Abstract
Mitochondrial Ca signaling contributes to the regulation of cellular energy metabolism, and mitochondria participate in cardiac excitation-contraction coupling (ECC) through their ability to store Ca, shape the cytosolic Ca signals and generate ATP required for contraction. The mitochondrial inner membrane is equipped with an elaborate system of channels and transporters for Ca uptake and extrusion that allows for the decoding of cytosolic Ca signals, and the storage of Ca in the mitochondrial matrix compartment. Controversy, however remains whether the fast cytosolic Ca transients underlying ECC in the beating heart are transmitted rapidly into the matrix compartment or slowly integrated by the mitochondrial Ca transport machinery. This review summarizes established and novel findings on cardiac mitochondrial Ca transport and buffering, and discusses the evidence either supporting or arguing against the idea that Ca can be taken up rapidly by mitochondria during ECC.
Keywords: excitation-contraction coupling, heart, intracellular calcium, mitochondrial Ca transport, Ca buffering
1. Introduction: Cardiac excitation-contraction coupling and mitochondrial Ca
Cardiac contraction is regulated by beat-to-beat elevations of cytosolic calcium ([Ca]i) by a process termed excitation-contraction coupling (ECC) [1] where membrane depolarization induced by an action potential leads to Ca entry through voltage-activated L-type Ca channels. Entering Ca triggers Ca release from the sarcoplasmic reticulum (SR) Ca store via ryanodine receptor (RyR) Ca release channels by a mechanism known as Ca-induced Ca release (CICR). CICR increases global [Ca]i which activates proteins of the contractile apparatus and initiates cell contraction. Subsequent relaxation occurs by removal of Ca from the cytosol via four main pathways including reuptake via the SR Ca-ATPase (SERCA), extrusion via sarcolemmal Na/Ca exchange (NCX) and the sarcolemmal Ca-ATPase. A fourth avenue of Ca sequestration potentially involves mitochondrial Ca uptake since mitochondria are equipped with an efficient machinery for Ca transport and are capable of storing large amounts of Ca. Mitochondria of cardiomyocytes are known to accumulate Ca during elevations in cytosolic [Ca]i (for reviews cf. [2–8]), however, the kinetics of mitochondrial Ca cycling and buffering during ECC have remained highly controversial [9]. The question of whether and how beat-to-beat changes of cytosolic [Ca]i during ECC translate into changes of mitochondrial matrix Ca concentration ([Ca]m) has important ramifications for cardiac physiology and pathophysiology. First, mitochondrial Ca uptake and buffering have the potential to shape the cytosolic Ca transient and therefore contribute to the regulation of contractile activity, and second, mitochondrial Ca uptake regulates cellular metabolism and energy supplies required for contraction. The latter occurs through the Ca-dependence of key enzymes of the tricarboxylic acid (TCA) cycle and possibly also Ca-dependent regulation of various sites of the electron transport chain (ETC) and the mitochondrial F1/F0 ATP synthase [10–12].
In this review we will briefly summarize the elements of the mitochondrial Ca transport machinery and recent novel findings on mitochondrial Ca buffering. Furthermore, we discuss how mitochondria encode rapid beat-to-beat cytosolic Ca oscillations and critically review arguments in favor and against rapid mitochondrial Ca uptake during ECC.
2. Mitochondrial Ca transport
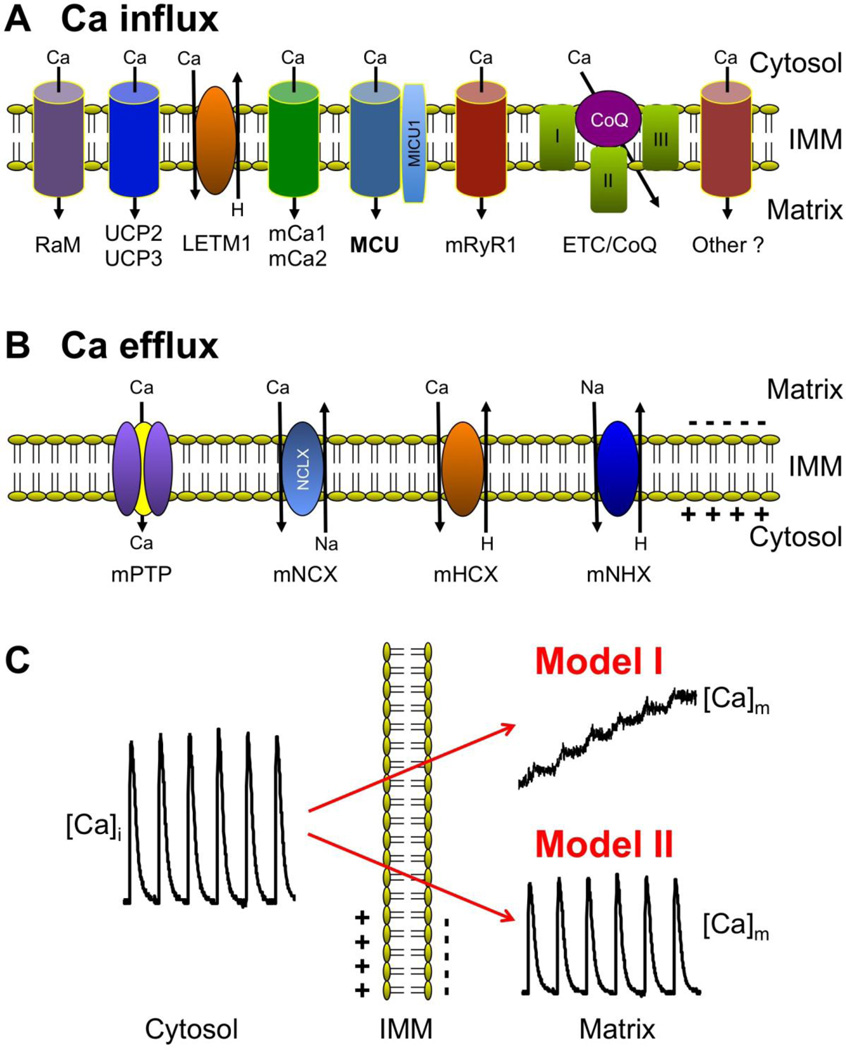
Mitochondria are cytosolic double-membrane organelles that have been dubbed 'power plants' of the cell [13, 14] for their ability to generate ATP to satisfy cellular energy demands. Mitochondria, however, participate in a myriad of other cellular processes such as ion homeostasis, redox signaling, apoptotic and necrotic cell death, as well as the control of cell cycle and cell growth [15]. Furthermore, mitochondria undergo remodeling in cardiac disease, including arrhythmia and heart failure, that has profound effects on mitochondrial structure-function that become important determinants of the course of the disease [6, 16–19]. In cardiac myocytes mitochondria occupy ~35% of the cell volume [20, 21] reflecting the high energy demands of these cells. Mitochondria possess an elaborate system of Ca uptake and extrusion mechanisms and pathways (Fig. 1) that allow for a fine-tuned regulation of [Ca]m [22, 23].
Fig. 1. Mitochondrial Ca transport and mitochondrial decoding of cytosolic Ca signals.
A) Mitochondrial Ca uptake mechanisms and pathways located at the inner mitochondrial membrane (IMM). From left: RaM, rapid mode of Ca uptake; UCP2 and UCP3, uncoupling proteins 2 and 3; LETM1, leucine-zipper-EF-hand-containing transmembrane protein 1; mCa1 and mCa2, Ca-selective IMM conductances 1 and 2; MCU, mitochondrial Ca uniporter, with the associated protein MICU1; mRyR1, mitochondrial ryanodine receptor type 1; CoQ, Coenzyme Q10; ETC, electron transport chain. B) Mitochondrial Ca extrusion mechanisms and pathways located at the IMM. mPTP, mitochondrial permeability transition pore; mNCX, mitochondrial Na/Ca exchange with NCLX as suggested molecular identity; mHCX, mitochondrial proton/Ca exchange; mNHX, mitochondrial Na/proton exchange. C) Models of transmission of fast cytosolic Ca transients to matrix [Ca]m: Model I, slow integration of cytosolic Ca spiking. Model II: rapid, beat-to-beat transmission of cytosolic Ca oscillations.
2.1. Mitochondrial Ca uptake
Several mechanisms for mitochondrial Ca uptake (Fig. 1A) have been described and proposed for cardiac myocytes - some well established, others still controversial or a matter of debate: the mitochondrial Ca uniporter (MCU) [24–27], a rapid mode of Ca uptake (RaM) [28–31], the mitochondrial ryanodine receptor type 1 (mRyR1) [32–35] and the recently proposed leucine-zipper-EF-hand-containing transmembrane protein 1 (LETM 1) [36–38], all, together with additional putative Ca uptake pathways (see discussion below), localized to the inner mitochondrial membrane (IMM).
The best established and most characterized pathway for mitochondrial Ca uptake is through the MCU [24–27], driven by the large electrochemical gradient (mitochondrial membrane potential ΔΨ ~ -180 mV) for Ca across the IMM. Many characteristics of the MCU (kinetic and electrophysiological [39] properties, Ca-dependence and ion selectivity) have been known for many years. MCU is regulated by a number of modulators (activators and inhibitors) that include divalent cations, lanthanides, adenine nucleotides, and ruthenium compounds (for review see [3, 40]). Among the latter RU-360 is preferentially used experimentally to inhibit MCU [41]. Nonetheless, it was not until recently that the molecular identity of the transporter has been revealed, essentially at the same time by two different laboratories [26, 27]. Both studies are in agreement that a transmembrane protein previously identified as CCDC109A is the molecular identity of the MCU (but see [42]). CCDC109A (MCU) has two transmembrane domains that are likely to form the Ca channel pore. The Ca binding protein MICU1 (mitochondrial calcium uptake 1) has been postulated to be required for mitochondrial Ca uptake [43], and may act as an auxiliary regulatory protein to MCU. Recently, MICU1 was shown to serve as a 'gatekeeper' for MCU that determines the Ca threshold for uptake and prevents mitochondrial Ca overload [44]. Furthermore, Ca/calmodulin-dependent protein kinase II (CaMKII), a key signaling molecule involved in cardiac pathologies including heart failure, promotes mPTP opening and myocardial cell death by increasing mitochondrial Ca uptake via MCU [45].
In addition to the MCU other avenues of Ca entry into mitochondria have been proposed. RaM (demonstrated in isolated heart mitochondria) operates transiently during the initial phase of pulsatile elevations of extramitochondrial [Ca] ([Ca]em) [28–31]. Ca uptake via RaM is several hundred times faster than uptake via MCU, however, the recovery of RaM following a Ca pulse in isolated heart mitochondria required more than 60 s [28] rendering this mechanism potentially inactivate during cardiac Ca oscillations.
Of more controversial nature are ryanodine receptors that have been located in the IMM and proposed to be one of the mitochondrial Ca uptake pathways [32–35]. Reconstitution into lipid bilayers yielded cesium-conducting, Ca-sensitive, large conductance (500–800 pS) channels [34] with characteristics similar to SR RyRs. The mitochondrial RyR exhibits biochemical, pharmacological, and functional properties similar to the skeletal muscle RyR (type 1, RyR1), and is therefore called mRyR1.
Additional putative mitochondrial Ca uptake mechanisms and pathways have been proposed, including two Ca-selective conductances referred to as mCa1 and mCa2 [46], Coenzyme Q10 (an element of the mitochondrial electron-transport chain; [47]), the uncoupling proteins UCP2 and UCP3 [48], and LETM1 [36–38]). A debated issue is whether the LETM1 protein can function as a high affinity mitochondrial Ca influx mechanism. LETM1 was identified first as a mitochondrial potassium/proton antiporter, but has since been argued to function as a Ca/H antiporter [37]. It remains to be determined, however, whether normal electrochemical gradients for protons and Ca would allow LETM1 to act as a Ca influx pathway, or whether LETM1 might actually act as Ca efflux rather than influx mechanism [38].
2.2. Mitochondrial Ca efflux
Ca extrusion from the mitochondrial matrix occurs by Na-dependent and Na-independent mechanisms (Fig. 1B). The predominant Ca extrusion pathway in cardiac mitochondria is Na-dependent [49, 50], while Na-independent efflux (particularly proton/Ca exchange, mHCX) plays little to no role [51, 52]. The Na-dependent extrusion occurs via mitochondrial Na/Ca exchange (mNCX, with NCLX (Na/Ca/Li exchanger) suggested as its molecular identity [53, 54]), i.e. mediated by an antiporter that utilizes the Na gradient across the inner membrane. The [Na]i dependence of mNCX is sigmoidal with a half-maximal activity at ~4–8 mM [55–58], i.e. in the range of resting [Na]i observed under physiological conditions in cardiomyocytes [59–62], making mNCX potentially sensitive to physiological fluctuations in cytosolic [Na]i [56]. However, no significant variations in bulk [Na]i are typically observed during the normal cardiac cycle, and only unphysiological increases in stimulation frequency or pathological conditions such as heart failure resulted in significant changes of bulk [Na]i [60, 63, 64]. mNCX plays an important role in modulating the steady-state balance between extra- and intramitochondrial Ca [65]. Cellular Na overload, as observed in heart failure, disrupts this equilibrium, resulting in changes of pyridine nucleotide redox potential and increase in reactive oxygen species (ROS) generation, with ultimately detrimental effects on mitochondrial bioenergetics and mismatch of cardiac energy supply and demand [63, 66, 67]. Na-dependent efflux via mNCX is inhibited by divalent cations and a number of drugs, including Ca channel blockers (e.g. diltiazem, verapamil) and benzodiazepine derivatives (e.g. clonazepam, CGP-37157) [3, 7, 40]. Furthermore, it has long remained elusive and controversial [2, 50] whether mNCX is an electroneutral or electrogenic [68, 69] antiporter. Nonetheless, the majority of data agree that mNCX exchanges 3 Na ions for 1 Ca, and thus is electrogenic [7, 70–72]. As an electrogenic exchanger, Ca extrusion would tend to depolarize ΔΨ, thereby reducing the electrical gradient for Ca uptake and favor net mitochondrial Ca extrusion. The IMM also hosts a Na/proton exchange system (mNHX) [73], which serves as the pathway for Na extrusion. Through this mechanism the energy requirement for Ca extrusion via mNCX is coupled to proton movement across the ETC during mitochondrial respiration.
Of considerable controversy remains whether the mitochondrial Permeability Transition Pore (mPTP) can function as a Ca efflux pathway [7, 74]. The mitochondrial permeability transition defines a sudden increase in the permeability of the IMM to ions and solutes with molecular weights up to ~1.5 kDa [75]. Elevated levels of matrix Ca and ROS are known inducers of mPTP opening [40, 76]. The permeability transition process is attributed to the opening of a voltage- and Ca-dependent, cyclosporin A (CsA)-sensitive [77], high-conductance channel. The molecular identity of mPTP is largely unknown (see [76, 78, 79] for recent reviews) and the original model of the mPTP proposed a multi-protein complex that included the adenine nucleotide translocase in the IMM, the voltage-dependent anion channel of the outer membrane (VDAC), the F1/F0 ATP-synthase, and the protein cyclophilin D (Cyp D) [80]. In addition, complex I of the ETC has also been suggested to be a part of the mPTP complex [81], and modulation of mPTP by complex I and Cyp D may share a common mechanism [82]. However, this mPTP model has been challenged by recent genetic studies [83, 84]. Electrophysiological studies revealed that the mPTP is a large-conductance (1.3 nS) channel with numerous subconductance states and may flicker rapidly between a fully closed and a subconductance state [85, 86]. mPTP is activated by high matrix [Ca], oxidative stress and depolarization. Repetitive opening and closing of the mPTP has been demonstrated in individual isolated heart mitochondria under conditions of oxidative stress [87, 88]. However, whether mPTP can serve as a mitochondrial Ca release channel [25] under physiological conditions has remained controversial. The existence of small and possibly ion-selective subconductance states of the mPTP [89] could potentially allow brief openings of the mPTP and serve as a mechanism for fast dissipation of ΔΨ and subsequent Ca efflux without causing dramatic changes to the matrix environment, but possibly acting as a Ca 'relief walve' and provide protection against cell injury under mitochondrial Ca overload conditions. However, experimental evidence for the participation of mPTP in mitochondrial Ca signaling under physiological conditions remains scarce [90–92] and controversial [93]. Under pathological conditions of heart failure mice lacking Cyp D exhibit a substantially more pronounced maladaptive phenotype and a reduction in myocardial function that was associated with altered mPTP-mediated Ca efflux which resulted in elevated matrix Ca, leading to a mismatch of energy metabolism and myocardial workload [91]. Under conditions of ΔΨ dissipation Ca can enter mitochondria through the mPTP [55] potentially maintaining a mitochondrial Ca sink for cytosolic Ca overload when MCU is inactivated.
3. Mitochondrial Ca signals during ECC: The controversy of beat-to-beat mitochondrial Ca transients
Historically, two different theories (Fig. 1C) have evolved on how mitochondria decode rapid cytosolic Ca transients (for review see for example refs. [2–4, 7, 9]). In model I, originally introduced by Crompton [94], Ca uptake into mitochondria is slow and balanced by an even slower release of accumulated Ca ions. Fast cytosolic beat-to-beat Ca oscillations are integrated by the Ca transport machinery of the IMM. The response to a train of cytosolic Ca transients is a gradual amplitude- and frequency-dependent increase of [Ca]m until a new steady-state is reached when the amount of Ca gained during a single cycle equals Ca removal from the matrix compartment. Consequently, beat-to-beat [Ca]m changes are small, and energetic requirements of mitochondrial Ca transport are minimal. In contrast, model II describes how fast cytosolic Ca oscillations are efficiently translated into rapid beat-to-beat changes of [Ca]m of considerable amplitude. For this to occur, rapid mitochondrial Ca uptake as well as fast Ca efflux mechanisms are mandatory prerequisites, with Ca uptake being large enough to overcome the buffering capacity of both the cytosol and the mitochondrial matrix compartment, thus requiring high SR Ca release fluxes. With this scenario, mitochondrial Ca uptake would effectively buffer cytosolic Ca transients and therefore play a critical role for shaping the cytosolic Ca transient during ECC, and thus potentially regulate contraction on a beat-to-beat basis. With approximately one third of cell volume being occupied by mitochondria in cardiac cells, the additional SR Ca fluxes (and energy requirement linked to them) would have to be substantial [2].
The controversy surrounding beat-to-beat changes in [Ca]m in the heart is certainly (at least in part) related to experimental limitations of available methods for reliable measurements of [Ca]m [4]. This caveat applies to many such studies irrespective whether the outcome favored model I or II. Below we discuss experimental data (and their limitations) supporting either model I or model II.
3.1. Model I: [Ca]m reflects the slow integration of cytosolic Ca transients
Evidence in support of slow mitochondrial integration of cytosolic Ca transients are based on studies utilizing electron probe microanalysis (EPMA) and fluorescence microscopy techniques. EPMA uses rapidly frozen tissue samples and has the advantages of a resolution close to electron microscopy, however it measures total mitochondrial Ca ([Ca]m,tot) instead of free [Ca]m. EPMA studies on hamster [95, 96] and rat [97] papillary muscles were unable to resolve fast changes in [Ca]m,tot, not even after β-adrenergic stimulation to increase Ca cycling [96, 98].
In a majority of studies, fluorescent dyes (such as the membrane permeable ester forms of indo-1, rhod-2 or fluo-3) known to compartmentalize into mitochondria were used to monitor [Ca]m directly. To eliminate the cytosolic component of the fluorescent signals, cells were treated with manganese [99–102] or cobalt [103], exposed to higher temperature [104, 105], membrane permeabilized [106, 107] or dialysed [66, 101]. Using indo-1 loaded rat [99, 105], hamster [100], ferret and cat [101] ventricular myocytes with subsequent Mn-quenching of cytosolic dye, it was shown that an increase in the stimulation frequency from 0.2 to 4 Hz in the presence of β-adrenergic stimulation [99, 104] or cellular Ca loading via sarcolemmal NCX [101] led to a slow rise of [Ca]m from ~100–200 to ~500–800 nM, however no beat-to beat changes in [Ca]m were observed. In the absence of β-adrenergic stimulation, only modest increases in [Ca]m could be achieved in rat myocytes by electrical stimulation at 2 Hz [100], which indicated that only large amplitude cytosolic Ca transients were sensed by mitochondria. Miyata et al. [99] demonstrated an exponential relationship between [Ca]m and [Ca]i, with a threshold for mitochondrial Ca uptake being at a [Ca]i of ~500 nM. Similarly, Zhou et al. [101] reported that under conditions of high cellular Ca load imposed by membrane depolarization in ferret and cat myocytes, phasic increases of [Ca]m could be detected, although they were slow and only observed at diastolic [Ca]i >400 nM. The authors concluded that mitochondria of intact cells did not take up detectible amounts of Ca during individual contractions. These findings are in agreement with a recent study where [Ca]m changes during cytosolic Ca transients were quantified [108]. Changes in [Ca]m during individual cytosolic Ca transients amounted to only approximately 2–10 nM per beat, but integrated gradually to a new steady-state. Total mitochondrial Ca uptake for larger [Ca]m transients amounted to only about 1% of the SR Ca uptake during a normal cytosolic Ca transient. However, there is evidence for species-dependent differences in the kinetics of [Ca]m as demonstrated in a comparison between rat and guinea pig cardiomyocytes where [Ca]m transients were observed in indo-1 loaded guinea-pig myocytes, while no changes in [Ca]m were observed in rat cardiomyocytes [105].
A general criticism of the experimental approach used in some of the studies outlined above is the possible interference of Mn with Ca transport across the IMM. Mn can be sequestered by the MCU, and Mn can potentially quench the fluorescence of the dye compartmentalized into mitochondria. While Miyata et al. [99] provided evidence that Mn had no effect on mitochondrial Ca uptake or cell shortening, other studies demonstrated that Mn significantly inhibited Ca efflux [109]. Nonetheless, removal of cytosolic indo-1 by heat treatment gave similar results to the Mn quench technique [104, 105]. Moreover, when fura-2 loaded rat heart mitochondria were exposed to Ca oscillations of 100 cycles/min, [Ca]m increased proportionally to the average rise in extramitochondrial [Ca], but independently of oscillation frequency [110].
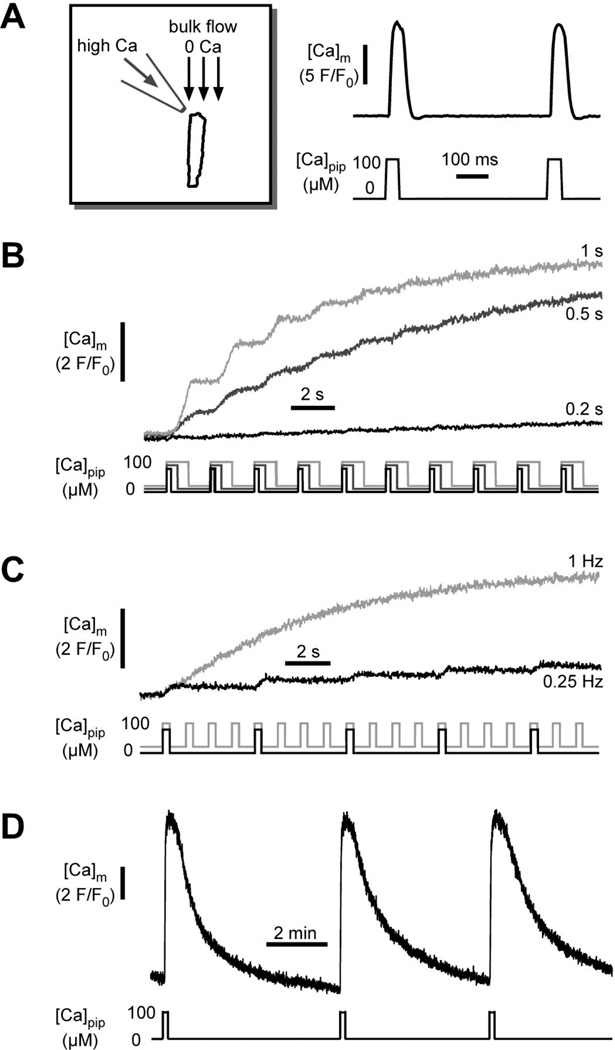
A novel experimental approach was developed in our laboratory bySedova et al. [106] to study [Ca]m kinetics in response to cytosolic Ca spikes (Fig. 2). The approach allowed the simulation of fast cytosolic Ca transients in membrane-permeabilized cells. [Ca]m was measured with fluo-3 entrapped inside mitochondria. Permeabilized cells have the unique advantage that the cytosolic environment can be controlled precisely (including complete removal of cytosolic Ca indicator dye) while the arrangements and interaction between intracellular membranes and organelles (SR, mitochondria) remain structurally and functionally intact [111, 112]. Control experiments indicated that mitochondrial Ca uptake was initiated only when [Ca]em was elevated above 0.5 µM, confirming the existence of a threshold for MCU activation [101, 107]. Ca entry via MCU exhibited a sigmoidal [Ca]em-dependence (half-maximal uptake rate at [Ca]em=4.4 µM). To simulate cytosolic Ca transients (Fig. 2A), cells were placed in the laminar flow of a Ca-free solution and then exposed to rapid ejections of a solution containing 100 µM Ca from a micropipette positioned upstream of the cell with regard to the direction of the bulk flow. Calculations revealed that with the applied technique cells were exposed to ~5 µM [Ca]em during each ejection pulse, which is in the range of physiological cytosolic Ca transient amplitudes and close to half-maximal activation of MCU. With this technique rapid beat-tobeat changes in [Ca]i were simulated by rapidly switching [Ca]em between low and high levels. The technique allowed to precisely vary pulse duration and pulse frequency. Exposure to a train of Ca transients evoked a gradual, but pulse duration- (Fig. 2B) and frequency-dependent (Fig. 2C) elevation of [Ca]m, however no [Ca]m oscillations were observed. As shown in Fig. 2D transient elevations of [Ca]m in response to a cytosolic Ca pulse could be observed, provided a sufficiently long time interval between pulses was allowed. The recovery time of the [Ca]m transients was in the range of minutes, i.e. orders of magnitude longer than the time interval between typical cytosolic Ca oscillations. Taken together, the data suggest that in cat ventricular myocytes fast cytosolic Ca transients are integrated by mitochondrial Ca transport systems resulting in a frequency- and pulse duration-dependent net accumulation of Ca in the matrix, thus supporting model I of mitochondrial decoding of fast cytosolic Ca signals. These data are complemented by the quantitative estimates of mitochondrial Ca uptake and its kinetics in response to SR Ca release by Andrienko et al., as discussed above [108].
Fig. 2. Measurements of [Ca]i to [Ca]m transmission in single permeabilized ventricular myocytes.
A) Technique used to simulate the effect of repetitive rapid cytosolic Ca transients on [Ca]m. Mitochondria were loaded with the fluorescent Ca indicator fluo-3. Cells were placed in the laminar flow of Ca-free solution (1 mM EGTA) and exposed to computer controlled pressure ejections of a 100 µM Ca containing solution from a micropipette. B) Effect of Ca pulse duration (0.2, 0.5 and 1 s) on [Ca]m at constant stimulation frequency (0.5 Hz). C) Effect of Ca pulse frequency (0.25 and 1 Hz) on [Ca]m at constant pulse duration (0.5 s). (Panels A, B and C from Sedova, Dedkova & Blatter, Am. J. Physiol. Cell Physiol., 291(5): C840-50, 2006). D) [Ca]m transients elicited by single Ca puffs applied at extended intervals. Extracellular [Na]o (20 mM) was set to allow for maximal Ca extrusion rates via mNCX.
3.2. Model II: Beat-to-beat oscillations of [Ca]m
Evidence in support of a beat-to-beat translation of cytosolic Ca transients into [Ca]m oscillations (model II; Fig. 1C) was first obtained in guinea pig myocytes using EPMA [113, 114]. Although changes [Ca]m,tot were resolved only in myocytes in which the SR Ca content and diastolic [Ca]i was enhanced, beat-to-beat variations in [Ca]m,tot, which followed cytosolic Ca transients with 15–25 ms delay, peaked within 30–45 ms, and reversed by 90 ms, could be demonstrated. The decline of the [Ca]m,tot transient was attributed to mNCX activity. In a later study [115] the same authors found that subsarcolemmal mitochondria responded to changes in [Ca]i with [Ca]m,tot transients, while central mitochondria did not.
Further support for fast mitochondrial [Ca]m transients came from studies using laser scanning confocal microscopy in combination with fluorescent Ca indicators (e.g. fluo-3, indo-1, rhod-2) entrapped in mitochondria and identifying mitochondrial locations by co-staining with 'mitochondrial markers' such as the voltage-sensitive dye TMRM or rhodamine 123 [116–119]. Recordings obtained during electrical or β-adrenergic stimulation revealed [Ca]m transients with an identical time course in mitochondria and cytosol. The lack of kinetic differences between the two signals raises the possibility that the mitochondrial signal was 'contaminated' to a significant degree with cytosolic signal due to the presence of indicator dye in the cytosol. Refining the dye loading protocol using different incubation temperatures it could be demonstrated that the majority of the signal arose from mitochondria since [Ca]m measured with rhod-2 was sensitive to ruthenium red, while [Ca]i measured with fluo-3 was only slightly affected [118, 119]. Moreover, Mackenzie et al. [120] were able to record mitochondrial [Ca]m transients in rat atrial myocytes with rhod-2 that were abolished by the mitochondrial inhibitors antimycin and oligomycin, while under identical experimental conditions cytosolic Ca transients could still be evoked. These authors also confirmed the importance of mitochondrial location, showing that during electrical stimulation peripherally located mitochondria sequestered most of the released Ca.
To circumvent the problem of signal contamination mentioned above, novel fluorescent probes were developed that could be genetically encoded and targeted specifically to the mitochondrial matrix compartment (for review see [4, 121]). Using the Ca-sensitive photoprotein aequorin and green fluorescent protein-based Ca indicator pericam as such probes [122], it could be demonstrated that spontaneous cytosolic Ca oscillations in cultured neonatal rat ventricular myocytes were synchronous with mitochondrial [Ca]m oscillations. Elevation of extracellular [Ca] or β-adrenergic stimulation resulted in a substantial increase in spike amplitude in both compartments, and increased inter-spike [Ca]m, suggesting that Ca extrusion from mitochondria was slower than mitochondrial Ca uptake, resulting in mitochondrial Ca accumulation. With a similar technique (adenoviral infection with aequorin targeted to cytosol and mitochondria) [Ca]m and [Ca]i were shown to increase simultaneously in adult rat myocytes in response to electrical stimulation, however the decay of [Ca]m was much slower [123], and the observation of [Ca]m transients required increased extracellular [Ca]o or β-adrenergic stimulation. The mitochondrial fluorescence signal was insensitive to the MCU inhibitor Ru360 (presumably due to poor membrane permeability of Ru360 in these cells), increased by the mNCX blocker clonazepam, and decreased by the mitochondrial uncoupler FCCP in combination with oligomycin.
Szalai et al. [124] showed that activation of RyRs by Ca, ryanodine or caffeine evoked cytosolic Ca oscillations that were synchronized with [Ca]m oscillations in cardiac H9c2 myotubes. However, the frequency of [Ca]m oscillations observed in this study was nearly an order of magnitude slower than physiological heart rates, and an increase in basal (inter-spike) [Ca]m was observed, suggesting that the rate of mitochondrial Ca removal was not fast enough to extrude all the Ca before the next cytosolic Ca spike. Addition of 30 µM Ca to the bath was required to match the rates of rise of the caffeine-induced [Ca]m transients, indicating that the local Ca in the microdomain near the mitochondria during SR Ca release is much higher than the average cytoplasmic concentration. In another study by the Hajnoczky group, more direct evidence of a local fast communication between the SR Ca release units and mitochondria was forwarded. Also in permeabilized H9c2 myotubes, local SR Ca release events in form of Ca sparks elicited [Ca]m transients in adjacent mitochondria, referred to as Ca marks (i.e. mitochondrial Ca sparks) [125]. This and other studies (e.g. [126, 127]) have provided strong evidence for the importance of a SR-mitochondria Ca signaling microdomain where a close physical association between the SR Ca release apparatus and mitochondria exists (for recent reviews see e.g. [128–130]). The functional and structural connection between SR and mitochondria is attributed to inter-organelle tether proteins [131–133], such as mitofusin 2 [134]. This microdomain enables elevations of local [Ca] possibly into the tens of micromolar range [135, 136] bringing it into the range of the relatively low Ca affinity of the uniporter [106, 129].
Evidence for fast mitochondrial Ca uptake also came from a recent study where cytosolic and mitochondrial Ca was monitored simultaneously in guinea-pig cardiomyocytes using a differential Ca dye loading technique [66]. The study showed that cytosolic Ca transients elicited by voltage-clamp depolarization were accompanied by fast [Ca]m transients, however the detection of [Ca]m transients required conditions of enhanced Ca cycling (β-adrenergic stimulation, elevated extracellular [Ca] or increased stimulation frequency). During β-adrenergic stimulation, the decay of [Ca]m was ~2.5-fold slower than of [Ca]i, leading to a stepwise accumulation [Ca]m during rapid pacing. Remarkably, the upstroke of the [Ca]m transient preceded the rise of [Ca]i. This raises the question of possible contamination of the mitochondrial Ca signal by cytosolic rhod-2 as traces of the indicator dye could have remained in the cytosol even after dialysis. Nonetheless, directionally opposite effects on the [Ca]m and [Ca]i signals were elicited by inhibitors of mitochondrial Ca uptake or efflux, underpinning the mitochondrial origin of the signal and indicating that a significant fraction of the Ca released by the SR was buffered by the mitochondria with every beat. Maack et al. [66] also presented a new computational model that included mitochondrial microdomains in cardiomyocytes, where pulses of high [Ca]em (10 or 20 µM) and 50 ms duration were simulated. This model predicted changes in [Ca]m reminiscent of both, model I and model II described in this review. Similarly to model II, beat-to-beat [Ca]m oscillations were simulated with extramitochondrial Ca pulses applied at 1 Hz, but with increasing frequency or amplitude of stimulation, diastolic [Ca]m rose slowly until a new steady-state level was reached (reminiscent of model I). Recently beat-to-beat mitochondrial [Ca]m transients were also reported using the Ca-sensitive inverse pericam Mitycam [137] targeted to mitochondria with a mitochondria-targeting sequence (subunit VIII of human cytochrome c oxidase) and adenovirally expressed in cardiomyocytes [138]. With the Mitycam probe sustained and transient phases of mitochondrial Ca signals were observed, which were dependent on [Ca]i levels and required a functional MCU. Furthermore, in rat neonatal cardiomvocytes cvtoplasmic Ca transients were reduced or enhanced by MCU overexpression or siRNA silencing, respectively, using novel targeted Ca probes. The data provide evidence that mitochondrial Ca uptake contributes to buffering of cytoplasmic Ca peaks in cardiomyocvtes [139].
3.3. Fast versus slow mitochondrial Ca uptake: mutually exclusive or requirement for both?
Whether mitochondria can sequester Ca, even in large quantities, is not at debate. The controversies center around the kinetics and magnitude of mitochondrial Ca uptake. Arguments have been made in favor of or against beat-to-beat mitochondrial Ca uptake and the question of energetic cost efficiency of the translation of cytosolic Ca oscillations into oscillations of [Calm have been raised [3, 9]. The mitochondrial Ca sink function that helps protect against cytosolic Ca overload requires high Ca buffering power, rather than fast uptake. From an ECC point of view, substantial beat-to-beat mitochondrial Ca sequestration would curtail the cytosolic Ca transient, and consequently, larger quantities of Ca are required to be shuttled, at increased energetic costs, between extracellular space, cytosol, SR and mitochondria to achieve [Ca]i, levels necessary for contraction. However, rapid and frequent changes in metabolic demands of the working heart may indeed necessitate a rapid mitochondrial Ca response and fast mitochondrial Ca uptake to stimulate Ca-dependent dehydrogenases of the TCA cycle [67], Future studies are likely to demonstrate that there is a role for both, rapid uptake in response to cytoslic Ca signals and a slow integration of changes in [Ca]i. Indeed, consistent with this notion a recent study demonstrated two modes of operation of MCU, one with a proposed role for energetic signaling, the other serving as a cellular Ca sink [140] (see also section 4).
4. Mitochondrial Ca and mPTP
Excessive mitochondrial Ca uptake and Ca accumulation bears the risk of mPTP activation [3], potentially leading to irreversible ΔΨ collapse, cessation of mitochondrial respiration and ultimately cell death. Activation of mPTP is facilitated by elevated levels of matrix [Ca]m and ROS. We have recently shown that a mitochondrial Ca-dependent nitric oxide synthase [141] can become uncoupled and turn into a significant source of ROS, which together with enhanced mitochondrial Ca accumulation, significantly increased the risk of deleterious mPTP opening. According to the redox-optimized ROS balance model proposed by Aon et al. [142] mitochondria are required to operate in an intermediate redox state to maximize energy output and keep ROS generation minimal. In an attempt to define the relationship between total mitochondrial Ca uptake, changes in mitochondrial matrix free Ca, and mPTP activation, Wei et al. [140] measured changes in [Ca]em and [Ca]m during Ca uptake in isolated cardiac mitochondria and identified two components of Ca influx, termed MCUmode1 and MCUmode2, with differential sensitivity to the MCU inhibitor Ru360, Ca transport kinetics and capacities, and Ca buffering associated with the respective pathway. Intra-mitochondrial Ca buffering has been assumed to be in the order of 100:1 (bound:free Ca) [108], however, according to Wei et al. [140] mitochondrial Ca buffering is highly dynamic, and the differential responses of [Ca]m to Ca entry results from a two-component buffer system comprised of static Ca buffers and dynamic Ca buffering by phosphate that enters together with Ca. The authors interpreted their results that the role of MCUmode1 might be to modulate oxidative phosphorylation in response to intracellular Ca signaling, whereas MCUmode2 and a dynamic high-capacity Ca buffering system through calcium-phosphate complex formation constitute a Ca sink function. Furthermore, evidence was provided that the trigger for mPTP activation is unlikely to be [Ca]m itself, but rather a downstream byproduct of total mitochondrial Ca loading. In agreement with this study separate components of mitochondrial Ca accumulations were also identified in brain and liver mitochondria that serve matrix dehydrogenase regulation, buffering of extramitochondrial free Ca, and mPTP activation [143].
5. Inorganic polyphosphate - a mediator of Ca-dependent mPTP activation?
A critical link between mitochondrial Ca influx, mitochondrial Ca buffering and activation of the mPTP has recently been proposed by Seidlmayer et al. [144, 145]. In this study we tested the hypothesis that the adverse effect of mitochondrial Ca accumulation followed by mPTP opening is mediated by its interaction with inorganic polyphosphate (polyP), a polymer of orthophosphates (average length of 25 orthophosphates) that is found in cardiac mitochondria in significant amounts (280 ± 60 pmol/mg of protein). Depletion of polyP in mitochondria of rabbit ventricular myocytes led to significant inhibition of mPTP opening. This effect was observed when mitochondrial Ca uptake was stimulated by increasing cytosolic [Ca]i in permeabilized myocytes mimicking mitochondrial Ca overload as observed e.g. during ischemia-reperfusion injury [19]. The results of this study indicate that inorganic polyP is a previously unrecognized major activator of mPTP and lend support to the hypothesis that the adverse effects of polyP might be caused by its ability to form stable complexes with Ca and directly contribute to IMM permeabilization, raising even the possibility that a Ca/PolyP complex forms an integral part of the mPTP.
6. Conclusions
While substantial efforts were undertaken in recent years to characterize the properties and kinetics of mitochondrial Ca cycling, the experimental approaches and techniques used to date have essentially failed to reach unequivocal conclusions whether and how cardiac mitochondria respond to cytosolic Ca oscillations in a rapid beat-to-beat fashion. Despite experimental and technical limitations and shortcomings, it is needless to say that Ca is a key second messenger for the regulation of mitochondrial tasks and represents a crucial link for the role of mitochondria for excitation-metabolism as well as excitation-contraction coupling in the heart. Recent findings indicate that mitochondrial Ca transport capacities and kinetics are more complex and variable than previously assumed. The coexistence of rapid Ca uptake capabilities and slower, but high-capacity Ca buffering properties simply underpins the versatility of Ca signaling that is genuine for these organelles. Furthermore (and only discussed with a few illustrative examples in this review) the development of advanced computational models of energy metabolism, mitochondrial ion dynamics, redox signaling and mPTP regulation has significantly advanced the field and generated unprecedented novel insights into these complexities of mitochondrial dynamics [146–153]. Combined computational-experimental approaches [66, 154] will increasingly help overcome experimental limitations, validate computational approaches and generate new hypotheses on energy metabolism and mitochondrial signaling.
Highlights.
-
-
Mitochondria occupy a third of the cell volume of cardiac myocytes
-
-
Mitochondria have an elaborate system of calcium uptake and extrusion
-
-
mechanisms
-
-
Mitochondrial Ca signaling is crucial for excitation-metabolism-contraction coupling
-
-
Mitochondria decode cytosolic Ca signals to respond to energy demands and buffer Ca
-
-
Beat-to-beat transmission or slow integration of cytosolic Ca signals are debated
Acknowledgements
This work was supported by the National Institutes of Health Grants HL62231, HL80101 and HL101235, the Leducq Foundation (to LAB), and the American Heart Association, National Scientist Development Grant AHA 0735071N and Rush University Medical Center New Investigator Grant-in-Aid 31196 (to END).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
None.
References
- 1.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415:198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 2.Huser J, Blatter LA, Sheu SS. Mitochondrial calcium in heart cells: beat-to- beat oscillations or slow integration of cytosolic transients? J Bioenerg Biomembr. 2000;32:27–33. doi: 10.1023/a:1005556227425. [DOI] [PubMed] [Google Scholar]
- 3.Dedkova EN, Blatter LA. Mitochondrial Ca(2+) and the heart. Cell Calcium. 2008;44:77–91. doi: 10.1016/j.ceca.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Dedkova EN, Blatter LA. Measuring mitochondrial function in intact cardiac myocytes. J Mol Cell Cardiol. 2012;52:48–61. doi: 10.1016/j.yjmcc.2011.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O-Uchi J, Pan S, Sheu SS. Perspectives on: SGP symposium on mitochondrial physiology and medicine: molecular identities of mitochondrial Ca2+ influx mechanism: updated passwords for accessing mitochondrial Ca2+- linked health and disease. J Gen Physiol. 2012;139:435–443. doi: 10.1085/jgp.201210795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akar FG, O'Rourke B. Mitochondria are sources of metabolic sink and arrhythmias. Pharmacol Ther. 2011;131:287–294. doi: 10.1016/j.pharmthera.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gunter TE, Sheu SS. Characteristics and possible functions of mitochondrial Ca(2+) transport mechanisms. Biochim Biophys Acta. 2009;1787:1291–1308. doi: 10.1016/j.bbabio.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walsh C, Barrow S, Voronina S, Chvanov M, Petersen OH, Tepikin A. Modulation of calcium signalling by mitochondria. Biochim Biophys Acta. 2009;1787:1374–1382. doi: 10.1016/j.bbabio.2009.01.007. [DOI] [PubMed] [Google Scholar]
- 9.O'Rourke B, Blatter LA. Mitochondrial Ca2+ uptake: tortoise or hare? J Mol Cell Cardiol. 2009;46:767–774. doi: 10.1016/j.yjmcc.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Balaban RS, Bose S, French SA, Territo PR. Role of calcium in metabolic signaling between cardiac sarcoplasmic reticulum and mitochondria in vitro. Am J Physiol Cell Physiol. 2003;284:C285–C293. doi: 10.1152/ajpcell.00129.2002. [DOI] [PubMed] [Google Scholar]
- 11.Territo PR, Mootha VK, French SA, Balaban RS. Ca(2+) activation of heart mitochondrial oxidative phosphorylation: role of the F(0)/F(1)-ATPase. Am J Physiol Cell Physiol. 2000;278:C423–C435. doi: 10.1152/ajpcell.2000.278.2.C423. [DOI] [PubMed] [Google Scholar]
- 12.Glancy B, Balaban RS. Role of mitochondrial Ca2+ in the regulation of cellular energetics. Biochemistry. 2012;51:2959–2973. doi: 10.1021/bi2018909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stanley WC, Recchia FA, Lopaschuk GD. Myocardial substrate metabolism in the normal and failing heart. Physiol Rev. 2005;85:1093–1129. doi: 10.1152/physrev.00006.2004. [DOI] [PubMed] [Google Scholar]
- 14.Saks V, Favier R, Guzun R, Schlattner U, Wallimann T. Molecular system bioenergetics: regulation of substrate supply in response to heart energy demands. J Physiol. 2006;577:769–777. doi: 10.1113/jphysiol.2006.120584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.McBride HM, Neuspiel M, Wasiak S. Mitochondria: more than just a powerhouse. Curr Biol. 2006;16:R551–R560. doi: 10.1016/j.cub.2006.06.054. [DOI] [PubMed] [Google Scholar]
- 16.Rosca MG, Hoppel CL. Mitochondria in heart failure. Cardiovasc Res. 2010;88:40–50. doi: 10.1093/cvr/cvq240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffiths EJ. Mitochondria and heart disease. Adv Exp Med Biol. 2012;942:249–267. doi: 10.1007/978-94-007-2869-1_11. [DOI] [PubMed] [Google Scholar]
- 18.Gustafsson AB, Gottlieb RA. Heart mitochondria: gates of life and death. Cardiovasc Res. 2008;77:334–343. doi: 10.1093/cvr/cvm005. [DOI] [PubMed] [Google Scholar]
- 19.Halestrap AP. Calcium, mitochondria and reperfusion injury: a pore way to die. Biochem Soc Trans. 2006;34:232–237. doi: 10.1042/BST20060232. [DOI] [PubMed] [Google Scholar]
- 20.Barth E, Stammler G, Speiser B, Schaper J. Ultrastructural quantitation of mitochondria and myofilaments in cardiac muscle from 10 different animal species including man. J Mol Cell Cardiol. 1992;24:669–681. doi: 10.1016/0022-2828(92)93381-s. [DOI] [PubMed] [Google Scholar]
- 21.Bers DM. Excitation-Contraction Coupling and Cardiac Contractile Force. 2nd ed. Dordrecht, Netherlands: Kluwer; 2001. [Google Scholar]
- 22.Drago I, Pizzo P, Pozzan T. After half a century mitochondrial calcium in- and efflux machineries reveal themselves. EMBO J. 2011;30:4119–4125. doi: 10.1038/emboj.2011.337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Csordas G, Varnai P, Golenar T, Sheu SS, Hajnoczky G. Calcium transport across the inner mitochondrial membrane: molecular mechanisms and pharmacology. Mol Cell Endocrinol. 2012;353:109–113. doi: 10.1016/j.mce.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gunter TE, Gunter KK, Sheu SS, Gavin CE. Mitochondrial calcium transport: physiological and pathological relevance. Am J Physiol. 1994;267:C313–C339. doi: 10.1152/ajpcell.1994.267.2.C313. [DOI] [PubMed] [Google Scholar]
- 25.Gunter TE, Pfeiffer DR. Mechanisms by which mitochondria transport calcium. Am J Physiol. 1990;258:C755–C786. doi: 10.1152/ajpcell.1990.258.5.C755. [DOI] [PubMed] [Google Scholar]
- 26.De Stefani D, Raffaello A, Teardo E, Szabo I, Rizzuto R. A forty-kilodalton protein of the inner membrane is the mitochondrial calcium uniporter. Nature. 2011;476:336–340. doi: 10.1038/nature10230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baughman JM, Perocchi F, Girgis HS, Plovanich M, Belcher-Timme CA, Sancak Y, et al. Integrative genomics identifies MCU as an essential component of the mitochondrial calcium uniporter. Nature. 2011;476:341–345. doi: 10.1038/nature10234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buntinas L, Gunter KK, Sparagna GC, Gunter TE. The rapid mode of calcium uptake into heart mitochondria (RaM): comparison to RaM in liver mitochondria. Biochim BiophysActa. 2001;1504:248–261. doi: 10.1016/s0005-2728(00)00254-1. [DOI] [PubMed] [Google Scholar]
- 29.Sparagna GC, Gunter KK, Sheu SS, Gunter TE. Mitochondrial calcium uptake from physiological-type pulses of calcium. A description of the rapid uptake mode. J Biol Chem. 1995;270:27510–27515. doi: 10.1074/jbc.270.46.27510. [DOI] [PubMed] [Google Scholar]
- 30.Gunter TE, Buntinas L, Sparagna G, Eliseev R, Gunter K. Mitochondrial calcium transport: mechanisms and functions. Cell Calcium. 2000;28:285–296. doi: 10.1054/ceca.2000.0168. [DOI] [PubMed] [Google Scholar]
- 31.Bazil JN, Dash RK. A minimal model for the mitochondrial rapid mode of Ca(2)+ uptake mechanism. PLoS One. 2011;6:e21324. doi: 10.1371/journal.pone.0021324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Beutner G, Sharma VK, Lin L, Ryu SY, Dirksen RT, Sheu SS. Type 1 ryanodine receptor in cardiac mitochondria: transducer of excitation-metabolism coupling. Biochim BiophysActa. 2005;1717:1–10. doi: 10.1016/j.bbamem.2005.09.016. [DOI] [PubMed] [Google Scholar]
- 33.Beutner G, Sharma VK, Giovannucci DR, Yule DI, Sheu SS. Identification of a ryanodine receptor in rat heart mitochondria. J Biol Chem. 2001;276:21482–21488. doi: 10.1074/jbc.M101486200. [DOI] [PubMed] [Google Scholar]
- 34.Altschafl BA, Beutner G, Sharma VK, Sheu SS, Valdivia HH. The mitochondrial ryanodine receptor in rat heart: a pharmaco-kinetic profile. Biochim Biophys Acta. 2007;1768:1784–1795. doi: 10.1016/j.bbamem.2007.04.011. [DOI] [PubMed] [Google Scholar]
- 35.Ryu SY, Beutner G, Kinnally KW, Dirksen RT, Sheu SS. Single channel characterization of the mitochondrial ryanodine receptor in heart mitoplasts. J Biol Chem. 2011;286:21324–21329. doi: 10.1074/jbc.C111.245597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Santo-Domingo J, Demaurex N. Calcium uptake mechanisms of mitochondria. Biochim BiophysActa. 2010;1797:907–912. doi: 10.1016/j.bbabio.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 37.Jiang D, Zhao L, Clapham DE. Genome-wide RNAi screen identifies Letm1 as a mitochondrial Ca2+/H+ antiporter. Science. 2009;326:144–147. doi: 10.1126/science.1175145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nowikovsky K, Pozzan T, Rizzuto R, Scorrano L, Bernardi P. Perspectives on: SGP symposium on mitochondrial physiology and medicine: the pathophysiology of LETM1. J Gen Physiol. 2012;139:445–454. doi: 10.1085/jgp.201110757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kirichok Y, Krapivinsky G, Clapham DE. The mitochondrial calcium uniporter is a highly selective ion channel. Nature. 2004;427:360–364. doi: 10.1038/nature02246. [DOI] [PubMed] [Google Scholar]
- 40.Bernardi P. Mitochondrial transport of cations: channels, exchangers, and permeability transition. Physiol Rev. 1999;79:1127–1155. doi: 10.1152/physrev.1999.79.4.1127. [DOI] [PubMed] [Google Scholar]
- 41.Matlib MA, Zhou Z, Knight S, Ahmed S, Choi KM, Krause-Bauer J, et al. Oxygen-bridged dinuclear ruthenium amine complex specifically inhibits Ca2+ uptake into mitochondria in vitro and in situ in single cardiac myocytes. J Biol Chem. 1998;273:10223–10231. doi: 10.1074/jbc.273.17.10223. [DOI] [PubMed] [Google Scholar]
- 42.Mallilankaraman K, Cardenas C, Doonan PJ, Chandramoorthy HC, Irrinki KM, Golenar T, et al. MCUR1 is an essential component of mitochondrial Ca(2+) uptake that regulates cellular metabolism. Nature cell biology. 2012;14:1336–1343. doi: 10.1038/ncb2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perocchi F, Gohil VM, Girgis HS, Bao XR, McCombs JE, Palmer AE, et al. MICU1 encodes a mitochondrial EF hand protein required for Ca(2+) uptake. Nature. 2010;467:291–296. doi: 10.1038/nature09358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallilankaraman K, Doonan P, Cardenas C, Chandramoorthy HC, Muller M, Miller R, et al. MICU1 Is an Essential Gatekeeper for MCU-Mediated Mitochondrial Ca(2+) Uptake that Regulates Cell Survival. Cell. 2012;151:630–644. doi: 10.1016/j.cell.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Joiner ML, Koval OM, Li J, He BJ, Allamargot C, Gao Z, et al. CaMKII determines mitochondrial stress responses in heart. Nature. 2012;491:269–273. doi: 10.1038/nature11444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Michels G, Khan IF, Endres-Becker J, Rottlaender D, Herzig S, Ruhparwar A, et al. Regulation of the human cardiac mitochondrial Ca2+ uptake by 2 different voltage-gated Ca2+ channels. Circulation. 2009;119:2435–2443. doi: 10.1161/CIRCULATIONAHA.108.835389. [DOI] [PubMed] [Google Scholar]
- 47.Bogeski I, Gulaboski R, Kappl R, Mirceski V, Stefova M, Petreska J, et al. Calcium binding and transport by coenzyme Q. J Am Chem Soc. 2011;133:9293–9303. doi: 10.1021/ja110190t. [DOI] [PubMed] [Google Scholar]
- 48.Trenker M, Malli R, Fertschai I, Levak-Frank S, Graier WF. Uncoupling proteins 2 and 3 are fundamental for mitochondrial Ca2+ uniport. Nat Cell Biol. 2007;9:445–452. doi: 10.1038/ncb1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gunter KK, Gunter TE. Transport of calcium by mitochondria. J Bioenerg Biomembr. 1994;26:471–485. doi: 10.1007/BF00762732. [DOI] [PubMed] [Google Scholar]
- 50.Gunter TE, Yule DI, Gunter KK, Eliseev RA, Salter JD. Calcium and mitochondria. FEBS Lett. 2004;567:96–102. doi: 10.1016/j.febslet.2004.03.071. [DOI] [PubMed] [Google Scholar]
- 51.Rosier RN, Tucker DA, Meerdink S, Jain I, Gunter TE. Ca2+ transport against its electrochemical gradient in cytochrome oxidase vesicles reconstituted with mitochondrial hydrophobic proteins. Arch Biochem Biophys. 1981;210:549–564. doi: 10.1016/0003-9861(81)90221-6. [DOI] [PubMed] [Google Scholar]
- 52.Crompton M, Kunzi M, Carafoli E. The calcium-induced and sodium-induced effluxes of calcium from heart mitochondria. Evidence for a sodium-calcium carrier. Eur J Biochem. 1977;79:549–558. doi: 10.1111/j.1432-1033.1977.tb11839.x. [DOI] [PubMed] [Google Scholar]
- 53.Palty R, Hershfinkel M, Sekler I. Molecular, Identity and Functional Properties of the Mitochondrial Na+/Ca2+ Exchanger. J Biol Chem. 2012;287:31650–31657. doi: 10.1074/jbc.R112.355867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Palty R, Silverman WF, Hershfinkel M, Caporale T, Sensi SL, Parnis J, et al. NCLX is an essential component of mitochondrial Na+/Ca2+ exchange. Proc Natl Acad Sci USA. 2010;107:436–441. doi: 10.1073/pnas.0908099107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Saotome M, Katoh H, Satoh H, Nagasaka S, Yoshihara S, Terada H, et al. Mitochondrial membrane potential modulates regulation of mitochondrial Ca2+ in rat ventricular myocytes. Am J Physiol Heart Circ Physiol. 2005;288:H1820–H1828. doi: 10.1152/ajpheart.00589.2004. [DOI] [PubMed] [Google Scholar]
- 56.Bers DM, Barry WH, Despa S. Intracellular Na+ regulation in cardiac myocytes. Cardiovasc Res. 2003;57:897–912. doi: 10.1016/s0008-6363(02)00656-9. [DOI] [PubMed] [Google Scholar]
- 57.Fry CH, Powell T, Twist VW, Ward JP. The effects of sodium, hydrogen and magnesium ions on mitochondrial calcium sequestration in adult rat ventricular myocytes. Proc R Soc Lond B Biol Sci. 1984;223:239–254. doi: 10.1098/rspb.1984.0092. [DOI] [PubMed] [Google Scholar]
- 58.Cox DA, Matlib MA. A role for the mitochondrial Na(+)-Ca2+ exchanger in the regulation of oxidative phosphorylation in isolated heart mitochondria. J Biol Chem. 1993;268:938–947. [PubMed] [Google Scholar]
- 59.Despa S, Bers DM. Na/K pump current and [Na](i) in rabbit ventricular myocytes: local [Na](i) depletion and Na buffering. Biophys J. 2003;84:4157–4166. doi: 10.1016/S0006-3495(03)75140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Despa S, Islam MA, Pogwizd SM, Bers DM. Intracellular [Na+] and Na+ pump rate in rat and rabbit ventricular myocytes. J Physiol. 2002;539:133–143. doi: 10.1113/jphysiol.2001.012940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Despa S, Kockskamper J, Blatter LA, Bers DM. Na/K pump-induced [Na]i gradients in rat ventricular myocytes measured with two-photon microscopy. Biophys J. 2004;87:1360–1368. doi: 10.1529/biophysj.103.037895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Blatter LA, McGuigan JA. Intracellular pH regulation in ferret ventricular muscle. The role of Na-H exchange and the influence of metabolic substrates. Circ Res. 1991;68:150–161. doi: 10.1161/01.res.68.1.150. [DOI] [PubMed] [Google Scholar]
- 63.Kohlhaas M, Liu T, Knopp A, Zeller T, Ong MF, Bohm M, et al. Elevated cytosolic Na+ increases mitochondrial formation of reactive oxygen species in failing cardiac myocytes. Circulation. 2010;121:1606–1613. doi: 10.1161/CIRCULATIONAHA.109.914911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pieske B, Houser SR. [Na+]i handling in the failing human heart. Cardiovasc Res. 2003;57:874–886. doi: 10.1016/s0008-6363(02)00841-6. [DOI] [PubMed] [Google Scholar]
- 65.Wei AC, Liu T, Cortassa S, Winslow RL, O'Rourke B. Mitochondrial Ca2+ influx and efflux rates in guinea pig cardiac mitochondria: low and high affinity effects of cyclosporine A. Biochimica et biophysica acta. 2011;1813:1373–1381. doi: 10.1016/j.bbamcr.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Maack C, Cortassa S, Aon MA, Ganesan AN, Liu T, O'Rourke B. Elevated cytosolic Na+ decreases mitochondrial Ca2+ uptake during excitation-contraction coupling and impairs energetic adaptation in cardiac myocytes. Circ Res. 2006;99:172–182. doi: 10.1161/01.RES.0000232546.92777.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Liu T, O'Rourke B. Enhancing mitochondrial Ca2+ uptake in myocytes from failing hearts restores energy supply and demand matching. Circ Res. 2008;103:279–288. doi: 10.1161/CIRCRESAHA.108.175919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Baysal K, Jung DW, Gunter KK, Gunter TE, Brierley GP. Na(+)-dependent Ca2+ efflux mechanism of heart mitochondria is not a passive Ca2+/2Na+ exchanger. Am J Physiol. 1994;266:C800–C808. doi: 10.1152/ajpcell.1994.266.3.C800. [DOI] [PubMed] [Google Scholar]
- 69.Jung DW, Baysal K, Brierley GP. The sodium-calcium antiport of heart mitochondria is not electroneutral. J Biol Chem. 1995;270:672–678. doi: 10.1074/jbc.270.2.672. [DOI] [PubMed] [Google Scholar]
- 70.Kim B, Matsuoka S. Cytoplasmic Na+-dependent modulation of mitochondrial Ca2+ via electrogenic mitochondrial Na+-Ca2+ exchange. The Journal of physiology. 2008;586:1683–1697. doi: 10.1113/jphysiol.2007.148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dash RK, Beard DA. Analysis of cardiac mitochondrial Na+-Ca2+ exchanger kinetics with a biophysical model of mitochondrial Ca2+ handling suggests a 3:1 stoichiometry. J Physiol. 2008;586:3267–3285. doi: 10.1113/jphysiol.2008.151977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Murphy E, Eisner DA. Regulation of intracellular and mitochondrial sodium in health and disease. Circ Res. 2009;104:292–303. doi: 10.1161/CIRCRESAHA.108.189050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jurkowitz MS, Brierley GP. H+-dependent efflux of Ca2+ from heart mitochondria. J Bioenerg Biomembr. 1982;14:435–449. doi: 10.1007/BF00743069. [DOI] [PubMed] [Google Scholar]
- 74.Bernardi P, von Stockum S. The permeability transition pore as a Ca(2+) release channel: new answers to an old question. Cell Calcium. 2012;52:22–27. doi: 10.1016/j.ceca.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bernardi P, Broekemeier KM, Pfeiffer DR. Recent progress on regulation of the mitochondrial permeability transition pore; a cyclosporin-sensitive pore in the inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:509–517. doi: 10.1007/BF00762735. [DOI] [PubMed] [Google Scholar]
- 76.Di Lisa F, Bernardi P. A CaPful of mechanisms regulating the mitochondrial permeability transition. J Mol Cell Cardiol. 2009;46:775–780. doi: 10.1016/j.yjmcc.2009.03.006. [DOI] [PubMed] [Google Scholar]
- 77.Crompton M, Ellinger H, Costi A. Inhibition by cyclosporin A of a Ca2+-dependent pore in heart mitochondria activated by inorganic phosphate and oxidative stress. Biochem J. 1988;255:357–360. [PMC free article] [PubMed] [Google Scholar]
- 78.Zorov DB, Juhaszova M, Yaniv Y, Nuss HB, Wang S, Sollott SJ. Regulation and pharmacology of the mitochondrial permeability transition pore. Cardiovasc Res. 2009;83:213–225. doi: 10.1093/cvr/cvp151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Halestrap AP. What is the mitochondrial permeability transition pore? J Mol Cell Cardiol. 2009;46:821–831. doi: 10.1016/j.yjmcc.2009.02.021. [DOI] [PubMed] [Google Scholar]
- 80.Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- 81.Fontaine E, Eriksson O, Ichas F, Bernardi P. Regulation of the permeability transition pore in skeletal muscle mitochondria. Modulation By electron flow through the respiratory chain complex i. J Biol Chem. 1998;273:12662–12668. doi: 10.1074/jbc.273.20.12662. [DOI] [PubMed] [Google Scholar]
- 82.Li B, Chauvin C, De Paulis D, De Oliveira F, Gharib A, Vial G, et al. Inhibition of complex I regulates the mitochondrial permeability transition through a phosphate-sensitive inhibitory site masked by cyclophilin D. Biochimica et biophysica acta. 2012;1817:1628–1634. doi: 10.1016/j.bbabio.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 83.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, et al. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 84.Baines CP, Kaiser RA, Sheiko T, Craigen WJ, Molkentin JD. Voltage-dependent anion channels are dispensable for mitochondrial-dependent cell death. Nat Cell Biol. 2007;9:550–555. doi: 10.1038/ncb1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zoratti M, Szabo I. Electrophysiology of the inner mitochondrial membrane. J Bioenerg Biomembr. 1994;26:543–553. doi: 10.1007/BF00762739. [DOI] [PubMed] [Google Scholar]
- 86.Zoratti M, Szabo I. The mitochondrial permeability transition. Biochim BiophysActa. 1995;1241:139–176. doi: 10.1016/0304-4157(95)00003-a. [DOI] [PubMed] [Google Scholar]
- 87.Huser J, Blatter LA. Fluctuations in mitochondrial membrane potential caused by repetitive gating of the permeability transition pore. Biochem J. 1999;343(Pt 2):311–317. [PMC free article] [PubMed] [Google Scholar]
- 88.Huser J, Rechenmacher CE, Blatter LA. Imaging the permeability pore transition in single mitochondria. Biophys J. 1998;74:2129–2137. doi: 10.1016/S0006-3495(98)77920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Broekemeier KM, Klocek CK, Pfeiffer DR. Proton selective substate of the mitochondrial permeability transition pore: regulation by the redox state of the electron transport chain. Biochemistry. 1998;37:13059–13065. doi: 10.1021/bi980820c. [DOI] [PubMed] [Google Scholar]
- 90.Ichas F, Jouaville LS, Mazat JP. Mitochondria are excitable organelles capable of generating and conveying electrical and calcium signals. Cell. 1997;89:1145–1153. doi: 10.1016/s0092-8674(00)80301-3. [DOI] [PubMed] [Google Scholar]
- 91.Elrod JW, Wong R, Mishra S, Vagnozzi RJ, Sakthievel B, Goonasekera SA, et al. Cyclophilin D controls mitochondrial pore-dependent Ca(2+) exchange, metabolic flexibility, and propensity for heart failure in mice. J Clin Invest. 2010;120:3680–3687. doi: 10.1172/JCI43171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Saotome M, Katoh H, Yaguchi Y, Tanaka T, Urushida T, Satoh H, et al. Transient opening of mitochondrial permeability transition pore by reactive oxygen species protects myocardium from ischemia-reperfusion injury. American journal of physiology Heart and circulatory physiology. 2009;296:H1125–H1132. doi: 10.1152/ajpheart.00436.2008. [DOI] [PubMed] [Google Scholar]
- 93.Hoek JB, Farber JL, Thomas AP, Wang X. Calcium ion-dependent signalling and mitochondrial dysfunction: mitochondrial calcium uptake during hormonal stimulation in intact liver cells and its implication for the mitochondrial permeability transition. Biochim Biophys Acta. 1995;1271:93–102. doi: 10.1016/0925-4439(95)00015-v. [DOI] [PubMed] [Google Scholar]
- 94.Crompton FM. The role of Ca2+ in the function and dysfunction of heart mitochondria. In: Langer GA, editor. Calcium and the Heart. New York: Raven Press; 1990. pp. 167–198. [Google Scholar]
- 95.Moravec CS, Bond M. Calcium is released from the junctional sarcoplasmic reticulum during cardiac muscle contraction. Am J Physiol. 1991;260:H989–H997. doi: 10.1152/ajpheart.1991.260.3.H989. [DOI] [PubMed] [Google Scholar]
- 96.Moravec CS, Bond M. Effect of inotropic stimulation on mitochondrial calcium in cardiac muscle. J Biol Chem. 1992;267:5310–5316. [PubMed] [Google Scholar]
- 97.Horikawa Y, Goel A, Somlyo AP, Somlyo AV. Mitochondrial calcium in relaxed and tetanized myocardium. Biophys J. 1998;74:1579–1590. doi: 10.1016/S0006-3495(98)77869-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Moravec CS, Desnoyer RW, Milovanovic M, Schluchter MD, Bond M. Mitochondrial calcium content in isolated perfused heart: effects of inotropic stimulation. Am J Physiol. 1997;273:H1432–H1439. doi: 10.1152/ajpheart.1997.273.3.H1432. [DOI] [PubMed] [Google Scholar]
- 99.Miyata H, Silverman HS, Sollott SJ, Lakatta EG, Stern MD, Hansford RG. Measurement of mitochondrial free Ca2+ concentration in living single rat cardiac myocytes. Am J Physiol. 1991;261:H1123–H1134. doi: 10.1152/ajpheart.1991.261.4.H1123. [DOI] [PubMed] [Google Scholar]
- 100.Di Lisa F, Gambassi G, Spurgeon H, Hansford RG. Intramitochondrial free calcium in cardiac myocytes in relation to dehydrogenase activation. Cardiovasc Res. 1993;27:1840–1844. doi: 10.1093/cvr/27.10.1840. [DOI] [PubMed] [Google Scholar]
- 101.Zhou Z, Matlib MA, Bers DM. Cytosolic and mitochondrial Ca2+ signals in patch clamped mammalian ventricular myocytes. J Physiol. 1998;507(Pt 2):379–403. doi: 10.1111/j.1469-7793.1998.379bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Schreur JH, Figueredo VM, Miyamae M, Shames DM, Baker AJ, Camacho SA. Cytosolic and mitochondrial [Ca2+] in whole hearts using indo-1 acetoxymethyl ester: effects of high extracellular Ca2+ Biophys J. 1996;70:2571–2580. doi: 10.1016/S0006-3495(96)79828-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Davidson SM, Yellon D, Duchen MR. Assessing mitochondrial potential, calcium, and redox state in isolated mammalian cells using confocal microscopy. Methods Mol Biol. 2007;372:421–430. doi: 10.1007/978-1-59745-365-3_30. [DOI] [PubMed] [Google Scholar]
- 104.Griffiths EJ, Stern MD, Silverman HS. Measurement of mitochondrial calcium in single living cardiomyocytes by selective removal of cytosolic indo 1. Am J Physiol. 1997;273:C37–C44. doi: 10.1152/ajpcell.1997.273.1.C37. [DOI] [PubMed] [Google Scholar]
- 105.Griffiths EJ. Species dependence of mitochondrial calcium transients during excitation-contraction coupling in isolated cardiomyocytes. Biochem Biophys Res Commun. 1999;263:554–559. doi: 10.1006/bbrc.1999.1311. [DOI] [PubMed] [Google Scholar]
- 106.Sedova M, Dedkova EN, Blatter LA. Integration of rapid cytosolic Ca2+ signals by mitochondria in cat ventricular myocytes. Am J Physiol Cell Physiol. 2006;291:C840–C850. doi: 10.1152/ajpcell.00619.2005. [DOI] [PubMed] [Google Scholar]
- 107.Fry CH, Powell T, Twist VW, Ward JP. Net calcium exchange in adult rat ventricular myocytes: an assessment of mitochondrial calcium accumulating capacity. Proc R Soc Lond B Biol Sci. 1984;223:223–238. doi: 10.1098/rspb.1984.0091. [DOI] [PubMed] [Google Scholar]
- 108.Andrienko TN, Picht E, Bers DM. Mitochondrial free calcium regulation during sarcoplasmic reticulum calcium release in rat cardiac myocytes. J Mol Cell Cardiol. 2009;46:1027–1036. doi: 10.1016/j.yjmcc.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Gavin CE, Gunter KK, Gunter TE. Manganese and calcium efflux kinetics in brain mitochondria. Relevance to manganese toxicity. Biochem J. 1990;266:329–334. doi: 10.1042/bj2660329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Leisey JR, Grotyohann LW, Scott DA, Scaduto RC., Jr Regulation of cardiac mitochondrial calcium by average extramitochondrial calcium. Am J Physiol. 1993;265:H1203–H1208. doi: 10.1152/ajpheart.1993.265.4.H1203. [DOI] [PubMed] [Google Scholar]
- 111.Saks VA, Veksler VI, Kuznetsov AV, Kay L, Sikk P, Tiivel T, et al. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol Cell Biochem. 1998;184:81–100. [PubMed] [Google Scholar]
- 112.Fiskum G, Kowaltowksi AJ, Andreyev AY, Kushnareva YE, Starkov AA. Apoptosis-related activities measured with isolated mitochondria and digitonin-permeabilized cells. Methods Enzymol. 2000;322:222–234. doi: 10.1016/s0076-6879(00)22023-5. [DOI] [PubMed] [Google Scholar]
- 113.Isenberg G, Han S, Schiefer A, Wendt-Gallitelli MF. Changes in mitochondrial calcium concentration during the cardiac contraction cycle. Cardiovasc Res. 1993;27:1800–1809. doi: 10.1093/cvr/27.10.1800. [DOI] [PubMed] [Google Scholar]
- 114.Wendt-Gallitelli MF, Isenberg G. Total and free myoplasmic calcium during a contraction cycle: x-ray microanalysis in guinea-pig ventricular myocytes. J Physiol. 1991;435:349–372. doi: 10.1113/jphysiol.1991.sp018514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Gallitelli MF, Schultz M, Isenberg G, Rudolf F. Twitch-potentiation increases calcium in peripheral more than in central mitochondria of guinea-pig ventricular myocytes. J Physiol. 1999;518(Pt 2):433–447. doi: 10.1111/j.1469-7793.1999.0433p.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Ohata H, Chacon E, Tesfai SA, Harper IS, Herman B, Lemasters JJ. Mitochondrial Ca2+ transients in cardiac myocytes during the excitation-contraction cycle: effects of pacing and hormonal stimulation. J Bioenerg Biomembr. 1998;30:207–222. doi: 10.1023/a:1020588618496. [DOI] [PubMed] [Google Scholar]
- 39.Chacon E, Ohata H, Harper IS, Trollinger DR, Herman B, Lemasters JJ. Mitochondrial free calcium transients during excitation-contraction coupling in rabbit cardiac myocytes. FEBS Lett. 1996;382:31–36. doi: 10.1016/0014-5793(96)00138-x. [DOI] [PubMed] [Google Scholar]
- 118.Trollinger DR, Cascio WE, Lemasters JJ. Selective loading of Rhod 2 into mitochondria shows mitochondrial Ca2+ transients during the contractile cycle in adult rabbit cardiac myocytes. Biochem Biophys Res Commun. 1997;236:738–742. doi: 10.1006/bbrc.1997.7042. [DOI] [PubMed] [Google Scholar]
- 119.Trollinger DR, Cascio WE, Lemasters JJ. Mitochondrial calcium transients in adult rabbit cardiac myocytes: inhibition by ruthenium red and artifacts caused by lysosomal loading of Ca(2+)-indicating fluorophores. Biophys J. 2000;79:39–50. doi: 10.1016/S0006-3495(00)76272-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Mackenzie L, Roderick HL, Berridge MJ, Conway SJ, Bootman MD. The spatial pattern of atrial cardiomyocyte calcium signalling modulates contraction. J Cell Sci. 2004;117:6327–6337. doi: 10.1242/jcs.01559. [DOI] [PubMed] [Google Scholar]
- 121.Contreras L, Drago I, Zampese E, Pozzan T. Mitochondria: the calcium connection. Biochim Biophys Acta. 2010;1797:607–618. doi: 10.1016/j.bbabio.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 122.Robert V, Gurlini P, Tosello V, Nagai T, Miyawaki A, Di Lisa F, et al. Beat-to-beat oscillations of mitochondrial [Ca2+] in cardiac cells. Embo J. 2001;20:4998–5007. doi: 10.1093/emboj/20.17.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Bell CJ, Bright NA, Rutter GA, Griffiths EJ. ATP regulation in adult rat cardiomyocytes: time-resolved decoding of rapid mitochondrial calcium spiking imaged with targeted photoproteins. J Biol Chem. 2006;281:28058–28067. doi: 10.1074/jbc.M604540200. [DOI] [PubMed] [Google Scholar]
- 124.Szalai G, Csordas G, Hantash BM, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria. J Biol Chem. 2000;275:15305–15313. doi: 10.1074/jbc.275.20.15305. [DOI] [PubMed] [Google Scholar]
- 125.Pacher P, Thomas AP, Hajnoczky G. Ca2+ marks: miniature calcium signals in single mitochondria driven by ryanodine receptors. Proc Natl Acad Sci USA. 2002;99:2380–2385. doi: 10.1073/pnas.032423699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Sharma VK, Ramesh V, Franzini-Armstrong C, Sheu SS. Transport of Ca2+ from sarcoplasmic reticulum to mitochondria in rat ventricular myocytes. J Bioenerg Biomembr. 2000;32:97–104. doi: 10.1023/a:1005520714221. [DOI] [PubMed] [Google Scholar]
- 127.Belmonte S, Morad M. 'Pressure-flow'-triggered intracellular Ca2+ transients in rat cardiac myocytes: possible mechanisms and role of mitochondria. J Physiol. 2008;586:1379–1397. doi: 10.1113/jphysiol.2007.149294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Olson ML, Chalmers S, McCarron JG. Mitochondrial organization and Ca2+ uptake. Biochem Soc Trans. 2012;40:158–167. doi: 10.1042/BST20110705. [DOI] [PubMed] [Google Scholar]
- 129.Dorn GW, Maack C. SR and mitochondria: Calcium cross-talk between kissing cousins. J Mol Cell Cardiol. 2013;55:42–49. doi: 10.1016/j.yjmcc.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 130.Csordas G, Thomas AP, Hajnoczky G. Calcium signal transmission between ryanodine receptors and mitochondria in cardiac muscle. Trends Cardiovasc Med. 2001;11:269–275. doi: 10.1016/s1050-1738(01)00123-2. [DOI] [PubMed] [Google Scholar]
- 131.de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- 132.Garcia-Perez C, Hajnoczky G, Csordas G. Physical coupling supports the local Ca2+ transfer between sarcoplasmic reticulum subdomains and the mitochondria in heart muscle. J Biol Chem. 2008;283:32771–32780. doi: 10.1074/jbc.M803385200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Garcia-Perez C, Schneider TG, Hajnoczky G, Csordas G. Alignment of sarcoplasmic reticulum-mitochondrial junctions with mitochondrial contact points. Am J Physiol Heart Circ Physiol. 2011;301:H1907–H1915. doi: 10.1152/ajpheart.00397.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, et al. Mitofusin 2-Containing Mitochondrial-Reticular Microdomains Direct Rapid Cardiomyocyte Bioenergetic Responses via Inter-Organelle Ca2+ Crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 136.Csordas G, Varnai P, Golenar T, Roy S, Purkins G, Schneider TG, et al. Imaging interorganelle contacts and local calcium dynamics at the ER- mitochondrial interface. Mol Cell. 2010;39:121–132. doi: 10.1016/j.molcel.2010.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Nagai T, Sawano A, Park ES, Miyawaki A. Circularly permuted green fluorescent proteins engineered to sense Ca2+ Proc Natl Acad Sci USA. 2001;98:3197–3202. doi: 10.1073/pnas.051636098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Kettlewell S, Cabrero P, Nicklin SA, Dow JA, Davies S, Smith GL. Changes of intra-mitochondrial Ca2+ in adult ventricular cardiomyocytes examined using a novel fluorescent Ca2+ indicator targeted to mitochondria. J Mol Cell Cardiol. 2009;46:891–901. doi: 10.1016/j.yjmcc.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 139.Drago I, De Stefani D, Rizzuto R, Pozzan T. Mitochondrial Ca2+ uptake contributes to buffering cytoplasmic Ca2+ peaks in cardiomyocytes. Proc Natl Acad Sci U S A. 2012;109:12986–12991. doi: 10.1073/pnas.1210718109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wei AC, Liu T, Winslow RL, O'Rourke B. Dynamics of matrix-free Ca2+ in cardiac mitochondria: two components of Ca2+ uptake and role of phosphate buffering. J Gen Physiol. 2012;139:465–478. doi: 10.1085/jgp.201210784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Dedkova EN, Blatter LA. Characteristics and function of cardiac mitochondrial nitric oxide synthase. J Physiol. 2009;587:851–872. doi: 10.1113/jphysiol.2008.165423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Aon MA, Cortassa S, O'Rourke B. Redox-optimized ROS balance: a unifying hypothesis. Biochimica et biophysica acta. 2010;1797:865–877. doi: 10.1016/j.bbabio.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chalmers S, Nicholls DG. The relationship between free and total calcium concentrations in the matrix of liver and brain mitochondria. The Journal of biological chemistry. 2003;278:19062–19070. doi: 10.1074/jbc.M212661200. [DOI] [PubMed] [Google Scholar]
- 144.Seidlmayer LK, Gomez-Garcia MR, Blatter LA, Pavlov E, Dedkova EN. Inorganic polyphosphate is a potent activator of the mitochondrial permeability transition pore in cardiac myocytes. J Gen Physiol. 2012;139:321–331. doi: 10.1085/jgp.201210788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Seidlmayer LK, Blatter LA, Pavlov E, Dedkova EN. Inorganic polyphosphate - an unusual suspect of the mitochondrial permeability transition mystery. Channels (Austin) 2012;6:463–467. doi: 10.4161/chan.21939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Magnus G, Keizer J. Minimal model of beta-cell mitochondrial Ca2+ handling. The American journal of physiology. 1997;273:C717–C733. doi: 10.1152/ajpcell.1997.273.2.C717. [DOI] [PubMed] [Google Scholar]
- 147.Nguyen MH, Dudycha SJ, Jafri MS. Effect of Ca2+ on cardiac mitochondrial energy production is modulated by Na+ and H+ dynamics. Am J Physiol Cell Physiol. 2007;292:C2004–C2020. doi: 10.1152/ajpcell.00271.2006. [DOI] [PubMed] [Google Scholar]
- 148.Cortassa S, Aon MA, Marban E, Winslow RL, O'Rourke B. An integrated model of cardiac mitochondrial energy metabolism and calcium dynamics. Biophys J. 2003;84:2734–2755. doi: 10.1016/S0006-3495(03)75079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Cortassa S, Aon MA, O'Rourke B, Jacques R, Tseng HJ, Marban E, et al. A computational model integrating electrophysiology, contraction, and mitochondrial bioenergetics in the ventricular myocyte. Biophys J. 2006;91:1564–1589. doi: 10.1529/biophysj.105.076174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Beard DA. A biophysical model of the mitochondrial respiratory system and oxidative phosphorylation. PLoS Comput Biol. 2005;1:e36. doi: 10.1371/journal.pcbi.0010036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Wu F, Yang F, Vinnakota KC, Beard DA. Computer modeling of mitochondrial tricarboxylic acid cycle, oxidative phosphorylation, metabolite transport, and electrophysiology. The Journal of biological chemistry. 2007;282:24525–24537. doi: 10.1074/jbc.M701024200. [DOI] [PubMed] [Google Scholar]
- 152.Korzeniewski B, Mazat JP. Theoretical studies on the control of oxidative phosphorylation in muscle mitochondria: application to mitochondrial deficiencies. The Biochemical journal. 1996;319(Pt 1):143–148. doi: 10.1042/bj3190143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhou L, Aon MA, Almas T, Cortassa S, Winslow RL, O'Rourke B. A reaction-diffusion model of ROS-induced ROS release in a mitochondrial network. PLoS Comput Biol. 2010;6:e1000657. doi: 10.1371/journal.pcbi.1000657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Wei AC, Aon MA, O'Rourke B, Winslow RL, Cortassa S. Mitochondrial energetics, pH regulation, and ion dynamics: a computational-experimental approach. Biophysical journal. 2011;100:2894–2903. doi: 10.1016/j.bpj.2011.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]