Abstract
Purpose
Laparoscopic gastrectomy has been adopted for the treatment of gastric cancer, and despite the technical difficulties, totally laparoscopic distal gastrectomy has been considered less invasive than laparoscopy-assisted distal gastrectomy. Although there have been many reports regarding the feasibility and safety of totally laparoscopic distal gastrectomy at large volume centers, few reports have been conducted at low-volume centers. The purpose of this study is to try to assess the feasibility and safety of totally laparoscopic distal gastrectomy at a low volume center through the analysis of short-term outcomes of totally laparoscopic distal gastrectomy compared with laparoscopy-assisted distal gastrectomy.
Materials and Methods
The clinical data and short-term surgical outcomes of 35 patients who had undergone laparoscopy-assisted distal gastrectomy between April 2007 and March 2010, and 37 patients who underwent totally laparoscopic distal gastrectomy between April 2010 and August 2012 were retrospectively reviewed.
Results
There was no significant difference in the demographic and clinical data. However the reconstruction method and extent of lymphadenectomy showed statistically significant differences. Operation time and estimated blood loss did not show significant differences. Surgical and medical complications did not show significant differences but postoperative courses including time-to-first oral intake and postoperative hospital stay were significantly increased.
Conclusions
Our study shows that totally laparoscopic distal gastrectomy is technically feasible at a low volume center. Therefore, totally laparoscopic distal gastrectomy can be considered as one of the surgical treatment for early gastric cancer. However the possibility that totally laparoscopic distal gastrectomy may have less benefit should also be considered.
Keywords: Stomach neoplasms, Laparoscopy, Gastrectomy
Introduction
Gastric cancer is still one of the most common malignancies in Korea1 and the incidence of early gastric cancer (EGC) has increased due to the implementation of national cancer screening program.
Since the report of Kitano et al.2 about good oncologic outcomes of laparoscopy-assisted gastrectomy (LAG), laparoscopic gastrectomy for EGC has been widely accepted and performed in Korea and Japan. Despite the technical difficulties of intracorporeal anastomosis, totally laparoscopic distal gastrectomy (TLDG) is known to have several advantages over laparoscopy-assisted distal gastrectomy (LADG) including smaller wounds, less invasiveness, better feasibility of securing the proximal margin and shorter bowel recovery.3,4 Moreover, there have been many reports about the feasibility and safety of the totally laparoscopic gastrectomy.3,5-7 However most of studies were conducted at large volume centers. In Korea, most patients with gastric cancer undergo surgical treatment at large volume centers. But there still are substantial patients who should take surgical treatment at low volume centers due to their financial situation or residential region.8,9 In addition, the number of patients who want to have minimally invasive surgery has increased recently, even in these low volume centers. However, there have been few reports on the feasibility and safety of TLDG at low volume centers.
Therefore, we conducted this study to assess the feasibility and safety of TLDG at low volume center through the analysis of short-term outcomes of TLDG compared with those of LADG performed at the same center and then compared the results at the low volume center with the published data from large volume centers.
Materials and Methods
1. Patients
We retrospectively reviewed the database of 35 patients who underwent LADG between April 2007 and March 2010, and 37 patients who underwent TLDG between April 2010 and August 2012 at the National Medical Center. All patients were thoroughly informed about the procedure and consents for surgery were obtained.
At our institution, indication for LADG was limited to pre-operative stage T1N0, T1N1 and T2N0 and as the surgeon's experience for LADG accumulated, the indication for TLDG was extended to pre-operative stage T1~3N0~2. Patients with an ASA score 4 or more points, serosal invasion in laparoscopic view, extensive lymph node metastasis and patient suitable for endoscopic submucosal dissection (ESD) were excluded. The indication for ESD was well-differentiated mucosal lesion smaller than 2 cm without ulceration.
The disease stage was classified according to the 7th International Union Against Cancer (UICC) TNM classification. The characteristics of patients, such as age, gender, body mass index (kg/m2), history of abdominal surgery, American Society of Anesthesiologists (ASA) score, co-morbidities, and surgical outcomes (operative method, combined operation, operative time, number of used stapler, blood loss, complications, pathologic results, time-to-first flatus, time-to-first oral intake, and postoperative hospital stay) were examined.
2. Surgical procedure
Patients were placed in the supine position and the operator stood on the right side of the patient. After general anesthesia, a 10 mm trocar was inserted at the infraumbilical area and pneumoperitoneum was formed by insufflations of carbon dioxide. The patient was placed in the reverse trendelenburg position and four or five additional trocars were inserted. The intraperitoneal pressure was maintained at 12 to 13 mmHg. A 12 mm trocar was inserted on the right side of the umbilicus on the lateral side of the rectus abdominis muscle. A 5 mm trocar was inserted in the right subcostal area on the lateral side of the rectus abdominis muscle for use by the surgeon. A 10 mm trocar was inserted in the left subcostal area on the lateral side of the rectus abdominis muscle. A 5 or 12 mm trocar was inserted on the left side of the umbilicus on the lateral side of the rectus abdominis muscle for use by the assistant in retracting organs. A 10 mm trocar was inserted at the subxiphoid area and used for insertion of a fan-shaped liver retractor but it was sometimes omitted. The EXERA® Laparo-thoraco videoscope (Olympus, Tokyo, Japan) was used. For dissection of tissues, an ultrasonic cautery Harmonic scalpel® (Ethicon Endo Surgery, Cincinnati, OH, USA) was used.
The greater omentum was divided from the mid-portion of the transverse colon toward the lower pole of the spleen. The right omentum was dissected and right gastroepiploic vessels were clipped and dissected. Then the lesser omentum was opened, and the right gastric artery was clipped and dissected. The duodenum was divided distal to the pyloric ring with a 60 mm linear stapler ECR60®B (Blue cartridge, Ethicon Endo Surgery). The left gastric artery was doubly ligated with clips then transected.
1) LADG
For Billroth-I gastroduodenostomy cases, a 4~6 cm transverse mini-laparotomy was made in the subcostal area. For other anastomoses, a vertical 4~6 cm mini-laparotomy was made in the subxiphoid area, and an ALEXIS® wound retractor (2.5~6 cm; Applied Medical, Rancho Santa Margarita, CA, USA) was installed.
For Billroth-I reconstruction, one 25 mm circular stapler CDH25® (Ethicon Endo Surgery,) was used for gastroduodenostomy and distal gastrectomy was performed with two or three 60 mm linear staplers ECR60®G (Gold cartridge, Ethicon Endo Surgery).
For Billroth-II reconstruction, gastrojejunostomy was performed using either one 60 mm linear stapler ECR60®B or hand-sawn closure. Two or three 60 mm linear staplers ECR60®G were used for artificial lesser curvature formation.
For Roux-en-Y gastrojejunostomy, the jejunum was transected 20 cm distal to the ligament of Treitz using 60 mm linear stapler ECR60®B. The transected distal Roux limb was positioned in an antecolic or retrocolic fashion and one 60 mm linear stapler ECR60®B was used for gastrojejunostomy. At 20~30 cm distal from the anastomosis site, hand-sawn end-to-side jejunojejunostomy was performed.
2) TLDG
For Billroth-II gastrojejunostomy, one 60 mm linear stapler ECR60®B was used for gastrojejunostomy and the common entry hole was horizontally closed with one 60 mm linear stapler ECR60®B. Two or three 60 mm linear staplers ECR60®G were used for artificial lesser curvature formation. Reinforcement sutures were interruptedly performed at the stapler line.
For Roux-en-Y gastrojejunostomy, the jejunum was transected 20 cm distal to the ligament of Treitz using a 60 mm linear stapler ECR60®B. The transected distal Roux limb was positioned in an antecolic or retrocolic fashion and one 60 mm linear stapler ECR60B was used for gastrojejunostomy. At 20~30 cm distal from the anastomosis site, side-to-side jejunojejunostomy was performed using a 60 mm linear stapler ECR60®B and common entry holes were also closed intracorporeally by using a 60 mm linear stapler ECR60®B. Reinforcement sutures also were interruptedly performed at the stapler line.
For specimen removal, a 4~5 cm vertical mini-laparotomy was made in the infraumbilical port. The mini-laparotomy was retracted and protected by the ALEXIS® wound retractor.
3. Statistical analysis
All continuous data are presented as the mean±standard deviation. Statistical analyses were performed using the Wilcoxon rank sum test for continuous variables and Fisher's exact test for categorical variables. A value of P<0.05 was regarded as significant. Statistical analyses were performed using the SAS 9.1 (SAS Institute Inc., Cary, NC, USA).
Results
1. Demographic and clinical data
In our study, LADG and TLDG were performed by a single operator. TLDG was performed after the operator got over the learning curve of the LADG.10,11 None of the procedures were converted to open gastrectomy and no intra-operative complications.
There was no significant difference in age, gender, body mass index, previous history of abdominal surgery, comorbidity or ASA score between the two groups. The characteristics of the patients are summarized in Table 1.
Table 1.
Characteristics of patients
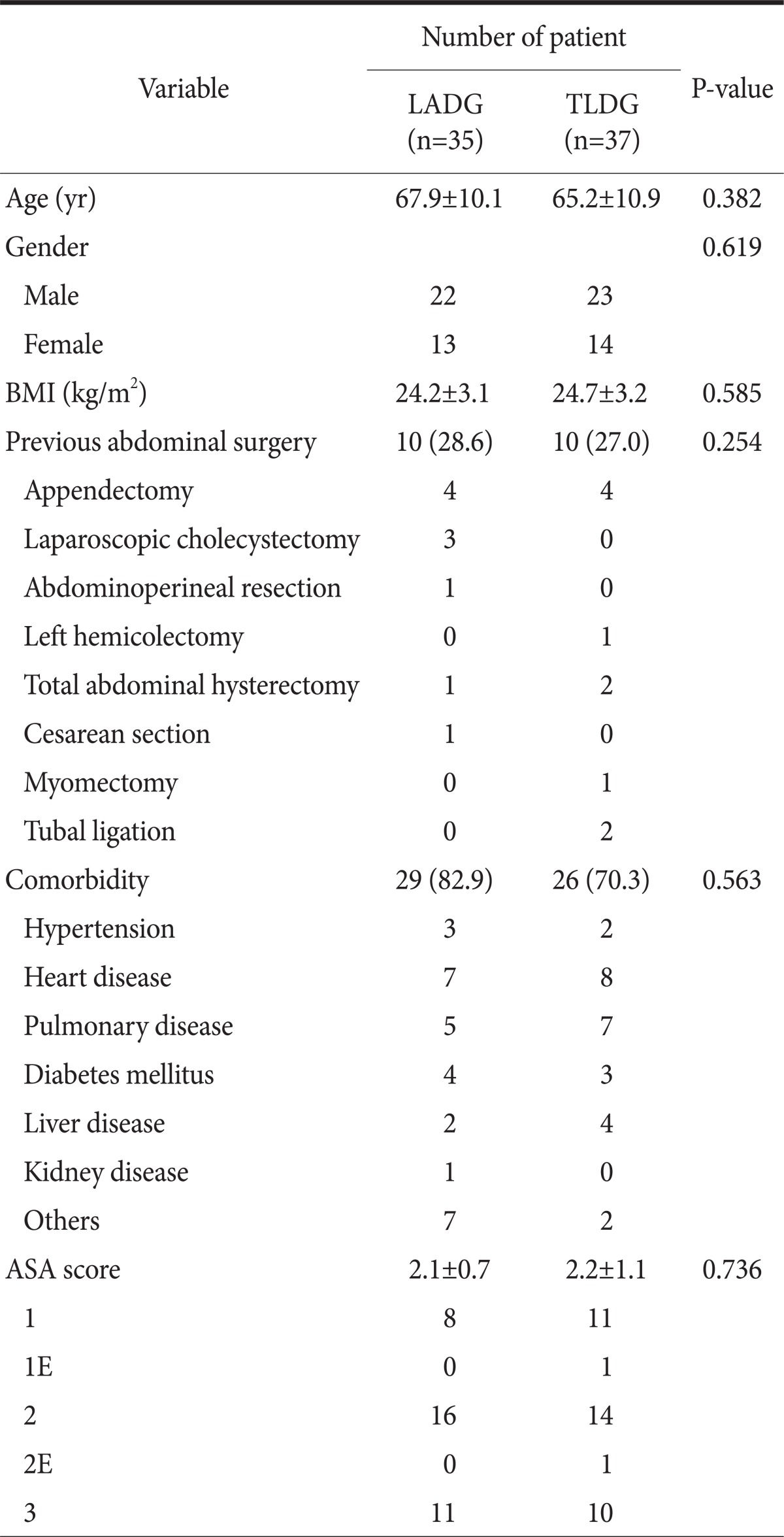
Values are presented as mean±standard deviation or number (%). LADG = laparoscopy-assisted distal gastrectomy; TLDG = totally laparoscopic distal gastrectomy; BMI = body mass index; ASA = American Society of Anesthesiologists.
There were some differences in operation methods. Billroth-II and Roux-en-Y gastrojejunostomy after distal gastrectomy were significantly increased in the TLDG group (P<0.05). Complete omentectomy, but not statistically significant (P=0.9872) was performed more in the TLDG group (17.1% vs. 35.1% in the LADG and TLDG, respectively, P=0.083).
There was no significant difference in tumor size, proximal resection margin, tumor location, depth of invasion, histology, number of harvested lymph nodes or TNM stage. There were 3 patients with serosal invasion (1 vs. 2 in LADG and TLDG, respectively), and serosal invasion was not detected in the operative field but diagnosed as serosal invasion on the pathologic report. The operation method and pathologic findings are summarized in Table 2.
Table 2.
Operation method and pathologic findings
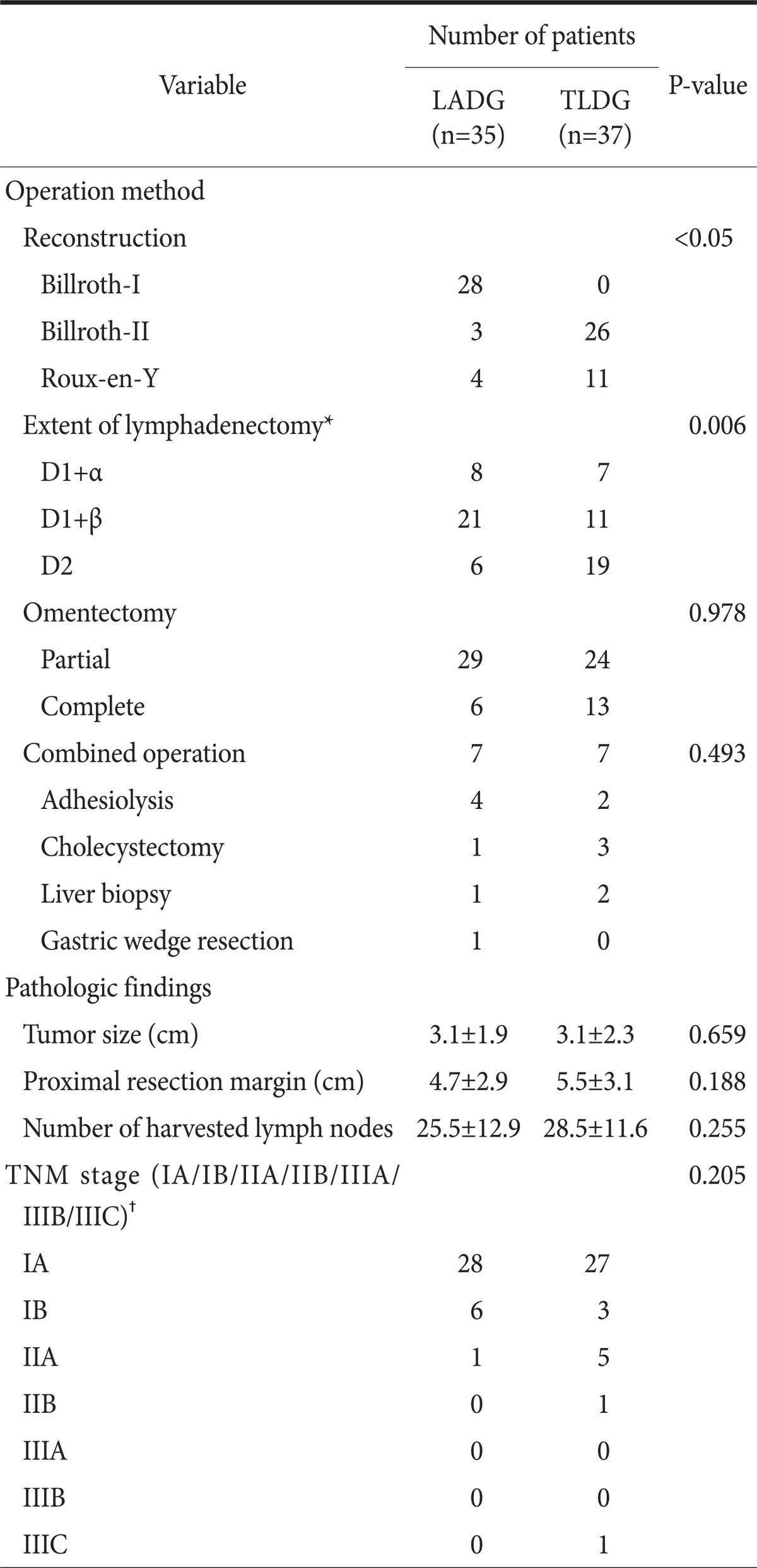
Values are presented as number or mean±standard deviation. LADG = laparoscopy-assisted distal gastrectomy; TLDG = totally laparoscopic distal gastrectomy. *Extent of lymphadenectomy classified according to the Guidelines of the Japanese Gastric Cancer Association; †Stage classified by the 7th edition of the International Union Against Cancer (UICC).
2. Surgical outcomes and postoperative courses
There was no significant difference in operation time and estimated blood loss between the two groups. The overall surgical and medical complication shows no significant difference. Among surgical complications, there was more leakage in the TLDG group but it was not statistically significant (1 vs. 4 in LADG and TLDG, respectively, P=0.3566). Most of the morbidities were well recovered with conservative treatment but one mortality case occurred in the TLDG group.
The postoperative course showed some differences. Time-to-first flatus was not significantly different. But time-to-first oral intake (3.1±0.5 vs. 3.5±0.9 in LADG and TLDG, respectively, P=0.0110) and postoperative hospital stay (9.1±3.6 vs. 14.3±9.7 in LADG and TLDG, respectively, P<0.0001) were significantly increased in the TLDG group. The surgical outcomes and postoperative courses are summarized in Table 3.
Table 3.
Surgical outcomes and postoperative courses
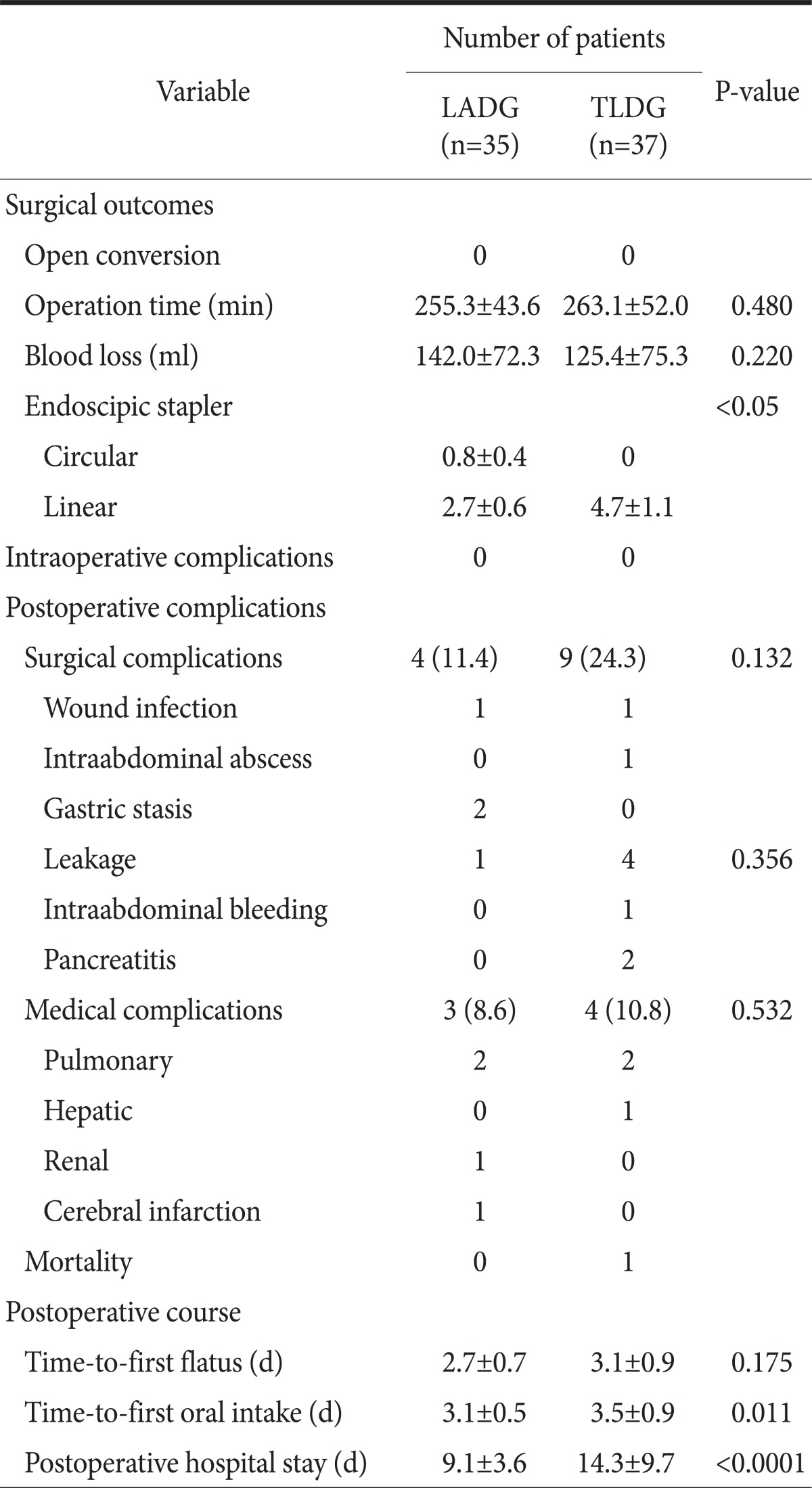
Values are presented as mean±standard deviation or number (%). LADG = laparoscopy-assisted distal gastrectomy; TLDG = totally laparoscopic distal gastrectomy.
Discussion
Despite the known advantage of TLDG compared with LADG, many surgeons are concerned about the technical difficulty of intracorporeal anastomosis, intraoperative localization of the tumor, oncologic aspects and additional costs of using many linear staplers.12
The safety and efficacy of LAG in the treatment of EGC have been demonstrated in many studies.13-15 Also, many authors have reported that intracorporeal anastomosis of the TLDG wound be feasible and safe.6,12 In this study, there was no significant difference in operation time, estimated blood loss, or surgical complication between LADG and TLDG. But there were four anastomosis leakage cases and one mortality case in the TLDG group. Anastomosis leakage was more in the TLDG group but it was not statistically significant (P=0.3566). All anastomosis leakages in TLDG group were duodenal stump leakages. Two of them were well recovered with conservative treatment including gut rest with nutritional support and the rest of them were managed and recovered by percutaneous drainage. The mortality case in the TLDG group was a 48-year-old man with alcoholic liver cirrhosis (Child-Pugh-Turcotte class B). The pre-operative TNM stage of the patient was expected to be T1N0M0 but he was not a candidate for ESD because the patient had signet-ring cell carcinoma. The patient was well informed about the risk of the surgery and an informed consent for surgery was obtained. After the operation, liver failure, renal failure and pneumonia occurred and despite the best treatment including continuous renal replacement therapy, the patient died 19 days after surgery.
EGC is not visible or palpable from outside of the stomach, which makes the localization of the tumor very difficult during TLDG.12 Of the many methods for tumor localization,4,16,17 we used preoperative endoscopic clipping if the lesion was located at the middle third of the stomach. In our study, the proximal resection margin in TLDG was not smaller than that of LADG.
The oncologic outcome, including number of retrieved lymph nodes and node station of LADG are known to be comparable with that of open distal gastrectomy.18 In this study we could not a find significant difference in the number of harvested lymph nodes and lymph node station between the two groups. Rather, the frequency of the D2 lymph node dissection was increased in the TLDG group (17.1% vs. 35.1% in LADG and TLDG respectively, P=0.0062). We think that this may be due to improved surgical skill during the surgeon's learning curve period. And during the study period for TLDG, the Japanese Classification of Gastric Carcinoma (JC) and the Japanese Gastric Cancer Treatment Guideline (JGL) was revised.19 D2 lymph node dissection became more easily achieved due to lymph node station No. 14v was excluded in the revised recommendation.20
After distal gastrectomy, we used linear staplers for reconstruction in the TLDG. The number of stapler cartridges that were used was more in the TLDG group than in the LADG group (4.7 vs. 3.5 in TLDG and LADG respectively). In this study, as shown in other studies,7,12 the higher cost for using additional stapling was also a problem in the TLDG group.
Postoperative courses including time-to-first oral intake and postoperative hospital stay turned out to be longer in the TLDG group. The data about time-to-first oral intake did not show much difference but there were statistically significant differences after using nonparametric statistical methods (3.1±0.5 vs. 3.5±0.9 in LADG and TLDG, respectively, P=0.0110). As for the cause of the increase in postoperative hospital stay, it is estimated that there were relatively more complications in the TLDG group that prolonged the duration of hospitalization such as leakage. In addition, an increase in the duration of hospitalization in the small number of patients have greater impact on the overall average due to the small population who were involved in this study. Furthermore, some patients with senility and much comorbidity had a longer hospital stay than expected because they showed delayed recovery from general conditions, even without the development of particular complications.21
The results described above are what had been performed at a single low volume center in Korea performing an average of 31 cases of gastric cancer surgery annually. The exact meaning of low volume center is hard to define. Based on some reports about hospital surgical volume and surgical outcomes of gastric cancer surgery, in case of the Korea and Japan where the incidence of gastric cancer is higher, the hospital where less than 70 to 112 surgeries take place a year is considered a low or very low volume center.
When compared with the data of large volume centers, surgical outcomes including operation time and blood loss were acceptable. But morbidity especially anastomosis leakage were more prevalent in our study. The ratio of anastomosis leakage in other reports ranges from 0 to 4.0%.3,6,12 The oncologic outcomes including proximal resection margin, number of harvested lymph nodes and lymph node station were comparable with the data of large volume centers.3,4,6,12 The postoperative course including time-to first flatus and time-to-first oral intake also were not greatly different. But compared with other studies, postoperative hospital stay of the TLDG group was somewhat longer. In other studies, the average postoperative hospital stay was 8.3 to 13.3 days.3,4,12 The amount of stapler cartridge usage was less than in other study. The number of cartridges used in other studies was on average 4.9 to 10.2.6,12,22
This study has some limitations. This report may have errors of retrospective studies. Reconstruction methods and frequency of D2 lymph node dissection differed between the two groups. In addition, the number of total patients was too small to perform adjustment of covariates such as the difference of reconstruction method and extent of lymphadenectomy. And TLDG was performed after overcoming the learning curve of the LADG. Therefore, these limitations might have influenced outcomes.
Despite these limitations, our study shows that TLDG is a technically feasible procedure. However there may be problems related to the increase in postoperative hospital stay, the safety issues including leakage even though they were not statistically significant and the relatively higher cost than LADG.
Therefore, TLDG can be considered as one of the surgical treatment for EGC if the surgeon is proficient at intracorporeal suturing and gets over the learning curve of the LADG. But the possibility that TLDG may have less benefit compared to LADG should be also considered. An additional study should be undertaken to further examine the benefit of TLDG at low volume centers.
References
- 1.Jung KW, Park S, Won YJ, Kong HJ, Lee JY, Park EC, et al. Prediction of cancer incidence and mortality in Korea, 2011. Cancer Res Treat. 2011;43:12–18. doi: 10.4143/crt.2011.43.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kitano S, Shiraishi N, Uyama I, Sugihara K, Tanigawa N Japanese Laparoscopic Surgery Study Group. A multicenter study on oncologic outcome of laparoscopic gastrectomy for early cancer in Japan. Ann Surg. 2007;245:68–72. doi: 10.1097/01.sla.0000225364.03133.f8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ikeda O, Sakaguchi Y, Aoki Y, Harimoto N, Taomoto J, Masuda T, et al. Advantages of totally laparoscopic distal gastrectomy over laparoscopically assisted distal gastrectomy for gastric cancer. Surg Endosc. 2009;23:2374–2379. doi: 10.1007/s00464-009-0360-3. [DOI] [PubMed] [Google Scholar]
- 4.Song KY, Park CH, Kang HC, Kim JJ, Park SM, Jun KH, et al. Is totally laparoscopic gastrectomy less invasive than laparoscopy-assisted gastrectomy?: prospective, multicenter study. J Gastrointest Surg. 2008;12:1015–1021. doi: 10.1007/s11605-008-0484-0. [DOI] [PubMed] [Google Scholar]
- 5.Kim KH, Kim MC, Jung GJ, Kim HH. Long-term outcomes and feasibility with laparoscopy-assisted gastrectomy for gastric cancer. J Gastric Cancer. 2012;12:18–25. doi: 10.5230/jgc.2012.12.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J, Kim D, Kim W. Comparison of laparoscopy-assisted and totally laparoscopic Billroth-II distal gastrectomy for gastric cancer. J Korean Surg Soc. 2012;82:135–142. doi: 10.4174/jkss.2012.82.3.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kinoshita T, Shibasaki H, Oshiro T, Ooshiro M, Okazumi S, Katoh R. Comparison of laparoscopy-assisted and total laparoscopic Billroth-I gastrectomy for gastric cancer: a report of short-term outcomes. Surg Endosc. 2011;25:1395–1401. doi: 10.1007/s00464-010-1402-6. [DOI] [PubMed] [Google Scholar]
- 8.Choi YY, Bae J, Hur KY, Choi D, Kim YJ. Reinforcing the staple line during laparoscopic sleeve gastrectomy: does it have advantages? A meta-analysis. Obes Surg. 2012;22:1206–1213. doi: 10.1007/s11695-012-0674-4. [DOI] [PubMed] [Google Scholar]
- 9.Kim M, Park J, Kim SG, Choi S, Yoon S, Lee S. Feasibility of gastric cancer surgery at low volume hospitals. J Gastric Cancer. 2010;10:234–240. doi: 10.5230/jgc.2010.10.4.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin SH, Kim DY, Kim H, Jeong IH, Kim MW, Cho YK, et al. Multidimensional learning curve in laparoscopy-assisted gastrectomy for early gastric cancer. Surg Endosc. 2007;21:28–33. doi: 10.1007/s00464-005-0634-3. [DOI] [PubMed] [Google Scholar]
- 11.Kim MC, Jung GJ, Kim HH. Learning curve of laparoscopy-assisted distal gastrectomy with systemic lymphadenectomy for early gastric cancer. World J Gastroenterol. 2005;11:7508–7511. doi: 10.3748/wjg.v11.i47.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kim JJ, Song KY, Chin HM, Kim W, Jeon HM, Park CH, et al. Totally laparoscopic gastrectomy with various types of intracorporeal anastomosis using laparoscopic linear staplers: preliminary experience. Surg Endosc. 2008;22:436–442. doi: 10.1007/s00464-007-9446-y. [DOI] [PubMed] [Google Scholar]
- 13.Lee SI, Choi YS, Park DJ, Kim HH, Yang HK, Kim MC. Comparative study of laparoscopy-assisted distal gastrectomy and open distal gastrectomy. J Am Coll Surg. 2006;202:874–880. doi: 10.1016/j.jamcollsurg.2006.02.028. [DOI] [PubMed] [Google Scholar]
- 14.Hayashi H, Ochiai T, Shimada H, Gunji Y. Prospective randomized study of open versus laparoscopy-assisted distal gastrectomy with extraperigastric lymph node dissection for early gastric cancer. Surg Endosc. 2005;19:1172–1176. doi: 10.1007/s00464-004-8207-4. [DOI] [PubMed] [Google Scholar]
- 15.Lee JH, Han HS, Lee JH. A prospective randomized study comparing open vs laparoscopy-assisted distal gastrectomy in early gastric cancer: early results. Surg Endosc. 2005;19:168–173. doi: 10.1007/s00464-004-8808-y. [DOI] [PubMed] [Google Scholar]
- 16.Kim HI, Hyung WJ, Lee CR, Lim JS, An JY, Cheong JH, et al. Intraoperative portable abdominal radiograph for tumor localization: a simple and accurate method for laparoscopic gastrectomy. Surg Endosc. 2011;25:958–963. doi: 10.1007/s00464-010-1288-3. [DOI] [PubMed] [Google Scholar]
- 17.Hyung WJ, Lim JS, Cheong JH, Kim J, Choi SH, Song SY, et al. Intraoperative tumor localization using laparoscopic ultrasonography in laparoscopic-assisted gastrectomy. Surg Endosc. 2005;19:1353–1357. doi: 10.1007/s00464-004-8196-3. [DOI] [PubMed] [Google Scholar]
- 18.Song KY, Kim SN, Park CH. Laparoscopy-assisted distal gastrectomy with D2 lymph node dissection for gastric cancer: technical and oncologic aspects. Surg Endosc. 2008;22:655–659. doi: 10.1007/s00464-007-9431-5. [DOI] [PubMed] [Google Scholar]
- 19.Kwon SJ. Evaluation of the 7th UICC TNM staging system of gastric cancer. J Gastric Cancer. 2011;11:78–85. doi: 10.5230/jgc.2011.11.2.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sano T, Aiko T. New Japanese classifications and treatment guidelines for gastric cancer: revision concepts and major revised points. Gastric Cancer. 2011;14:97–100. doi: 10.1007/s10120-011-0040-6. [DOI] [PubMed] [Google Scholar]
- 21.Park JM, Jin SH, Lee SR, Kim H, Jung IH, Cho YK, et al. Complications with laparoscopically assisted gastrectomy: multivariate analysis of 300 consecutive cases. Surg Endosc. 2008;22:2133–2139. doi: 10.1007/s00464-008-9962-4. [DOI] [PubMed] [Google Scholar]
- 22.Kim JJ, Kim SK, Jun KH, Kang HC, Song KY, Chin HM, et al. Clinical usefulness of a totally laparoscopic gastrectomy. J Korean Gastric Cancer Assoc. 2007;7:132–138. [Google Scholar]


