Abstract
This study assessed the association between second-generation antipsychotic medications and risk of pneumonia requiring hospitalization in patients with schizophrenia because the evidence is limited in the population. We enrolled a nationwide cohort of 33 024 inpatients with schizophrenia ranged in age from 18 to 65 years, who were derived from the National Health Insurance Research Database in Taiwan from 2000 to 2008. Cases (n = 1741) were defined as patients who developed pneumonia after their first psychiatric admissions. Risk set sampling was used to match each case with 4 controls by age, sex, and the year of the first admission based on nested case-control study. Antipsychotic exposure was categorized by type, duration, and daily dose, and the association between exposure and pneumonia was assessed using conditional logistic regression. We found that current use of clozapine (adjusted risk ratio = 3.18, 95% CI: 2.62–3.86, P < .001) was associated with a dose-dependent increase in the risk. Although quetiapine, olanzapine, zotepine, and risperidone were associated with increased risk, there was no clear dose-dependent relationship. Amisulpride was associated with a low risk of pneumonia. The use of clozapine combined with another drug (olanzapine, quetiapine, zotepine, risperidone, or amisulpride), as assessed separately, was associated with increased risk for pneumonia. In addition, with the exception of amisulpride, each drug was associated with increased risk for pneumonia at the beginning of treatment. Clinicians who prescribe clozapine to patients with schizophrenia should closely monitor them for pneumonia, particularly at the start of therapy and when clozapine is combined with other antipsychotics.
Keywords: second-generation antipsychotics, clozapine, pneumonia, dose-dependent
Introduction
Second-generation antipsychotics introduced in the early 1990s were initially associated with superior quality of life, better tolerability, and lower risk of relapse than first-generation antipsychotics.1–3 Subsequent studies questioned the clinical superiority of second-generation antipsychotics,4,5 and currently, there is widespread concern about the adverse effects associated with these medications.5 In particular, several of the second-generation antipsychotics have been shown to be more likely to cause weight gain and metabolic syndrome than first-generation antipsychotics, despite having fewer extrapyramidal effects.6–8 In addition, second-generation antipsychotics sometimes cause serious side effects, including pneumonia.9,10 In 2005, the US Food and Drug Administration (FDA) issued a warning against the use of second-generation antipsychotics for the treatment of elderly patients with behavioral disturbances because use of those agents was associated with increased mortality.11 In the FDA report, the specific causes of death were either due to heart-related events or infections (mostly pneumonia). In 2008, the FDA further requested that boxed warnings be placed on the packaging of first-generation antipsychotics stating that older people with dementia who take these drugs are at increased risk of death.12
Several studies have evaluated the association between the antipsychotics and risk of developing pneumonia in elderly populations. Two studies conducted in the Netherlands9,10 reported that the use of first-generation antipsychotics and the use of second-generation antipsychotics in elderly patients were both associated with increased risk for pneumonia. A study conducted in the United States13 reported that use of first-generation antipsychotics but not use of second-generation antipsychotics was associated with an increased risk of inhospital mortality in acutely ill pneumonia patients.
The marked increase in use of second-generation antipsychotics worldwide has resulted in a sharp rise in the number of safety alerts issued by international regulatory agencies in the last decade.14,15 Although the association between second-generation antipsychotics and pneumonia had been reported in studies of elderly populations,9,10 studies of schizophrenic populations were limited by small size or being case reports.16,17 One exception is a recent UK study18 that established outcomes for 529 consecutive patients with schizophrenia who received clozapine. The authors found that death in 5 of the 21 patients was due to pneumonia. Schizophrenic patients with pneumonia deserve intensive clinical attention due to their poor clinical outcomes,19 with greater risks of intensive care unit admission, acute respiratory failure, and necessity of mechanical ventilation. Pneumonia is an illness in which hospitalization is potentially avoidable.20 As a basis for prevention efforts, further studies were required for improving the quality of care of such patients.
Previous studies9,10 proposed that the possible mechanisms that mediate the association between pneumonia and antipsychotics are based on the affinities of said drugs for neurotransmitter receptors, particularly the histaminergic-1 (H1) receptor and the muscarinic-1 (M1) receptor. We hypothesized that second-generation antipsychotics with high affinity for the H1 receptor or anticholinergic action, such as clozapine, would be associated with increased risk for pneumonia. This study conducted a nested case-control study in a large cohort with schizophrenia derived from a nationwide dataset in Taiwan. The objective was to explore various dimensions of the associations between each second-generation antipsychotic drug and the risk of pneumonia.
Methods
Study Subjects
Details of the data source were described elsewhere21,22 and briefly summarized here. Taiwan introduced a single-payer National Health Insurance program on March 1, 1995, 98% of the 23 million Taiwanese population were enrolled. In 1996, the National Health Research Institute in Taiwan established the National Health Insurance Research Database. The database includes medical claim files representative of the entire population in Taiwan. All investigators signed an agreement guaranteeing patient confidentiality before using the database.
We used the Psychiatric Inpatient Medical Claims database, a subset of the National Health Insurance Research Database, comprising a cohort of patients hospitalized for any psychiatric disorder between 1996 and 2008 (n = 187 117). The database included patients with at least one psychiatric inpatient record and one discharge diagnosis of mental illness coded by the International Classification of Diseases, Ninth Revision (ICD-9) codes 290–319. The database includes patients’ demographic characteristics, diagnoses, medical expenditures, and prescription claims data.23 Each prescription record contains the type of medication, dosage, time of prescription, and duration of drug supply. Information that could be used to identify beneficiaries and medical care providers were scrambled by the Bureau of National Health Insurance.24
We selected patients with at least one psychiatric admission between 2000 and 2008 but no psychiatric admissions between 1996 and 1999 (n = 125 225) (see online supplementary figure 1). The inclusion criteria for the study cohort was that one’s diagnosis at each discharge fulfilled the principal diagnosis of schizophrenia (ICD-9 code 295.**) if a patient had several psychiatric admissions. The age of patients at first admission was restricted to 18–65 years. Subsequently, 33 024 patients with schizophrenia as the cohort was included, and all of their medical records during 1996 and 2008 were retrieved.
Case and Control Definition
Using the patients with pneumonia requiring hospitalization after first psychiatric admission as cases (n = 1741), we conducted a nested case-control study derived from the cohort. Pneumonia was defined as the primary or any-listed discharge diagnosis with ICD-9 codes 480–486 and 507. In this study, all types of pneumonia were included because subcategorization of pneumonia is not always recorded properly, and the criterion was adopted in a prior study.9
The date of hospitalization for pneumonia was defined as the index date. Each case was matched by sex, age (±5 y), and the year of the first psychiatric admission to 4 controls or fewer (ie, no hospitalization for pneumonia) who had been randomly selected from the cohort by risk set sampling. Controls were assigned the same index date as their corresponding case. The corresponding index date was later than the first psychiatric admission in each control. In addition, each control had at least one claim record after the corresponding index date to confirm that patients were covered by the National Health Insurance program.
Cases that were identified later during the follow-up were eligible to serve as controls for earlier cases. During the study period, 281 subjects were first selected as controls and later became cases, while another 922 controls were selected more than twice for the analysis. Finally, this study included a total of 1739 case-control pairs (ie, 1739 cases and 6949 controls) due to unavailability of controls for 2 cases.
Measurement of Exposure
We obtained data on antipsychotic drug use from prescription files and calculated the duration of treatment on the basis of the dispensed number of units and the dosing regimen for each patient. As reported in a previous study,10 we defined the use of a single antipsychotic drug as “current” if the prescription duration covered the index date or ended at most 30 days before the index date. Drug use was categorized as “recent” if usage ended 31–180 days before the index date and was catagorized as “past” if the last prescription ended more than 180 days before the index date.
Second-generation antipsychotics used in this study comprised clozapine, olanzapine, quetiapine, zotepine, risperidone, amisulpride, ziprasidone, aripiprazole, and paliperidone. However, ziprasidone, aripiprazole, and paliperidone, which were marketed in Taiwan in 2002, 2004, and 2007, respectively, were not included in the analysis of individual drug use because the proportion of current users was low (less than 1.2% for each).
First-generation antipsychotics comprised chlorpromazine, levomepromazine, fluphenazine, perphenazine, trifluoperazine, thioridazine, pipotiazine, haloperidol, moperone, flupentixol, clopenthixol, chlorprothixene, pimozide, loxapine, sulpiride, clotiapine, and penfluridol. This study focused on the associations between the individual second-generation antipsychotic drug and the risk of pneumonia; thus we grouped all first-generation antipsychotic drugs into one category.
We studied the risk of developing pneumonia for each second-generation antipsychotic drug separately by duration and daily dose within 30 days before the index date. Those who were noncurrent users for a specified drug contributed zero duration and daily dose of the drug within 30 days before the index date. Drugs were classified according to the Anatomical Therapeutic Chemical (ATC) classification system.25 The daily dose was based on the international standard defined daily dose (ATC/DDD Index 2009. http://www.whocc.no/atc_ddd_index/ [accessed May 1, 2009]). For example, 300 mg of clozapine was equivalent to one DDD. Duration of antipsychotic treatment was calculated based on the purchased DDD.26
We estimated the effect of continuous antipsychotic exposure on pneumonia onset risk. In current users, the number of days of antipsychotic exposure beginning with the prescribing date most proximal to the index date were added together; 30 days between 2 prescriptions was considered a treatment gap and previous exposures were not cumulated.
Covariates
Age and gender were controlled by the matching process of the study design. Covariates for adjustment comprised Charlson comorbidity score at first psychiatric admission and number of psychiatric admissions, physical illnesses, and concomitant medications prescribed within 180 days before the index date. We assessed general health status using the Charlson comorbidity index, which is the sum of the weighted scores of 31 comorbid conditions.27 The index is widely used to control for confounding variables in epidemiological studies.28
Statistical Analysis
The crude incidence of subsequent pneumonia was calculated as the number of incident cases divided by the contributed person-years for each individual in the cohort. Group comparisons between cases and controls were performed using univariate conditional logistic regressions initially. Covariates with reasonable associations with pneumonia (P < .1) were then entered into the final adjusted models. Multivariate regression was used to estimate the effect of individual antipsychotic drug (past, recent, and current use) on the risk of pneumonia. Another multivariate regression model was used to estimate the associations between pneumonia and the daily dose and duration of drug use within 30 days before the index date, respectively. Multivariate models were conducting using SAS software, version 9.2 (SAS Institutes Inc., Cary, NC). A P value of .01 was considered significant.
Sensitivity Analysis
The assignment of an antipsychotic drug for the study subjects was not randomized because of the nonexperimental design of this study. The assignment could be, therefore, determined based on the presence of comorbid physical illnesses and concomitant medications (table 1). Propensity score methods, as formalized by Rosenbaum and Rubin,29 are standard techniques for controlling confounding variables in nonexperimental studies.30 We conducted a propensity score-adjusted regression as a sensitivity analysis to examine how the covariates might influence our estimates of the associations between each antipsychotic drug and pneumonia. Further details are provided in the online supplementary table 2.
Table 1.
Characteristics of Incident Pneumonia Case Subjects and Controls Derived From a Nationwide Cohort With Schizophrenia, From 2000 to 2008 (N = 33 024)
| Characteristic N (%) | Case (N = 1739) | Controls (N = 6949) | Unadjusted Risk Ratioa | 95% CI |
| N (%) | N (%) | |||
| At first admission | ||||
| Men | 1088 (62.9) | 4350 (62.9) | — | — |
| Age, mean (SD) (y) | 42.8 (12.6) | 42.7 (12.6) | 1.47** | 1.24–1.75 |
| Charlson comorbidity index | ||||
| 1 | 1361 (78.3) | 5818 (83.7) | Reference | |
| 2 | 286 (16.5) | 946 (13.6) | 1.30** | 1.12–1.50 |
| ≥3 | 92 (5.3) | 185 (2.7) | 2.16** | 1.67–2.80 |
| Within 180 d before the index date | ||||
| Number of psychiatric hospital admissions, mean (SD) | 0.7 (0.9) | 0.4 (0.6) | 1.95** | 1.80–2.11 |
| Physical illnesses | ||||
| Cardiovascular disease | 574 (33.0) | 1308 (18.8) | 2.32** | 2.05–2.63 |
| Diabetes mellitus | 262 (15.1) | 595 (8.6) | 1.95** | 1.66–2.28 |
| Cerebrovascular disease | 110 (6.3) | 92 (1.3) | 5.33** | 3.98–7.15 |
| Chronic hepatic disease | 177 (10.2) | 360 (5.2) | 2.09** | 1.73–2.53 |
| Cancer | 91 (5.2) | 76 (1.1) | 5.12** | 3.74–7.03 |
| Asthma | 115 (6.6) | 117 (1.7) | 4.17** | 3.20–5.44 |
| Upper respiratory tract infection | 722 (41.5) | 2134 (30.7) | 1.62** | 1.45–1.81 |
| Deliriumb | 24 (1.38) | 27 (0.39) | 3.62** | 2.08–6.32 |
| Concomitant drugs | ||||
| Cardiovascular drugs | ||||
| Antihypertensive agents | 104 (6.0) | 165 (2.4) | 2.67** | 2.07–3.44 |
| Beta blocking agents | 707 (40.7) | 2126 (30.6) | 1.57** | 1.40–1.75 |
| Calcium channel blockers | 341 (19.6) | 662 (9.5) | 2.46** | 2.12–2.86 |
| Agents acting on the renin-angiotensin system | 184 (10.6) | 393 (5.7) | 2.06** | 1.70–2.49 |
| Lipid modifying agents | 81 (4.7) | 261 (3.8) | 1.26 | 0.97–1.62 |
| Respiratory drugsc | 1606 (92.4) | 3548 (51.1) | 11.60** | 9.63–13.98 |
| Drugs used in diabetes | 278 (16.0) | 527 (7.6) | 2.40** | 2.05–2.82 |
| Antithrombotic agents | 238 (13.7) | 372 (5.4) | 2.98** | 2.49–3.57 |
| Corticosteroids for systemic use | 541 (31.1) | 670 (9.6) | 4.30** | 3.77–4.92 |
| Anti-Parkinson drugs | 1265 (72.7) | 4484 (64.5) | 1.49** | 1.32–1.67 |
Estimated using univariate conditional logistic regression, **P < .001.
Based on ICD-9 code, including presenile dementia with delirium, senile dementia with delirium, arteriosclerotic dementia with delirium, alcohol withdrawal delirium, drug-induced delirium, acute delirium, subacute delirium.
Based on Anatomical Therapeutic Chemical code, including nasal preparations, throat preparations, drugs for obstructive airway diseases, cough and cold preparations, antihistamines for systemic use, other respiratory system products.
To estimate how the potential misclassification between subcategories of pneumonia influence the findings of this study, we conducted subgroup analyses by restricting the outcomes to pneumonia, organism unspecificed (ICD-9 code 485.**), and bronchopneumonia, organism unspecified (ICD-9 code 486.**), respectively, the subcategories that comprise the largest (n = 1132) and second largest (n = 149) parts of total pneumonia cases in this study.
Results
The incidence of pneumonia requiring hospitalization was 1.12 cases per 100 person-years (95% CI: 1.07–1.18, based on Poisson distribution).
Comorbid Physical Illnesses and Concomitant Medications
The characteristics of patients with pneumonia and controls are shown in table 1. Case subjects had higher Charlson comorbidity scores at the first psychiatric admission than controls. In addition, case subjects had a greater number of physical illnesses and concomitant medications 180 days before the index date than controls.
Temporal Relationship
Nearly all of the patients in both groups had received at least one first-generation antipsychotic drug (table 2). After adjustment for covariates (all variables in table 1, except for gender and age), there was no significant difference in the risk of developing pneumonia between nonusers and current, recent, and past users.
Table 2.
Association Between Pneumonia and the Use of Antipsychotic Drugs in Case Subjects and Controls Stratified by Current Use, Recent Use, Past Use, and No Use (Reference Group)
| Cases | Controls | Adjusted | Modela | |
| (n = 1739), N (%) | (n = 6949), N (%) | Risk Ratio | 95% CI | |
| Any use of second-generation antipsychotics | ||||
| No use | 330 (19.0) | 1856 (26.7) | Reference | — |
| Past use | 235 (13.5) | 1322 (19.0) | 1.04 | 0.83–1.30 |
| Recent use | 119 (6.8) | 563 (8.1) | 0.90 | 0.68–1.18 |
| Current use | 1055 (60.7) | 3208 (46.2) | 1.69** | 1.43–2.01 |
| Clozapine | ||||
| No use | 1271 (73.1) | 6039 (86.9) | Reference | — |
| Past use | 112 (6.4) | 332 (4.8) | 1.47* | 1.12–1.93 |
| Recent use | 20 (1.2) | 68 (1.0) | 0.93 | 0.52–1.67 |
| Current use | 336 (19.3) | 510 (7.3) | 3.18** | 2.62–3.86 |
| Olanzapine | ||||
| No use | 1217 (80.0) | 5383 (77.5) | Reference | — |
| Past use | 262 (15.1) | 885 (12.7) | 1.09 | 0.90–1.31 |
| Recent use | 61 (3.5) | 192 (2.8) | 1.04 | 0.72–1.50 |
| Current use | 199 (11.4) | 489 (7.0) | 1.83** | 1.48–2.28 |
| Quetiapine | ||||
| No use | 1253 (72.1) | 5501 (79.2) | Reference | — |
| Past use | 221 (12.7) | 845 (12.2) | 1.04 | 0.85–1.26 |
| Recent use | 67 (3.9) | 181 (2.6) | 1.03 | 0.72–1.46 |
| Current use | 198 (11.4) | 422 (6.1) | 1.63** | 1.31–2.04 |
| Zotepine | ||||
| No use | 1317 (75.7) | 5733 (82.5) | Reference | — |
| Past use | 238 (13.7) | 742 (10.7) | 1.14 | 0.93–1.39 |
| Recent use | 54 (3.1) | 141 (2.0) | 1.02 | 0.69–1.50 |
| Current use | 130 (7.5) | 333 (4.8) | 1.48* | 1.15–1.91 |
| Risperidone | ||||
| No use | 650 (37.4) | 3110 (44.8) | Reference | — |
| Past use | 492 (28.3) | 1945 (28.0) | 1.16 | 0.98–1.36 |
| Recent use | 129 (7.4) | 468 (6.7) | 0.81 | 0.62–1.04 |
| Current use | 468 (26.9) | 1426 (20.5) | 1.32** | 1.12–1.56 |
| Amisulpride | ||||
| No use | 1567 (90.1) | 6408 (92.2) | Reference | — |
| Past use | 87 (5.0) | 262 (3.8) | 1.07 | 0.78–1.46 |
| Recent use | 29 (1.7) | 90 (1.3) | 0.82 | 0.48–1.39 |
| Current use | 56 (3.2) | 189 (2.7) | 1.14 | 0.79–1.65 |
| Any use of first-generation antipsychotics | ||||
| No use | 41 (2.4) | 239 (3.4) | Reference | — |
| Past use | 468 (26.9) | 2808 (40.4) | 0.88 | 0.59–1.32 |
| Recent use | 212 (12.2) | 1037 (14.9) | 0.67 | 0.44–10.4 |
| Current use | 1018 (58.5) | 2865 (41.2) | 1.38 | 0.92–2.07 |
Adjusted for Charlson comorbidity index at the first admission, and the following variables within 180 d before the index date, including the number of psychiatric hospital admissions, physical illnesses, and concomitant medications (listed in table 1).
*P < .01, **P < .001.
As for second-generation antipsychotics, adjusted analysis showed that current use of any second-generation antipsychotic drug was significantly associated with pneumonia (adjusted risk ratio = 1.69, P < .001) and that recent and past use of the drugs were not associated with pneumonia. Adjusted models showed that current use of clozapine was associated with the highest risk of pneumonia, followed by current use of olanzapine, quetiapine, zotepine, and risperidone. Amisulpride was not associated with pneumonia.
Dose-Dependent Relationship
After adjusting for covariates, the analysis revealed that case subjects with pneumonia had significantly longer durations of clozapine use within 30 days before the index date than control patients (see the supplementary table 1). As for the cumulative DDD, only clozapine showed an increased risk for pneumonia.
Single- and Polypharmacy in Association With Pneumonia
We classified the polypharmacy of antipsychotics (ie, several antipsychotic medications) used within 30 days before the index date. Polypharmacy was classified into 4 subgroups: single drug, combined use with clozapine, combined use with nonclozapine antipsychotics (including second-generation and first-generation antipsychotics), and noncurrent use (reference group) (table 3). Current users of clozapine alone were 2 times more likely to develop pneumonia than patients who did not currently use clozapine (adjusted risk ratio = 2.05, P < .001). Current use of other drugs was not associated with pneumonia. In addition, use of clozapine combined with olanzapine, quetiapine, zotepine, risperidone, or amisulpride was also associated with greater risk of developing pneumonia. Nearly all combinations with nonclozapine antipsychotics showed increased risks for pneumonia, except for the combinations with amisulpride or zotepine.
Table 3.
Association Between Pneumonia and the Use of Individual Second-Generation Antipsychotic Drugs Within 30 D Before the Index Date Stratified by Single Drug Use, the Specified Drug Combined With Clozapine, the Specified Drug Combined With Nonclozapine Antipsychotic(s), and Noncurrent Use (Reference Group)
| Single Antipsychotic Drug | Combined With Clozapine | Combined With Nonclozapine Antipsychotics | ||||
| Adjusted Risk Ratioa | 95% CI | Adjusted Risk Ratioa | 95% CI | Adjusted Risk Ratioa | 95% CI | |
| Clozapine | 2.01** | 1.54 –2.61 | — | — | 4.78** | 3.68–6.23 |
| Olanzapine | 0.73 | 0.51–1.06 | 22.40** | 9.43–53.18 | 2.33** | 1.71–3.18 |
| Quetiapine | 0.86 | 0.61–1.23 | 14.76** | 6.44–33.84 | 1.87** | 1.37–2.56 |
| Zotepine | 0.97 | 0.65–1.47 | 4.80** | 2.17–10.64 | 1.57 | 1.09–2.27 |
| Risperidone | 0.65** | 0.53–0.80 | 7.49** | 4.47–12.56 | 2.41** | 1.93–3.00 |
| Amisulpride | 0.51 | 0.27–0.98 | 21.44** | 4.49–102.30 | 1.36 | 0.80–2.29 |
Adjusted for Charlson comorbidity index at the first admission, and the following variables within 180 d before the index date, including the number of psychiatric hospital admissions, physical illnesses, and concomitant medications (listed in table 1)
*P < .01, **P < .001.
Cumulative Days of Continuous Treatment in Association With Pneumonia
Among current users, the median durations (days) of continuous treatment for clozapine, olanzapine, quetiapine, zotepine, risperidone, and amisulpride were 106, 38, 61.5, 41.5, 59.5, and 47 in the case patients, which were shorter than those in the controls (413, 169, 136.5, 124.0, 161.0, and 98.0).
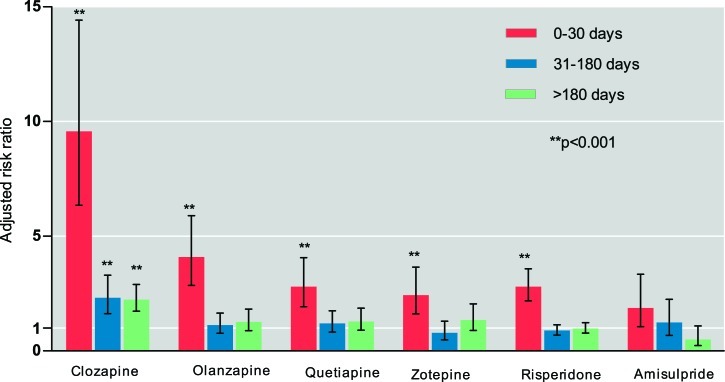
Figure 1 shows the association between cumulative days of continuous antipsychotic treatment and risk of pneumonia. All of the drugs with the exception of amisulpride were significantly associated with increased risk of pneumonia in the first 30 days of use, and clozapine was associated with the highest risk (adjusted risk ratio = 9.57, P < .001). With longer use of antipsychotics, the risk of pneumonia decreased, except for clozapine. Significantly increased risk of pneumonia remained for patients receiving treatment with clozapine for more than 31 days.
Fig. 1.
Risk of pneumonia and effect of cumulative days of continuous antipsychotic treatment (noncurrent use as the reference group). Adjusted risk ratio: adjusted for Charlson comorbidity index at the first admission, and the following variables within 180 d before the index date, including the number of psychiatric hospital admissions, physical illnesses, and concomitant medications (listed in table 1).
Sensitivity Analysis
The sensitivity analysis, which controlled for the confounding effect of assignment of individual antipsychotic drugs based on propensity scores, revealed no substantial difference in the association between individual drugs and pneumonia (see online supplementary table 2). For example, compared with patients who did not currently use clozapine, the adjusted risk ratios for current users of clozapine were 3.09 (95% CI: 2.55–3.74, P < .001) in the multivariate-adjusted model and 3.14 (95% CI: 2.59–3.80, P < .001) in the propensity score-adjusted model.
Furthermore, subgroup analyses showed that current use of clozapine was associated with risk of developing pneumonia, organism unspecified (adjusted risk ratio, 3.43; 95% CI: 2.69–4.36; P < .0001) and risk of bronchopneumonia, organism unspecified (adjusted risk ratio, 2.36; 95% CI: 1.21–4.60; P = .0114). Both results were similar to those obtained for the association with total pneumonia cases.
Discussion
To our knowledge, this is the first study to investigate the association between use of second-generation antipsychotics and risk of pneumonia in a nationwide cohort of patients with schizophrenia. The large sample size allowed us to estimate the risk that a single antipsychotic drug would induce pneumonia. In this nested case-control study, we found that the use of second-generation antipsychotic drugs was associated with a 69% greater risk (adjusted risk ratio = 1.69) of developing pneumonia, after taking confounding factors into account, and that the magnitude of association was highest for clozapine.
Previous studies have shown that first-generation antipsychotics are significantly associated with pneumonia.9,10 In this study, however, we did not find a significant association between first-generation antipsychotics and risk of developing pneumonia requiring hospitalization. The heterogeneity of the drugs may explain, at least in part, the lack of association in our study. Further studies are, therefore, needed to investigate the effect of individual first-generation antipsychotics on the development of pneumonia.
Evidence of the Association in This Study
The major findings of this study are summarized in table 4. The causal relationship between each antipsychotic drug and the risk for pneumonia was determined by investigating whether a temporal relationship existed, whether a dose-dependent relationship existed, and whether the risk of pneumonia existed when the drug was used alone. An antipsychotic drug fulfilling all 3 criteria was taken as strong evidence that the drug was associated with risk for pneumonia; the drug fulfilling 1 or 2 of the 3 criteria was taken as moderate evidence that the drug was associated with the development of pneumonia; and a drug that did not fulfill any of the criteria was taken as inadequate evidence. Accordingly, we found that clozapine was associated with high risk of pneumonia, that olanzapine, quetiapine, zotepine, and risperidone were associated with moderate risk, and that amisulpride was associated with low risk of developing pneumonia.
Table 4.
Summary of Various Dimensions of the Associations Between the Use of Individual Second-Generation Antipsychotic Drugs and Pneumonia
| Dimensions of the Association | Clozapine | Olanzapine | Quetiapine | Zotepine | Risperidone | Amisulpride |
| Temporal relationship | +++ | + | + | + | + | – |
| Duration and dose within 30 d before the index date | ||||||
| Duration | + | – | – | – | – | – |
| Dose | + | – | – | – | – | – |
| Single vs polypharmacy | ||||||
| Single drug | ++ | – | – | – | – – | – |
| Combined with clozapine | np | ++++ | ++++ | ++++ | ++++ | ++++ |
| Combined with nonclozapine antipsychotics | ++++ | ++ | + | – | ++ | – |
| Cumulative days of continuous use | ||||||
| 0–30 (short-term use) | ++++ | +++ | +++ | ++ | ++ | – |
| 31–180 (medium-term use) | ++ | – | – | – | – | – |
| >180 (long-term use) | ++ | – | – | – | – | – |
| Evidence of the association in this study | Strong | Moderate | Moderate | Moderate | Moderate | Inadequate |
Note: Classification of effect size of adjusted risk ratio with statistical significance: +, between 1.00 and 1.99; ++, between 2.00 and 2.99; +++, between 3.00 and 3.99; ++++, ≥4.00; –, without statistical significance; – –, <1.0 with statistical significance; np, not applicable.
Clozapine was associated with increased risk of pneumonia when used individually or when used in combination with other antipsychotics. Each of the other antipsychotics was associated with increased risk of pneumonia when used concomitantly with antipsychotic drugs, especially clozapine.
In the present study, we provide robust evidence in support of the causal relationship between clozapine and pneumonia. The relationship between clozapine and pneumonia is indeed complex. Clozapine has the potential to cause agranulocytosis31 and increases the risk for infection.32 Based on neurotransmitter receptor affinities (see online supplementary table 3), the affinity of clozapine for muscarinic-1 receptors (M1) was remarkably high, olanzapine, quetiapine, and zotepine showed moderate affinity, and risperidone and amisulpride had minimal affinity for those receptors. In this study, current use of clozapine had the highest adjusted risk ratio for pneumonia (3.18), followed by olanzapine (1.83), quetiapine (1.63), zotepine (1.48), risperidone (1.32), and amisulpride (1.14), findings that are compatible with those from the affinity analysis, indicating a clear association between the risk of pneumonia and a given drug’s affinity for muscarinic-1 receptors. In addition, the affinity for histaminergic-1 (H1) receptors was similar. Three drugs (clozapine, olanzapine, and quetiapine) with high affinity for histaminergic-1 receptors had the highest risk ratios (>1.5), followed by zotepine (moderate affinity), risperidone, and amisulpride (minimal affinity). The findings provided some association between pneumonia and histaminergic-1 receptors. Affinities for histaminergic-1 and muscarinic-1, therefore, are the most plausible explanations for the association between a given antipsychotic medication and the risk for pneumonia. Our findings are similar to those reported previously.9,10 In addition, the anticholinergic effect of antipsychotic drugs with muscarinic-1 action has been shown to contribute to aspiration pneumonia through dryness of mouth and esophageal dilatation and hypomotility.33 Sedation34 as a result of histaminergic-1-receptor blocking in the central nervous system might also facilitate aspiration pneumonia.
In this study, we found that many of the patients, especially those in the case-subject group, took anti-Parkinson drugs. A possible explanation was provided in our prior study,14 which indicated that although the risk of extrapyramidal syndrome is lower among patients who take second-generation antipsychotics than in patients who take first-generation antipsychotics, the considerably high rate of extrapyramidal syndrome in patients who take some of second-generation antipsychotics (such as risperidone) warrants clinical attention. The higher rate of use in case subjects than in controls indicates the potential association between anti-Parkinson drugs and pneumonia.
Use of Combined Antipsychotic Drugs
One of the major findings in this study was the identification that combined use of antipsychotic drug(s), especially clozapine, was associated with high risk for pneumonia. Our study revealed that drugs used in combination had a synergistic effect, thereby increasing the risk of developing pneumonia. The synergistic effect was particularly striking in patients who took clozapine in combination with another antipsychotic medication. We hypothesized that polypharmacy of antipsychotics could lead to the occupation of more domains of neurotransmitter receptors and thus increase the risk for pneumonia. Further research is needed to clarify the mechanism(s) mediating the synergistic effect of antipsychotic medications on the risk for pneumonia.
High Risk With Short-Term Use
We found that initial treatment with second-generation antipsychotic drugs was associated with high risk for pneumonia. Our results show that, with the exception of amisulpride, all of the tested drugs increased the risk of developing pneumonia at the start of therapy and that the risk decreased over time. Clozapine, however, was associated with high risk for pneumonia even with medium- and long-term use. Clinicians who start patients on clozapine treatment should closely monitor them for clinical symptoms and signs of pneumonia, particularly at the start of therapy, and especially in patients who receive high doses.
Other Possible Mechanisms of Pneumonia
Underlying physical illnesses and severe psychiatric conditions might also be factors contributing to the increased risk of developing pneumonia in patients who take second-generation antipsychotic medications. In this study, we found that case subjects were more likely to have underlying illness (table 1), including cardiovascular disease and diabetes mellitus, than controls. Trifiro et al10 reported similar findings. In addition, there was a higher proportion of antihistamines use among case subjects than among controls (36.2% vs 59.2%, P < .001). Antihistamines are a category of respiratory drugs that are used to relieve symptoms of upper respiratory infection. Studies have shown that pneumonia can be followed by upper respiratory infections.35 Indeed, we found that the incidence of upper respiratory infection was higher in case patients than in controls.
Clozapine is indicated for treatment-resistant schizophrenia.36,37 Thus, the severity of schizophrenia might be associated with the development of pneumonia. The only component of the severity in this study was hospitalization. We found that the rate of hospitalization within 180 days before the index date was higher among cases than among controls. Further research is needed to clarify whether the severity of schizophrenia predisposes the patients to pneumonia.
Although amisulpride is primarily recommended for treating both positive and negative symptoms in adults with schizophrenia, there are reports of its use for treating other conditions such as dysthymia.38,39 Enrollment criteria used in this study included a principal diagnosis of schizophrenia; therefore, the number of patients with dysthymia was low. The proportions (percentage, %) of comorbid dysthymia within 180 days before the index date between cases and controls were 4.6 and 3.4. The proportions (%) between cases and controls among the current users of clozapine, olanzapine, quetiapine, zotepine, risperidone, and amisulpride were 2.4 and 2.9, 4.0 and 4.1, 6.6, and 6.2, 4.2, and 4.2, 4.1, and 4.0, 8.9 and 3.7, respectively. There were no significant differences in the proportions of comorbid dysthymia between cases and controls for each drug. Thus, the infrequent occasions that a drug was used for dysthymia did not confound our results.
In addition, the proportions of previous use (excluding current use) of first-generation antipsychotics among cases with current use of clozapine, olanzapine, quetiapine, zotepine, risperidone, or amisulpride were 43.5% (146/336), 36.7% (73/199), 43.4% (86/198), 35.4% (46/130), 40.6% (190/468), and 46.4% (26/56), respectively. The proportions were lower than those among patients in the control group with current use of these drugs (73.3% [374/510], 71.0% [347/489], 68.0% [287/422], 64.3% [214/333], 73.7% [1051/1426], and 73.0% [138/189], respectively). The reasons why the proportions of switching from first-generation antipsychotics to second-generation antipsychotics were higher in the control group deserve further study.
Limitations
This study is limited in several ways. First, we were unable to assess adherence to the prescribed medication because drug use data were obtained from claims databases. However, nonadherence would most likely result in nondifferentiated misclassification of exposure, which would lead to underestimation of the actual risk.
Second, a number of potential confounding factors that might affect pneumonia risk, such as body mass index, alcohol use, and malnutrition, were not available in the database. Information on tobacco smoking exposure, a potentially strong confounder for pneumonia,40 was also unavailable in the study. Residual confounding due to unmeasured covariates is, therefore, possible.
Third, this study did not include the long-acting injectable form of risperidone in the analysis. However, the risk estimates would unlikely change because of the low rate of current use of the injectable form and because there were no significant difference in the proportions of current use between case subjects (21/1739, 1.21%) and controls (69/6949, 0.99%).
Fourth, this study enrolled nonelderly patients with schizophrenia. Therefore, generalizability of the findings to elderly patients with schizophrenia is limited. In addition, a substantial portion of patients with pneumonia in the cohort could not be hospitalized (especially young patients), and the findings, therefore, cannot be generalized to patients with pneumonia not requiring hospitalization. However, the resulting bias, if it exists, would be more likely to cause underestimation of the risk ratios (ie, toward the null) because a small fraction of control patients had pneumonia that did not require hospitalization.
Implications
In conclusion, clozapine treatment was highly associated with pneumonia among patients with schizophrenia. Olanzapine, quetiapine, zotapine, and risperidone were moderately associated with risk for pneumonia, while amisulpride was associated with a low risk of developing pneumonia. Clinicians who start patients on clozapine should closely monitor them for signs of pneumonia, particularly at the start of therapy, and when combined with another antipsychotic drug.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
National Science Council (NSC 99-2314-B-532-003-MY3, NSC 99-2314-B-532-002-MY2) of Taiwan; the Taipei City Hospital (99001-62-049, 99001-62-004,10001-62-017, 10001-62-005), Taipei, Taiwan.
Supplementary Material
Acknowledgments
The authors thank Chien-Wei Lin and Yen-Chung Chen for data management and statistical analyses. This study is based in part on data from the National Health Insurance Research Database provided by the Bureau of National Health Insurance, Department of Health and managed by National Health Research Institutes. The interpretation and conclusions contained herein do not represent those of Bureau of National Health Insurance, Department of Health or National Health Research Institutes. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Leucht S, Barnes TR, Kissling W, Engel RR, Correll C, Kane JM. Relapse prevention in schizophrenia with new-generation antipsychotics: a systematic review and exploratory meta-analysis of randomized, controlled trials. Am J Psychiatry. 2003;160:1209–1222. doi: 10.1176/appi.ajp.160.7.1209. [DOI] [PubMed] [Google Scholar]
- 2.Leucht S, Wahlbeck K, Hamann J, Kissling W. New generation antipsychotics versus low-potency conventional antipsychotics: a systematic review and meta-analysis. Lancet. 2003;361:1581–1589. doi: 10.1016/S0140-6736(03)13306-5. [DOI] [PubMed] [Google Scholar]
- 3.Awad AG, Voruganti LN. Impact of atypical antipsychotics on quality of life in patients with schizophrenia. CNS Drugs. 2004;18:877–893. doi: 10.2165/00023210-200418130-00004. [DOI] [PubMed] [Google Scholar]
- 4.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 5.Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63:1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 6.Bai YM, Lin CC, Chen JY, Chen TT, Su TP, Chou P. Association of weight gain and metabolic syndrome in patients taking clozapine: an 8-year cohort study. J Clin Psychiatry. 2011;72:751–756. doi: 10.4088/JCP.09m05402yel. [DOI] [PubMed] [Google Scholar]
- 7.Remington G. Schizophrenia, antipsychotics, and the metabolic syndrome: is there a silver lining? Am J Psychiatry. 2006;163:1132–1134. doi: 10.1176/ajp.2006.163.7.1132. [DOI] [PubMed] [Google Scholar]
- 8.Saha S, Chant D, McGrath J. A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry. 2007;64:1123–1131. doi: 10.1001/archpsyc.64.10.1123. [DOI] [PubMed] [Google Scholar]
- 9.Knol W, van Marum RJ, Jansen PA, Souverein PC, Schobben AF, Egberts AC. Antipsychotic drug use and risk of pneumonia in elderly people. J Am Geriatr Soc. 2008;56:661–666. doi: 10.1111/j.1532-5415.2007.01625.x. [DOI] [PubMed] [Google Scholar]
- 10.Trifiro G, Gambassi G, Sen EF, et al. Association of community-acquired pneumonia with antipsychotic drug use in elderly patients: a nested case-control study. Ann Intern Med. 2010;152:418–425. doi: 10.7326/0003-4819-152-7-201004060-00006. W139–440. [DOI] [PubMed] [Google Scholar]
- 11.U.S. Food and Drug Administration. Public Health Advisory: Deaths with Antipsychotics in Elderly Patients with Behavioural Disturbances. Silver Spring, MD: U.S. Food and Drug Administration; 2005. http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/DrugSafetyInformationforHeathcareProfessionals/PublicHealthAdvisories/ucm053171.htm. [Google Scholar]
- 12.U.S. Food and Drug Administration. FDA Requests Boxed Warnings on Older Class of Antipsychotic Drugs. Silver Spring, MD: U.S. Food and Drug Administration; 2008. http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/2008/ucm116912.htm. [Google Scholar]
- 13.Barnett MJ, Perry PJ, Alexander B, Kaboli PJ. Risk of mortality associated with antipsychotic and other neuropsychiatric drugs in pneumonia patients. J Clin Psychopharmacol. 2006;26:182–187. doi: 10.1097/01.jcp.0000203598.43314.34. [DOI] [PubMed] [Google Scholar]
- 14.Yang SY, Kao Yang YH, Chong MY, Yang YH, Chang WH, Lai CS. Risk of extrapyramidal syndrome in schizophrenic patients treated with antipsychotics: a population-based study. Clin Pharmacol Ther. 2007;81:586–594. doi: 10.1038/sj.clpt.6100069. [DOI] [PubMed] [Google Scholar]
- 15.Trifiro G. Antipsychotic drug use and community-acquired pneumonia. Curr Infect Dis Rep. 2011;13:262–268. doi: 10.1007/s11908-011-0175-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McElroy SL, Nelson EB, Welge JA, Kaehler L, Keck PE., Jr Olanzapine in the treatment of pathological gambling: a negative randomized placebo-controlled trial. J Clin Psychiatry. 2008;69:433–440. doi: 10.4088/jcp.v69n0314. [DOI] [PubMed] [Google Scholar]
- 17.Cordes J, Streit M, Loeffler S, von Wilmsdorff M, Agelink M, Klimke A. Reversible neutropenia during treatment with olanzapine: three case reports. World J Biol Psychiatry. 2004;5:230–234. doi: 10.1080/15622970410029938. [DOI] [PubMed] [Google Scholar]
- 18.Taylor DM, Douglas-Hall P, Olofinjana B, Whiskey E, Thomas A. Reasons for discontinuing clozapine: matched, case-control comparison with risperidone long-acting injection. Br J Psychiatry. 2009;194:165–167. doi: 10.1192/bjp.bp.108.051979. [DOI] [PubMed] [Google Scholar]
- 19.Chen YH, Lin HC. Poor Clinical Outcomes Among Pneumonia Patients With Schizophrenia. Schizophr Bull. 2011;37:1088–1194. doi: 10.1093/schbul/sbq019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weissman JS, Gatsonis C, Epstein AM. Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland. JAMA. 1992;268:2388–2394. [PubMed] [Google Scholar]
- 21.Wu CS, Wang SC, Cheng YC, Gau SS. Association of cerebrovascular events with antidepressant use: a case-crossover study. Am J Psychiatry. 2011;168:511–521. doi: 10.1176/appi.ajp.2010.10071064. [DOI] [PubMed] [Google Scholar]
- 22.Gau SS, Chao PF, Lin YJ, Chang CJ, Gau CS. The association between carbamazepine and valproate and adverse cutaneous drug reactions in patients with bipolar disorder: a nested matched case-control study. J Clin Psychopharmacol. 2008;28:509–517. doi: 10.1097/JCP.0b013e3181845610. [DOI] [PubMed] [Google Scholar]
- 23.Gau CS, Chang CJ, Tsai FJ, Chao PF, Gau SS. Association between mood stabilizers and hypothyroidism in patients with bipolar disorders: a nested, matched case-control study. Bipolar Disord. 2010;12:253–263. doi: 10.1111/j.1399-5618.2010.00814.x. [DOI] [PubMed] [Google Scholar]
- 24.Gau SS, Chung CH, Gau CS. A pharmacoeconomic analysis of atypical antipsychotics and haloperidol in first-episode schizophrenic patients in Taiwan. J Clin Psychopharmacol. 2008;28:271–278. doi: 10.1097/JCP.0b013e3181723713. [DOI] [PubMed] [Google Scholar]
- 25.WHO Collaborating Centre for Drug Statistic Methodology. ATC index with DDDs. Oslo, Norway: WHO; 2009. [Google Scholar]
- 26.Mantel-Teeuwisse AK, Klungel OH, Verschuren WM, Porsius A, de Boer A. Comparison of different methods to estimate prevalence of drug use by using pharmacy records. J Clin Epidemiol. 2001;54:1181–1186. doi: 10.1016/s0895-4356(01)00396-1. [DOI] [PubMed] [Google Scholar]
- 27.Quan H, Sundararajan V, Halfon P, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43:1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 28.Quail JM, Lix LM, Osman BA, Teare GF. Comparing comorbidity measures for predicting mortality and hospitalization in three population-based cohorts. BMC Health Serv Res. 2011;11:146. doi: 10.1186/1472-6963-11-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;79:516–524. [Google Scholar]
- 30.Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol. 2006;163:1149–1156. doi: 10.1093/aje/kwj149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alvir JM, Lieberman JA, Safferman AZ, Schwimmer JL, Schaaf JA. Clozapine-induced agranulocytosis. Incidence and risk factors in the United States. N Engl J Med. 1993;329:162–167. doi: 10.1056/NEJM199307153290303. [DOI] [PubMed] [Google Scholar]
- 32.Nielsen J, Foldager L, Meyer JM. Increased use of antibiotics in patients treated with clozapine. Eur Neuropsychopharmacol. 2009;19:483–486. doi: 10.1016/j.euroneuro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Maddalena AS, Fox M, Hofmann M, Hock C. Esophageal dysfunction on psychotropic medication. A case report and literature review. Pharmacopsychiatry. 2004;37:134–138. doi: 10.1055/s-2004-818993. [DOI] [PubMed] [Google Scholar]
- 34.Hinkes R, Quesada TV, Currier MB, Gonzalez-Blanco M. Aspiration pneumonia possibly secondary to clozapine-induced sialorrhea. J Clin Psychopharmacol. 1996;16:462–463. doi: 10.1097/00004714-199612000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Fauci AS, Braunwald E, Kasper DL, et al. Harrison's Principles of Internal Medicine. 17th ed. New York, NY: McGraw-Hill Professional; 2008. [Google Scholar]
- 36.Kane J, Honigfeld G, Singer J, Meltzer H. Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry. 1988;45:789–796. doi: 10.1001/archpsyc.1988.01800330013001. [DOI] [PubMed] [Google Scholar]
- 37.Kane JM, Correll CU. Past and present progress in the pharmacologic treatment of schizophrenia. J Clin Psychiatry. 2010;71:1115–1124. doi: 10.4088/JCP.10r06264yel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boyer P, Lecrubier Y, Stalla-Bourdillon A, Fleurot O. Amisulpride versus amineptine and placebo for the treatment of dysthymia. Neuropsychobiology. 1999;39:25–32. doi: 10.1159/000026556. [DOI] [PubMed] [Google Scholar]
- 39.Smeraldi E. Amisulpride versus fluoxetine in patients with dysthymia or major depression in partial remission: a double-blind, comparative study. J Affect Disord. 1998;48:47–56. doi: 10.1016/s0165-0327(97)00139-0. [DOI] [PubMed] [Google Scholar]
- 40.Almirall J, Gonzalez CA, Balanzo X, Bolibar I. Proportion of community-acquired pneumonia cases attributable to tobacco smoking. Chest. 1999;116:375–379. doi: 10.1378/chest.116.2.375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.