Abstract
Background and Purpose
EGFR-inhibitor Cetuximab (C225) improves the efficacy of radiotherapy in only a subgroup of HNSCC patients. Identification of predictive tumor characteristics is essential to improve patient selection.
Material and Methods
Response to C225 and/or radiotherapy was assessed with tumor growth delay assays in 4 HNSCC xenograft models with varying EGFR-expression levels. Hypoxia and proliferation was quantified with immunohistochemistry and expression of proteins involved in C225-resistance with western blot.
Results
EGFR-expression did not predict response to C225 and/or radiotherapy. Reduction of hypoxia by C225 was only observed in SCCNij202, which was highly sensitive to C225. Proliferation changes correlated with response to C225 and C225 combined with radiotherapy, as proliferation decreased after C225 treatment in C225-sensitive SCCNij202 and after combined treatment in SCCNij185, which showed a synergistic effect to combined C225-radiotherapy. Furthermore, C225-resistant SCCNij153 tumors expressed high levels of (activated) HER3 and MET.
Conclusions
EGFR-expression is needed for C225-response, but is not sufficient to predict response to C225 with or without radiotherapy. However, basal expression of additional growth factor receptors and effects on proliferation, but not hypoxia, correlated with response to combined C225-radiotherapy treatment and are potential clinically relevant predictive biomarkers.
Keywords: head and neck cancer, EGFR-inhibition, tumor microenvironment, radiotherapy, tyrosine kinase receptors
Introduction
Ionizing radiation activates the epidermal growth factor receptor (EGFR) and its downstream signaling pathways, including the phospatidylinositol-3-kinase (PI3-K)/protein kinase B (AKT) pathway [1]. The PI3-K/AKT pathway promotes DNA repair, cell growth, and proliferation and is, therefore, associated with major radioresistance mechanisms [2]. Accordingly, patients with EGFR-overexpressing head and neck squamous cell carcinomas (HNSCC) have a worse prognosis after radiotherapy [3]. Given this important role for EGFR in HNSCC, strategies to block EGFR signaling using monoclonal antibodies or tyrosine kinase inhibitors have been developed. Cetuximab (C225), a chimeric monoclonal antibody against the extracellular part of EGFR, is a successful example of these new molecular targeted therapies, since a phase III study of Bonner et al. [4] has shown that addition of C225 treatment to radiotherapy improves clinical outcome in HNSCC patients. However, despite these successes, a significant proportion of patients does not benefit from the addition of this targeted therapy. In addition, the expression level of EGFR assessed by immunohistochemistry does not correlate with clinical response [5]. To allow a better selection of patients, biological tumor characteristics that predict the response to these intensified treatments need to be identified [6].
In search of these predictive biological tumor characteristics, we treated 4 human head and neck xenograft lines (SCCNij) with radiotherapy, C225, and concurrent C225 and radiotherapy. These 4 tumor models were selected from a panel of primary xenografts to represent the diversity of EGFR expression patterns observed in the clinic. Effects on tumor growth delay were correlated with effects on the important radiation resistance mechanisms hypoxia and proliferation. Moreover, the expression levels of various proteins of the EGFR-signaling network shown to be involved in resistance to EGFR-inhibitors [5] were determined in the different xenograft models.
Material and Methods
Xenograft tumor models and treatment
Tumor models SCCNij202, SCCNij167 and SCCNij153 were derived from human larynx carcinomas and SCCNij185 from a human hypopharynx carcinoma. Viable 1 mm3 tumor pieces were implanted subcutaneously in 6–10 week-old athymic BALB/c nu/nu mice and passaged at a diameter of 1 cm. Tumors with a mean diameter of 5–8 mm transplanted at the hind leg were used in the experiments. Animals were treated with 1 mg i.p. C225 (Erbitux ™, Merck, Darmstadt, Germany), 10 or 20 Gy single dose radiotherapy (16 MV photon beam, dose rate 2 Gy/min with 2 cm Perspex build-up material), or a combination of C225 and radiotherapy (C225 was given 24h before radiotherapy).
For effects on tumor growth delay, tumor diameters were measured twice a week in three perpendicular directions and tumor volumes were determined by the formula: V= (a*b*c*π)/6. Endpoint was reached when the tumor volume tripled compared to the start volume. Maximal follow up was 120 days. Number of animals per group varied from 7 to 14.
For effects on proliferation and hypoxia, tumors were harvested 1 or 4 days after 10 Gy radiotherapy and/or 1, 2 or 5 days after C225. One hour before euthanization, animals were injected with 80 mg/kg of the hypoxia marker pimonidazole (Natural Pharmaceuticals International Inc., Research Triangle Park, NC, USA) and 15 minutes before euthanization with 50 mg/kg of the proliferation marker bromodeoxyuridine (BrdUrd) (Sigma, St Louis, MO, USA). Tumors were snap frozen in liquid nitrogen immediately after euthanizing the animals. Number of animals per group varied from 4 to 6.
Animals were kept in a specific pathogen-free unit in accordance with institutional guidelines. All experiments were approved by the Animal Experiments Committee of the Radboud University Nijmegen Medical Centre.
Multiplex ligation-dependent probe amplification (MLPA)
To identify EGFR copy number changes and EGFR variant III (EGFRVIII) in the tumor models, MLPA analysis was performed as described by Jeuken et al [7].
K-RAS mutation analysis
To detect mutations in codon 12 and 13 of K-RAS, mutation analysis was performed as described by Knijn et al [8].
Immunohistochemical staining, image acquisition and analysis of tumor sections
Frozen tumor sections (5 μm) were stained for BrdUrd, pimonidazole, vessels and all nuclei or for EGFR, pimonidazole and vessels. The antibody against pimonidazole was a gift from J.A. Raleigh (University of North Carolina). 9F1, a rat monoclonal antibody against mouse endothelium, was a gift from the Department of Pathology, Radboud University Nijmegen Medical Centre. The antibodies against EGFR and BrdUrd were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and Genetex (Irvine, CA, USA), respectively. Primary antibodies were detected by appropriate Cy3-conjugated (Jackson Immuno Research Laboratories Inc., West Grove, PA, USA) Alexa488-conjugated or Alexa647-conjugated (Molecular Probes, Leiden, The Netherlands) secondary antibodies. All nuclei were stained with Hoechst 33342 (Sigma).
Stained tumor sections were scanned with a fluorescence microscope (Axioskop, Zeiss, Göttingen, Germany). Each section was sequentially scanned at 100x magnification to yield images of the different fluorescent signals. Thresholds for the fluorescence signals were interactively set above the background and the grey value images were converted to binary images. Using ImageJ software (NIH, Bethesda, MD, USA), the hypoxic fraction (HF) was calculated by dividing the tumor area positive for pimonidazole by the total tumor area and the BrdUrd labeling index (LI) by dividing the nuclear area positive for BrdUrd by the total nuclear area of the tumor. Necrotic areas and staining artifacts were excluded from the total (nuclear) tumor area and further analyses.
Western blot analysis
Tumor sections were lysed in NP-40 lysis buffer and protein was quantitated using a standard Bradford absorbance assay. Proteins were separated by SDS-PAGE and blotted onto PVDF membranes. Membranes were incubated with the appropriate primary antibodies followed by incubation with HRP-conjugated antibodies. Finally, proteins were detected with an ECL chemiluminescence system. Antibodies against the following antigens were used: EGFR, pEGFR(Y1173), HER2, HER3, E-cadherin, vimentin, AKT, STAT3 and HRP-conjugated goat-anti-rabbit IgG, goat-anti-mouse IgG and donkey-anti-goat IgG were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). pHER2(Y1221/1222), pHER3(Y1289), MET, pMET(Y1234/1235), B-RAF, p-B-RAF(S445), pAKT(S473), ERK1/2, pERK1/2(T202/Y204), pSTAT3(Y705) were purchased from Cell Signaling Technology (Beverly, MA, USA) and α-tubulin was obtained from Calbiochem (San Diego, CA, USA).
Statistics
Changes in HF and BrdUrd LI were tested for significance using the Mann-Whitney test. Tumor growth delay data was analyzed using Kaplan-Meier survival curves and Cox proportional-hazards regression. To determine synergism between C225 and radiotherapy, the relative excess risk due to interaction (RERI) was calculated as described by Andersson et al [9] RERI values above 0 indicate biological interaction between treatments. Prism 4.0c (GraphPad Software, Inc., LA Jolla, CA, USA) and the statistical package R (R version 2.14.1, The R Development Core Team) with library “survival” and “eha” were used and p-values <0.05 were considered significant.
Results
Differential EGFR expression in 4 HNSCC xenograft models
The 4 human HNSCC xenograft models were selected on the basis of their EGFR expression levels to represent the range of phenotypes found in HNSCC patients. SCCNij202 showed high levels of EGFR, SCCNij153 and SCCNij185 showed intermediate levels, while EGFR expression was hardly detectable in SCCNij167 (Fig. 1). These expression levels correspond to the copy number of EGFR as assessed by MLPA-analysis; SCCNij202 had a gene amplification, SCCNij153 and SCCNij185 a (partial) gain, and SCCNij167 a hemizygous loss of the EGFR gene. The mutant form EGFRVIII or K-RAS mutations in codon 12 and 13 were not detectable in any of the models.
Figure 1. EGFR expression in 4 HNSCC xenograft models.
A) Expression of EGFR and pEGFR assessed with western blot analysis in 4 SCCNij lines. Two tumors per line were analyzed. B) Expression of EGFR in relation to hypoxia and vessels assessed with immunohistochemical staining in 4 SCCNij lines. EGFR (red), hypoxia (green), vessels (blue). Scale bars represent 200 μm. Magnification: 200X.
Using immunohistochemistry, EGFR expression was predominantly observed at the membranes of cells near blood vessels, which were mostly non-hypoxic, based on pimonidazole staining (Fig. 1B). However, some colocalization of EGFR-expression and hypoxia was observed (Fig. 1B, yellow).
Sensitivity for Cetuximab (C225) and radiotherapy correlates partly with EGFR-expression
Sensitivity for C225 and/or radiotherapy was assessed by growth delay assays, whereby the endpoint was a tripling of the tumor volume compared to the start volume (Fig. 2). High EGFR-expressing SCCNij202 tumors were highly sensitive to EGFR inhibition by C225 (p<0.001), but were relatively radioresistant as even 20 Gy induced only a small, albeit significant delay in tumor growth (p<0.001) (Fig. 2A). Combining C225 with either 10 Gy or 20 Gy did also result in a large significant growth delay (both p<0.001), which was comparable to the effect of C225 alone. Although the combined treatment induced a larger growth delay than radiotherapy alone no synergism was observed, as the effect on growth delay was comparable with the effect of C225 alone. SCCNij167 did not respond to C225, while radiotherapy induced a significant dose-dependent growth delay (p<0.001) (Fig. 2B). Again no interaction between the treatments was observed. The absence of response to C225 is in accordance with the low EGFR-expression and hemizygous loss of the EGFR gene in this latter tumor model.
Figure 2. Effects of EGFR-inhibition and/or radiotherapy on tumor growth delay.

Kaplan-Meier survival curves showing the effect of C225 and/or radiotherapy on the tumor growth of 4 SCCNij lines: A) SCCNij202, B) SCCNij167, C) SCCNij153, D) SCCNij185. Events were scored when the tumor volume tripled compared to the start volume and % tumor response represents the percentage of tumors that did not reach the event. Tumors were treated with C225 (1 mg i.p.), 10 or 20 Gy radiotherapy, or concurrent treatment of C225 and radiotherapy. C225 was given 24h before radiotherapy. Number of animals per group varied from 7 to 14.
SSCNij185 (Fig. 2D) and SCCNij153 (Fig. 2C) tumors, both with intermediate EGFR expression, were insensitive to C225 alone, but showed opposing effects to concurrent treatment: there was no synergistic effect of C225 with radiotherapy in SCCNij153, while a synergistic effect was observed in the SCCNij185 tumors when C225 was combined with radiotherapy (p=0.001). To quantify this interaction, we calculated a RERI (relative excess risk due to interaction) of 12.7, confirming a significant synergistic effect of C225 and radiotherapy in this tumor model.
Thus, EGFR-expression is needed for C225-response, but is not sufficient to predict response to C225 alone or combined with radiotherapy. EGFR expression was also not correlated with radiosensitivity as low EGFR-expressing SCCNij167 tumors showed an intermediate radioresponse in the growth delay experiments.
Changes in proliferation, but not hypoxia, correlate with response to C225 and C225 combined with radiotherapy
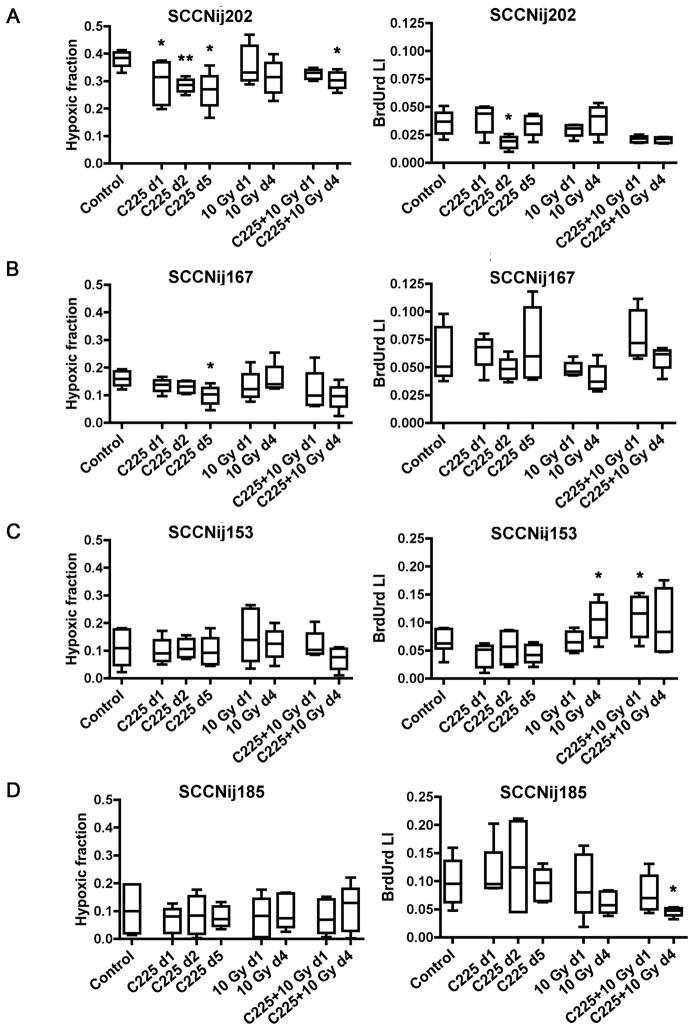
Preclinical studies suggest that EGFR-inhibition enhances radiosensitivity by reduction of hypoxia and tumor cell proliferation rate [10]. In our study, significant changes in both the hypoxic fraction (HF) and BrdUrd labeling index (LI) after C225 alone were only observed in SCCNij202 (Fig. 3), which is in accordance with the large effect on tumor growth by C225 in this tumor model. Hypoxia was significantly reduced at all time points (p<0.04), while proliferation was only reduced at 2 days after C225 treatment (p=0.02) (Fig. 3A). Combined C225 and radiotherapy also induced a significant reduction in hypoxia at 4 days after treatment (p=0.02), while a trend towards reduction of proliferation was observed at both time points (p=0.056). In SCCNij185, where there was an interaction between C225 and radiotherapy, no effects on hypoxia were observed, while proliferation was significantly reduced at 4 days after combined treatment (p=0.02) (Fig. 3D). At time of radiation, 1 day after C225, proliferation was not reduced in SCCNij185 tumors. In contrast, proliferation was significantly increased at 1 day after combined treatment in SCCNij153 (p=0.01) (Fig. 3C), in which no interaction between C225 and radiotherapy was observed. An overview of the effects of the different treatments on growth delay and on hypoxia and proliferation is shown in Table 1. Overall, changes in proliferation, but not in hypoxia, correlated with response to C225 alone and to C225 combined with radiotherapy. The basal level of hypoxia correlated with radiosensitivity as radioresistant SCCNij202 tumors had the highest HF and radiosensitive SCCNij153 and SCCNij185 had the lowest HF (Fig. 3).
Figure 3. Effects of EGFR-inhibition and/or radiotherapy on hypoxia and proliferation.
Effects of C225 and/or radiotherapy on the hypoxic fraction (HF) and BrdUrd labeling index (LI) of 4 SCCNij lines: A) SCCNij202, B) SCCNij167, C) SCCNij153, D) SCCNij185. Tumors were treated with C225 (1 mg i.p.) and/or 10 Gy radiotherapy and harvested 1 or 4 days after radiotherapy and/or 1, 2 or 5 days after C225. Tumors harvested 2 or 5 days after C225 correspond with tumors harvested 1 or 4 days after combined C225-radiotherapy, respectively. Differences between treated tumors and controls were tested for significance using Mann-Whitney tests, *: p<0.05, **: p<0.01. Error bars represent SEM. Number of animals per group varied from 4 to 6.
Table 1.
Overview of treatment response and significant changes in hypoxia and proliferation after treatment with C225 and/or radiotherapy
| Model | Response to treatment | Changes in hypoxia | Changes in proliferation |
|---|---|---|---|
| SCCNij202 | Sensitive to C225 | Decrease in hypoxia by C225 | Decrease in proliferation by C225 |
| Radioresistant | No effect of RT | No effect of RT | |
| No synergism C225+RT | Decrease in hypoxia by C225+RT | Trend towards decreased proliferation by C225+RT | |
|
| |||
| SCCNij167 | Resistant to C225 | Decrease in hypoxia by C225 | No effect of C225 |
| Intermediate radioresistant | No effect of RT | No effect of RT | |
| No synergism C225+RT | No effect of C225+RT | No effect of C225+RT | |
|
| |||
| SCCNij153 | Resistant to C225 | No effect of C225 | No effect of C225 |
| Radiosensitive | No effect of RT | Increase in proliferation by RT | |
| No synergism C225+RT | No effect of C225+RT | Increase in proliferation by C225+RT | |
|
| |||
| SCCNij185 | Resistant to C225 | No effect of C225 | No effect of C225 |
| Radiosensitive | No effect of RT | No effect of RT | |
| Synergism C225+RT | No effect of C225+RT | Decrease in proliferation by C225+RT | |
RT: radiotherapy, C225: Cetuximab
Expression of tyrosine kinase receptors associated with C225-resistance
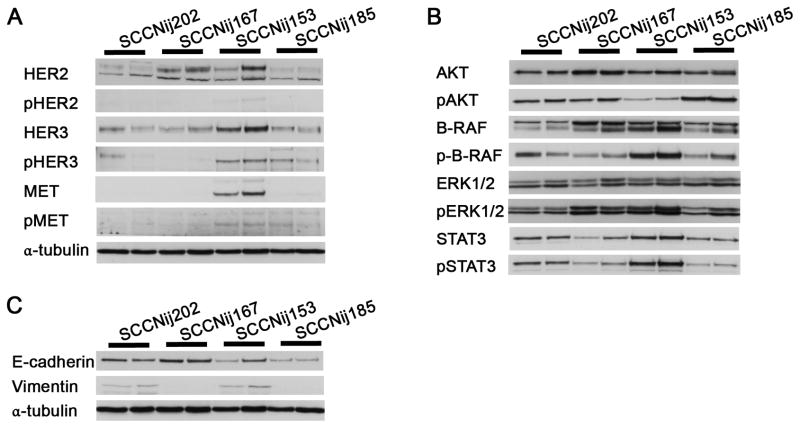
To further investigate resistance mechanisms, we studied expression of receptor tyrosine kinases, kinases downstream of EGFR, and markers for epithelial-mesenchymal transition (EMT). Next to EGFR, additional tyrosine kinase receptors were present in all tumor lines (Fig. 4A), but C225-resistant SCCNij153 tumors had relatively the highest levels of the (activated) tyrosine kinase receptors (p)HER3 and (p)MET. Expression of the activated kinases pAKT, p-B-RAF, pERK1/2 and pSTAT3 was relatively abundant in all tumor lines (Fig. 4B). Expression of pAKT was lowest and p-B-RAF and pSTAT3 expression highest in C225-resistant SCCNij153 tumors. Also in C225-sensitive SCCNij202 tumors pSTAT3 expression was relatively high compared to the other models. However, changes were small and no correlation was observed with response to C225 and/or radiotherapy. EMT characteristics could also not explain differences in treatment sensitivity, as expression of vimentin was present in both C225-sensitive, radioresistant SCCNij202 as in C225-resistant, radiosensitive SCCNij153 tumors (Fig. 4C). Thus, only overexpression of additional growth factor receptors were involved in C225-resistance in the tumor models investigated.
Figure 4. Expression of tyrosine kinase receptors, activated kinases and EMT markers.
A) Expression of tyrosine kinase receptors (p)HER2, (p)HER3 and (p)MET. B) Expression of kinases (p)AKT, (p-)B-Raf, (p)ERK1/2 and (p)STAT3. C) Expression of EMT markers E-cadherin and vimentin. Expression levels were determined with western blot analysis in 4 SCCNij lines. Two tumors per line were analyzed.
Discussion
We investigated the response to EGFR-inhibition and/or radiotherapy in 4 HNSCC xenograft models with differing EGFR-expression levels, which represent the variation observed in the clinic. The tumor models showed a diverse response to C225 and/or radiotherapy, again representing the clinical situation. High EGFR-expressing SCCNij202 tumors were highly sensitive to C225, but radioresistant, whereas SCCNij167 tumors with low EGFR were C225-resistant, but more sensitive to radiotherapy. The low expression level of EGFR in SCCNij167 is associated with a lack of response to C225 in this model. In contrast EGFR is amplified in SCCNij202, constituting oncogene addiction as inhibition of EGFR alone even cured tumors in this model. On the other hand, high-EGFR expressing SCCNij153 tumors did not show any growth delay upon C225 treatment. This difference in response between high EGFR-expressing tumors is also seen in the clinic, where EGFR copy number, but not EGFR expression assessed by immunohistochemistry, has been found to be predictive for response to EGFR-inhibition [5]. Surprisingly, SCCNij185, which has a lower expression of EGFR than SCCNij153, shows a synergistic response when C225 is combined with radiotherapy, possibly representing the responding patients in the clinic [4].
EGFR-expression correlates with poor survival after radiotherapy in head and neck cancer patients [3]. We did not find a similar correlation between radiosensitivity and EGFR expression, which is probably due to the number of tumor models investigated.
The observed variation in response was also not due to the presence of EGFRvIII or activating K-RAS mutations, which have been implicated in resistance to both EGFR-inhibition and radiation [11–13]. Both these genetic changes were not detected in the tumor models investigated in this study. Also, in contrast to others, EMT characteristics did not correlate with response to EGFR-inhibition [14,15].
The amount of hypoxia in our models was a good predictor for radiosensitivity, which has already been found in preclinical and clinical studies [16–21]. However, changes in hypoxia did not correlate with response to radiotherapy, C225 or both. Changes in proliferation did correlate with response to C225 and to C225 combined with radiotherapy, as proliferation was decreased after C225 treatment in C225-sensitive SCCNij202 tumors and after combined C225-radiotherapy in SCCNij185 tumors, which showed a synergistic effect when C225 was combined with radiotherapy. These results implicate that changes in proliferation after EGFR-inhibition and radiotherapy could be used to predict response in an early phase of treatment. Imaging proliferation by 3′-deoxy-3′-18F-fluorothymidine (18F-FLT) has already been shown to detect proliferation changes after (chemo-)radiotherapy and EGFR-inhibition [22–24] and could be an attractive way to asses early treatment response in the clinic. Moreover, the reduction of proliferation after combined treatment in SCCNij185 suggests that C225 radiosensitizes these tumors by reducing their ability to repopulate after radiotherapy. However, we did not use a fractionated irradiation scheme and can, therefore, not assess to what extent a decrease in repopulation by C225 contributed to the increase in tumor response to radiotherapy observed in this model. Nonetheless, our data are in agreement with a study by Krause et al. [10] that showed that decreased repopulation by C225 contributes to improved local control after fractionated radiotherapy in a HNSCC xenograft model.
Interestingly, proliferation significantly increased after combined treatment in SCCNij153, where no interaction was observed between these treatments. This is in line with data from Gurtner et al [25], who established an increase in pERK1/2 expression, a regulator of proliferation, in a C225-resistant xenograft model after combined treatment. This increased tumor cell proliferation rate might constitute a compensatory mechanism triggered by radiotherapy that cannot be blocked by EGFR- inhibition in this tumor model.
Changes in proliferation were thus predictive for response to C225, either alone or combined with radiotherapy. However, the described experiments were performed with single dose radiotherapy and further research is warranted to determine whether proliferation can be used to assess early treatment response in a fractionated setting in the clinic.
We also investigated the expression of multiple proteins implicated in resistance to C225 and radiotherapy. C225-resistant SCCNij153 had relatively high levels of (p)HER3 and (p)MET, two receptor tyrosine kinases that have been implicated in C225-resistance [26,27]. There is extensive crosstalk between these receptors and they activate downstream signaling pathways important for cell survival similar to pathways activated by EGFR, including the PI3K-AKT and RAS/RAF pathways [28]. Due to this overlap in signaling networks, cells overexpressing multiple growth factor receptors can sustain survival signaling when one of the receptors is blocked. This bypass resistance mechanism is supported by the observations that combining EGFR-inhibition with a HER3- or MET-inhibitor overcomes resistance to EGFR-inhibition [27,29].
Either activation via alternate tyrosine kinase receptors, or constitutive activation of downstream signaling pathways could represent another resistance mechanism. The expression of pAKT, pERK1/2, p-B-RAF, and pSTAT3 was relatively abundant in all tumors without any relation with sensitivity to C225 and/or radiotherapy. Expression of pAKT, an important activator of radiation resistance mechanisms [2], was even lowest in C225-resistant SCCNij153 tumors and highest in SCCNij185 tumors, which showed similar growth delay after radiotherapy. The activation of B-RAF and STAT3, both implicated in C225-resistance [30,31], were indeed highest in C225-resistant SCCNij153 tumors. However, the differences with the other models were small, and activated STAT3, pSTAT3, was also relative high in C225-sensitive SCCNij202 tumors. In contrast to other studies [30,32], our data indicate that basal expression levels of these kinases were not predictive for response to C225. This difference can be due to the use of in vitro models in these studies as data of our own lab shows that in vivo expression of these kinases can be substantially affected by the tumor microenvironment and consequently does not necessarily correlate with in vitro expression [33].
In conclusion, we studied 4 HNSCC xenograft models that represent the variation in biological characteristics and consequently treatment response observed in the clinic. EGFR-expression is needed for C225-response, but is not sufficient to predict response to C225 with or without radiotherapy. Effects on tumor cell proliferation correlated with response to C225, either alone or combined with radiotherapy. Importantly, the C225 resistant model had relative high basal expression of HER3 and MET. Further research is warranted to confirm that overexpression of HER3 and MET is involved in resistance to EGFR-inhibition combined with radiotherapy and whether early changes in proliferation could be used to assess early treatment response. Such knowledge will be instrumental for the optimization and customization of targeted therapies in combination with radiotherapy.
Supplementary Material
Acknowledgments
We thank P. Rijken, J. Lok, M. Verheijen and A. Grotenhuis for their excellent technical assistance. This project was financially supported by the Dutch Cancer Society, grant number 2008–4000, and, in part by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427 (DLW). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Conflict of interest statement
No conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rodemann HP, Dittmann K, Toulany M. Radiation-induced EGFR-signaling and control of DNA-damage repair. Int J Radiat Biol. 2007;83:781–791. doi: 10.1080/09553000701769970. [DOI] [PubMed] [Google Scholar]
- 2.Bussink J, vander Kogel AJ, Kaanders JH. Activation of the PI3-K/AKT pathway and implications for radioresistance mechanisms in head and neck cancer. Lancet Oncol. 2008;9:288–296. doi: 10.1016/S1470-2045(08)70073-1. [DOI] [PubMed] [Google Scholar]
- 3.Ang KK, Berkey BA, Tu X, et al. Impact of epidermal growth factor receptor expression on survival and pattern of relapse in patients with advanced head and neck carcinoma. Cancer Res. 2002;62:7350–7356. [PubMed] [Google Scholar]
- 4.Bonner JA, Harari PM, Giralt J, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. 2006;354:567–578. doi: 10.1056/NEJMoa053422. [DOI] [PubMed] [Google Scholar]
- 5.Wheeler DL, Dunn EF, Harari PM. Understanding resistance to EGFR inhibitors-impact on future treatment strategies. Nat Rev Clin Oncol. 2010;7:493–507. doi: 10.1038/nrclinonc.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bussink J, Kaanders JH, van der Kogel AJ. Microenvironmental transformations by VEGF- and EGF-receptor inhibition and potential implications for responsiveness to radiotherapy. Radiother Oncol. 2007;82:10–17. doi: 10.1016/j.radonc.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 7.Jeuken J, Sijben A, Alenda C, et al. Robust detection of EGFR copy number changes and EGFR variant III: technical aspects and relevance for glioma diagnostics. Brain Pathol. 2009;19:661–671. doi: 10.1111/j.1750-3639.2009.00320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Knijn N, Mekenkamp LJ, Klomp M, et al. KRAS mutation analysis: a comparison between primary tumours and matched liver metastases in 305 colorectal cancer patients. Br J Cancer. 2011;104:1020–1026. doi: 10.1038/bjc.2011.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andersson T, Alfredsson L, Kallberg H, Zdravkovic S, Ahlbom A. Calculating measures of biological interaction. Eur J Epidemiol. 2005;20:575–579. doi: 10.1007/s10654-005-7835-x. [DOI] [PubMed] [Google Scholar]
- 10.Krause M, Ostermann G, Petersen C, et al. Decreased repopulation as well as increased reoxygenation contribute to the improvement in local control after targeting of the EGFR by C225 during fractionated irradiation. Radiother Oncol. 2005;76:162–167. doi: 10.1016/j.radonc.2005.06.032. [DOI] [PubMed] [Google Scholar]
- 11.Allegra CJ, Jessup JM, Somerfield MR, et al. American Society of Clinical Oncology provisional clinical opinion: testing for KRAS gene mutations in patients with metastatic colorectal carcinoma to predict response to anti-epidermal growth factor receptor monoclonal antibody therapy. J Clin Oncol. 2009;27:2091–2096. doi: 10.1200/JCO.2009.21.9170. [DOI] [PubMed] [Google Scholar]
- 12.Sok JC, Coppelli FM, Thomas SM, et al. Mutant epidermal growth factor receptor (EGFRvIII) contributes to head and neck cancer growth and resistance to EGFR targeting. Clin Cancer Res. 2006;12:5064–5073. doi: 10.1158/1078-0432.CCR-06-0913. [DOI] [PubMed] [Google Scholar]
- 13.Weppler SA, Li Y, Dubois L, et al. Expression of EGFR variant vIII promotes both radiation resistance and hypoxia tolerance. Radiother Oncol. 2007;83:333–339. doi: 10.1016/j.radonc.2007.04.025. [DOI] [PubMed] [Google Scholar]
- 14.Fuchs BC, Fujii T, Dorfman JD, et al. Epithelial-to-mesenchymal transition and integrin-linked kinase mediate sensitivity to epidermal growth factor receptor inhibition in human hepatoma cells. Cancer Res. 2008;68:2391–2399. doi: 10.1158/0008-5472.CAN-07-2460. [DOI] [PubMed] [Google Scholar]
- 15.Theys J, Jutten B, Habets R, et al. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol. 2011;99:392–397. doi: 10.1016/j.radonc.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Janssens GO, Rademakers SE, Terhaard CH, et al. Accelerated Radiotherapy With Carbogen and Nicotinamide for Laryngeal Cancer: Results of a Phase III Randomized Trial. J Clin Oncol. 2012;30:1777–1783. doi: 10.1200/JCO.2011.35.9315. [DOI] [PubMed] [Google Scholar]
- 17.Kaanders JH, Wijffels KI, Marres HA, et al. Pimonidazole binding and tumor vascularity predict for treatment outcome in head and neck cancer. Cancer Res. 2002;62:7066–7074. [PubMed] [Google Scholar]
- 18.Rischin D, Hicks RJ, Fisher R, et al. Prognostic significance of [18F]-misonidazole positron emission tomography-detected tumor hypoxia in patients with advanced head and neck cancer randomly assigned to chemoradiation with or without tirapazamine: a substudy of Trans-Tasman Radiation Oncology Group Study 98. 02. J Clin Oncol. 2006;24:2098–2104. doi: 10.1200/JCO.2005.05.2878. [DOI] [PubMed] [Google Scholar]
- 19.Toustrup K, Sorensen BS, Nordsmark M, et al. Development of a hypoxia gene expression classifier with predictive impact for hypoxic modification of radiotherapy in head and neck cancer. Cancer Res. 2011;71:5923–5931. doi: 10.1158/0008-5472.CAN-11-1182. [DOI] [PubMed] [Google Scholar]
- 20.Yaromina A, Thames H, Zhou X, et al. Radiobiological hypoxia, histological parameters of tumour microenvironment and local tumour control after fractionated irradiation. Radiother Oncol. 2010;96:116–122. doi: 10.1016/j.radonc.2010.04.020. [DOI] [PubMed] [Google Scholar]
- 21.Yaromina A, Zips D, Thames HD, et al. Pimonidazole labelling and response to fractionated irradiation of five human squamous cell carcinoma (hSCC) lines in nude mice: the need for a multivariate approach in biomarker studies. Radiother Oncol. 2006;81:122–129. doi: 10.1016/j.radonc.2006.08.010. [DOI] [PubMed] [Google Scholar]
- 22.Atkinson DM, Clarke MJ, Mladek AC, et al. Using fluorodeoxythymidine to monitor anti-EGFR inhibitor therapy in squamous cell carcinoma xenografts. Head Neck. 2008;30:790–799. doi: 10.1002/hed.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Troost EG, Bussink J, Hoffmann AL, Boerman OC, Oyen WJ, Kaanders JH. 18F-FLT PET/CT for early response monitoring and dose escalation in oropharyngeal tumors. J Nucl Med. 2010;51:866–874. doi: 10.2967/jnumed.109.069310. [DOI] [PubMed] [Google Scholar]
- 24.Hoeben BAW, Troost EGC, van Herpen CM, Bussink J, Oyen WJ, Kaanders JHAM. 18F-FLT PET is an Early Predictor of Long-term Outcome during (Chemo)Radiotherapy in Head and Neck Carcinoma Patients. J Nucl Med. doi: 10.2967/jnumed.112.105999. In press. [DOI] [PubMed] [Google Scholar]
- 25.Gurtner K, Deuse Y, Butof R, et al. Diverse effects of combined radiotherapy and EGFR inhibition with antibodies or TK inhibitors on local tumour control and correlation with EGFR gene expression. Radiother Oncol. 2011;99:323–330. doi: 10.1016/j.radonc.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler DL, Huang S, Kruser TJ, et al. Mechanisms of acquired resistance to cetuximab: role of HER (ErbB) family members. Oncogene. 2008;27:3944–3956. doi: 10.1038/onc.2008.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xu H, Stabile LP, Gubish CT, Gooding WE, Grandis JR, Siegfried JM. Dual blockade of EGFR and c-Met abrogates redundant signaling and proliferation in head and neck carcinoma cells. Clin Cancer Res. 2011;17:4425–4438. doi: 10.1158/1078-0432.CCR-10-3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo A, Villen J, Kornhauser J, et al. Signaling networks assembled by oncogenic EGFR and c-Met. Proc Natl Acad Sci U S A. 2008;105:692–697. doi: 10.1073/pnas.0707270105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaefer G, Haber L, Crocker LM, et al. A two-in-one antibody against HER3 and EGFR has superior inhibitory activity compared with monospecific antibodies. Cancer Cell. 2011;20:472–486. doi: 10.1016/j.ccr.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 30.Bonner JA, Yang ES, Trummell HQ, Nowsheen S, Willey CD, Raisch KP. Inhibition of STAT-3 results in greater cetuximab sensitivity in head and neck squamous cell carcinoma. Radiother Oncol. 2011;99:339–343. doi: 10.1016/j.radonc.2011.05.070. [DOI] [PubMed] [Google Scholar]
- 31.Siena S, Sartore-Bianchi A, Di Nicolantonio F, Balfour J, Bardelli A. Biomarkers predicting clinical outcome of epidermal growth factor receptor-targeted therapy in metastatic colorectal cancer. J Natl Cancer Inst. 2009;101:1308–1324. doi: 10.1093/jnci/djp280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pernas FG, Allen CT, Winters ME, et al. Proteomic signatures of epidermal growth factor receptor and survival signal pathways correspond to gefitinib sensitivity in head and neck cancer. Clin Cancer Res. 2009;15:2361–2372. doi: 10.1158/1078-0432.CCR-08-1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Stegeman H, Kaanders JH, Wheeler DL, et al. Activation of AKT by hypoxia: a potential target for hypoxic tumors of the head and neck. BMC Cancer. 2012;12:463. doi: 10.1186/1471-2407-12-463. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.