15 (5.12) | 23.60 (4.91) | 24.59 (6.42) | 23.94 (4.99) | F = 0.771, P = .567 | F = 0.779, P = .555 | F = 0.781, P = .560 | N/A | 0.07 |
| PANSS negative subscale | 21.99 (5.16) | 21.10 (5.03) | 22.81 (5.11) | 22.03 (5.01) | F = 0.810, P = .560 | F = 0.800, P = .558 | F = 0.774, P = .566 | N/A | 0.19 |
| PANSS total score | 91.97 (11.09) | 91.41 (11.32) | 90.46 (12.78) | 90.09 (11.90) | F = 0.799, P = .559 | F = 0.792, P = .560 | F = 0.794, P = .559 | N/A | 0.11 |
Note: Treatment × Time interaction was not significant, therefore estimated linear slope to determine significant differences within group was not estimated. FEIT, Facial Emotional Identification Task; FEDT, Facial Emotional Discrimination Task; MCCB, MATRICS Consensus Cognitive Battery; PSP, Personal and Social Performance; PANSS, Positive and Negative Syndrome Scale.
aBased on maximum likelihood parameter estimates using General Linear Mixed Model.
bCohen’s d was used to compute effect size using the final mean (±SD) in the CR Alone group and the CR + Mind Reader group as follows ((Mean CR + Mind Reader − Mean CR)/pooled SD). This procedure to compute Cohen’s d is presented further in McGough & Faraone (2009).
*P ≤ .05, **P ≤ .001.
Of the 59 patients enrolled in the study, 54.24% (n = 32; CR = 15; CR + MRIGE = 17) were on 1 or more antipsychotic medication at baseline. As patients on more than 1 medication may have been on oral atypicals plus typicals (ie, n = 6), 2 atypicals (n = 12), intramuscular medications plus 1 or more typicals (n = 8), or intramuscular medications plus 1 or more atypicals (n = 6), it was not possible to assess type of medication. Therefore CPZ equivalent doses were computed. Daily CPZ (mg/day) was 415.76mg/d (46.11) for the patients on only 1 antipsychotic, and 923.47mg/d (47.22) for the patients on more than 1 antipsychotic. The CPZ equivalency distribution per group was as follows: CR alone [CPZ = 589.12mg/d (56.11)] and CR + MRIGE [CPZ = 597.59mg/d (59.99)]; no significant differences in CPZ equivalency were observed between groups (F(1,57) = 1.236, P = .647).
There was a significant treatment × time effect on FEIT scores (F = 11.509, P = .004); CR + MRIGE patients demonstrated significantly greater improvement over time than patients in the CR alone group (CR + MRIGE, Z = 1.89, P = .05; CR alone Z = 0.57, P = .13). There was a significant treatment × time effect on FEDT scores (F = 5.663, P = .022); CR + MRIGE patients demonstrated significantly greater improvement over time than patients in the CR alone group (CR + MRIGE, Z = 1.90, P = .05; CR alone Z = 0.67, P = .21).
There was also a significant treatment × time effect for social cognition as measured by the MSCEIT (F = 5.473, P = .050): CR + MRIGE patients demonstrated significantly greater improvement over time than patients in the CR alone group (CR + MRIGE, Z = 1.98, P = .02; CR alone, Z = 1.00, P = .05).
Of the 55 patients who completed both baseline and endpoint testing, there was a significant difference at baseline in both groups between correct recognition of positive emotions (happy, and surprised) vs negative emotions (ashamed, sad, angry, and afraid) (Chi Square = 13.579, P = .027), with patients in both groups being able to more accurately recognize positive emotions (CR alone: baseline, 92.00% of patients; CR + MRIGE: baseline, 90.00% of patients). Similar results were observed at endpoint (CR alone: endpoint, 92.00% of patients; CR + MRIGE: endpoint, 96.67% of patients). Greater but nonsignificant improvements were observed at endpoint for all emotions for the CR + MRIGE group compared with the group receiving CR alone (CR alone: negative emotions, 56.00% of patients identified angry, 44.00% identified ashamed, 56.00% identified afraid, and 36.00% identified sad at endpoint; CR + MRIGE, 80.00% of patients identified angry, 63.33% identified ashamed, 63.33% identified afraid, and 56.67% identified sad at endpoint).
Both CR alone and CR + MRIGE groups were associated with significant improvements across 3 MCCB cognitive domains, including attention/vigilance (F = 5.786, P = .030), speed of processing (F = 5.644, P = .041) and working memory (F = 5.674, P = .040). The change in the CR + MRIGE group was greater than the change in the CR alone group in the domains of attention/vigilance (change from baseline, CR alone 3.18±5.01; CR + MRIGE 5.69±8.11; F = 5.644; P = .041), speed of processing (change from baseline, CR alone 2.73±7.01; CR + MRIGE 4.98±8.11; F = 5.768; P = .03), and working memory (change from baseline, CR alone 3.31±6.54, CR + MRIGE 5.44±5.99; F = 5.674; P = .040). The change of the MCCB composite measure was also significantly greater for the CR + MRIGE group compared with CR alone group (F = 5.512, P = .050) with a change from baseline for the CR alone group of 5.64±5.91 and 7.52±5.33 for the CR + MRIGE group. Estimated linear slopes are presented in table 2.
There was a statistically significant between-group difference favoring the CR + MRIGE group based on the change from baseline PSP (ie, 95% CI) total score (F = 4.238, P = .038; see table 2). There were no significant improvements over time on the PANSS positive subscale (F = 0.781, P = .559), negative subscale (F = 0.774, P = .563), or the total score (F = 0.789, P = .568). The group-by-time interaction was not significant for the PANSS subscales or total score, suggesting that CR and CR+ MRIGE did not appear to affect the psychopathology symptoms over the study period.
Using residual change scores for both treatment groups, change in speed of processing, working memory, verbal learning, and the MCCB composite score were significantly correlated with the change in FEDT and FEIT measures for both the CR + MRIGE and CR alone group (see table 3).
Table 3.
Correlations Between Residual Change Scores on the MCCB Neurocognitive Domains and Emotion Perception Measures
| CR Alone (n = 27) | CR + MRIGE (n = 32) | |||||
|---|---|---|---|---|---|---|
| FEIT | FEDT | MCCB-MATRICS Social Cognition | FEIT | FEDT | MCCB MATRICS Social Cognition | |
| Speed of processing | 0.38* | 0.38* | 0.21* | 0.39* | 0.36* | 0.25* |
| Attention pigilance | 0.13 | 0.12 | 0.24* | 0.14 | 0.16 | 0.24* |
| Working memory | 0.26* | 0.27* | 0.31* | 0.28* | 0.28* | 0.30* |
| Verbal learning | 0.20** | 0.22* | 0.14 | 0.20** | 0.20** | 0.21** |
| Visual learning | 0.18 | 0.15 | 0.14 | 0.18 | 0.19 | 0.19 |
| Reasoning and problem solving | 0.15 | 0.14 | 0.14 | 0.15 | 0.19 | 0.19 |
| MCCB composite | 0.29* | 0.30* | N/A | 0.30* | 0.31* | N/A |
Note: Mean of the seven MATRICS Consensus Cognitive Battery (MCCB) neurocognitive T-scores. Correlations with the MCCB MSCEIT was not performed with the MCCB Composite score as the MCCB MSCEIT is included in the computation of the MCCB composite score. MSCEIT, Mayer-Salovey-Caruso Emotion Intelligence Test.
*P ≤ .001, **P ≤ .05.
Discussion
Our study, to the best of our knowledge, is the first controlled trial evaluating a novel emotion perception computerized intervention developed for patients with autism in patients with chronic schizophrenia. The results of the study indicate the feasibility of this computerized social cognition program in schizophrenia in that 87% of the patients completed the treatment program. In addition, the study provides evidence of the efficacy of the MRIGE program on emotion perception compared with CR alone. Significant improvements were seen on the emotion identification task and the emotion discrimination task. It appeared that the improvements occurred both for positive and for negative emotions to a similar extent. These findings were further complemented by significant improvement in the social cognition measure of the MCCB. Results also demonstrate that improvements in social cognition were not related to improvements in clinical status, as PANSS subscale and total scores did not significantly change overtime. There were also significantly greater neurocognitive improvements in the combined group on processing speed, attention/vigilance and working memory for patients enrolled compared with patients receiving CR alone. We also found greater improvement in social functioning in the combined treatment group, which further supports findings of social cognitive improvements and provides evidence for generalization of our combined social cognition training to improvement of broader social functioning.
Our study findings demonstrate that CR alone has limited ability to impact emotion recognition and discrimination5,8,9 and that neurocognitive ability may represent a “necessary but not sufficient” prerequisite for social cognitive ability.44 The finding of “domain-specific” effects of practice indicates that the provision of cognitive practice improves neurocognition, but has only modest ability to improve other aspects of functioning, such as social cognition, unless interventions targeting those areas of functioning are combined with the cognitive program.17,45 One exception is the study by Bell et al. (2001),21 which showed improvement in facial and voice tone affect recognition with a CR intervention (NET). The addition of MRIGE to CR training in our study may have “boosted” the benefits of emotion perception training, as indicated by the relatively large effect sizes in the social perception measures (1.26 for emotion identification and 1.23 for emotion discrimination). The lack of a MRIGE alone group limits our ability to know whether the improvements in social cognition associated with the combined group may have been obtained by MRIGE alone, which we will address in future studies.
Our results are also consistent with a recent meta-analysis of social cognition interventions.6 These authors found that these programs “produced improvements on facial affect recognition in the moderate-large range” and also moderate-large effect size improvement on observer-rated measures of functioning. Our results in a group of patients with chronic and extended length of illness support their findings showing that longer duration of illness “predicted greater response to social cognitive training”. This supports the use of CR and social cognition interventions in patients with a chronic course of illness and significant social impairments.
The greater improvements observed in the CR + MRIGE group in aspects of neurocognition compared with CR alone supports the close relationship of neuro- and social cognition; specifically, the provision of practice of social cognition imparted some additional benefits to neurocognitive functioning.13,46 Also, tasks on the MRIGE program practice reaction time, which may have contributed to the benefits to processing speed.
The combined CR + MRIGE intervention appeared to generalize to social functioning, whereas CR alone did not. These findings support the idea that social cognition may have greater potential to impact functional outcomes than neurocognition.5,16,47 It is, however, possible that our CR + MRIGE group may have differentially benefitted from the added exposure to the Treatment Mall program although both groups had equal exposure to this general rehabilitative program, into which both interventions were embedded. We may need to further complement the MRIGE program with other interventions that address more distal “real world” functions, such as work functions. We consider the MRIGE program as an entry door into more comprehensive social skills interventions. An important research question would be to explore the effect of combining a computerized social cognition program like MRIGE with a group based social cognition intervention. Another next step will be the expansion of the program into outpatient settings and to assess the persistence of the effects on social functions.
Our study demonstrates that the use of a program developed for patients with autism can be implemented in patients with schizophrenia using a relatively low staff to patient ratio. Other social cognition programs, such as the Social Cognition and Interaction Training (SCIT)7,48 and the Targeting Affective Recognition program9 entail staff-intensive training that is often difficult to accomplish and maintain in facilities with limited staff support although we realize that SCIT addresss the broad range of social cognition domains. Importantly, the MRIGE program was effective for our chronically ill, long stay patients who are heavily burdened with symptoms of schizophrenia and significant educational disadvantages (eg, an average of 8.9 years). In addition, the program was engaging and capable of retaining patients demonstrated by a very low discontinuation rate. The fact that we did not pay patients for their participation in the training sessions further contributes to its generalizability beyond research settings.
Our findings have to be interpreted within the context of some limitations. First, although we had adequate power to detect group differences in our primary analyses and all statistical tests yielded small to moderate effect sizes,49 our supplemental analyses were underpowered. We did however obtain convergent results from 3 independent sources of measurements: the 2 emotion recognition/discrimination measures, the social cognition measure from the MCCB-MATRICS, and the social function scale results, which all favored the CR + MRIGE intervention, strengthening our findings. A second limitation may have arisen in that raters were not blinded as to the group assignments of patients although none of the raters were involved with the implementation of the remediation interventions and were not aware of the hypothesis of the study. However, rater bias may have been least prominent for the primary outcome variables, the FEIT, and the FEDT measures. The scoring of these measures are based on categorical “yes” or “no” answers by the subject in the stimulus presentation. There is no room for rater interpretation in scoring the subjects’ answers. Rater bias could have been greater in the secondary outcome variable, the improvement in the PSP. However, the meta-analysis of Wykes et al. (2011)17 did not find that methodological issues acted as moderators on the strengths of the outcome findings. The recent meta-analysis of social cognitive training, which examined among several moderator variables design features of the included studies, found that this moderator variable did not show a significant effect.6 We were not able to examine the moderating effect of medication on our outcome measures as both groups were comparable in their mean total medication dosages.
Conclusions
Our findings suggest that an integrated combination of CR with the Mind Reading program used in patients with autism improves emotion recognition and emotion discrimination enabling improvements in social functioning in patients with chronic schizophrenia. In addition, CR together with our social cognition training program appeared to be associated with greater improvement in selected cognitive functions compared with CR alone. Our results of this combined approach will need to be replicated by larger controlled studies including the testing of the persistence of these gains after completion of the intervention.
References
- 1. Green MF, Horan WP. Social cognition in schizophrenia Curr. Dir. Psychol 2010. 19 243–248 [Google Scholar]
- 2. Harvey PD, Penn D. Social cognition: the key factor predicting social outcome in people with schizophrenia? Psychiatry 2010. 7 41–44 [PMC free article] [PubMed] [Google Scholar]
- 3. Horan WP, Kern RS, Shokat-Fadai K, et al. Social cognitive skills training in schizophrenia: an initial efficacy study of stabilized outpatients Schizophr Res 2009. 107 47–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roberts DL, Penn DL, Corrigan P, Lipkovich I, Kinon B, Black RA. Antipsychotic medication and social cue recognition in chronic schizophrenia Psychiatry Res 2010. 178 46–50 [DOI] [PubMed] [Google Scholar]
- 5. Horan WP, Kern RS, Tripp C, et al. Efficacy and specificity of social cognitive skills training for outpatients with psychotic disorders J Psychiatr Res 2011. 45 1113–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kurtz MM, Richardson CL. 2011. Social cognitive training for schizophrenia: a meta-analytic investigation of controlled research Schizophr Bull. doi:10.1093/schbul/sbr036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Combs DR, Adams SD, Penn DL, Roberts D, Tiegreen J, Stern P. Social Cognition and Interaction Training (SCIT) for inpatients with schizophrenia spectrum disorders: preliminary findings Schizophr Res 2007. 91 112–116 [DOI] [PubMed] [Google Scholar]
- 8. Frommann N, Steit M, Wolwer W. Remediation of facial affect recognition impairments in patients with schizophrenia: a new training program Psychiatry Res 2003. 117 281–284 [DOI] [PubMed] [Google Scholar]
- 9. Wolwer W, Frommann N, Halfmann S, Piaszek A, Streit M, Gaebel W. Remediation of impairments in facial affect recognition in schizophrenia: efficacy and specificity of a new training program Schizophr Res 2005. 80 295–303 [DOI] [PubMed] [Google Scholar]
- 10. Addington J, Saeedi H, Addington D. Facial affect recognition: a mediator between cognitive and social functioning in psychosis? Schizophr Res 2006. 85 142–150 [DOI] [PubMed] [Google Scholar]
- 11. Brekke J, Kay DD, Lee KS, Green MF. Biosocial pathways to functional outcome in schizophrenia Schizophr Res 2005. 80 213–225 [DOI] [PubMed] [Google Scholar]
- 12. Meyer MB, Kurtz MM. Elementary neurocognitive function, facial affect recognition and social-skills in schizophrenia Schizophr Res 2009. 110 173–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Sergi MJ, Rassovsky Y, Widmark C, et al. Social cognition in schizophrenia: relationships with neurocognition and negative symptoms Schizophr Res 2007. 90 316–324 [DOI] [PubMed] [Google Scholar]
- 14. Vaskinn A, Sundet K, Friis S, et al. Emotion perception and learning potential: mediators between neurocognition and social problem-solving in schizophrenia? J Int Neuropsychol Soc 2008. 14 279–288 [DOI] [PubMed] [Google Scholar]
- 15. Vauth R, Rusch N, Wirtz M, Corrigan PW. Does social cognition influence the relation between neurocognitive deficits and vocational functioning in schizophrenia? Psychiatry Res 2004. 128 155–165 [DOI] [PubMed] [Google Scholar]
- 16. Bell M, Tsang HW, Greig TC, Bryson GJ. Neurocognition, social cognition, perceived social discomfort, and vocational outcomes in schizophrenia Schizophr Bull 2009. 35 738–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wykes T, Huddy V, Cellard C, McGurk SR, Czobor P. A meta-analysis of cognitive remediation for schizophrenia: methodology and effect sizes Am J Psychiatry 2011. 168 472–485 [DOI] [PubMed] [Google Scholar]
- 18. Grynszpan O, Perbal S, Pelissolo A. Efficacy and specificity of computer-assisted cognitive remediation in schizophrenia: a meta-analytical study Psychol Med 2011. 41 163–173 [DOI] [PubMed] [Google Scholar]
- 19. Roder V, Mueller DR, Mueser KT, Brenner HD. Integrated psychological therapy (IPT) for schizophrenia: is it effective? Schizophr Bull 2006. 32(Suppl 1):S81–S93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hogarty GE, Flesher S, Ulrich R, et al. Cognitive enhancement therapy for schizophrenia: effects of a 2-year randomized trial on cognition and behavior. Arch Gen Psychiatry 2004. 61 866–876 [DOI] [PubMed] [Google Scholar]
- 21. Bell M, Bryson G, Greig T, Corcoran C, Wexler BE. Neurocognitive enhancement therapy with work therapy: effects on neuropsychological test performance. Arch Gen Psychiatry 2001. 58 763–768 [DOI] [PubMed] [Google Scholar]
- 22. McGurk SR, Mueser KT, DeRosa TJ, Wolfe R. Work, recovery, and comorbidity in schizophrenia: a randomized controlled trial of cognitive remediation. Schizophr Bull 2009. 35 319–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Couture SM, Penn DL, Losh M, Adolphs R, Hurley R, Piven J. Comparison of social cognitive functioning in schizophrenia and high functioning autism: more convergence than divergence. Psychol Med 2010. 40 569–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Baron-Cohen S, Golan O, Wheelwright S, Hill JJ.Mind Reading: The interactive guide to emotionsLondon, UK: Jessica Kingsley Limited; 2004. [Google Scholar]
- 25. Golan O, Baron-Cohen S. Systemizing empathy: teaching adults with Asperger syndrome or high-functioning autism to recognize complex emotions using interactive multimedia. Dev Psychopathol 2006. 18 591–617 [DOI] [PubMed] [Google Scholar]
- 26. Lindenmayer JP, McGurk SR, Mueser KT, et al. A randomized controlled trial of cognitive remediation among inpatients with persistent mental illness. Psychiatr Serv 2008. 59 241–247 [DOI] [PubMed] [Google Scholar]
- 27.Rose D, Hooker C, Verosky S, Miyakawa A, Vinogradov S. 2006. www.schizophrenia.com/pdfs/social.cognition.schizophrenia.DROSE.pdf www.schizophrenia.com/pdfs/social.cognition.schizophrenia.DROSE.pdf Social cognition and schizophrenia: a pilot intervention combining auditory processing, working memory, affect and theory of mind training.
- 28. Vita A, De Peri L, Barlati S, et al. Effectiveness of different modalities of cognitive remediation on symptomatological, neuropsychological, and functional outcome domains in schizophrenia: a prospective study in a real-world setting. Schizophr Res 2011. 133 223–231 [DOI] [PubMed] [Google Scholar]
- 29. Bender S, Dittmann-Balcar A, Prehn G, Thienel R, Peters S, Gastpar M. Subjective experience of a computer-assisted cognitive training by patients with schizophrenia. Nervenarzt 2004. 75 44–50 [DOI] [PubMed] [Google Scholar]
- 30. McGurk SR, Mueser KT, Pascaris A. Cognitive training and supported employment for persons with severe mental illness: one-year results from a randomized controlled trial. Schizophr Bull 2005. 31 898–909 [DOI] [PubMed] [Google Scholar]
- 31. Nuechterlein KH, Green MF.MATRICS Consensus Cognitive BatteryLos Angeles, CA: MATRICS Assessment, Inc; 2006. [Google Scholar]
- 32. Kerr SL, Neale JM. Emotion perception in schizophrenia: specific deficit or further evidence of generalized poor performance? J Abnorm Psychol 1993. 102 312–318 [DOI] [PubMed] [Google Scholar]
- 33. Mayer JD, Salovey P, Caruso D.MSCEIT User’s ManualToronto, ON: Multi-Health Systems; 2002. [Google Scholar]
- 34. Kern RS, Nuechterlein KH, Green MF, et al. The MATRICS Consensus Cognitive battery, part 2: co-norming and standardization. Am J Psychiatry 2008. 165 214–220 [DOI] [PubMed] [Google Scholar]
- 35. Nuechterlein KH, Green MF, Kern RS, et al. The MATRICS consensus cognitive battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008. 165 203–213 [DOI] [PubMed] [Google Scholar]
- 36. Kay SR, Fiszbein A, Opler LA. The positive and negative syndrome scale (PANSS) for schizophrenia. Schizophr Bull 1987. 13 261–276 [DOI] [PubMed] [Google Scholar]
- 37. Morosini PL, Magliano L, Brambilla L, Ugolini S, Pioli R. Development, reliability and acceptability of a new version of the DSM-IV Social and occupational functioning assessment scale (SOFAS) to assess routine social functioning. Acta Psychiatr Scand 2000. 101 323–329 [PubMed] [Google Scholar]
- 38. Hsieh PC, Huang HY, Wang HC, et al. Intercorrelations between the personal and social performance scale, cognitive function, and activities of daily living. J Nerv Ment Dis 2011. 199 513–515 [DOI] [PubMed] [Google Scholar]
- 39. American Psychiatric Association Practice guideline for the treatment of patients with schizophrenia Am J Psychiatry 1997. 154 1–63 [DOI] [PubMed] [Google Scholar]
- 40. Woods SW. Chlorpromazine equivalent doses for the newer atypical antipsychotics. J Clin Psychiatry 2003. 64 663–667 [DOI] [PubMed] [Google Scholar]
- 41. Chue P, Eerdekens M, Augustyns I, et al. Comparative efficacy and safety of long-acting risperidone and risperidone oral tablets. Eur Neuropsychopharmacol 2005. 15 111–117 [DOI] [PubMed] [Google Scholar]
- 42. Dempster AP, Laird NM, Rubin DB. Maximum likelihood from incomplete data via the EM algorithm J R Stat Soc 1997; series B 39 1–38 [Google Scholar]
- 43. McGough JJ, Faraone SV. Estimating the size of treatment effects: moving beyond p values. Psychiatry 2009. 6 21–29 [PMC free article] [PubMed] [Google Scholar]
- 44. Penn DL, Spaulding W, Reed D, Sullivan M, Mueser KT, Hope DA. Cognition and social functioning in schizophrenia Psychiatry 1997. 60 281–291 [DOI] [PubMed] [Google Scholar]
- 45. McGurk SR, Twamley EW, Sitzer DI, McHugo GJ, Mueser KT. A meta-analysis of cognitive remediation in schizophrenia Am J Psychiatry 2007. 164 1791–1802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Sachs G, Steger-Wuchse D, Kryspin-Exner I, Gur RC, Katschnig H. Facial recognition deficits and cognition in schizophrenia Schizophr Res 2004. 68 27–35 [DOI] [PubMed] [Google Scholar]
- 47. Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review Schizophr Bull 2006. 32(Suppl 1):S44–S63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Combs DR, Elerson K, Penn DL, et al. Stability and generalization of Social Cognition and Interaction Training (SCIT) for schizophrenia: six-month follow-up results Schizophr Res 2009. 112 196–197 [DOI] [PubMed] [Google Scholar]
- 49. Cohen J. Statistical Power Analysis of the Behavioral Sciences 2nd ed. New York, NY: Academic Press; 1988. [Google Scholar]