Abstract
Schizophrenic patients suffer from many deficits including visual, attentional, and cognitive ones. Visual deficits are of particular interest because they are at the fore-end of information processing and can provide clear examples of interactions between sensory, perceptual, and higher cognitive functions. Visual deficits in schizophrenic patients are often attributed to impairments in the dorsal (where) rather than the ventral (what) stream of visual processing. We used a visual-masking paradigm in which patients and matched controls discriminated small vernier offsets. We analyzed the evoked electroencephalography (EEG) responses and applied distributed electrical source imaging techniques to estimate activity differences between conditions and groups throughout the brain. Compared with controls, patients showed strongly reduced discrimination accuracy, confirming previous work. The behavioral deficits corresponded to pronounced decreases in the evoked EEG response at around 200 ms after stimulus onset. At this latency, patients showed decreased activity for targets in left parietal cortex (dorsal stream), but the decrease was most pronounced in lateral occipital cortex (in the ventral stream). These deficiencies occurred at latencies that reflect object processing and fine shape discriminations. We relate the reduced ventral stream activity to deficient top-down processing of target stimuli and provide a framework for relating the commonly observed dorsal stream deficiencies with the currently observed ventral stream deficiencies.
Keywords: visual perception, electroencephalography (EEG), dorsal stream, N1, backward masking, discrimination
Introduction
Schizophrenic patients are seriously impaired in most behavioral paradigms, including visual, cognitive, executive, and memory tests. Visual processing deficits are of considerable importance because of their good replicability, their relatively well-known neurobiological underpinnings, and their contributions to higher cognitive impairments.1,2 In addition, visual paradigms are potential endophenotypes of schizophrenia reflecting the genetic underpinnings of the disease rather than the current state.3,4
For these reasons, several previous electroencephalography (EEG) studies have investigated the visual processing deficits in schizophrenic patients. These studies have demonstrated robust decreases in the evoked neural response at around 100 ms after stimulus onset related to visual processing in the dorsal stream.5–10 The dorsal, or “where” stream, is located in parietal cortex and is dedicated to the processing of motion and spatial location.11 Higher-level object recognition and detailed representation of stimuli, on the other hand, are localized in the lateral occipital and inferotemporal cortex which constitute the ventral stream of visual processing. Functional effects in these areas are typically observed at somewhat longer latencies (150–250 ms after stimulus onset). At these latencies, processes in the ventral stream reflect both bottom-up processing from primary visual areas and top-down recurrent processing from higher-level brain regions.12,13 Ventral stream processing was often found to be mostly intact in schizophrenic patients,7,10 although their deficiencies in face-processing point to ventral stream deficiencies.14–16 In addition, a few recent studies using masking paradigms17,18 and challenging perceptual organization tasks19 have found ventral stream deficits using functional magnetic resonance Imaging (fMRI).
We predicted that ventral stream deficits would become most evident when task demands on the ventral stream are high. More specifically, these deficits should become apparent at latencies of around 200 ms after stimulus onset when fine shape discriminations are processed. To increase the demands on ventral stream areas, we used the shine-through masking paradigm which combines strong demands on both spatial and temporal processing. The shine-through paradigm has previously been shown to be a potential endophenoytpe of schizophrenia.3,20 In this paradigm, the target stimulus consists of 2 vertical bars with a slight offset to the left or right called a vernier stimulus (figure 1). Observers indicate the perceived offset direction of this vernier. Because of the small offset, the task requires precise spatial processing. The verniers were followed by a grating, limiting processing time, and making the spatial offset discrimination task even more demanding. Hence, successful performance in the shine-through paradigm depends on both good spatial and temporal processing. Correlates of unmasked vernier discrimination thresholds can readily be observed in the EEG, and the evoked voltage topography generated by verniers suggests that their processing involves extrastriate visual areas.21 To make sure that any of our effects were specific to the processing of the vernier, we included a control condition in which only the mask, but no vernier, was presented. We combined EEG and distributed electrical source imaging (DESI) to investigate the neurophysiological underpinnings of the shine-through paradigm in schizophrenia patients and healthy controls. Whereas visual deficits in schizophrenia patients are usually related to dorsal processing deficits, we found, in addition, strong processing deficits in lateral occipital areas.
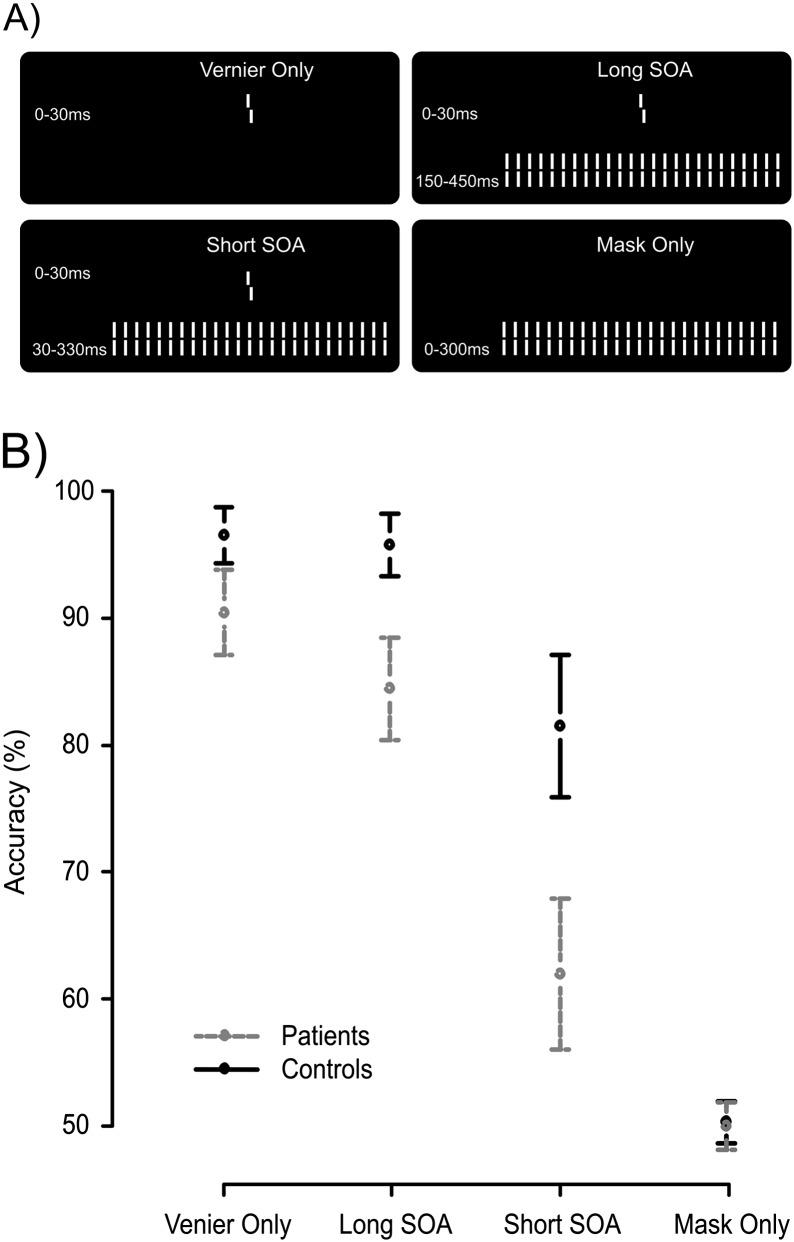
Fig. 1.
(A) The 4 stimulus conditions. In the first condition, a vernier was presented for 30 ms. Observers discriminated the offset direction (here, a right offset is shown). In the second and third condition, the 30 ms vernier was followed by a grating either immediately (short [stimulus onset asynchrony] SOA) or after a blank screen, ie, an interstimulus interval, of 120 ms (long SOA). We aimed for comparable performance of controls in the short SOA condition and patients in the long SOA condition. Fourth, we presented only the masking grating as a control. (B) Accuracy data. Patients were less accurate at discriminating the vernier offset in general, but the largest difference was seen in the short SOA condition. Error bars represent 95% CIs around the mean.
Methods and Materials
Observers
Two groups of observers from a Georgian sample participated. The patient group consisted of 22 individuals diagnosed with schizophrenia. Diagnosis was made according to Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, (DSM-IV) criteria, based on SCID-CV (Structured Clinical Interview for DSM-IV, Clinician Version). Psychopathological symptoms were assessed by means of Scale for the Assessment of Negative Symptoms (SANS) and Scale for the Assessment of Positive Symptoms (SAPS). Fourteen patients were inpatients; 8 were outpatients at the time of the study. All patients were on medication taking either clozapine, haloperidole, trifluoperazine, risperidone, olanzapine, fluphenazine, or zuclopenthixol, some of them took more than one antipsychotic drug. Four patients received mood stabilizers, 5 amitriptyline, and 7 diazepam. The controls were 20 observers recruited from the general population to match the patients as closely as possible with respect to age, education, and gender (table 1).
Table 1.
Average Statistics (±SD) of Schizophrenia Patients and Healthy Controls
| Schizophrenic Patients | Healthy Controls | |
| Gender (F/M) | 7/15 | 8/12 |
| Age | 33.5 ± 7.9 | 35.4 ± 10.5 |
| Education | 13 ± 2.4 | 15 ± 2.9 |
| Illness duration | 8.2 ± 6.4 | |
| SANS | 11 ± 5.4 | |
| SAPS | 13.4 ± 17.4 | |
| Degraded CPT (D′) | 2.8 ± 0.98 | 3.7 ± 0.72 |
| WCST (categories) | 2.4 ± 1.6 | 4.9 ± 1.5 |
| Handedness (R/L) | 21/1 | 18/2 |
| Visual acuity | 1.40 ± 0.29 | 1.56 ± 0.47 |
Abbreviations: SANS, Scale for the Assessment of Negative Symptoms; SAPS, Scale for the Assessment of Positive Symptoms; WCST, Wisconsin Card Sorting Test.
All observers had good visual acuity of at least 0.8 when using both eyes, as measured by the Freiburg Visual Acuity Test. Observers gave informed consent before the experiment. All procedures complied with the Declaration of Helsinki and were approved by the local ethics committee.
Stimuli and Apparatus
A vernier stimulus consisted of 2 vertical line segments of 10′ (arc minutes) separated by a gap of 1′, with a fixed horizontal offset of about 1.2′. The gratings consisted of 25 verniers without horizontal offset, separated horizontally by 3. 33′. Verniers were presented for 30 ms, gratings for 300 ms, in white on a black background (see figure 1A).
In the Vernier Only condition, only the vernier was presented. In the Long stimulus onset asynchrony (SOA) condition, a grating followed the vernier with a 150 ms SOA. In the Short SOA condition, the SOA was 30 ms. In previous studies, we found that with an SOA of 150 ms patients performed as well as controls with an SOA of 30 ms.3,20 In the Mask Only condition, only a grating was presented. Vernier offset direction (left/right) was chosen pseudorandomly such that half the trials had a left/right offset. In the Mask Only condition, accuracy was calculated by comparing the response (left/right offset) to a randomly chosen notional offset (not presented).
The stimuli appeared on a cathode ray tube screen (Siemens Fujitsu P796-1) with a 100 Hz refresh rate and maximal luminance of around 100 cd/m2 (measured with a GretagMacbeth Eye-One Display 2 colorimeter). Background luminance was below 1 cd/m2. Observers sat in a dimly lit room at 3.5 m from the monitor, so that one pixel comprised about 18″.
Procedure
Observers reported the perceived offset direction by pushing 1 of 2 buttons and guessed when they were not sure. Accuracy was emphasized over speed. The intertrial pause varied randomly between 1000 and 1500 ms. Stimulus conditions were presented randomly interleaved in blocks of 80 trials, in which each condition appeared 20 times. Eight blocks were presented, with a total of 160 repetitions per stimulus condition.
EEG Recording and Data Processing
The EEG was recorded at the Asatiani Psychiatric Hospital in Tbilisi, Georgia, using a BioSemi Active 2 system (BioSemi) with 64 Ag-AgCl sintered active electrodes positioned in a cap according to the 10–20 system, referenced to the common mode sense (CMS) electrode. The electrooculogram (EOG) was recorded with electrodes positioned 1 cm above and below the right eye and 1 cm lateral to the outer canthi. The recording sampling rate was 2048 Hz. Off-line data were downsampled to 512 Hz, DC corrected, and band-pass filtered between 1 and 40 Hz using a Butterworth filter (−12 db/octave roll off). Notch filters removed 50 Hz and monitor noise.
We did semiautomatic artifact detection between −100 and 400 ms around stimulus onset with a hard threshold of 75 μV on the EEG and EOG signals. Trials were rejected when amplitudes exceeded this threshold or when artifacts resulting from muscle tension or eye-movements were clearly present. Noisy electrodes were excluded from the artifact rejection procedure and later interpolated when necessary. One control observer was excluded from further analysis due to excessive blinks and muscle activity. For patients, 7.6% of epochs were rejected on average and for controls, 5.6%. Data were analyzed irrespective of the behavioral response.
The averaged epochs for each observer where time-locked to stimulus onset and re-referenced to the average reference. No correction for prestimulus amplitudes was applied. We interpolated noisy electrodes using 3D spline interpolation. We interpolated on average 0.9 electrodes for patients and 0.3 for controls. For display purposes, grand averages were computed per stimulus condition after normalizing the individual averages to their global field power (GFP) across the entire epoch. This normalization assures even contributions of individual averages to the grand-average amplitudes.
GFP Analysis
The GFP is an instantaneous reference-independent measure of the neural response strength throughout the brain, calculated as the SD of the electrical potentials across all electrodes.22 Especially in patients with possible frontal and parietal deficits,23 it is important to take into account all concurrent EEG sources. After the GFP analysis, we used source localization to identify the underlying EEG sources that differentiate between groups and conditions.
We computed a grand-average GFP for patients and controls in each of the 4 stimulus conditions (figure 1A) and identified the peak latencies for each condition. The peak latencies differed because the mask onset latency depended on condition. We statistically compared the GFP at the peak latencies across subjects with a 2-way repeated measures ANOVA of the factors Group (patients and controls) and Condition (Vernier Only, Long SOA, Short SOA, and Mask Only).
Distributed Electrical Source Imaging
From the individually averaged EEG data, we estimated current densities throughout the brain’s gray matter using a Local Auto-Regressive Average (LAURA) inverse solution.24 We defined a source space of 4022 points equally spaced throughout the gray matter of the Montreal Neurological Institute's 152 template brain. We used a 3-shell spherical head model to calculate the lead field after transforming the volume to a best-fitting sphere (Spherical Model with Anatomical Constraints, SMAC). A detailed description of DESI methods, and their accuracy, can be found in previous work.25,26
We statistically compared current density scalar amplitudes with a 2-way repeated measures ANOVA of the factors Group and Condition, with observers as a random factor. This analysis was done at the peak latency of the grand-average GFP for each group and condition, thus comparing the largest evoked responses across conditions. We controlled for multiple testing across the 4022 source points by setting the false discovery rate (FDR) to 0.05. The ANOVA and statistical thresholds were calculated using R (R-Development-Core-Team, 2011, http://www.R-project.org/, accessed November 9, 2011). EEG data processing and analyses were performed with the Cartool software (http://sites.google.com/site/fbmlab/cartool, accessed November 9, 2011).
Results
Accuracy
Patients showed decreased vernier offset discrimination accuracy in each of the 3 conditions that contained the target vernier. A repeated measures ANOVA of the factors Group (patient and control) and Condition (Vernier Only, Long SOA, and Short SOA) showed significant effects of Group, F 1,39 = 22.64, P < .0001, Condition, F 2,78 = 181.23, P < .0001, and a significant interaction effect F 2,78 = 14. 15, P < .0001. The interaction indicates that Group differences depend on stimulus condition. This interaction effect is displayed in figure 1B. Patients performed worse than controls in the Vernier Only and Long SOA condition (90.4 ± 7.6 SD % vs 96.5 ± 4.7, and 84.4 ± 9.0 vs 95.8 ± 5.2, respectively) but the largest deficit occurred in the Short SOA condition (62.0 ± 13.4 vs 81.5 ± 12.0). In the Long SOA condition, patients performed at about the same level as healthy controls in the Short SOA condition, as aimed for.
GFP
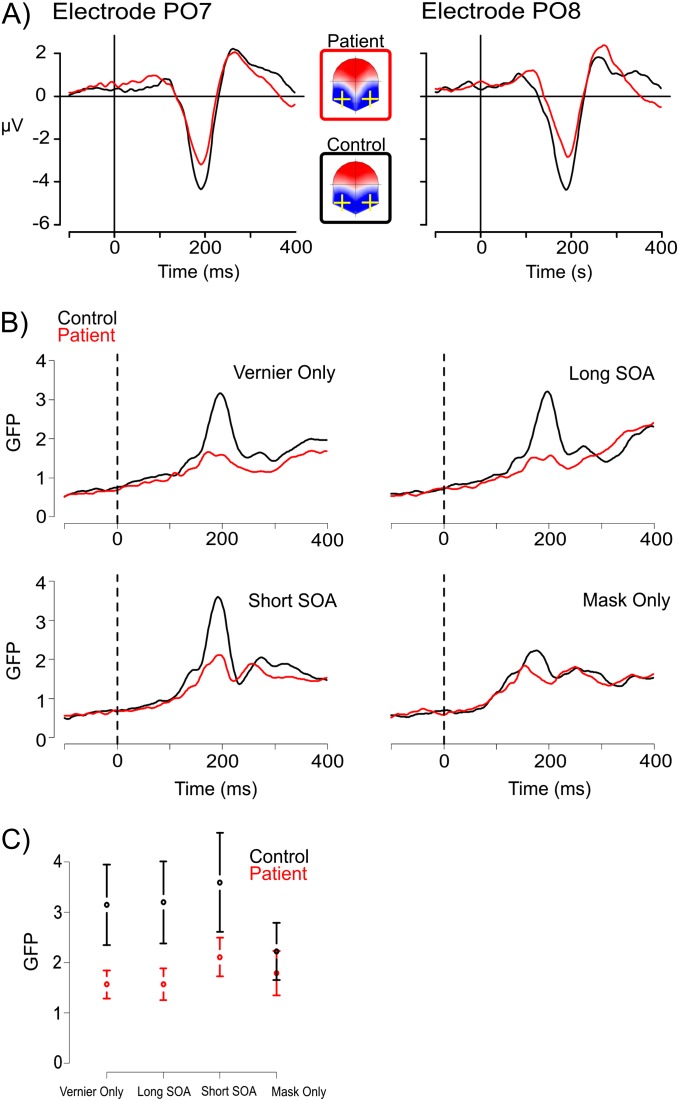
Figure 2 shows the grand-average EEG results. Patients and controls showed a prominent negative deflection at occipital electrodes PO7 and PO8 (panel A), and a corresponding peak in the GFP ranging between 160 ms (patients, Mask Only) and 205 ms (patients, Vernier Only/Long SOA) after stimulus onset (panel B). An ANOVA of the peak GFP amplitudes showed statistically significant main effects of Group, F 1,39 = 11.83, P = .001, Condition, F 1,117 = 14.16, P = .0001, and an interaction effect, F 1,117 = 10.30, P = .0001. Figure 2C displays this interaction, showing that patients had decreased GFP in all conditions with a vernier target; while in the Mask Only condition, their GFP was comparable to controls (P = .2).
Fig. 2.
(A) Voltage amplitudes of the occipital electrodes PO7 and PO8 for patients and controls in the short SOA condition. At around 200 ms, evoked voltage amplitudes were strongly reduced for the patients, while the instantaneous voltage topographies showed a bilateral posterior negativity resembling an N1 component. (B) The global field power (GFP) time series for patients and controls in the 4stimulus conditions showed differences in peak amplitude between groups and conditions, at around 200 ms. (C) The interaction effect of GFP amplitudes at the peak latencies indicates that the GFP decrease for patients is not as large in the Mask Only condition as compared with the other conditions, which contain a target vernier. Error bars indicate 95% CIs.
Distributed Electrical Source Imaging
To localize the GFP differences to underlying generators, we estimated current densities throughout the brain at the peak latencies of the grand-average GFP and calculated a repeated measures ANOVA. To identify areas where patients process the stimuli differently from controls, we located regions with an interaction effect between Group and Condition.
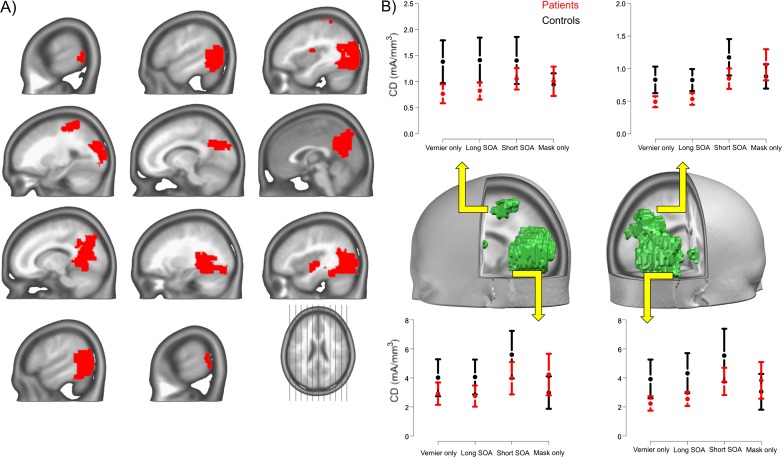
Figure 3 shows the locations of statistically significant interaction effects after correction for multiple testing. The regions were located in the left and right lateral occipital cortex (LOC) and more medially in the precuneus. In the left parietal cortex, the postcentral sulcus also showed an interaction effect. In addition, 2 isolated regions close to the left and right posterior insula showed an interaction effect. Table 2 lists the Talairach coordinates of the centers of mass for these areas. In each of the areas, similar interaction effects occurred, showing smaller differences between patients and controls in the Mask Only condition than in the 3 other conditions (figure 3B).
Fig. 3.
Source imaging results. Regions of significant Group × Condition effects (panel A, FDR = 0.05) are indicated in red. The interaction effects identify brain areas where patients show differences in the processing of specific visual stimuli. Panel B shows the direction of the interaction effects at the centers of mass (table 2) for 4 of the regions of panel A, the left parietal and left occipital cortex, and the right precuneus and occipital cortex. Error bars denote 95% CIs around the mean. The interactions indicate that patients and controls showed comparable activity in the Mask Only condition but not on the other 3 conditions. The effects were thus specific for conditions where a target vernier stimulus was presented. This effect held true in both the left parietal (dorsal stream) and the lateral occipital regions (ventral stream), showing that patients have both dorsal and ventral stream deficits at around 200 ms, but only when the target stimulus was presented.
Table 2.
Locations of the Center of Mass for the Significant Interaction Effects
| Label | x | y | z |
| Left postcentral gyrus | −28 | −34 | 58 |
| Left insula | −38 | −6 | 14 |
| Left precuneus | −15 | −70 | 29 |
| Left middle occipital gyrus | −49 | −67 | 6 |
| Right insula | 37 | −12 | 0 |
| Right middle temporal gyrus | 47 | −60 | 6 |
| Right precuneus | 3 | −64 | 23 |
Discussion
We studied the neurophysiological underpinnings of visual processing in schizophrenic patients and healthy controls by combining a masking paradigm with EEG and electrical source imaging. Patients showed strongly decreased electrophysiological responses, particularly, at around 200 ms after stimulus onset, ie, around the N1 component (figure 2). This effect was predominantly localized to the LOC, that is, to the ventral stream of visual processing. Patients also showed decreased activity in the left parietal cortex, which is part of the dorsal stream. All effects were specific for the target vernier and did not occur when only the mask was presented. This suggests that the deficits manifest only when fine shape discriminations are required and strongly indicates that top-down processes are responsible for the observed deficits in the ventral stream.
Evidence for dorsal stream impairments in patients comes from the observed decreased P1 amplitudes at around 100 ms.9,10 Although our small stimuli did not evoke a strong P1 component and we consequently saw no evidence in our data for a P1 reduction, such reductions have been repeatedly reported in schizophrenic patients and their siblings, making dorsal stream deficits, a candidate endophenotype.4 Reduced P1 amplitudes can be considered in line with the proposal that schizophrenic patients suffer from M-system deficiencies because the P1 reflects dorsal more than ventral stream processing,27 and the dorsal system receives predominantly M-pathway input.
Ventral stream processing is best observed at lateral occipital electrode locations between 150 and 200 ms after stimulus onset at the N1 component, reflecting higher-order object processing.13,28 In contrast to the well-known deficiencies in the dorsal stream, the first 250 ms of ventral stream processing have often been found to be intact in schizophrenic patients.7,10 For example, the N1 component of patients was comparable to that of controls for illusory contour stimuli, which, however, evoked a decreased P1 in patients at around 100 ms, indicating deficits in dorsal stream processing.10 When ventral stream deficits were observed, they occurred at latencies beyond 250 ms after stimulus onset.7,10 The N1 amplitudes were likely comparable to controls due to the specific stimuli and task used in these experiments. The illusory contour stimuli in Foxe et al10 seem to require little detailed processing and so does the recognition task based on degraded stimuli used in Doniger et al.7 When the experimental task requires no fine spatial discrimination, ventral stream activity in patients may appear similar to that of controls because the task demands are low. However, we expect impaired processing in the ventral stream when task demands are higher. Face stimuli, for example, elicit strong responses in ventral areas and, consequently, N1 deficiencies are evident in schizophrenic patients for these stimuli.14,15 Likewise, we found strong reductions of the response in LOC in a difficult vernier offset discrimination task.
Another goal of the current study was to investigate whether neural processing of patients and controls is similar under conditions of similar behavioral performance. For this reason, we aimed to equate performance by adjusting the SOA based on previous work.3 In the current work, patients responded as accurately in the Long SOA condition as controls in the Short SOA condition (figure 3). If patients need more time for processing the target vernier, we expected EEG signals to be similar in peak amplitudes but delayed. However, we still found strongly reduced GFP for patients around the N1 latency in the long SOA condition. This shows that the longer SOA needed for patients to reach normal performance levels does not come from a slowing of neural processing but rather from a reduced response in parietal and lateral occipital areas. This result suggests that patients have problems discriminating a target, not because of slowed processing but because of reduced activity in dorsal and ventral stream areas.
It seems unlikely that the results reflect general decreased attention to the stimuli in patients. If this were the case, similar decreases in more widespread areas would be expected, including the frontal areas that reflect attention, such as the frontal eye fields. Although our analyses included these areas, we did not observe effects in frontal areas related to attention.
The reduced activity in LOC most likely results from reduced top-down processing of the target stimuli. Our data show that the processing of the mask is mostly intact, while processing of the vernier target (Vernier alone, Short, and Long SOA condition) leads to strongly reduced GFP. If bottom-up processing was impaired, similar deficits would have been found for the mask only condition. This is in good agreement with previous studies where subtle variations in the mask had comparable effects in patients and controls,29,30 again pointing to deficient target processing rather than a general visual deficit. It seems that recurrent or top-down processing is impaired in the patients while bottom-up processing is intact.20 This result is in line with fMRI work showing comparable V1 activity for patients and controls in a perceptual grouping task while LOC activity was decreased for patients.31 Work by Green et al17,18 using fMRI furthermore shows that LOC activity is reduced in masking paradigms. Our results replicate these findings using EEG in a fine shape discrimination task, but also show that the effects, at around 200 ms after stimulus onset at least, may not be specifically related to masking but rather to detailed representations of task-relevant shapes. An explanation for the large behavioral deficit in the Short SOA condition may thus be that briefly presented targets cannot be efficiently transferred into a stable representation and are hence more vulnerable to masking.18
Top-down influence typically correlates well with activity in the frontoparietal attention network. Within this network, patients only showed decreased activity in parietal cortex, at around the same latency as reductions in ventral areas. We therefore hypothesize that the ventral stream impairments at least partly depend on impaired dorsal stream processing.7,10 The mechanisms for this are still speculative, but the following account of the data is in line with previously observed impairments in top-down processing in schizophrenic patients32 and could provide a unified framework for understanding both dorsal and ventral stream deficits as closely tied together. One important function of parietal cortex (dorsal stream) is to code for spatial location and attending to stimuli.11,33,34 Accurate encoding of location is a prerequisite for detailed inspection of a visual stimulus. When dorsal stream processing in schizophrenic patients fails to encode the exact location of the target object, this makes fine discriminations about, for example, vernier offsets difficult. It has recently been shown that inferior parietal cortex (dorsal stream), together with the frontal eye fields, exerts top-down control over earlier visual areas (V3) in the case of attention.35 This opens up the possibility that deficiencies in dorsal visual stream may lead to deficiencies in the ventral stream. If we assume that dorsal areas make available information about the spatial location of the stimulus to areas in the ventral stream, then dorsal stream information will help computations for fine shape discriminations. On this account, healthy controls more successfully encode the target location, whereas schizophrenic patients have trouble establishing a spatial reference and consequently show decreased ventral stream activity and impaired discrimination performance. This interaction could be both direct,11,36 but also via frontal cortex, as a recent fMRI study suggests.37
In addition to reduced ventral stream activity, patients also showed decreased activity in the posterior insula. The insula reflects several functions, but one important role is the higher-level integration of information from different modalities and brain areas.38 Although the localization precision of EEG source imaging is necessarily limited, the results correspond well to previous EEG work that found that decreased insula activity follows decreased activity in ventral stream areas.25 The decrease in schizophrenic patients may therefore reflect an impairment in collecting visual evidence for a subsequent decision, resulting from the deficient activity in lateral occipital areas, but direct test of this idea are so far lacking.
Taken together, our results show that in addition to the well-documented deficits in the dorsal stream, similar deficits are present in the ventral stream of schizophrenic patients. This corroborates previously reported deficits in ventral areas,17,31 as well as morphological changes in the ventral stream.39,40 Our results furthermore demonstrate that ventral stream deficiencies can occur at short latencies after stimulus onset, ie, before 210 ms. Because they are specific to the processing of targets, we relate these deficiencies to failures of top-down processes. The results are in line with the idea that dysfunction in the dorsal visual pathway of patients fails to provide a stable coding of spatial location, which hinders fine shape discriminations in the ventral stream. Such imprecise visual representations may also contribute to subsequent distortions in thought processes, decisions, and behavior.
Funding
This work was supported by the Volkswagen Foundation project “Between Europe and the Orient—A Focus on Research and Higher Education in/on Central Asia and the Caucasus.” Michael Herzog is a member of the Swiss National Science Foundation NCCR project “Synapsy.” The Cartool software (http://sites.google.com/site/fbmlab/cartool, accessed November 9, 2011) has been programmed by Denis Brunet, from the Functional Brain Mapping Laboratory, Geneva, Switzerland, and is supported by the Center for Biomedical Imaging (CIBM) of Geneva and Lausanne.
Acknowledgments
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Silverstein SM, Keane BP. Vision science and schizophrenia research: toward a re-view of the disorder. Editors' introduction to special section. Schizophr Bull. 2011;37:681–689. doi: 10.1093/schbul/sbr053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schechter I, Butler PD, Zemon VM, et al. Impairments in generation of early-stage transient visual evoked potentials to magno- and parvocellular-selective stimuli in schizophrenia. Clin Neurophysiol. 2005;116:2204–2215. doi: 10.1016/j.clinph.2005.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chkonia E, Roinishvili M, Makhatadze N, et al. The shine-through masking paradigm is a potential endophenotype of schizophrenia. PloS One. 2010;5:e14268. doi: 10.1371/journal.pone.0014268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yeap S, Kelly SP, Sehatpour P, et al. Early visual sensory deficits as endophenotypes for schizophrenia: high-density electrical mapping in clinically unaffected first-degree relatives. Arch Gen Psychiatry. 2006;63:1180–1188. doi: 10.1001/archpsyc.63.11.1180. [DOI] [PubMed] [Google Scholar]
- 5.Butler PD, Javitt DC. Early-stage visual processing deficits in schizophrenia. Curr Opin Psychiatry. 2005;18:151–157. doi: 10.1097/00001504-200503000-00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butler PD, Zemon V, Schechter I, et al. Early-stage visual processing and cortical amplification deficits in schizophrenia. Arch Gen Psychiatry. 2005;62:495–504. doi: 10.1001/archpsyc.62.5.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Doniger GM, Foxe JJ, Murray MM, Higgins BA, Javitt DC. Impaired visual object recognition and dorsal/ventral stream interaction in schizophrenia. Arch Gen Psychiatry. 2002;59:1011–1020. doi: 10.1001/archpsyc.59.11.1011. [DOI] [PubMed] [Google Scholar]
- 8.Donohoe G, Morris DW, De Sanctis P, et al. Early visual processing deficits in dysbindin-associated schizophrenia. Biol Psychiatry. 2008;63:484–489. doi: 10.1016/j.biopsych.2007.07.022. [DOI] [PubMed] [Google Scholar]
- 9.Foxe JJ, Doniger GM, Javitt DC. Early visual processing deficits in schizophrenia: impaired P1 generation revealed by high-density electrical mapping. Neuroreport. 2001;12:3815–3820. doi: 10.1097/00001756-200112040-00043. [DOI] [PubMed] [Google Scholar]
- 10.Foxe JJ, Murray MM, Javitt DC. Filling-in in schizophrenia: a high-density electrical mapping and source-analysis investigation of illusory contour processing. Cereb Cortex. 2005;15:1914–1927. doi: 10.1093/cercor/bhi069. [DOI] [PubMed] [Google Scholar]
- 11.Kravitz DJ, Saleem KS, Baker CI, Mishkin M. A new neural framework for visuospatial processing. Nat Rev. 2011;12:217–230. doi: 10.1038/nrn3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Plomp G, Leeuwen C, Ioannides AA. Functional specialization and dynamic resource allocation in visual cortex. Hum Brain Mapp. 2010;31:1–13. doi: 10.1002/hbm.20840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel EK, Luck SJ. The visual N1 component as an index of a discrimination process. Psychophysiology. 2000;37:190–203. [PubMed] [Google Scholar]
- 14.Turetsky BI, Kohler CG, Indersmitten T, Bhati MT, Charbonnier D, Gur RC. Facial emotion recognition in schizophrenia: when and why does it go awry? Schizophr Res. 2007;94:253–263. doi: 10.1016/j.schres.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Herrmann MJ, Ellgring H, Fallgatter AJ. Early-stage face processing dysfunction in patients with schizophrenia. Am J Psychiatry. 2004;161:915–917. doi: 10.1176/appi.ajp.161.5.915. [DOI] [PubMed] [Google Scholar]
- 16.Onitsuka T, Niznikiewicz MA, Spencer KM, et al. Functional and structural deficits in brain regions subserving face perception in schizophrenia. Am J Psychiatry. 2006;163:455–462. doi: 10.1176/appi.ajp.163.3.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Green MF, Lee J, Cohen MS, et al. Functional neuroanatomy of visual masking deficits in schizophrenia. Arch Gen Psychiatry. 2009;66:1295–1303. doi: 10.1001/archgenpsychiatry.2009.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Green MF, Lee J, Wynn JK, Mathis KI. Visual masking in schizophrenia: overview and theoretical implications. Schizophr Bull. 2011;37:700–708. doi: 10.1093/schbul/sbr051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Silverstein SM, Berten S, Essex B, Kovacs I, Susmaras T, Little DM. An fMRI examination of visual integration in schizophrenia. J Integr Neurosci. 2009;8:175–202. doi: 10.1142/s0219635209002113. [DOI] [PubMed] [Google Scholar]
- 20.Herzog MH, Kopmann S, Brand A. Intact figure-ground segmentation in schizophrenia. Psychiatry Res. 2004;129:55–63. doi: 10.1016/j.psychres.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Srebro R, Osetinsky MV. The localization of cortical activity evoked by vernier offset. Vision Res. 1987;27:1387–1390. doi: 10.1016/0042-6989(87)90215-x. [DOI] [PubMed] [Google Scholar]
- 22.Murray MM, Brunet D, Michel CM. Topographic ERP analyses: a step-by-step tutorial review. Brain Topogr. 2008;20:249–264. doi: 10.1007/s10548-008-0054-5. [DOI] [PubMed] [Google Scholar]
- 23.Meyer-Lindenberg A. From maps to mechanisms through neuroimaging of schizophrenia. Nature. 2010;468:194–202. doi: 10.1038/nature09569. [DOI] [PubMed] [Google Scholar]
- 24.Grave de Peralta Menendez R, Murray MM, Michel CM, Martuzzi R, Gonzalez Andino SL. Electrical neuroimaging based on biophysical constraints. Neuroimage. 2004;21:527–539. doi: 10.1016/j.neuroimage.2003.09.051. [DOI] [PubMed] [Google Scholar]
- 25.Plomp G, Mercier MR, Otto TU, Blanke O, Herzog MH. Non-retinotopic feature integration decreases response-locked brain activity as revealed by electrical neuroimaging. Neuroimage. 2009;48:405–414. doi: 10.1016/j.neuroimage.2009.06.031. [DOI] [PubMed] [Google Scholar]
- 26.Plomp G, Michel CM, Herzog MH. Electrical source dynamics in three functional localizer paradigms. Neuroimage. 2010;53:257–267. doi: 10.1016/j.neuroimage.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 27.Martinez A, Di Russo F, Anllo-Vento L, Hillyard SA. Electrophysiological analysis of cortical mechanisms of selective attention to high and low spatial frequencies. Clin Neurophysiol. 2001;112:1980–1998. doi: 10.1016/s1388-2457(01)00660-5. [DOI] [PubMed] [Google Scholar]
- 28.Itier RJ, Taylor MJ. Source analysis of the N170 to faces and objects. Neuroreport. 2004;15:1261–1265. doi: 10.1097/01.wnr.0000127827.73576.d8. [DOI] [PubMed] [Google Scholar]
- 29.Schutze C, Bongard I, Marbach S, Brand A, Herzog MH. Collinear contextual suppression in schizophrenic patients. Psychiatry Res. 2007;150:237–243. doi: 10.1016/j.psychres.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 30.Roinishvili M, Chkonia E, Brand A, Herzog MH. Contextual suppression and protection in schizophrenic patients. Eur Arch Psychiatry Clin Neurosci. 2008;258:210–216. doi: 10.1007/s00406-007-0780-9. [DOI] [PubMed] [Google Scholar]
- 31.Silverstein SM, Keane BP. Perceptual organization impairment in schizophrenia and associated brain mechanisms: review of research from 2005 to 2010. Schizophr Bull. 2011;37:690–699. doi: 10.1093/schbul/sbr052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dima D, Dietrich DE, Dillo W, Emrich HM. Impaired top-down processes in schizophrenia: a DCM study of ERPs. Neuroimage. 2010;52:824–832. doi: 10.1016/j.neuroimage.2009.12.086. [DOI] [PubMed] [Google Scholar]
- 33.Corbetta M, Patel G, Shulman GL. The reorienting system of the human brain: from environment to theory of mind. Neuron. 2008;58:306–324. doi: 10.1016/j.neuron.2008.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mishkin M, Ungerleider LG, Macko KA. Object vision and spatial vision: two cortical pathways. Trends Neurosci. 1983;6:414–417. [Google Scholar]
- 35.Bressler SL, Tang W, Sylvester CM, Shulman GL, Corbetta M. Top-down control of human visual cortex by frontal and parietal cortex in anticipatory visual spatial attention. J Neurosci. 2008;28:10056–10061. doi: 10.1523/JNEUROSCI.1776-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Webster MJ, Bachevalier J, Ungerleider LG. Connections of inferior temporal areas TEO and TE with parietal and frontal cortex in macaque monkeys. Cereb Cortex. 1994;4:470–483. doi: 10.1093/cercor/4.5.470. [DOI] [PubMed] [Google Scholar]
- 37.Sehatpour P, Dias EC, Butler PD, et al. Impaired visual object processing across an occipital-frontal-hippocampal brain network in schizophrenia: an integrated neuroimaging study. Arch Gen Psychiatry. 2010;67:772–782. doi: 10.1001/archgenpsychiatry.2010.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Craig AD. How do you feel–now? The anterior insula and human awareness. Nature Rev. 2009;10:59–70. doi: 10.1038/nrn2555. [DOI] [PubMed] [Google Scholar]
- 39.McDonald B, Highley JR, Walker MA, et al. Anomalous asymmetry of fusiform and parahippocampal gyrus gray matter in schizophrenia: a postmortem study. Am J Psychiatry. 2000;157:40–47. doi: 10.1176/ajp.157.1.40. [DOI] [PubMed] [Google Scholar]
- 40.Lee CU, Shenton ME, Salisbury DF, et al. Fusiform gyrus volume reduction in first-episode schizophrenia: a magnetic resonance imaging study. Arch Gen Psychiatry. 2002;59:775–781. doi: 10.1001/archpsyc.59.9.775. [DOI] [PubMed] [Google Scholar]