Abstract
The objective of this study is to compare the effectiveness among sulpiride, risperidone, olanzapine, and haloperidol by evaluating the persistence of drug use. A retrospective cohort study was conducted by analyzing the National Health Insurance Research Database of Taiwan. Patients with schizophrenia aged 18–65 years and newly prescribed with a single oral antipsychotic medication between years 2003 and 2008 were included. The primary outcome was the persistence of antipsychotic agents by calculating the treatment duration till treatment changed. All defined treatment changes were also analyzed separately, including discontinuation, switching, augmentation, and hospitalization. A total of 1324 eligible patients were included, with an average age of 36 years old and approximately 45% of them were female. The most prevalent antipsychotic use was risperidone (42.1%), followed by sulpiride (36.0%), haloperidol (14.2%), and olanzapine (7.7%). After adjusting for patient demographics, mental illness characteristics, and propensity score, the Cox regression models found that the risk of nonpersistence was significantly higher in patients receiving risperidone (hazard ratio [HR], 1.22; 95% CI, 1.06–1.40), haloperidol (HR, 1.98; 95% CI, 1.63–2.40), and olanzapine (HR, 1.34; 95% CI, 1.07–1.68), as compared with sulpiride, suggesting the effectiveness of sulpiride was better than the other 3 antipsychotics. Therefore, this study would provide strong grounds for a properly conducted randomized controlled trial of the clinical- and cost-effectiveness of sulpiride vs atypical antipsychotics.
Keywords: schizophrenia, antipsychotics, sulpiride, effectiveness, persistence, pharmacoepidemiology
Introduction
Antipsychotics are the essential treatment for schizophrenic symptom control, and poor adherence could precipitate clinical relapse and impede the therapeutic benefit.1 On the other hand, patients with good persistence may indicate that they can consistently stay on their antipsychotic regimen. Measuring persistence integrates patients’ and clinicians’ judgments of efficacy, safety, and tolerability into a global measurement of effectiveness that reflects their evaluation of therapeutic benefits in relation to undesirable effects.2 Therefore, persistence has been used as a proxy to measure treatment effectiveness in several large randomized controlled trials (RCTs) including the Clinical Antipsychotic Trials of Intervention Effectiveness2 (CATIE), the European First Episode Schizophrenia Trial3,4 (EUFEST), and also some observational studies.5–13
The atypical antipsychotics (AAs) have been used widely because they came into market in the 1990s with a claim of better efficacy in reducing negative symptoms and fewer extrapyramidal symptoms as compared with typical antipsychotics (TAs). However, there might be some misperceptions as this has been disproven in efficacy comparisons between AAs and TAs by several studies since then.2,14–16 Furthermore, since AAs are much more expensive than TAs, they could be a greater financial burden on the health care system. In the Cost Utility of the Latest Antipsychotic drugs in Schizophrenia Study (CUtLASS),15 TAs were found to be noninferior to AAs in terms of quality of life, overall (total/positive/negative) symptom control, and associated cost. Notably, most subjects in the TAs arm received sulpiride. A recent meta-analysis of several RCTs reported sulpiride had similar efficacy and fewer side effects as compared with the other TAs, such as haloperidol, chlorpromazine, and perphenazine.17,18 Sulpiride is a benzamide-derived TA and has been widely used for patients with schizophrenia in some European and Asian countries for decades but remains unavailable in North America. Owing to its relatively low price, it is potentially a viable option for patients with schizophrenia. Although there were some studies comparing the clinical efficacy and side effects among sulpiride and several antipsychotic agents, no study has provided a direct comparison of effectiveness between AAs and sulpiride. Thus, this study aimed to compare the effectiveness among sulpiride, haloperidol, and the 2 most prevalently used AAs, risperidone and olanzapine, by evaluating the persistence of drug use.
Methods
Data Source
Electronic data sets for this study were derived from the National Health Insurance Research Database (NHIRD), which is maintained by the National Health Research Institute and made accessible for research purposes. Taiwan launched a single-payer and compulsory National Health Insurance (NHI) program on March 1, 1995, and by 2007, nearly 99% of the population was enrolled in this program. The NHIRD compiles information on the demographics of enrollees, information regarding health care professionals and facilities, and service claims from inpatient, ambulatory care, and contracted pharmacies for reimbursement purposes. Personal identities have been encrypted for privacy protection, but all data sets can be linked with the unique and anonymous identifiers created by the National Health Research Institute for research purposes. Using NHIRD without cross linkage to other health data is therefore exempt from Institutional Review Board in Taiwan. We used 2 Longitudinal Health Insurance Databases, LHID2000 and LHID2005, each of which was a cohort of 1 million beneficiaries, randomly sampled from the year 2000 and the year 2005 registry, respectively. There was no significant difference in distribution of the age, gender, annual births, and average premium of beneficiaries, using chi-square tests at alpha level .05, between the patients in the sampled databases and the original NHIRD. The details of sampling process are published online by the Taiwan National Health Research Institutes.19
Study Cohort Assembly and Follow-up
This study identified a cohort of patients with schizophrenia by International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) code 295.XX between 2003 and 2008, who were 18–65 years old and new to single oral antipsychotic agents defined as N05AL01 sulpiride, N05AD01 haloperidol, N05AH03 olanzapine, and N05AX08 risperidone in the Anatomical Therapeutic Chemical classification system.20
We considered the first prescribed antipsychotic as the index agent, and its prescription date was the index date. Antipsychotic users who did not receive any antipsychotic prescription 6 years prior to the index date were considered new users. Patients without over 6 year of prior eligibility via NHI before index date were excluded to ensure there were sufficient data for identifying new users. We excluded patients who were pregnant within 1 year either before or after the index date. Because antipsychotics may be used for dementia-related psychotic symptoms and patients with dementia may have different persistence, we excluded patients who had dementia defined by ICD-9-CM code 290 to avoid potential confounding. Since low-dose sulpiride (150 mg/day) was commonly used for gastrointestinal upset and prochlorperazine for nausea and vomiting in Taiwan, sulpiride with a daily dose of 150 mg and lower and all prochlorperazine prescriptions were not considered as antipsychotic regimens.
All eligible patients were classified into the following 4 groups according to their index agents: sulpiride, haloperidol, risperidone, and olanzapine. Although previous studies suggest that long-acting injectable (LAI) antipsychotic agents might improve adherence to treatment,21,22 these medications were not included in this study. The exclusion decision was based on 2 reasons. Firstly, the current study population was antipsychotics-naïve patients, and the objective of the study was to understand the effectiveness of initial antipsychotic treatment. LAI preparations are rarely used as the first antipsychotics for treatment-naïve patients. Secondly, the small group of patients who used LAI preparations as the first antipsychotics might have different characteristics than those treated with oral formulation.
Definition of Outcomes and Treatment Changes
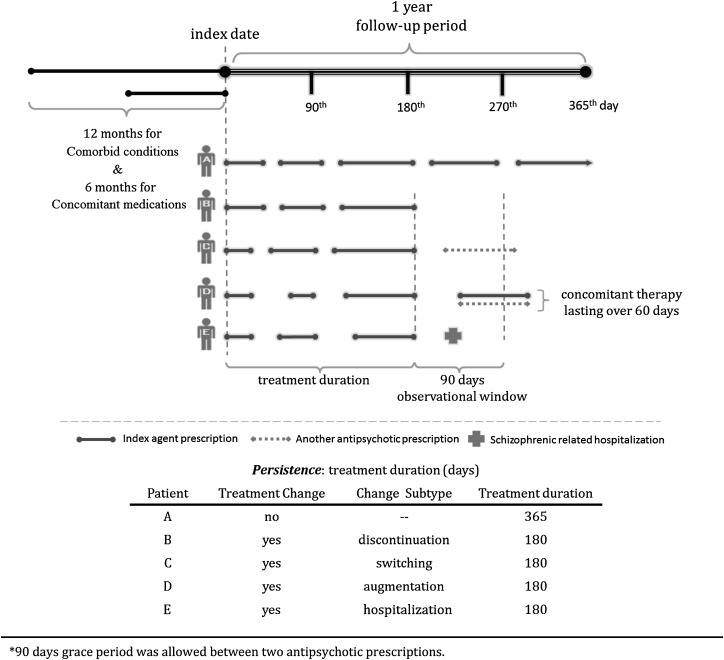
We primarily aimed to evaluate the persistence of each index agent by calculating the treatment duration from the index date to the end of a 1-year follow-up period or to the end of the last prescription supply for those who had encountered a treatment change as defined. All the changes that happened within the 90-day observational window starting at the end of the last prescription supply were classified into 4 mutually exclusive categories (whichever came first): (1) “Discontinuation” was defined as patients who no longer received any antipsychotic prescription within the observational window because patients with a gap exceeding 90 days rarely restart7; (2) “Switching” was defined as patients who stopped using the index agent and received another antipsychotic prescription; (3) “Augmentation” was defined as patients who started concomitant antipsychotic therapy that lasted over 60 days; (4) “Hospitalization” was defined when patients were admitted for schizophrenic episodes (Appendix 1). All defined treatment changes were analyzed separately. In addition, patients who lost their eligibility from NHI after the index date were censored from the date of disenrollment.
Covariates
The following patient demographics, mental illness characteristics, and approximately 30 covariates of comorbid conditions and concomitant medications other than mental-related were included to assess the study outcomes (table 1). Patient demographics included patient’s age, gender, year of index date, and NHI premium level. In the NHI of Taiwan, premiums are mostly determined by the insured’s wage, and hence, insurance premiums are income related, which can be taken as a proxy for patients’ socioeconomic status. Mental illness characteristics included the relative dosage of antipsychotics, subtype of first diagnosed schizophrenia, alcohol/substance abuse, and other mental disorder such as episodic mood disorders, nonschizopsychosis, personality disorder, anxiety disorder, and other mental-related medications, such as lithium, antiepileptic drugs, extrapyramidal symptom relief (anti-extrapyramidal syndrome [EPS]) medications, benzodiazepines, and antidepressants. Two indicators, defined daily dose (DDD) and prescribed daily dose (PDD), were computed for relative dosages of antipsychotics. The DDD assignment was based on dose information obtained from the World Health Organization collaborating center,20 and the PDD was calculated from prescription data of hospital visits. The PDD/DDD ratio of an antipsychotic agent, thus, indicates the relative dosage of any given drug as compared with what has been recommended and may reflect one’s schizophrenic severity. We retrieved all the ICD-9-CM codes for all comorbid conditions within 365 days before the index date and any prescription within 180 days before the index date as concomitant medications.
Table 1.
Baseline Characteristics of Eligible Patients
| Sulpiride | Haloperidol | Olanzapine | Risperidone | |
| (n = 476) | (n = 188) | (n = 103) | (n = 557) | |
| Demographics | ||||
| Age, mean (SD), y | 36.16 (12.06) | 37.31 (11.09) | 35.68 (11.79) | 36.25 (12.43) |
| Patient distribution by age, n (%), y | ||||
| 18–24 | 97 (20.38) | 23 (12.23) | 18 (17.48) | 111 (19.93) |
| 25–34 | 131 (27.52) | 58 (30.85) | 31 (30.10) | 161 (28.90) |
| 35–44 | 120 (25.21) | 61 (32.45) | 27 (26.21) | 138 (24.78) |
| 45–54 | 91 (19.12) | 35 (18.62) | 21 (20.39) | 87 (15.62) |
| 55–65 | 37 (7.77) | 11 (5.85) | 6 (5.83) | 60 (10.77) |
| Female, n (%) | 195 (40.97) | 76 (40.43) | 48 (46.60) | 248 (44.52) |
| Patient distribution by index year, n (%), y | ||||
| 2003 | 146 (30.67) | 56 (29.79) | 36 (34.95) | 96 (17.24) |
| 2004 | 99 (20.80) | 47 (25.00) | 26 (25.24) | 114 (20.47) |
| 2005 | 76 (15.97) | 31 (16.49) | 10 (9.71) | 106 (19.03) |
| 2006 | 61 (12.82) | 18 (9.57) | 13 (12.62) | 72 (12.93) |
| 2007 | 54 (11.34) | 16 (8.51) | 9 (8.74) | 86 (15.44) |
| 2008 | 40 (8.40) | 20 (10.64) | 9 (8.74) | 83 (14.90) |
| aPatient distribution by NHI premium levels, n (%), NT$ | ||||
| >25 000 | 22 (4.62) | 9 (4.79) | 6 (5.83) | 47 (8.44) |
| 15 000–25 000 | 224 (47.06) | 85 (45.21) | 63 (61.17) | 279 (50.09) |
| <15 000 | 230 (48.32) | 94 (50.00) | 34 (33.010) | 231 (41.47) |
| Patient distribution by datasets, n (%) | ||||
| LHID2000 | 250 (52.52) | 86 (45.74) | 50 (48.54) | 296 (53.14) |
| LHID2005 | 226 (47.42) | 102 (54.26) | 53 (51.46) | 261 (46.86) |
| Mental illness characteristics | ||||
| Relative dosages of antipsychotics, n (%), PDD/DDD ratio | ||||
| 0.0–0.2 | 7 (1.47) | 33 (17.55) | 0 (0.00) | 27 (4.85) |
| 0.2–0.4 | 119 (25.00) | 30 (15.96) | 5 (4.85) | 205 (36.80) |
| 0.4–0.6 | 192 (40.34) | 12 (6.38) | 39 (37.86) | 177 (31.78) |
| 0.6–0.8 | 99 (20.80) | 45 (23.94) | 2 (1.94) | 75 (13.46) |
| >0.8 | 59 (12.39) | 68 (36.17) | 57 (55.34) | 73 (13.11) |
| Subtype of first diagnosed schizophrenia, n (%) | ||||
| Acute schizophrenic episode | 32 (6.72) | 7 (3.72) | 14 (13.59) | 47 (8.44) |
| Schizoaffective type | 20 (4.20) | 1 (0.53) | 3 (2.91) | 9 (1.62) |
| Catatonic type | 1 (0.21) | 3 (1.60) | 1 (0.97) | 4 (0.72) |
| Latent schizophrenia | 11 (2.31) | 4 (2.13) | 1 (0.97) | 8 (1.44) |
| Disorganized type | 23 (4.83) | 10 (5.32) | 3 (2.91) | 20 (3.59) |
| Other specified types | 12 (2.52) | 5 (2.66) | 0 (0.00) | 20 (3.59) |
| Paranoid type | 259 (54.41) | 113 (60.11) | 53 (51.46) | 299 (53.68) |
| Residual schizophrenia | 11 (2.31) | 6 (3.19) | 0 (0.00) | 8 (1.44) |
| Simple type | 36 (7.56) | 11 (5.85) | 7 (6.80) | 41 (7.36) |
| Unspecified schizophrenia | 71 (14.92) | 28 (14.89) | 21 (20.39) | 101 (18.13) |
| Other mental disorder, n (%) | ||||
| Alcohol/substance abuse | 8 (1.68) | 4 (2.13) | 0 (0.00) | 6 (1.08) |
| Episodic mood disorder | 31 (6.51) | 6 (3.19) | 7 (6.80) | 40 (7.18) |
| Nonschizopsychosis | 10 (2.10) | 7 (3.72) | 3 (2.91) | 17 (3.05) |
| Personality disorder | 6 (1.26) | 1 (0.53) | 0 (0.00) | 5 (0.90) |
| Anxiety disorder | 70 (14.71) | 26 (13.83) | 19 (18.45) | 80 (14.36) |
| Other mental-related medications, n (%) | ||||
| Lithium | 0 (0.00) | 2 (1.06) | 0 (0.00) | 2 (0.36) |
| Benzodiazepines | 214 (44.96) | 85 (45.21) | 48 (46.60) | 238 (42.73) |
| Antidepressants | 93 (19.54) | 24 (12.77) | 14 (13.59) | 95 (17.06) |
| Antiepileptic drugs | 40 (8.40) | 20 (10.64) | 12 (11.65) | 52 (9.34) |
| Anti-EPS medications | 51 (10.71) | 36 (19.15) | 15 (14.56) | 74 (13.29) |
| Comorbid conditions, n (%) | ||||
| Hypertension | 28 (5.88) | 11 (5.85) | 4 (3.88) | 26 (4.67) |
| Diabetes mellitus | 17 (3.57) | 10 (5.32) | 1 (0.97) | 9 (1.62) |
| Hyperlipidemia | 11 (2.31) | 12 (6.38) | 3 (2.91) | 4 (0.72) |
| Cardiovascular disease | 25 (5.25) | 6 (3.19) | 3 (2.91) | 20 (3.59) |
| Pneumonia | 23 (4.83) | 6 (3.19) | 6 (5.83) | 24 (4.31) |
| COPD | 29 (6.09) | 11 (5.85) | 4 (3.88) | 25 (4.49) |
| Liver disorders | 21 (4.41) | 7 (3.72) | 3 (2.91) | 16 (2.87) |
| Peptic ulcer | 21 (4.41) | 6 (3.19) | 2 (1.94) | 22 (3.95) |
| Gastric disorders | 81 (17.02) | 23 (12.23) | 12 (11.65) | 72 (12.93) |
| Colitis | 52 (10.92) | 17 (9.04) | 7 (6.80) | 48 (8.62) |
| Renal disease | 2 (0.42) | 2 (1.06) | 0 (0.00) | 6 (1.08) |
| Concomitant medications, n (%) | ||||
| Diabetes medications | 17 (3.57) | 10 (5.32) | 1 (0.97) | 12 (2.15) |
| Diuretics | 14 (2.94) | 9 (4.79) | 3 (2.91) | 14 (2.51) |
| Beta-blockers | 57 (11.97) | 25 (13.30) | 13 (12.62) | 73 (13.11) |
| CCBs | 17 (3.57) | 9 (4.79) | 4 (3.88) | 25 (4.49) |
| RAS inhibitors | 13 (2.73) | 9 (4.79) | 3 (2.91) | 15 (2.69) |
| Lipid lowering medications | 7 (1.47) | 7 (3.72) | 2 (1.94) | 4 (0.72) |
| COPD medications | 131 (27.52) | 50 (26.60) | 24 (23.30) | 105 (18.85) |
| Antihistamines | 228 (47.90) | 78 (41.49) | 33 (32.04) | 212 (38.06) |
| Hormone agents | 28 (5.88) | 9 (4.79) | 0 (0.00) | 28 (5.03) |
| Systemic steroid | 88 (18.49) | 27 (14.36) | 12 (11.65) | 77 (13.82) |
| Antibacterial agents | 233 (48.95) | 83 (44.15) | 47 (45.63) | 222 (39.86) |
| NSAIDs | 259 (54.41) | 90 (47.87) | 47 (45.63) | 253 (45.42) |
| Gout medications | 8 (1.68) | 10 (5.32) | 0 (0.00) | 15 (2.69) |
| GERD medications | 79 (16.60) | 20 (10.64) | 11 (10.68) | 70 (12.57) |
| Propulsives | 109 (22.90) | 37 (19.68) | 10 (9.71) | 92 (16.52) |
| Antiplatelets | 16 (3.36) | 9 (4.79) | 2 (1.94) | 13 (2.33) |
| Antithrombotic agents | 22 (4.62) | 15 (7.98) | 6 (5.83) | 32 (5.75) |
| Anemic medications | 11 (2.31) | 4 (2.13) | 5 (4.85) | 17 (3.05) |
| Antiarrhythmic agents | 5 (1.05) | 0 (0.00) | 1 (0.97) | 2 (0.36) |
| bPropensity score | ||||
| Mean (SD) | 0.38 (0.08) | 0.36 (0.08) | 0.35 (0.08) | 0.34 (0.08) |
| Median (IQR) | 0.37 (0.30–0.44) | 0.36 (0.30–0.44) | 0.36 (0.30–0.40) | 0.35 (0.29–0.40) |
| Treatment duration, in days | ||||
| Mean (SD) | 170.76 (144.95) | 81.78 (95.26) | 130.64 (133.47) | 136.22 (134.74) |
| Median (IQR) | 95 (32–245) | 32 (8–145) | 63 (19–262) | 74 (20–252) |
| Days of antipsychotics supplied of each prescription, in days | ||||
| Mean (SD) | 22.17 (8.70) | 20.44 (9.89) | 21.67 (9.63) | 21.11 (9.51) |
| Median (IQR) | 28 (14–28) | 21 (14–28) | 28 (14–28) | 28 (14–28) |
Note: LHID, longitudinal health insurance database; DDD, defined daily dose; PDD, prescribed daily dose; EPS, extrapyramidal symptom; Anti-EPS medications, extrapyramidal symptom relief medication, such as trihexyphenidyl and biperiden; COPD, chronic obstructive pulmonary disease; RAS, renin-angiotensin system; CCB, calcium channel blocker; NSAID, nonsteroidal anti-inflammatory drug; GERD, gastroesophageal reflux disease; NHI, National Health Insurance; IQR, interquartile range.
1 NT dollar is approximately equal to 0.034 US dollar.
Propensity score was derived from comorbid conditions and concomitant medications by using multinomial logistic regression.
Sensitivity Analyses
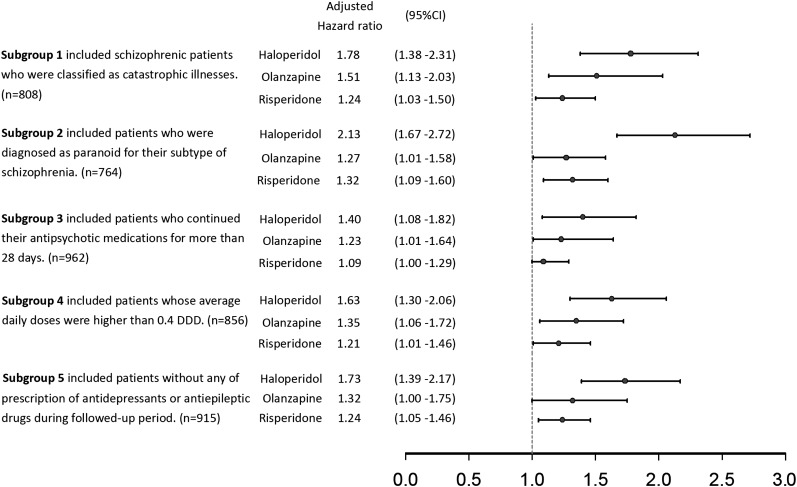
We conducted several sensitivity analyses by selecting 5 subcohorts with more homogenous patients to test the robustness of our study. “Subgroup 1” only included patients with schizophrenia who were classified as “catastrophic illnesses.” Patients with catastrophic illness as defined by the Department of Health, Taiwan, are exempt from copayments in the NHI. Therefore, a diagnosis of schizophrenia in patients with a catastrophic illness certificate is more accurate and severe. To explore whether subtypes of schizophrenia would affect antipsychotic persistence, “Subgroup 2” included patients who were diagnosed as paranoid for their subtype of schizophrenia, which was the most prevalent subtype in Taiwan.23 “Subgroup 3” included patients who continued their antipsychotic medications for more than 28 days. This criterion would have excluded patients who used antipsychotic medications only for short-term control of psychotic symptoms. Since the relative dosage of antipsychotic was correlated with disease severity, “Subgroup 4” included patients whose PDD/DDD ratios were higher than 0.4. To explore whether concomitant use of other mood stabilizing drugs including antidepressants and antiepileptic drugs would affect antipsychotic persistence, “Subgroup 5” included patients without any prescription of antidepressants or antiepileptic drugs during the followed-up period.
Statistical Analysis
We performed ANOVA tests and chi-square tests to compare baseline characteristics for continuous variables and categorized variables, respectively. Kaplan–Meier survival curves were used to estimate the time to the treatment changes. Hazard ratios (HRs) and 95% CIs derived from Cox proportional hazards regression models were used to estimate the persistence of antipsychotic groups. Covariates including patient’s demographics, mental illness characteristics, and propensity score (PS) derived from comorbid conditions, and concomitant medications were added to the final adjusted model. PS was a predicted probability of getting one treatment vs other treatments obtained from the logistic regression model. We also assessed interactions between covariates added to the model to minimize the degree of multicolinearity. Any correlated covariate was eliminated from the final adjusted model. All statistical analyses were performed using SAS 9.2 version software (SAS Institute, Cary, NC).
Results
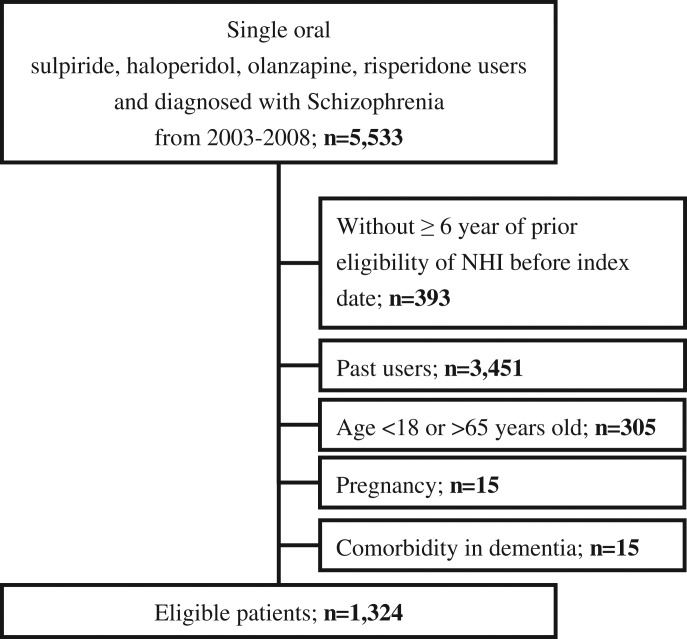
We identified 5533 individuals who were diagnosed with schizophrenia and used one of the oral study antipsychotics from a population of 2 million patients. We excluded 393 who did not have over 6 years of prior eligibility in the NHI before the index date and 3451 who were not incident users. Then we excluded 305 who were younger than 18 or older than 65 years of age at the index date, 15 who were pregnant, and 15 with dementia. The study cohort assembly flowchart is shown in figure 1. A total of 1324 patients constituted the study cohort, with a mean age of 36, and approximately 40% were female. Of this cohort, 557 (42.1%) received risperidone, 476 (36.0%) sulpiride, 188 (14.2%) haloperidol, and 103 (7.7%) olanzapine. Regarding mental illness characteristics, most of the patients were diagnosed with paranoid type of schizophrenia, with anxiety and episodic mood disorder, and had concomitant used of benzodiazepines and antidepressants, respectively (table 1).
Fig. 1.
Study cohort assembly flowchart.
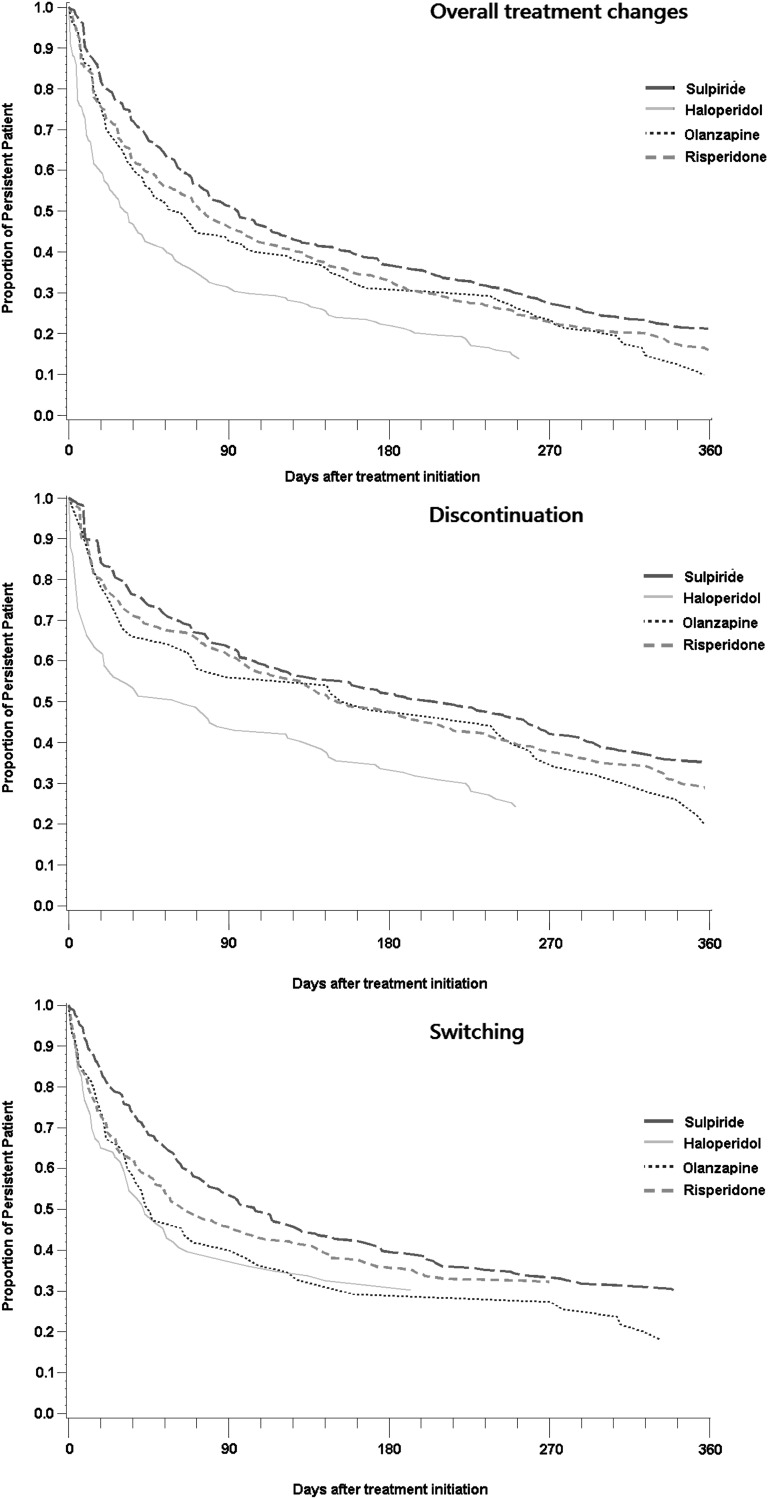
Almost 85% of patients in every group encountered a treatment change as shown in table 2. The mean treatment duration was 171 (±145) days for sulpiride, 82 (±95) days for haloperidol, 131 (±133) days for olanzapine, and 136 (±135) days for risperidone. Nonadjusted comparisons of treatment changes for 4 groups over time are shown in Kaplan–Meier survival curves in figure 2. Persistence in patients receiving sulpiride was significantly better than haloperidol (hazard ratio [HR], 1.98; 95% CI, 1.63–2.40), olanzapine (HR, 1.34; 95% CI, 1.07–1.68), and risperidone (HR, 1.22; 95% CI, 1.06–1.40) after adjusting for patients demographics, mental illness characteristics, and PS (table 2).
Table 2.
Cox Proportional Hazard Regression Models for Time to First Treatment Change Among 4 Antipsychotics
| Number of Patient-Encountered Treatment Change (%) | Hazard Ratio (95% CIs) | ||
| Crude | Adjusteda | ||
| Overall treatment changes | |||
| Sulpiride (n = 476) | 407 (85.50) | 1.00 (—) | 1.00 (—) |
| Haloperidol (n = 188) | 162 (86.17) | 1.82 (1.51−2.19) | 1.98 (1.63−2.40) |
| Olanzapine (n = 103) | 93 (90.29) | 1.31 (1.05−1.65) | 1.34 (1.07−1.68) |
| Risperidone (n = 557) | 469 (84.20) | 1.18 (1.03−1.35) | 1.22 (1.06−1.40) |
| Subgroup analyses by specific treatment changesb | |||
| Discontinuation | |||
| Sulpiride (n = 276) | 207 (75.00) | 1.00 (—) | 1.00 (—) |
| Haloperidol (n = 107) | 81 (75.70) | 1.96 (1.51−2.56) | 2.25 (1.70−2.98) |
| Olanzapine (n = 50) | 40 (80.00) | 1.33 (0.95−1.88) | 1.36 (0.96−1.91) |
| Risperidone (n = 315) | 227 (72.06) | 1.17 (0.96−1.42) | 1.21 (1.00−1.48) |
| Switching | |||
| Sulpiride (n = 236) | 167 (70.76) | 1.00 (—) | 1.00 (—) |
| Haloperidol (n = 86) | 60 (69.77) | 1.35 (1.00−1.81) | 1.65 (1.21−2.25) |
| Olanzapine (n = 55) | 45 (81.82) | 1.43 (1.03−1.99) | 1.48 (1.06−2.05) |
| Risperidone (n = 273) | 185 (67.77) | 1.17 (0.94−1.44) | 1.22 (1.01−1.52) |
| Augmentationc | |||
| Sulpiride (n = 70) | 1 (1.43) | — | — |
| Haloperidol (n = 31) | 5 (16.13) | — | — |
| Olanzapine (n = 10) | 0 (0.00) | — | — |
| Risperidone (n = 97) | 9 (9.28) | — | — |
| Hospitalization | |||
| Sulpiride (n = 101) | 32 (31.68) | 1.00 (—) | 1.00 (—) |
| Haloperidol (n = 42) | 16 (38.10) | 1.50 (0.82−2.77) | 1.47 (0.80−2.73) |
| Olanzapine (n = 18) | 8 (44.44) | 1.59 (0.73−3.46) | 1.59 (0.73−3.47) |
| Risperidone (n = 136) | 48 (35.29) | 1.24 (0.79−1.96) | 1.22 (0.77−1.94) |
Note: NHI, National Health Insurance.
Adjusted for age, gender, year of index date, NHI premium levels, datasets, relative dosages of antipsychotics, subtype of schizophrenia, alcohol/substance abuse, episodic mood disorders, nonschizopsychosis, personality disorder, anxiety disorder, lithium, antiepileptic drugs, extrapyramidal symptom relief medications, benzodiazepines, antidepressants, and propensity score derived from other comorbid conditions and concomitant medications.
For each analysis, subgroup comprised only patients with specific treatment change or those without any treatment change.
Cox-regression in subgroup analyses of augmentation was not performed because of small sample size.
Fig. 2.
Kaplan–Meier survival curves of crude antipsychotic effectiveness comparisons.
Of all patients who encountered treatment changes, 50% discontinued treatment, 40% switched, 9% were hospitalized, and 1% received augmentation. In the discontinuation subgroup analysis, patients receiving sulpiride had better persistence than those receiving haloperidol (HR, 2.25; 95% CI, 1.70–2.98), olanzapine (HR, 1.36; 95% CI, 0.96–1.91), and risperidone (HR, 1.21; 95% CI, 1.00–1.48), but no significant difference was found when compared with olanzapine. The risk of switching among sulpiride users was significantly lower as compared with that of haloperidol (HR, 1.65; 95% CI, 1.21–2.25), olanzapine (HR, 1.48; 95% CI, 1.06–2.05), and risperidone users (HR, 1.22; 95% CI, 1.01–1.52). Patients receiving sulpiride also had a lower risk of schizophrenia-related hospitalization as compared with the other 3 groups, although statistically insignificant. Analyses of discontinuation and switching of the antipsychotics in nonadjusted comparisons for 4 index agents over time are also shown in Kaplan–Meier survival curves in figure 2.
The results of 5 sensitivity analyses showed similar trends. Persistence in patients receiving sulpiride was significantly better than those receiving haloperidol, olanzapine, and risperidone (figure 3).
Fig. 3.
Sensitivity analyses for antipsychotic effectiveness comparisons.
Discussion
This population-based retrospective cohort study addressed the comparative effectiveness of antipsychotics by evaluating persistence. Similar to concepts of time to all-cause treatment discontinuation in CATIE,2 as well as loss of retention in EUFEST,3,4 our primary outcome encompassed patients’ and clinicians’ judgments on efficacy and tolerability.24 These studies2–4 have given some insight into why, in the course of real-world treatment, patients with schizophrenia discontinue medications or ask to be switched to other medications.25 Our findings do not support that AAs are better than TAs in effectiveness as reported in some previous studies,5,10–13 possibly because sulpiride was not included in their TAs arm. Some physicians might hold the belief that TAs or sulpiride were poorer antipsychotics and so had a low threshold for taking patients off these medications or augmenting them. This will further lead to a biased impression that sulpiride is not as effective because it is often discontinued or augmented. Nonetheless, we found the persistence of using sulpiride was better than AAs such as risperidone and olanzapine in this study. There were some differences between the groups in the baseline characteristics; these variables were adjusted in multiple regressions to minimize potential bias. Five specific subcohorts with more homogenous patients were also selected for comparisons, and all the results were consistent with the main analysis, indicating the robustness of our findings.
Similar to the CATIE study,2 the survival curves (figure 2) suggest that over 50% of patients appeared to have some treatment changes during the first 3 months after treatment initiation and the persistence became relatively stable thereafter. Because the side effects of antipsychotics often appear earlier than the treatment benefit, the significant deterioration in persistence in the initial treatment stage might largely due to the side effects rather than the lack of treatment benefit.26
Good clinical efficacy in controlling schizophrenic symptoms plus lower side effects could be possible reasons for better effectiveness of sulpiride. Some superiority for using sulpiride compared with placebo in clinical benefits was reported by a recent meta-analysis.18 To date, although there is no study directly comparing the efficacy of sulpiride with AAs, the result of CUtLASS had implied the noninferiority of sulpiride to AAs.15 Mauri et al27 had reported the clinical benefits of sulpiride in not only improvement of negative defective symptoms but also partial activity against positive symptoms. They suggested sulpiride could be regarded as an AA because of its characteristics such as its antipsychotic mechanism that selectively acts on dopamine D2-like receptors. Sulpiride being more effective in controlling negative symptoms than TAs such as chlorpromazine and haloperidol had also been reported by Gerlach16 and Azorin28 et al. It is understandable that improvements in negative symptoms may increase interpersonal interactions, including with their health care providers, and more willingness to be treated, which may further contribute to the favorable effectiveness of sulpiride.7
In addition to clinical efficacy, antipsychotic side effects are another concern in prescribing selection, especially EPS. EPS can affect the activities of daily living and is commonly encountered earlier than the timing of other side effects.29 Accordingly, patients who suffer from EPS may lose confidence in the specific antipsychotic treatment and are more likely to discontinue its use. Although anticholinergic agents are used to relieve this problem, their benefit is limited by insufficient effectiveness and frequent side effects, such as decreased saliva production and constipation. From literature review, sulpiride had fewer EPS than other TAs,14,16 which might explain why sulpiride showed better effectiveness. The most troubling side effects of sulpiride were weight gain– and endocrine-related symptoms, which may also affect patients’ persistence; however, they usually occur later than EPS.18,26,29
In analyzing the risk of switching, we found patients receiving sulpiride had a higher likelihood of continuously staying on the sulpiride regimen during the 1-year follow-up period, which was significantly better than the other 3 groups. Since the switching of antipsychotics was a decision made by the clinicians, it is possible that “switching” might be a better indicator than discontinuation (which could be initiated by either the clinicians or the patients) to evaluate the clinical effect. A lower incidence of switching of sulpiride further confirmed that this medication has higher effectiveness that the other antipsychotics. As for discontinuation, however, we found approximately 3-quarters of patients discontinued their antipsychotic medications within a 1-year period, whatever they received. This finding is consistent with previous reports.30 Different from antipsychotic switching, treatment discontinuation implied that patient was no longer followed-up, and the factors become relatively more complex. The possible explanations may not be associated with antipsychotic medication, but rather result from social factors, such as patient refusing to take the drug because of the lack of insight, a feeling of personal and/or family stigma, or patient seeking traditional Chinese therapies. Further investigations will be needed to explore the associations.
Most subgroup analyses showed a result consistent with the main analyses, except in the few analyses of the specific treatment change (augmentation and hospitalization). The nonsignificant findings are likely due to the small sample size of the subgroup (n = 208 and n = 297 in the subgroup of augmentation and hospitalization, respectively) and the reduced statistical power in the subgroup analyses. Nevertheless, the point estimates of HR of these subgroup analyses suggest a lower likelihood of sulpiride to experience treatment changes. On the other hand, our study is designed to demonstrate some treatments’ superiority by evaluating persistence, statistically nonsignificant results should not be misinterpreted as evidence of equivalence.31 Further research is needed for analyzing augmentation and hospitalization, which could be important indicators to evaluate the clinical effect of antipsychotics.
The global antipsychotic market has grown from less than $1 billion annually in 1993 to more than $10 billion now.32 The consumption of expensive AAs increased dramatically and can be a great economic burden for the medical care system.33 Therefore, issues concerning the cost-effectiveness of antipsychotics become more important. Glimer et al34 reported that patients with good adherence had lower hospital costs but higher pharmacy-related costs. As a result, the total annual expenditures of the adherent group were higher than the nonadherent group. Becker et al35 examined costs associated with adherence rates by different antipsychotic classes. Patients with good adherence would reduce approximately 30% of total cost in the TAs group, but the degree of cost reduction in adherent patients receiving AAs would be quite minor, resulting from the much higher drug-cost expenditures. Therefore, an antipsychotic agent with similar effectiveness and a cheaper price would become an appropriate alternative. It was evident that sulpiride was better in effectiveness than olanzapine and risperidone, so its cost, on average one-tenth of AAs in the reimbursement scheme in NHI, would play a crucial role in curbing the high and rising cost of antipsychotic treatment.
Using a large nationwide sample was one of the strengths of the current study, which well represented the entire population of Taiwan. Because antipsychotics were reimbursed under the Taiwan NHI system, all antipsychotics usage of schizophrenia patients was recorded in NHIRD. Additionally, because the costs of antipsychotics were not a concern to patients, evaluating persistence focuses precisely on drug effect. Relative to clinical trials, this study provides comparisons for the effectiveness among antipsychotics in a real-world setting. Moreover, many potential confounders, such as patient demographics, mental illness characteristics, comorbid conditions, and concomitant medications, were adjusted in our study, and the results remained consistent throughout the series of adjusted processes and sensitivity analyses. To our knowledge, there is no study comparing the effectiveness of sulpiride, which was a rather affordable effective traditional antipsychotic agent, with an atypical one.
As in all observational studies using electronic databases, we were unable to confirm whether the patients actually took their dispensed medicines. Nevertheless, we believed that all treatment changes we defined could reflect clinicians’ assessments and decisions on schizophrenic symptoms that would likely result from poor antipsychotic adherence and unsatisfactory outcomes (eg, patient hospitalized). The NHIRD does not have information on certain important clinical variables, such as disease severity or duration of illness. To reduce the possible confounding effect from these missing variables, we included only new users in this study as an attempt to create a relatively homogenous cohort. Additionally, Cox regression models were also used to adjust for several variables that may reflect disease severity, including patients’ mental illness characteristics and PS derived from comorbidities and concomitant medications. The consistent results from the sensitivity analyses based on the 5 subcohorts also support the robustness for effectiveness comparisons. We believed the bias could be minimized. Several inclusion and exclusion criteria were setup to increase the internal validity of the study; however, this might decrease the generalizability of this study. Patients who were past users aged fewer than 18 or over 65, with pregnancy or with dementia, were not discussed in this study. Because low-dose sulpiride is commonly indicated for gastrointestinal upset in the clinical practice in Taiwan, sulpiride users were only included in this study if they received a daily dose of more than 150. By excluding sulpiride users with less than 150 mg daily dose, it is possible that some patients with mild psychotic symptoms (and thus used lower dose of sulpiride) might have been excluded in sulpiride group, which could slant our results toward null; however, this would also made sulpiride group more comparable to other groups in terms of severity. Although a 6-year observational periods was set to ensure the selected patient were new users, reapplication users may still exist in the present study and could confound the results because their persistence may differ from a new user. Among the 3451 patients with antipsychotics history, 81% had the last claim of antipsychotics within 1 year before the index date, 15% within 2–3 years, and only 4% within 4–6 year. Therefore, we considered a baseline period of 6 years should be sufficient to exclude patients who were in fact not naïve patients but were reapplication of antipsychotics after a lengthy period of time.
In conclusion, the present study indicated that the effectiveness of sulpiride was better than haloperidol, as well as the 2 most prevalent AAs, olanzapine and risperidone. This finding would provide strong grounds for a properly conducted RCT of the clinical- and cost-effectiveness of sulpiride vs AAs, and other TAs using the kind of pragmatic outcome measures that have been used in recent trials2,3,15,36 and taking into account the need for blind allocation and assessment if discontinuation is an outcome to avoid problems seen in EUFEST, where clinicians discontinued TA preferentially given equal side-effect burdens.3,4 If such an ambitious RCT with an economic component were to show a cost-effectiveness benefit for sulpiride, this could make a tremendous difference to patients and to services around the world.
Funding
This study was supported in part by grants from the Multidisciplinary Center of Excellence for Clinical Trial and Research (DOH100-TD-B-111-002), Department of Health, Executive Yuan, Taiwan.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Appendix 1. Overview for study design and treatment changes definition.
References
- 1.Ahn J, McCombs JS, Jung C, et al. Classifying patients by antipsychotic adherence patterns using latent class analysis: characteristics of nonadherent groups in the California Medicaid (Medi-Cal) program. Value Health. 2008;11:48–56. doi: 10.1111/j.1524-4733.2007.00214.x. [DOI] [PubMed] [Google Scholar]
- 2.Lieberman JA, Stroup TS, McEvoy JP, et al. Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med. 2005;353:1209–1223. doi: 10.1056/NEJMoa051688. [DOI] [PubMed] [Google Scholar]
- 3.Fleischhacker WW, Keet IP, Kahn RS. The European First Episode Schizophrenia Trial (EUFEST): rationale and design of the trial. Schizophr Res. 2005;78:147–156. doi: 10.1016/j.schres.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Kahn RS, Fleischhacker WW, Boter H, et al. Effectiveness of antipsychotic drugs in first-episode schizophrenia and schizophreniform disorder: an open randomised clinical trial. Lancet. 2008;371:1085–1097. doi: 10.1016/S0140-6736(08)60486-9. [DOI] [PubMed] [Google Scholar]
- 5.Ascher-Svanum H, Zhu B, Faries D, Landbloom R, Swartz M, Swanson J. Time to discontinuation of atypical versus typical antipsychotics in the naturalistic treatment of schizophrenia. BMC Psychiatry. 2006;6:8. doi: 10.1186/1471-244X-6-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ascher-Svanum H, Zhu B, Faries DE, Lacro JP, Dolder CR, Peng X. Adherence and persistence to typical and atypical antipsychotics in the naturalistic treatment of patients with schizophrenia. Patient Prefer Adherence. 2008;2:67–77. doi: 10.2147/ppa.s2940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gianfrancesco FD, Rajagopalan K, Sajatovic M, Wang RH. Treatment adherence among patients with schizophrenia treated with atypical and typical antipsychotics. Psychiatry Res. 2006;144:177–189. doi: 10.1016/j.psychres.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 8.Gianfrancesco FD, Rajagopalan K, Sajatovic M, Wang RH. Treatment adherence among patients with bipolar or manic disorder taking atypical and typical antipsychotics. J Clin Psychiatry. 2006;67:222–232. doi: 10.4088/jcp.v67n0208. [DOI] [PubMed] [Google Scholar]
- 9.Gianfrancesco FD, Sajatovic M, Rajagopalan K, Wang RH. Antipsychotic treatment adherence and associated mental health care use among individuals with bipolar disorder. Clin Ther. 2008;30:1358–1374. doi: 10.1016/s0149-2918(08)80062-8. [DOI] [PubMed] [Google Scholar]
- 10.Menzin J, Boulanger L, Friedman M, Mackell J, Lloyd JR. Treatment adherence associated with conventional and atypical antipsychotics in a large state Medicaid program. Psychiatr Serv. 2003;54:719–723. doi: 10.1176/appi.ps.54.5.719. [DOI] [PubMed] [Google Scholar]
- 11.Haro JM, Suarez D, Novick D, Brown J, Usall J, Naber D. Three-year antipsychotic effectiveness in the outpatient care of schizophrenia: observational versus randomized studies results. Eur Neuropsychopharmacol. 2007;17:235–244. doi: 10.1016/j.euroneuro.2006.09.005. [DOI] [PubMed] [Google Scholar]
- 12.Tiihonen J, Wahlbeck K, Lonnqvist J, et al. Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: observational follow-up study. BMJ. 2006;333:224. doi: 10.1136/bmj.38881.382755.2F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tunis SL, Faries DE, Nyhuis AW, Kinon BJ, Ascher-Svanum H, Aquila R. Cost-effectiveness of olanzapine as first-line treatment for schizophrenia: results from a randomized, open-label, 1-year trial. Value Health. 2006;9:77–89. doi: 10.1111/j.1524-4733.2006.00083.x. [DOI] [PubMed] [Google Scholar]
- 14.Leucht S, Corves C, Arbter D, Engel RR, Li C, Davis JM. Second-generation versus first-generation antipsychotic drugs for schizophrenia: a meta-analysis. Lancet. 2009;373:31–41. doi: 10.1016/S0140-6736(08)61764-X. [DOI] [PubMed] [Google Scholar]
- 15.Jones PB, Barnes TR, Davies L, et al. Randomized controlled trial of the effect on Quality of Life of second- vs first-generation antipsychotic drugs in schizophrenia: Cost Utility of the Latest Antipsychotic Drugs in Schizophrenia Study (CUtLASS 1) Arch Gen Psychiatry. 2006;63:1079–1087. doi: 10.1001/archpsyc.63.10.1079. [DOI] [PubMed] [Google Scholar]
- 16.Gerlach J. New antipsychotics: classification, efficacy, and adverse effects. Schizophr Bull. 1991;17:289–309. doi: 10.1093/schbul/17.2.289. [DOI] [PubMed] [Google Scholar]
- 17.Omori IM, Wang J. Sulpiride versus other antipsychotics for schizophrenia. Cochrane Database Syst Rev. 2009;(4):CD007811. doi: 10.1002/14651858.CD007811. [DOI] [PubMed] [Google Scholar]
- 18.Soares BG, Fenton M, Chue P. Sulpiride for schizophrenia. Cochrane Database Syst Rev. 2010;(1):CD001162. doi: 10.1002/14651858.CD001162. [DOI] [PubMed] [Google Scholar]
- 19.National Health Insurance Research Database, Taiwan. http://w3.nhri.org.tw/nhird//en/Data_Subsets.html#S3. Accessed January 24, 2012.
- 20.World Health Organization Collaborating Centre for Drug Statistics Methodology. Anatomical therapeutic chemical code classification index with defined daily doses. http://www.whocc.no/atc_ddd_index/. Accessed September 23, 2010. [Google Scholar]
- 21.Zhu B, Ascher-Svanum H, Shi L, Faries D, Montgomery W, Marder SR. Time to discontinuation of depot and oral first-generation antipsychotics in the usual care of schizophrenia. Psychiatr Serv. 2008;59:315–317. doi: 10.1176/ps.2008.59.3.315. [DOI] [PubMed] [Google Scholar]
- 22.Hosalli P, Davis JM. Depot risperidone for schizophrenia. Cochrane Database Syst Rev. 2003;(4):CD004161. doi: 10.1002/14651858.CD004161. [DOI] [PubMed] [Google Scholar]
- 23.Gau SS, Chung CH, Gau CS. A pharmacoeconomic analysis of atypical antipsychotics and haloperidol in first-episode schizophrenic patients in taiwan. J Clin Psychopharmacol. 2008;28:271–278. doi: 10.1097/JCP.0b013e3181723713. [DOI] [PubMed] [Google Scholar]
- 24.McEvoy JP. An overview of the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) study. CNS Spectr. 2006;11(7 suppl 7):4–8. doi: 10.1017/s1092852900026626. [DOI] [PubMed] [Google Scholar]
- 25.Falkai P. Limitations of current therapies: why do patients switch therapies? Eur Neuropsychopharmacol. 2008;18(suppl 3):S135–S139. doi: 10.1016/j.euroneuro.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 26.Weiden PJ. Understanding and addressing adherence issues in schizophrenia: from theory to practice. J Clin Psychiatry. 2007;68(suppl. 14):14–19. [PubMed] [Google Scholar]
- 27.Mauri MC, Bravin S, Bitetto A, Rudelli R, Invernizzi G. A risk-benefit assessment of sulpiride in the treatment of schizophrenia. Drug Saf. 1996;14:288–298. doi: 10.2165/00002018-199614050-00003. [DOI] [PubMed] [Google Scholar]
- 28.Azorin JM, Dassa D, Jalfre M. [The atypical neuroleptic concept] Encephale. 1992;18 Spec No. 3:453–457. [PubMed] [Google Scholar]
- 29.Tschoner A, Engl J, Laimer M, et al. Metabolic side effects of antipsychotic medication. Int J Clin Pract. 2007;61:1356–1370. doi: 10.1111/j.1742-1241.2007.01416.x. [DOI] [PubMed] [Google Scholar]
- 30.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 31.Kraemer HC, Glick ID, Klein DF. Clinical trials design lessons from the CATIE study. Am J Psychiatry. 2009;166:1222–1228. doi: 10.1176/appi.ajp.2009.08121809. [DOI] [PubMed] [Google Scholar]
- 32.Snyder EM, Murphy MR. Schizophrenia therapy: beyond atypical antipsychotics. Nat Rev Drug Discov. 2008;7:471–472. doi: 10.1038/nrd2571. [DOI] [PubMed] [Google Scholar]
- 33.Al-Zakwani IS, Barron JJ, Bullano MF, Arcona S, Drury CJ, Cockerham TR. Analysis of healthcare utilization patterns and adherence in patients receiving typical and atypical antipsychotic medications. Curr Med Res Opin. 2003;19:619–626. doi: 10.1185/030079903125002270. [DOI] [PubMed] [Google Scholar]
- 34.Gilmer TP, Dolder CR, Lacro JP, et al. Adherence to treatment with antipsychotic medication and health care costs among Medicaid beneficiaries with schizophrenia. Am J Psychiatry. 2004;161:692–699. doi: 10.1176/appi.ajp.161.4.692. [DOI] [PubMed] [Google Scholar]
- 35.Becker MA, Young MS, Ochshorn E, Diamond RJ. The relationship of antipsychotic medication class and adherence with treatment outcomes and costs for Florida Medicaid beneficiaries with schizophrenia. Adm Policy Ment Health. 2007;34:307–314. doi: 10.1007/s10488-006-0108-5. [DOI] [PubMed] [Google Scholar]
- 36.Rosenheck RA, Leslie DL, Sindelar J, et al. Cost-effectiveness of second-generation antipsychotics and perphenazine in a randomized trial of treatment for chronic schizophrenia. Am J Psychiatry. 2006;163:2080–2089. doi: 10.1176/ajp.2006.163.12.2080. [DOI] [PubMed] [Google Scholar]