Abstract
In recently completed Japanese genome-wide association studies (GWAS) of schizophrenia (JPN_GWAS) one of the top association signals was detected in the region of VAV3, a gene that maps to the chromosome 1p13.3. In order to complement JPN_GWAS findings, we tested the association of rs1410403 with brain structure in healthy individuals and schizophrenic patients and performed exon resequencing of VAV3. We performed voxel-based morphometry (VBM) and mutation screening of VAV3. Four independent samples were used in the present study: (1) for VBM analysis, we used case-control sample comprising 100 patients with schizophrenia and 264 healthy controls, (2) mutation analysis was performed on a total of 321 patients suffering from schizophrenia, and 2 case-control samples (3) 729 unrelated patients with schizophrenia and 564 healthy comparison subjects, and (4) sample comprising 1511 cases and 1517 healthy comparison subjects and were used for genetic association analysis of novel coding variants with schizophrenia. The VBM analysis suggests that rs1410403 might affect the volume of the left superior and middle temporal gyri (P = .011 and P = .013, respectively), which were reduced in patients with schizophrenia compared with healthy subjects. Moreover, 4 rare novel missense variants were detected. The mutations were followed-up in large independent sample, and one of the novel variants (Glu741Gly) was associated with schizophrenia (P = .02). These findings demonstrate that VAV3 can be seen as novel candidate gene for schizophrenia in which both rare and common variants may be related to increased genetic risk for schizophrenia in Japanese population.
Keywords: resequencing, MRI, Japanese population, axon guidance, rare variant, GWAS
Introduction
Schizophrenia is a severe mental disorder with a lifetime risk of about 1%, characterized by hallucinations, delusions, and cognitive deficits, with heritability estimated at up to 80%. Recently, there have been a few major advances in identifying common variants associated with schizophrenia by genome-wide association studies (GWAS). The GWAS approach has both highlighted genes previously identified by the candidate gene approach studies or by basic biological investigation and illuminated novel genomic loci clearly associated with schizophrenia that were previously unsuspected.1–3
In recently completed Japanese GWAS of schizophrenia (JPN_GWAS),4 one of the top association signals based on the meta-analysis for Japanese sample (rs1410403, P CMH = 9.3 × 10−4, OR = 0.86) was detected in the region of VAV3 (see online supplementary table 1). VAV3 is closely related to the axon guidance pathways, which are implicated in etiology of schizophrenia.5 During axon guidance, ephrin binding to Ephs triggers VAV-dependent endocytosis of the ligand-receptor complex, converting an initially adhesive interaction into a repulsive event. In the absence of VAVs, ephrin-Eph endocytosis is blocked, leading to defects in growth cone collapse in vitro and significant defects in the ipsilateral retinogeniculate projections in vivo.6 Therefore, VAV family guanine nucleotide exchange factors (GEFs) may play an important role as regulators of ligand-receptor endocytosis and determinants of repulsive signaling during axon guidance. The additional findings implicating the relevance of this locus for pathogenesis of schizophrenia came from genomewide linkage analysis of 236 Japanese families.7 Specifically, they located the strongest evidence of linkage at rs2048839 (LOD [logarithm (base 10) of odds] = 3.39) and 95% CI comprises VAV3 locus (Chr1: 102.0–111.9 Mbp, based on NCBI36 annotation).
Based on genetic evidence from the JPN_GWAS, the meta-analysis for Japanese sample, biological studies, and linkage evidence VAV3 can be seen as novel candidate gene for schizophrenia. Therefore, to follow-up JPN_GWAS findings, we tested the association of rs1410403 with brain structure in healthy individuals and schizophrenic patients. Because biological phenotypes (eg, brain structure and function) are thought to more closely reflect the effects of genetic variation as compared with manifest psychiatric illness, endophenotype studies have proven to be more robust and require vastly smaller sample sizes than purely diagnosis-based studies.8 Furthermore, statistical genetic association studies can provide a link between genes and complex polygenetic constructs like schizophrenia, but this approach does not illuminate the possible underlying pathophysiology impacted or the mechanisms of association. Here, we used imaging approach to examine the impact of variation in VAV3 on risk for schizophrenia and function and structure in human brain of neural circuitries implicated in the pathophysiology of schizophrenia. Furthermore, in terms of genetic architecture, liability to schizophrenia is related to the number of loci involved and the effect size of each risk variant, and on the population level, these 2 factors combine to form an “allelic spectrum” which is bounded by “common disease/common variant” and “multiple rare variant” models.9 Based on the results of recent schizophrenia GWAS, it was suggested that common variants can explain at least one-third of the total variation in liability, and genetic transmission patterns in schizophrenia may be a complex hybrid of common, low-penetrant alleles and rare, highly penetrant variants.1 Therefore, in order to complement JPN_GWAS findings and search for novel rare variants with larger effect, we performed exon resequencing of VAV3.
Methods
Sample
Four independent samples were used in the present study: (1) for the voxel-based morphometry (VBM) analysis, we used case-control sample comprising 100 patients with schizophrenia (38.3 ± 13.0 y) and 264 healthy comparison subjects (36.7 ± 11.9 y), (2) mutation analysis was performed on a total of 321 patients suffering from schizophrenia (54.3 ± 14.1 y) (JMut sample) (3) JPN_GWAS comprised of 729 unrelated patients with schizophrenia (45.4 ± 15.1 y) and 564 healthy comparison subjects (44.0 ± 14.4 y) and (4) Rep_JPN comprising 1511 cases (45.9 ± 14.0 y) and 1517 healthy comparison subjects (46.0 ± 14.6 y). JPN_GWAS and Rep_JPN were used for genetic association analysis of novel coding variants with schizophrenia. The individuals with personal or family history of psychiatric disorders (first-degree relatives only based on the subject’s interview) were not included in the healthy comparison group. After complete description of the study to the subjects, written informed consent was obtained. A general characterization and psychiatric assessment of subjects are available elsewhere.10
Voxel-Based Morphometry
All magnetic resonance (MR) studies were performed on a 1.5T GE Sigma EXCITE system. A 3-dimensional volumetric acquisition of a T1-weighted gradient echo sequence produced a gapless series of 124 sagittal sections using a spoiled gradient recalled acquisition in the steady state sequence (TE [Eecho time]/TR [repetition time], 4.2/12.6 ms; flip angle, 15°; acquisition matrix, 256 × 256; 1NEX [number of excitations], FOV [Field of view], 24 × 24 cm; and slice thickness, 1.4 mm). Statistical analyses were performed with Statistical Parametric Mapping 5 (SPM5) software (http//www.fil.ion.ucl.ac.uk/spm) running on MATLAB R2007a (MathWorks, Natick, MA). MR images were processed using optimized VBM in SPM5 according to VBM5.1-Manual (http://dbm.neuro.uni-jena.de/vbm/vbm5-for-spm5/manual/) as described in detail previously.11,12 Each image was confirmed to eliminate images with artifacts and then anterior commissure-posterior commissure line was adjusted. The normalized segmented images were modulated by multiplication with Jacobian determinants of the spatial normalization function to encode the deformation field for each subject as tissue density changes in the normal space. Finally, images were smoothed with a 12-mm full-width half-maximum of isotropic Gaussian kernel.
In first stage of the analysis, we performed whole brain search to explore the effects of diagnosis, genotype, and their interaction on gray matter (GM) volume in patients with schizophrenia and controls. These effects on GM volume were assessed statistically using the full factorial model for a 2 × 2 ANOVA in SPM5. We contrasted GM volumes between the genotype groups (individuals with A/A genotype and G-carriers), the diagnosis groups (smaller volume region in patients with schizophrenia relative to controls), and the diagnosis-genotype interaction. Age, sex, and education years were included to control for confounding in all analyses. Because it is desirable to adjust for each subject’s global GM volume,11 adjustment was performed by entering the global GM values as a covariate. Nonsphericity estimation was used. These analyses yielded statistical parametric maps (SPM (t)) based on a voxel-level height threshold of P < .001 (uncorrected for multiple comparisons). Only the clusters of more than 100 contiguous voxels were considered in the analyses. Additionally, small volume correction (SVC) was applied in order to protect against type I error using family wise error (FWE). The significance level was set P < 0.05 (FWE corrected) after SVC, spheres with radius 10 mm around the peak.
In second stage of the analysis, we extracted a sphere of 10 mm volume of interest (VOI)-radius on left superior temporal gyrus and left middle temporal gyrus to compare regions of the genotype effects. Anatomic localization was according to both MNI coordinates and Talairach coordinates, obtained from M. Brett’s transformations (http://www.mrccbu.cam.ac.uk/Imaging/Common/mnispace.shtml) and presented as Talairach coordinates.
Statistical analyses were performed using PASW Statistics 18.0 software (SPSS Japan Inc., Tokyo, Japan). Differences in clinical characteristics between patients and controls or between genotypes were analyzed using χ2 tests for categorical variables and the Mann-Whitney U test for continuous variables. We extracted the “y” values from the left superior temporal gyrus and left middle temporal gyrus maxima voxel and used these values in the VOI analysis using PASW. The effects of the variant in VAV3 on extracted VOI were tested using the 2-way ANOVA without covariates because the extraction of VOI was performed after confounding factors, including age, sex, education years, and total GM volumes, were included in the whole-brain search analyses. Statistical significance was defined as P < .05.
Mutation Screening
For the purpose of mutation screening, we have designed a custom resequencing microarray, based on NCBI36 build (Affymetrix, Santa Clara, CA), NAGOYA_DESIGN, which primarily focuses on the genes selected, based on the JPN_GWAS findings. The sequences tiled on the microarray included the sequences of all VAV3 exons totaling 4933 bps (consensus CDS transcripts ENST00000370056 and ENST00000343258). Because the principle of the resequencing microarray is based on sequencing by hybridization, it was crucially important to avoid cross-hybridization to increase the accuracy of resequencing. For this purpose, we conducted an in-silico screening to compare the tiled sequences with a sliding 25-nucleotide window to detect the sequences with an identity exceeding 22 bases in the tiled sequences and optimized the design of the microarrays and polymerase chain reaction (PCR) primers. Initially, the arrays were run according to the manufacturer’s protocol. Briefly, long-range PCR conditions for the LA TaKaRa Polymerase (Takara, Japan) were: TaKaRa LA Taq 0.05 U/μl, 1X LA PCR Buffer II, 400 μM (each) deoxynucleotide triphosphate, 0.3 μM (each) primers, 4 ng/μl genomic DNA in a 25 μl reaction volume. Modifications using standard approaches to PCR optimization were made for some difficult reactions. All PCR assays were quantified using PicoGreen (Molecular Probes, Eugene, OR) and then pooled in equimolar amounts. The PCR products were then purified, fragmented, labeled, and hybridized to the array. Finally, affymetrix GSEQ 4.0 Software (default settings) was used to process raw data and analyze the nucleotide sequences. SeqC Ver. 3.2.1.5 (JSI-medisys, www.jsi-medisys.de) was used to reanalyze the acquired datasets and assign annotation (based on NCBI 36 build). Novel variants with frequency of less than 5% were validated by cycle sequencing on ABI 3130xl DNA Analyzer (Applied Biosystems) according to standard manufacturer protocol. Allelic discrimination was performed using Taqman (Applied Biosystems) custom probes (details about DNA sequences, and PCR conditions are available upon request). Each 384 microtiter plate contained at least 3 nontemplate controls and the sample(s) in which novel variant was observed. Analysis was performed on HT7500 instrument (Applied Biosystems) according to the standard protocol. In-silico analysis of deleterious effect of amino acid substitution was done by algorithms implemented in LRT,13 PMUT,14 and PANTHER.15 All these tools operate using approximately the same principles, that is, they are all supervised and employ features based on protein sequence, sequence conservation, and/or protein structure. The interpretation of the results was done based on score of the likelihood that missense variants, which cause a single amino acid substitution within a protein sequence, may or may not lead to altered protein function.
As basis for a more detailed functional interpretation of the novel rare variants, we performed ab-initio structure predictions based on I-TASSER algorithm.16 This automated pipeline predicts secondary and tertiary protein structure based on sequence homology between protein/domain of interest and the proteins/domains with experimentally determined structures. The output of I-TASSER is analyzed and visualized using UCSF Chimera.17
All allele-wise association analyses were carried out by calculating the P values for each SNP using Fisher’s exact test. In meta-analysis (JPN_GWAS and Rep_JPN sample), P values were generated by Cochran-Mantel-Haenszel stratified analysis. Two-tailed P values of less than 0.05 were considered significant. Calculations were done using Plink v1.07.18
Results
Voxel-Based Morphometry
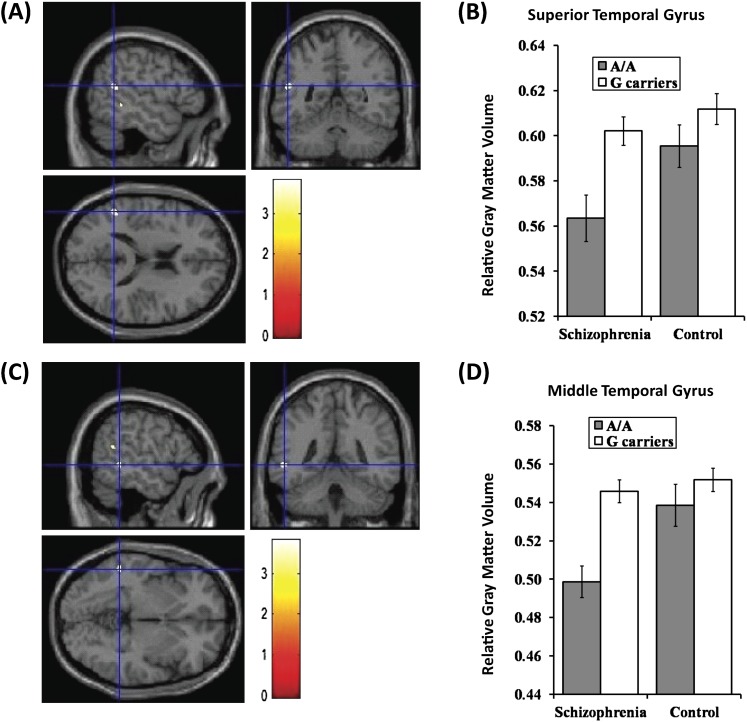
We investigated effects of diagnosis, genotype, and their interaction on GM volumes in the whole brain analyses. There was no difference in demographic variables between VAV3 genotype groups, except for scores of positive symptom between the genotype groups in patients with schizophrenia and sex between the genotype groups in healthy controls (table 1). Patients with schizophrenia showed reduced GM volumes compared with controls mainly in the frontal lobe and temporal lobe (data not shown), consistent with previous studies.19,20 Genotype effects on GM volume in several brain regions were found in all subjects (uncorrected P < .001, table 2). Individuals with A/A genotype had reduced GM volumes of left superior and middle temporal gyri than G-carriers, while individuals with A/A genotype had larger GM volumes of cerebellum anterior lobe (culmen) and right medial frontal gyrus than G-carriers (uncorrected P < .001, table 2). Additionally, we found significant diagnosis-genotype interaction of GM volume in the right medial frontal gyrus (uncorrected P < .001, table 2). These results remained positive after SVC for multiple tests (FWE corrected P < .05 after SVC).
Table 1.
Demographic Information for Patients With Schizophrenia and Healthy Controls Included in the VBM Analysis
| Variablesa | Schizophrenia (N = 100) | Control (N = 264) | Group Difference | ||||||||
| A/A | G-carrier | A/A | G-carrier | P values (z )b | |||||||
| (N = 52) | (N = 48) | P values (z )b | (N = 131) | (N = 133) | P values (z )b | ||||||
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | ||||
| Age (years) | 37.7 | 12.5 | 39 | 13.5 | 0.91 (−0.12) | 36.2 | 11.9 | 37.2 | 11.3 | 0.35 (−0.93) | 0.37 (−0.89) |
| Sex (male/female) | 25/27 | 28/20 | 0.30 (1.05) | 51/80 | 69/64 | 0.035 (4.46) | 0.20 (1.66) | ||||
| Education (years) | 14.1 | 2.5 | 13.7 | 2.2 | 0.38 (−0.87) | 14.9 | 2.1 | 15.3 | 2.4 | 0.21 (−1.26) | <0.001 (− 4.13) |
| Estimated premorbid IQ | 102.7 | 10.8 | 99.9 | 9.2 | 0.23 (−1.20) | 107.4 | 8.6 | 106.9 | 7.6 | 0.58 (−0.55) | <0.001 (− 5.07) |
| Gray matter volume (mm3) | 666.2 | 73.1 | 688 | 82.6 | 0.23 (−1.20) | 702.4 | 71.7 | 706.2 | 81.3 | 0.79 (−0.26) | 0.003 (− 2.96) |
| CPZ-eq (mg/day)c | 584.4 | 510.2 | 639.5 | 610.7 | 0.75 (−0.32) | — | — | — | — | — | — |
| Age at onset (years) | 25.5 | 10.6 | 25.1 | 10.9 | 0.91 (−0.11) | — | — | — | — | — | — |
| Duration of illness (years) | 12.2 | 10 | 13.9 | 10.7 | 0.37 (−0.90) | — | — | — | — | — | — |
| PANSS positive symptoms | 20.1 | 5.7 | 17.1 | 5.9 | 0.009 (− 2.61) | — | — | — | — | — | — |
| PANSS negative symptoms | 19.8 | 6.4 | 18.3 | 6.2 | 0.13 (−1.50) | — | — | — | — | — | — |
Note: PANSS, Positive and Negative Syndrome Scale.
Some demographic information was obtained in part of subjects (estimated premorbid IQ and PANSS in patients: A/A, N = 49; G-carriers, N = 46, Estimated premorbid IQ in controls: A/A, N = 130).
Significant results are bolded and underlined.
CPZ-eq: chlorpromazine equivalent of total antipsychotics.
Table 2.
Effects of the VAV3 Genotype on Brain Morphology in All Subjects
| Brain regions | R/L | BA | Cluster Size | T 356 | P valuesa | Talairach Coordinates | ||
| x | y | z | ||||||
| A/A < G-carriers | ||||||||
| Superior temporal gyrus | L | 13 | 194 | 3.8 | 0.011 | −50 | −48 | 18 |
| Middle temporal gyrus | L | 22 | 124 | 3.8 | 0.013 | −53 | −42 | 0 |
| A/A > G-carriers | ||||||||
| Cerebellum anterior lobe (culmen) | R | NA | 1533 | 4.1 | 0.004 | 7 | −40 | −12 |
| Medial frontal gyrus | R | 25 | 286 | 3.8 | 0.012 | 6 | 6 | −17 |
| VAV3 genotype × diagnosis interaction | ||||||||
| Medial frontal gyrus | R | 25 | 271 | 3.9 | 0.009 | 6 | 7 | −18 |
Note: R, right; L, left; BA, brodmann area.
Significant results (P < .05 [FWE corrected]) are indicated with bold and underline.
Systematic searches for VBM studies have reported that smaller volumes of the left temporal gyrus were found in patients with schizophrenia than those in healthy subjects. Based on the JPN_GWAS data, individuals with A/A genotype of the rs1410403 were enriched in patients with schizophrenia. Therefore, we focused on the individuals with A/A genotype of the rs1410403 in VAV3 as they may have a reduction of GM in the left superior temporal and left middle temporal gyri. Two-way ANOVA revealed significant effects of diagnosis (F 1,360 = 5.77, η2 = 0.016, P = .017) and genotype (F 1,360 = 10.04, η2 = 0.027, P = .0017) in the extracted region centering the left superior temporal gyrus (−50, −48, 18) (figure 1A and B). No interaction was found in the left superior temporal gyrus (F 1,360 = 1.65, η2 = 0.0046, P = .20). Individuals with homozygous A had smaller GM volumes of the left superior temporal gyrus than G-carriers. We also found significant effects of diagnosis (F 1,360 = 8.03, η2 = 0.022, P = .0049) and genotype (F 1,360 = 14.04, η2 = 0.038, P < .001) and their interaction (F 1,360 = 4.40, η2 = 0.012, P = .037) in the extracted region centering the left middle temporal gyrus (−53, −42, 0) (figure 1C and D). As the genotype-diagnosis interaction was found in the left middle temporal gyrus, we analyzed the effects of genotype on the region in patients and controls separately. Patients with schizophrenia showed that patients with A/A genotype had smaller GM volumes of the region than G-carriers (F 1,98 = 12.00, η2 = 0.11, P < .001). In contrast, controls showed no genotype effect on GM volumes of the region (F 1,262 = 2.46, η2 = 0.0093, P = .12).
Fig. 1.
Voxel-based morphometry. Impacts of VAV3 genotype on gray matter (GM) volumes of left superior temporal gyrus and left middle temporal gyrus. (A and C) Anatomical localizations were displayed on coronal, sagittal, and axial sections of a normal magnetic resonance imaging spatially normalized into the Montreal Neurological Institute template (uncorrected P < .001, cluster size >100). Significant clusters of genotype effect were in the left superior temporal gyrus (Talairach cordinate; −50, −48, 18) (A) and in the left middle temporal gyrus (−53, −42, 0) (C). These regions were showed as cross-hairline. The color bars showed t values corresponding to color in the figure. (B and D) Each column showed relative GM volumes extracted from left superior temporal gyrus (−50, −48, 18) (B) and left middle temporal gyrus (−53, −42, 0) (D). Data represent means ± SEM.
Mutation Screening
We detected 4 novel nonsynonymous heterozygous variants within the JMut sample (321 schizophrenic patients). Protein homology analysis showed that VAV3 is highly conserved between species (∼95% identical amino acids between human and mouse), accordingly the identified point mutations affected conserved residues (see online supplementary table 2). All the variants detected were located in the C-terminal region of VAV3 (see online supplementary figure 1).
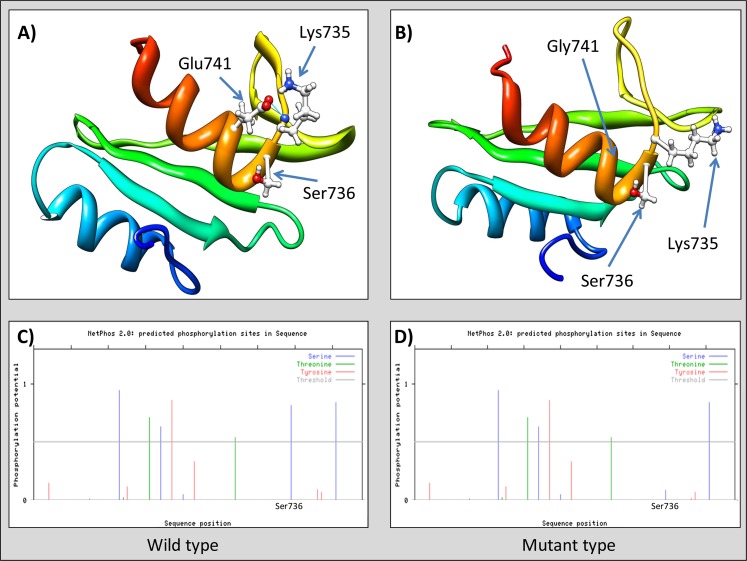
The identified novel variants were reconfirmed by cycle sequencing and followed-up in 2 large independent schizophrenia case-control samples (Rep_JPN and JPN_GWAS sample). Only one rare variant (Glu741Gly) showed statistical evidence for association in meta-analysis (P CMH = .020, OR = 0.58), while the others were observed at similar frequencies both in case and control samples (table 3). In-silico analysis of the missense variants applying 3 different algorithms predicted Glu741Gly as variant of functional relevance (table 4). Detailed 3-dimensional structural analysis of SH2 domain (wild type—figure 2a) indicated specific interaction (hydrogen bond) between Glu741 (side chain) and Lys735 (main chain). Point mutation at position 741 (Glu→Gly) would abolish hydrogen bonding because glycine does not contain a side chain (figure 2b). Moreover, beta strand extending into Lys735 is lost in the model of VAV3 mutant. The functional consequence of the associated point mutation is disappearance of casein kinase 2 phosphorylation site (SXXE)21 as shown on figure 2c and d.
Table 3.
Resequencing Results
| Chr | Variant | Physical Positiona | Protein Domain | M | JMut (Minor Allele Count) | m | JPN_GWAS (MAF) | Rep_JPN (MAF) | Meta-Analysis | |||||||
| Cases | Control | P allele | OR | Cases | Control | P allele | OR | P CMH | OR | |||||||
| 1 | p.Asp623Val | 107.986.810 | N-SH3 | A | 2 | T | 0.0006964 | 0.0008993 | 0.8561 | 0.7742 | 0.0003344 | 0 | 0.3171 | NA | 0.6649 | 1.662 |
| 1 | p.Glu685Lys | 107.947.271 | SH2 | G | 1 | A | 0.0006974 | 0.001821 | 0.4151 | 0.3824 | 0.0003336 | 0 | 0.3168 | NA | 0.8415 | 0.8246 |
| 1 | p.Glu705Lys | 107.947.211 | SH2 | G | 3 | A | 0.0007022 | 0.0009074 | 0.8557 | 0.7737 | 0.0006658 | 0.0003311 | 0.5605 | 2.011 | 0.7354 | 1.355 |
| 1 | P.Glu741Gly | 107.940.485 | SH2 | A | 7 | G | 0.004972 | 0.01087 | 0.09038 | 0.4547 | 0.0074480 | 0.0117400 | 0.0897 | 0.6314 | 0.02065 | 0.5821 |
Note: M, major allele; m, minor allele; MAF, minor allele frequency; OR, odds ratio; P CMH, Cochran-Mantel-Hentzel test.
NCBI 36 build.
Table 4.
In-Silico Analysis
| Variant | Protein Domain | Genomic Data | Impact on Protein Structure/Function | ||||
| Physical Positiona | Strand | Alleles M/m | PMUT (Prediction Score)b | Panther (subPSEC Score)c | LRT (P value)d | ||
| p.Asp623Val | N-SH3 | Chr1: 107986810 | −1 | A/T | Yes (0.97) | Yes (−3.99) | Yes (4.29 × 10−8) |
| p.Glu685Lys | SH2 | Chr1: 107947271 | −1 | G/A | Yes (0.93) | No (−2.04) | Yes (7.08 × 10−10) |
| p.Glu705Lys | SH2 | Chr1: 107947211 | −1 | G/A | Yes (0.75) | No (−1.74) | Yes (1.83 × 10−7) |
| p.Glu741Gly | SH2 | Chr1: 107940485 | −1 | A/G | Yes (0.89) | Yes (−3.24) | Yes (1.64 × 10−6) |
Note: M/m, major/minor allele.
NCBI36 build.
>0.5 is interpreted as nonneutral substitution.
Less than −3 is interpreted as nonneutral substitution.
<0.001 interpreted as nonneutral substitution.
Fig. 2.
Ab-initio 3D modeling of SH2 domain (VAV3). (a) Wild type, note hydrogen bond between Glu741 and Lys735 (blue line); (b) Mutant type, hydrogen bond between Gly741 and Lys735 cannot be formed; (c) and (d) posphorylation potential of serine (blue), threonine (green), and tyrosine (red). Threshold is marked by horizontal gray line (phosphorylation analysis performed by NetPhos 2.0).
Discussion
Our study reports the systematic genetic evaluation of VAV3, as a candidate gene for schizophrenia based on our genome wide screening.4 Specifically, meta-analysis of the JPN_GWAS and follow-up sample provided genetic evidence for the involvement of VAV3 locus in schizophrenia in the Japanese population. Mutation screening of all VAV3 coding exons did not reveal evidence for the existence of a common (minor allele frequency >5%) nonsynonymous variant that explains the association signal in JPN_GWAS.
The VBM analysis showed that the associated common SNP (rs1410403) might affect the volume of the left superior and middle temporal gyri, which were reduced in patients with schizophrenia compared with healthy subjects. VBM analysis suggests that VAV3’s influence is focal to the aforementioned regions. Furthermore, the Allen Brain Atlas (http://human.brain-map.org) records relatively high levels of expression of the human VAV3 gene and lower expression levels of other VAV family GEFs (VAV1 or VAV2) in left superior and middle temporal gyri. Considering VAV3 biological function (developmental processes in particular), we speculate that our macroscopic observation using VBM approach is result of neuronal distribution and differential activity of VAV3 protein associated with rs1410403 genotypes.
Systematic searches for VBM studies have reported that reduced volumes of the left temporal gyrus were found in patients with schizophrenia.19 Furthermore, the data from previous study of the superior temporal gyrus22 and a study of the middle temporal gyrus in which patients with schizophrenia who were predisposed to auditory hallucinations showed reduced activation of the left middle temporal gyrus when imagining sentences in another person’s voice.23 Several other functional magnetic resonance imaging studies have reported decreased left and increased right middle temporal gyrus activation in schizophrenia during auditory verbal hallucinations.24,25 In our exploratory analysis (see online supplementary material), positive symptoms scores of Positive and Negative Syndrome Scale (PANSS) were not able to predict the VOIs in left superior and left middle temporal gyri (P > .05). It is of note that VBM sample size in the current study might not be sufficient to detect the effect of positive symptoms score of PANSS on VOIs in left superior and left middle temporal gyri because positive symptoms score of PANSS is derived from symptomatology that is characterized by excess or distortion of the individual’s normal functioning and therefore may not reflect only auditory hallucinations.
We detected several rare variants close to original association signal and in case of one rare variant (Glu741Gly), we observed nonsignificant association trend. When the evidence was combined across the 2 samples (JPN_GWAS and Rep_JPN sample), we found that the P values had strengthen. VAV3 is composed of 8 domains: calponin homology (CH), Acidic (Ac), Dbl homology (DH), pleckstrin homology (PH), zinc finger (ZF), Src homology 3 (SH3), Src homology 2 (SH2), and a second SH3 (see online supplementary figure 1).26 Interestingly, the associated common variant (rs1410403) is located in LD block that encompass coding exons of SH2 domain of vav3 (see online supplementary figure 2), and the associated rare variant (Glu741Gly) is located within the exon that is translated into the SH2 domain of vav3 (see online supplementary figure 1).
VAV family GEFs have been implicated as regulators of Eph receptor endocytosis, the event which is required for efficient cell detachment.27 This can provide a complex and dynamic set of cues that either repel or attract axons toward their synaptic targets, converting initially adhesive interaction into a repulsive force. Several studies had shown that SH2 domain of the VAV3 binds to phosphorylated tyrosine residue(s) on the EphA receptors,6,28 which triggers endocytosis of EphA receptors and growth cone collapse.27 Although the mechanisms by which Glu741Gly contributes to schizophrenia pathogenesis remain to be explored, we note that 3 different bioinformatics algorithms had predicted functional effect, and Glu741Gly have stronger protective effects on schizophrenia risk (OR = 0.58) than does the associated common SNP (OR = 0.81). Moreover, analysis of phosphorylation sites showed that point mutation at position 741 (Glu→Gly) would abolish phosphorylation of Ser736 by casein kinase II. The substitution of glutamic acid by Glycine at position 741 might have as a consequence alteration of the biological function of VAV3 because a substrate with n phosphorylation sites has an exponential number (2n) of phosphoforms and each phosphoform may have distinct properties.29 Our genetic association results suggested that the rare variant, which is predicted to alter function of the VAV3, would decrease the risk of schizophrenia, whereas normal function is associated with schizophrenia. Same protective allelic effect was observed for common variant identified by JPN_GWAS.
As the conclusion, our results showed that in case of schizophrenia, the “rare high-risk variant” vs the “common variant with low effect” hypotheses should not be viewed as 2 mutually exclusive hypotheses. Therefore, direct resequencing of candidate genes and copy number variants on the one side and GWAS analyses on the other side could be viewed as complementary approaches to analyze the genetic susceptibilities to schizophrenia.
Funding
Funding for this study was provided by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Ministry of Health, Labor and Welfare of Japan; Grant-in-Aid for “Integrated research on neuropsychiatric disorders” carried out under the Strategic Research Program for Brain Sciences by the Ministry of Education, Culture, Sports, Science and Technology of Japan; Grant-in-Aid for Scientific Research on Innovative Areas (Comprehensive Brain Science Network) from the Ministry of Education, Science, Sports and Culture of Japan; The Academic Frontier Project for Private Universities, Comparative Cognitive Science Institutes, Meijo University; the Core Research for Evolutional Science and Technology and SENSHIN Medical Research.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors have no conflicts to declare.
References
- 1.Purcell SM, Wray NR, Stone JL, et al. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi J, Levinson DF, Duan J, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stefansson H, Ophoff RA, Steinberg S, et al. Common variants conferring risk of schizophrenia. Nature. 2009;460:744–747. doi: 10.1038/nature08186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ikeda M, Aleksic B, Kinoshita Y, et al. Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry. 2011;69:472–478. doi: 10.1016/j.biopsych.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 5.Yaron A, Zheng B. Navigating their way to the clinic: emerging roles for axon guidance molecules in neurological disorders and injury. Dev Neurobiol. 2007;67:1216–1231. doi: 10.1002/dneu.20512. [DOI] [PubMed] [Google Scholar]
- 6.Cowan CW, Shao YR, Sahin M, et al. Vav family GEFs link activated Ephs to endocytosis and axon guidance. Neuron. 2005;46:205–217. doi: 10.1016/j.neuron.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 7.Arinami T, Ohtsuki T, Ishiguro H, et al. Genomewide high-density SNP linkage analysis of 236 Japanese families supports the existence of schizophrenia susceptibility loci on chromosomes 1p, 14q, and 20p. Am J Hum Genet. 2005;77:937–944. doi: 10.1086/498122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Glahn DC, Thompson PM, Blangero J. Neuroimaging endophenotypes: strategies for finding genes influencing brain structure and function. Hum Brain Mapp. 2007;28:488–501. doi: 10.1002/hbm.20401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Manolio TA, Collins FS, Cox NJ, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ikeda M, Aleksic B, Kirov G, et al. Copy number variation in schizophrenia in the Japanese population. Biol Psychiatry. 2010;67:283–286. doi: 10.1016/j.biopsych.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Good CD, Johnsrude IS, Ashburner J, et al. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- 12.Ashburner J, Friston KJ. Voxel-based morphometry–the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 13.Chun S, Fay JC. Identification of deleterious mutations within three human genomes. Genome Res. 2009;19:1553–1561. doi: 10.1101/gr.092619.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferrer-Costa C, Gelpi JL, Zamakola L, et al. PMUT: a web-based tool for the annotation of pathological mutations on proteins. Bioinformatics. 2005;21:3176–3178. doi: 10.1093/bioinformatics/bti486. [DOI] [PubMed] [Google Scholar]
- 15.Thomas PD, Kejariwal A. Coding single-nucleotide polymorphisms associated with complex vs. Mendelian disease: evolutionary evidence for differences in molecular effects. Proc Natl Acad Sci U S A. 2004;101:15398–15403. doi: 10.1073/pnas.0404380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roy A, Kucukural A, Zhang Y. I-TASSER: a unified platform for automated protein structure and function prediction. Nat Protoc. 2010;5:725–738. doi: 10.1038/nprot.2010.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pettersen EF, Goddard TD, Huang CC, et al. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 18.Purcell S, Neale B, Todd-Brown K, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chan RC, Di X, McAlonan GM, Gong QY. Brain anatomical abnormalities in high-risk individuals, first-episode, and chronic schizophrenia: an activation likelihood estimation meta-analysis of illness progression. Schizophr Bull. 2011;37:177–188. doi: 10.1093/schbul/sbp073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ellison-Wright I, Bullmore E. Anatomy of bipolar disorder and schizophrenia: a meta-analysis. Schizophr Res. 2010;117:1–12. doi: 10.1016/j.schres.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 21.Aroor AR, Denslow ND, Singh LP, O'Brien TW, Wahba AJ. Phosphorylation of rabbit reticulocyte guanine nucleotide exchange factor in vivo. Identification of putative casein kinase II phosphorylation sites. Biochemistry. 1994;33:3350–3357. doi: 10.1021/bi00177a028. [DOI] [PubMed] [Google Scholar]
- 22.Barta PE, Pearlson GD, Powers RE, Richards SS, Tune LE. Auditory hallucinations and smaller superior temporal gyral volume in schizophrenia. Am J Psychiatry. 1990;147:1457–1462. doi: 10.1176/ajp.147.11.1457. [DOI] [PubMed] [Google Scholar]
- 23.McGuire PK, Silbersweig DA, Wright I, et al. Abnormal monitoring of inner speech: a physiological basis for auditory hallucinations. Lancet. 1995;346:596–600. doi: 10.1016/s0140-6736(95)91435-8. [DOI] [PubMed] [Google Scholar]
- 24.Woodruff P, Brammer M, Mellers J, et al. Auditory hallucinations and perception of external speech. Lancet. 1995;346:1035. doi: 10.1016/s0140-6736(95)91715-2. [DOI] [PubMed] [Google Scholar]
- 25.Lennox BR, Park SB, Medley I, Morris PG, Jones PB. The functional anatomy of auditory hallucinations in schizophrenia. Psychiatry Res. 2000;100:13–20. doi: 10.1016/s0925-4927(00)00068-8. [DOI] [PubMed] [Google Scholar]
- 26.Zugaza JL, Lopez-Lago MA, Caloca MJ, et al. Structural determinants for the biological activity of Vav proteins. J Biol Chem. 2002;277:45377–45392. doi: 10.1074/jbc.M208039200. [DOI] [PubMed] [Google Scholar]
- 27.Bashaw GJ, Klein R. Signaling from axon guidance receptors. Cold Spring Harb Perspect Biol. 2010;2:a001941. doi: 10.1101/cshperspect.a001941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hunter SG, Zhuang G, Brantley-Sieders D, et al. Essential role of Vav family guanine nucleotide exchange factors in EphA receptor-mediated angiogenesis. Mol Cell Biol. 2006;26:4830–4842. doi: 10.1128/MCB.02215-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thomson M, Gunawardena J. Unlimited multistability in multisite phosphorylation systems. Nature. 2009;460:274–277. doi: 10.1038/nature08102. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.