Abstract
Despite the consistent presence of performance deficits on the Wisconsin Card Sorting Test (WCST) in schizophrenia patients, whether poorer performance is also present in their nonpsychotic relatives is not certain. This study aimed to estimate both the recurrence risk ratio (λs) and the heritability of WCST scores in simplex and multiplex families, respectively, and to examine the influence of familial loading on these estimates. Participants were patients with schizophrenia and their nonpsychotic first-degree relatives from 168 simplex families and 653 multiplex families as well as 440 normal comparisons. On the basis of adjusted z scores, both the λs at a series of cutoff points and heritability estimates based on variance component modeling in the nonpsychotic relatives of schizophrenia patients were estimated. The WCST deficits in schizophrenia patients were more prominent in multiplex families than in simplex ones. Among relatives, WCST deficits were limited to parents of multiplex families for most WCST scores and siblings from multiplex families for total errors, perseverative responses, and perseverative errors. Pertaining to λs, the estimates for multiplex families (highest estimates ranging from 7.9 to 11.0) were greater than those for simplex ones (<2.5). Nevertheless, the heritability estimates were very similar between simplex (ranging from 0% to 17%) and multiplex (ranging from 0% to 21%) families, with the latter having slightly greater values than the former. There is only a small-to-modest familial aggregation on part of WCST scores in families of schizophrenia patients, and this may limit its use as endophenotypic markers to schizophrenia susceptibility.
Key words: executive function, recurrence risk ratio, heritability, endophenotype, familial aggregation, prefrontal cortex
Introduction
The Wisconsin Card Sorting Test (WCST) is one of the most widely used neurocognitive tests for assessing executive function that has been strongly linked to prefrontal cortical function,1,2 reflecting an integrated ability for the purpose of planning and executing goal-directed activities.3 WCST performance deficits have been consistently detected in patients with schizophrenia, with a mean effect size of 0.95 in a meta-analysis of 43 studies.4 An increasing number of studies have applied the WCST in exploring the association of genetic variants to the neurocognitive deficits of schizophrenia.5–7 Despite the popular use of the WCST, the empirical results on whether its performance deficits represent endophenotypic markers for schizophre-nia susceptibility have been conflicting. Early studies using small sample sizes examining the WCST performance among nonpsychotic relatives of patients with schizophrenia indicated a deficit of moderate effect size around 0.26–0.40, as revealed in 3 meta-analytic studies.8–10 A subsequent study of a large sample failed to support the presence of WCST deficits among relatives of schizophrenia patients,11 whereas another study in relatives of first-episode schizophrenia patients reported greater effect sizes of 0.61 (categories achieved) to 0.87 (perseverative errors).12 Thus, it remains uncertain if there is familial aggregation of WCST deficits among families of patients with schizophrenia.
To evaluate the magnitude of familial aggregation, either recurrence risk ratios or heritability can be used. The recurrence risk ratio (λ) is a commonly used index for binary outcomes that is often used as an indicator of genetic effect.13 For executive dysfunction to be a useful endophenotype, the magnitude of its λ should be greater than that of the schizophrenia diagnosis itself. To date, only one study reported λ estimates for WCST scores,14 with a sibling recurrence risk ratio of 2.2–4.9 for perseverative errors and 2.4–7.5 for categories achieved, depending on 1 or 2 SDs below the reference mean were used as the threshold.
Estimates of heritability, the proportion of the variance explained by genetic factors, are usually derived for continuous outcomes from either twin or family studies. Two studies among discordant monozygotic twins of schizophrenia patients found no evidence of genetic influence on WCST performance,15,16 whereas another study found that unaffected monozygotic cotwins of schizophrenia patients had more perseverative errors than unaffected dizygotic twins.17 Although these twin studies provide useful clues, they are limited by small sample sizes. The only family study examining the heritability of WCST performance reported a heritability of 0.14 for categories achieved and 0.28 for perseverative errors, though the estimates became nonsignificant after adjustment for intelligence quotient.18
An important factor that might influence the magnitude of familial aggregation for a schizophrenia-related trait is the familial loading of schizophrenia, with the rationale that patients with negative family history (sporadic cases) would have less genetic loading for schizophrenia than those with positive family history (familial cases).19,20 The utility of this approach has been demonstrated in detecting the association of higher genetic loading with more severe neurocognitive deficits21–23 or impaired flush response to niacin.24 In the 2 studies that examined nonpsychotic relatives of familial vs nonfamilial schizophrenia on WCST performance, one failed to show any deficits in both groups of relatives,21 whereas the other one reported deficits on 2 scores for familial relatives as well as 1 score for nonfamilial relatives.25 However, the numbers of families in these 2 studies were small.
To address the gap in literature on the magnitude of familial aggregation in WCST performance, we examined families having different familial loadings for schizophrenia. We hypothesized that families with a high familial loading for schizophrenia would show a stronger familial aggregation on WCST performance. In this study, we aimed to estimate both the recurrence risk ratio and the heritability of WCST scores in simplex and multiplex families, respectively, and to examine the influence of familial loading on these estimates.
Methods
Subjects
Participants in this study consisted of 3 groups: (1) patients with schizophrenia and their nonpsychotic first-degree relatives from 168 simplex families (having 149 patients, 205 nonpsychotic parents, and 77 nonpsychotic siblings with information on the WCST), (2) 653 multiplex families (having 985 patients, 601 nonpsychotic parents, and 283 nonpsychotic siblings with information on the WCST), and (3) 440 unrelated, normal comparison subjects. The schizophrenia probands and their relatives were recruited from 3 programs: the Multidimensional Psychopathology Study of Schizophrenia (MPSS)23 from 1998 to 2001, the Taiwan Schizophrenia Linkage Study (TSLS)26 from 1998 to 2002, and the Study on Etiological Factors of Schizophrenia (SEFOS) from 2002 to 2005. Both the MPSS and the TSLS aimed to collect schizophrenia families with at least 2 siblings fulfilling the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV), criteria for schizophrenia or schizoaffective disorder depressive type. The affected sib-pair probands were identified from either the inpatient wards or the outpatient clinics of the National Taiwan University Hospital and the Provincial Tao-Yuan Psychiatric Center for the MPSS or 6 data collection field research centers throughout Taiwan for the TSLS. The third program, SEFOS, aimed to recruit families of schizophrenia patients of different familial loadings, including both simplex (ie, without affected siblings) and multiplex families (ie, at least 2 affected siblings), from National Taiwan University Hospital and Ju-Shan Psychiatric Hospital (a private hospital in Tao-Yuan County, Taiwan). Thus, our simplex families were recruited mainly from northern Taiwan, whereas our multiplex families were recruited from throughout the country. In terms of the normal comparison subjects, they were recruited via different venues: subjects attending health checkup at National Taiwan University Hospital in 2000, hospital or university staff members as well as factory employees from 2002 to 2005, and senior high school students in 2007. All normal subjects reported a negative history of major psychiatric disorders.
All participants in this study, regardless of the multiplex, simplex, or control group, were Taiwanese Han Chinese in ethnic origin and had to meet the following criteria to be eligible for taking the WCST: no history of alcohol and drug abuse, no neurologic disease or damage, no brain surgery, no mental retardation, and no medical illnesses that may significantly impair neurocognitive function. All participants aged 18 years or older were included in the present study. This study was approved by the institutional review boards of the participating hospitals and College of Public Health, National Taiwan University. Written informed consent was obtained from all subjects after complete description.
Interview Instruments and Diagnostic Procedure
All participants were interviewed by well-trained assistants using the Chinese version of the Diagnostic Interview for Genetic Studies (DIGS),27 which was designed specifically for family-genetic studies of schizophrenia and bipolar disorder with good interrater reliabilities.28 The Chinese version of the Family Interview for Genetic Studies (FIGS)29 was used to collect relevant information on relatives who were not interviewed for the study. Meanwhile, the screening sections of the DIGS as well as the FIGS were used in the screening for normal comparison subjects. Best estimate final diagnosis according to DSM-IV criteria was made independently by 2 board-certified psychiatrists using all available information, including the DIGS, the FIGS, the hospital records, and the interviewers’ notes.
Wisconsin Card Sorting Test
A computerized version of the WCST30,31 was administered to each participant, who was instructed to match a ‘‘response’’ card to 1 of the 4 ‘‘stimulus’’ cards on the basis of 3 dimensions (color, form, or number) by pressing 1 of the 1–4 number keys on the computer keyboard. Subjects neither were informed of the correct sorting principle nor were they told when the principle would shift during the test, but they were given feedback (‘‘Right’’ or ‘‘Wrong’’) on the screen after each trial. The testing continued until all 128 cards were sorted. According to the WCST manual,2 the following performance indexes can be derived—(1) total errors: total number of perseverative and nonperseverative errors, (2) perseverative responses: number of responses that were perseverative, regardless of whether they were correct or not, (3) perseverative errors: number of errors that were perseverative, reflecting tendency toward perseveration, (4) nonperseverative errors: number of errors that were not perseverative, (5) categories achieved: number of times 10 correct responses in a row were made, reflecting overall success, (6) conceptual level response: proportion of consecutive correct responses occurring in runs of 3 or more, reflecting insight into the correct sorting principles, (7) trials to complete first category: number of trials to successfully complete the first category (counted as 129 if no category was completed), reflecting initial conceptual ability, (8) failure to maintain set: number of times subject makes between 5 and 9 correct responses in a row, reflecting efficiency of sorting, and (9) learning to learn: average difference in percent errors between successive categories, reflecting the average change in conceptual efficiency during the test. The adjusted z scores of the WCST scores for the subjects from the simplex and multiplex families were obtained with adjustments for sex, age, and education level against the group of 440 normal comparison subjects following the method of Chen et al27 Briefly, the predictive score of a subject was calculated by use of the regression coefficients obtained from the regression of the scores on these covariates among the normal comparison subjects. The difference between the raw score and the predictive score was then standardized by the root mean error of the regression and was defined as the adjusted z score of the subject.
Statistical Analysis
Group comparisons in demographic features were conducted using the χ2 test for sex and mixed-effect models for age and education level. We also used mixed-effect models to compare WCST scores among different groups (ie, patients, parents, siblings, and normal comparisons) with control for familial dependence.
For both recurrence risk ratio and heritability estimation, we used the adjusted z scores of the WCST scores to eliminate the influence of sex, age, and education level simultaneously against an external norm. For recurrence risk ratio analyses, affection status was defined as having WCST deficit, operationally as with an adjusted z score of the WCST score below a prespecified cutoff value. The λ coefficient was calculated as the ratio of the recurrence risk among a certain type of relatives to the prevalence of the general population as follows:13,32
| λ = Pr(relatives with the WCST deficit\having a proband with the WCST deficit)/Pr (the general population with the WCST deficit). |
Because the original sample was recruited on the basis of at least one sibling affected with schizophrenia, the recurrence risk in relatives was estimated using the methods with correction for complete ascertainment.33 According to this method, a family has at least one person with the specified WCST deficit. Thus, only relatives of a proband with the specified deficit on the WCST would be included for the recurrence risk estimation. Because the cutoff value moved, the number of families included for the recurrence risk estimation changed. If a family had 2 schizophrenia patients with the specified deficit on the WCST, the family would be repeated twice; each time 1 patient with the specified WCST deficit was treated as the proband and the other patient counted as relatives in the estimation of the recurrence risk. Using sibling recurrence risk (K s) as an example,
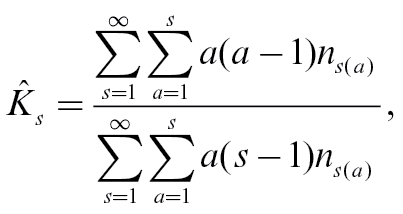 |
where n s(a) is the number of sibships of size s with a affected with the specified WCST deficit. This estimator of K s has been shown to be unbiased and consistent when the ascertainment is complete.33 For example, among 985 schizophrenia patients of multiplex families, 625 were impaired at or beyond the cutoff value of−1 for total errors, including 282 families with only 1 deficit patient, 158 families with 2 deficit patients, and 9 families with 3 deficit patients. If a family had n patients with the specified deficit on the WCST, the family would be repeated n times (in this example, n = 1, 2, or 3); each time 1 patient with the specified WCST deficit was treated as the proband and the other patients counted as relatives in the estimation the recurrence risk. Of these included relatives, 720/1371 (ie, 52.5%) demonstrated such impairment in comparison to the 15.5% of controls. Thus, λ was estimated to be 3.4. In the estimation of recurrence risk, the estimates of some cutoff points were not presented if the numerator < 6 or the denominator < 30 because they could not provide a stable estimate.
In terms of heritability, the continuous adjusted z scores of individual WCST performance indexes were subjected to estimation using the variance component methods34 as implemented in software package SOLAR (version 4.2.0. for Linux; Southwest Foundation for Biomedical Research). By dividing the overall variance of the trait into portions due to genetic factors determined by the pedigree relationships and environmental factors, the program uses an iterative process in which genetic and environmental variances are changed until a combination is found which has the maximum likelihood value. Under this circumstance, the heritability of a WCST performance index is the proportion of overall variance due to the summed additive genetic effects of genes at unspecified loci throughout the genome. The SOLAR-based heritability estimation was performed with ascertainment correction by specifying the proband status in each family. In this case, the patient with schizophrenia of simplex families and the affected sibling-pair of multiplex families were designated as the proband. We did not include age, sex, or educational level as covariate in the SOLAR models to avoid overcorrection 18 because the adjusted z scores had been corrected for these demographic features. All the statistical analyses except heritability estimates were performed using the SAS statistical package (version 9.1.3 for Windows; SAS Institute Inc).
Results
Participants from simplex families, multiplex families, and normal comparisons were examined for differences in demographic features (table 1). There were greater proportions of males for schizophrenia patients and their nonpsychotic siblings compared with normal comparisons. Participants from multiplex families were older than their counterparts from simplex ones. Parents had lower educational levels than their offspring, and both parents and siblings of multiplex families had lower educational level than their counterparts of simplex families. The distributions of raw WCST scores are displayed in the lower part of table 1. Schizophrenia patients and their nonpsychotic parents from both simplex and multiplex families appeared to have poorer performance than their nonpsychotic siblings or normal comparisons on most WCST scores. Furthermore, participants from multiplex families also showed poorer performance than their counterparts from simplex ones.
Table 1.
Demographic Features and Raw Scores on the Wisconsin Card Sorting Test (WCST) for Schizophrenia Patients, Their Nonpsychotic Relatives, and Normal Comparisons
| Multiplex Families | Simplex Families | Normal Comparisons (n = 440) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Variable | Patients (n = 985) | Parents (n = 601) | Siblings (n = 283) | Patients (n = 149) | Parents (n = 205) | Siblings (n = 77) | |||
| Male gender | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | ||
| 616 (63) | 268 (45) | 149 (53) | 90 (60) | 84 (41) | 40 (52) | 191 (43) | |||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Age (years) | 35.0 (7.7) | 63.0 (8.7) | 36.7 (8.7) | 31.7 (8.0) | 56.3 (7.8) | 33.4 (9.2) | 39.9 (15.7) | ||
| Education (years) | 10.9 (3.2) | 5.5 (4.2) | 11.7 (3.5) | 12.6 (3.0) | 9.6 (4.3) | 14.0 (3.5) | 12.8 (4.2) | ||
| WCST raw score | |||||||||
| Total errors | 71.1 (25.1) | 75.1 (22.5) | 49.3 (24.9) | 58.0 (26.3) | 56.7 (23.3) | 39.9 (22.3) | 45.1 (22.4) | ||
| Perseverative responses | 52.6 (38.6) | 50.1 (36.2) | 34.4 (29.7) | 39.3 (30.9) | 37.9 (27.7) | 24.1 (18.0) | 26.6 (21.3) | ||
| Perseverative errors | 42.7 (28.0) | 41.1 (26.0) | 29.1 (21.9) | 33.0 (22.6) | 32.2 (20.5) | 21.1 (14.2) | 23.4 (16.2) | ||
| Nonperseverative errors | 28.4 (24.1) | 34.0 (27.5) | 20.2 (15.3) | 25.0 (20.2) | 24.5 (16.2) | 18.8 (13.4) | 21.7 (13.9) | ||
| Categories achieved | 2.1 (2.7) | 1.6 (2.1) | 4.6 (3.3) | 3.6 (3.2) | 3.6 (3.0) | 6.2 (3.3) | 5.0 (3.3) | ||
| Conceptual level responses (%) | 28.5 (25.1) | 25.0 (21.8) | 50.6 (25.7) | 41.6 (27.2) | 42.5 (24.2) | 61.0 (23.7) | 54.7 (23.7) | ||
| Trials to complete first category | 69.8 (54.1) | 70.2 (54.2) | 36.5 (40.2) | 45.0 (47.8) | 38.9 (41.3) | 27.7 (31.7) | 34.5 (36.5) | ||
| Failure to maintain set | 0.8 (1.3) | 1.0 (1.4) | 1.3 (1.4) | 1.1 (1.3) | 1.2 (1.4) | 0.8 (1.1) | 1.3 (1.4) | ||
| Learning to learna | −4.7 (10.6) | −8.7 (11.4) | −2.2 (8.1) | −2.0 (7.5) | −5.2 (9.9) | 0.4 (4.3) | −0.5 (7.4) | ||
aParticipants whose scores could be derived for this index include 409 patients, 199 parents, and 207 siblings for multiplex families; 88 patients, 133 parents, and 64 siblings for simplex families; and 112 for normal comparisons.
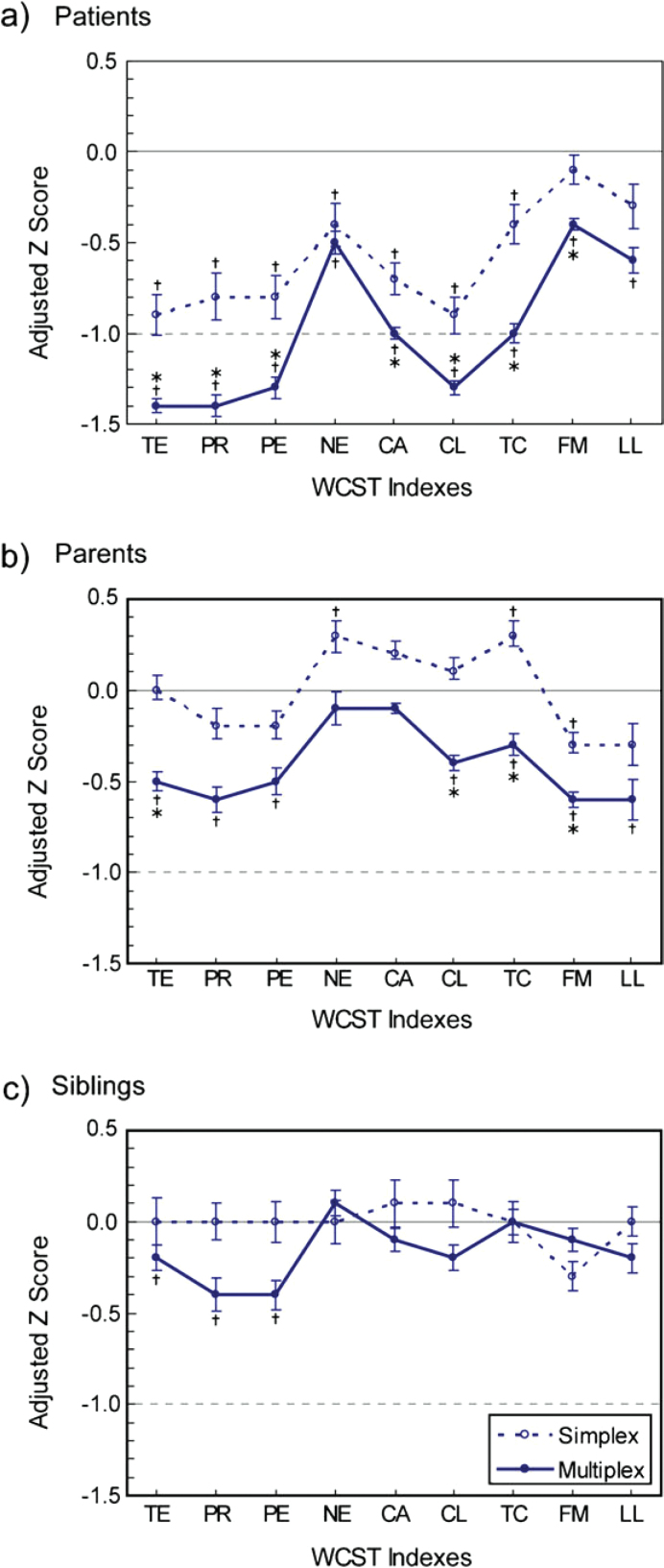
In making group comparisons, we used adjusted z scores to adjust for the demographic differences among groups (figure 1). The patients from both multiplex and simplex families had deficits on most WCST scores compared with normal comparisons, with varying magnitudes of deficit. If the 2 types of patients were compared, those from multiplex families had greater deficits on most scores. In particular, the deficits of multiplex patients were greater than 1.5 SDs on total errors, perseverative responses, perseverative errors, and conceptual level response, whereas the deficits of simplex patients on these scores were less than 1 SD. For nonpsychotic parents, those from multiplex families showed significant deficits in a pattern similar to that of patients but with a less magnitude of deficit, whereas those of simplex families did not show any deficits (even better performance than normal comparisons on nonperseverative errors and trials to complete first category). For nonpsychotic siblings, only those from multiplex families showed deficits on 3 scores (total errors, perseverative responses, and perseverative errors) as compared with normal comparisons.
Fig. 1.
Adjusted z Scores of the Wisconsin Card Sorting Test (WCST) Indexes for Simplex and Multiplex Families of Schizophrenia, Respectively: (a) Patients, (b) Parents, and (c) Siblings. Scores are presented in the direction of deficit against normal comparisons, in which TE = − total errors, PR = − perseverative responses, PE = − perseverative errors, NE = − nonperseverative errors, CA = categories achieved, CL = conceptual level response, TC = − trials to complete first category, FM = failure to maintain set, and LL = learning to learn. A vertical bar indicates the standard error of the mean WCST index. †P <.006 for the comparison with normal comparisons, indicating a significant difference with adjustment for the multiple testing of 9 indexes (ie, 0.05/9). *P <.006 for the comparison between simplex and multiplex groups, indicating a significant difference with adjustment for the multiple testing of 9 indexes (ie, 0.05/9).
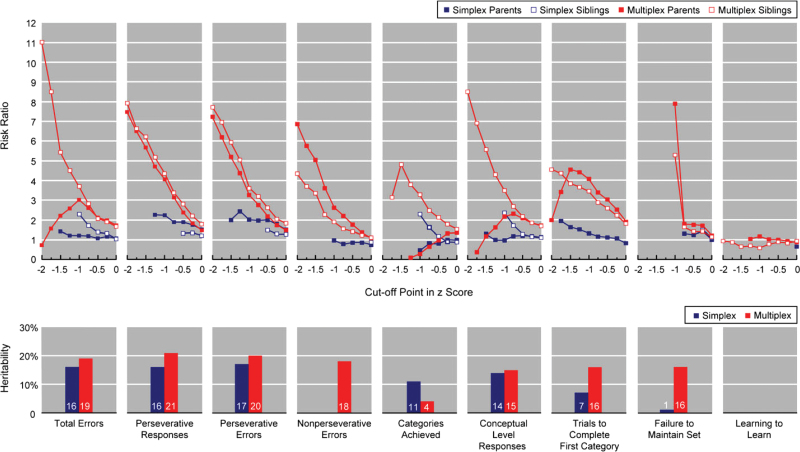
The recurrence risk ratios of WCST scores for parents and siblings, respectively, were estimated for a series of cutoff points (figure 2, upper panel). For multiplex families, the risk ratios of siblings were greater than those of parents on most WCST scores, with the 3 greatest ratios of siblings being 11.0 (using the cutoff point of −2 for total errors), 8.5 (using −2 for conceptual level response), and 7.9 (using −2 for perseverative responses). For simplex families, most risk ratios ranged from 1 to 2 for both parents and siblings, with the greatest one being 2.4 (using −1.25 for perseverative errors in parents). When 2 types of families were compared, most risk ratios for the parents from multiplex families were higher than their counterparts from simplex ones. The differences were most striking for perseverative responses, perseverative errors, and nonperseverative errors when a cutoff point of less than −1 was adopted. More detailed results of the recurrence risk ratios by different cutoff points are provided in Supplementary table S1.
Fig. 2.
Recurrence Risk Ratios at Various Cutoff Points in Adjusted z Score and Heritability Estimates on the Wisconsin Card Sorting Test for Simplex and Multiplex Families, Respectively. Scores are presented in the direction of deficit against normal comparisons. The estimates of the recurrence risk at some cutoff points were not presented if the numerator <6 or the denominator <30 because they could not provide a stable estimate.
When the whole family was subjected to heritability estimation, the estimates ranged from 0% (nonperseverative errors and learning to learn) to 17% (total errors) in simplex families and from 0% (learning to learn) to 21% (perseverative responses) in multiplex ones (figure 2, lower panel). Intriguingly, for those scores that showed relatively greater deficits in schizophrenia patients, ie, total errors, perseverative responses, perseverative errors, and conceptual level response, their heritability estimates were among the greatest ones and both multiplex and simplex families had very similar estimates, with the former having slightly greater values than the latter.
For comparison, age and education level were added as covariates in the SOLAR models for the heritability estimation of the adjusted z scores of WCST performance indexes (model 2 in Supplementary table S2). In general, the estimates were very similar to those shown in figure 1 (listed as model 1 in Supplementary table S2).
Discussion
To our knowledge, this is the largest sample size study that has assessed the familial aggregation on the WCST performance for both simplex and multiplex families of patients with schizophrenia. Through comprehensive evaluations of all 9 WCST scores, our findings suggest that there exists a small-to-modest familial aggregation for WCST scores in these families and that a higher familial loading of schizophrenia is associated with greater recurrence risk ratio and heritability. These findings have important implications for the application of WCST performance deficits as endophenotypic markers for schizophrenia susceptibility.
Despite a strong genetic contribution to schizophrenia suggested by evidence from family, twin, and adoption studies,35,36 the distinction between sporadic and familial subtypes of schizophrenia is complicated by the low pen-etrance of disease genes as well as the thoroughness of diagnostic assessment.37,38 One challenging issue in the familial-sporadic grouping of schizophrenia is the misclassification due to small number of relatives or unavailable information.19 In this study, patients’ familial loading was classified based mainly on direct interviews in relatives. In contrast, many previous family studies of WCST in schizophrenia patients did not indicate whether patients had a family history, which is around 10% for the morbidity risk among first-degree relatives.39 Intermingling of these 2 groups of relatives in varying proportions might account for the inconsistency in previous family studies, in which some reported impairment on the WCST in relatives,8,9 whereas the others did not.11,40 Another important issue is how to correct for ascertainment. In this study, a correction for complete ascertainment was adopted in the estimation of recurrence risk, and a conditional likelihood on each family’s probands was used in the estimation of heritability. These formal corrections for ascertainment in the analysis of familial aggregation help render our results more robust.
Before comparing the WCST performance between relatives of 2 types of families, it is important to note that there were differences in demographic features between them, particularly in educational level. Owing to the limited educational resources for the parents’ generation when it came of age in Taiwan, their educational levels were lower than that of their offspring as in the general population. Meanwhile, educational attainment might also be part of the predisposition to psychopathology because the educational levels of multiplex families were lower than those of simplex families. Nevertheless, the comparisons in the WCST performance were made using the adjusted z scores that were obtained against an external normative sample to eliminate the influence of such demographic features. Despite the consistent presence of WCST performance deficits on most indexes in schizophrenia patients, in which the deficit in multiplex families was more prominent than those for simplex ones, similar deficits do not appear universally in relatives of both types of families. Such deficits were limited to parents from multiplex families on most WCST scores, as well as siblings from multiplex families on a small number of indexes, including total errors, perseverative responses, and perseverative errors. Meanwhile, neither parents nor siblings of simplex families exhibited any WCST deficits as compared with normal comparisons, except for parents’ failure to maintain set. The differential familiality of WCST scores is further supported by the λs, with greater estimates for multiplex families than those for simplex families and the heritability estimates were also slightly higher for multiplex families than for simplex ones. Thus, our results demonstrate that the familial aggregation on WCST performance depends on several factors, including type of indexes, type of relatives, and familial loading of schizophrenia patients.
Among 2 previous family studies of WCST performance that did classify patients into simplex vs multiplex, one failed to find any deficits in either probands or relatives,21 whereas the other reported deficits in both pro-bands and relatives of either type of families on categories achieved.25 It needs to be noted that the sample sizes of these 2 studies were small (about 30–50 subjects in each type of families); particularly, the number of controls that were used for the standardization of z score was relatively small (40 and 100, respectively, vs 440 in this study). Furthermore, the administration of the WCST in Birkett et al25 was different from conventional way in that the examiners told the subject when the matching principle changed. Whether this change of test administration of the WCST helps increase the discrimination of simplex relatives from normal comparisons needs further investigation.
Among the 9 WCST scores examined, our results suggest that indexes such as perseverative response or perseverative errors are more useful in terms of familial aggregation. Deficits on these indexes are not only greater than the other indexes in schizophrenia patients but also consistently present in both parents and siblings of multiplex families. This is further supported by the stable estimates of their heritability across 2 types of families. In contrary, another traditional index, categories achieved, did not show much familial aggregation in this study. One possible explanation is that categories achieved have a very limited range of score (from 0 to 12), which may not fully capture the variability in different individuals’ performance.
Although the λs for several WCST scores were found to be significantly greater than 1, the trends depend highly on the cutoff points chosen. Some of the estimates are equivalent to those reported by a former study in the United States.14 Even under a liberal choice of cutoff points, the magnitudes of these λs do not indicate that incorporation of them in phenotype definition might increase the statistical power in genetic dissection for schizophrenia because these ratios did not exceed that of schizophrenia alone (around 10).41 Furthermore, for those λs with greater values at more deficit cutoff points of WCST indexes, their 95% CIs tended to be very wide because the number of subjects with these deficits was small. In comparison, the λs of several neurocognitive tests were higher than those of the WCST, such as the Continuous Performance Test (CPT) of sustained attention42 and Trails B of oculomotor scanning/psychomotor speed.14 For example, the λs of the degraded CPT d′ at a cutoff point of −2.5 SD was 12.4 for parents and 8.6 for siblings in simplex families.42 The low level of familial aggregation on the WCST is further supported by the heritability estimates (<24%), which is similar to that of 1 recent study in multiplex families of schizophrenia.18 The lack of familial aggregation on WCST performance has also been reported in many twin studies among nonclinical populations, in which no significant difference in correlations between monozygotic and dizygotic twins43–45 or a low level of contribution from either genetic or shared environmental factors46 was found.
Taken together, there is only a small-to-modest familial aggregation on part of WCST scores in families of patients with schizophrenia, particularly in multiplex families. One possible explanation is that assessment using the WCST might be complicated by the problem of task impurity because it is a very complex and multi-determined test.44,47 Although the associations of worsened deficits as well as increased λs or heritability with familial loading do indicate that there is some genetic contribution to certain WCST performance indexes, the low level of the familial aggregation may limit their use as endophenotypic markers for schizophrenia susceptibility. This does not necessarily suggest lack of genetic influences on the construct of executive functions in general because many other tests that consist of simpler tasks in executive function do have empirical evidence of genetic contribution.48,49
Some limitations of this study should be kept in mind in interpreting our results. First, a mean age of 33 years in siblings of simplex families in our sample implied that some of them had not passed through the age of risk for schizophrenia, and this might lead to misclassification of family type. Second, the relatively low participation rate of the relatives in the multiplex families might bias the mean WCST performance in this group of relatives. Third, most of the patients with schizophrenia in this study were receiving medication treatment, and this might influence our results because some antipsychotics may be associated with WCST performance. Fourth, the adjustment for age and education level on WCST performance might not be adequate for the parents because relatively few controls were at the age and educational level seen in the parents, particularly for the multiplex group. Thus, the adjusted z scores from the sibling group, while smaller in sample size, might be more robust and reliable than that from the parent group. Finally, because our analyses did not distinguish patients with first episode or not, we do not know whether relatives of first-episode schizophrenia patients would increase the magnitude of familial aggregation on the WCST performance, as indicated in a previous study.12
Funding
National Research Program for Genomic Medicine; National Science Council, Taiwan (NSC-91-3112-B- 002-011, NSC-92-3112-B-002-019, NSC-93-3112-B-002- 012, NSC-94-3112-B-002); National Health Research Institute, Taiwan (NHRI-90-8825PP, NHRI-EX91, 92, 93, 94-9113PP, NHRI-EX98-9511PP); National Institute of Mental Health, USA (1R01 MH59624-01, 1R01 MH59624-02).
Supplementary Material
Supplementary material is available at http:// schizophreniabulletin.oxfordjournals.org.
Acknowledgments
The authors thank other participants in the MPSS, TSLS, and SEFOS who helped in the recruitment and evaluation of schizophrenia patients, including Drs Shi K. Liu, Ching-Jui Chang, Hung-Jung Chang, Hai Ho, Ping-Ju Chang, Shi-Chin Guo, Hsien-Yuan Lane, Su-Kuan Lin, Fu-Chuan Wei, and Joseph J. Cheng.
References
- 1. Robinson AL, Heaton RK, Lehman RA, Stilson DW. The utility of the Wisconsin Card Sorting Test in detecting and localizing frontal lobe lesions. J Consult Clin Psychol. 1980; 48: 605–614 [DOI] [PubMed] [Google Scholar]
- 2. Heaton RK, Chelune GI, Talley JL, Kay GG, Curtiss G. Wis-consin Card Sorting Test Manual: Revised and Expanded. Odessa, FL: Psychological Assessment Resources; 1993; [Google Scholar]
- 3. Alvarez JA, Emory E. Executive function and the frontal lobes: a meta-analytic review. Neuropsychol Rev. 2006; 16: 17–42 [DOI] [PubMed] [Google Scholar]
- 4. Heinrichs RW, Zakzanis KK. Neurocognitive deficit in schizophrenia: a quantitative review of the evidence. Neuro-psychology. 1998; 12: 426–445 [DOI] [PubMed] [Google Scholar]
- 5. Egan MF, Goldberg TE, Kolachana BS, et al. Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proc Natl Acad Sci U S A. 2001; 98: 6917–6922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Barnett JH, Scoriels L, Munafo MR. Meta-analysis of the cognitive effects of the catechol-O-methyltransferase gene Val158/108Met polymorphism. Biol Psychiatry. 2008; 64: 137–144 [DOI] [PubMed] [Google Scholar]
- 7. Lin S-H, Liu CM, Liu YL, et al. Clustering by neurocognition for fine-mapping of the schizophrenia susceptibility loci on chromosome 6p. Genes Brain Behav. 2009; 8: 785–794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sitskoorn M, Aleman A, Ebisch S, Appels M, Kahn R. Cognitive deficits in relatives of patients with schizophrenia: a meta-analysis. Schizophr Res. 2004; 71: 285–295 [DOI] [PubMed] [Google Scholar]
- 9. Szoke A, Schurhoff F, Mathiew F, Meary A, Ionescu S, Leboyer M. Tests of executive functions in first-degree relatives of schizophrenic patients: a meta analysis. Psychol Med. 2005; 35: 771–781 [DOI] [PubMed] [Google Scholar]
- 10. Snitz BE, MacDonald AW, Carter CS., III Cognitive deficits in unaffected first-degreerelatives of schizophrenia patients: a meta-analytic review of putative endophenotypes. Schizophr Bull. 2006; 32: 179–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Szoke A, Schurhoff F, Golmard J-L, et al. Familial resemblance for executive functions in families of schizophrenic and bipolar patients. Psychiatry Res. 2006; 144: 131–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ma X, Wang Q, Sham PC, et al. Neurocognitive deficits in first-episode schizophrenic patients and their first-degree relatives. Am J Med Genet B Neuropsychiatr Genet. 2007; 144: 407–416 [DOI] [PubMed] [Google Scholar]
- 13. Risch N. Linkage strategies for genetically complex traits, I: multilocus models. Am J Hum Genet. 1990; 46: 222–228 [PMC free article] [PubMed] [Google Scholar]
- 14. Egan MF, Goldberg TE, Gscheidle T, et al. Relative risk for cognitive impairments in siblings of patients with schizophrenia. Biol Psychiatry. 2001; 50: 98–107 [DOI] [PubMed] [Google Scholar]
- 15. Goldberg TE, Ragland JD, Torrey EF, Gold JM, Bigelow LB, Weinberger DR. Neuropsychological assessment of monozygotic twins discordant for schizophrenia. Arch Gen Psychiatry. 1990; 47: 1066–1072 [DOI] [PubMed] [Google Scholar]
- 16. Goldberg TE, Torrey EF, Gold JM, et al. Genetic risk of neuropsychological impairment in schizophrenia: a study of monozygotic twins discordant and concordant for the disorder. Schizophr Res. 1995; 17: 77–84 [DOI] [PubMed] [Google Scholar]
- 17. Pardo PJ, Knesevich MA, Vogler GP, et al. Genetic and state variables of neurocognitive dysfunction in schizophrenia: a twin study. Schizophr Bull. 2000; 26: 459–477 [DOI] [PubMed] [Google Scholar]
- 18. Husted JA, Lim S, Chow EWC, Greenwood C, Bassett AS. Heritability of neurocognitive traits in familial schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2009; 150: 845–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lewis SW, Reveley AM, Reveley MA, Chitkara B, Murray RM. The familial/sporadic distinction as a strategy in schizophrenia research. Br J Psychiatry. 1987; 151: 306–313 [DOI] [PubMed] [Google Scholar]
- 20. Farmer AE, McGuffin P, Gottesman II. Problems and pitfalls of the family history positive and negative dichotomy: response to Dalen. Schizophr Bull. 1990; 16: 367–370 [DOI] [PubMed] [Google Scholar]
- 21. Faraone SV, Seidman LJ, Kremen WS, Toomey R, Pepple JR, Tsuang MT. Neuropsychologic functioning among the nonpsychotic relatives of schizophrenic patients: the effect of genetic loading. Biol Psychiatry. 2000; 48: 120–126 [DOI] [PubMed] [Google Scholar]
- 22. Tuulio-Henriksson A, Arajarvi R, Partonen T, et al. Familial loading associates with impairment in visual span among healthy siblings of schizophrenia patients. Biol Psychiatry. 2003; 54: 623–628 [DOI] [PubMed] [Google Scholar]
- 23. Tsuang H-C, Lin S-H, Liu SK, et al. More severe sustained attention deficits in nonpsychotic siblings of multiplex schizophrenia families than in those of simplex ones. Schiz-ophr Res. 2006; 87: 172–180 [DOI] [PubMed] [Google Scholar]
- 24. Chang S-S, Liu C-M, Lin S-H, et al. Impaired flush response to niacin skin patch among schizophrenia patients and their nonpsychotic relatives: the effect of genetic loading. Schizophr Bull. 2009; 35: 213–221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Birkett P, Sigmundsson T, Sharma T, et al. Executive function and genetic predisposition to schizophrenia-the Maudsley family study. Am J Med Genet B Neuropsychiatr Genet. 2008; 147: 285–293 [DOI] [PubMed] [Google Scholar]
- 26. Hwu HG, Faraone SV, Liu CM, et al. Taiwan schizophrenia linkage study: the field study. Am J Med Genet B Neuropsychiatr Genet. 2005; 134: 30–36 [DOI] [PubMed] [Google Scholar]
- 27. Chen WJ, Liu SK, Chang C-J, Lien Y-J, Chang Y-H, Hwu H-G. Sustained attention deficit and schizotypal personality features in nonpsychotic relatives of schizophrenic patients. Am J Psychiatry. 1998; 155: 1214–1220 [DOI] [PubMed] [Google Scholar]
- 28. Nurnberger JI, Blehar MC, Jr, Kaufmann CA, et al. Diagnostic interview for genetic studies. Rationale, unique features, and training. NIMH Genetics Initiative. Arch Gen Psychiatry. 1994; 51: 849–859 [DOI] [PubMed] [Google Scholar]
- 29. NIMH Genetics Initiative Family Interview for Genetic Studies. 1992; Rockville, MD: National Institute of Mental Health; [Google Scholar]
- 30. Lin CCH, Chen WJ, Yang HJ, Hsiao CK, Tien AY. Performance on the Wisconsin Card Sorting Test among adolescents in Taiwan: norms, factorial structure, and relation to schizotypy. J Clin Exp Neuropsychol. 2000; 22: 69–79 [DOI] [PubMed] [Google Scholar]
- 31. Tien AY, Spevack TV, Jones DW, Pearlson GD, Schlaepfer TE, Strauss ME. Computerized Wisconsin Card Sorting Test: comparison with manual administration. Kaohsiung J Med Sci. 1996; 12: 479–485 [PubMed] [Google Scholar]
- 32. James JW. Frequency in relatives for an all-or-none trait. Ann Hum Genet. 1971; 35: 47–49 [DOI] [PubMed] [Google Scholar]
- 33. Olson JM, Cordell HJ. Ascertainment bias in the estimation of sibling genetic risk parameters. Genet Epidemiol. 2000; 18: 217–235 [DOI] [PubMed] [Google Scholar]
- 34. Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998; 62: 1198–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Tsuang M. Schizophrenia: genes and environment. Biol Psychiatry. 2000; 47: 210–220 [DOI] [PubMed] [Google Scholar]
- 36. Sullivan PF. The genetics of schizophrenia. PLoS Med. 2005; 2: 614–618 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Goldin LR, Gershon ES. Unravelling the relationship between genetic and environmental risk factors in psychiatric disorders. Br J Psychiatry. 1987; 151: 302–305 [DOI] [PubMed] [Google Scholar]
- 38. Kendler KS. The sporadic v. familial classification given aetiological heterogeneity: II. Power analyses. Psychol Med. 1988; 18: 991–999 [DOI] [PubMed] [Google Scholar]
- 39. Chang C-J, Chen WJ, Liu S-K, et al. Morbidity risk of psychiatric disorders among the first degree relatives of schizophrenia patients in Taiwan. Schizophr Bull. 2002; 28: 379–392 [DOI] [PubMed] [Google Scholar]
- 40. Stratta P, Daneluzzo E, Mattei P, Bustini M, Casacchia M, Rossi A. No deficit in Wisconsin Card Sorting Test performance of schizophrenic patients’ first-degree relatives. Schiz-ophr Res. 1997; 26: 147–151 [DOI] [PubMed] [Google Scholar]
- 41. Faraone SV, Kremen WS, Lyons MJ, Pepple JR, Seidman LJ, Tsuang MT. Diagnostic accuracy and linkage analysis: how useful are schizophrenia spectrum phenotypes?. Am J Psychiatry. 1995; 152: 1286–1290 [DOI] [PubMed] [Google Scholar]
- 42. Chen WJ, Chang C-H, Liu SK, Hwang TJ, Hwu H-G. Collaborators from the Multidimensional Psychopathology Group Research Project. Sustained attention deficits in nonpsychotic relatives of schizophrenic patients: a recurrence risk ratio analysis. Biol Psychiatry. 2004; 55: 995–1000 [DOI] [PubMed] [Google Scholar]
- 43. Campana A, Macciardi F, Gambini O, Scarone S. The Wisconsin Card Sorting Test (WCST) performance in normal subjects: a twin study. Neuropsychobiology. 1996; 34: 14–17 [DOI] [PubMed] [Google Scholar]
- 44. Kremen WS, Eisen SA, Tsuang MT, Lyons MJ. Is the Wisconsin Card Sorting Test a useful neurocognitive endo-phenotype?. Am J Med Genet B Neuropsychiatr Genet. 2007; 144B: 403–406 [DOI] [PubMed] [Google Scholar]
- 45. Taylor J. Heritability of Wisconsin Card Sorting Test (WCST) and Stroop Color-Word Test performance in normal individuals: implications for the search for endophenotypes. Twin Res Hum Genet. 2007; 10: 829–834 [DOI] [PubMed] [Google Scholar]
- 46. Chou LN, Kuo PH, Lin CCH, Chen WJ. Genetic and environmental influences on the Wisconsin Card Sorting Test performance in healthy adolescents: a twin/sibling study. Behav Genet. 2010; 40: 22–30 [DOI] [PubMed] [Google Scholar]
- 47. Miyake A, Friedman NP, Emerson MJ, Witzki AH, Howerter A, Wager TD. The unity and diversity of executive functions and their contributions to complex ‘‘frontal lobe’’ tasks: a latent variable analysis. Cognit Psychol. 2000; 41: 49–age100 [DOI] [PubMed] [Google Scholar]
- 48. Kremen WS, Xian H, Jacobson KC, et al. Storage and executive components of working memory: integrating cognitive psychology and behavior genetics in the study of aging. J Gerontol B Psychol Sci Soc Sci. 2008; 63: 84–91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Friedman NP, Miyake A, Young SE, Defries JC, Corley RP, Hewitt JK. Individual differences in executive functions are almost entirely genetic in origin. J Exp Psychol Gen. 2008; 137: 201–225 [DOI] [PMC free article] [PubMed] [Google Scholar]