Abstract
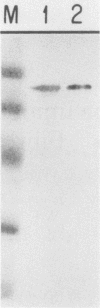
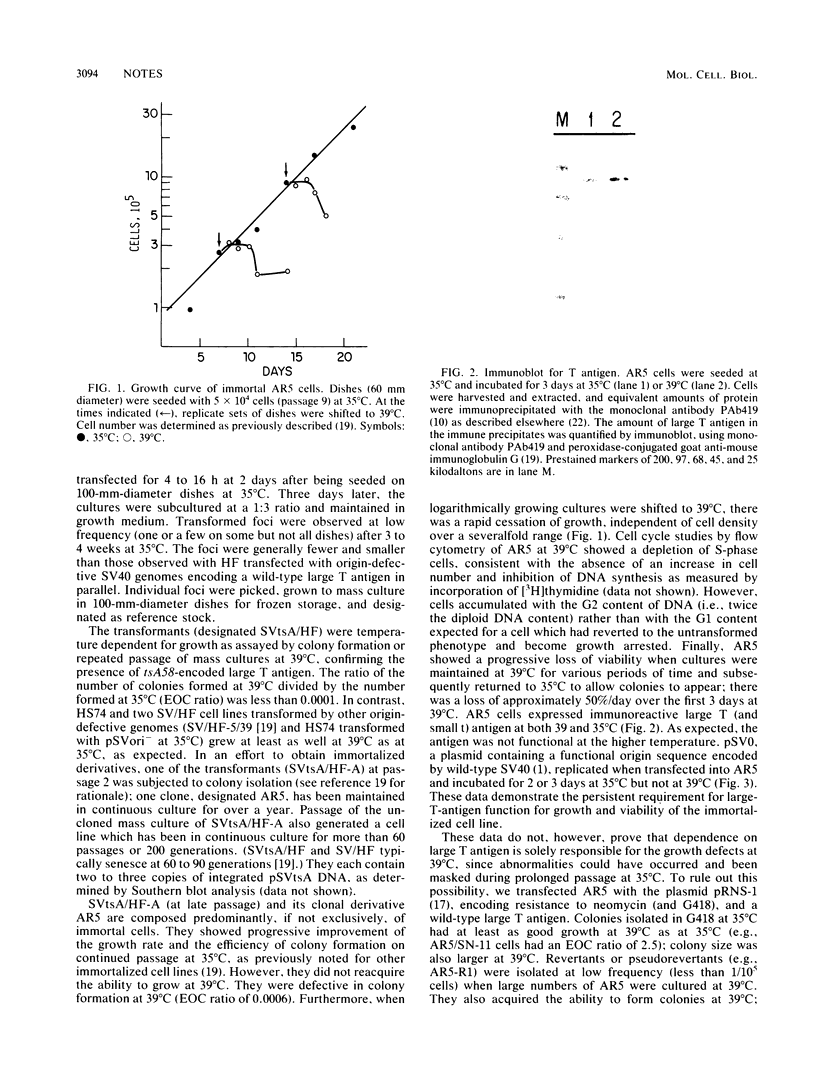
Simian virus 40 (SV40)-mediated transformation of human fibroblasts offers an experimental system for studying both carcinogenesis and cellular aging, since such transformants show the typical features of altered cellular growth but still have a limited life span in culture and undergo senescence. We have previously demonstrated (D. S. Neufeld, S. Ripley, A. Henderson, and H. L. Ozer, Mol. Cell. Biol. 7:2794-2802, 1987) that transformants generated with origin-defective mutants of SV40 show an increased frequency of overcoming senescence and becoming immortal. To clarify further the role of large T antigen, we have generated immortalized transformants by using origin-defective mutants of SV40 encoding a heat-labile large T antigen (tsA58 transformants). At a temperature permissive for large-T-antigen function (35 degrees C), the cell line AR5 had properties resembling those of cell lines transformed with wild-type SV40. However, the AR5 cells were unable to proliferate or form colonies at temperatures restrictive for large-T-antigen function (39 degrees C), demonstrating a continuous need for large T antigen even in immortalized human fibroblasts. Such immortal temperature-dependent transformants should be useful cell lines for the identification of other cellular or viral gene products that induce cell proliferation in human cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Auborn K. J., Markowitz R. B., Wang E., Yu Y. T., Prives C. Simian virus 40 (SV40) T antigen binds specifically to double-stranded DNA but not to single-stranded DNA or DNA/RNA hybrids containing the SV40 regulatory sequences. J Virol. 1988 Jun;62(6):2204–2208. doi: 10.1128/jvi.62.6.2204-2208.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbis D. P., Schultz R. A., Friedberg E. C. Isolation and partial characterization of virus-transformed cell lines representing the A, G and variant complementation groups of xeroderma pigmentosum. Mutat Res. 1986 May;165(3):175–184. doi: 10.1016/0167-8817(86)90052-0. [DOI] [PubMed] [Google Scholar]
- Canaani D., Naiman T., Teitz T., Berg P. Immortalization of xeroderma pigmentosum cells by simian virus 40 DNA having a defective origin of DNA replication. Somat Cell Mol Genet. 1986 Jan;12(1):13–20. doi: 10.1007/BF01560723. [DOI] [PubMed] [Google Scholar]
- Chang S. E. In vitro transformation of human epithelial cells. Biochim Biophys Acta. 1986;823(3):161–194. doi: 10.1016/0304-419x(86)90001-6. [DOI] [PubMed] [Google Scholar]
- Daya-Grosjean L., James M. R., Drougard C., Sarasin A. An immortalized xeroderma pigmentosum, group C, cell line which replicates SV40 shuttle vectors. Mutat Res. 1987 Mar;183(2):185–196. doi: 10.1016/0167-8817(87)90061-7. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gluzman Y. SV40-transformed simian cells support the replication of early SV40 mutants. Cell. 1981 Jan;23(1):175–182. doi: 10.1016/0092-8674(81)90282-8. [DOI] [PubMed] [Google Scholar]
- Gluzman Y., Sambrook J. F., Frisque R. J. Expression of early genes of origin-defective mutants of simian virus 40. Proc Natl Acad Sci U S A. 1980 Jul;77(7):3898–3902. doi: 10.1073/pnas.77.7.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harlow E., Crawford L. V., Pim D. C., Williamson N. M. Monoclonal antibodies specific for simian virus 40 tumor antigens. J Virol. 1981 Sep;39(3):861–869. doi: 10.1128/jvi.39.3.861-869.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurlin P. J., Fry D. G., Maher V. M., McCormick J. J. Morphological transformation, focus formation, and anchorage independence induced in diploid human fibroblasts by expression of a transfected H-ras oncogene. Cancer Res. 1987 Nov 1;47(21):5752–5757. [PubMed] [Google Scholar]
- Hurlin P. J., Maher V. M., McCormick J. J. Malignant transformation of human fibroblasts caused by expression of a transfected T24 HRAS oncogene. Proc Natl Acad Sci U S A. 1989 Jan;86(1):187–191. doi: 10.1073/pnas.86.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jat P. S., Sharp P. A. Cell lines established by a temperature-sensitive simian virus 40 large-T-antigen gene are growth restricted at the nonpermissive temperature. Mol Cell Biol. 1989 Apr;9(4):1672–1681. doi: 10.1128/mcb.9.4.1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Bella F., Ozer H. L. Differential replication of SV40 and polyoma DNAs in Chinese hamster ovary cells. Virus Res. 1985 Jun;2(4):329–344. doi: 10.1016/0168-1702(85)90029-2. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Litzkas P., Jha K. K., Ozer H. L. Efficient transfer of cloned DNA into human diploid cells: protoplast fusion in suspension. Mol Cell Biol. 1984 Nov;4(11):2549–2552. doi: 10.1128/mcb.4.11.2549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murnane J. P., Fuller L. F., Painter R. B. Establishment and characterization of a permanent pSV ori--transformed ataxia-telangiectasia cell line. Exp Cell Res. 1985 May;158(1):119–126. doi: 10.1016/0014-4827(85)90437-9. [DOI] [PubMed] [Google Scholar]
- Neufeld D. S., Ripley S., Henderson A., Ozer H. L. Immortalization of human fibroblasts transformed by origin-defective simian virus 40. Mol Cell Biol. 1987 Aug;7(8):2794–2802. doi: 10.1128/mcb.7.8.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozer H. L., Slater M. L., Dermody J. J., Mandel M. Replication of simian virus 40 DNA in normal human fibroblasts and in fibroblasts from xeroderma pigmentosum. J Virol. 1981 Aug;39(2):481–489. doi: 10.1128/jvi.39.2.481-489.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit C. A., Gardes M., Feunteun J. Immortalization of rodent embryo fibroblasts by SV40 is maintained by the A gene. Virology. 1983 May;127(1):74–82. doi: 10.1016/0042-6822(83)90372-0. [DOI] [PubMed] [Google Scholar]
- Radna R. L., Foellmer B., Feldman L. A., Francke U., Ozer H. L. Restriction of human adenovirus replication in Chinese hamster cell lines and their hybrids with human cells. Virus Res. 1987 Nov;8(4):277–299. doi: 10.1016/0168-1702(87)90001-3. [DOI] [PubMed] [Google Scholar]
- Sager R., Tanaka K., Lau C. C., Ebina Y., Anisowicz A. Resistance of human cells to tumorigenesis induced by cloned transforming genes. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7601–7605. doi: 10.1073/pnas.80.24.7601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small M. B., Gluzman Y., Ozer H. L. Enhanced transformation of human fibroblasts by origin-defective simian virus 40. Nature. 1982 Apr 15;296(5858):671–672. doi: 10.1038/296671a0. [DOI] [PubMed] [Google Scholar]
- Tubo R. A., Rheinwald J. G. Normal human mesothelial cells and fibroblasts transfected with the EJras oncogene become EGF-independent, but are not malignantly transformed. Oncogene Res. 1987 Sep-Oct;1(4):407–421. [PubMed] [Google Scholar]
- Wright W. E., Pereira-Smith O. M., Shay J. W. Reversible cellular senescence: implications for immortalization of normal human diploid fibroblasts. Mol Cell Biol. 1989 Jul;9(7):3088–3092. doi: 10.1128/mcb.9.7.3088. [DOI] [PMC free article] [PubMed] [Google Scholar]