Abstract
Introduction: Antipsychotic drugs exert antipsychotic effects by blocking dopamine D2 receptors in the treatment of schizophrenia. However, effects of D2 receptor blockade on neurocognitive function still remain to be elucidated. The objective of this analysis was to evaluate impacts of estimated dopamine D2 receptor occupancy with antipsychotic drugs on several domains of neurocognitive function in patients with schizophrenia in the Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE) trial. Methods: The dataset from the CATIE trial was used in the present analysis. Data were extracted from 410 subjects who were treated with risperidone, olanzapine, or ziprasidone, received assessments for neurocognitive functions (verbal memory, vigilance, processing speed, reasoning, and working memory) and psychopathology, and provided plasma samples for the measurement of plasma antipsychotic concentrations. D2 receptor occupancy levels on the day of neurocognitive assessment were estimated from plasma antipsychotic concentrations, using population pharmacokinetic analysis and our recently developed model. A multivariate general linear model was used to examine effects of clinical and demographic characteristics, including estimated D2 occupancy levels, on neurocognitive functions. Results: D2 occupancy levels showed significant associations with the vigilance and the summary scores. Neurocognitive functions, including vigilance, were especially impaired in subjects who showed D2 receptor occupancy level of >77%. Discussion: These findings suggest a nonlinear relationship between prescribed antipsychotic doses and overall neurocognitive function and vigilance. This study shows that D2 occupancy above approximately 80% not only increases the risk for extrapyramidal side effects as consistently reported in the literature but also increases the risk for cognitive impairment.
Keywords: antipsychotic, cognition, dopamine, olanzapine, risperidone, schizophrenia, ziprasodine
Introduction
Neurocognitive impairment is considered to be a core feature in schizophrenia and has a strong correlation with real-world functioning of patients.1 While antipsychotic drugs play a principal role in the treatment of schizophrenia, high doses of antipsychotic drugs have been associated with negative consequences on global neurocognitive function2 as well as specific cognitive domains, including processing speed,3 visual memory, delayed recall, performance IQ, and executive function.4 Antipsychotic drugs, including atypical antipsychotics, have been related with mixed results in terms of effects on the neurocognitive impairment due to this illness5,6; however, many of these studies involved methodological shortcomings, including small sample sizes and insufficient neurocognitive measures.7–9 Therefore, effects of antipsychotic drugs on neurocognitive function still remain to be elucidated.10
Accumulated evidence has shown that the dopaminergic system in the central nervous system is profoundly associated with cognition.11 For example, the availability of dopamine D2 receptors has been reported to have a significant impact on neurocognitive function, including attention and executive function, in healthy subjects.11 Similarly, the blockade of dopamine D2 receptors by risperidone has a negative correlation with attention in patients with schizophrenia.12 Furthermore, neurocognitive performance, including verbal fluency, spatial span, planning, and sequence generation, was found to be positively correlated with both dopamine D1 and D2 receptor binding levels, but mainly with D2 binding levels in patients with Huntington’s disease.13 Animal studies have also endorsed the pivotal role of the dopaminergic system in neurocognitive function; in mutant mice, the absence of D2 receptors has been demonstrated to impair performance in spatial working memory and perceptual discrimination.14,15
We have recently reported that striatal dopamine D2 receptor occupancy by antipsychotic drugs, including risperidone, olanzapine, and ziprasidone, can be reliably estimated from plasma concentrations of these drugs.16 In addition, recent advances in nonlinear mixed-effects population pharmacokinetic methods have made it possible to predict individual pharmacokinetic parameters for antipsychotic drugs, including peak and trough plasma concentrations, using 2 or more sparsely collected blood samples in a real-world setting.17 By combining these models, the dopamine D2 receptor occupancy levels at peak and trough can be reliably estimated using the measurement of antipsychotic plasma concentrations at 2 separate time points.18
For the purpose of elucidating the relationship between neurocognitive function and estimated dopamine D2 receptor blockade by antipsychotics, the Clinical Antipsychotic Trials in Intervention Effectiveness (CATIE) trial provides an ideal dataset in light of its unprecedented large sample size, comprehensive neurocognitive assessments, and assessment of plasma antipsychotic concentrations with which population pharmacokinetic models have already been developed for risperidone, olanzapine, and ziprasidone.19–21 The objective of this report was to evaluate impacts of estimated dopamine D2 receptor occupancy with risperidone, olanzapine, and ziprasidone on several domains of neurocognitive function in patients with schizophrenia in the CATIE trial. Our working hypothesis was that the relations between cognitive functions and D2 occupancy would be U-shaped. That is, cognition remains impaired at low D2 occupancy, and medium D2 occupancy may improve cognition; however, high occupancy of D2 receptors impairs cognition. This hypothesis came from the following observations in the literature: (1) a moderate amount of antipsychotic drugs generally improves cognitive functions,5 (2) an excessive blockade of dopamine D2 receptors by antipsychotics is associated with worsening in cognitive functions,12 and (3) an insufficient blockade of dopamine D2 receptors by antipsychotics does not fully exert its therapeutic effect.22
Methods
Study Design
The CATIE trial was funded by the National Institute of Mental Health to compare the effectiveness of atypical antipsychotics and a single conventional antipsychotic medication in patients with schizophrenia; the details of the study were reported elsewhere.23 Briefly, the study was performed between January 2001 and December 2004 at 57 clinical sites in the United States. One thousand four hundred and ninety-three patients between ages 18 and 65 years with a diagnosis of schizophrenia on the basis of the Structured Clinical Interview of the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition participated in the CATIE trial. Patients were initially randomized to olanzapine (7.5–30 mg/day), risperidone (1.5–6.0 mg/day), ziprasidone (40–160 mg/day), quetiapine (200–800 mg/day), or perphenazine (8–32 mg/day) under double-blind conditions and received treatments for up to 18 months or until treatment was discontinued for any reason (phase 1).
Data used in the present analysis were derived from subjects who were receiving risperidone, olanzapine, or ziprasidone, completed assessments for psychopathology and neurocognitive function at months 1 and 2, respectively, and provided plasma samples for the assessment of plasma antipsychotic concentrations. These 3 drugs were included in the present study because the nonlinear mixed-effect models were already established for them using the data from the CATIE studies.19–21 All participants gave written informed consent to participate in the protocols approved by the local institutional review boards.
Assessments for Cognition, Psychopathology, and Extrapyramidal Symptoms
Neurocognitive assessment was performed at month 2. The neurocognitive tests were chosen by a group of advisors based upon the following considerations; sensitivity to neurocognitive impairment in schizophrenia, relation to functional outcome, potential sensitivity to treatment, and practicality for various antipsychotic clinical trials for schizophrenia.7,24,25 The following 5 neurocognitive domain scores were calculated from 9 neurocognitive test summary scores and standardized to create z scores for each domain in the CATIE trial: verbal memory, vigilance, processing speed, reasoning, and working memory. The verbal memory domain score was calculated from the Hopkins Verbal Learning Test, which assesses verbal learning and memory. The vigilance domain score was calculated from the Continuous Performance Test, which assesses attention. The processing speed domain score was obtained from category instances, the Grooved Pegboard, and the Revised Wechsler Adult Intelligence Scale Digit Symbol Test, which represents processing speed. The reasoning domain score was derived from the Wisconsin Card Sorting Test and the Revised Wechsler Intelligence Scale for Children Mazes. The working memory domain score was calculated from the Letter-number test of auditory working memory and a computerized test of visuospatial working memory. A neurocognitive summary score was calculated by creating a z score of the average of the 5 standardized domain scores. The following information was also collected: age, sex, years of education, and concomitant mediations. The Positive and Negative Syndrome Scale (PANSS) and Simpson-Angus Scale (SAS) were also conducted at month 1.
Population Pharmacokinetic Analysis
Subjects who participated in the CATIE trial provided plasma samples for the measurement of concentrations of risperidone plus 9-hydroxyrisperidone (active moiety), olanzapine, or ziprasidone at more than one time points. Using these samples, plasma antipsychotic concentrations at peak and trough that corresponded to the dose given on the day of cognitive assessment were calculated for each individual using the established population pharmacokinetic models and extracting the Empirical Bayes Estimates for the pharmacokinetic parameters from each of these individuals.26,27 These parameters were utilized to calculate the expected peak and trough concentrations for each of risperidone, 9-hydroxyrisperidone, ziprasidone, and olanzapine for the dosage regimen on the day of the neurocognitive assessments. Thus, the model-predicted values of the plasma concentrations were used to calculate the peak and trough dopamine D2 receptor occupancy levels for each individual on the day of neurocognitive assessments. The precision and reliability of this estimation has recently been confirmed in our population pharmacokinetic study.28 The nonlinear mixed-effect models for olanzapine, risperidone, and ziprasidone were previously established using the CATIE data.19–21 These original studies used to establish the population pharmacokinetic models comprised 1236 risperidone and 9-hydroxyrisperidone concentrations from 490 subjects, 1527 olanzapine concentrations from 523 subjects, and 568 ziprasidone concentrations from 233 subjects, respectively. All 3 compounds were adequately described using a one-compartment linear model with first-order absorption. The previously established models utilized exponentiated or log-normal interindividual variability on each pharmacokinetic parameters, a mixture distribution to assign the tri-modal distribution of clearance as CYP 2D6 genotype was not available for risperidone, an age effect on clearance of the 9-hydroxyrisperidone moiety, and sex, race, and age effects on olanzapine disposition.
Estimation of Dopamine D2 Receptor Occupancy
By using the predicted plasma concentrations of antipsychotics at peak and trough on the day of cognitive assessment, corresponding dopamine D2 receptor occupancy levels were estimated, using our recently developed model.16 Briefly, dopamine D2 receptor occupancy levels were estimated by incorporating the predicted plasma concentration of risperidone active moiety, olanzapine, or ziprasidone into the following one-site binding model: occupancy (%) = a × (plasma level/[plasma level + ED50]), where a is the maximum receptor occupancy attributable to the antipsychotic drug, and ED50 is the estimated plasma concentration of the antipsychotic drug associated with 50% of receptor occupancy, which was obtained in the systematic review and pooled analysis (Risperidone active moiety: a = 88.0%, ED50 = 4.9 ng/ml; olanzapine: a = 90.7%, ED50 = 7.1 ng/ml; and ziprasidone: a = 88.2%, ED50 = 32.9 ng/ml).16 Mean values of those peak and trough dopamine D2 receptor occupancy levels were obtained for further analyses.
Statistical Analysis
Statistical Analyses Were Carried Out Using SPSS Version 19.0 (SPSS Inc., Chicago). To test the hypothesis, subjects were divided into an equal number of 4 groups (ie, 102 or 103) based on the predicted dopamine D2 receptor occupancy on the day of cognitive testing (low D2 occupancy group: 15.5–62.7%, n = 102; slightly low D2 occupancy group: 62.7–71.8%, n = 102; slightly high D2 occupancy group: 71.9–77.2%, n = 103; and high D2 occupancy group: 77.2–85.8%, n = 103). A multivariate general linear model was used to examine effects of antipsychotic drugs (ie, risperidone, olanzapine, or ziprasidone), dopamine D2 receptor occupancy levels (ie, those 4 groups), age, education years, PANSS total score, SAS mean score, and the use of anticholinergics on 5 neurocognitive domain and summary scores. In addition, to exclude a possibility of potential interaction between age and estimated dopamine D2 receptor occupancy, we performed additional analysis, using the data from subjects aged less than 50; another multivariate general linear model was used to examine effects of the above demographic and clinical characteristics other than age on 5 neurocognitive domain and summary scores. Variables of interest were compared among the 4 dopamine D2 receptor occupancy groups, using a one-way ANOVA for parametric data and chi-square test for categorical variables. When appropriate, we also examined group differences with pairwise comparisons using Turkey-Kramer HSD (honestly significant difference). A P value of <.05 was considered statistically significant (2-tailed).
Results
Subject Characteristics
Four hundred and ten subjects who provided plasma samples of risperidone plus 9-hydroxyrisperidone, olanzapine, or ziprasidone and received neurocognitive assessments at month 2 and the PANSS and SAS at month 1 were identified. Demographic and clinical characteristics of these subjects were summarized in table 1. Mean ± SD daily doses of risperidone, olanzapine, and ziprasidone on the day of neurocognitive assessments were 3.9 ± 1.3 mg, 19.7 ± 7.0 mg, and 100.5 ± 57.9 mg, respectively. Mean ± SD PANSS total and SAS mean scores were 69.9 ± 18.3 and 0.19 ± 0.29, respectively. Characteristics of groups stratified by dopamine D2 receptor occupancy levels are detailed in table 2. No significant difference was found in any of the total, positive, negative, or general psychopathology scale PANSS score.
Table 1.
Demographic and Clinical Characteristics of 410 Subjects
| Characteristics | Values |
| Age, years, mean ± SD (range) | 40.9 ± 10.3 (18–62) |
| Male, n (%) | 303 (73.9) |
| Ethnicity, n (%) | |
| White | 259 (63.2) |
| Others | 151 (36.8) |
| Duration of education, years, mean ± SD (range) | 12.3 ± 2.0 (3–21) |
| Duration of treatment, years, mean ± SD (range) | 16.5 ± 10.9 (0–56) |
| Marital status | |
| Married, n (%) | 39 (9.5) |
| Previously married, n (%) | 130 (31.7) |
| Never married, n (%) | 241 (58.8) |
| Employment status | |
| Unemployed, n (%) | 349 (85.1) |
| Antipsychotics | |
| Risperidone, n (%) | 150 (36.6) |
| Olanzapine, n (%) | 184 (44.9) |
| Ziprasidone, n (%) | 76 (18.5) |
| Use of anticholinergics, n (%) | 69 (16.8) |
| PANSS total score, mean ± SD (range) | 69.9 ± 18.3 (32–131) |
| SAS mean score, mean ± SD (range) | 0.19 ± 0.29 (0–1.83) |
Note: PANSS, Positive and Negative Syndrome Scale; SAS, Simpson-Angus Scale.
Table 2.
Characteristics of Subjects Stratified by Dopamine D2 Occupancy
| Characteristics | Low (n = 102) | Slightly Low (n = 102) | Slightly High (n = 103) | High (n = 103) |
| D2 occupancy, %, range (mean ± SD) | 15.5–62.7 (49.0 ± 11.4) | 62.7–71.8 (67.6 ± 2.6) | 71.9–77.2 (74.4 ± 1.6) | 77.2–85.8 (80.1 ± 1.9) |
| Age, years, mean ± SD (range)a | 39.2 ± 10.6 (20–62) | 39.8 ± 10.0 (20–62) | 41.5 ± 9.4 (21–59) | 43.2 ± 10.9 (18–62) |
| Male, n (%) | 78 (76.5) | 77 (75.5) | 79 (76.7) | 69 (67.0) |
| Duration of education, years, mean ± SD (range) | 12.2 ± 2.4 (3–21) | 12.2 ± 1.8 (6–18) | 12.4 ± 1.8 (7–21) | 12.3 ± 1.9 (3–18) |
| Duration of treatment, years, mean ± SD (range) | 14.8 ± 10.0 (0–40) | 16.0 ± 11.2 (0–39) | 17.9 ± 11.7 (0–56) | 17.3 ± 10.4 (0–42) |
| Antipsychoticsb | ||||
| Risperidone, n (%) | 16 (15.7) | 42 (41.2) | 53 (51.5) | 39 (37.9) |
| Olanzapine, n (%) | 20 (19.6) | 50 (49.0) | 50 (48.5) | 64 (62.1) |
| Ziprasidone, n (%) | 66 (64.7) | 10 (9.8) | 0 (0) | 0 (0) |
| Use of anticholinergics, n (%) | 14 (13.7) | 16 (15.7) | 17 (16.5) | 22 (21.4) |
| PANSS total score, mean ± SD (range) | 68.0 ± 18.8 (32–108) | 68.7 ± 17.3 (32–113) | 71.7 ± 18.3 (34–131) | 71.1 ± 18.6 (33–120) |
| PANSS positive score, mean ± SD (range) | 16.1 ± 5.9 (7–33) | 16.4 ± 5.4 (7–31) | 16.8 ± 5.5 (7–35) | 17.1 ± 5.6 (7–31) |
| PANSS negative score, mean ± SD (range) | 18.4 ± 6.7 (7–36) | 18.2 ± 6.0 (8–35) | 19.7 ± 6.3 (7–38) | 19.7 ± 6.8 (7–38) |
| PANSS general score, mean ± SD (range) | 33.6 ± 9.7 (16–56) | 34.0 ± 9.2 (16–57) | 35.1 ± 9.4 (18–58) | 34.3 ± 9.3 (16–62) |
| SAS mean score, mean ± SD (range)c | 0.16 ± 0.26 (0–1.5) | 0.13 ± 0.19 (0–0.8) | 0.25 ± 0.34 (0–1.83) | 0.22 ± 0.31 (0–1.5) |
Note: Abbreviations are explained in the first footnote to table 1. No statistically significant differences were found in any of the other variables.
F 3, 406 = 3.15, P = .03 by the one-way ANOVA; no further statically significant difference was found by the Turkey-Kramer HSD (honestly significant difference).
χ2 6 = 200.8, P < .001.
F 3, 406 = 3.58, P = .01 by the one-way ANOVA; no further statically significant difference was found by the Turkey-Kramer HSD.
Association Between D2 Receptor Occupancy and Neurocognitive Function
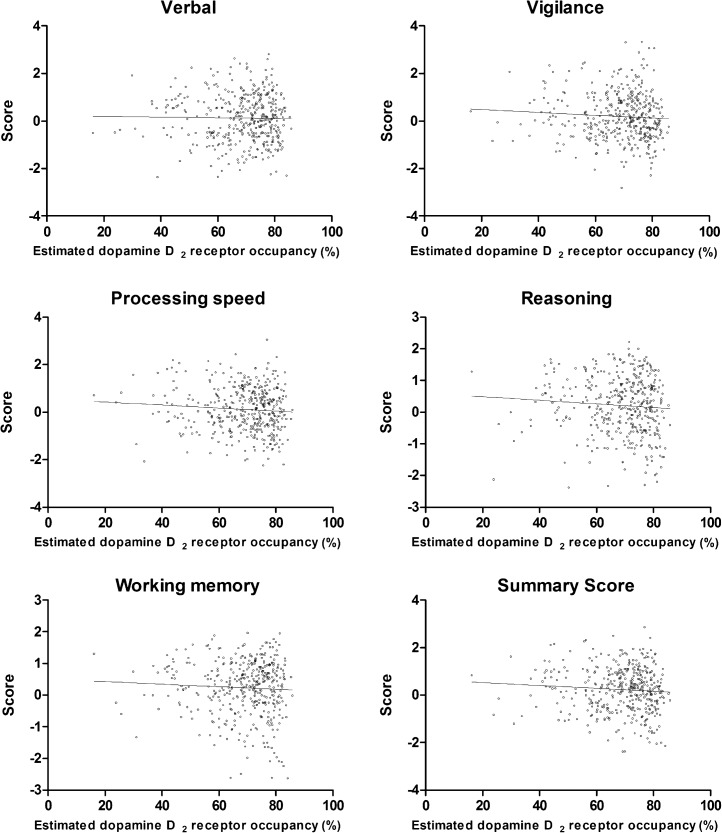
Results of the multivariate general linear model are detailed in table 3. While age and education years were found to have significant effects on all of the subscale scores, dopamine D2 receptor occupancy levels also showed significant association with the vigilance and the summary scores. Mean values of neurocognitive scores, including the vigilance and the summary scores, and dopamine D2 receptor occupancy levels demonstrated a nonlinear relationship, where there seemed to be a cliff-fall-off at higher occupancies (table 4 and figure 1). We therefore conducted additional analyses to try to find the threshold. Subjects were divided into the 2 groups based on consecutive cutoff points in increments of 1% between 70% and 85% in the dopamine D2 receptor occupancy. Then, other general linear models that included the same clinical and demographic variables as the original model were built to examine which threshold(s) resulted in the presence of significant effects of dopamine D2 receptor occupancy on vigilance and summary scores. The results revealed that dichotomization of subjects based on 77%, 78%, or 79% and 77%, 78%, 79%, 80%, or 81% were associated with the presence of statistically significant effects of dopamine D2 receptor occupancy on vigilance and summary scores, respectively. Another significant finding was an association between the processing speed score and the PANSS total score (table 3).
Table 3.
Relationship Between Neurocognitive Scores and Subjects’ Characteristics (n = 410)
| Characteristics | Verbal Memory | Vigilance | Processing Speed | Reasoning | Working Memory | Summary Score | ||||||
| F df1,df2 | P Value | F df1,df2 | P Value | F df1,df2 | P Value | F df1,df2 | P Value | F df1,df2 | P Value | F df1,df2 | P Value | |
| D2 occupancy level | 0.583,399 | .63 | 5.523,399 | .002 | 1.813,399 | .14 | 1.273,399 | .28 | 1.373,399 | .25 | 3.023,399 | .03 |
| Age | 8.301,399 | .004 | 11.551,399 | .001 | 51.331,399 | <.001 | 63.911,399 | <.001 | 31.081,399 | <.001 | 51.281,399 | <.001 |
| Education year | 43.991,399 | <.001 | 16.191,399 | <.001 | 31.751,399 | <.001 | 28.361,399 | <.001 | 28.221,399 | <.001 | 53.261,399 | <.001 |
| PANSS | 2.681,399 | .10 | 0.601,399 | .44 | 5.111,399 | .02 | 0.441,399 | .51 | 1.631,399 | .20 | 3.211,399 | .07 |
| SAS | 0.521,399 | .47 | 0.171,399 | .68 | 0.781,399 | .38 | 0.071,399 | .79 | 0.471,399 | .49 | 0.321,399 | .57 |
| Antipsychotic medication | 1.472,399 | .23 | 0.952,399 | .39 | 1.572,399 | .21 | 1.692,399 | .19 | 2.432,399 | .09 | 1.702,399 | .19 |
| Use of anticholinergics | 0.411,399 | .52 | 1.421,399 | .23 | 2.951,399 | .09 | 3.271,399 | .07 | 3.061,399 | .08 | 3.581,399 | .06 |
Note: Abbreviations are explained in the first footnote to table 1. Statistics for these general linear models are as follows: verbal memory: F 10, 399 = 5.97, P < .001, R 2 = .13; vigilance: F 10, 399 = 4.10, P < .001, R 2 = .10; processing speed: F 10, 399 = 7.03, P < .001, R 2 = .20; reasoning: F 10, 399 = 6.99, P < .001, R 2 = .19; working memory: F 10, 399 = 4.64, P < .001, R 2 = .13; and summary score: F 10, 399 = 8.22, P < .001, R 2 = .22. Statistically significant effects with P value of <.05 were described in bold.
Table 4.
Neurocognitive Scores Stratified by Dopamine D2 Occupancy
| D2 Occupancy Level | D2 Occupancy Range (%) | z Score (Mean ± SD) | |||||
| Verbal Memory | Vigilance | Processing Speed | Reasoning | Working Memory | Summary Score | ||
| Low (n = 102) | 15.5–62.7 | 0.084 ± 1.077 | 0.174 ± 0.964 | 0.159 ± 0.958 | 0.311 ± 0.866a | 0.293 ± 0.787 | 0.275 ± 0.920 |
| Slightly low (n = 102) | 62.7–71.8 | 0.188 ± 1.015 | 0.306 ± 0.992b | 0.223 ± 0.889 | 0.259 ± 0.899 | 0.229 ± 0.880 | 0.323 ± 0.937 |
| Slightly high (n = 103) | 71.9–77.2 | 0.180 ± 0.865 | 0.385 ± 0.951c | 0.167 ± 0.913 | 0.285 ± 0.853 | 0.309 ± 0.736 | 0.354 ± 0.839d |
| High (n = 103) | 77.2–85.8 | 0.025 ± 0.990 | −0.110 ± 0.934 | −0.085 ± 0.903 | −0.013 ± 0.949 | 0.095 ± 1.017 | −0.008 ± 0.951 |
Note: Significant differences were found in vigilance score, reasoning score, and summary score (F 3, 406 = 5.21, P = .002; F 3, 406 = 2.90, P = .04; F 3, 406 = 3.36, P = .02, respectively) by the one-way ANOVA.
P =.049 by the Turkey-Kramer HSD (honestly significant difference), vs high D2 group.
P = .012 by the Turkey-Kramer HSD, vs high D2 group.
P = .002 by the Turkey-Kramer HSD, vs high D2 group.
P = .025 by the Turkey-Kramer HSD, vs high D2 group.
Fig. 1.
Neurocognitive domain scores and estimated dopamine D2 receptor occupancy. Pearson’s correlation analysis did not find any statistically significant linear correlation between neurocognitive scores and dopamine D2 occupancy. Note that trend lines are shown only for descriptive purposes; refer to table 4 for detailed comparison to test the nonlinear relationship between neurocognitive scores and dopamine D2 occupancy.
Since the mean differences across the D2 groups may account for other factors that were entered into the model, especially age, another model was generated, using the data from 327 subjects aged less than 50 (see online s upplementary table 1) Estimated dopamine D2 receptor occupancy levels showed significant association with the vigilance, processing speed, working memory, and the summary scores. Again, a nonlinear relationship was found between mean values of these neurocognitive scores and dopamine D2 receptor occupancy levels (see online supplementary table 2).
Discussion
To our knowledge, this is the largest study to investigate neurocognitive function in relation to dopamine D2 receptor occupancy with antipsychotic drugs in patients with schizophrenia. The results demonstrated a nonlinear relationship between prescribed antipsychotic doses and overall neurocognitive function and vigilance; they were especially impaired in subjects who showed D2 receptor occupancy level of >77%. Thus, our hypothesis regarding the association of very high D2 receptor occupancy with impaired cognitive functions was supported, while there was no evidence that supported the relationship between very low dopamine D2 occupancy and cognitive dysfunction.
The association between a high dopamine D2 receptor occupancy of >77% by antipsychotic drugs and impaired neurocognitive function was observed in this study, which is in line with the findings in the literature. Patients with schizophrenia who were treated with high doses of antipsychotic drugs (ie, 1134 ± 840 mg/day of chlorpromazine equivalents [mean ± SD]) showed significantly poorer performance than those with standard doses (ie, 473 ± 268 mg/day of chlorpromazine equivalents [mean ± SD]) on visual memory, delayed recall, performance IQ, and executive function.4 Similarly, one clinical positron emission tomography (PET) study demonstrated that the attentional deficits were observed above 74% blockade of dopamine D2 receptor by risperidone in patients with schizophrenia.12 In animal experiments, the 5-choice serial reaction time task (5CSRTT) provides substantial validity as a direct measure of attention and bears a good analogy to the continuous performance test29 that was adopted in the CATIE trial to measure vigilance. The neurochemical lesion of nucleus accumbens septi (NAS) induced by intracerebral infusions of neurotoxin 6-hydroxydopamine (6-OHDA) in rats produced an 87% depletion of dopamine in the NAS, which attenuated both speed and impulsivity of responding on the 5CSRTT.30 In human cortex, long-term potentiation (LTP) of synaptic efficacy is considered as a fundamental mechanism of learning and memory. A single oral dose of dopamine antagonist, haloperidol, depressed significantly the paired associative stimulation–induced LTP-like plasticity at the systems level of human cortex in 8 healthy subjects.31 Thus, excessive blockade of dopamine D2 receptor by antipsychotic drugs or relative paucity of dopamine may have detrimental effects on neurocognitive function in patients with schizophrenia.
On the other hand, dopamine D2 receptor blockade with antipsychotic drugs has also been shown to improve cognitive functions. Keefe et al5 conducted a meta-analysis of 15 studies and found that atypical antipsychotic drugs improved attention, executive function, working memory, visuospatial analysis, verbal fluency, and digit symbol substitution in patients with schizophrenia. Similarly, in a systemic review of 20 previous reports that examined changes in neurocognitive function followed by the treatment with atypical antipsychotic drugs in patients with schizophrenia, significant improvements in overall neurocognitive function were observed7; especially, effects for domains related with vigilance were consistently large. Combined with the observation that an excessive blockade of dopamine D2 receptor by antipsychotic drugs was associated with impaired neurocognitive function as described above, these findings may suggest that a moderate degree of dopamine D2 receptor blockade may provide amelioration in neurocognitive dysfunction in patients with schizophrenia.
The efficacy and tolerability of all available dopamine antagonist antipsychotics have been linked to their binding to dopamine D2 receptors.32 For most antipsychotic drugs, PET studies have suggested the presence of a therapeutic window of striatal dopamine D2 receptor occupancy (65%–80%) in younger patients,33,34 with extrapyramidal side effects more likely at more than 80% dopamine D2 receptor occupancy.34,35 Our recent pooled analysis also supports the presence of the therapeutic window in young adults with schizophrenia.22 Interestingly, the results of this study may also endorse the upper limit of this established therapeutic window of dopamine D2 receptor occupancy in terms of neurocognitive function. If the observations in the present study are confirmed in future studies with a specific focus on the causal relationship between dopamine D2 receptor blockade with antipsychotics and cognitive function, the therapeutic window could also be used to predict the therapeutic dose range of antipsychotic drugs to maximize therapeutic effects and minimize detrimental effects of antipsychotic drugs from a perspective of neurocognitive function.
The impairment of processing speed has been consistently observed in patients with schizophrenia.36,37 A recent meta-analysis has shown that patients with schizophrenia presented the most profound impairment on a digit symbol coding test that measures processing speed among various common neuropsychological measures.38 A reduced processing speed is known to be observed in patients with schizophrenia prior to the onset of the illness and is associated with clinical and functional outcomes.36,37 These data suggest that the decline of processing speed represents an important behavioral marker of the pathophysiology of schizophrenia. The significant association between the processing speed and the PANSS total score that we observed in this study is compatible with these findings.
In the present study, dopamine D2 receptor occupancy was not measured, using brain imaging techniques, but estimated with our recently developed model; the prediction performance of the model has been shown to be reliable.16 Furthermore, in theory, dopamine D2 receptor occupancy is not determined by plasma drug concentrations but by free and unbound concentrations. We therefore compared the estimated dopamine D2 receptor occupancy levels between the model that we used in our study16 and the method using the following formula: f = C/(K + C), where f is the fraction of D2 receptors occupied, where C is free concentration in the plasma samples, and where K is the drug dissociation constant at D2. This additional analysis was not performed for risperidone because of the following reason. It was possible to estimate dopamine D2 receptor occupancy for either of risperidone or 9-hydroxyrisperidone; however, it was unclear how to estimate the combined effects of risperidone plus 9-hydroxyrisperidone in terms of dopamine D2 receptor occupancy. In addition, the percentage of free and unbound concentration of ziprasidone has been reported in the literature; however, it has consistently been reported as “greater than 99%.”39 This lack of an exact value also discouraged us from including ziprasidone in the additional analysis. Thus, this additional analysis was performed, using the data from subjects on olanzapine (K = 2.3 ng/ml40; percentage of free and unbound drug concentration = 7%41). Although the values obtained from those 2 models were found to be closely correlated (Pearson’s r = .98 and P < .0001), the values obtained from the formula were generally lower by 10%–20% than those from the Uchida prediction model. Because we did not actually measure dopamine D2 receptor occupancy levels in the present study, it was impossible to compare the precision of prediction performance between those 2 methods. Therefore, we decided to perform an additional analysis, using the dataset of measured plasma drug concentrations and corresponding dopamine D2 receptor occupancy levels that were used when the Uchida prediction model was developed. We estimated dopamine D2 receptor occupancy levels (n = 42), using the formula, and compared the results with those obtained from the prediction model by Uchida et al. As shown in online supplementary figure 1, the values from the model by Uchida et al look more comparable to the measured values than those from the formula; in fact, the mean prediction error (%) and squared mean prediction error (%)42 also corroborate this finding (−0.1 [95% CI: −1.2–1.2] vs 23.1 [95% CI: 16.8–29.4]; 4.6 [95% CI: 3.5–5.8] vs 24.5 [95% CI: 19.3–29.7], respectively). However, this finding does not always mean that the prediction model by Uchida et al that was used in this study is superior to the formula that has a robust theoretical basis. In theory, again, dopamine D2 receptor occupancy is dependent upon free and unbound drug concentrations. This modeling issue clearly warrants further investigations. Moreover, the region of interest in 97% of the PET data used for the development of this prediction model by Uchida et al. was striatum. Although a potential difference in D2 receptor blockade by antipsychotics between striatal and extrastriatal regions could be attributable to the methodology used,43 the findings in the present study need to be replicated in future investigations, using radiotracers that can assess extrastriatal dopamine receptors. In addition, dopamine D2 receptor occupancy levels were calculated with an unconstrained one-site occupancy model in this study. Therefore, for example, 77% D2 occupancy corresponds to 81%, 79%, and 83% that were estimated with a maximum constrained occupancy of 100% for risperidone, olanzapine, and ziprasidone, respectively.16 Even though the differences are small, it is important to remember that the estimated D2 receptor occupancy levels are not absolute values.
Our focus on the dopaminergic system is not intended to insist that neurocognitive function in schizophrenia is solely related to effects in the dopaminergic system. Clearly, this relationship is far more complex, and we certainly do not exclude the involvement of other systems. For example, manipulations of the central serotoninergic system can produce specific changes in cognitive functioning.29,44,45 We therefore estimated 5-HT2 receptor occupancy levels, using the ED50 values reported in the literature.46,47 5-HT2 receptors were almost saturated in a majority of the subjects; in fact, 71.5% of the subjects showed >90% occupancy and 92.4% showed >80% occupancy, which may suggest a limited impact of the serotonergic system on cognition in this study. However, potential confounding effects through this system clearly warrant further investigations. In addition, anticholinergic effects have been reported to impair cognitive function both globally48 as well as in specific domains, including memory49 and executive functioning.50,51 The association between anticholinergic activity and cognitive performance are also strongly supported by the studies that measured serum anticholinergic activity.52,53 Overall, anticholinergic burden due to all prescribed medications was not evaluated in the present study although the use of antiparkinsonian anticholinergic drugs was taken into consideration, which has to be acknowledged in light of the exposure-dependent detrimental effects of those medications on cognition.48
Several other limitations qualify our conclusions. First, mean values of predicted peak and trough dopamine D2 receptor occupancy levels on the day of cognitive assessment were used in this analysis; however, they did not always represent the levels at the time of neurocognitive assessments. Second, subjects were divided into 4 groups according to their estimated dopamine D2 receptor occupancy levels in order to test the hypothesis; however, this classification could be considered arbitrary, In our hypothesis, we expected that effects of dopamine D2 receptor occupancy on cognitive functions would not be simply linear but inverted U-shaped. To test this hypothesis while taking other clinical and demographic variables into consideration, we decided to divide subjects to 4 groups based on their dopamine D2 receptor occupancy levels and then examined the effects of dopamine D2 receptor occupancy on cognition, using a multivariate general linear model. Still, it would have been ideal to handle dopamine D2 receptor occupancy as a continuous variable from a statistical perspective. Third, extrapyramidal symptoms are also expected to affect cognition; we therefore included the SAS mean score in the model but failed to find any statistically significant effect on cognition in this study. However, this does not always exclude any possibility of potential effects of extrapyramidal symptoms on cognition. Similarly, sedative effects of antipsychotic drugs could also affect cognition, which was not taken into consideration in the present study. These limitations clearly emphasize the need for prospective studies with more comprehensive assessments. Finally, it would have been ideal to include perphenazine that demonstrated comparable clinical effects to newer antipsychotic drugs although any model for the prediction of dopamine D2 receptor occupancy for this drug is not available.
In conclusion, the degree of dopamine D2 receptor occupancy levels estimated from plasma concentrations of antipsychotic drugs were associated with overall neurocognitive function and vigilance in patients with schizophrenia. This study shows that D2 occupancy above 80% not only increases the risk for extrapyramidal side effects as consistently reported in the literature but also increases the risk for cognitive impairment.
Supplementary Material
Supplementary material is available at http://schizophreniabulletin.oxfordjournals.org.
Funding
Data used in the preparation of this article were obtained from the limited access datasets distributed from the National Institutes of Health (NIH)-supported “Clinical Antipsychotic Trials of Intervention Effectiveness in Schizophrenia” (CATIE-Sz). This is a multisite clinical trial of persons with schizophrenia comparing the effectiveness of randomly assigned medication treatment. The study was supported by National Institute of Mental Health (NIMH) Contract #N01MH90001 to the University of North Carolina at Chapel Hill. The ClinicalTrials.gov identifier is NCT00014001. The version of the dataset used was 1.0. This study was also supported by grant R01MH064173 from the NIMH and was ancillary to CATIE, N01MH90001, from the NIMH. The present analysis was also funded by Grant-in-Aid for Young Scientists-B from the Ministry of Education, Culture, Sports, Science, and Technology (HU); this organization had no role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Supplementary Material
Acknowledgments
Previous presentation: None. Conflicts of interest and financial disclosure: Dr R.R.B. has received National Institutes of Health (NIH), Centre for Addiction and Mental Health, Lilly, and Indiana University–based grant funding. Dr S.S. has received grants from National Institute of Mental Health (NIMH) and the Foundation for the NIH. He has received consulting income from Janssen and Lilly. Dr R.S.E.K. currently or in the past 5+ years has received investigator-initiated research funding support from the Allon, AstraZeneca, Department of Veterans Affairs, Eli Lilly, GlaxoSmithKline, NIMH, Novartis, Organon Pharmaceuticals, Pfizer, Psychogenics, Research Foundation for Mental Hygiene, Inc., and the Singapore National Medical Research Council. He currently or in the past 5+ years has received honoraria, served as a consultant or advisory board member for Abbott, Acadia, Astellas, Astra-Zeneca, BiolineRx, BrainCells, Bristol-Myers-Squibb, Cephalon, CHDI, Cortex, Cypress Bioscience, Eli Lilly, EnVivo, Johnson & Johnson, Lundbeck, Memory Pharmaceuticals, Merck, NeuroSearch, Orexigen, Oganon Pharmaceuticals, Orion, Otsuka, Pfizer, Roche, Sanofi-Aventis, Shering-Plough, Shire, Solvay, Sunovion, Takeda, Wyeth, and Xenoport. Dr R.S.E.K. receives royalties from the Brief Assessment of Cognition in Schizophrenia (BACS) testing battery and the Measurement and Treatment Research to Improve Cognition in Schizophrenia Battery (BACS Symbol Coding). He is also a shareholder in NeuroCog Trials, Inc., Durham NC. Duke University holds the copyright for the SCoRS, and licenses are issued by NeuroCog Trials, Inc. There is currently no license fee to use the SCoRS. Dr T.K.R. has received Brain and Behavior Research Foundation, Canadian Foundation for Innovation, Canadian Institutes of Health Research (CIHR), NIH, Ontario Ministry for Health and Long-Term Facilities, and Ontario Ministry for Innovation. Dr D.C.M. has received investigator-initiated grant support from Pfizer within the past 5 years. Dr T.S. has received fellowship grants from the Japanese Society of Clinical Neuropsychopharmacology, Government of Canada Post-Doctoral Research Fellowships, Kanae Foundation, and Mochida Memorial Foundation, and manuscript fees from Kyowa Hakko Kirin and Dainippon Sumitomo Pharma within the past 5 years. Dr B.G.P. receives research support from the NIH and the CIHR. Within the past 5 years, he has been a member of the advisory board of Lundbeck Canada (final meeting was May 2009) and Forest Laboratories (final meeting was March 2008). Dr B.G.P. has served one time as a consultant for Wyeth (October 2008) and Takeda (July 2007). He was also a faculty member of the Lundbeck International Neuroscience Foundation (final meeting was April 2010). Dr K.W. has received grants, or consultant fees from Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, and Pfizer, and received speaker’s honoraria from Astellas Pharma, Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Meiji, Otsuka Pharmaceutical, Pfizer, and Yoshitomiyakuhin within the past 5 years. Dr M.M. has received grants, or consultant fees from Eisai, Astellas Pharma, GlaxoSmithKline and Meiji, and received speaker’s honoraria from Astellas Pharma, Dainippon Sumitomo Pharma, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutical, Meiji, Otsuka Pharmaceutical, Pfizer, and Yoshitomiyakuhin within the past 5 years. Dr H.U. has received grants from Pfizer, speaker’s honoraria from Otsuka Pharmaceutical, Janssen Pharmaceutical, and Shionogi, and manuscript fees from Dainippon Sumitomo Pharma within the past 5 years. Dr H.S. has nothing to disclose. Disclaimer: This manuscript reflects the views of the authors and may not reflect the opinions or views of the CATIE-Sz Study Investigators or the NIH.
References
- 1.Green MF. What are the functional consequences of neurocognitive deficits in schizophrenia? Am J Psychiatry. 1996;153:321–330. doi: 10.1176/ajp.153.3.321. [DOI] [PubMed] [Google Scholar]
- 2.Elie D, Poirier M, Chianetta J, Durand M, Gregoire C, Grignon S. Cognitive effects of antipsychotic dosage and polypharmacy: a study with the BACS in patients with schizophrenia and schizoaffective disorder. J Psychopharmacol. 2010;24:1037–1044. doi: 10.1177/0269881108100777. [DOI] [PubMed] [Google Scholar]
- 3.Knowles EE, David AS, Reichenberg A. Processing speed deficits in schizophrenia: reexamining the evidence. Am J Psychiatry. 2010;167:828–835. doi: 10.1176/appi.ajp.2010.09070937. [DOI] [PubMed] [Google Scholar]
- 4.Hori H, Noguchi H, Hashimoto R, et al. Antipsychotic medication and cognitive function in schizophrenia. Schizophr Res. 2006;86:138–146. doi: 10.1016/j.schres.2006.05.004. [DOI] [PubMed] [Google Scholar]
- 5.Keefe RS, Silva SG, Perkins DO, Lieberman JA. The effects of atypical antipsychotic drugs on neurocognitive impairment in schizophrenia: a review and meta-analysis. Schizophr Bull. 1999;25:201–222. doi: 10.1093/oxfordjournals.schbul.a033374. [DOI] [PubMed] [Google Scholar]
- 6.Minzenberg MJ, Poole JH, Benton C, Vinogradov S. Association of anticholinergic load with impairment of complex attention and memory in schizophrenia. Am J Psychiatry. 2004;161:116–124. doi: 10.1176/appi.ajp.161.1.116. [DOI] [PubMed] [Google Scholar]
- 7.Harvey PD, Keefe RS. Studies of cognitive change in patients with schizophrenia following novel antipsychotic treatment. Am J Psychiatry. 2001;158:176–184. doi: 10.1176/appi.ajp.158.2.176. [DOI] [PubMed] [Google Scholar]
- 8.Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8:457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]
- 9.Keefe RS, Bilder RM, Davis SM, et al. Neurocognitive effects of antipsychotic medications in patients with chronic schizophrenia in the CATIE Trial. Arch Gen Psychiatry. 2007;64:633–647. doi: 10.1001/archpsyc.64.6.633. [DOI] [PubMed] [Google Scholar]
- 10.Harvey PD, Rabinowitz J, Eerdekens M, Davidson M. Treatment of cognitive impairment in early psychosis: a comparison of risperidone and haloperidol in a large long-term trial. Am J Psychiatry. 2005;162:1888–1895. doi: 10.1176/appi.ajp.162.10.1888. [DOI] [PubMed] [Google Scholar]
- 11.Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry. 1998;155:344–349. doi: 10.1176/ajp.155.3.344. [DOI] [PubMed] [Google Scholar]
- 12.Uchida H, Rajji TK, Mulsant BH, et al. D2 receptor blockade by risperidone correlates with attention deficits in late-life schizophrenia. J Clin Psychopharmacol. 2009;29:571–575. doi: 10.1097/JCP.0b013e3181bf4ea3. [DOI] [PubMed] [Google Scholar]
- 13.Lawrence AD, Weeks RA, Brooks DJ, et al. The relationship between striatal dopamine receptor binding and cognitive performance in Huntington's disease. Brain. 1998;121(pt 7):1343–1355. doi: 10.1093/brain/121.7.1343. [DOI] [PubMed] [Google Scholar]
- 14.Glickstein SB, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors have spatial working memory deficits. J Neurosci. 2002;22:5619–5629. doi: 10.1523/JNEUROSCI.22-13-05619.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Glickstein SB, Desteno DA, Hof PR, Schmauss C. Mice lacking dopamine D2 and D3 receptors exhibit differential activation of prefrontal cortical neurons during tasks requiring attention. Cereb Cortex. 2005;15:1016–1024. doi: 10.1093/cercor/bhh202. [DOI] [PubMed] [Google Scholar]
- 16.Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Predicting dopamine D2 receptor occupancy from plasma levels of antipsychotic drugs: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011;31:318–325. doi: 10.1097/JCP.0b013e318218d339. [DOI] [PubMed] [Google Scholar]
- 17.Bigos KL, Bies RR, Pollock BG. Population pharmacokinetics in geriatric psychiatry. Am J Geriatr Psychiatry. 2006;14:993–1003. doi: 10.1097/01.JGP.0000224330.73063.6c. [DOI] [PubMed] [Google Scholar]
- 18.Uchida H, Pollock BG, Bies RR, Mamo DC. Predicting age-specific dosing of antipsychotics. Clin Pharmacol Ther. 2009;86:360–362. doi: 10.1038/clpt.2009.133. [DOI] [PubMed] [Google Scholar]
- 19.Feng Y, Pollock BG, Coley K, et al. Population pharmacokinetic analysis for risperidone using highly sparse sampling measurements from the CATIE study. Br J Clin Pharmacol. 2008;66:629–639. doi: 10.1111/j.1365-2125.2008.03276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wessels AM, Bies RR, Pollock BG, et al. Population pharmacokinetic modeling of ziprasidone in patients with schizophrenia from the CATIE study. J Clin Pharmacol. 2011;51:1587–1591. doi: 10.1177/0091270010387604. [DOI] [PubMed] [Google Scholar]
- 21.Bigos KL, Pollock BG, Coley KC, et al. Sex, race, and smoking impact olanzapine exposure. J Clin Pharmacol. 2008;48:157–165. doi: 10.1177/0091270007310385. [DOI] [PubMed] [Google Scholar]
- 22.Uchida H, Takeuchi H, Graff-Guerrero A, Suzuki T, Watanabe K, Mamo DC. Dopamine D2 receptor occupancy and clinical effects: a systematic review and pooled analysis. J Clin Psychopharmacol. 2011;31:497–502. doi: 10.1097/JCP.0b013e3182214aad. [DOI] [PubMed] [Google Scholar]
- 23.Stroup TS, McEvoy JP, Swartz MS, et al. The National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project: schizophrenia trial design and protocol development. Schizophr Bull. 2003;29:15–31. doi: 10.1093/oxfordjournals.schbul.a006986. [DOI] [PubMed] [Google Scholar]
- 24.Keefe RS, Mohs RC, Bilder RM, et al. Neurocognitive assessment in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) project schizophrenia trial: development, methodology, and rationale. Schizophr Bull. 2003;29:45–55. doi: 10.1093/oxfordjournals.schbul.a006990. [DOI] [PubMed] [Google Scholar]
- 25.Keefe RS, Bilder RM, Harvey PD, et al. Baseline neurocognitive deficits in the CATIE schizophrenia trial. Neuropsychopharmacology. 2006;31:2033–2046. doi: 10.1038/sj.npp.1301072. [DOI] [PubMed] [Google Scholar]
- 26.Sheiner LB, Rosenberg B, Marathe VV. Estimation of population characteristics of pharmacokinetic parameters from routine clinical data. J Pharmacokinet Biopharm. 1977;5:445–479. doi: 10.1007/BF01061728. [DOI] [PubMed] [Google Scholar]
- 27.Cha TA, Beall E, Irvine B, et al. At least five related, but distinct, hepatitis C viral genotypes exist. Proc Natl Acad Sci U S A. 1992;89:7144–7148. doi: 10.1073/pnas.89.15.7144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ismail Z, Wessels AM, Uchida H, et al. Age and sex impact clozapine plasma concentrations in inpatients and outpatients with schizophrenia. Am J Geriatr Psychiatry. 2012;20:53–60. doi: 10.1097/JGP.0b013e3182118318. [DOI] [PubMed] [Google Scholar]
- 29.Boulougouris V, Tsaltas E. Serotonergic and dopaminergic modulation of attentional processes. Prog Brain Res. 2008;172:517–542. doi: 10.1016/S0079-6123(08)00925-4. [DOI] [PubMed] [Google Scholar]
- 30.Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- 31.Korchounov A, Ziemann U. Neuromodulatory neurotransmitters influence LTP-like plasticity in human cortex: a pharmaco-TMS study. Neuropsychopharmacology. 2011;36:1894–1902. doi: 10.1038/npp.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapur S, Mamo D. Half a century of antipsychotics and still a central role for dopamine D2 receptors. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:1081–1090. doi: 10.1016/j.pnpbp.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 33.Farde L, Nyberg S, Oxenstierna G, Nakashima Y, Halldin C, Ericsson B. Positron emission tomography studies on D2 and 5-HT2 receptor binding in risperidone-treated schizophrenic patients. J Clin Psychopharmacol. 1995;15(1 suppl 1):19–23. doi: 10.1097/00004714-199502001-00004. [DOI] [PubMed] [Google Scholar]
- 34.Kapur S, Zipursky R, Jones C, Remington G, Houle S. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–520. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 35.Farde L, Nordstrom AL, Wiesel FA, Pauli S, Halldin C, Sedvall G. Positron emission tomographic analysis of central D1 and D2 dopamine receptor occupancy in patients treated with classical neuroleptics and clozapine. Relation to extrapyramidal side effects. Arch Gen Psychiatry. 1992;49:538–544. doi: 10.1001/archpsyc.1992.01820070032005. [DOI] [PubMed] [Google Scholar]
- 36.Leeson VC, Barnes TR, Harrison M, et al. The relationship between IQ, memory, executive function, and processing speed in recent-onset psychosis: 1-year stability and clinical outcome. Schizophr Bull. 2010;36:400–409. doi: 10.1093/schbul/sbn100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niendam TA, Bearden CE, Rosso IM, et al. A prospective study of childhood neurocognitive functioning in schizophrenic patients and their siblings. Am J Psychiatry. 2003;160:2060–2062. doi: 10.1176/appi.ajp.160.11.2060. [DOI] [PubMed] [Google Scholar]
- 38.Dickinson D, Ramsey ME, Gold JM. Overlooking the obvious: a meta-analytic comparison of digit symbol coding tasks and other cognitive measures in schizophrenia. Arch Gen Psychiatry. 2007;64:532–542. doi: 10.1001/archpsyc.64.5.532. [DOI] [PubMed] [Google Scholar]
- 39.Caley CF, Cooper CK. Ziprasidone: the fifth atypical antipsychotic. Ann Pharmacother. 2002;36:839–851. doi: 10.1345/aph.1A053. [DOI] [PubMed] [Google Scholar]
- 40.Seeman P. All roads to schizophrenia lead to dopamine supersensitivity and elevated dopamine D2(high) receptors. CNS Neurosci Ther. 2011;17:118–132. doi: 10.1111/j.1755-5949.2010.00162.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kassahun K, Mattiuz E, Nyhart E, Jr, et al. Disposition and biotransformation of the antipsychotic agent olanzapine in humans. Drug Metab Dispos. 1997;25:81–93. [PubMed] [Google Scholar]
- 42.Sheiner LB, Beal SL. Some suggestions for measuring predictive performance. J Pharmacokinet Biopharm. 1981;9:503–512. doi: 10.1007/BF01060893. [DOI] [PubMed] [Google Scholar]
- 43.Agid O, Mamo D, Ginovart N, et al. Striatal vs extrastriatal dopamine D2 receptors in antipsychotic response–a double-blind PET study in schizophrenia. Neuropsychopharmacology. 2007;32:1209–1215. doi: 10.1038/sj.npp.1301242. [DOI] [PubMed] [Google Scholar]
- 44.Schmitt JA, Wingen M, Ramaekers JG, Evers EA, Riedel WJ. Serotonin and human cognitive performance. Curr Pharm Des. 2006;12:2473–2486. doi: 10.2174/138161206777698909. [DOI] [PubMed] [Google Scholar]
- 45.Gonzalez-Burgos I, Feria-Velasco A. Serotonin/dopamine interaction in memory formation. Prog Brain Res. 2008;172:603–623. doi: 10.1016/S0079-6123(08)00928-X. [DOI] [PubMed] [Google Scholar]
- 46.Mamo D, Kapur S, Shammi CM, et al. A PET study of dopamine D2 and serotonin 5-HT2 receptor occupancy in patients with schizophrenia treated with therapeutic doses of ziprasidone. Am J Psychiatry. 2004;161:818–825. doi: 10.1176/appi.ajp.161.5.818. [DOI] [PubMed] [Google Scholar]
- 47.Kapur S, Zipursky RB, Remington G. Clinical and theoretical implications of 5-HT2 and D2 receptor occupancy of clozapine, risperidone, and olanzapine in schizophrenia. Am J Psychiatry. 1999;156:286–293. doi: 10.1176/ajp.156.2.286. [DOI] [PubMed] [Google Scholar]
- 48.Heinik J. Effects of trihexyphenidyl on MMSE and CAMCOG scores of medicated elderly patients with schizophrenia. Int Psychogeriatr. 1998;10:103–108. doi: 10.1017/s1041610298005195. [DOI] [PubMed] [Google Scholar]
- 49.Gelenberg AJ, Van Putten T, Lavori PW, et al. Anticholinergic effects on memory: benztropine versus amantadine. J Clin Psychopharmacol. 1989;9:180–185. [PubMed] [Google Scholar]
- 50.Bottiggi KA, Salazar JC, Yu L, et al. Long-term cognitive impact of anticholinergic medications in older adults. Am J Geriatr Psychiatry. 2006;14:980–984. doi: 10.1097/01.JGP.0000224619.87681.71. [DOI] [PubMed] [Google Scholar]
- 51.Kay GG, Abou-Donia MB, Messer WS, Jr, Murphy DG, Tsao JW, Ouslander JG. Antimuscarinic drugs for overactive bladder and their potential effects on cognitive function in older patients. J Am Geriatr Soc. 2005;53:2195–2201. doi: 10.1111/j.1532-5415.2005.00537.x. [DOI] [PubMed] [Google Scholar]
- 52.Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60:198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 53.Chew ML, Mulsant BH, Pollock BG. Serum anticholinergic activity and cognition in patients with moderate-to-severe dementia. Am J Geriatr Psychiatry. 2005;13:535–538. doi: 10.1176/appi.ajgp.13.6.535. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.