Abstract
Context:
The process of diagnosing temporal arteritis remains controversial. Although temporal artery biopsy has long been the standard tool of evaluation, its poor sensitivity has prompted investigation of other methods to aid in diagnosis. Improved clinical evaluation and various imaging techniques have been suggested as ways to establish the diagnosis through noninvasive means and to improve biopsy yield.
Objective:
To retrospectively report and evaluate the process and experience of the Kaiser Permanente Northwest Region in implementing a new protocol that includes an enhanced clinical evaluation as well as the incorporation of color duplex ultrasonography in addition to biopsy when appropriate for temporal arteritis evaluation.
Results:
A 38% reduction in the number of temporal artery biopsies performed was achieved through the new protocol, which was created by a multidisciplinary process, including stakeholders from all departments involved. The percentage of abnormal biopsy results rose from 8.5% at baseline to 24%. No cases of the disease were missed after careful evaluation of clinical and medical-legal records.
Conclusions:
Adding specialist clinical evaluation and color duplex ultrasonography to the standard diagnostic workup for temporal arteritis creates a rapid, noninvasive, resource-sensible means to diagnose giant cell arteritis, to improve temporal artery biopsy yield, and to decrease the total number of biopsies done. The diagnosis can be made in some cases by clinical evaluation and color duplex ultrasonography alone, thereby saving the patient an unnecessary surgical procedure. Protocols such as this can be implemented by multidisciplinary cooperation in a patient-centered, integrated system.
Introduction
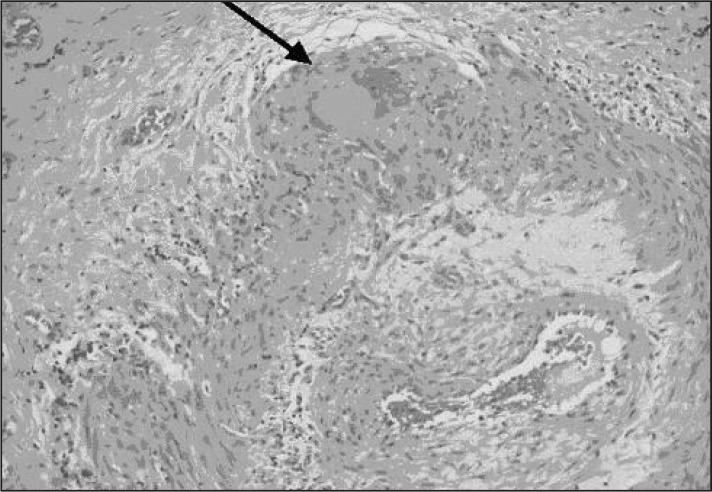
Few clinical dilemmas have proved as vexing as the diagnosis of temporal arteritis, also known as giant cell arteritis (GCA). An arterial inflammatory condition most commonly affecting the temporal arteries, GCA is characterized by arterial infiltration of so-called giant cells (Figure 1).1 Failure to identify the disease in its early state can lead to lack of treatment and ultimately irreversible blindness. GCA is thought to occur in 10 to 20 per 100,000 patients older than age 50 years, more often in elderly patients and in women.2 The treatment consists of long-term administration of corticosteroids, which nearly immediately arrest the progression of the disease but carry a number of known risks (eg, electrolyte disturbances, hypertension, psychiatric disturbances, diabetes, osteoporosis, and adrenal suppression).3
Figure 1.
Histologic specimen of giant cell arteritis. Arrow points to giant cell in arterial wall.
Reprinted with permission from: Mansoor O, Majeed T. A 90 year old woman with painless vision loss. Digital Journal of Ophthalmology [serial on the Internet] 2005 Feb 10 [cited 2012 Oct 31];11(7): Figure 2. Available from: www.djo.harvard.edu/site.php?url=/physicians/gr/728&page=GR_TT.
Attempts at standardization of diagnostic protocols have been controversial. Measures such as careful clinical evaluation based on specific criteria, temporal artery biopsy, magnetic resonance imaging, positron emission tomography, and color duplex ultrasonography, alone or in combination, have been proposed.4,5 No universally accepted algorithm has emerged, although temporal artery biopsy has remained the traditional cornerstone of diagnosis. Because the arterial inflammation is characterized by “skip lesions” (areas of inflammation intermixed with a normal artery), evaluating the sensitivity of detection by any method, including biopsy, is limited and difficult to determine. The false-negative rate of biopsy is estimated to vary between 10% and 61%.6 The reference criteria against which this sensitivity is determined is typically clinical suspicion, itself an imprecise measure.
A weakness of any diagnostic modality, whether imaging or biopsy, is that it must clearly identify the actively inflamed artery segment. Clinicians have remained frustrated that even pathologic diagnosis by temporal artery biopsy, which is limited to the surgically accessible part of the artery, demonstrates a limited sensitivity when compared against clinical suspicion because, even in active disease, there remain segments of unaffected artery. For this reason, some authors have recommended trying to obtain longer segments of artery (>2 cm) at biopsy.7 However, longer artery segments require longer incisions and an increased complexity of the procedure.
In 1990, the American College of Rheumatology, in an attempt to standardize the diagnosis of GCA, created a classification system consisting of 5 specific criteria that predict the presence of temporal arteritis.8 The group of patients studied was composed of those assumed to have the disease compared with control patients (who had other vascular conditions) on 30 discrete variables.8 By factor analysis, an abnormal result of a temporal artery biopsy emerged as 1 of 5 equally weighted final criteria that contribute evidence to the diagnosis (Table 1).8 This guideline recognized that, even with a normal biopsy result, a patient might still have a high likelihood of having GCA, assuming that his/her clinical picture is consistent with the disease. In fact, of the patients studied, even when highly screened to meet clinical criteria for temporal arteritis, 12 of 214 patients had normal biopsy results. The presence of any 3 of the 5 criteria (even if abnormal biopsy result is not one of them) imparted a sensitivity of 93.5% and a specificity of 91.2%.
Table 1.
American College of Rheumatology classification for giant cell arteritis1
| Criterion | Definition |
|---|---|
| 1. Age at disease onset ≥50 years | Development of symptoms or findings beginning at age 50 years or older |
| 2. New headache | New onset of or new type of localized pain in the head |
| 3. Temporal artery abnormality | Temporal artery tenderness to palpation or decreased pulsation, unrelated to arteriosclerosis of cervical arteries |
| 4. Elevated erythrocyte sedimentation rate | Erythrocyte sedimentation rate ≥50 mm/hour by the Westergren method |
| 5. Abnormal artery biopsy | Biopsy specimen with artery showing vasculitis characterized by a predominance of mononuclear cell infiltration or granulomatous inflammation, usually with multinucleated giant cells |
Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum 1990 Aug;33(8):1122–8. DOI: http://dx.doi.org/10.1002/art.1780330810
Regardless of its poor sensitivity, temporal artery biopsy has remained the hallmark of diagnosis because, when the result is abnormal, it defines the disease. It is an outpatient surgical procedure, performed with either sedation and local anesthesia or local anesthesia alone. Although the procedure is generally safe, rare complications such as facial nerve injury can occur.9 Additionally, it is resource intensive, typically requiring the use of the ambulatory surgical center operating room (OR).
Perhaps most frustrating is the inability of temporal artery biopsy to definitively make the diagnosis in most cases because a normal biopsy result does not rule out GCA. Most clinicians are aware of this. In fact, when retrospective reviews have evaluated whether treatment decisions are altered by biopsy result in the case of a normal biopsy, the findings demonstrate clearly that they are not.10 Because the consequence to the patient of a missed case of GCA is so high, clinicians will treat on the basis of probability of disease without regard to the biopsy result unless the biopsy result is abnormal, at which point the need for treatment is clear.
As a department we wondered, with the result of so few biopsies yielding abnormal results, should temporal artery biopsy continue to be the initial screening measure for GCA in all patients, or is there a more select group of patients for whom biopsy result would most influence treatment decisions? How could we improve our yield of abnormal biopsy results? Are there some patients for whom biopsy is unnecessary, because the pathologic result would not influence treatment decisions?
Methods/Results
Phase 1: Pilot Protocol
The investigation was begun by examining our own experience. From June 2000 until September 2003, the department found that 143 unilateral temporal artery biopsies were performed in the Kaiser Permanente Northwest (KPNW) Region. Of these, 8.5% showed an abnormal pathologic result (inflammatory mononuclear cell infiltrates within the vessel wall with presence of giant cells). The regional protocol at that time was to initiate corticosteroid therapy immediately in a patient that the clinician suspected of having GCA and to refer that patient to the General Surgery Department for urgent unilateral temporal artery biopsy on the most symptomatic side. These biopsies were performed at the first surgical opportunity (ideally 1 to 3 days). There were no established criteria for referral other than clinical suspicion. Most referrals came from primary care clinicians. The yield of abnormal biopsy results did not meet published norms reported in the professional literature,11 prompting a closer look at the process.
Initial Revisions to the Protocol—Adding Color Duplex Ultrasonography
In an effort to improve our rate of abnormal biopsy results, we introduced temporal artery color duplex ultrasonography as the first diagnostic measure in the algorithm for GCA workup, judging it to be the most promising of the noninvasive diagnostic modalities being studied. We believed this would help us target our biopsy at the particular segment of artery that was abnormal as well as allow us to evaluate the entire artery on both sides rather than being limited to the surgically accessible portion.12 At that time, color duplex ultrasonography was emerging as a promising modality for evaluation of arteritis of various types.
The additional objective, by evaluating the correlation between ultrasound result and biopsy result was that, if this technique was successful (had increased sensitivity and specificity compared with clinical suspicion), duplex ultrasonography could eventually replace temporal artery biopsy as the diagnostic modality of choice for suspected cases of GCA. Duplex ultrasonography has the following advantages: it is noninvasive (thereby eliminating the risks associated with surgery); it does not require altering the anticoagulation regimen for patients receiving these medications; it is less expensive than biopsy; it is more readily available in KPNW (given the premium on available OR time); and it can be done more expeditiously.
Bringing duplex ultrasonography into the protocol required some technical training of duplex ultrasound technicians. However, their core training in noninvasive vascular imaging made the additional training a natural progression of their scope of practice rather than an entirely new undertaking. Learning the technique did not prove difficult (5- to 15-MHz linear probe depending on the depth of the vessel, bilateral evaluation of the entirety of the temporal arteries). Vascular surgeons already had the expertise to read these noninvasive studies. A commitment was negotiated with the Radiology Department staff to perform the study within 48 hours of referral and with vascular surgeons to read the studies on the same day as they were performed whenever possible, and at most by the end of the following day.
The General Surgery Department agreed to biopsy all patients with any abnormality on the duplex ultrasound image (an inflammatory halo resulting from periarterial edema or arterial stenosis), using the specific site of the finding on the duplex ultrasound image as the physical target for the biopsy. The site on the patient’s temple was marked with permanent marker at the time of duplex ultrasonography and kept in place until the biopsy was performed. Patients with a normal result of duplex ultrasonography were treated clinically by the referring physician on the basis of their pretest suspicion of GCA (Figure 2). This choice was made under the assumption, based on a literature review, that there would be few cases in which the duplex ultrasound result was normal that the subsequent biopsy result would be abnormal.13
Figure 2.
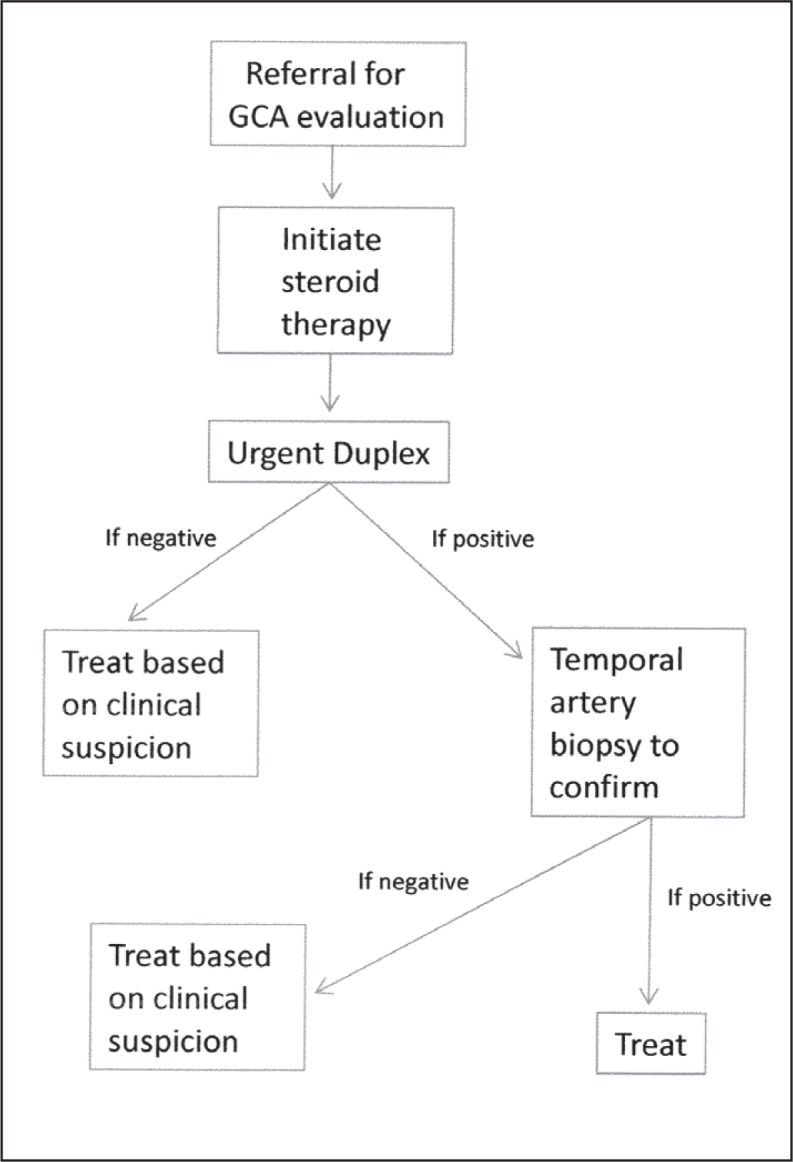
Initial protocol revision.
GCA = giant cell arteritis; Duplex = duplex ultrasound imaging.
All patients with abnormality found on the duplex ultrasound image ultimately received treatment, regardless of the eventual biopsy result.
Three questions arose and were addressed:
Did a normal duplex ultrasound result have the same impact on treatment decisions as a normal biopsy result had in the past? In other words, since most studies of either type, biopsy or duplex ultrasonography, will be normal, did it make a difference in treatment planning which study had been done?
Did an abnormal duplex ultrasound image result correlate with an abnormal biopsy result? If so, perhaps biopsy would not be necessary after an abnormal ultrasound result.
Were any cases of GCA missed with the new protocol?
Results for Pilot Protocol
Satisfactory answers to all three questions suggested that duplex ultrasound imaging was filling the same role in treatment planning as biopsy and that perhaps duplex ultrasonography could replace biopsy as the diagnostic test of choice for GCA.
In the first year of the protocol, 55 duplex ultrasound image studies were ordered, all of which, before the use of color duplex ultrasonography, would likely instead have been biopsies initially. In 6 of the 55 duplex ultrasound images, results were either abnormal (halo present) or equivocal (arterial stenosis or non-diagnostic). The rest had normal findings. Of the 6 biopsies performed because of an abnormal duplex ultrasound image, 3 showed pathologic signs of arteritis and 3 did not. The number of biopsies performed was markedly reduced, and 50% of the small number of biopsies had abnormal results rather than the prior rate of 8.5%.
The impact of normal duplex ultrasound results was reviewed by comparing the first 147 patients who had a normal duplex ultrasound image against a historic control group of 143 patients who had had a normal result of a unilateral temporal artery biopsy but no color duplex ultrasonography in the 3 years before initiating the new protocol.14 It was found that referring clinicians demonstrated no differences in treatment decisions based on whether the normal result came from a biopsy in the historic group or duplex ultrasound image in the experimental group. (Corticosteroid treatment was stopped in 80% of patients after normal biopsy results in the historic group and in 87% of patients after normal duplex ultrasound results in the experimental group.) No cases of GCA were missed, as determined by reviewing both the diagnostic database for this condition and medical-legal files for claims or potentially compensable events.
Phase 2: New Algorithm
The department was aware at the time that no protocol such as this (to biopsy only those patients with an abnormal duplex ultrasound result) had been tested in a prospective randomized way. Also, there was no consensus support by the American College of Rheumatology for moving away from temporal artery biopsy as the primary means of evaluation for GCA, although a variety of imaging modalities were being actively discussed in leading rheumatology journals as well as at the national congresses.15 Regardless, it seemed that the prior “biopsy only” protocol had not been optimal.
The task was to determine—using a combination of the Region’s clinical experience, the scientific data, a desire to provide the safest and most effective care for patients, and mindfulness of resource availability—which protocol was best for evaluation of GCA. The goal was to do so in a way that was acceptable to all clinical stakeholders who evaluate and treat this disease.
Working Toward Consensus
A multidisciplinary group was convened in October 2007, that included representation from the Departments of Rheumatology, Neurology, Ophthalmology, General Surgery, Primary Care, Information Technology, Evidence-Based Medicine, Population Care, and Medical/Legal. There were three explicit goals in designing a final protocol:
ensure the rapid and appropriate evaluation of GCA without missing cases
reduce the number of temporal artery biopsies from the original baseline
increase the rate of abnormal biopsy results (what we called the biopsy “positive” rate) by improving the quality of referrals for temporal artery biopsy.
The department did not limit consideration to only certain modalities or processes. Instead it tried to elicit the specific concerns posed by each stakeholder. The discussion was not without contention. It was unclear whether duplex ultrasonography of the temporal artery could justifiably be considered appropriate screening for GCA given the controversy in the medical literature despite favorable findings. Yet it was also unclear why a rate of abnormal biopsy results far below the rate in the published literature had occurred in the Region’s diagnostic workup before starting the ultrasound protocol. There was general agreement to consider changes that would benefit KPNW’s patients.
Changes to the Algorithm
The first point of consensus was that the low rate of abnormal biopsy results was being influenced by the appropriateness of patient selection for GCA workup. It was agreed that the first step would be to add specific clinical expertise to the patient evaluation process before referral for further diagnostic evaluation. This “screen” would likely decrease the number of patients who required any additional diagnostic test. The Department of Rheumatology agreed to provide real-time feedback by telephone to any clinician who was evaluating a patient suspected of having GCA. To facilitate this process, a broad communication was made to all primary care clinicians about the new workflow. A new internal referral in HealthConnect for temporal artery biopsy was made so that any referral option for biopsy, rather than initiating a referral to surgery, led to specific advice to page the rheumatologist on call. The rheumatologist would discuss the case, review the chart, and give guidance regarding any further workup. This clinician-to-clinician communication also allowed a consideration of differential diagnoses for patients who were unlikely to have the disease. The ability to directly order diagnostic studies for GCA was removed from all but the rheumatologists.
Initially there was some trepidation that the influx of advice calls would overwhelm the capacity of the Rheumatology Department to handle these in a timely fashion. Fortunately, this did not occur. In fact, both rheumatologists and primary care physicians found this to be a professionally satisfying exchange of information and a benefit to the patient. It was agreed, therefore, that only the rheumatologist would determine additional workup (either duplex ultrasonography or biopsy, or both) after discussion with the referring physicians. After several months into the protocol, it was agreed that other specialists with experience evaluating patients with GCA (neurologists and ophthalmologists) would also be given access to order duplex ultrasonography or biopsy without rheumatology review. In exchange, the General Surgery Department agreed to biopsy any patient requested by this group of clinicians as expeditiously as possible if that was the choice of the referring specialist.
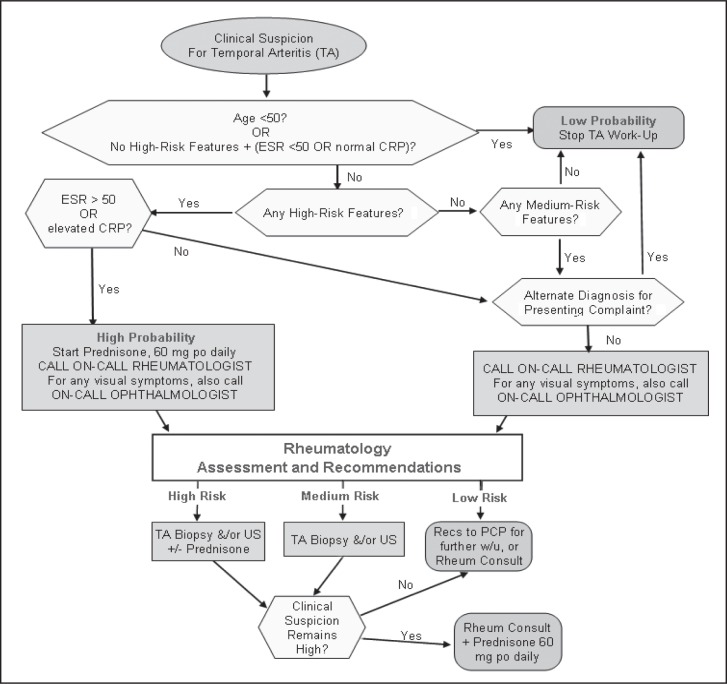
The second point of consensus proved more difficult. Should temporal artery duplex ultrasonography, or any other imaging modality, remain as part of the diagnostic algorithm, and if so, for which patients, given that it had not been accepted as standard practice by the medical community? Through compromise and literature review, it was agreed that it would be left to the discretion of the referring specialist (rheumatologist, neurologist, or ophthalmologist) to determine the next diagnostic modality. The referring specialist would first stratify patients into low, medium, or high risk of GCA on the basis of chart review and discussion with the referring clinician. Then, on the basis of this determination of risk, the specialist would decide the type of evaluation (duplex ultrasonography, biopsy, or both) that would best guide treatment decisions. The new algorithm is shown in Figure 3.
Figure 3.
Current algorithm that includes clinical risk stratification.
CRP = C-reactive protein; ESR = erythrocyte sedimentation rate; po = orally; PCP = primary care provider; Recs = recommendations; Rheum = rheumatology; US = ultrasound image; w/u = workup; +/− = with or without.
The dilemma of temporal arteritis is its diagnosis. If clinicians were able to accurately identify the disease when present, the treatment (corticosteroids) would clearly outweigh the risk of the disease (blindness).
Results of New Algorithm
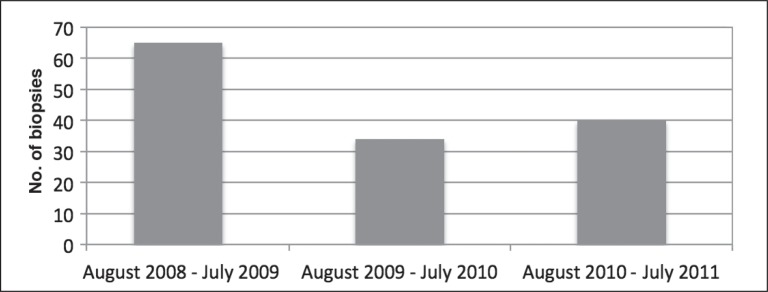
The results of the new algorithm, which included a specialist’s evaluation and color duplex ultrasonography when thought to be appropriate, and biopsy if requested, demonstrated an impressive reduction in the number of temporal artery biopsies performed (Figure 4). Of perhaps greater importance was that no known cases of temporal arteritis were missed.
Figure 4.
Total number of temporal artery biopsies done for first 3 years after starting new protocol (August 2008–July 2011).
With the declining number of biopsies, the rate of abnormal biopsy results rose from the baseline of 8.5% to 24%, more in keeping with the rate reported in the published literature. The rate of congruence between duplex ultrasound and biopsy results, when both were obtained, also was consistent with published expected results. In 42 of 60 cases (70%), when both duplex ultrasonography and biopsy were performed, the ultrasound result agreed with the biopsy result (Table 2). In 5 cases, the ultrasound finding was reported as normal, but the biopsy result was ultimately abnormal; whereas in 6 cases, the ultrasound result was abnormal, but the biopsy was normal. It was concluded that, although not perfect, the addition of 2 measures, careful clinical evaluation and the option of ultrasonography before, or instead of, biopsy, was accomplishing our goals. These two measures continue to be used.
Table 2.
Rate of congruence between duplex ultrasound and biopsy result
| Month/year | Counts | Fractions of total (%) | ||||
|---|---|---|---|---|---|---|
| Congruent | Equivocal | Discordant | Congruent | Equivocal | Discordant | |
| August 2008–July 2009 | 21 | 2 | 6 | 72 | 7 | 21 |
| August 2009–July 2010 | 13 | 1 | 3 | 76 | 6 | 18 |
| August 2010–July 2011 | 8 | 4 | 2 | 57 | 29 | 14 |
| Total/average | 42 | 7 | 11 | 70 | 12 | 18 |
Discussion
The dilemma of temporal arteritis is its diagnosis. If clinicians were able to accurately identify the disease when present, the treatment (corticosteroids) would clearly outweigh the risk of the disease (blindness). However, without a singular diagnostic test of acceptable sensitivity and specificity for GCA, it must ultimately remain a diagnosis made by careful clinical evaluation in conjunction with judicious use of other diagnostic modalities. Temporal artery biopsy, the traditional mainstay of diagnosis, is of value only when the result is abnormal, an occurrence that happens in less than half (often much less than half) of the biopsies under the best of circumstances.16
The primary goal was to improve the diagnostic process for both patients and clinicians. This was accomplished by reducing the total number of invasive procedures (biopsies) performed through limiting them only to the patients for whom the result would be meaningful, and by increasing the use of noninvasive imaging that might accomplish the same goal. It was critical that, in changing the protocol, no patient with treatable disease was missed. It was accepted that the protocol runs counter to the current practice in most institutions, but the department was willing to challenge the widely held pattern of temporal artery biopsy for all patients suspected of having GCA.
Our multidisciplinary discussions resulted in two changes to the patient evaluation protocol, which continues to the present:
Direct, real-time assessment by a rheumatologist, by chart review and conversation with the referring clinician (with the exception of ophthalmologists and neurologists), for every patient referred by a primary care physician for GCA evaluation
Temporal artery color duplex ultrasonography for patients in whom, in combination with the level of baseline clinical suspicion, GCA could safely be ruled in or out on the basis of duplex ultrasound result (typically high clinical suspicion/abnormal duplex ultrasound result or low clinical suspicion/normal duplex ultrasound result, respectively), and the patient therefore could avoid a biopsy. Biopsy remains available at the specialist’s discretion, typically for duplex ultrasound results that conflict with clinical suspicion.
Which change was most responsible for the reduction in biopsies was not specifically evaluated. It was neither anticipated nor expected that duplex ultrasonography would completely replace temporal artery biopsy or clinical evaluation, only to supplement them and allow biopsy to be used when the result would be useful for treatment decisions.
Our primary use of duplex ultrasonography has been to rule out patients with low clinical suspicion of GCA. Evidence exists to support ruling out “low probability” patients who have a normal duplex ultrasound result.17 For these patients, who would not be subjected to treatment without an abnormal test result, duplex ultrasonography may play a key role. Other institutions have adopted this practice.18
There is increasing evidence, however, that duplex ultrasonography can be used to rule in the disease. The most recent data show that the “halo sign” on duplex ultrasound image is highly specific for GCA (81% for unilateral halo and 100% for bilateral halo), and therefore biopsy adds nothing to the evaluation in these cases.12,19 In fact, it would be difficult to imagine the justification not to treat a patient with an abnormal duplex ultrasound result, regardless of biopsy result, given biopsy’s high false-negative rate. With greater limitation of surgical resources, it becomes difficult to defend continuing to perform invasive procedures such as biopsy if the result will not influence the treatment decision. Some European rheumatology centers have preceded this institution in adjusting their algorithm to include duplex ultrasonography as the primary means for evaluation of GCA. It has been less accepted in the US. In fact, the American College of Rheumatology, in its formal information to patients,20 makes no mention of duplex ultrasonography.
Wolfgang Schmidt, MD, a leading international advocate of duplex ultrasonography for the diagnosis of GCA, practicing in Berlin, has used this modality as the primary diagnostic tool for several years. He communicated the following (WA Schmidt, MD, personal communication, 2012 May 6)a:
We offer a daily ultrasound clinic. Physicians who are suspecting temporal arteritis/giant cell arteritis can call our Ultrasound Department and patients receive an appointment within 24 hours of a working day (Monday to Friday). Here patients first see an experienced rheumatologist. We do a standardized clinical history and short clinical examination (eg, palpation of temporal arteries). We review previous examinations (eg, for ESR [erythrocyte sedimentation rate] or CRP [C-reactive protein]) and then examine the temporal and axillary arteries with ultrasound. At the end more than 95% receive a clear yes or no with regard to the diagnosis of giant cell arteritis. We send only very few patients with ambivalent findings to biopsy. We have 15 minutes for an appointment. In general, this is enough time to confirm or exclude giant cell arteritis.
This institution has not yet adopted such an aggressive protocol or investigated adding axillary artery ultrasound imaging to its workup. However, there is ample evidence to suggest that temporal artery duplex may be an important and expedient tool which, when coupled with clinical evaluation, could make the diagnosis of GCA a nearly immediate and painless experience for patients. KPNW has a unique opportunity to use its integrated care delivery system and electronic medical record to increase efficiencies and safety for its patients. This may be an excellent example of patient-centered care that challenges the established practice and sets a new community standard. Ultimately, the success of this program relies on the multidisciplinary collaboration that comes from thoughtful discussion, testing new and innovative practices, and a review of available scientific and clinical evidence. Through these methods the prevailing practice to improve the care of patients could change and improve.
Acknowledgments
The author acknowledges Ariel K Hill, MS, who provided research assistance for this project.
Kathleen Louden, ELS, of Louden Health Communications provided editorial assistance.
Footnotes
Professor of Medicine, Medical Center for Rheumatology, Berlin-Buch, Germany
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Mansoor O, Majeed T. A 90 year old woman with painless vision loss. Digital Journal of Ophthalmology. [serial on the Internet] 2005 Feb 10 [cited 2012 Oct 31];11(7): Figure 2. Available from: www.djo.harvard.edu/site.php?url=/physicians/gr/728&page=GR_TT. [Google Scholar]
- 2.González-Gay MA, Garcia-Porrua C, Rivas MJ, Rodriguez-Ledo P, Llorca J. Epidemiology of biopsy proven giant cell arteritis in northwestern Spain: trend over an 18 year period. Ann Rheum Dis. 2001 Apr;60(4):367–71. doi: 10.1136/ard.60.4.367. DOI: http://dx.doi.org/10.1136/ard.60.4.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Butteriss DJ, Clarke L, Dayan M, Birchall D. Use of colour duplex ultrasound to diagnose giant cell arteritis in a case of visual loss of uncertain aetiology. Br J Radiol. 2004 Jul;77(919):607–9. doi: 10.1259/bjr/22460193. DOI: http://dx.doi.org/10.1259/bjr/22460193. [DOI] [PubMed] [Google Scholar]
- 4.Schmidt WA, Blockmans D. Use of ultrasonography and positron emission tomography in the diagnosis and assessment of large-vessel vasculitis. Curr Opin Rheumatol. 2005 Jan;17(1):9–15. doi: 10.1097/01.bor.0000147282.02411.c6. DOI: http://dx.doi.org/10.1097/01.bor.0000147282.02411.c6. [DOI] [PubMed] [Google Scholar]
- 5.Narváez J, Narváez JA, Nolla JM, Sirvent E, Reina D, Valverde J. Giant cell arteritis and polymyalgia rheumatica: usefulness of vascular magnetic resonance imaging studies in the diagnosis of aortitis. Rheumatology (Oxford) 2005 Apr;44(4):479–83. doi: 10.1093/rheumatology/keh513. DOI: http://dx.doi.org/10.1093/rheumatology/keh513. [DOI] [PubMed] [Google Scholar]
- 6.Ashton-Key MR, Gallagher PJ. False-negative temporal artery biopsy. Am J Surg Pathol. 1992 Jun;16(6):634–5. doi: 10.1097/00000478-199206000-00014. DOI: http://dx.doi.org/10.1097/00000478-199206000-00014. [DOI] [PubMed] [Google Scholar]
- 7.van der Straaten D, Rajakulenthiran M, McKelvie PA, O’Day J.A case of biopsy-negative temporal arteritis—diagnostic challenges Surv Ophthalmol 2004November–Dec496603–7.DOI: http://dx.doi.org/10.1016/j.survoph-thal.2004.08.005 [DOI] [PubMed] [Google Scholar]
- 8.Hunder GG, Bloch DA, Michel BA, et al. The American College of Rheumatology 1990 criteria for the classification of giant cell arteritis. Arthritis Rheum. 1990 Aug;33(8):1122–8. doi: 10.1002/art.1780330810. DOI: http://dx.doi.org/10.1002/art.1780330810. [DOI] [PubMed] [Google Scholar]
- 9.Bhatti MT, Goldstein MH. Facial nerve injury following superficial temporal artery biopsy. Dermatol Surg. 2001 Jan;27(1):15–7. DOI: http://dx.doi.org/10.1046/j.1524-4725.2001.00258.x. [PubMed] [Google Scholar]
- 10.Lenton J, Donnelly R, Nash JR. Does temporal artery biopsy influence the management of temporal arteritis? QJM. 2006 Jan;99(1):33–6. doi: 10.1093/qjmed/hci141. DOI: http://dx.doi.org/10.1093/qjmed/hci141. [DOI] [PubMed] [Google Scholar]
- 11.Taylor-Gjevre R, Vo M, Shukla D, Resch L. Temporal artery biopsy for giant cell arteritis. J Rheumatol. 2005 Jul;32(7):1279–82. [PubMed] [Google Scholar]
- 12.Karahaliou M, Vaiopoulos G, Papaspyrou S, Kanakis MA, Revenas K, Sfikakis PP. Colour duplex sonography of temporal arteries before decision for biopsy: a prospective study in 55 patients with suspected giant cell arteritis. Arthritis Res Ther. 2006 Jul;8(4):R116. doi: 10.1186/ar2003. DOI: http://dx.doi.org/10.1186/ar2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.LeSar CJ, Meier GH, DeMasi RJ, et al. The utility of color duplex ultrasonography in the diagnosis of temporal arteritis. J Vasc Surg. 2002 Dec;36(6):1154–60. doi: 10.1067/mva.2002.129648. DOI: http://dx.doi.org/10.1067/mva.2002.129648. [DOI] [PubMed] [Google Scholar]
- 14.Alberts MS, Mosen DM. Diagnosing temporal arteritis: duplex vs biopsy. QJM. 2007 Dec;100(12):785–9. doi: 10.1093/qjmed/hcm103. DOI: http://dx.doi.org/10.1093/qjmed/hcm103. [DOI] [PubMed] [Google Scholar]
- 15.Brodmann M, Lipp RW, Passath A, Seinost G, Pabst E, Pilger E. The role of 2-18F-fluoro-2-de-oxy-D-glucose positron emission tomography in the diagnosis of giant cell arteritis of the temporal arteries. Rheumatology (Oxford) 2004 Feb;43(2):241–2. doi: 10.1093/rheumatology/keh025. DOI: http://dx.doi.org/10.1093/rheumatology/keh025. [DOI] [PubMed] [Google Scholar]
- 16.Goslin BJ, Chung MH. Temporal artery biopsy as a means of diagnosing giant cell arteritis: is there over-utilization? Am Surg. 2011 Sep;77(9):1158–60. [PubMed] [Google Scholar]
- 17.Karassa FB, Matsagas MI, Schmidt WA, Ioannidis JP. Meta-analysis: test performance of ultrasonography for giant-cell arteritis. Ann Intern Med. 2005 Mar 1;142(5):359–69. doi: 10.7326/0003-4819-142-5-200503010-00011. [DOI] [PubMed] [Google Scholar]
- 18.Meisner RJ, Labropoulos N, Gasparis AP, Tassiopoulos AK. How to diagnose giant cell arteritis. Int Angiol. 2011 Feb;30(1):58–63. [PubMed] [Google Scholar]
- 19.Habib HM, Essa AA, Hassan AA. Color duplex ultrasonography of temporal arteries: role in diagnosis and follow-up of suspected cases of temporal arteritis. Clin Rheumatol. 2012 Feb;31(2):231–7. doi: 10.1007/s10067-011-1808-0. DOI: http://dx.doi.org/10.1007/s10067-011-1808-0. [DOI] [PubMed] [Google Scholar]
- 20.Atlanta, GA: American College of Rheumatology; 2012. Giant cell arteritis [monograph on the Internet] [cited 2012 Oct 31]. Available from: www.rheumatology.org/practice/clinical/patients/diseases_and_conditions/giantcellarteritis.asp. [Google Scholar]