Abstract
Drug abuse and addiction are excessively common in schizophrenia. Chronic antipsychotic treatment might contribute to this comorbidity by inducing supersensitivity within the brain’s dopamine system. Dopamine supersensitivity can enhance the incentive motivational properties of reward cues, and reward cues contribute to the maintenance and severity of drug addiction. We have shown previously that rats withdrawn from continuous haloperidol (HAL) treatment (via subcutaneous minipump) develop dopamine supersensitivity and pursue reward cues more vigorously than HAL-naive rats following an amphetamine (AMPH) challenge. Atypical antipsychotic drugs are thought to be less likely than typicals to produce dopamine supersensitivity. Thus, we compared the effects of HAL and the atypical antipsychotic olanzapine (OLZ) on the pursuit of reward cues. Rats were trained to associate a light-tone cue with water then treated with HAL or OLZ. Following antipsychotic withdrawal, we assessed AMPH-induced enhancement of lever pressing for the cue. Withdrawal from HAL, but not from OLZ, enhanced this effect. HAL, but not OLZ, also enhanced AMPH-induced psychomotor activation and c-fos mRNA expression in the caudate-putamen. Thus, prior HAL, but not OLZ, enhanced conditioned reward following an AMPH challenge, and this was potentially linked to enhanced behavioral sensitivity to AMPH and AMPH-induced engagement of the caudate-putamen. These findings suggest that HAL, but not an atypical like OLZ, modifies reward circuitry in ways that increase responsiveness to reward cues. Because enhanced responsiveness to reward cues can promote drug-seeking behavior, it should be investigated whether atypical antipsychotics might be a preferential option in schizophrenic patients at risk for drug abuse or addiction.
Key words: antipsychotic drug, conditioned reward, Pavlovian conditioning, incentive motivation, dopamine supersensitivity, immediate early gene
Introduction
Of all individuals with a lifetime diagnosis of schizophrenia, up to half have a substance abuse problem.1 This subpopulation of schizophrenia patients are more difficult to treat2 and have a poorer long-term outcome.3 Schizophrenia patients might take drugs to self-medicate4 and/or because the neuropathology of schizophrenia includes neural changes that increase the vulnerability to drug addiction.5 A complementary hypothesis is that chronic antipsychotic treatment might alter brain reward circuits in ways that could contribute to the abnormally high rates of drug abuse and addiction in schizophrenia.6–8 This idea is supported by a number of observations. First, both schizophrenic and nonschizophrenic psychiatric patients treated with antipsychotic compounds exhibit higher rates of psychostimulant drug addiction than the general population.8 Second, the onset of drug abuse follows the onset of schizophrenia (and of antipsychotic treatment) in a considerable proportion of patients (38%)9, and substance abuse is more prevalent in schizophrenia than in many other psychiatric populations.1 In laboratory animals, chronic antipsychotic treatment increases sensitivity to the place-conditioning effects of cocaine10 and heroin,11 increases cocaine self-administration,12 and enhances brain self-stimulation.13
It is not known how prolonged exposure to antipsychotic medication might enhance reward function. One potential mechanism is by making dopamine receptors supersensitive to agonist stimulation. Antipsychotic-induced dopamine supersensitivity is documented in both humans and laboratory animals. For example, medicated schizophrenic patients given doses of psychostimulant drugs that are subpsychotogenic in nonschizophrenic individuals show an increase in the occurrence of psychosis relative to untreated schizophrenics.14 In rats, chronic antipsychotic treatment can augment the psychomotor response to dopamine agonists.6,15,16 At the neurobiological level, antipsychotic-induced dopamine receptor sensitivity is linked to increases in the density and sensitivity of striatal D2 receptors.15,17,18
A supersensitive dopamine system can increase sensitivity to the psychotogenic and psychomotor effects of drugs but also to the motivational properties of reward cues. Such cues can play a significant role in the maintenance and severity of drug addiction. In both humans and laboratory animals, drug cues can generate motivational states that can elicit or energize drug-seeking behaviors19,20 and precipitate drug craving and relapse to drug use following abstinence.21,22 We have shown previously that withdrawal from continuous (via subcutaneous [SC] osmotic minipumps), but not from intermittent (via daily SC injections) haloperidol (HAL) treatment enhances the ability of a small dose of amphetamine (AMPH) to potentiate the operant pursuit of reward cues.6 Would chronic treatment with an atypical antipsychotic drug have similar effects? There are currently no published studies examining how a history of exposure to clinically pertinent doses of an atypical antipsychotic might influence reward function. It is important to address this issue for three principal reasons. First, atypical antipsychotics are now more widely used in the management of schizophrenia. Second, it is thought that atypical antipsychotics are less likely to induce dopamine receptor supersensitivity,23,24 suggesting that they might thus be less likely to perturb reward function. Third, given the disproportionate rates of drug abuse in schizophrenia, factors that might promote or protect from this comorbidity need to be studied. Here, we evaluated the hypothesis that unlike HAL, withdrawal from chronic olanzapine (OLZ) treatment would not alter AMPH-induced potentiation of conditioned reward (CR). To assess neuronal activation by AMPH following antipsychotic treatment, we also compared AMPH-induced expression of striatal c-fos mRNA following HAL versus OLZ treatment.
Methods
Subjects
Male Sprague-Dawley rats (Charles River Labora tories, Montréal, QC, Canada; 200–225g) were housed 2/cage in a climate-controlled colony room (12-h reverse light/dark cycle; lights off at 8 am). Food and water were available ad libitum unless noted otherwise. Testing occurred during the dark phase of the animals’ circadian cycle. Procedures complied with principles outlined by the Canadian Council on Animal Care. The Animal Ethics Committee of the Université de Montréal approved all experiments (Protocol Number: 10-106). Experimenters made all reasonable efforts to minimize animal suffering.
Drugs
HAL (Sabex, Boucherville, Canada) was dissolved in a 0.5% acetic acid/distilled water solution (pH adjusted to ~5 with sodium hydroxide [1M NaOH]) for treatment via minipump (Alzet model 2ML2, 19-day drug delivery; Durect Corporation, Cupertino, CA). This HAL/acetic acid/water formulation produces stable striatal D2 receptor occupancy levels for at least 2 weeks when delivered via minipump.15 OLZ (Toronto Research Chemicals, Toronto, Canada) was dissolved in a 2% acetic acid/distilled water solution (pH adjusted to ~5 with 1M NaOH) for treatment via minipump. An OLZ/acetic acid formulation delivered via minipump can lead to declining plasma levels of the antipsychotic 14 days into treatment.25,26 However, striatal D2 occupancy remains within the clinical range (74% ± 7% SD) at the 14-day time point.26 For SC injections, OLZ was dissolved in a 20 mmol/L phosphate-buffered saline (PBS) vehicle instead of an acetic acid solution. This is because the rats were injected daily for 17 days (see below) and an acetic acid/water solution can cause irritation. The OLZ/PBS solution was prepared daily (pH adjusted to ~7 with 1M NaOH). d-Amphetamine sulfate ( Sigma-Aldrich, Dorset, UK) was dissolved in 0.9% saline and given SC. All injections were in a volume of 1ml/kg.
Antipsychotic Treatment
Our goal was to compare the effects of HAL and OLZ using equivalent and clinically pertinent doses and modes of administration. To select appropriate antipsychotic doses in animal studies, a straightforward approach using the same mg/kg dose in rats as is used in humans would be inadequate, given the important pharmacokinetic differences that distinguish the two species.27 Instead, we have used a validated approach based on in vivo occupancy of a critical neurobiological target; the dopamine D2 receptor in the striatum.27
For many antipsychotics, doses that produce 65%–75% striatal D2 receptor occupancy are efficacious and unlikely to produce extrapyramidal side effects.28–30 The HAL and OLZ doses used here produce striatal D2 occupancy levels that lie within this range and that are equivalent. We used 0.5mg/kg/day HAL for administration via minipump because it produces 73% D2 occupancy ex vivo (±14 SD; see online supplementary material for a color version of this figure), as assessed using the procedures described in Samaha et al.15 We used 10mg/kg/day OLZ for administration via minipump based on our previous work15 and on work showing that a similar dose (7.5mg/kg/day) produces 74% (±7% SD) D2 receptor occupancy 2 weeks into treatment.26 We used 1mg/kg/day OLZ for administration via daily SC injection because it yields peak levels of 74% (±4.6% SD) D2 occupancy.30 In addition, in humans, a single dose of antipsychotic can maintain high striatal D2 receptor occupancy levels for several days.31 In rats, striatal D2 occupancy declines significantly 24h following a single SC injection but is continuous if antipsychotics are given via SC osmotic minipump.27 Thus, to achieve the relatively continuous D2 receptor occupancy that is produced by clinical antipsychotic treatment regimens, we administered HAL or OLZ via minipump. We also treated a group of rats with daily SC injections of OLZ because we have shown previously that withdrawal from continuous (via SC minipumps), but not from intermittent (via daily SC injections), HAL treatment enhances CR.6 Thus, four groups were generated as follows: CONT-HAL, receiving 0.5mg/kg/day HAL via SC minipump; CONT-OLZ, receiving 10mg/kg/day OLZ via SC minipump; INT-OLZ, receiving 1mg/kg OLZ via daily SC injection; VEH, receiving PBS via daily SC injection.
Under 1.5% isoflurane anesthesia, CONT-HAL and CONT-OLZ rats were implanted with minipumps as in Samaha et al.15 The INT-OLZ and VEH animals received sham surgery (an incision closed with wound clips). The next day and for 17 consecutive days, the INT-OLZ group was injected daily with OLZ. All remaining animals were injected daily with PBS. Thus, all animals were subjected to equivalent surgical, handling, and injection procedures. On the 18th day, under 1.5% isoflurane anesthesia, the minipumps were removed. INT-OLZ and VEH animals received a sham surgery.
Experiment 1: Effects of HAL or OLZ on Responding for Conditioned Reward and AMPH-Induced Locomotion
Here, we determined the effects of HAL or OLZ pretreatment on operant responding for a CR. We also assessed the locomotor response to AMPH to determine whether operant responding for a CR was linked to the development of supersensitivity to dopamine receptor stimulation.
Pavlovian Conditioning and Operant Responding for Conditioned Reward. Figure 1A shows the sequence of manipulations. Rats were restricted to 2h/day of access to water. For 10 daily sessions (~35min/session) in standard operant cages (MED Associates, St. Albans, VT, USA), the rats were trained to associate the delivery of 0.1ml tap water (the unconditioned stimulus; UCS) into a receptacle with a light-tone stimulus (the conditioned stimulus; CS), as in Bedard et al.6
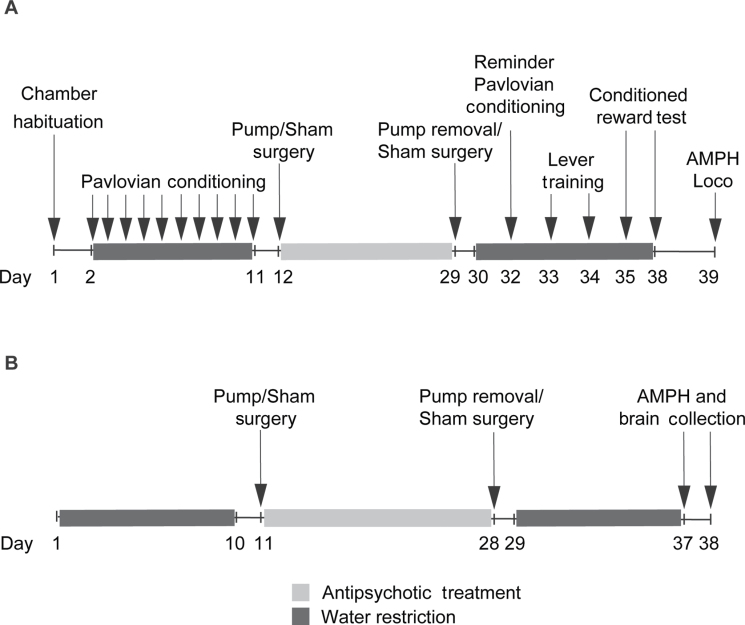
Fig. 1.
The sequence of experimental procedures for Experiment 1 (A), where operant responding for a conditioned reward was assessed following haloperidol/olanzapine treatment cessation, and Experiment 2 (B), where amphetamine (AMPH)-induced c-fos mRNA expression was measured. Loco, locomotion.
Nose-pokes into the receptacle during the 5-s CS presentation (CSR) and during the 5-s preceding CS presentation (PCSR) were recorded. A CSR/PCSR ratio served as an index of the learning of the CS-UCS association. Animals were then assigned to the VEH, CONT-HAL, CONT-OLZ, and INT-OLZ groups such that the average CSR/PCSR ratios were comparable across groups. Following antipsychotic treatment cessation, rats received an additional CS-UCS training session followed by two lever training sessions during which two levers were present. Pressing the left (active) lever produced the CS (now the CR) according to a random-ratio 2 schedule. Pressing the right lever (inactive) had no programmed consequences. The session ended following 40 min or 10 active-lever presses. No water was delivered. Next, two CR tests were given under the same conditions, except that lever presses were not limited and the rats were injected SC with saline (first test) or 0.5mg/kg AMPH (second test) before testing.
AMPH-Induced Locomotion. One day following CR testing, locomotor activity was measured for 30 min before and 60 min after AMPH (1.5mg/kg, SC), in Plexiglas cages, as in Samaha et al.15
Experiment 2: Effects of HAL or OLZ on c-fos mRNA Expression
In a separate set of rats, we examined the influence of CONT-HAL or CONT-OLZ treatment on the cells engaged by AMPH. To this end, we mapped AMPH-induced c-fos mRNA expression in cortical and striatal regions following antipsychotic treatment. We did not include INT-OLZ rats because they showed similar AMPH-induced responding for a CR and locomotion compared with VEH (see figures 2 and 3).
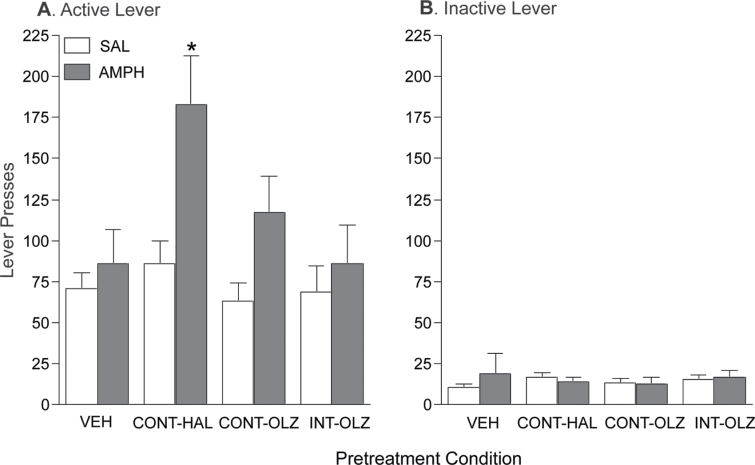
Fig. 2.
Continuous haloperidol (0.5mg/kg/day via minipump; CONT-HAL), but not continuous olanzapine (10mg/kg/day via minipump; CONT-OLZ) or intermittent olanzapine (1mg/kg/day via subcutaneous [SC] injection; INT-OLZ) treatment enhanced the ability of amphetamine (AMPH) to potentiate operant responding for a conditioned reward. n′s = 14/condition. *P < .05 compared with vehicle (VEH) and INT-OLZ. Data are means ± SEM. VEH, group injected daily with SC phosphate-buffered saline. SAL, saline.
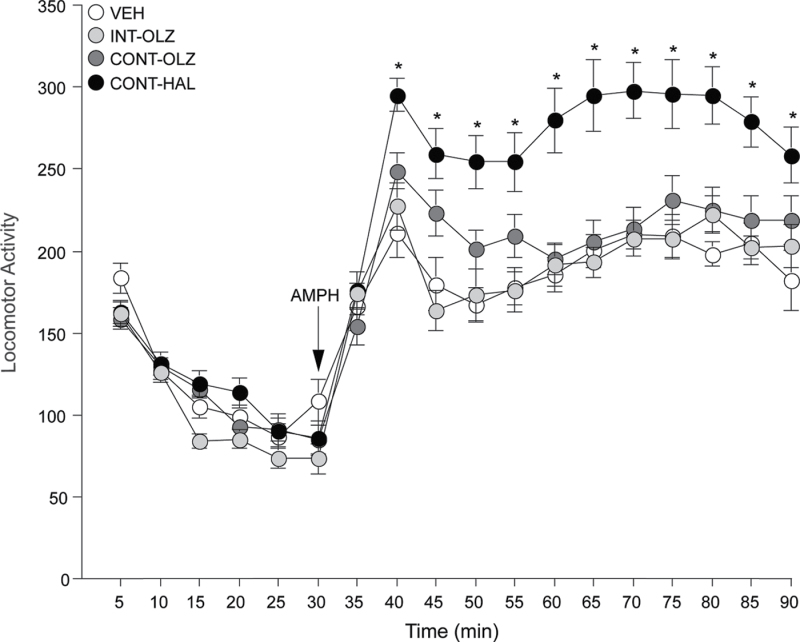
Fig. 3.
Continuous haloperidol (0.5mg/kg/day via minipump; CONT-HAL), but not continuous olanzapine (10mg/kg/day via minipump; CONT-OLZ) or intermittent olanzapine (1mg/kg/day via subcutaneous [SC] injection; INT-OLZ) treatment enhanced amphetamine-induced locomotion. n′s = 14/condition. *P < .05 compared with vehicle (VEH). Data are means ± SEM. VEH, group injected daily with SC phosphate-buffered saline. AMPH, amphetamine.
Antipsychotic Treatment and In Situ Hybridization. Figure 1B
shows the sequence of events. Antipsychotic treatment was as above and three groups were generated as follows: VEH, CONT-OLZ, and CONT-HAL.
On the 9th or 10th day following treatment cessation, rats from each group received a SC injection of saline, 0.5 or 1.5mg/kg AMPH in the home cage. Rats were sacrificed 1h following the injection. Brains were extracted, plunged into isopentane (−50°C) for 7 s and stored at −80°C until processing. c-fos mRNA expression was labeled on 12-μm-thick coronal brain sections using a [35S]-UTP-labeled riboprobe complementary to c-fos, as in Bedard et al.6 Brain sections were then apposed against X-ray film (Kodak Biomax-MR; Kodak, New Haven, CT, USA) for 4 days. mRNA was quantified on autoradiographs using ImageJ software (National Institutes of Health [NIH], Bethesda, MD, USA). ImageJ translates optical gray densities into µCi/g of tissue using a 14C standard curve (ARC-146A; American Radiolabeled Chemicals, St-Louis, MI, USA). A background value, obtained from the corpus callosum of each section, was subtracted from analysis. c-fos mRNA levels were quantified in the dorsomedial (DM), dorsolateral (DL), ventromedial (VM), and ventrolateral (VL) quadrants of the caudate-putamen (+1.6, +1.2, +0.8, +0.4, and 0.0mm relative to Bregma), in the core (AcbC) and shell (AcbSh) of the nucleus accumbens (+1.6, +1.2, and +0.8mm relative to Bregma), in the anterior cingulate (Cg1 and Cg3) and infralimbic (IL) cortices (+2.6mm relative to Bregma), and in the ventrolateral (VLO) and lateral (LO) orbitofrontal cortices (+3.0mm relative to Bregma). Anatomical regions were identified according to Paxinos and Watson.32 Sections were analyzed without awareness of group membership.
Statistics
Lever presses were analyzed using three-way ANOVA (group × lever type × AMPH dose). Significant interactions were investigated with the Bonferroni test. AMPH-induced locomotion and striatal c-fos mRNA levels were analyzed using two-way ANOVA (group × time or rostrocaudal level). Cortical c-fos mRNA levels were analyzed using one-way ANOVA followed by Tukey’s Multiple Comparison Test.
Results
Experiment 1: Effects of HAL or OLZ on Responding for Conditioned Reward and AMPH-Induced Locomotion
All rats were given an additional Pavlovian conditioning session following antipsychotic treatment cessation. During this session, all animals nose-poked more into the magazine containing the water receptacle during the 5-s CS period (CSR) than in the 5-s period before the onset on the CS (PCSR), and there were no group differences in this behavior (data not shown; one-way ANOVA on average CSR/PCSR ratios; F(3,52) = 0.00001, P = 1.00). Thus, all groups retained the CS-UCS contingency during the antipsychotic or VEH treatment period.
Figure 2 shows active (A) and inactive (B) lever presses during CR tests. All groups pressed more on the active than on the inactive lever (F(1,52) = 122.19, P = 0.000). Thus, all groups showed lever discrimination and spontaneously learned a novel operant behavior reinforced only by the CR. There was a significant group × injection × lever interaction (F(3, 52) = 3.90, P = 0.014). Post hoc analysis of this interaction revealed that following an injection of AMPH, CONT-HAL rats pressed significantly more on the active lever compared with VEH (P = .043) or INT-OLZ rats (P = .044). There were no other group differences following AMPH. In all groups, AMPH did not affect inactive lever presses (all P > .05). There were no group differences in active or inactive lever pressing following saline (all P > .05).
CONT-HAL rats showed greater AMPH-induced locomotion than VEH and CONT-OLZ rats (figure 3). Main effect of group on minutes 40–90 of the test session; versus VEH, F(1,26) = 24.04; versus CONT-OLZ, F(1, 26) = 11.01, all P < .003). CONT-OLZ and INT-OLZ rats did not differ from VEH rats (all P > .05).
Experiment 2: Effects of HAL or OLZ on c-fos mRNA Expression
In all groups, 0.5mg/kg AMPH did not increase c-fos mRNA levels relative to saline in any caudate-putamen quadrant at +0.8, +0.4, or 0.0mm relative to Bregma (data not shown; all P > .05). This is consistent with previous work showing that 0.5mg/kg AMPH does not increase the density of c-fos immunoreactive cells in striatal subregions.33
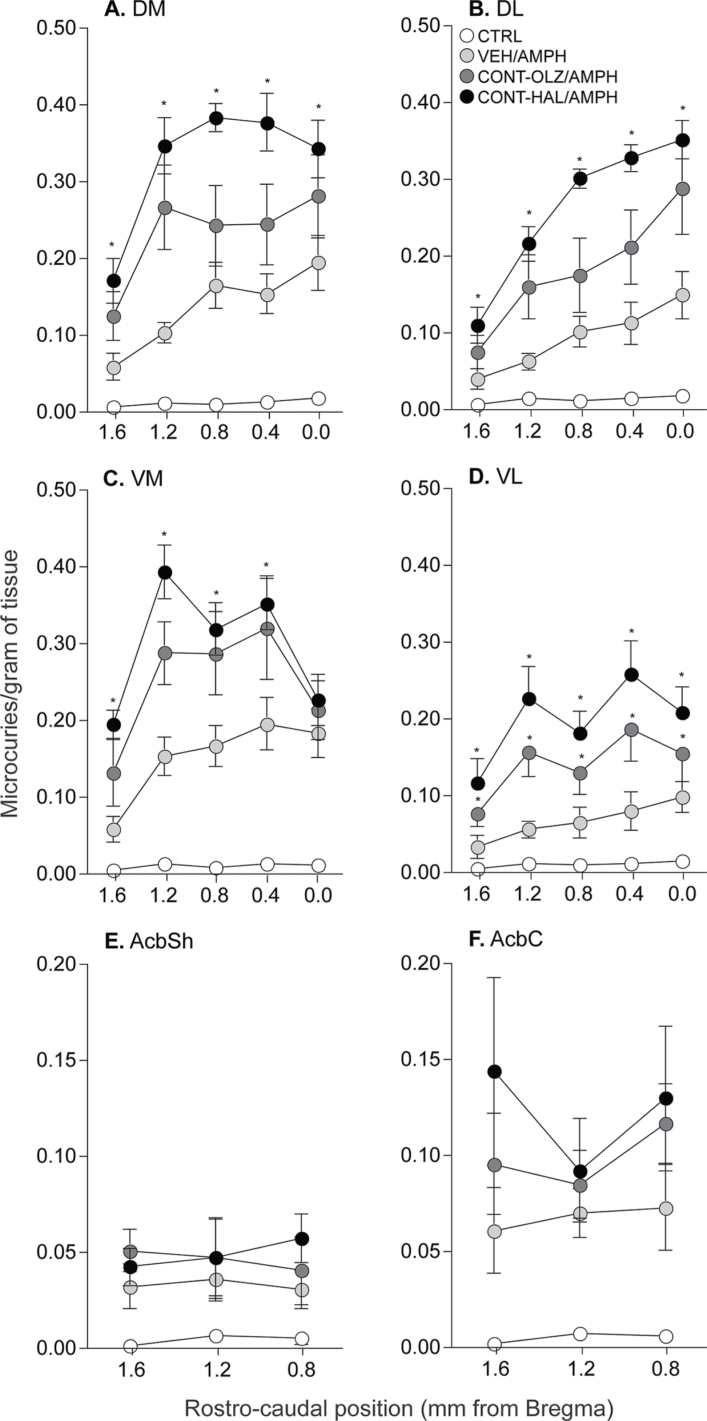
Saline-induced c-fos mRNA levels in VEH, CONT-OLZ, and CONT-HAL rats were similar in all striatal and cortical regions analyzed (data not shown; all P > .05). Thus, for each region (figures 4A–F and 5A–D), we pooled all saline-treated animals into one control (CTRL) group. In all caudate-putamen quadrants and in the nucleus accumbens shell and core (figures 4A–F), all AMPH-treated groups had greater c-fos mRNA expression compared with CTRL (all P < .0005). In addition, antipsychotic treatment altered the c-fos mRNA response to AMPH in the caudate-putamen (figures 4A–D; main effects of group; A, F(3,24) = 40.14; B, F(3,24) = 32.41; C, F(3,24) = 35.17; D, F(3,24) =18.47; group × rostrocaudal level interaction effects; A, F(12,24) = 4.149; B, F(12,24) = 9.037; C, F(12,24) = 4.845; D, F(12,24) = 2.929; all Ps < .05), but not in the nucleus accumbens. In all caudate-putamen quadrants and across the rostrocaudal gradient, CONT-HAL treatment potentiated AMPH-induced c-fos mRNA expression relative to VEH (A, F(1,11) = 43.01; B, F(1,11) = 54.87; C, F(1,11) = 19.28; D, F(1,11) = 12.32; all Ps < .005). In all caudate-putamen quadrants but the VL (figure 4D, where CONT-OLZ > VEH; F(1,11) = 7.16, P = .02), AMPH-induced c-fos mRNA levels were similar in CONT-OLZ and VEH rats (all P > .05). No other comparisons were significant. There were no group differences in AMPH-induced c-fos mRNA in the nucleus accumbens (figures 4E–F; all P > .05).
Fig. 4.
Continuous haloperidol treatment (0.5mg/kg/day via minipump; CONT-HAL) enhanced the c-fos mRNA response to amphetamine in the quadrants of the caudate-putamen (A–D) but not in the nucleus accumbens shell (E) or core (F). n′s = 6–9/condition. *P < .05 compared with VEH. Data are means ± SEM. VEH, group injected daily with subcutaneous phosphate-buffered saline. CONT-OLZ, group treated with 10mg/kg/day olanzapine via osmotic minipump; CTRL, combined control group receiving saline; AMPH, amphetamine; AcbSh, nucleus accumbens shell; AcbC, nucleus accumbens core; DM, dorsomedial; DL, dorsolateral; VM, ventrolateral; VL, ventrolateral.
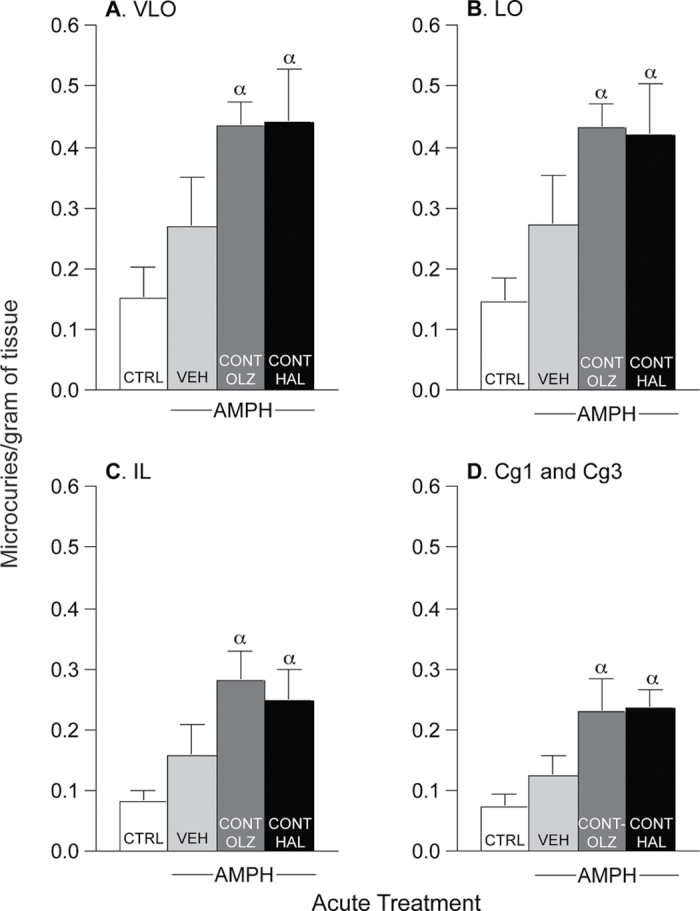
Fig. 5.
Continuous treatment with either haloperidol (0.5mg/kg/day via minipump; CONT-HAL) or olanzapine (10mg/kg/day via minipump; CONT-OLZ) enhanced amphetamine-induced c-fos mRNA expression in the ventrolateral (A: VLO) and lateral (B: LO) orbitofrontal cortices and in the infralimbic (C: IL) and anterior cingulate (D: Cg1 and Cg3) cortices when compared with the CTRL group. n′s = 6–9/condition. α: P < .05 compared with CTRL. Data are means ± SEM. VEH, group injected daily with subcutaneous phosphate-buffered saline; CTRL, combined control group receiving saline; AMPH, amphetamine.
Figure 5 shows c-fos mRNA levels in the ventrolateral (A; VLO) and lateral (B; LO) orbitofrontal cortex, as well as in the infralimbic (C; IL) and anterior cingulate (D; Cg1 + Cg3) cortices. In all of these areas, AMPH increased c-fos mRNA expression above CTRL levels only in CONT-HAL and CONT-OLZ rats (A, F(3,22) = 5.03; B, F(3,22) = 5.10; C, F(3,23) = 5.45; D, F(3,23) = 5.96; all P < .01). In the VEH group, AMPH did not induce c-fos mRNA expression above saline (ie, CTRL; all P > .05).
Discussion
A history of continuous exposure to HAL enhances the ability of AMPH to potentiate the operant pursuit of a CR, to produce psychomotor activation, and to augment immediate early gene expression in the caudate-putamen.6 Here, we asked whether a history of exposure to a clinically pertinent dose of the atypical antipsychotic OLZ would have the same effects. We found that it does not. Consistent with our previous work,6 withdrawal from HAL augmented the ability of AMPH to potentiate instrumental responding for a reward cue (a light-tone cue associated with water), to elicit locomotor activity, and to induce c-fos mRNA expression across the rostrocaudal gradient of the caudate-putamen. These effects were either absent or significantly less pronounced following a history of continuous or intermittent treatment with OLZ. HAL and OLZ led to different outcomes in spite of the fact that we used comparable and clinically representative doses (as measured by striatal D2 occupancy levels) and held constant the route and the duration of antipsychotic drug administration. Thus, our findings suggest that a history of continuous exposure to a typical antipsychotic such as HAL, but not to an atypical antipsychotic such as OLZ, can produce sensitization-like changes in incentive motivation and CR processes that persist after the discontinuation of antipsychotic treatment.
Why does a history of HAL, but not OLZ, treatment enhance the ability of AMPH to potentiate CR? We compared doses of OLZ and HAL that produce similar levels of D2 occupancy, and when administered to rats via minipump, both HAL15 and OLZ26 maintain high striatal D2 receptor occupancy for the duration of the ~2-week treatment used here. As such, both treatments likely produce continual disruption of D2 receptor-mediated dopamine neurotransmission. However, the degree of dopaminergic disruption might be different under the two conditions. Atypical antipsychotics including OLZ are more loosely bound to the D2 receptor compared with typical antipsychotics.34 As such, OLZ might be more easily displaced from dopamine receptors by endogenous dopamine, therefore permitting a greater degree of endogenous dopaminergic stimulation.34 This in turn could reduce the likelihood or magnitude of compensatory neuroadaptations within the dopamine system produced by prolonged antipsychotic exposure. As the present findings suggest, the functional consequences of such neuroadaptations could include hypersensitivity to reward and psychomotor activation following dopaminergic stimulation (achieved here with an AMPH challenge), and altered AMPH-induced gene regulation within the caudate-putamen. Alternatively, the present findings might involve the fact that OLZ, but not HAL, has potent inverse agonist/antagonist actions at the 5-HT2C receptor.35,36 5-HT2C receptor inverse agonists/antagonists enhance striatal dopamine release in vivo.37 As such, OLZ could increase endogenous striatal dopamine levels via its actions at 5-HT2C receptors. This in turn would displace some of the OLZ that is bound to D2 receptors, thereby partially restoring D2-mediated dopamine neurotransmission during antipsychotic treatment and preventing or minimizing compensatory neuroadaptations. A number of additional pharmacological properties distinguish OLZ from typical antipsychotics. For example, OLZ has effects on D4, D1, muscarinic, and histaminergic receptors.38 Any of these actions could contribute to the effects seen here.
What implications might the current findings have for understanding the high rates of drug abuse and addiction in schizophrenia? To begin to address this question, we must consider the role of reward cues in drug-seeking and drug-taking behaviors. Environmental and interoceptive cues that predict drug reward contribute in powerful ways to the initiation and persistence of drug addiction.39 As stated by Levison40 30 years ago, “When stimuli in the environment indicate availability to the addict, steps to taking a substance or engaging in an activity appear to be inexorable” (p. 29). Indeed, clinical observations suggest that compulsive drug use can be highly stimulus bound.41 Many psychological mechanisms contribute to the ability of reward cues to control behavior. For example, drugs cues can elicit strong incentive motivational states that can in turn drive drug-seeking behavior. Indeed, in addicts, stimuli that predict drug availability elicit attention and approach42,43 and invoke states (eg, conditioned craving) that can support compulsive drug seeking and induce relapse during abstinence.22,39 In laboratory animals, drug-associated cues also elicit attention and approach,44 precipitate the reinstatement of previously extinguished drug-seeking behaviors,21,45 and promote drug-taking behaviors.46 The current findings show that a history of continuous treatment with HAL, but not with OLZ, can markedly enhance the ability of reward cues to gain control over behavior. Thus, combined with our previous work,6 these results suggest that prolonged exposure to HAL can change incentive motivational processes in ways that could contribute to the compulsive and persistent nature of drug addiction and that exposure to OLZ might be less likely to induce such changes.
An implication of the present work is that the likelihood and/or severity of drug addiction would be less in individuals treated with atypical antipsychotics such as OLZ than in those treated with typicals like HAL. Is there evidence supporting this? First, it should be noted that in the clinic, antipsychotic treatment is an especially dynamic process. Many schizophrenia patients frequently switch medications, take more than one medication at a time and are exposed to both typical and atypical antipsychotics throughout their lifetime.47 This reality in mind, prolonged exposure to a typical antipsychotic like HAL versus an atypical like OLZ is associated with greater sensitivity to certain effects of drugs and drug cues that are relevant for addiction. A small but rigorous double-blind study found that HAL-treated schizophrenic individuals have significantly higher scores on the energy subcomponent of cue-elicited cocaine craving compared with OLZ-treated individuals.48 A study examining the outcome of schizophrenia patients before and following a switch from a typical antipsychotic to OLZ found that compared with baseline (but not to a group of patients who remained on typical antipsychotics), alcohol and other drug use decreased following the medication switch.49 Finally, both schizophrenic and nonschizophrenic psychiatric patients prescribed typical antipsychotics have high rates of psychostimulant drug abuse, suggesting a link between neuroleptic treatment and vulnerability to drug use8. In animal studies, chronic exposure to a typical versus atypical antipsychotic is more likely to potentiate the psychomotor-activating effects of drugs such as methamphetamine,50 AMPH (Samaha et al.15; and present results), and apomorphine.51 Finally, the present findings show that long-term exposure to HAL versus OLZ also enhances AMPH-induced potentiation of CR.
A number of considerations must be noted when interpreting our findings. First, we have evaluated the effects of HAL and OLZ on CR in neurologically healthy animals. This has allowed us to better understand the impact of chronic antipsychotic treatment on an otherwise unaltered reward system. An important next step is to extend the current finding to animal models of schizophrenia-like symptoms. Second, we have assessed operant responding for CR following antipsychotic treatment cessation. It remains to be determined how ongoing treatment might influence this behavior. In this regard, it is worthy to note that administration of either OLZ or HAL (via intraperitoneal injection) disrupts conditioned approach behavior to reward-predicting cues in rats.52 Nevertheless, assessing reward-directed behavior during withdrawal from antipsychotic has clear clinical implications. This is because frequent cessation of treatment is widespread in schizophrenia, particularly in individuals who abuse drugs.2 Third, we have assessed only one effect of a reward cue on motivated behavior—the ability to support the spontaneous learning of a new action. Reward cues have other effects that are relevant for addiction, including the ability to elicit approach and invigorate or trigger reward-seeking behavior. Additional work is needed to determine whether chronic antipsychotic treatment influences these effects. Fourth, we have compared rats treated with continuous HAL or OLZ. Is continuous antipsychotic exposure clinically pertinent? Current oral antipsychotic dosing regimens consist of daily medication intake, and this can result in high levels of D2 receptor occupancy in the brain that can last for several days following a dose.31,53,54 This is because antipsychotics have a very long half-life in humans (eg, 24h for HAL).55 Thus, the long half-life of antipsychotics in humans makes it such that standard treatment regimens can result in the continuous presence of antipsychotic in the brain. Fifth, how do these findings reconcile with data showing that schizophrenia patients treated with antipsychotics—particularly typical antipsychotics—show what might be a neurobiological correlate of reduced rather than enhanced reward function (blunted activation of the ventral striatum during reward anticipation)?56,57 It is possible that in subjects with altered dopamine systems (such as in schizophrenia, but not in our neurologically intact rats), treatment with a typical antipsychotic might indeed blunt reward function. This possibility must be investigated. However, it must also be noted that our rats were treated with either a typical or an atypical antipsychotic, while subjects in the previous imaging studies were often exposed to both medication classes in their lifetimes.56,57 Another possibility is that HAL-induced dopamine supersensitivity might be potentially advantageous in some schizophrenia patients, particularly those with anhedonia-related symptoms. However, this remains speculative and not supported by clinical observations. Finally, we have assessed the effects of a systemic injection of AMPH on CR in antipsychotic-exposed animals. This leaves open the question of where in the brain a history of exposure to HAL and an acute exposure to AMPH might interact to potentiate CR. Dopamine neurotransmission in the nucleus accumbens and in the caudate-putamen modulates AMPH-induced potentiation of CR.58,59 Our data show a correspondence between AMPH-induced potentiation of CR and induction of c-fos mRNA in the caudate-putamen, but not in the nucleus accumbens (albeit separate groups of rats were used for the behavioral and c-fos measures). This prompts the speculation that AMPH might be acting in the caudate-putamen to potentiate CR following chronic HAL treatment. This hypothesis can be evaluated in the future using intracerebral infusion techniques.
In summary, the present findings show that (1) exposure to a clinically pertinent dose and mode of HAL treatment subsequently enhances the operant pursuit of a reward cue following an AMPH challenge, (2) this is potentially related to the development of dopamine supersensitivity (as indicated by enhanced AMPH-induced locomotion) and an enhanced ability of AMPH to recruit caudate-putamen cells, and (3) these changes in brain and behavior are either absent or markedly weaker following a history of exposure to OLZ. The implication is that compared with atypicals, typical antipsychotics can promote forms of neural plasticity that underlie the ability of reward cues to gain control over behavior. Because enhanced responsiveness to reward cues can prompt and exacerbate drug-taking behavior, it should be investigated whether atypical antipsychotics might be a better option in schizophrenic patients at risk for drug abuse or addiction.
Funding
National Science and Engineering Research Council of Canada (355923); Canadian Foundation for Innovation (24326); Fonds de la Recherche en Santé du Québec (16193 to A.-N.S.). J.M. holds a Michael Smith honorific fellowship for research on schizophrenia from the Canadian Institutes for Health Research (CIHR).
Supplementary Material
Footnotes
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
References
- 1. Regier DA, Farmer ME, Rae DS, et al. Comorbidity of mental disorders with alcohol and other drug abuse. Results from the Epidemiologic Catchment Area (ECA) Study J Am Med Assoc. 1990;264:2511–2518 [PubMed] [Google Scholar]
- 2. Owen RR, Fischer EP, Booth BM, Cuffel BJ. Medication noncompliance and substance abuse among patients with schizophrenia Psychiatr Serv 1996;47:853–858 [DOI] [PubMed] [Google Scholar]
- 3. Kerfoot KE, Rosenheck RA, Petrakis IL, et al. Substance use and schizophrenia: Adverse correlates in the CATIE study sample Schizophr Res 2011;132:177–182 [DOI] [PubMed] [Google Scholar]
- 4. Schneier FR, Siris SG. A review of psychoactive substance use and abuse in schizophrenia. Patterns of drug choice J Nerv Ment Dis 1987;175:641–652 [DOI] [PubMed] [Google Scholar]
- 5. Chambers RA, Krystal JH, Self DW. A neurobiological basis for substance abuse comorbidity in schizophrenia Biol Psychiatry 2001;50:71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bedard AM, Maheux J, Levesque D, Samaha AN. Continuous, but not intermittent, antipsychotic drug delivery intensifies the pursuit of reward cues Neuropsychopharmacology. 2011;36:1248–1259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kosten TA, DeCaprio JL, Nestler EJ. Long-term haloperidol administration enhances and short-term administration attenuates the behavioral effects of cocaine in a place conditioning procedure Psychopharmacology 1996;128:304–312 [DOI] [PubMed] [Google Scholar]
- 8. LeDuc PA, Mittleman G. Schizophrenia and psychostimulant abuse: a review and re-analysis of clinical evidence Psychopharmacology 1995;121:407–427 [DOI] [PubMed] [Google Scholar]
- 9. Hambrecht M, Hafner H. Substance abuse and the onset of schizophrenia Biol Psychiatry 1996;40:1155–1163 [DOI] [PubMed] [Google Scholar]
- 10. Fukushiro DF, Alvarez Jdo N, Tatsu JA, de Castro JP, Chinen CC, Frussa-Filho R. Haloperidol (but not ziprasidone) withdrawal enhances cocaine-induced locomotor activation and conditioned place preference in mice Prog Neuropsychopharmacol Biol Psychiatry 2007;31:867–872 [DOI] [PubMed] [Google Scholar]
- 11. Stinus L, Nadaud D, Deminiere JM, Jauregui J, Hand TT, Le Moal M. Chronic flupentixol treatment potentiates the reinforcing properties of systemic heroin administration Biol Psychiatry 1989;26:363–371 [DOI] [PubMed] [Google Scholar]
- 12. Roberts DC, Vickers G. The effect of haloperidol on cocaine self-administration is augmented with repeated administrations Psychopharmacology 1987;93:526–528 [DOI] [PubMed] [Google Scholar]
- 13. Seeger TF, Gardner EL. Enhancement of self-stimulation behavior in rats and monkeys after chronic neuroleptic treatment: evidence for mesolimbic supersensitivity Brain Res 1979;175:49–57 [DOI] [PubMed] [Google Scholar]
- 14. Lieberman JA, Kane JM, Alvir J. Provocative tests with psychostimulant drugs in schizophrenia Psychopharmacology 1987;91:415–433 [DOI] [PubMed] [Google Scholar]
- 15. Samaha AN, Seeman P, Stewart J, Rajabi H, Kapur S. “Breakthrough” dopamine supersensitivity during ongoing antipsychotic treatment leads to treatment failure over time J Neurosci 2007;27:2979–2986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Smith RC, Davis JM. Behavioral supersensitivity to apomorphine and amphetamine after chronic high dose haloperidol treatment Psychopharmacol Commun 1975;1:285–293 [PubMed] [Google Scholar]
- 17. Samaha AN, Reckless GE, Seeman P, Diwan M, Nobrega JN, Kapur S. Less is more: antipsychotic efficacy is greater with transient rather than continuous drug delivery Biol Psychiatry 2008;64:145–152 [DOI] [PubMed] [Google Scholar]
- 18. Ginovart N, Wilson AA, Hussey D, Houle S, Kapur S. D2-receptor upregulation is dependent upon temporal course of D2-occupancy: a longitudinal [11C]-raclopride PET study in cats Neuropsychopharmacology 2009;34:662–671 [DOI] [PubMed] [Google Scholar]
- 19. Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: Effects of conditioned cues and continuous access to cocaine Psychopharmacology 1999;140:331–344 [DOI] [PubMed] [Google Scholar]
- 20. Panlilio LV, Weiss SJ, Schindler CW. Cocaine self-administration increased by compounding discriminative stimuli Psychopharmacology 1996;125:202–208 [DOI] [PubMed] [Google Scholar]
- 21. de Wit H, Stewart J. Reinstatement of cocaine-reinforced responding in the rat Psychopharmacology 1981;75:134–143 [DOI] [PubMed] [Google Scholar]
- 22. Ehrman RN, Robbins SJ, Childress AR, O'Brien CP. Conditioned responses to cocaine-related stimuli in cocaine abuse patients Psychopharmacology 1992;107:523–529 [DOI] [PubMed] [Google Scholar]
- 23. Creese I, Snyder SH. Chronic neuroleptic treatment and dopamine receptor regulation Adv Biochem Psychopharmacol 1980;24:89–94 [PubMed] [Google Scholar]
- 24. Severson JA, Robinson HE, Simpson GM. Neuroleptic-induced striatal dopamine receptor supersensitivity in mice: relationship to dose and drug Psychopharmacology (Berl) 1984;84:115–119 [DOI] [PubMed] [Google Scholar]
- 25. van der Zwaal EM, Luijendijk MC, Adan RA, la Fleur SE. Olanzapine-induced weight gain: chronic infusion using osmotic minipumps does not result in stable plasma levels due to degradation of olanzapine in solution Eur J Pharmacol 2008;585:130–136 [DOI] [PubMed] [Google Scholar]
- 26. McCormick PN, Kapur S, Graff-Guerrero A, Raymond R, Nobrega JN, Wilson AA. The antipsychotics olanzapine, risperidone, clozapine, and haloperidol are D2-selective ex vivo but not in vitro Neuropsychopharmacology 2010;35:1826–1835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kapur S, VanderSpek SC, Brownlee BA, Nobrega JN. Antipsychotic dosing in preclinical models is often unrepresentative of the clinical condition: a suggested solution based on in vivo occupancy J Pharmacol Exp Therap 2003;305:625–631 [DOI] [PubMed] [Google Scholar]
- 28. Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects Arch Gen Psychiatry 1992;49:538–544 [DOI] [PubMed] [Google Scholar]
- 29. Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia Am J Psychiatry 2000;157:514–520 [DOI] [PubMed] [Google Scholar]
- 30. Wadenberg ML, Soliman A, VanderSpek SC, Kapur S. Dopamine D(2) receptor occupancy is a common mechanism underlying animal models of antipsychotics and their clinical effects Neuropsychopharmacology 2001;25:633–641 [DOI] [PubMed] [Google Scholar]
- 31. Tauscher J, Jones C, Remington G, Zipursky RB, Kapur S. Significant dissociation of brain and plasma kinetics with antipsychotics Mol Psychiatry 2002;7:317–321 [DOI] [PubMed] [Google Scholar]
- 32. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates 2nd ed New York: Academic Press; 1986. [Google Scholar]
- 33. Vanderschuren LJ, Schoffelmeer AN, Van Leeuwen SD, Hof L, Jonker AJ, Voorn P. Compartment-specific changes in striatal neuronal activity during expression of amphetamine sensitization are the result of drug hypersensitivity Eur J Neurosci 2002;16:2462–2468 [DOI] [PubMed] [Google Scholar]
- 34. Seeman P, Corbett R, Van Tol HH. Atypical neuroleptics have low affinity for dopamine D2 receptors or are selective for D4 receptors Neuropsychopharmacology 1997;16:93–110; discussion 111–135 [DOI] [PubMed] [Google Scholar]
- 35. Rauser L, Savage JE, Meltzer HY, Roth BL. Inverse agonist actions of typical and atypical antipsychotic drugs at the human 5-hydroxytryptamine(2C) receptor J Pharmacol Exp Ther 2001;299:83–89 [PubMed] [Google Scholar]
- 36. Zhang JY, Kowal DM, Nawoschik SP, Lou Z, Dunlop J. Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms Biochem Pharmacol 2006;71:521–529 [DOI] [PubMed] [Google Scholar]
- 37. Egerton A, Ahmad R, Hirani E, Grasby PM. Modulation of striatal dopamine release by 5-HT2A and 5-HT2C receptor antagonists: [11C]raclopride PET studies in the rat Psychopharmacology (Berl) 2008;200:487–496 [DOI] [PubMed] [Google Scholar]
- 38. Bymaster FP, Hemrick-Luecke SK, Perry KW, Fuller RW. Neurochemical evidence for antagonism by olanzapine of dopamine, serotonin, alpha 1-adrenergic and muscarinic receptors in vivo in rats Psychopharmacology (Berl) 1996;124:87–94 [DOI] [PubMed] [Google Scholar]
- 39. O’Brien CP, Childress AR, McLellan AT, Ehrman R. Classical conditioning in drug-dependent humans Ann N Y Acad Sci 1992;654:400–415 [DOI] [PubMed] [Google Scholar]
- 40. Levison PK. An analysis of commonalities in substance abuse and habitual behavior NIDA Res Monogr 1981;37:27–41 [PubMed] [Google Scholar]
- 41. Tiffany ST, Carter BL. Is craving the source of compulsive drug use? J Psychopharmacol 1998;12:23–30 [DOI] [PubMed] [Google Scholar]
- 42. Duka T, Townshend JM. The priming effect of alcohol pre-load on attentional bias to alcohol-related stimuli Psychopharmacology 2004;176:353–361 [DOI] [PubMed] [Google Scholar]
- 43. Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences Drug Alcohol Depend 2008;97:1–20 [DOI] [PubMed] [Google Scholar]
- 44. Uslaner JM, Acerbo MJ, Jones SA, Robinson TE. The attribution of incentive salience to a stimulus that signals an intravenous injection of cocaine Behav Brain Res 2006;169:320–324 [DOI] [PubMed] [Google Scholar]
- 45. Shaham Y, Shalev U, Lu L, De Wit H, Stewart J. The reinstatement model of drug relapse: history, methodology and major findings Psychopharmacology 2003;168:3–20 [DOI] [PubMed] [Google Scholar]
- 46. Arroyo M, Markou A, Robbins TW, Everitt BJ. Acquisition, maintenance and reinstatement of intravenous cocaine self-administration under a second-order schedule of reinforcement in rats: effects of conditioned cues and continuous access to cocaine Psychopharmacology (Berl) 1998;140:331–344 [DOI] [PubMed] [Google Scholar]
- 47. Leslie DL, Rosenheck RA. From conventional to atypical antipsychotics and back: dynamic processes in the diffusion of new medications Am J Psychiatry 2002;159:1534–1540 [DOI] [PubMed] [Google Scholar]
- 48. Smelson DA, Ziedonis D, Williams J, Losonczy MF, Steinberg ML, Kaune M. The efficacy of olanzapine for decreasing cue-elicited craving in individuals with schizophrenia and cocaine dependence: a preliminary report J Clin Psychopharmacol 2006;26:9–12 [DOI] [PubMed] [Google Scholar]
- 49. Noordsy DL, O'Keefe C, Mueser KT, Xie H. Six-month outcomes for patients who switched to olanzapine treatment Psychiatr Serv 2001;52:501–507 [DOI] [PubMed] [Google Scholar]
- 50.Tadokoro S, Okamura N, Sekine Y, Kanahara N, Hashimoto K, Iyo M. Chronic treatment with aripiprazole prevents development of dopamine supersensitivity and potentially supersensitivity psychosis. Schizophr Bull. 2011 doi: 10.1093/schbul/sbr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Rupniak NM, Hall MD, Mann S, et al. Chronic treatment with clozapine, unlike haloperidol, does not induce changes in striatal D-2 receptor function in the rat Biochem Pharmacol 1985;34:2755–2763 [DOI] [PubMed] [Google Scholar]
- 52. Danna CL, Elmer GI. Disruption of conditioned reward association by typical and atypical antipsychotics Pharmacol Biochem Behav 2010;96:40–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Farde L, Wiesel FA, Nordstrom AL, Sedvall G. D1- and D2-dopamine receptor occupancy during treatment with conventional and atypical neuroleptics Psychopharmacology 1989;99, Suppl S28–S31 [DOI] [PubMed] [Google Scholar]
- 54. Seeman P. Atypical antipsychotics: mechanism of action Can J Psychiatry 2002;47:27–38 [PubMed] [Google Scholar]
- 55. Bezchlibnyk-Butler KZ, Jeffries JJ. Clinical Handbook of Psychotropic Drugs Toronto: Hogrefe & Huber Publishers; 1999. [Google Scholar]
- 56. Kirsch P, Ronshausen S, Mier D, Gallhofer B. The influence of antipsychotic treatment on brain reward system reactivity in schizophrenia patients Pharmacopsychiatry 2007;40:196–198 [DOI] [PubMed] [Google Scholar]
- 57. Schlagenhauf F, Juckel G, Koslowski M, et al. Reward system activation in schizophrenic patients switched from typical neuroleptics to olanzapine Psychopharmacology 2008;196:673–684 [DOI] [PubMed] [Google Scholar]
- 58. Kelley AE, Delfs JM. Dopamine and conditioned reinforcement. I. Differential effects of amphetamine microinjections into striatal subregions Psychopharmacology 1991;103:187–196 [DOI] [PubMed] [Google Scholar]
- 59. Parkinson JA, Olmstead MC, Burns LH, Robbins TW, Everitt BJ. Dissociation in effects of lesions of the nucleus accumbens core and shell on appetitive pavlovian approach behavior and the potentiation of conditioned reinforcement and locomotor activity by D-amphetamine J Neurosci 1999;19:2401–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.