Abstract
Introduction
“Navigation in surgery” spans a broad area, which, depending on the clinical challenge, can have different meanings. Over the past decade, navigation in surgery has evolved beyond imaging modalities and bulky systems into the rich networking of the cloud or devices that are pocket-sized.
Discussion
This article will review various aspects of navigation in the operating room and beyond. This includes a short history of navigation, the evolution of surgical navigation, as well as technical aspects and clinical benefits with examples from neurosurgery, spinal surgery, and orthopedics.
Conclusion
With improved computer technology and a trend towards advanced information processing within hospitals, navigation is quickly becoming an integral part in the surgical routine of clinicians.
Keywords: Neuronavigation, Orthopedic surgery, Image-guided surgery, Digital OR, CAS
Introduction
Over the last three decades, technical advances have significantly changed the way we live. From computers to smartphones, from single purpose to multipurpose devices, technology has become an intrinsic part of our daily routine. Navigation in surgery is an important example of today’s technological capabilities being applied to medicine. It has emerged as one of the most reliable representatives of technology as it continues to transform surgical interventions into safer and less invasive procedures. In surgery, navigation has spurred technical progress, enabled more daring procedures, and unlocked new synergies. What was once a simple localization tool has evolved into a centerpiece of technology in the surgical theater.
“Navigation in surgery” spans a broad area, which, depending on the clinical challenge, may have various interpretations. The meaning of navigation in surgery is most accurately defined by the questions posed: “Where is my (anatomical) target?”, “How do I reach my target safely?”, “Where am I (anatomically)?”, or “Where and how shall I position my implant?”. Apart from these important anatomical orientation questions, surgical navigation is also used as a measurement tool and an information center for providing surgeons with the right information at the right time.
There are examples of technological advances in the medical field, whose benefit to the patient became immediately evident which were rapidly adopted and integrated into the clinical routine—without the need for proper randomized clinical trials. Examples range from the introduction of anesthesia to enable safer surgery and the introduction of microscopy enabling microsurgery. Surgical navigation and its wide range of benefits could be next.
Navigation is a relatively recent addition (in the last 20 years) to the surgeon’s tool box. Navigation in surgery was born from the desire to perform safer and less invasive procedures. This progress allowed for newer and more challenging surgical approaches, which in turn resulted in the need for better and more effective technical tools. Navigation in surgery is an important surgical decision-making tool, which has evolved hand-in-hand with the fresh approaches it enables surgeons to perform.
The inception of navigation in surgery
The first serious experiments to precisely localize specific anatomical structures within the human body can be traced back to the late nineteenth century [1]. Much has changed since, but the main challenge to specifically target an anatomical structure in safer and less invasive ways remained the same. It was only with the advent of medical imaging in connection with the exponential growth of computer processing capabilities that made precise and safe targeting of anatomy a reality. Medical imaging was an important prerequisite to enable navigation. However, pioneering surgeons remain the driving force behind the development of surgical navigation. These clinicians pushed for the development of new technology to solve their surgical challenges. In essence, three key factors pushed the development of navigation in surgery as we know it today: neurosurgery, stereotaxy, and medical imaging.
Neurosurgery
The symbioses of technology and surgery seem to be the strongest when faced with the challenge to operate on the most delicate organ of the human body, the brain. The entire history of neurosurgery reflects an epic quest to conduct brain surgery as minimally invasively as possible. The reason being that neurosurgery is the art of surgery on and in an organ abundant with sensitive or eloquent areas, which directly affect a patient’s mental and physical state. The brain is confined in a tight space, packed together with other vital structures, like vessels and cranial nerves, which themselves can cause major functional deficits if damaged. Due to the abundance of risk structures, eloquent cortical and subcortical areas, surgical access can be limited. The intraoperative view of the target area is often constrained and lacks anatomical landmarks for orientation. Therefore, neurosurgeons are often early adopters of new technology, which holds the promise of mitigating surgical risks and enhancing patient outcome.
Stereotaxy
Stereotaxy is a neurosurgical procedure which requires the exact localization and targeting of intracranial structures for the placement of electrodes, needles, or catheters. Initially, this problem was addressed using anatomical drawings as an atlas for intracranial target planning and with the help of mechanical head frames attached to the patient’s skull. The planned target could then be transferred onto the actual intraoperative patient setup. This was most advantageous, as once the surgical trajectory was defined, only a burr hole was required and an electrode or a needle could be advanced with minimal brain trauma. This type of minimally invasive procedure was termed stereotaxy. The name stems from Greek for “stereo” (solid) and “taxis” (arrangement, order). Other surgical interventions which utilize the concept of stereotaxy are ablation, biopsy, injection, stimulation, implantation, and radiosurgery. In the 1950s, E.A. Spiegel and H.T. Wycis invented the first stereotactic instruments for clinical use for humans and initiated the modern era of stereotactic neurosurgery. However, using the anatomical atlases to plan surgeries spawned many inaccuracies as one could not take into account a patient’s individual anatomy. Such issues were further exacerbated when anatomy was altered due to pathology like a growing or infiltrating tumor. This is where medical imaging was able to bridge the gap and enables the use of patient-specific anatomy for stereotactical planning.
Medical imaging
The discovery of the X-ray by Wilhelm Roentgen in 1895 opened the path for an entirely new era of medical diagnosis and treatment. It was the first time surgeons were able to see inside a patient’s body without opening it. This constituted a revolution for medical technology starting in the military section to locate bullets in extremities followed by radiography of the stomach. Shortly thereafter, the first radiographs of the skull were made to support stereotactic targeting. However, radiographs, which are simple X-ray images, could not display any intracranial soft tissue; therefore, clinicians experimented with other methods to overcome this problem. Walter Dandy, for example, fortuitously discovered ventriculography in 1918, when he was performing a radiograph on a patient with an open, penetrating head injury and the ventricles filled up with air. Based on the idea of ventriculography, pneumoencephalography was developed where most of the cerebrospinal fluid (CSF) was drained from around the brain and replaced with air or other gases. This enabled a better image of structures in the brain on an X-ray image and allowed the calculation of stereotactic coordinates for targets in the basal ganglia and thalamus because of their definite and stable relationship to the third ventricle.
With the advent of computers, it was possible to calculate a 3D image from a set of 2D X-ray images. In the 1970s, Sir Hounsfield introduced the very first computer tomography (CT) imaging device, which he called “computerized axial tomography.” As CT images allowed 3D targeting, it evoked a developmental leap in stereotactic head frame design. Stereotactic procedures using rigid head frames fixed to the skull proved to be extremely accurate and are still currently used in clinical practice.
The CT remains an important workhorse for the neurosurgeon and the initial patient assessment, but it was the introduction of the magnetic resonance imaging (MRI) in the1980s, which not only allowed the imaging of soft tissue in greater detail, but also enabled the imaging of functional brain areas, like motoric or speech regions.
The introduction of the MRI marked another important milestone towards navigation in surgery. MRI images not only show more soft tissue detail, but also allow visualizing a lesion in relation to other risk structures enabling the preoperative planning of an optimal surgical route or radiosurgery plan.
From frame-based stereotaxy to frameless navigation
Frame-based stereotactical procedures in neurosurgery had a limited application. Only burr hole procedures such as biopsies, electrode placements, or the resection of small intracranial tumors were possible. Other disadvantages of frame-based procedures include significant patient discomfort from scanning to surgery, the inability to visualize the biopsy needle pass, a very limited view of the surgical field through the burr hole, and no intraoperative control over the stereotactic pathway or awareness of complications, like rupturing a vessel.
It was in the 1990s when David Roberts first developed the concept of frameless stereotaxy for neurosurgery to overcome the limitations of frame-based stereotaxy [1]. The biggest advantage of frameless stereotaxy is the capability to track a surgical instrument in “real-time” and constantly visualize its position on the preoperative CT or MRI. This marked the inception of navigation in surgery as we know it today. Navigation is a successor or natural evolution of frame-based stereotaxy. It is not only used to guide the surgeon to find a specific anatomical target, avoid areas of risk, and offer intraoperative orientation in the absence of anatomical landmarks, but it can also support the optimal alignment of implants and act as a 3D measurement system.
To summarize, the adoption and integration of technology such as medical imaging and stereotaxy have fostered the development of surgical navigation allowing surgeons to conduct procedures that are truly effective and minimally invasive. Currently, navigation in surgery is not exclusive to neurosurgery and can be found in many other surgical disciplines like ENT, CMF, Trauma, and Orthopedics supporting a wide variety of surgical interventions.
Principles of navigation in surgery
A surgical navigation system is in some way the same as a commonly used navigation system in a car, for example. Both attempt to localize or determine a position in space in the context of its surroundings. The actual localization technology, however, differs as surgical navigation is not using triangulation like a global positioning system with the help of several geostationary satellites. Modern surgical navigation systems use a stereoscopic camera emitting infrared light which can determine a 3D position of prominent structures, like reflective marker spheres. This allows for real-time tracking of the marker spheres.
For the basic setup, the requirements are a stereoscopic camera, a computer platform with screen, and the respective navigation software. During the surgery, the marker spheres are attached to the patient and at surgical instruments (using reference arrays) to enable an exact localization in space and hence navigation in the operating room (OR).
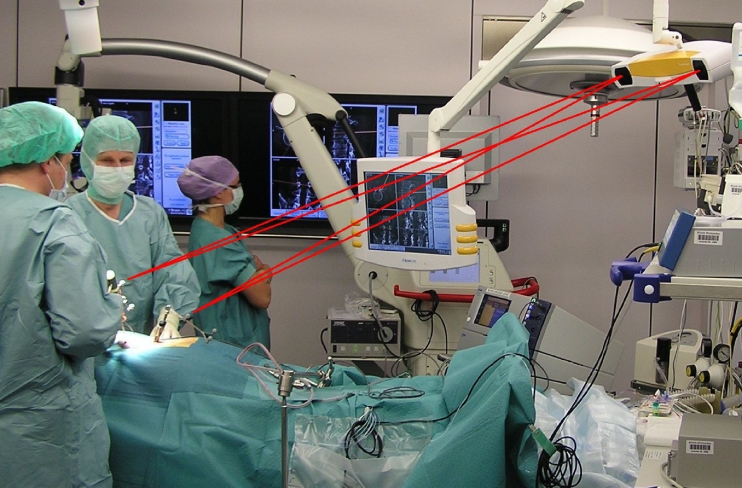
With each reference array comprising of at least three marker spheres, the computer can calculate the position and orientation of each instrument. A correct localization and virtual display of the instrument on the computer screen is ensured by firmly attaching a reference array to the patient, e.g., in the bone or via a head clamp (see Fig. 1 for a common OR setup in spinal surgery). Movements of the camera intraoperatively are possible because only the relative position of the tracked instruments to the tracked patient reference is relevant.
Fig. 1.
Spinal OR setup: The common OR setup involving surgical navigation consists of a stereotactic camera (upper right corner) and a computer screen (center)—both are mounted at the ceiling in the OR here. Further marker spheres are rigidly attached via a reference array to the patient and to surgical instruments
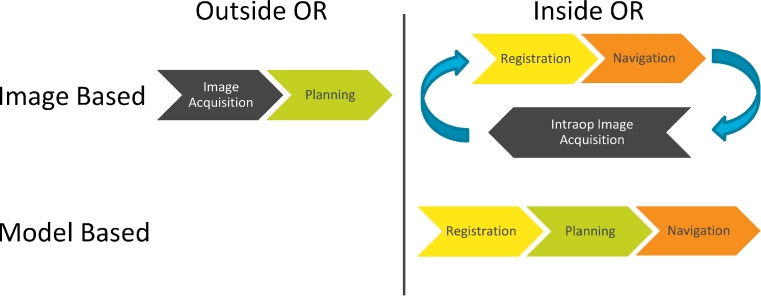
In the field of neurosurgery and spinal surgery, navigation is usually “image-based,” meaning that imaging data for example preoperative CT or MRI images are required and can be used for navigation in the OR (image acquisition). Before surgery, objects and areas of interest may be planned within the images and hence enrich the data sets (planning). Before the first cut is made, the preoperative image data need to be matched to the current patient position via a registration process (registration). This is the process to establish a relation between the “real” coordinate system as defined by the patient’s reference array and the “virtual” coordinate system of the imaging data. Registration can be paired point-based or use surface matching routines. The surgeon then virtually sees both the current situation and the imaging datasets overlapped and may then navigate on both (navigation). If required, the surgeon may obtain additional images during the surgery and register and include them in the running navigation (intraoperative image acquisition). Figure 2 shows the basic navigation workflow for neurosurgery and spinal surgery.
Fig. 2.
Basic workflows of image-based and model-based navigation. Image-based navigation requires preoperative images which need to be registered to the patient setup, typically employed for cranial or spinal surgery. Model-based navigation requires no imaging data and the process of registration matches the patients anatomy to a virtual model, typically employed for orthopedic surgery
Modern orthopedic navigation systems are “model-based” and work almost exclusively without information from external image sources. The patients do not need to be exposed to additional radiation, e.g., through CT or X-ray. Instead the navigation software calculates an individual model of the patients’ anatomy based on defined landmarks on the bone which are acquired using a navigated instrument (registration). After an optional planning on the model (planning, e.g., virtual orientation and placement of the joint implant), the actual procedure follows where the surgeon gets supported by relevant information added through the navigation system (navigation). Figure 2 shows the basic orthopedic navigation workflow.
Each surgical discipline and each individual hospital and surgeon have different navigation requirements for their workflow, amount of flexibility, and needed functionality. A wide variety of navigation platforms is available to accommodate all surgical needs: The systems can be installed permanently mounted at the OR ceiling, require minimal OR footprint, or minimize cable clutter and be mobile platforms to be used in several ORs at different times or even be carried around between hospitals for maximum flexibility (see Fig. 3).
Fig. 3.
The Brainlab platform family serves the needs of each discipline: Curve in two different configurations: a ceiling-mounted and b dual display; c Kick, more portable and with a smaller footprint; and d Dash, the smart mobile solution. Copyright: Brainlab AG
Navigation for neurosurgery
The various applications of navigation for neurosurgery, or “neuronavigation,” have been widely reported and published for almost two decades. Neurosurgery was the first surgical discipline to adopt navigation and integrate it successfully in clinical routine. The neurosurgical procedures supported by neuronavigation range widely from intracranial tumor resections to frameless biopsies to pedicle screw placement and stabilizations in the spine. Below is an overview of the main benefits and challenges of neuronavigation in general.
Minimally invasive surgery
Neuronavigation displays anatomical structures along a tracked instrument’s virtually axis, and in the majority of neurosurgical interventions, this information is used to optimize the craniotomy, e.g., skull opening. Neuronavigation helps to visualize the location of underlying tumor borders in relation to the skull and resulting in shorter surgical operation time and smaller craniotomies. Smaller and better-centered craniotomies are associated with reduced blood loss, minimized trauma, and brain retraction. This reduces the risk of postoperative swelling and/or hematomas, and this results in shorter hospitalization of the patient and decreased hospital costs [2–4]. For spinal surgery, neuronavigation enables minimal invasive percutaneous procedures and leads to less pedicle breaches and significant reduction of radiation for the surgeon [5].
Increased confidence
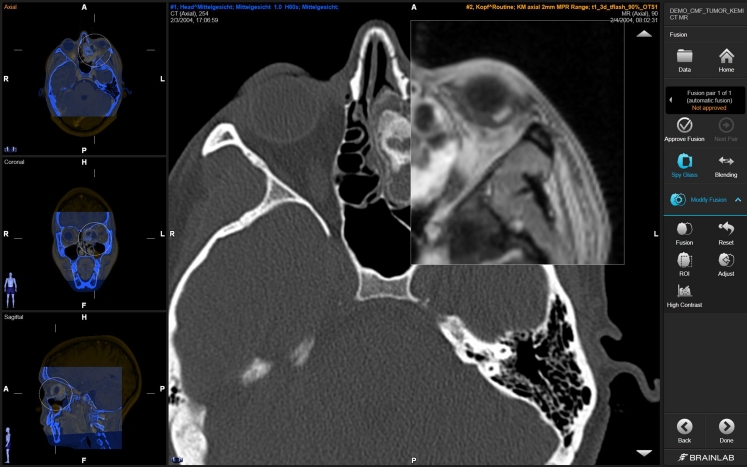
Operating in the brain is a very demanding surgical discipline, allowing only for little room of error and demanding maximum preparation and concentration. Prior to surgery, the neurosurgeon usually has the option to prepare an optimal treatment plan for the neuronavigation. Modern planning systems allow the surgeon to outline the tumor and use multimodal images, like CT for bone and MRI for soft tissue (see Fig. 4). Planning can be based on the original 2D image slices, on arbitrary reconstructions of the tomographic images as well as on virtual 3D models. The goal of preoperative planning is to find an optimal surgical route, and these computer-based models allow assessing various surgical routes outside the OR without time pressure.
Fig. 4.
Multimodal image fusion is an important preoperative planning step to combine various imaging information for optimal surgical route planning. Copyright: Brainlab AG
Younger and less experienced neurosurgeons embrace preoperative virtual planning and intraoperative navigation as an additional learning tool to become more confident with the complex anatomy of the brain [6]. Even among experienced neurosurgeons, there is increased concern with procedures approaching deep brain structures. Navigation allows the real-time display of a tracked instrument on the multiplanar reconstructed images to match exactly the surgical perspective. This increases anatomic appreciation and enhances the confidence of surgeons and their perception of safety [7, 8].
Improved patient outcome
The link of neuronavigation to improving patient outcome is the strongest for glioma surgery. Gliomas constitute roughly one third of all brain tumors and the surgical goal is to safely remove or resect as much of the tumor mass as possible to avoid recurrence. Neuronavigation has been shown in clinical studies to improve the extent of resection which in turn correlates with improved patient outcome [9–12].
Preservation of function
Despite the surgical goal to remove as much tumor mass or tumor cells (cytoreduction) as possible, it can be in conflict with another even more important surgical goal, namely the preservation of neurological function. Functional preservation and quality of patient life after tumor resection have in the last decade become the primary surgical goals. For benign brain tumors, which can be cured, the patient expects to leave the surgery in the same or better neurological state than before. For malignant brain tumors, the patient also expects maximum possible quality of life since they cannot be cured and life expectancy is short.
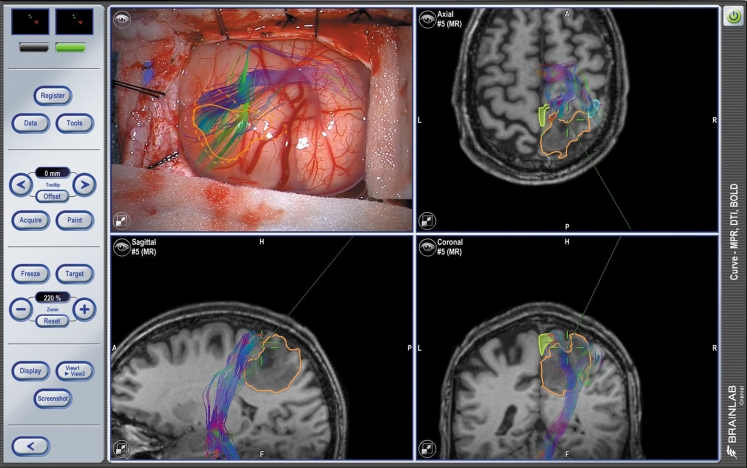
Preservation of function surgery requires the exact localization of functional or eloquent areas. Functional imaging is possible for the exact mapping of a variety of eloquent cortical functions like motoric, sensoric, speech, and language as well as for visualizing subcortical pathways. This information can be integrated into the preoperative planning to avoid any critical areas along the surgical route, and neuronavigation helps to adhere to this planned route. Neuronavigation can also augment the microscope view with preoperatively defined objects, such as tumor and critical structures, to support minimally invasive surgery (see Fig. 5). Image-guided neuronavigation has advanced to functional neuronavigation, which has been shown to improve surgical outcomes for complex surgeries [13].
Fig. 5.
Exemplary neuronavigation screenshot showing microscope-based navigation and the overlay of functional information, e.g., eloquent cortical areas (light blue outline), subcortical fibers (colorful fibers) in relation to the tumor (yellow outline) allowing to navigate to the tumor avoiding critical risk structures. Copyright: Brainlab AG
Intraoperative imaging
The main limitation of current neuronavigation systems is that they rely on preoperative images for accurate navigation. The brain is a semi-rigid mass surrounded by CSF and susceptible to a phenomenon called brainshift, which is the result of either CSF leakage or the collapse or movement of certain parts of the brain. Brainshift can also occur after the drainage of an intracranial cyst or the resection of tumor mass, which initially compacted parts of the brain. Even the opening of the skull and dura incision can introduce brainshift due to the change of the physical environment within the skull. Due to brainshift, the accurate presentation of functional as well as anatomical structures from preoperative images declines. Hence, navigation accuracy is reduced for exact localization of targets but still remains valuable for intraoperative orientation.
To address the problem of brainshift, intraoperative imaging was employed over the last decade to provide the navigation with up-to-date images. Intraoperative imaging also allows evaluating if the surgical goal has been achieved avoiding potential revision operations.
Intraoperative imaging solutions can range from the integration of live ultrasound images to the integration of intraoperative MRI or CT in the operating room, thereby intraoperative MRI (iMRI) offers the best soft tissue contrast for tumor surgery. It also enables an update of functional information during surgery, and in combination with navigation, iMRI is a powerful tool to preserve function and achieve optimal resection control [14, 15]. Modern iMRI suites are designed as a multi-room configuration to allow for a higher utilization, as it can be not only utilized for surgical disciplines but also for diagnostic scanning. This way return-of-investment can be achieved faster. But intraoperative MRI remains the most expensive imaging option and requires significant building constructions.
As a compromise of soft tissue image quality, versatility, and affordability, intraoperative CT (iCT) has emerged. iCT allows for minimal interruption of the surgical workflow since scan time is significantly shorter than iMRI and patient positioning is less limited, especially newer generations of portable iCT scanners are designed specifically for intraoperative use and enable the surgeon to verify their surgical progress and automatically update the navigation (see Fig. 6). For spinal interventions, iCT brings the advantage of having the patient in prone positioning both during image acquisition and surgery, meaning there is no need to compensate for differences of the spine providing more accuracy and reducing the surgeon’s radiation exposure [16].
Fig. 6.
Intraoperative imaging of the future with a portable, multi-slice CT scanner tightly integrated with navigation optimized for use in surgery. Copyright: Brainlab AG
Navigation for orthopedic surgery
In orthopedic surgery, although each patient is comprised of individual anatomy, the surgeries are quite similar regarding the intraoperative workflow. In each case, a joint must be replaced, with the goal of reproducing nature as well as possible. For both hip and knee replacement, the surgeon aims for precise and accurate placement and alignment of the implants. In contrast to neurosurgery, where the focus lies on precise localization and avoidance of areas of risk, in orthopedic surgery, just a precise measurement tool is required.
There are more than 1.5 million estimated knee procedures per year worldwide. The surgical challenges for total knee replacement are to restore function and to achieve implant longevity. In the cases where a revision of the implant is required, it occurs within 2 years (55 %) or within 5 years (63 %) after the primary total knee replacement. The main causes of this early revision are instability (21–27 %), polyethylene wear and aseptic loosening (10–28 %), and patellofemoral problems (8 %) [17, 18].
The key challenges during hip replacement surgery are the restoration of length and offset of the leg as well as the accurate positioning of the implant. Leg length discrepancy is the second and total hip dislocation is the fifth most cited source of medical malpractice litigation among American Association of Hip and Knee Surgeons [19, 20].
The aim of navigation is to make joint replacement surgery more accurate and more reproducible, not only in highly specialized institutions, but also at average care facilities where 80 % of all joints are implanted. To meet the expectance criteria on the user side, the navigations system needs to seamlessly integrate into the conventional surgical workflow and add little extra cost and effort.
Improved implant placement
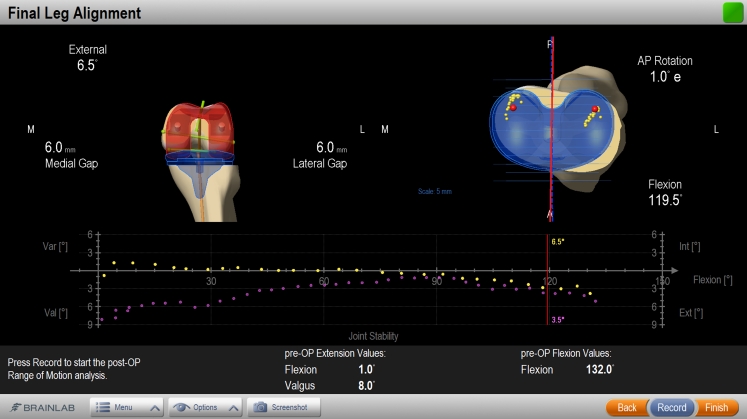
For knee replacement surgery, navigation offers preoperative planning, perioperative intervention, and accurate implant positioning. The exact mechanical axis can be visualized to optimize the alignment of the implant (see Fig. 7), as straight alignment is difficult to see during surgery and manual alignment guides fail to deliver 3D measurements. This can lead to implants placed with improved overall long leg alignment and less outliers outside the scientifically accepted deviation of ±3° from the mechanical axis [21, 22]. Furthermore, the navigation system is able to collect data regarding the laxity of the knee joint while moving through its range of motion. Here the benefit of navigation for the surgeon is to review soft tissue behavior and assess soft tissue balancing intraoperatively with quantifiable values, with the aim of optimizing functional performance of the knee joint and outcome of the patient [23–25].
Fig. 7.
Knee navigation screenshot showing how navigation adds valuable information for orthopedic surgeons. It enables a gap optimization and delivers information on the laxity of the knee joint over the whole range-of-motion. This allows an analysis of the initial and final biomechanical situation during a knee replacement surgery (graph on bottom of image; purple: initial situation, yellow: final situation). Copyright: Brainlab AG
For hip replacement surgery, navigation supports a reproducible and accurate component positioning [26–29], even during minimally invasive techniques [20, 30]. The surgeon may control the limb length and achieve an accurate restoration of leg length and joint offset [20, 31–33].
However, there are certain drawbacks and limitations currently in orthopedic navigation, which prevent it becoming a standard of care. The usability and ease-of-use is still improvable and considered the prevailing limiting factor for mass acceptance [22, 34]. There is a learning curve the surgeon and the surgical team need to go through for each indication supported by navigation. The goal is to change the perception of the computer to that of a “partner” during surgery, rather than an obstacle [35]. Other factors are the space required for the navigation system in the OR and the difficult hand–eye coordination required, if the computer screen is in different orientation than the surgical field.
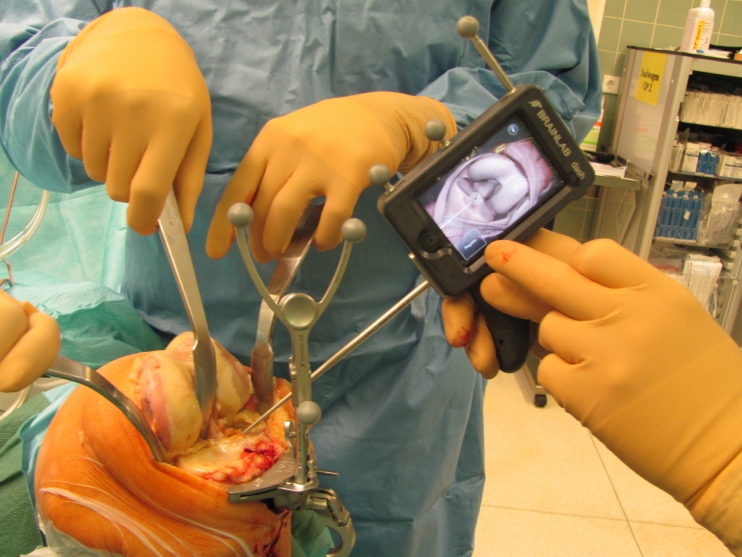
Taking this feedback into consideration, a product was developed in which the computer screen attaches to the manual instruments, rather than increasing the component footprint in the OR. This removes any need to draw the surgeon’s attention away from the surgical field. This product further represents a streamlining of former navigation systems as its capabilities are condensed to what the surgeon really requires. This offers an intuitive use of the software with the addition of only minimal surgical time [36]. Dash® by Brainlab uses an iPod touch® as its screen, which is intraoperatively draped in a sterile bag (see Fig. 8). The communication between camera, computer, and iPod is wireless and connections may even be established to an iPhone® or iPad® during surgery. The “navigation screen” (i.e., the iPod display) can, therefore, be streamed to other devices, facilitating observers to accompany the surgery, even in the nonsterile environment.
Fig. 8.
Use of Brainlab® Dash navigation system during total knee replacement surgery: the surgeon can intuitively navigate the bone resection with the iPod screen alongside the surgical field
From image-guided navigation to navigation of information
Image-guided navigation yields clinical examples of treatments that only become practical through the benefits of computer-centric patient data, image, and sometimes video-management (e.g., microscope–video integration). A further and natural evolution surrounding navigation systems over the last decade on the high-end has been the digital ORs of neurosurgery suites. These are centered on an image-guided surgery system and integrated with an intraoperative modality. Here, in the treatment of a brain tumor, e.g., an intraoperative MRI is acquired to visualize the progress of a tumor resection. This additional intraoperative information is critical and can be highly beneficial for the remainder of the surgical treatment and the resulting patient outcome [37, 38]. However, to make this feasible, a large amount of data has to be managed adequately in the OR during the procedure—several preoperative scans (e.g., CT and MR), possibly enriched with preoperative planning information—and the intraoperative datasets which undergo further data enrichment from intelligent algorithms like image fusion are automatically registered with the integrated surgical navigation system. In such cases, the additional information becoming available by active and intelligent data management in the OR can be just as relevant as the surgical navigation (transforming the scan data into clinically relevant information by putting it into the correct clinical context, e.g., by image fusion with a preoperative dataset, for example).
It is important to note that such neurosurgery suites with intraoperative imaging are only one of the highest end incarnations of what is generally referred to as “integrated” (and sometimes also touted as “digital”) OR. Generally, integrated ORs have been around for the last two decades [39]. Initially, the key motivation in their creation was to provide additional value to the surgeon and OR staff by making endoscopy video and control more accessible and to enhance these with adjacent functionalities—with the goal of optimizing space, efficiency, and intraoperative decision-making. Thus, integration aspects in ORs to this day still mostly center around central control of endoscope video and parameters, accessed on a joint control panel or touch screen together with surgical equipment like tables, lights, etc. Together, this summarized medical device integration. Only lately, the term “digital OR” has been increasingly used along with an increased focus on digital handling of elements such as endoscope video. Typically, digital video components are used to make a video available on various displays in the OR and allow for enhanced setup flexibility and better OR staff coordination. In addition, streaming or recording of video can be done for teaching purposes in an auditorium, for example, or for documentation purposes. Independent from video management, a typical OR today often comprises a special OR-suitable workstation perhaps wall-mounted with special hygienic measures implemented for the keyboard and mouse user interfaces. This computer is typically intended to access preoperative digital medical image data, such as X-ray or CT scans, which are stored in the facilities’ Picture Archiving and Communication System (PACS), and provide an interface to the Hospital Information System (HIS) by running SAP-client software—summarized as IT access systems.
Thus, in the modern OR, the three realms of medical device integration, image and video distribution and IT access systems are often segregated, as they have their origin in different domains. However, this comes at significant cost: for example (1) hospitals are being locked into OEM-specific solutions for medical device integration and/or video distribution sides which are hard or impossible to alter and expand at a later point; (2) intraoperative image and video data are typically unrelated to patient-specific information making systematic documentation, for example, often a manual and tedious process; and (3) several inconsistent human–machine interfaces inhibit users exploiting available functionalities to their full potential (see Fig. 9).
Fig. 9.
Typical partitioning of needs in an integrated OR
Nowadays, computer and IP networks can be utilized to tear down existing barriers between medical devices, video distribution, and IT systems, thus enabling better access and improved management of information to facilitate and maximize productivity in the operating room—both for the benefit of the user and patient as well as for the financial benefit of the hospital.
As an example, these benefits become especially apparent when trying to maximize the usefulness of preoperative radiology images. With the widespread adoption of PACS driven by radiology, preoperative medical images had been separated further from the surgeon and staff in the OR: What was formerly available on a physical film for direct interaction in the OR, is now often only accessible via a computer system through a more or less OR-suitable viewing software. Often, there is little to no optimization available for usage in the OR, and only recently, have truly digital ORs emerged, which are optimized for the navigation of information to support on-the-fly clinical decision-making. The widespread adoption of products like Digital Lightbox® from Brainlab [40] show that it can be very beneficial to make preoperative image data available in a simple and intuitive way in the OR—such that the surgeon can ideally immerse in and interact with the data available and is not hindered by the technological shortcomings of an office-viewing solution brought to the OR environment. Furthermore, additional intelligent algorithms such as 3D volume rendering [41] or image fusion [42] can also be deployed to intuitively enrich the raw medical image data, such that critical information becomes more readily available to the surgeon in the OR compared to the usual 2D slice view representations of large 3D CT/MR datasets.
While similar intelligent assistance would also be desirable in real-time for any surgical videos available in an OR, this is technologically far more challenging due to dynamic real-time requirements. Only for certain disciplines do special solutions exist nowadays, for example, in the form of the image-guided surgery systems discussed above.
Furthermore, a truly digital OR also means that all image and video data are fully available for further computer processing and, during this processing, can always be united with any data such as patient demographics retrieved from a connected HIS. Beneficiaries can be intelligent algorithms which further enrich intraoperative image/video data thereby also taking into account initial diagnosis information. Already implemented today is the proper storage of documentation such as screenshots or recordings into digital archives which are being filed automatically under the correct patient ID, etc. An exemplary computer- and IP-centric product is Buzz by Brainlab in which all information processed is fully computer-integrated and thus automatically linked to patient demographics. Additionally, only in such computer-driven information systems can any access to patient-related image and video data including live video streams from the OR be restricted according to state-of-the-art privacy standards, as defined by the Health Insurance Portability and Accountability Act (HIPAA). HIPAA defines the national standards for electronic health care transactions and national identifiers for providers, health insurance plans, and employers in the USA.
From local to global navigation of information
The concept of a “digital OR” works quite well for the local navigation of information. But often surgeons and other medical professionals need a more global interconnection. Medical information (e.g., digital medical images), naturally, may not be widely shared, as it is sensitive and personal information.
In days of the aforementioned privacy standards like HIPAA, a secure network needs to be installed, for example, before images may be passed online from an external radiologist to a hospital. Brainlab has developed a clinical online network called Quentry™.
The clinical online network
When using the clinical online network for exchange of medical data, doctors can quickly examine medical images like CT, X-ray, MR data, or other medical results that are sent by peripheral clinics or medical practices. The immediate and comprehensive assessment of the situation allows doctors to make decisions regarding measures to be taken, having discussed it with the referring physician. Consequently, the patient is treated on-site or, after a short journey (elective), on the campus of a distant hospital. The relating necessary medical images can be immediately and directly downloaded onto the surgical navigation device in the OR.
The introduction of a clinical online network replaces this previously time-consuming task, during which patient data were burned onto a CD and sent to the hospital by taxi. This resulted in delayed operation dates and repeat exams, and in emergencies, interventions had to be carried out without previously viewing the medical images relevant for planning. The result was a potential risk for patients, unnecessary costs, and more work for the hospital. The cloud-based network solution integrates into the routine clinical workflow, enables cost efficiency, and provides the hospitals with an efficient technological basis to offer their medical expertise as a service for other doctors and hospitals.
The Brainlab clinical online network is used as a web-based service and enables connection with any number of hospitals via the cloud, with no infrastructure investment. All of the basic functionality is browser-based. The hospital neither has to provide hardware components nor incur investment costs. Maintenance costs are also minimal.
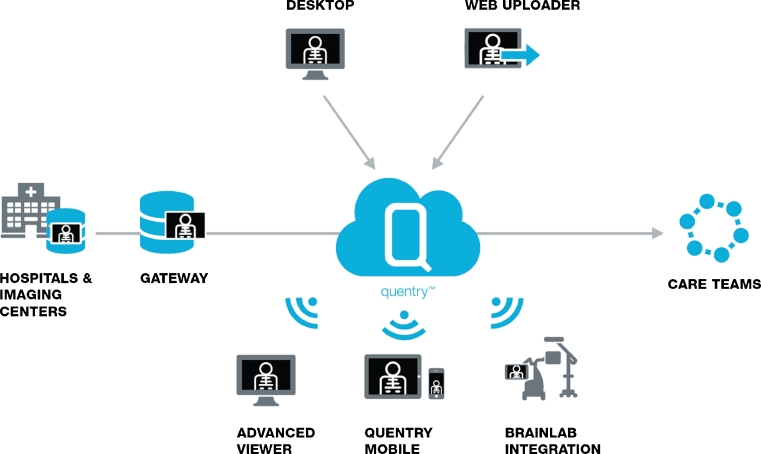
An additional advantage in particular is the scalability and flexibility of the cloud solution. Because of automatic updates, users always access the latest version without having to install any updates (see Fig. 10).
Fig. 10.
A clinical online network like Quentry™ enables uploading of medical images into the cloud to single user or defined departmental accounts, the so-called CareTeams. The uploaded medical data can then be accessed on mobile, desktop, and surgical navigation devices
Use case of a clinical online network
The benefits and possibilities of this clinical online network can be best described on the basis of a use case. The Grosshadern campus of the Ludwig-Maximilians-Universität hospital in Munich, Germany, is an expert specialist medical center, receiving referrals from numerous clinics and medical practitioners in the vicinity. For the doctors at the specialist center, this primarily means time-critical diagnoses and regular referrals of acute cases from the outer surrounding regions. For example, cardiology and cardiac surgery particularly depend upon fast access to image data, so that even before the referral, treatments for common patients can be planned and checked, having necessary information available beforehand.
In practice, for example, the senior physician on duty in the cardiac surgery clinic carries an official mobile phone that informs him or her of new requests from colleagues that come in via e-mail. Using any PC with internet access, the physician can log into the clinical online network with their password and view the patient’s medical image. They may then contact the referring physician by phone, give their assessment regarding the indicated treatment, and—after agreeing on a treatment strategy—can arrange for transfer of the patient if necessary.
Security measures
Each user has a personal password with which they log into the clinical cloud. Access by individuals can be assigned to departmental accounts and removed again, if a doctor leaves the hospital, for example. The patient data are transferred with SSL encryption and saved in encrypted form according to the AES standard. It can be transferred anonymously or with personal information. The user that uploads the data is responsible for it; they can revoke the viewing rights at any time and specify whether the recipient can view the data only or whether they can also download it to the hospital’s own PACS, where it is subject to the particular internal data storage routines. The system complies with the German Federal Data Protection Act (BDSG (Bundesdatenschutzgesetz) and fulfills the American US HIPAA standards.
Interconnection to come
Parallel to the implementation of the clinical online network, Brainlab will provide mobile applications for tablets and smartphones for flexible access to patient medical data and integration with PACS systems for easier uploading and downloading. Cloud-based clinical planning applications will be deployed via the clinical cloud platform to enrich medical images online. Furthermore, all Brainlab devices (such as surgical navigation systems) will be connected to the Brainlab cloud, providing doctors access to medical data from the cloud right in the operating room.
Conclusion
The surgeon’s quest for safer, less invasive, and more cost-efficient procedures has come a long way and continues to move forward at an unprecedented pace. What started as basic localization technique has followed the growth of modern technology beyond specialized uses. Over the past decade, navigation in surgery has evolved beyond imaging modalities and bulky systems into the rich networking of the cloud or devices that are pocket-sized. Surgical plans for navigation can now be reviewed from an iPad on the sofa at home or easily shared and discussed with colleagues abroad. Such advances have only been made possible by close collaboration between technologically companies and surgeons. Navigation in surgery is already the standard of care for a variety of disciplines. With improved computer technology and a trend towards advanced information processing, navigation will soon be increasingly integrated into surgical routines.
Acknowledgments
We would like to thank the following people for supporting the writing and reviewing and for their valuable input: Dr. Daniel Denzler, Nadja Heindl, and Martin Pregler.
Conflicts of interest
All authors are employees of Brainlab AG.
Footnotes
Uli Mezger and Claudia Jendrewski equally contributed to the article.
References
- 1.Enchev Y. Neuronavigation: geneology, reality, and prospects. Neurosurg Focus. 2009;27(3):E11. doi: 10.3171/2009.6.FOCUS09109. [DOI] [PubMed] [Google Scholar]
- 2.Paleologos TS, Wadley JP, Kitchen ND, Thomas DG. Clinical utility and cost-effectiveness of interactive image-guided craniotomy: clinical comparison between conventional and image-guided meningioma surgery. Neurosug. 2000;47(1):40–48. doi: 10.1097/00006123-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 3.Omay SB, Barnett GH. Surgical navigation for meningioma surgery. J Neurooncol. 2010;99(3):357–364. doi: 10.1007/s11060-010-0359-6. [DOI] [PubMed] [Google Scholar]
- 4.Maciuanas RJ. Computer-assisted neurosurgery. Clin Neurosurg. 2006;53:267–271. [PubMed] [Google Scholar]
- 5.Kraus MD, Krischak G, Keppler P, Gebhard FT, Schuetz UH. Can computer-assisted surgery reduce the effective dose for spinal fusion and sacroiliac screw insertion? Clin Orthop Relat Res. 2010;468(9):2419–2429. doi: 10.1007/s11999-010-1393-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferroli P, Tringali G, Acerbi F, Schiariti M, Broggi M, Aquino D, Broggi G. Advanced 3-dimensional planning in neurosurgery. Neurosurg Suppl. 2013;1:A54–A62. doi: 10.1227/NEU.0b013e3182748ee8. [DOI] [PubMed] [Google Scholar]
- 7.Jung TY, Jung S, Kim IY, Park SJ, Kang SS, Kim S, Lim SC. Application of neuronavigation system to brain tumor surgery with clinical experience of 420 cases. Minim Invasive Neurosurg. 2006;49(4):210–215. doi: 10.1055/s-2006-948305. [DOI] [PubMed] [Google Scholar]
- 8.Wadley J, Dorward N, Kitchen N, Thomas D. Pre-operative planning and intra-operative guidance in modern neurosurgery: a review of 300 cases. Ann R Coll Surg Engl. 1999;81(4):217–225. [PMC free article] [PubMed] [Google Scholar]
- 9.Kurimoto M, Hayashi N, Kamiyama H, Nagai S, Shibata T, Asahi T, Matsumura N, Hirashima Y, Endo S. Impact of neuronavigation and image-guided extensive resection for adult patients with supratentorial malignant astrocytomas: a single-institution retrospective study. Minim Invasive Neurosurg. 2004;47(5):278–283. doi: 10.1055/s-2004-830093. [DOI] [PubMed] [Google Scholar]
- 10.Wirtz CR, Albert FK, Schwaderer M, Heuer C, Staubert A, Tronnier VM, Knauth M, Kunze S. The benefit of neuronavigation for neurosurgery analyzed by its impact on glioblastoma surgery. Neurol Res. 2000;22(4):354–360. doi: 10.1080/01616412.2000.11740684. [DOI] [PubMed] [Google Scholar]
- 11.Sanai N, Berger MS. Glioma extent of resection and its impact on patient outcome. Neurosurg. 2008;62(4):753–766. doi: 10.1227/01.neu.0000318159.21731.cf. [DOI] [PubMed] [Google Scholar]
- 12.Schulz C, Waldeck S, Mauer UM (2012) Intraoperative image guidance in neurosurgery: development, current indications, and future trends. Radiol Res Pract 197364. doi:10.1155/2012/197364 [DOI] [PMC free article] [PubMed]
- 13.Nimsky C, Ganslandt O, Fahlbusch R. Implementation of fiber tract navigation. Neurosurg. 2006;58(4 Suppl 2):ONS-292–ONS-303. doi: 10.1227/01.NEU.0000204726.00088.6D. [DOI] [PubMed] [Google Scholar]
- 14.Hatiboglu MA, Weinberg JS, Suki D, Rao G, Prabhu SS, Shah K, Jackson E, Sawaya R. Impact of intraoperative high-field magnetic resonance imaging guidance on glioma surgery: a prospective volumetric analysis. Neurosurg. 2009;64(6):1073–1081. doi: 10.1227/01.NEU.0000345647.58219.07. [DOI] [PubMed] [Google Scholar]
- 15.Nimsky C, Ganslandt O, Hastreiter P, Wang R, Benner T, Sorensen AG, Fahlbusch R. Preoperative and intraoperative diffusion tensor imaging-based fiber tracking in glioma surgery. Neurosurg. 2005;56(1):130–138. doi: 10.1227/01.neu.0000144842.18771.30. [DOI] [PubMed] [Google Scholar]
- 16.Scheufler KM, Franke J, Eckardt A, Dohmen H. Accuracy of image-guided pedicle screw placement using intraoperative computed tomography-based navigation with automated referencing, part I: cervicothoracic spine. Neurosurg. 2011;69(4):782–795. doi: 10.1227/NEU.0b013e318222ae16. [DOI] [PubMed] [Google Scholar]
- 17.Fehring TK, Odum S, Griffin WL, Mason JB, Nadaud M. Early failures in total knee arthroplasty. Clin Orthop Relat Res. 2001;392:315–318. doi: 10.1097/00003086-200111000-00041. [DOI] [PubMed] [Google Scholar]
- 18.Sharkey PF, Hozack WJ, Rothman RH, Shastri S, Jacoby SM (2002) Why are total knee arthroplasties failing today? Clin Orthop Relat Res (404)7-13. [DOI] [PubMed]
- 19.Upadhyay A, York S, Macaulay W, McGrory B, Robbennolt J, Bal BS. Medical malpractice in hip and knee arthroplasty. J Arthroplasty. 2007;22(6 Suppl 2):2–7. doi: 10.1016/j.arth.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 20.Kelley TC, Swank ML. Role of navigation in total hip arthroplasty. J Bone Joint Surg Am. 2009;91(Suppl 1):153–158. doi: 10.2106/JBJS.H.01463. [DOI] [PubMed] [Google Scholar]
- 21.Mason JB, Fehring TK, Estok R, Banel D, Fahrbach K. Meta-analysis of alignment outcomes in computer-assisted total knee arthroplasty surgery. J Arthroplasty. 2007;8:1097–1106. doi: 10.1016/j.arth.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Blakeney WG, Khan RJ, Wall SJ. Computer-assisted techniques versus conventional guides for component alignment in total knee arthroplasty: a randomized controlled trial. J Bone Joint Surg Am. 2011;93:1377–1384. doi: 10.2106/JBJS.I.01321. [DOI] [PubMed] [Google Scholar]
- 23.Choong PF, Dowsey MM, Stoney JD. Does accurate anatomical alignment result in better function and quality of life? Comparing conventional and computer-assisted total knee arthroplasty. J Arthroplasty. 2009;24(4):560–569. doi: 10.1016/j.arth.2008.02.018. [DOI] [PubMed] [Google Scholar]
- 24.Peterlein CD, Schofer MD, Fuchs-Winkelmann S, Scherf FG. Clinical outcome and quality of life after computer-assisted total knee arthroplasty: results from a prospective, single-surgeon study and review of the literature. Chir Organi Mov. 2009;93(3):115–122. doi: 10.1007/s12306-009-0042-2. [DOI] [PubMed] [Google Scholar]
- 25.Luring C, Oczipka F, Grifka J, Perlick L (2008) The computer-assisted sequential lateral soft-tissue release in total knee arthroplasty for valgus knees. 32(2):229–235. [DOI] [PMC free article] [PubMed]
- 26.Ryan JA, Jamali AA, Bargar WL. Accuracy of computer navigation for acetabular component placement in THA. Clin Orthop Relat Res. 2010;468(1):169–177. doi: 10.1007/s11999-009-1003-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petrella AJ, Stowe JQ, D’Lima DD, Rullkoetter PJ, Laz PJ. Computer-assisted versus manual alignment in THA—a probabilistic approach to range of motion. Clin Orthop Relat Res. 2009;467:50–55. doi: 10.1007/s11999-008-0561-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalteis T, Handel M, Baethis H, Perlick L, Tingart M, Grifka J. Imageless navigation for insertion of the acetabular component in total hip arthroplasty—is it as accurate as CT-based navigation? J Bone Joint Surg Br. 2006;88(2):163–167. doi: 10.1302/0301-620X.88B2.17163. [DOI] [PubMed] [Google Scholar]
- 29.Reininga IH, Zijlstra W, Wagenmakers R, Boerboom AL, Huijbers BP, Groothoff JW, Bulstra SK, Stevens M. Minimally invasive and computer-navigated total hip arthroplasty: a qualitative and systematic review of the literature. BMC Musculoskelet Disord. 2010;11:92. doi: 10.1186/1471-2474-11-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wixson RL, MacDonald MA. Total hip arthroplasty through a minimal posterior approach using imageless computer-assisted hip navigation. J Arthroplasty. 2005;20(7 suppl 3):51–56. doi: 10.1016/j.arth.2005.04.024. [DOI] [PubMed] [Google Scholar]
- 31.Manzotti A, Cerveri P, De Momi E, Pullen C, Confalonieri N. Does computer-assisted surgery benefit leg length restoration in THA? Navigation versus conventional freehand. Int Orthop. 2009;35(1):19–24. doi: 10.1007/s00264-009-0903-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy SB, Ecker TM. Evaluation of a new leg length measurement algorithm in hip arthroplasty. Clin Orthop Relat Res. 2007;463:85–89. doi: 10.1097/BLO.0b013e318126c08f. [DOI] [PubMed] [Google Scholar]
- 33.Renkawitz T, Schuster T, Grifka J, Kalteis E, Sendtner E. Leg length and offset measures with a pinless femoral reference array during THA. Clin Orthop Relat Res. 2010;468(7):1862–1868. doi: 10.1007/s11999-009-1086-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lehnen K, Giesinger K, Warschkow R, Porter M, Koch E, Kuster MS. Clinical outcome using a ligament referencing technique in CAS versus conventional technique. Knee Surg Sports Traumatol Arthrosc. 2010;19(6):887–892. doi: 10.1007/s00167-010-1264-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivkin G, Liebergall M. Challenges of technology integration and computer-assisted surgery. J Bone Joint Surg Am. 2009;91(Suppl 1):13–16. doi: 10.2106/JBJS.H.01410. [DOI] [PubMed] [Google Scholar]
- 36.Schnurr C, Eysel P, König DP. Displays mounted on cutting blocks reduce the learning curve in navigated total knee arthroplasty. Comput Aided Surg. 2011;16:249–256. doi: 10.3109/10929088.2011.603750. [DOI] [PubMed] [Google Scholar]
- 37.Kuhnt D, Becker A, Ganslandt O, Bauer M, Buchfelder M, Nimsky C. Correlation of the extent of tumor volume resection and patient survival in surgery of glioblastoma multiforme with high-field intraoperative MRI guidance. Neuro Oncol. 2011;13(12):1339–1348. doi: 10.1093/neuonc/nor133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Senft C, Bink A, Franz K, Vatter H, Gasser T, Seifert V. Intraoperative MRI guidance and extent of resection in glioma surgery: a randomised, controlled trial. Lancet Oncol. 2011;12(11):997–1003. doi: 10.1016/S1470-2045(11)70196-6. [DOI] [PubMed] [Google Scholar]
- 39.Stryker. On http://www.europe.stryker.com/i-suite. Accessed 5 May 2012.
- 40.Denzler DN (2010) Brainlab AG, internal analysis of functionalities and user surveys of “Brainsuite Net” (Brainlab 1st generation OR integration) vs “Digital Lightbox” (Brainlab OR-optimized surgical DICOM viewer with intuitive user interface and intelligent algorithms), Mar 2010
- 41.de Yang L, Xu QW, Che XM, Wu JS, Sun B. Clinical evaluation and follow-up outcome of presurgical plan by Dextroscope: a prospective controlled study in patients with skull base tumors. Surg Neurol. 2009;72(6):682–689. doi: 10.1016/j.surneu.2009.07.040. [DOI] [PubMed] [Google Scholar]
- 42.Thani NB, Bala A, Swann GB, Lind CR. Accuracy of postoperative computed tomography and magnetic resonance image fusion for assessing deep brain stimulation electrodes. Neurosurg. 2011;69(1):207–214. doi: 10.1227/NEU.0b013e318218c7ae. [DOI] [PubMed] [Google Scholar]