Abstract
Introduction
Empathic deficits in schizophrenia may lead to social dysfunction, but previous studies of schizophrenia have not modeled empathy through paradigms that (1) present participants with naturalistic social stimuli and (2) link brain activity to “accuracy” about inferring other’s emotional states. This study addressed this gap by investigating the neural correlates of empathic accuracy (EA) in schizophrenia.
Methods
Fifteen schizophrenia patients and 15 controls were scanned while continuously rating the affective state of another person shown in a series of videos (ie, targets). These ratings were compared with targets’ own self-rated affect, and EA was defined as the correlation between participants’ ratings and targets’ self-ratings. Targets’ self-reported emotional expressivity also was measured. We searched for brain regions whose activity tracked parametrically with (1) perceivers’ EA and (2) targets’ expressivity.
Results
Patients showed reduced EA compared with controls. The left precuneus, left middle frontal gyrus, and bilateral thalamus were significantly more correlated with EA in controls compared with patients. High expressivity in targets was associated with better EA in controls but not in patients. High expressivity was associated with increased brain activity in a large set of regions in controls (eg, fusiform gyrus, medial prefrontal cortex) but not in patients.
Discussion
These results use a naturalistic performance measure to confirm that schizophrenic patients demonstrate impaired ability to understand others’ internal states. They provide novel evidence about a potential mechanism for this impairment: schizophrenic patients failed to capitalize on targets’ emotional expressivity and also demonstrate reduced neural sensitivity to targets’ affective cues.
Keywords: social cognition, empathic accuracy, emotional expressivity, schizophrenia, functional imaging
Introduction
Empathy, the ability to share and understand what other people are intending, thinking, and feeling,1 is crucial for maintaining successful social relationships.2 Difficulties in responding empathically may lead to social dysfunctions, including those that characterize severe mental illnesses such as schizophrenia and autism.3,4 While schizophrenia research has already explored some social cognitive and emotional constructs that may impact empathic abilities in patients (eg, facial emotion recognition),5–7 fewer studies on schizophrenia have directly investigated empathy per se.
While empathy is a multifaceted ability, in recent years, behavioral and neuroscience work identified 2 key facets: mental state attribution and experience sharing.1,8–10 Mental state attribution (also referred to as mentalizing, theory of mind, or cognitive empathy9) is the conscious and effortful process by which perceivers (individuals focusing on someone else) use all pieces of available information (verbal, nonverbal, etc.) in the service of nuanced flexible inferences about targets’ states and dispositions.11,12 Experience sharing (also referred to as emotional empathy1,13) refers to the tendency of perceivers to experience emotions like those experienced by social targets that they are observing. This process is thought to depend on the engagement of many of the cognitive and somatic processes perceivers would engage if they were experiencing themselves the states that targets are experiencing.13,14 Neuroimaging studies in healthy subjects have identified distinct neural correlates for mental state attribution (ie, medial prefrontal cortex [mPFC], temporo-parietal junction, posterior cingulate cortex, and temporal poles11,15–17) and experience sharing (ie, premotor, inferior frontal, inferior parietal, somatosensory, anterior insula, and anterior cingulate cortex18–21). A model of empathy recently published by Zaki and Ochsner10 suggests that these 2 components of empathy contribute to empathic accuracy (EA), which is defined as the ability to accurately judge the amount and kind of emotions or thoughts experienced by another person22,23. According to this model, cues (eg, content of speech, prosody, facial expression, etc.) that are produced by a social target are received and processed by a perceiver, who will then engage in both experience sharing and mental state attribution processes. The information drawn from these processes will impact EA, and ultimately, adaptive interpersonal outcomes (see online supplementary figure 1). In other words, the extent to which one has empathically accurate perceptions of a target is a downstream consequence of a number of processing steps, including mental state attribution and experience sharing.
Neuroimaging studies investigating empathy-related processes in schizophrenia have mostly focused on mental state attribution. Such studies have found mixed results thus far: 2 found reduced bilateral prefrontal activity in patients during mental state attributions made about comic strips24 or photographs of eyes,25 1 found significantly reduced activity in the mPFC and temporoparietal junction,26 1 found significantly reduced activity in the right temporal pole,27 and another observed larger responses in the right superior temporal gyrus.28 The neural correlates of experience sharing in schizophrenia have not received much attention thus far, though studies in this area are currently ongoing. Although neuroimaging studies that focus on characterizing the 2 main components of empathy in schizophrenia provide valuable initial insights into neural abnormalities associated with empathic deficits in patients, it is still uncertain whether brain activity measured in these studies predicts how well patients understand others minds. Hence, there is a need to complement these findings with studies that directly link brain activity to behavioral measures of patients’ ability to be empathically accurate.
The current neuroimaging study used a naturalistic EA paradigm adapted from Zaki and colleagues23,29,30 and validated in social psychological research.23,31 This paradigm involves complex stimuli (videos) that depict actual social targets experiencing internal states that vary dynamically across time. Further, it allows for a continuous measure of empathy performance. When the video stimuli were created, targets watched the videos of themselves just after being filmed and continuously rated how positive or negative they felt while discussing these events. Perceivers subsequently watch these videos and continuously rate how positive or negative they believe targets felt while talking. Time series correlations between perceivers’ inferences and targets’ self-ratings are used as a measure of EA. According to our model of empathy, EA is not solely a measure of mental state attribution or of experience sharing. Instead, it is the product of these 2 processes. This paradigm recently was validated in the scanner in healthy subjects. EA scores for each video (ie, block) were used as parametric modulators to determine the statistical weight of each block, and resulting activation maps reflected brain activity that varied with EA. Regions classically involved in mental state attribution and experience sharing, including mPFC and superior temporal sulcus, and other regions (eg, inferior parietal lobule and premotor cortex) tracked with the accuracy of inferences made about the emotions expressed by targets in these complex social stimuli.30 Furthermore, prior work by our group found significantly reduced EA in schizophrenia patients compared with healthy controls.32 These findings together suggested that exploring the neural correlates of EA in schizophrenia would be both novel and feasible.
EA depends on characteristics of both perceivers and targets.33,34 For example, behavioral studies have shown that targets who report high dispositional emotional expressivity (indexed with the Berkeley Expressivity Questionnaire—BEQ35) are more readable, that is, they produce higher levels of EA regardless of the perceiver viewing them.34 High expressivity targets generate social cues that communicate corresponding internal states more clearly than cues from low expressivity targets.36 In the current study, we tested whether targets’ trait expressivity would affect the neural processes of perceivers. Furthermore, in prior work, we found that EA in schizophrenia patients does not benefit as much from expressive targets as does EA in controls,32 suggesting that patients fail to take advantage of targets’ expressive cues.
The main focus of this study was to investigate the neural correlates of EA and targets’ expressivity in schizophrenia. Our first objective was to explore whether schizophrenia patients engage the same neural network as controls when being empathically accurate. Our second objective was to determine if different levels of expressivity in social targets influence patterns of neural activity in the same way in patients and controls. For both objectives, we expected to find group differences in brain regions especially linked to social cognition, notably the mPFC.
Methods
Participants
Fifteen right-handed patients with schizophrenia and 15 right-handed healthy controls participated in the study. Subjects had normal or corrected to normal vision. Schizophrenia patients were 18–60 years of age and recruited from outpatient clinics at the VA Greater Los Angeles Healthcare System and through local board and care facilities. Patients were clinically stable and received the Structural Clinical Interview for Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Axis I Disorders (SCID)37 to confirm diagnosis of schizophrenia. We assessed clinical symptoms using the expanded Brief Psychiatric Rating Scale (BPRS)38 and examined the BPRS total score as well as BPRS mean subscales for positive symptoms, negative symptoms, and depression anxiety. Negative symptoms were also assessed using the Scale for the Assessment of Negative Symptoms (SANS).39 All patients except one were taking antipsychotic medication at the time of testing: 2 with quetiapine, 5 with risperidone, 3 with aripiprazole, 3 with clozapine, and 1 with fluphenazine. The mean dose of antipsychotic medication was equivalent to 210 mg/day of chlorpromazine (SD = 142).40 Exclusion criteria for patients included (1) substance abuse or dependence in the last 6 months, (2) IQ < 70, (3) history of loss of consciousness > 1 hour, (4) identifiable neurological disorder, and (5) not sufficiently fluent in English. Healthy control participants were recruited through flyers posted in the local community, newspaper advertisements, and website postings. Exclusion criteria for control participants included (1) history of schizophrenia or other psychotic disorder, bipolar disorder, recurrent depression, history of substance dependence, or any substance abuse in the last 6 months based on the SCID,37 (2) avoidant, paranoid, schizoid, and schizotypal disorders based on the SCID for Axis II disorders,41 (3) history of loss of consciousness > 1 hour, (4) schizophrenia or other psychotic disorder in a first-degree relative, (5) significant neurological disorder or head injury, and (5) not sufficiently fluent in English. Both patients and healthy controls were comparable in terms of age, education, and parental education (see table 1).
Table 1.
Demographic and Clinical Data
| Group; Mean (SD) | ||||
| Characteristic | Schizophrenia, n = 15 | Control, n = 15 | Statistical Test | P value |
| Age, y | 42.4 (11.8) | 42.9 (8.6) | t 28 = 0.12 | .90 |
| Education, y | 12.7 (2.1) | 14.1 (1.7) | t 28 = 2.00 | .06 |
| Parental education, y | 13.2 (3.0) | 13.9 (2.2) | t 28 = 0.85 | .40 |
| Sex, male:female | 13:2 | 13:2 | χ2 1 = 0.00 | 1.00 |
| Ethnicity | ||||
| African American | 7 | 5 | ||
| Asian | 1 | 0 | ||
| Hispanic | 2 | 0 | ||
| White | 5 | 10 | ||
| Other | 0 | 0 | ||
| BPRS total score | 40.1 (5.7) | |||
| BPRS positivea | 2.3 (0.8) | |||
| BPRS negativea | 1.9 (0.6) | |||
| BPRS depression/anxietya | 1.3 (0.4) | |||
| SANS total score | 27.1 (9.7) | |||
| Age of onset, y | 21.3 (8.6) | |||
| Number of hospitalizations | 2.5 (1.1) | |||
Note: BPRS, Brief Psychiatric Rating Scale; SANS, Scale for the Assessment of Negative Symptoms.
Mean score of each BPRS subscale was calculated by dividing the total score by the number of items included in the subscale.
All interviewers were trained through the Treatment Unit of the VA Desert Pacific Mental Illness Research, Education, and Clinical Center. SCID interviewers were trained to a minimum kappa of 0.75 for key psychotic and mood items, and symptom raters were trained to a minimum intraclass correlation of .80. Participants were evaluated for their capacity to give informed consent and provided written informed consent after all procedures were fully explained, according to procedures approved by the institutional review board at University of California, Los Angeles (UCLA).
EA Task
The EA task presented participants with video clips lasting from 1 to 3 minutes each. A detailed explanation of the development of these videos is provided elsewhere.23,29,30 Briefly, the head and shoulders of an individual (referred to as “target”) were videotaped while he/she discussed a positive or negative autobiographical event. Immediately after the videos were filmed, targets: (1) provided continuous ratings of their own emotional experience while watching their own videos using a 9-point scale and (2) completed the 10-item BEQ,35 which assesses tendencies to experience and express strong emotions in general.
Sixteen video clips (8 positive valence and 8 negative valence) were shown to each participant in the scanner in counterbalanced pseudorandomized order. Social targets expressed joy and happiness in the positively valenced videos and a mix of sadness, anger, embarrassment, or fear/anxiety in the negatively-valenced videos. Each video was associated with 1 of 2 possible conditions. For each video trial, a cue (either “OTHER” or “EYES”) was presented for 5 seconds, followed by a fixation cross, presented for 2 seconds, and then the video clip. Participants were instructed that if the OTHER cue appeared before a clip, they were required to continuously rate how positive or negative they believed that target felt at each moment while talking about events. Participants’ ratings were made using a 9-point scale (1, very negative; 5, neutral; and 9, very positive) presented below the video. If the EYES cue appeared before a clip, participants were instructed to continuously rate how far to the left or right the target’s eyes were directed, relative to the screen, using a 9-point scale (1, far left; 5, middle; 9, far right). The EYES condition constitutes a comparison condition that matches motor behavior and attention to targets but does not require inferences about target affect. Each video started with the number 5 selected and participants pressed the left or right button of a key box to move the number upward (toward positive or right) or downward (toward negative of left). The selected number on the scale was always highlighted so that participants could monitor their responses. Participants watched 8 videos in the OTHER condition and 8 in the EYES condition. These videos were split across 4 functional runs (2 OTHER and 2 EYES video clips per run). The entire scanning session lasted 60 minutes. The EA task was generated using Presentation software (Neurobehavioral Systems) and video stimuli were presented using MR-compatible LCD goggles (Resonance Technology, Northridge, CA). During a prescan session, participants watched and rated 3 practice videos (2 in the OTHER condition and 1 in the EYES condition, and not from the pool of videos presented in the scanner), and the experimenter interviewed them to verify that they understood each task.
Two versions of the task were developed, each using different combinations of video clips. Each subject received only one version. For each version, the 8 video clips (4 positive and 4 negative) assigned to the OTHER condition were matched to the 8 video clips assigned to the EYES condition in terms of length and emotional expression. The 8 positive and 8 negative videos used in this study had equal number of male and female targets.
Behavioral Analysis
Data reduction and time series correlations were performed by using Matlab (Mathworks). Continuous affect ratings during the OTHER condition were converted into a time series of sequential values with one number for the average every 2-second epoch of video for each participant. These values served as data points in subsequent time series analyses. To calculate EA, participants’ continuous ratings across these 2-second epochs were correlated with the target’s own continuous ratings across the same epochs for each video. The resulting correlation coefficient (r) between 2 time series is the measure of EA. All correlation coefficients were r- to Z-transformed for subsequent analyses. For each subject, we also computed the mean affect rating for each video presented in the OTHER condition.
To compare groups on the EA task, z scores were summed for positive and negative valence separately and a 2 × 2 repeated measures ANOVA was performed with valence as a within-subject factor and group as a between-subject factor. To ensure that any group difference in EA was not confounded with the sheer number of ratings perceivers made, we included the mean number of button presses as a covariate (ie, mean number of affect ratings made during the OTHER videos). We also compared groups on number of button presses during the entire EA task by conducting a 2 (condition) × 2 (group) repeated measures ANOVA with condition as a within-subject factor and group as a between-subject factor. Similarly, a 2 (valence) × 2 (group) repeated measures ANOVA was conducted to compare groups for mean affect ratings (ie, mean affect value calculated for each video and each participant). To examine the effect of targets’ expressivity on EA, a mixed linear model was used with expressivity (BEQ score associated with each video) and group as fixed effects and subject as a random effect. We also conducted a complementary analysis to examine how EA differs between high expressivity videos and low expressivity videos in both groups. For each subject, we calculated a mean EA value for the 4 videos with the lowest target’s expressivity and another mean EA value for the 4 videos with the highest target’s expressivity. Then, for each group separately, we conducted a paired t test that directly compared EA for low expressivity videos vs high expressivity videos.
Imaging Data Acquisition
Scanning was conducted on a 3-T scanner (Siemens Allegra, Germany) in the UCLA Ahmanson-Lovelace Brain Mapping Center. A T2*-weighted gradient-echo sequence was used to detect blood-oxygen level-dependent signal. Each volume comprised 33 axial slices of 4.0-mm thickness and a 3.4 × 3.4-mm in-plane resolution. Volumes were acquired continuously every 2 s (echo time [TE] = 30 ms; flip angle = 75°; field of view = 220 mm; matrix size = 64 × 64). Four functional runs were acquired from each subject. Because the videos differed in length and were pseudorandomized across runs, the length of each run varied. At the end of the scanning session, a T1-weighted MPRAGE structural image (repetition time [TR] = 1900 ms; TE = 3.4 ms; 160 slices with a thickness of 1 mm) was acquired for each subject.
fMRI Data Analysis
All functional volumes from each run were realigned to the first volume to correct for interscan movement, spatially normalized to the Montreal Neurological Institute space (normalized voxel size: 2 × 2 × 2 mm) and smoothed with an 6-mm full-width half-maximum Gaussian kernel. After this processing, all 4 runs were concatenated into 1 consecutive time series for the regression analysis. Low-frequency temporal drifts were removed by applying a high-pass filter. Data were analyzed using Statistical Parametric Mapping software 8 (Wellcome Department of Imaging Neuroscience, UK). Data were analyzed by the general linear model, in which individual events were modeled by a canonical haemodynamic response function (HRF). To search for neural activity corresponding to EA, regressors were constructed by using time-course correlation EA scores as parametric modulators determining the weight of each block (ie, OTHER video). As such, the resulting activation maps reflect brain activity differences corresponding to the varying accuracy across videos within subjects. We identified brain regions whose increased brain activity was associated with higher EA. To ensure that the neural correlates of EA were not confounded by the sheer number of button presses, the analysis included a parametric regressor of no interest representing the number of button presses per video. In a separate model, we also explored the neural activity associated with target expressivity by using BEQ expressivity scores as parametric modulators. The resulting activation maps reflect brain activity differences corresponding to the varying expressivity across videos within subjects. We identified brain regions whose increased brain activity was associated with higher expressivity. One-sample t tests and 2-sample t tests were conducted to identify brain activity associated with EA and expressivity in each group and to compare groups. Resulting activation maps were thresholded at P < .01, uncorrected for multiple comparisons, with an extent threshold of 53 contiguous voxels, corresponding to a false-positive discovery rate of <5% across the whole brain as estimated by Monte Carlo simulation (10 000 simulations).42 With this technique, the overall family-wise error (FWE) rate is controlled by simulating null data sets with the same spatial autocorrelation as found in the residual images and creating a frequency distribution of different cluster sizes. Clusters with a size that exceeds the minimum cluster size corresponding to the a priori chosen FWE are reported. This relatively new thresholding method has been successfully employed by different research groups in recently published neuroimaging studies.30,43 For the purpose of the current study, we especially focus on brain activity associated with EA and expressivity. The results of the comparison between the OTHER condition and the EYES condition are presented in the online supplementary table S2.
Results
Behavioral Results
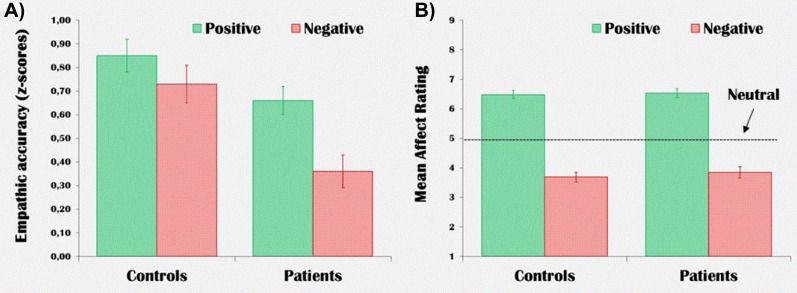
While patients had lower accuracy for rating targets’ emotions compared with healthy controls (main effect of group: F 1,28 = 11.21, P = .002), groups were not significantly different from each other for their mean number of button presses at each condition (main effect of group: F 1,28 = 1.81, P = .19). Moreover, groups were not significantly different in their mean affect ratings (main effect of group: F 1,28 = 0.38, P = .54). Both groups rated positive videos significantly above the neutral value (ie, “5”), and negative videos significantly below the neutral value. Figure 1 illustrates EA score and affect rating for each group and valence. Online supplementary table S1 provides complete behavioral and statistical test results.
Fig. 1.
Mean empathic accuracy scores (A) and affect ratings (B) for each group and valence during the OTHER condition.
A mixed linear model was used to examine the effect of targets’ expressivity on EA. We found a significant group by expressivity interaction (F 1,209 = 4.34, P = .03), indicating that the expressivity of the target had a different effect on EA in controls compared with patients (see online supplementary figures S2 and S3). Increasing target expressivity promoted higher EA in controls (R 2 linear = .28) but not in patients (R 2 linear = .03). These findings were confirmed by a complementary analysis: controls showed significantly increased EA for the videos involving high expressivity targets (EA: 0.92, SD = 0.30) compared with videos involving low expressivity targets (EA: 0.67, SD = 0.23) (t = 3.32, df = 14, P = .005). n contrast, patients did not show significantly increased EA for high expressivity targets (EA: 0.56, SD = 0.34) compared with low expressivity targets (EA: 0.46, SD = 0.19) (t = 1.35, d f = 14, P = .20).
fMRI Results
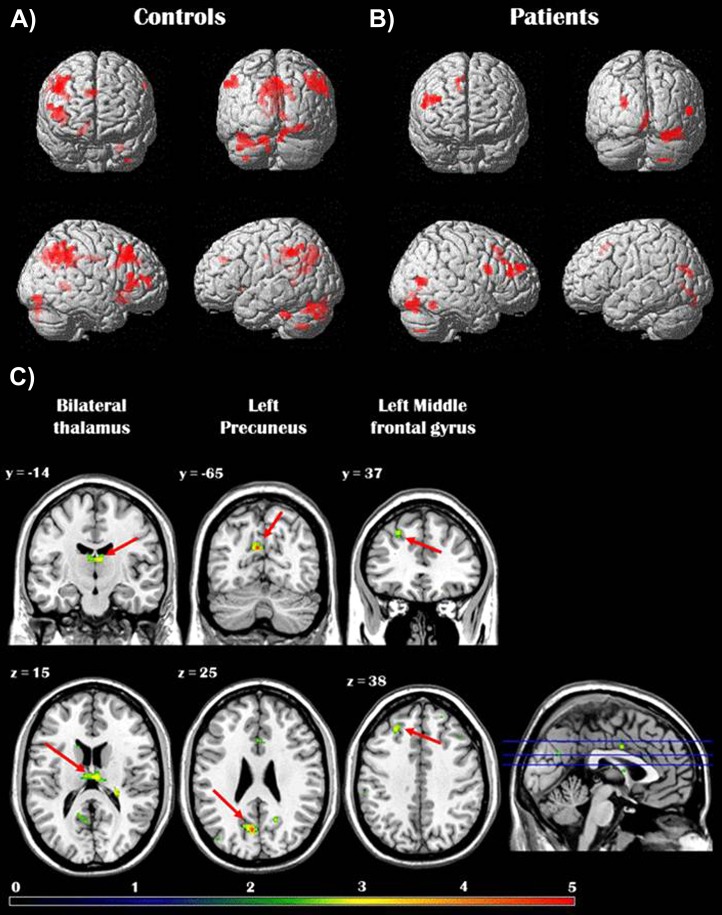
We examined brain regions where activity predicted better accuracy during the OTHER condition. In other words, higher activity in these regions was associated with higher EA. In keeping with prior work in healthy adults,30 for controls activity in several frontal (ie, medial, inferior, and middle frontal gyri) and parietal (ie, precuneus, inferior parietal lobule) regions as well as in the parahippocampal gyrus and middle occipital gyrus was significantly and positively correlated with EA performance. In patients, only the lateral PFC and some occipital regions showed a significant correlation with EA. Direct group comparison revealed that activity in the left precuneus, left middle frontal gyrus, and bilateral thalamus was significantly more correlated with EA in controls than in patients. No brain regions were found to be more correlated with EA in patients compared with controls (see table 2 and figure 2).
Table 2.
Brain Regions Whose Activity Predicted Empathic Accuracy in Healthy Subjects and Patients With Schizophreniaa
| Talairach Coordinates | ||||||
| Brain Region | H | x | y | z | t value | Cluster Size |
| Controls | ||||||
| Posterior cingulate (BA 23) | L | −1 | −27 | 25 | 4.83 | 1082b |
| Cingulate/precuneus (BA 31) | R | −3 | −38 | 34 | 3.82 | 1082b |
| Middle occipital gyrus | R | 29 | −82 | −3 | 4.88 | 178 |
| Putamen/caudate | R | 15 | 12 | −7 | 4.49 | 81 |
| Middle frontal gyrus (BA 8/9) | R | 41 | 24 | 39 | 4.46 | 402 |
| Parahippocampal gyrus (BA 30) | R | 12 | −46 | 4 | 4.24 | 113 |
| Inferior frontal gyrus (BA 46/13) | R | 40 | 24 | 9 | 4.34 | 212 |
| Precuneus (BA 7/31) | L | −11 | −61 | 31 | 4.37 | 591 |
| Inferior parietal lobe (BA 39) | R | 47 | −63 | 36 | 4.08 | 531 |
| Inferior parietal lobe (BA 40) | L | −48 | −46 | 38 | 4.04 | 179 |
| Medial frontal gyrus (BA 9/10) | R | 11 | 30 | 31 | 3.03 | 82 |
| Patients | ||||||
| Middle frontal gyrus (BA 9) | R | 48 | 10 | 27 | 4.71 | 77 |
| Superior frontal gyrus (BA 10) | R | 37 | 54 | 24 | 4.82 | 61 |
| Middle frontal gyrus (BA 46) | R | 53 | 38 | 21 | 4.91 | 62 |
| Lingual gyrus (BA 18) | R | 2 | −76 | 0 | 4.08 | 82 |
| Middle occipital gyrus (BA 18) | R | 29 | −82 | −3 | 4.30 | 218 |
| Cuneus (BA 18) | L | −24 | −77 | 17 | 4.29 | 71 |
| Anterior cingulate (BA 32) | R | 12 | 25 | 37 | 3.93 | 60 |
| Middle temporal gyrus (BA 39) | R | 53 | −68 | 15 | 3.42 | 65 |
| Controls > Patients | ||||||
| Precuneus (BA 31) | L | −5 | −65 | 25 | 4.22 | 77 |
| Middle frontal gyrus (BA 8/9) | L | −23 | 37 | 38 | 3.33 | 92 |
| Thalamus | B | 6 | −14 | 15 | 3.23 | 97 |
| Patients > Controls | ||||||
| No significant activation | ||||||
Note: L, Left; R, Right; B, Bilateral; H, Hemisphere; BA, Brodmann’s Area.
The cluster size represents the number of voxels. Talairach coordinates represent the peak voxel of each cluster, where x, y, and z indicate the distance measured in millimeters from the anterior commissure in the sagittal, coronal, and horizontal planes, respectively. All clusters of activation reported in table 2 were significant at P < .01, uncorrected for multiple comparisons, with a minimum cluster size of 53 contiguous voxels, corresponding to a false-positive discovery rate of <5% across the whole brain as estimated by Monte Carlo simulation.
These peaks belong to the same cluster of activation.
Fig. 2.
Panels A and B show the brain regions whose activity during affect rating of a target (OTHER condition) was significantly correlated with “empathic accuracy (EA)” in healthy controls (A) and schizophrenia patients (B) separately. Panel C depicts the brain regions showing greater association with EA in healthy controls compared with patients with schizophrenia. Reported activations were thresholded at P < .01, uncorrected for multiple comparisons, with an extent threshold of 53 contiguous voxels, corresponding to a false-positive discovery rate of <5% across the whole brain.
Next, we examined the neural correlates of target expressivity in both groups. In controls, several brain regions demonstrated activity that tracked with targets’ expressivity. Many of these regions—including mPFC and precuneus—overlapped with those tracking accuracy (see online supplementary table S3 and figure S4). However, no brain regions in patients were significantly linked to targets’ expressivity. Direct group comparison showed that mPFC and lateral PFC regions, inferior parietal lobule, and several visual processing areas were significantly more active in controls compared with patients when the target was more expressive (see table 3 and online supplementary figure S5). In fact, the analysis on targets’ expressivity revealed many more brain regions that significantly differentiated groups compared with the analysis on EA.
Table 3.
Brain Regions Whose Activity During Affect Rating of a Target (OTHER condition) Showed Greater Association With Target’s Expressivity in Healthy Controls Compared With Patients With Schizophreniaa
| Talairach Coordinates | ||||||
| Brain Region | H | x | y | z | t value | Cluster Size |
| Inferior parietal lobule (BA 40) | R | 37 | −51 | 36 | 5.31 | 3246 |
| Middle frontal gyrus (BA 9) | R | 35 | 40 | 27 | 5.21 | 296 |
| Inferior frontal gyrus (BA 46) | R | 47 | 43 | 13 | 4.90 | 157 |
| Anterior cingulate (BA 24) | R | 9 | 19 | 21 | 4.49 | 1641 |
| Superior frontal gyrus (BA 9) | L | −37 | 36 | 32 | 4.30 | 365 |
| Cingulate (BA 31) | R | 18 | −34 | 32 | 4.11 | 252 |
| Posterior cingulate (BA 30) | R | 18 | −54 | 16 | 4.09 | 175 |
| Middle occipital gyrus (BA 37) | L | −53 | −68 | 5 | 4.08 | 239 |
| Middle frontal gyrus (BA 9) | R | 49 | 17 | 32 | 3.75 | 369 |
| Lingual gyrus (BA 18) | L | −7 | −76 | −4 | 3.60 | 90 |
| Middle frontal gyrus (BA 6) | L | −40 | 7 | 53 | 3.53 | 80 |
| Inferior frontal gyrus (BA 45) | L | −35 | 29 | 9 | 3.52 | 321 |
| Fusiform gyrus (BA 19) | R | 25 | −75 | −12 | 3.41 | 74 |
| Lingual gyrus (BA 18) | L | −21 | −57 | 2 | 3.37 | 129 |
| Cuneus (BA 18) | L | −13 | −69 | 14 | 3.35 | 111 |
| Thalamus | R | 1 | −5 | 13 | 3.30 | 154 |
| Paracentral lobule (BA 6) | R | 1 | −30 | 61 | 2.97 | 69 |
Note: Abbreviations are explained in the first footnote to table 2.
The cluster size represents the number of voxels. Talairach coordinates represent the peak voxel of each cluster, where x, y, and z indicate the distance measured in millimeters from the anterior commissure in the sagittal, coronal, and horizontal planes, respectively. All clusters of activation reported in table 3 were significant at P < .01, uncorrected for multiple comparisons, with a minimum cluster size of 53 contiguous voxels, corresponding to a false-positive discovery rate of <5% across the whole brain as estimated by Monte Carlo simulation.
Discussion
This study is the first, to our knowledge, to explore the neural correlates of EA in schizophrenia. The objectives of the current report focused on whether schizophrenia patients engage the same neural network as controls when being empathically accurate and when encountering expressive social targets. At the behavioral level, we replicated 2 previous findings from our group: (1) that schizophrenia patients are impaired in their capacity to accurately judge the emotions of social targets and (2) that schizophrenia patients do not benefit from expressive social targets as much as controls.32
The impaired EA in schizophrenia patients compared with controls is not easily explained by attention or motor deficits because no significant group differences were observed for the mean number of button presses at the OTHER condition. At the neural level, we identified brain regions whose activity tracked parametrically with EA. Results in controls were consistent with prior findings for healthy adults reported by Zaki and colleagues30: greater EA was associated with increased activity in brain regions typically linked to cognitive effort (ie, lateral PFC), visual attention (ie, parietal and occipital cortices), and socioemotional processes, including mental state attribution (eg, mPFC, precuneus, posterior cingulate), experience sharing (eg, inferior frontal, inferior parietal), and social context processing (ie, parahippocampal gyrus).30 These results in healthy controls support the idea that both mental state attribution and experience sharing processes contribute to EA. By contrast, patients demonstrated a relatively sparse pattern of accuracy-related brain activity. One may suggest that this is explained by an overall reduced activation in patients during the OTHER condition. Indeed, the contrast between the OTHER condition and the control EYES condition (see online supplementary materials) did not reveal as much significant activations in patients compared with controls when groups were analyzed separately. However, the direct group comparison (OTHER vs EYES × Controls vs Patients) identified only the parahippocampal gyrus as being significantly more active in controls compared with patients during affect rating of the target, irrespective of EA (see online supplementary table S2). The parahippocampal gyrus is a medial temporal region that has been linked not only to memory encoding and recognition but also to social context processing, including paralinguistic elements of verbal communication.44 An overall reduced activity of the parahippocampal gyrus in schizophrenia during the OTHER condition may partly explain why this region has not been linked to changes in EA in patients even if this region is thought to be important for decoding social information. While greater EA in patients was significantly associated with activity in some lateral PFC and mPFC, temporal and occipital regions, direct group comparison showed that the left precuneus, left middle frontal gyrus, and bilateral thalamus were the only regions whose activity was significantly more predictive of EA in controls compared with patients. Parametric analyses using target expressivity—as opposed to EA—revealed a similar pattern: increasing levels of target expressivity elicited increasing activity in a network of brain regions typically associated with social cognition in controls but not in patients. Direct between-group comparisons demonstrated that expressivity more powerfully elicited activity in these regions in controls as compared with patients.
A recent meta-analysis of neuroimaging studies of the social cognitive processes contributing to empathy identified the precuneus as part of a putative mental state attribution network, along with the mPFC, superior temporal sulcus, some anterior temporal regions, and posterior cingulate cortex.45 Converging data suggest that the precuneus supports the imagery and imagination processes required to infer the mental states of another, integrating inputs from a wide variety of other brain regions that support memory, motor, and somatosensory processing.45 Other neuroimaging studies have shown increased activity of the precuneus in schizophrenia patients compared with controls during facial expression recognition.5,6 This hyperactivity of the precuneus is thought to reflect compensatory processes triggered to counterbalance the deficit in emotion recognition and face processing. Here, the reduced association between precuneus activity and EA found in schizophrenia patients may reflect ineffectiveness in using external and internal cues to mentally put themselves “in the shoes” of targets while evaluating their affective state.
The middle frontal gyrus and thalamus are key parts of working memory and cognitive control circuitry, important for maintaining representations of context and task goals.46–48 Behavioral evidence suggests that context processing may be selectively impaired in patients with schizophrenia.49 The fact that these 2 regions in patients did not track EA as much as in controls could suggest that patients had problems maintaining context during the EA task. Other studies have suggested that alterations in the empathic response may reflect impairment in the ability to shift a course of thought or action according to the demands of the social situation (ie, cognitive flexibility). Such flexibility, typically related to PFC functioning,50 may be important for EA, which may require flexible consideration and integration of different interpretations of another person’s emotions and point of view.51 Although cognitive flexibility may be part of the basic neurocognitive abilities required to accurately evaluate mental states in others, these abilities on their own are not sufficient to produce interpersonal accuracy. As suggested by the model of empathy presented in the introduction,10 an EA deficit in schizophrenia may be the downstream consequence of multiple deficits interacting together. Future investigations of the link between EA and neurocognitive deficits in schizophrenia will shed more light on the specific impact of cognitive flexibility on EA deficit.
As mentioned, expressiveness of the target is an important factor in predicting EA, presumably because expressive targets display more cues, making it easier for perceivers to identify their mood. Our results demonstrate that targets’ expressivity had a significantly different impact on EA in controls compared with patients. Elevated expressivity in targets improves EA in controls but not at all in patients. The idea that patients did not use expressivity as much as controls also is supported by the lack of association in patients between neural activity and targets’ expressivity. In fact, there were no brain regions in patients whose activity significantly tracked with targets’ expressivity during affect rating. In contrast, a large set of brain regions, including the mPFC and lateral PFC regions, inferior parietal lobule, and several visual processing areas, showed a significantly stronger association with targets’ expressivity in controls compared with patients. A study in healthy controls showed that greater target expressivity improved EA of perceivers with high emotional empathy (ie, experience sharing component) more than that of perceivers with low emotional empathy.23 Based on this observation in healthy subjects, it is possible that the lack of association between targets’ expressivity and EA in schizophrenia is explained by a reduced capacity in patients to share the emotional experience expressed by targets.
Emotional expressivity refers to both verbal and nonverbal cues. Zaki et al29 have recently demonstrated that more verbal cues are produced by expressive targets who are reporting positively valenced events, as opposed to more nonverbal cues when negatively valenced events are being reported. Our behavioral data suggest, at a trend level, that patients were more impaired on EA (vs controls) for negative videos compared with positive videos (group by valence interaction: P = .10). One could suggest that patients failed to use nonverbal visual cues, such as facial expressions, to accurately evaluate affective states in targets. This suggestion would be consistent with several studies demonstrating that schizophrenia patients have an impaired ability in identifying emotional expressions in faces.7 While our paradigm did not allow us to precisely address this possibility and isolate the specific contribution of visual (nonverbal) vs auditory (verbal and paraverbal) input to EA, indirect evidence suggest that the EA deficit in patients is not simply the by-product of a basic facial emotion recognition deficit. The model of empathy presented in the introduction proposes that facial expressions produced by social targets are one of the cues that are received and processed by perceivers (along with contextual/situational and other kinds of verbal and nonverbal information), who then engage in several processes such as mental state attribution and experience sharing. EA is viewed as the downstream consequence of these processing steps. While it could be theoretically possible that an EA deficit is only explained by an impaired ability to decode facial expressions in targets, extensive evidence shows that schizophrenia patients are also impaired in decoding other types of cues (eg, prosody) as well as in their ability to attribute mental states in paradigms that do not involve decoding facial expressions.52 Hence, while our paradigm did not allow us to precisely identify the cause of the EA deficit in patients, it is unlikely that a primary deficit in recognition of emotional facial expressions explains it. In support with this idea, our groups were not significantly different in their mean affect ratings of the videos, suggesting that patients perceived similar levels of emotional intensity compared with controls while watching the videos.
While this study provides new insights into the nature of empathic ability in schizophrenia, some important questions remain to be addressed in future research. Our model of EA proposes that both mental state attribution and experience sharing processes contribute to accuracy. While our functional magnetic resonance imagine (fMRI) findings support this idea, our EA paradigm did not specifically include a behavioral measure of experience sharing during the ratings. Our study does not rule out the possibility that the EA task was performed via emotion recognition and mental state attribution alone. Future studies using the EA paradigm would benefit from acquiring psychophysiological data (eg, skin conductance) from perceivers during the rating of the targets as a way to evaluate both components of empathy. Another limitation of this study involves antipsychotic medication. All but one patient was taking antipsychotic medications, which may have altered both the behavioral performance at the EA task and brain activity. Using a similar paradigm in unmedicated patients will allow researchers to avoid the potential confound of pharmacological treatment on the neural correlates of EA in schizophrenia. It should be mentioned, however, that the mean dose of chlorpromazine-equivalent antipsychotic was not significantly correlated with EA scores and with symptoms severity (ie, BPRS and SANS total scores). Additionally, our protocol did not include nonsocial neurocognitive measures or assess community and social functioning. Past studies have already shown that adequate social-cognitive abilities in patients are associated with better social functioning.53 However, it would be interesting to explore whether the specific construct of EA is a better predictor of social and community functioning in schizophrenia. Also, only 2 female participants were included in each group, which limits our ability to generalize the findings to female patients. Because women tend to show higher levels of empathy compared with males,54,55 it would be interesting to test whether gender impacts EA deficits in schizophrenia. Another limitation of this study is the small sample size. Although we found significant neural substrates of EA and group differences using acceptable statistical threshold methods, we may have missed additional group difference due to statistical power. We verified whether our main fMRI results reported in table 2 (ie, neural correlates of EA) survive a more conservative statistical threshold of P < .001. In healthy controls, regions that survive include the bilateral posterior cingulate, right middle occipital gyrus, right middle frontal gyrus, and left precuneus. When using a threshold of P < .005, every region reported in table 2 for healthy controls survive except the right medial frontal gyrus. Regarding the group comparison, the difference in precuneus activation survived a statistical threshold of P < .001. Using a threshold of P < .005 does not change the findings at all as all 3 regions reported in table 2 (ie, precuneus, middle frontal gyrus, and thalamus) remain significantly more predictive of EA in controls compared with patients. Findings not surviving more stringent thresholds should be interpreted with more caution and should be replicated in other studies on schizophrenia with larger sample sizes. Finally, videos were not highly varied in terms of race and age. Among the 16 videos that were used for our EA task, 2 included an African American target and 14 involved a Caucasian. Future versions of the EA paradigm should employ ethnically diverse social targets.
In summary, our findings suggest, in the context of an EA task, that schizophrenia patients fail to mobilize additional neural processes when targets are being more expressive. This in turn likely explains the lack of association between expressivity and EA, and ultimately, EA deficits when targets are expressive. The findings of the current study provide new insights for future studies. Notably, our findings on targets’ expressivity suggest that it is crucial to take this external variable into account when investigating EA in schizophrenia. Further investigation may attempt to differentiate the impact of verbal vs nonverbal social cues on EA and brain activity during affect rating.
Funding
This work was supported by pilot grants from the Desert Pacific Mental Illness Research, Education, and Clinical Center and the UCLA Brain Mapping Center. This work was also supported by grant MH043292 to Dr M.F.G. Poorang Nori assisted in data collection. Dr P.O.H. is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Acknowledgments
For generous support of the UCLA Brain Mapping Center, we also thank the Brain Mapping Medical Research Organization, Brain Mapping Support Foundation, Pierson-Lovelace Foundation, The Ahmanson Foundation, William M. and Linda R. Dietel Philanthropic Fund at the Northern Piedmont Community Foundation, Tamkin Foundation, Jennifer Jones-Simon, Capital Group Companies Charitable Foundation, Robson Family, and Northstar Fund. Financial Disclosures: The authors have no financial disclosure to report. The authors have declared that there are no conflicts of interest in relation to the subject of this study.
References
- 1.Decety J, Lamm C. Human empathy through the lens of social neuroscience. ScientificWorldJournal. 2006;6:1146–1163. doi: 10.1100/tsw.2006.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eisenberg N, Miller PA. The relation of empathy to prosocial and related behaviors. Psychol Bull. 1987;101:91–119. [PubMed] [Google Scholar]
- 3.Blair RJ. Responding to the emotions of others: dissociating forms of empathy through the study of typical and psychiatric populations. Conscious Cogn. 2005;14:698–718. doi: 10.1016/j.concog.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Henry JD, Bailey PE, Rendell PG. Empathy, social functioning and schizotypy. Psychiatry Res. 2008;160:15–22. doi: 10.1016/j.psychres.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 5.Fakra E, Salgado-Pineda P, Delaveau P, Hariri AR, Blin O. Neural bases of different cognitive strategies for facial affect processing in schizophrenia. Schizophr Res. 2008;100:191–205. doi: 10.1016/j.schres.2007.11.040. [DOI] [PubMed] [Google Scholar]
- 6.Habel U, Chechko N, Pauly K, et al. Neural correlates of emotion recognition in schizophrenia. Schizophr Res. 2010;122:113–123. doi: 10.1016/j.schres.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Kohler CG, Walker JB, Martin EA, Healey KM, Moberg PJ. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36:1009–1019. doi: 10.1093/schbul/sbn192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Epley N, Waytz A. Mind perception. In: Fiske S, Gilbert D, Lindzey G, editors. The Handbook of Social Psychology. New York, NY: Oxford University Press; 2009. pp. 498–541. [Google Scholar]
- 9.Shamay-Tsoory SG. The neural bases for empathy. Neuroscientist. 2011;17:18–24. doi: 10.1177/1073858410379268. [DOI] [PubMed] [Google Scholar]
- 10.Zaki J, Ochsner K. Re-integrating the study of accuracy into social cognition research. Psychol Inq. 2011;22:159–182. [Google Scholar]
- 11.Castelli F, Happé F, Frith U, Frith C. Movement and mind: a functional imaging study of perception and interpretation of complex intentional movement patterns. Neuroimage. 2000;12:314–325. doi: 10.1006/nimg.2000.0612. [DOI] [PubMed] [Google Scholar]
- 12.Saxe R, Carey S, Kanwisher N. Understanding other minds: linking developmental psychology and functional neuroimaging. Annu Rev Psychol. 2004;55:87–124. doi: 10.1146/annurev.psych.55.090902.142044. [DOI] [PubMed] [Google Scholar]
- 13.Davis MH. Empathy: A Social Psychological Approach. Boulder, CO: Westview Press; 1994. [Google Scholar]
- 14.Preston SD, de Waal FB. Empathy: its ultimate and proximate bases. Behav Brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. discussion 20–71. [DOI] [PubMed] [Google Scholar]
- 15.Ochsner KN, Knierim K, Ludlow DH, et al. Reflecting upon feelings: an fMRI study of neural systems supporting the attribution of emotion to self and other. J Cogn Neurosci. 2004;16:1746–1772. doi: 10.1162/0898929042947829. [DOI] [PubMed] [Google Scholar]
- 16.Olsson A, Ochsner KN. The role of social cognition in emotion. Trends Cogn Sci. 2008;12:65–71. doi: 10.1016/j.tics.2007.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Peelen MV, Atkinson AP, Vuilleumier P. Supramodal representations of perceived emotions in the human brain. J Neurosci. 2010;30:10127–10134. doi: 10.1523/JNEUROSCI.2161-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Iacoboni M. Imitation, empathy, and mirror neurons. Annu Rev Psychol. 2009;60:653–670. doi: 10.1146/annurev.psych.60.110707.163604. [DOI] [PubMed] [Google Scholar]
- 19.Jackson PL, Meltzoff AN, Decety J. How do we perceive the pain of others? A window into the neural processes involved in empathy. Neuroimage. 2005;24:771–779. doi: 10.1016/j.neuroimage.2004.09.006. [DOI] [PubMed] [Google Scholar]
- 20.Keysers C, Kaas JH, Gazzola V. Somatosensation in social perception. Nat Rev Neurosci. 2010;11:417–428. doi: 10.1038/nrn2833. [DOI] [PubMed] [Google Scholar]
- 21.Rizzolatti G, Sinigaglia C. The functional role of the parieto-frontal mirror circuit: interpretations and misinterpretations. Nat Rev Neurosci. 2010;11:264–274. doi: 10.1038/nrn2805. [DOI] [PubMed] [Google Scholar]
- 22.Ickes W, Stinson L, Bissonnette V, Garcia S. Naturalistic social cognition: empathic accuracy in mixed-sex dyads. J Pers Soc Psychol. 1990;59:730–742. [Google Scholar]
- 23.Zaki J, Bolger N, Ochsner K. It takes two: the interpersonal nature of empathic accuracy. Psychol Sci. 2008;19:399–404. doi: 10.1111/j.1467-9280.2008.02099.x. [DOI] [PubMed] [Google Scholar]
- 24.Brunet E, Sarfati Y, Hardy-Baylé M-C, Decety J. Abnormalities of brain function during a nonverbal theory of mind task in schizophrenia. Neuropsychologia. 2003;41:1574–1582. doi: 10.1016/s0028-3932(03)00119-2. [DOI] [PubMed] [Google Scholar]
- 25.Russell TA, Rubia K, Bullmore ET, et al. Exploring the social brain in schizophrenia: left prefrontal underactivation during mental state attribution. Am J Psychiatry. 2000;157:2040–2042. doi: 10.1176/appi.ajp.157.12.2040. [DOI] [PubMed] [Google Scholar]
- 26.Lee J, Quintana J, Nori P, Green MF. Theory of mind in schizophrenia: exploring neural mechanisms of belief attribution. Soc Neurosci. 2011;6:569–581. doi: 10.1080/17470919.2011.620774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee SJ, Kang DH, Kim C-W, et al. Multi-level comparison of empathy in schizophrenia: an fMRI study of a cartoon task. Psychiatry Res. 2010;181:121–129. doi: 10.1016/j.pscychresns.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 28.Benedetti F, Bernasconi A, Bosia M, et al. Functional and structural brain correlates of theory of mind and empathy deficits in schizophrenia. Schizophr Res. 2009;114:154–160. doi: 10.1016/j.schres.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 29.Zaki J, Bolger N, Ochsner K. Unpacking the informational bases of empathic accuracy. Emotion. 2009;9:478–487. doi: 10.1037/a0016551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zaki J, Weber J, Bolger N, Ochsner K. The neural bases of empathic accuracy. Proc Natl Acad Sci U S A. 2009;106:11382–11387. doi: 10.1073/pnas.0902666106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levenson RW, Ruef AM. Empathy: a physiological substrate. J Pers Soc Psychol. 1992;63:234–246. [PubMed] [Google Scholar]
- 32.Lee J, Zaki J, Harvey P-O, Ochsner K, Green MF. Schizophrenia patients are impaired in empathic accuracy. Psychol Med. 2011;41:2297–2304. doi: 10.1017/S0033291711000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kenny DA, Albright L. Accuracy in interpersonal perception: a social relations analysis. Psychol Bull. 1987;102:390–402. [PubMed] [Google Scholar]
- 34.Snodgrass SE, Hecht MA, Ploutz-Snyder R. Interpersonal sensitivity: expressivity or perceptivity? J Pers Soc Psychol. 1998;74:238–249. doi: 10.1037//0022-3514.74.1.238. [DOI] [PubMed] [Google Scholar]
- 35.Gross J. The Berkeley Expressivity Questionnaire. In: Lewis CA, Maltby J, Hill A, editors. Commissioned Reviews on 300 Psychological Tests. Lampeter, UK: Edwin Mellen Press; 2000. pp. 465–467. [Google Scholar]
- 36.Gross JJ, John OP. Revealing feelings: facets of emotional expressivity in self-reports, peer ratings, and behavior. J Pers Soc Psychol. 1997;72:435–448. doi: 10.1037//0022-3514.72.2.435. [DOI] [PubMed] [Google Scholar]
- 37.First MB. Structured Clinical Interview for DSM-IV Axis I Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1997. [Google Scholar]
- 38.Ventura J, Lukoff D, Nuechterlein KH, et al. Brief Psychiatric Rating Scale (BPRS) expanded version: scales, anchor points, and administration manual. Int J Methods Psychiatr Res. 1993;3:227–243. [Google Scholar]
- 39.Andreasen NC. Scale for the Assessment of Negative Symptoms (SANS) Iowa City, IA: University of Iowa; 1984. [Google Scholar]
- 40.Andreasen NC, Pressler M, Nopoulos P, Miller D, Ho B- C. Antipsychotic dose equivalents and dose-years: a standardized method for comparing exposure to different drugs. Biol Psychiatry. 2010;67:255–262. doi: 10.1016/j.biopsych.2009.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.First MB. Structured Clinical Interview for DSM-IV Axis II Personality Disorders. New York, NY: Biometrics Research Department, New York State Psychiatric Institute; 1996. [Google Scholar]
- 42.Slotnick SD, Moo LR, Segal JB, Hart J. Distinct prefrontal cortex activity associated with item memory and source memory for visual shapes. Brain Res Cogn Brain Res. 2003;17:75–82. doi: 10.1016/s0926-6410(03)00082-x. [DOI] [PubMed] [Google Scholar]
- 43.Johnstone T, van Reekum CM, Urry HL, Kalin NH, Davidson RJ. Failure to regulate: counterproductive recruitment of top-down prefrontal-subcortical circuitry in major depression. J Neurosci. 2007;27:8877–8884. doi: 10.1523/JNEUROSCI.2063-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rankin KP, Salazar A, Gorno-Tempini ML, et al. Detecting sarcasm from paralinguistic cues: anatomic and cognitive correlates in neurodegenerative disease. Neuroimage. 2009;47:2005–2015. doi: 10.1016/j.neuroimage.2009.05.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mar RA. The neural bases of social cognition and story comprehension. Annu Rev Psychol. 2011;62:103–134. doi: 10.1146/annurev-psych-120709-145406. [DOI] [PubMed] [Google Scholar]
- 46.Block AE, Dhanji H, Thompson-Tardif SF, Floresco SB. Thalamic-prefrontal cortical-ventral striatal circuitry mediates dissociable components of strategy set shifting. Cereb Cortex. 2007;17:1625–1636. doi: 10.1093/cercor/bhl073. [DOI] [PubMed] [Google Scholar]
- 47.Holmes AJ, MacDonald III A, Carter CS, Barch DM, Stenger VA, Cohen JD. Prefrontal functioning during context processing in schizophrenia and major depression: an event-related fMRI study. Schizophr Res. 2005;76:199–206. doi: 10.1016/j.schres.2005.01.021. [DOI] [PubMed] [Google Scholar]
- 48.Wager TD, Smith EE. Neuroimaging studies of working memory: a meta-analysis. Cogn Affect Behav Neurosci. 2003;3:255–274. doi: 10.3758/cabn.3.4.255. [DOI] [PubMed] [Google Scholar]
- 49.Brambilla P, MacDonald III A, Sassi RB, et al. Context processing performance in bipolar disorder patients. Bipolar Disord. 2007;9:230–237. doi: 10.1111/j.1399-5618.2007.00398.x. [DOI] [PubMed] [Google Scholar]
- 50.Grattan LM, Bloomer RH, Archambault FX, Eslinger PJ. Cognitive flexibility and empathy after prefrontal lobe lesion. Neuropsychiatry Neuropsychol Behav Neurol. 1994;7:251–257. [Google Scholar]
- 51.Shamay-Tsoory SG, Shur S, Harari H, Levkovitz Y. Neurocognitive basis of impaired empathy in schizophrenia. Neuropsychology. 2007;21:431–438. doi: 10.1037/0894-4105.21.4.431. [DOI] [PubMed] [Google Scholar]
- 52.Leitman DI, Ziwich R, Pasternak R, Javitt DC. Theory of Mind (ToM) and counterfactuality deficits in schizophrenia: misperception or misinterpretation? Psychol Med. 2006;36:1075–1083. doi: 10.1017/S0033291706007653. [DOI] [PubMed] [Google Scholar]
- 53.Couture SM, Penn DL, Roberts DL. The functional significance of social cognition in schizophrenia: a review. Schizophr Bull. 2006;32(suppl 1):S44–S63. doi: 10.1093/schbul/sbl029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derntl B, Finkelmeyer A, Eickhoff A, et al. Multidimensional assessment of empathic abilities: neural correlates and gender differences. Psychoneuroendocrinology. 2010;35:67–82. doi: 10.1016/j.psyneuen.2009.10.006. [DOI] [PubMed] [Google Scholar]
- 55.Rueckert L, Naybar N. Gender differences in empathy: the role of the right hemisphere. Brain Cogn. 2008;67:162–167. doi: 10.1016/j.bandc.2008.01.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.