Abstract
Angiocentric glioma is a recently described tumor recognized since 2007 by the World Health Organization Classification of Tumours of the Central Nervous System. We present the only case of angiocentric glioma at our institution in the last 15 years and review the literature in an attempt to establish prognostic parameters. Our search revealed only 27 cases of angiocentric glioma in the literature. The most common presenting symptom of angiocentric glioma was seizures. Gross total resection of the lesion was curative, without need for radiation or chemotherapy.
Introduction
Angiocentric glioma is a newly described brain tumor1 initially identified in 2005 in two separate case reports.2,3 In 2007, it was classified in the World Health Organization Classification of Tumours of the Central Nervous System.1 Since its initial description, only seven more reports have been described in the literature.4–10 This tumor has unique clinical, radiographic, and histopathologic features that distinguish it from other pediatric brain tumors, mostly with respect to prognosis and quality of life.
Angiocentric glioma has been described as a slow-growing neoplasm found primarily in a supratentorial location. This lesion appears very similar to a low-grade glioma on radiographs. Seizures are often the first clinical sign. On histologic examination, this entity is found to be composed of relatively cytologically bland, monomorphic spindle cells with a diffusely infiltrative and characteristic perivascular growth pattern, quite similar in many respects to an ependymoma. It also has variable immunoreactivity to glial fibrillary acidic protein.
Clinical data regarding this rare entity are lacking, and this limits our understanding of the symptoms and the necessary treatment and long-term follow-up. A more thorough review of the literature was performed to accurately predict the appropriate treatment and follow-up for these rare tumors. We present the case of a 12-year-old girl who presented with intractable seizures and an angiocentric glioma within her expressive speech area in the left temporal lobe. We also performed a literature review in an effort to establish prognostic parameters.
History and Examination
A 12-year-old, right-handed girl presented with history of seizures consisting of mumbling episodes and postictal confusion. The seizures occurred several times per month. The physical examination was unremarkable. Magnetic resonance imaging (MRI) of the brain with and without contrast revealed a left frontotemporal, nonenhancing lesion (T1 hypointense, fluid-attenuated inversion recovery [FLAIR] hyperintense; Figure 1). The child underwent functional MRI, which showed that the tumor was growing within the speech area, dividing it into anterior and posterior portions. Low-grade glioma was diagnosed after biopsy. Because the patient continued to have seizures refractory to medication, awake craniotomy was performed to resect the lesion. At the time of the operation, we noticed that the tumor was discolored and soft and distinctly different from the surrounding brain tissue. The tumor was easily dissected from the brain. During the surgery, an Ojemann stimulator was used to locate the patient’s expressive speech areas. Surprisingly, the tumor straddled the expressive speech region, and it was evident during surgery that the patient had two separate areas controlling expressive speech. Both were located in the inferior frontal gyrus. Once gross total resection was achieved, a grid with surface electrodes was placed for invasive electroencephalogram monitoring to further investigate the seizure foci. No further seizures were identified and the grid was removed after 4 days. The patient’s postoperative course was uncomplicated, without speech impairments. At 3-month follow-up, the patient continued to be seizure free and without speech difficulties. Postoperative MRI revealed gross total resection of the tumor.
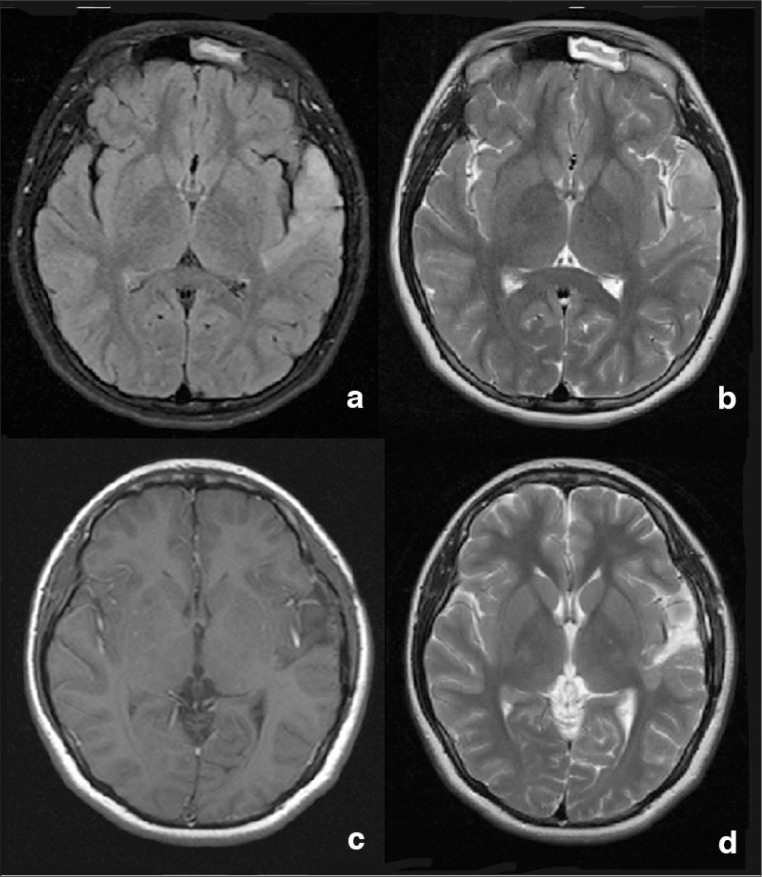
Figure 1.
Magnetic resonance imaging of the brain.
a) axial fluid-attenuated inversion recovery (FLAIR) MR image showing tumor signal; b) axial T2-weighted MR image obtained at the same level, demonstrating the hyperintense tumor signal; c) postoperative T1-weighted MR image with contrast; and d) postoperative T2 MR image showing complete resection of the tumor.
Histopathologic Examination
The final pathologic assessment revealed a neoplasm consistent with angiocentric glioma. On histopathologic analysis, the tumor exhibited a diffusely infiltrating pattern, with variable glial fibrillary acidic protein immunohistochemistry. Staining with epithelial membrane antigen demonstrated microlumens. The tumor also had cytologically bland, monomorphic spindle cells and a characteristic perivascular growth pattern, similar in some respects to ependymoma. These features were very consistent with angiocentric glioma (Figure 2).
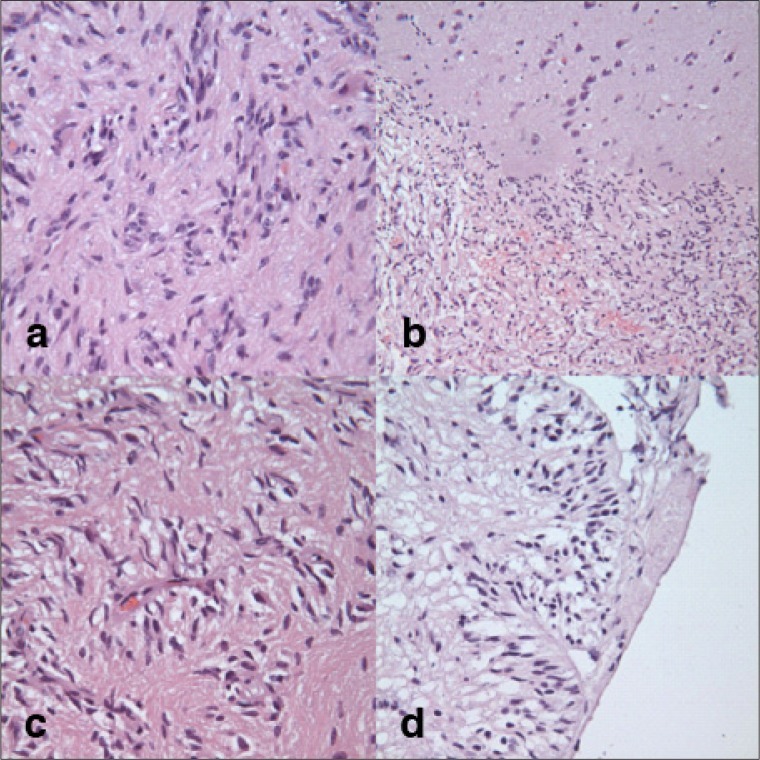
Figure 2.
Pathology evaluation of tumor.
a) Hematoxylin and eosin stain showing tumor cells; b) interface between the brain and the tumor; c) tumor cells that form pseudorosette type structures around the blood vessels; and d) tumor cells in subpial locations arranged in radial arrays perpendicular to the pial surface.
Discussion
This unique case is the first angiocentric glioma treated at our institution and the first reported case of angiocentric glioma within a speech area. The patient presented with intractable seizure, which affected her quality of life. Surgical resection of the tumor led to excellent seizure control.
A literature review revealed 27 cases of angiocentric glioma (Table 1).3,9–11 Ninety percent of these patients presented with intractable seizures; only 3 patients had different symptoms. One patient had decreased vision and headaches,10 another patient had otalgia,4 and the third patient was ataxic.6 In the majority of cases, it appeared that the tumor was in a supratentorial location: 10 tumors in the frontal lobe, 8 in the temporal lobe, 7 in the parietal lobe, and 1 in the occipital lobe. In 1 case the tumor originated in the brainstem.6 Our patient presented with a tumor in her expressive speech area. Age at surgery, as reported in the literature, ranged from 2 to 14 years.
Table 1.
Summary of reported cases of angiocentric glioma
| Case report | Age, years | Sex | Symptoms | Tumor location | Surgery | Follow-up, month | Seizure recurrence | Adjuvant therapy |
|---|---|---|---|---|---|---|---|---|
| Present case | 12 | F | Seizures | Left frontotemporal lobe speech area | GTR | 3 | N | None |
| Rho et al4 | 10 | F | Dizziness, otalgia | Right medial frontal cortex | GTR | 9 | N | None |
| Fulton et al5 | 2 | M | Seizures | Right frontoparietal parasagittal lesion | STR | 14 | N | None |
| Covington et al6 | 5 | F | Ataxia | Brainstem exophytic tumor | STR | 24 | N | None |
| Shakur et al10 Case 1 | 10 | M | Headache, decreasing vision | Left posterior temporal lobe | GTR | 24 | N | None |
| Shakur et al10 Case 2 | 10 | M | Seizures | Left middle temporal lobe | GTR | 9 | N | None |
| Shakur et al10 Case 3 | 13 | F | Seizures | Left anterior temporal lobe | GTR | 6 | N | None |
| Sugita et al11 | 6 | M | Seizures | Right occipitoparietal lobe | GTR | 9 | N | None |
| Lum et al8 | 5 | M | Seizures, headaches | Right inferomedial posterior frontal lobe | GTR | Unknown | N | None |
| Preusser et al9 Case 1 | 6 | M | Seizures | Medial temporal lobe | STR | 60 | N | None |
| Preusser et al9 Case 2 | 8 | F | Seizures | Medial inferior temporal lobe | STR | 60 | N | None |
| Preusser et al9 Case 3 | 3 | M | Seizures | Precuneus | STR | 60 | N | None |
| Preusser et al9 Case 4 | 12 | F | Seizures | Precuneus | STR | 7 | N | Radiation |
| Preusser et al9 Case 5 | 14 | M | Seizures | Frontoparietal lobe | Biopsy | 24 | N | Radiation |
| Lellouch-Tubiana et al2 Case 1 | 6.5 | M | Seizures | Left frontoparietal lobe | GTR | 168 | N | None |
| Lellouch-Tubiana et al2 Case 2 | 2 | M | Seizures | Right frontoparietal lobe | STR | 36 | Y | None |
| Lellouch-Tubiana et al2 Case 3 | 4 | F | Seizures | Left medial temporal lobe | GTR | 72 | N | None |
| Lellouch-Tubiana et al2 Case 4 | 4.5 | M | Seizures | Right parietal lobe | GTR | 60 | N | None |
| Lellouch-Tubiana et al2 Case 5 | 9.5 | F | Seizures | Left frontal lobe | GTR | 2.3 | N | None |
| Lellouch-Tubiana et al2 Case 6 | 13 | F | Seizures | Right orbitofrontal lobe, gyrus rectus, insula | STR | 24 | Y | None |
| Lellouch-Tubiana et al2 Case 7 | 12 | M | Seizures | Right parietal lobe | GTR | 24 | N | None |
| Lellouch-Tubiana et al2 Case 8 | 3 | F | Seizures | Left frontal lobe | STR | 14 | Y | None |
| Lellouch-Tubiana et al2 Case 9 | 10 | M | Seizures | Left frontolateral lobe, premotor cortex | STR | 12 | Y | None |
| Wang et al3 Case 1 | 11 | F | Seizures | Right medial temporal lobe | GTR | 12 | N | None |
| Wang et al3 Case 2 | 4 | F | Seizures | Right parietal lobe | GTR | 48 | N | None |
| Wang et al3 Case 3 | 3 | M | Seizures | Left occipital lobe | STR | 36 | N | None |
| Wang et al3 Case 4 | 10 | M | Seizures | Right inferior frontal lobe | GTR | 24 | N | None |
| Wang et al3 Case 5 | 3 | F | Seizures | Left inferior frontal lobe | STR | 24 | N | None |
GTR = gross total resection; STR = subtotal resection
The literature indicates that gross total resection of this lesion is curative. Sixteen of the 27 patients underwent gross total re-section, and they all had complete seizure control without tumor recurrence. Reviewing the data, we also identified 9 patients who had only subtotal resection. Of these, 4 patients had seizure recurrence. The addition of radiation therapy to subtotal resection appeared to result in complete control of seizures.9 Only 1 of 27 patients had biopsy followed by radiation. At 2-year follow-up, the patient continued to be seizure free.
Angiocentric gliomas have a very indolent clinical course. Even after subtotal tumor resection, the lesion did not increase in size. One case report described subtotal resection of a brainstem angiocentric glioma in a 5 year old. Two years after resection, without chemotherapy or radiation, the patient was doing well, and MRI showed that the tumor remained stable.6 In one case the tumor recurred and the histopathologic evaluation revealed an anaplastic (World Health Organization grade III) lesion that was ultimately fatal.3
All reviewed cases of angiocentric glioma were hyperintense on T2-weighted sequences and did not enhance with contrast on T1-weighted MRI. Lellouch-Tubiana et al2 and Preusser et al9 described a cortical rim of hyperintensity on T1-weighted images. They also reported stalklike extensions to the adjacent ventricle on T2 imaging. These findings were asserted to be pathognomonic of angiocentric glioma.10 However, these hallmarks were not reported in other cases.
On histopathologic examination, angiocentric gliomas are composed of diffusely infiltrating, monomorphic, bipolar spindle cells arranged around blood vessels in concentric sleeves and pseudorosettes, demonstrating an angiocentric pattern. Immunohistochemical staining results are typically positive for glial fibrillary acidic protein, S-100, and vimentin and are consistent with a dotlike pattern for epithelial membrane antigen. This tumor is also very similar to other benign brain tumors such as focal cortical dysplasias, gangliogliomas, and dysembryoplastic neuroepithelial tumors. The lineage of this tumor is unclear. Wang et al proposed that the tumor could be from both astrocytic and ependymal lineages.3 Lellouch-Tubiana et al postulated that the tumor could arise from radial glia or be of a neuronal origin.2 This tumor has been observed to behave as a low-grade neoplasm. This is probably why gross total resection has been curative in most documented cases. An analysis of the data clearly shows that the extent of resection appears to determine survival as well as complete control of seizures.
Conclusions
Angiocentric glioma is a very rare entity with unique histologic features. This tumor is associated with a more favorable prognosis than other gliomas. On the basis of our review of the literature, it appears that surgery is curative for this tumor. A gross total resection can achieve complete control of seizures and also prevent recurrence. However, longer follow-up periods are needed to accurately establish the time to recurrence and to determine what additional treatment is needed, such as adjuvant chemotherapy or radiation, for this newly described brain tumor.
Acknowledgments
Leslie Parker, ELS, provided editorial assistance.
Footnotes
Disclosure Statement
The author(s) have no conflicts of interest to disclose.
References
- 1.Brat DJ, Scheithauer BW, Fuller GN, Tihan T. Newly codified glial neoplasms of the 2007 WHO Classification of Tumours of the Central Nervous System: angiocentric glioma, pilomyxoid astrocytoma and pituicytoma. Brain Pathol. 2007 Jul;17(3):319–24. doi: 10.1111/j.1750-3639.2007.00082.x. DOI: http://dx.doi.org/10.1111/j.1750-3639.2007.00082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lellouch-Tubiana A, Boddaert N, Bourgeois M, et al. Angiocentric neuroepithelial tumor (ANET): a new epilepsy-related clinicopathological entity with distinctive MRI. Brain Pathol. 2005 Oct;15(4):281–6. doi: 10.1111/j.1750-3639.2005.tb00112.x. DOI: http://dx.doi.org/10.1111/j.1750-3639.2005.tb00112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang M, Tihan T, Rojiani AM, et al. Monomorphous angiocentric glioma: a distinctive epileptogenic neoplasm with features of infiltrating astrocytoma and ependymoma. J Neuropathol Exp Neurol. 2005 Oct;64(10):875–81. doi: 10.1097/01.jnen.0000182981.02355.10. DOI: http://dx.doi.org/10.1097/01.jnen.0000182981.02355.10. [DOI] [PubMed] [Google Scholar]
- 4.Rho GJ, Kim H, Kim HI, Ju MJ. A case of angiocentric glioma with unusual clinical and radiological features. J Korean Neurosurg Soc. 2011 Jun;49(6):367–9. doi: 10.3340/jkns.2011.49.6.367. DOI: http://dx.doi.org/10.3340/jkns.2011.49.6.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fulton SP, Clarke DF, Wheless JW, Ellison DW, Ogg R, Boop FA. Angiocentric glioma-induced seizures in a 2-year-old child. J Child Neurol. 2009 Jul;24(7):852–6. doi: 10.1177/0883073808331078. DOI: http://dx.doi.org/10.1177/0883073808331078. [DOI] [PubMed] [Google Scholar]
- 6.Covington DB, Rosenblum MK, Brathwaite CD, Sandberg DI. Angiocentric glioma-like tumor of the midbrain. Pediatr Neurosurg. 2009;45(6):429–33. doi: 10.1159/000277616. DOI: http://dx.doi.org/10.1159/000277616. [DOI] [PubMed] [Google Scholar]
- 7.Louis DN, Ohgaki H, Wiestler OD, et al. The 2007 WHO classification of tu-The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol. 2007 Aug;114(2):97–109. doi: 10.1007/s00401-007-0243-4. DOI: http://dx.doi.org/10.1007/s00401-007-0243-4 Erratum in: Acta Neuropathol 2007 Nov;114(5):547. DOI: http://dx.doi.org/10.1007/s00401-007-0278-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lum DJ, Halliday W, Watson M, Smith A, Law A. Cortical ependymoma or monomorphous angiocentric glioma? Neuropathology. 2008 Feb;28(1):81–6. doi: 10.1111/j.1440-1789.2007.00831.x. DOI: http://dx.doi.org/10.1111/j.1440-1789.2007.00831.x. [DOI] [PubMed] [Google Scholar]
- 9.Preusser M, Hoischen A, Novak K, et al. Angiocentric glioma: report of clinico-pathologic and genetic findings in 8 cases. Am J Surg Pathol. 2007 Nov;31(11):1709–18. doi: 10.1097/PAS.0b013e31804a7ebb. DOI: http://dx.doi.org/10.1097/PAS.0b013e31804a7ebb. [DOI] [PubMed] [Google Scholar]
- 10.Shakur SF, McGirt MJ, Johnson MW, et al. Angiocentric glioma: a case series. J Neurosurg Pediatr. 2009 Mar;3(3):197–202. doi: 10.3171/2008.11.PEDS0858. DOI: http://dx.doi.org/10.3171/2008.11.PEDS0858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sugita Y, Ono T, Ohshima K, et al. Brain surface spindle cell glioma in a patient with medically intractable partial epilepsy: a variant of monomorphous angiocentric glioma? Neuropathology. 2008 Oct;28(5):516–20. doi: 10.1111/j.1440-1789.2007.00849.x. DOI: http://dx.doi.org/10.1111/j.1440-1789.2007.00849.x. [DOI] [PubMed] [Google Scholar]