Histone deacetylase inhibitors (HDACi) represent a novel class of anticancer drugs, with two members of the group, vorinostat and romidepsin, approved for the therapy of cutaneous T-cell lymphoma (CTCL). HDACi modulate chromatin structure, and have been shown to promote growth arrest, differentiation, and apoptosis of tumor cells.(1) Specifically, vorinostat has been shown to cause selective apoptosis of malignant T cells by increasing expression of pro-apoptotic factors p21WAF1, bax, and caspase-3, and decreasing expression of anti-apoptotic factors such as Stat-6 in CTCL cell lines and patients’ peripheral blood mononuclear cells (PBMC).(2)
Additionally, there is emerging evidence for the potent immunosuppressive properties of HDACi. Animal studies have shown that these agents can have a therapeutic benefit in several autoimmune disease models(3–5) perhaps owing to the enhancement of regulatory T-cell functions.(6) Moreover, we have reported a patient with CTCL with refractory bullous pemphigoid, who experienced rapid resolution of this autoimmune blistering disorder following initiation of therapy with vorinostat.(7) In addition, in vitro studies have shown anti-inflammatory properties of HDACi via suppression of cytokines such as TNF-α and IL-1β.(8) As attempts at preservation of cellular immunity are critical in the management of CTCL, we examined effects of vorinostat on multiple arms of the immune system in a patient undergoing therapy with this agent and in healthy volunteers.
Our patient is a 65 year old female with a past history of erythroderma, lymphadenopathy and circulating atypical cells diagnosed with Sezary syndrome in 2001. She initially experienced a complete clinical response following three years of therapy with photopheresis, interferon gamma, bexarotene and PUVA. She was in clinical remission from 2004 until 2007 when she relapsed with recurrent skin lesion and blood involvement. Her disease progressed despite reinstitution of photopheresis, interferon gamma, bexarotene and topical nitrogen mustard. In February 2011 she was started on vorinostat 400 mg daily with improvement in her skin erythema within one month. Blood was obtained from the patient for study 30 days following initiation of vorinostat.
As the reciprocal stimulation between natural killer (NK) cells and antigen presenting cells (APCs) is critical in the maintenance of antitumor immunity(9), we examined effects of vorinostat on these cellular functions. NK cell cytotoxicity from our patient’s frozen peripheral blood mononuclear cell (PBMC) specimen obtained in October 2010 prior to the initiation of vorinostat therapy was compared to a current specimen obtained while the patient was undergoing therapy with vorinostat. After culturing PBMCs for 48hrs, NK cytolytic activity was assessed in a standard 4-hour Cr51-release assay using human lymphoblastoma K562 cells as targets.(10) To assess NK cell activity, the patient’s PBMCs were stimulated with 10 µg/mL of a known APC activator Toll-like receptor (TLR) 7/8 agonist, known to indirectly activate NK cells. While stimulation with TLR 7/8 agonist resulted in a significant increase in NK cell activity from 5.4 to 17.6 % K562 lysis in the baseline sample, responsiveness of NK cytotoxicity upon stimulation was impaired in the sample obtained during vorinostat therapy, increasing only from 3.2 to 6.1% K562 lysis (Fig. 1A). These results indicate that compared to baseline, vorinostat treatment resulted in a significant blunting of functional NK activity.
Figure 1. Suppression of NK cell cytotoxicity by vorinostat.
A: Peripheral blood mononuclear cells from the patient presented in the case report were collected prior to initiation of systemic CTCL therapy (baseline) and while undergoing treatment with vorinostat (vorinostat) were cultured in medium alone or treated with 10 µg/mL of a synthetic TLR 7/8 agonist 3M-007 for 48h, followed by a 4 hour Cr51 release assay using K562 cells as targets. Data represent mean of triplicate of percentage of specific lysis per single NK cell at E:T ratio 50:1. Percentage NK cells/total PBMC defined by flow cytometric analysis. B: Highly purified NK cells from healthy volunteers (n=3) were cultured in medium alone or treated with 0.4 µM and 1µM vorinostat for 48h, followed by a 4 hour Cr51 release assay using K562 cells as targets. Data represent mean ±SD of tested individuals and are presented as a percentage of specific lysis at E:T ratio 2:1. *p <0.05 when compared to medium.
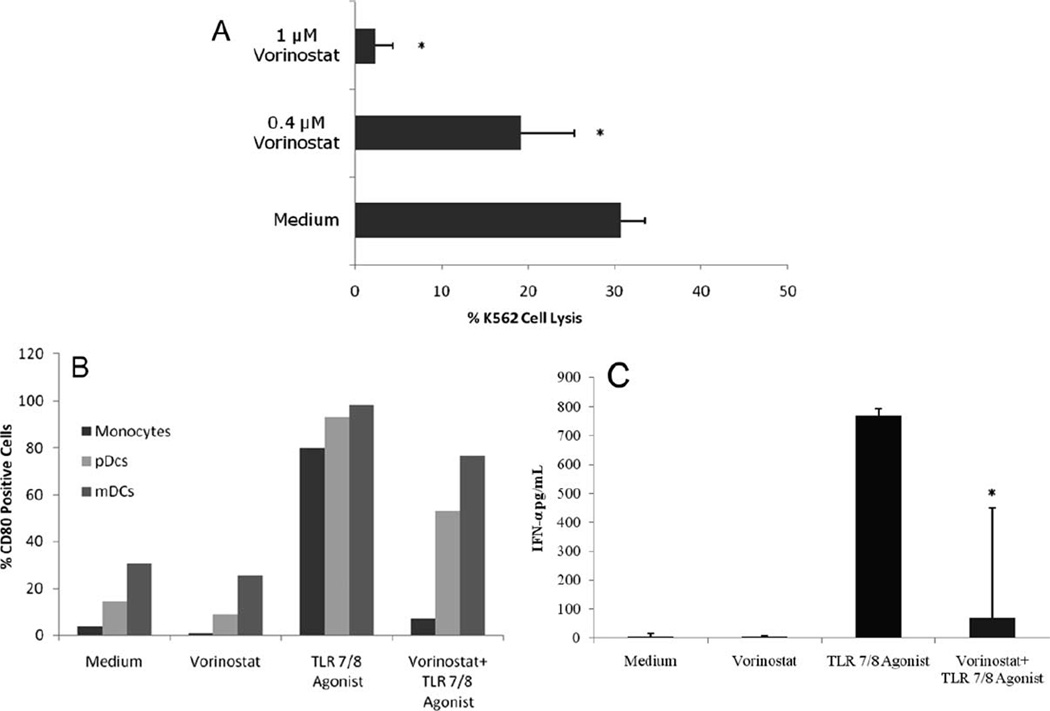
Subsequent in vitro studies of effects of vorinostat on the immunity of healthy volunteers revealed that this drug can profoundly suppress multiple arms of the cellular immune response. Purified CD56+ NK cells isolated from PBMCs from three healthy donors (Dynabeads Untouched Human NK cell isolation kit, Invitrogen) were cultured in medium with 1µM or 0.4 µM vorinostat, doses yielding serum concentrations in the range of those achieved with a standard vorinostat regimen of 400 mg daily used in clinical practice. Assessment of NK cytotoxicity after 48hr incubation with vorinostat, showed that vorinostat significantly suppressed NK cell cytotoxicity in a dose dependent manner (Fig. 1B). While untreated NK cells mediated 30.7% lysis of K562 cells, treatment with 0.4µM vorinostat reduced lysis of K562 cells to 19.2%, and it was further diminished to 2.4% after treatment with 1µM vorinostat. Thus, functional NK cell lytic activity of healthy volunteers was virtually eliminated following a short exposure to therapeutic levels of vorinostat.
In addition to depressed NK cell function, other important cellular immune functions appear to be inhibited by vorinostat. We observed that activation of antigen presenting cells measured by the expression of the inducible co-stimulatory molecule CD80 by flow cytometry(11) on monocytes, plasmacytoid and myeloid dendritic cells (DC)s collected from healthy volunteers was markedly inhibited by vorinostat. While stimulation with TLR 7/8 agonist (10µg/ml for 48 hrs) led to significant upregulation of CD80, and, thus activation, across all three cell lineages (79.9% for monocytes, 93.3% pDCs, and 98.4% mDCs) this activation was suppressed by vorinostat to 7.0%, 52.9%, and 76.8%, respectively (Fig. 2A).
Figure 2. Vorinostat inhibits APC activation and IFN-α production by plasmacytoid dendritic cells.
A: Peripheral blood mononuclear cells from healthy volunteers were cultured in 24-well plates at a density of 2×106 /ml/well in media alone, with 1µM vorinostat, or 10µg/ml synthetic TLR 7/8 agonist 3M-007 for 48h, or primed with 1µM vorinostat for 4h followed by activation with TLR 7/8 agonist for further 48h. Cells were then harvested, and analyzed for the expression of CD80. Monocytes were identified by anti-CD64 and anti-CD14 staining. Plasmacytoid dendritic cells (pDCs) were defined as lineage-negative cells (Lin 1-FITC cocktail) that co-express HLADR and CD123. Myeloid dendritic cells (mDCs) were defined as lineage-negative cells co-expressing HLADR and CD11c. The figure illustrated one of three representative experiments. B: Peripheral blood mononuclear cells from healthy volunteers were cultured in 24-well plates at a density of 2×106 /ml/well in media alone, with 1µM vorinostat, or 10µg/ml synthetic TLR 7/8 agonist 3M-007 for 48h, or primed with 1µM vorinostat for 4h followed by activation with TLR 7/8 agonist for further 48h. Cell free supernatants were then collected, and IFN-α levels were measured in cell-free supernatants by ELISA. Data represent mean ±SD of tested individuals. *p <0.05 compared to the TLR 7/8 agonist sample.
Importantly, we observed that interferon-α (IFN-α) production measured by ELISA assay following stimulation of healthy volunteer PBMC with a TLR 7/8 agonist was greatly inhibited by 1µM vorinostat (Fig. 2B). TLR 7/8 stimulation led to significant (766.6 pg/mL) IFN-α production by pDCs, which was markedly inhibited (68.4 pg/mL) by vorinostat. As NK cells require interaction with dendritic cells and monocytes for optimal activation, impairment of APC activation in turn diminishes NK functionality.
In summary, while HDACi vorinostat has been shown to have a significant response rate and a high rate of pruritus relief in heavily pretreated, refractory CTCL patients, our findings suggest that vorinostat also potently suppresses multiple arms of the immune system which may contribute to disease progression and lead to greater susceptibility of these patients to opportunistic infections(12). Our findings highlight the complexity of the effects of vorinostat and the need to balance its anti-tumor effects and immunosuppressive capabilities. Further studies are necessary to determine if the present findings of immune suppression are representative of the HDACi class as a whole, or solely of vorinostat.
Acknowledgments
Funding/Support: This work was supported in part by research grants from the National Cancer Institute R01CA122569 and R01CA132098, and a Translational Research Grant from the Leukemia and Lymphoma Society.
The sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions: Dr. Stephen and Dr. Rook had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Stephen, Showe, Wysocka, Rook. Acquisition of Data: Stephen, Morrissey, Benoit, Nasta. Analysis and Interpretation: Stephen, Morrissey, Kim, Vittorio, Wysocka, Rook. Drafting of the Manuscript: Stephen, Morrissey, Showe, Wysocka, Rook. Critical revision of the manuscript for important intellectual content: Stephen, Morrissey, Benoit, Kim, Vittorio, Nasta Statistical Analysis: Stephen, Wysocka Obtained funding: Rook Administrative, technical, or material support: Benoit, Showe, Wysocka, Rook Study supervision: Rook
Financial Disclosure: None reported
REFERENCES
- 1.Xu WS, Parmigiani RB, Marks PA. Histone deacetylase inhibitors: molecular mechanisms of action. Oncogene. 2007;26(37):5541–5552. doi: 10.1038/sj.onc.1210620. [DOI] [PubMed] [Google Scholar]
- 2.Zhang C, Richon V, Ni X, Talpur R, Duvic M. Selective induction of apoptosis by histone deacetylase inhibitor SAHA in cutaneous T-cell lymphoma cells: relevance to mechanism of therapeutic action. J Invest Dermatol. 2005;125(5):1045–1052. doi: 10.1111/j.0022-202X.2005.23925.x. [DOI] [PubMed] [Google Scholar]
- 3.Glauben R, Batra A, Fedke I, et al. Histone hyperacetylation is associated with amelioration of experimental colitis in mice. J Immunol. 2006;176(8):5015–5022. doi: 10.4049/jimmunol.176.8.5015. [DOI] [PubMed] [Google Scholar]
- 4.Reddy P, Maeda Y, Hotary K, et al. Histone deacetylase inhibitor suberoylanilide hydroxamic acid reduces acute graft-versus-host disease and preserves graft-versusleukemia effect. Proc Natl Acad Sci. 2004;101(11):3921–3926. doi: 10.1073/pnas.0400380101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mishra N, Reilly CM, Brown DR, Ruiz P, Gilkeson GS. Histone deacetylase inhibitors modulate renal disease in the MRL-lpr/lpr mouse. J Clin Invest. 2003;111(4):539–552. doi: 10.1172/JCI16153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tao R, de Zoeten EF, Ozkaynak E, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med. 2007;13(11):1299–1307. doi: 10.1038/nm1652. [DOI] [PubMed] [Google Scholar]
- 7.Gardner JM, Evans KG, Goldstein S, Kim EJ, Vittorio CC, Rook AH. Vorinostat for the treatment of bullous pemphigoid in the setting of advanced, refractory cutaneous T-cell lymphoma. Arch Dermatol. 2009;145(9):985–988. doi: 10.1001/archdermatol.2009.229. [DOI] [PubMed] [Google Scholar]
- 8.Leoni F, Fossati G, Lewis EC, et al. The histone deacetylase inhibitor ITF2357 reduces production of pro-inflammatory cytokines in vitro and systemic inflammation in vivo. Mol Med. 2005;11(1–12):1–15. doi: 10.2119/2006-00005.Dinarello. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195(3):327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rook AH, Kubin M, Cassin M, et al. IL-12 reverses cytokine and immune abnormalities in Sezary syndrome. J Immunol. 1995;154(3):1491–1498. [PubMed] [Google Scholar]
- 11.Wysocka M, Newton S, Benoit BM, et al. Synthetic imidazoquinolines potently and broadly activate the cellular immune response of patients with cutaneous T-cell lymphoma: synergy with interferon-gamma enhances production of interleukin-12. Clin Lymphoma Myeloma. 2007;7(8):524–534. doi: 10.3816/clm.2007.n.037. [DOI] [PubMed] [Google Scholar]
- 12.Ritchie D, Piekarz RL, Blombery P, et al. Reactivation of DNA viruses in association with histone deacetylase inhibitor therapy: a case series report. Haematologica. 2009;94(11):1618–1622. doi: 10.3324/haematol.2009.008607. [DOI] [PMC free article] [PubMed] [Google Scholar]