Abstract
It was recently demonstrated that mechanical shearing of synovial fluid (SF), induced during joint motion, rapidly activates latent transforming growth factor β (TGF-β). This discovery raised the possibility of a physiological process consisting of latent TGF-β supply to SF, activation via shearing, and transport of TGF-β into the cartilage matrix. Therefore, the two primary objectives of this investigation were to characterize the secretion rate of latent TGF-β into SF, and the transport of active TGF-β across the articular surface and into the cartilage layer. Experiments on tissue explants demonstrate that high levels of latent TGF-β1 are secreted from both the synovium and all three articular cartilage zones (superficial, middle, and deep), suggesting that these tissues are capable of continuously replenishing latent TGF-β to SF. Furthermore, upon exposure of cartilage to active TGF-β1, the peptide accumulates in the superficial zone (SZ) due to the presence of an overwhelming concentration of nonspecific TGF-β binding sites in the extracellular matrix. Although this response leads to high levels of active TGF-β in the SZ, the active peptide is unable to penetrate deeper into the middle and deep zones of cartilage. These results provide strong evidence for a sequential physiologic mechanism through which SZ chondrocytes gain access to active TGF-β: the synovium and articular cartilage secrete latent TGF-β into the SF and, upon activation, TGF-β transports back into the cartilage layer, binding exclusively to the SZ.
Introduction
Transforming growth factor β (TGF-β) is a multifunctional cytokine that modulates the differentiation, proliferation, and extracellular matrix (ECM) production of various biological tissues (1). Traditionally, investigators have been particularly interested in TGF-β’s role in the development and maintenance of articular cartilage due to its high native levels in synovial joints (2,3) and its well-characterized ability to modulate the biosynthesis of a large number of cartilage ECM products (4–8). However, efforts to elucidate TGF-β’s native role in the tissue have focused primarily on ex situ experimental models that exclusively describe the downstream effects of TGF-β on chondrocytes. These studies typically did not address a host of TGF-β events that occur in the tissue ECM, such as TGF-β activation and binding interactions. As a result, the extracellular regulation of TGF-β activity and the physiologic mechanisms through which chondrocytes access the growth factor remain unclear.
In cartilage, as in other tissues, TGF-β is synthesized in an inactive latent complex that is unable to bind to cellular membrane receptors, and thus initially unable to induce a biological response (9). In this latent complex, the 25 kDa mature TGF-β peptide is linked noncovalently to a 70 kDa latency-associated peptide (LAP), and together they form the small latent complex (SLC). This complex may be disulfide-bonded to a latent TGF-β binding protein (LTBP, ∼180 kDa), constituting a configuration termed the large latent complex (LLC). Although latent TGF-β is present in synovial fluid (SF) at relatively high concentrations (10–40 ng/mL (10–12)), the mature peptide must first undergo activation (release from LAP) before it can modulate the metabolic activity of cartilage.
Mechanisms behind the activation of latent TGF-β in healthy SF have not been addressed extensively in the literature. In a recent experimental investigation, we demonstrated that mechanical shearing of SF can serve as a critical mediator of latent TGF-β activation (13). During joint motion, opposing articular surfaces slide relative to one another, producing high levels of fluid shear in the SF. The shearing produced during physiologic levels of joint motion rapidly activates a large fraction of the latent TGF-β that is present, which remains stable in SF for at least several hours. Furthermore, TGF-β activation does not occur spontaneously in the absence of shearing, which strongly suggests that mechanical shearing forces (as opposed to chemical mediators) serve as the dominant mechanism of TGF-β activation in healthy SF.
Considering the well-established effect of active TGF-β on chondrocyte biosynthesis, shear-induced activation of latent TGF-β is likely to have a strong influence on the metabolic activity of articular cartilage. However, the physiologic role that this mechanism plays in the complex native environment of the synovial joint remains unclear. The metabolic impact of this mechanism requires a continuous presence of latent TGF-β in SF and the ability of activated TGF-β to act on surrounding cells.
Physiologic levels of SF shearing can rapidly activate latent TGF-β at a rate of ∼0.5 ng/mL per hour (13). Therefore, the level of active TGF-β in the joint may heavily depend on the rate at which latent TGF-β is secreted and replenished into SF. In previous studies, TGF-β was shown to undergo synthesis from synoviocytes (14) and chondrocytes (2,3,15–18). These studies were primarily conducted on isolated cells or for short culture durations. Therefore, the rate at which latent TGF-β is secreted in situ remains unclear.
In principle, upon activation, the mature TGF-β peptide is free to diffuse from SF into the adjoining articular cartilage ECM. However, in most tissues, TGF-β is believed to act locally in an autocrine or paracrine fashion, in the immediate proximity of its region of activation (19). Therefore, the transport of active TGF-β through a dense ECM has not been previously characterized. Evidence suggests that this transport may be a complex process due to extracellular interactions with matrix proteins and cellular receptors. Active TGF-β can bind to a large variety of molecules present in the cartilage ECM, such as proteoglycans (20–22), collagens (23,24), and glycoproteins (25,26). As demonstrated previously for other growth factors (27), these binding interactions can potentially influence the transport rate and spatial distribution of active TGF-β in the tissue.
Based on this understanding, the primary goals of this investigation were to: 1), characterize the rate of secretion of latent TGF-β into SF; and 2), characterize the transport of active TGF-β across the articular surface and into cartilage tissue.
Materials and Methods
To characterize the physiologic secretion of latent TGF-β into SF, we measured its synthesis and secretion from explants of synovium and different zones of articular cartilage into conditioned media (study 1). To characterize the physiologic rate of transport of activated TGF-β from SF into articular cartilage, we adopted an experimental transport system (study 2). Here, cartilage explants were exposed to a bath of 10 ng/mL exogenous active TGF-β1 and its uptake across the articular surface was monitored over time. We selected this exogenous concentration based on our earlier shear-activation study, in which low physiologic levels of SF shearing activated the TGF-β1 isoform at a rate of 0.5 ng/mL/h (13). Considering that additional isoforms (TGF-β2 and TGF-β3) are present in SF, the 10 ng/mL exogenous bath concentration may be representative of activated levels during a 1–2 day period of normal joint activity. Furthermore, to assess whether TGF-β can transport from SF into articular cartilage before activation, we measured the uptake of commercially available SLC TGF-β1 in explants. Lastly, to interpret and validate the experimental results of active TGF-β uptake, we conducted a finite-element simulation to theoretically assess the transport behavior (study 3).
Materials
Cylindrical explants of articular cartilage (with an intact articular surface) and sections of synovial tissue were obtained from the femoral condyles and capsule of 2-month-old calf knee joints within 24 h of sacrifice. In total, nine joints were used in this investigation, with samples evenly distributed from three joints for each study. For tissue culture experiments, samples were maintained sterilely in an ITS-media formulation consisting of Dulbecco’s modified Eagle’s medium (DMEM), insulin-transferrin-selenous acid (1% ITS+ Premix), 50 μg/mL L-proline, 1% antibiotic-antimycotic (100 U/mL penicillin, 100 μg/mL streptomycin, and 0.25 μg/mL amphotericin-B), 0.1 μM dexamethasone, 10 μg/mL sodium pyruvate, and 50 μg/mL ascorbate-2-phosphate. For experiments with devitalized tissue, samples were frozen and stored at −30°C for 1–2 weeks until testing. Active TGF-β1, active TGF-β3, and latent TGF-β1 (SLC) were supplied in human recombinant form from R&D Systems. Experiments were conducted in untreated polystyrene well plates and tissue/solution samples were collected in silanized polypropylene Eppendorf tubes. In all experiments (for both live and devitalized tissue), growth factors were reconstituted in the ITS media. The use of this media reconstitution was found to prevent the often-problematic occurrence of active TGF-β binding to the aforementioned plasticware (28).
TGF-β measurement
For all experiments (studies 2 and 3), at the completion of testing, cartilage explants were weighed, minced, and treated with a solution of 4 M guanidine hydrochloride, supplemented with protease inhibitors, at 4°C for 2 days to extract their TGF-β content, as reported previously (2). TGF-β1 concentrations were determined through an enzyme-linked immunosorbent assay (ELISA) kit specific for TGF-β1 (R&D Systems) using recombinant human active TGF-β1 as standards. This kit specifically detects the TGF-β1 isoform (human and bovine), but only recognizes it in the active form. Therefore, for TGF-β synthesis testing, conditioned media samples were assayed before and after an acid activation treatment (acidification for 15 min with 20% of 1 N HCl, followed by neutralization with an equal volume of 1.2 N NaOH/0.5 M HEPES), allowing for independent measures of their active and total (active + latent) TGF-β1 content. In explants, guanidine hydrochloride treatment activates and extracts all TGF-β from the tissue. Therefore, measures in explants represent their total (endogenous bovine + exogenous human recombinant) TGF-β1 content. TGF-β1 concentrations are expressed based on the fluid volume of the explant (given an experimentally determined sample water content: wet weight fraction of 0.87 ± 0.02). Guanidine extracts were diluted at least 20-fold in ITS-DMEM before assays were performed. A separate test confirmed that assay measurements were not affected by the presence of ECM constituents in guanidine extracts (see Supporting Material).
For the latent TGF-β1 uptake experiment (study 2), concentrations of the TGF-β1 SLC in explants and the external bath were determined through an ELISA for LAP with recombinant human LAP as standards. Mouse monoclonal IgG antibodies (R&D Systems) were used for capture (clone #9005, 2 μg/mL) and detection (clone #27240, 4 μg/mL). All other reagents were implemented as recommended in the TGF-β1 Duoset kit. This assay is specific for human LAP and therefore detects only exogenous human SLC while ignoring endogenous levels in bovine samples.
Study 1: TGF-β synthesis rate from cartilage and synovium explants
Live cylindrical cartilage explants (∅3 × 0.5 mm) from the superficial zone (SZ; 0 mm below the articular surface), middle zone (0.5 mm below surface), and deep zone (1.5 mm below surface), and patches of synovial tissue (≈5 mg) were individually cultured in ITS media (300 μL per sample, n = 5 per tissue type) for up to 8 days. Further, to examine the autoinduction of TGF-β synthesis, an additional set of SZ explants were individually cultured in ITS media with varying levels of exogenous active TGF-β3 (0, 1 ng/mL, or 10 ng/mL). TGF-β3 was used here to distinguish between exogenous and endogenous TGF-β content, since assays only detect TGF-β1. The media were changed daily. Conditioned media were frozen and subsequently tested for the mass of active and latent TGF-β1 released from each explant and expressed as the concentration based on the volume of the explant (approximated from mass measurements). At the end of the culture period, all live cartilage and synovial tissue explants were digested in 0.5 mg/mL Proteinase-K (MP Biomedicals) in 50 mM Tris-buffered saline containing 1 mM ethylenediaminetetraacetic acid, 1 mM iodoacetamide, and 10 mg/mL pepstatin A, at 56°C for 16 h. The DNA content of digested samples was quantified with the PicoGreen assay (Invitrogen), as described previously for cartilage (29), with bacteriophage λ DNA standards. Furthermore, the endogenous TGF-β1 content of an additional set of SZ explants was determined before and after a 12 day culture period (n = 6 for each).
Study 2: Experimental rate of penetration of TGF-β1 into articular cartilage
Active TGF-β1
Cylindrical devitalized cartilage explants (∅4 × 2.5 mm) were inserted into thin sections of Tygon tubing (4 mm inner diameter, 3 mm long) and fixed to the bottom of a polystyrene dish with cyanoacrylate glue. This configuration laterally confines each explant, ensuring that solute transport occurs only in one dimension across the solution-exposed articular surface. The absence of fluid leakage between the tubing and explant lateral surface was confirmed with the use of a fluorescent dye (results not shown). Samples were exposed to 10 ng/mL exogenous active TGF-β1 in ITS media (10 mL per sample) and after 0.5, 20, and 40 h the bath samples and explants were removed and frozen (n = 6 per time point). The frozen explants were sectioned with a freezing stage sledge microtome into 160 μm slices from the solution-exposed articular surface (up to 800 μm deep). The slices were then processed for their TGF-β1 content to measure the concentration at discrete positions through the depth of the tissue. Because the aforementioned TGF-β1 ELISA detects both bovine and human recombinant TGF-β1, both endogenous and exogenous TGF-β1 are detected through this analysis. Therefore, control samples exposed to TGF-β-free ITS DMEM were utilized to independently assess endogenous TGF-β1 levels through the depth of the tissue after 0.5 and 40 h (n = 6 per time point). All experiments were performed on an orbital shaker at 22°C.
Latent TGF-β1
Cylindrical devitalized cartilage explants (∅3 × 0.5 mm) were exposed to a bath of exogenous latent TGF-β1 (SLC) at 100 ng/mL or 1000 ng/mL (n = 6 per concentration). After 24 h, explants and bath samples were collected, frozen, and processed for their exogenous SLC content.
Study 3: Theoretical analysis of active TGF-β1 uptake into articular cartilage
To help interpret and validate our experimental uptake results, we performed a finite-element (computational) simulation to theoretically predict the rate of penetration of active TGF-β into articular cartilage. This simulation required knowledge about the transport and binding properties of active TGF-β in the cartilage ECM, i.e., the diffusivity, D; partition coefficient, κ; the concentration of binding sites, Nt; and the dissociation constant, KD. To characterize these parameters, we employed an experimental system in which TGF-β levels were monitored in thin devitalized cartilage explants that were exposed to a bath of exogenous active TGF-β. The following sections describe the experimental and theoretical techniques implemented for this characterization.
Theoretical model
The required TGF-β binding parameters can be independently determined using a Langmuir model, where it is assumed that free active TGF-β can reversibly bind to a finite number of uniformly distributed binding sites in the ECM of cartilage:
According to the law of mass action, under the assumption of elementary reaction kinetics, the binding rate of free active TGF-β can be described by
| (1) |
where CF and CB are the respective concentrations of free and bound active TGF-β, Nt is the total concentration of binding sites in the tissue, and kf and kr are the respective forward and reverse reaction rates.
When cartilage explants are exposed to a solution of exogenous active TGF-β, due to steric volume effects and/or long-range electrostatic interactions, active TGF-β is partially excluded from its interstitial pore space, which limits its free concentration in the tissue. At equilibrium, this partitioning can be described through the relation:
| (2) |
where κ is the partition coefficient of active TGF-β in the tissue and CBath is its concentration in the bathing solution. From Eqs. 1 and 2, at equilibrium (dCF/dt = 0), the normalized TGF-β uptake ratio RU can be expressed as
| (3) |
where KD = kr/kf is the dissociation constant. This equation expresses a sigmoidal relation between the free TGF-β concentration in the tissue, CF or κCBath, and the TGF-β uptake ratio. When the free TGF-β concentration is much lower than the dissociation constant (CF ≪ KD), RU approaches the value κ(Nt/KD + 1). As CF approaches KD, the binding sites begin to saturate and RU decreases in a sigmoidal fashion. Finally, when CF ≫ KD, the available binding sites become saturated with TGF-β, and RU approaches κ.
Upon exposure of an active TGF-β bath solution to cartilage, the bath concentration will decrease as free TGF-β binds to the tissue. Initially, for a thin tissue section (where diffusion time into the tissue is negligible) and when all binding sites are unoccupied (CB = 0), from Eqs. 1 and 2, the bath concentration can be approximated by
| (4) |
where NBath is the number of binding sites in the tissue normalized to the volume of the bath. NBath can be multiplied by the ratio of tissue to bath volume to recover Nt.
Equilibrium binding of TGF-β1
We formulated an experiment to determine the binding parameters Nt and KD by measuring the bound TGF-β uptake ratio RU in explants at equilibrium and curve-fitting the data to Eq. 3. According to Eq. 3, to extract a unique solution from the data, it is necessary to measure RU over a wide range of TGF-β bath concentrations (including ranges where CF ≪ KD and CF ≫ KD). Therefore, thin explant slices (∅3 × 0.5 mm) were exposed to a 10 mL bath of exogenous active TGF-β1 over the wide range of 0, 5, 10, 20, 65, 95, or 450 ng/mL for 24 h (n = 6 per concentration). The explant size was selected to ensure that active TGF-β1 would equilibrate over the time course of the experiment (assumption of Eq. 3). The suitability of the 24 h incubation period for achieving equilibrium was assessed by additional exposure of slices to a 10 ng/mL TGF-β1 bath for 0, 0.5, 5, 24, and 48 h. Experiments were conducted on an orbital shaker at 22°C. After the exposure period, explants and bath samples were frozen for a subsequent measure of their TGF-β1 content. The concentration of exogenous TGF-β1 in explants (CB + CF) was determined by measuring the total explant TGF-β1 content and subtracting the endogenous content as determined from control explants. For all samples, the normalized uptake ratio RU was determined and curve-fitted to Eq. 3.
Transient binding of TGF-β1
We formulated another experiment to determine the product Ntkf by measuring the loss of the active TGF-β content of a bathing solution as a result of its binding to the ECM of an immersed cartilage explant, and then curve-fitting the data to Eq. 4. Thin explant slices (∅4 × 0.16 mm, n = 5) were exposed to a 300 μL bath of 6.8 ng/mL exogenous active TGF-β1. The tissue thickness was selected to minimize the diffusion time of TGF-β1 into the tissue (since Eq. 4 assumes that diffusion time is negligible). The bath volume was selected to yield a measurable decrease in the bath TGF-β1 concentration during the experiment. Experiments were also conducted on an orbital shaker at 22°C. Initially and after 10, 30, 60, and 120 min of exposure, a small aliquot of the bath was acquired and frozen for subsequent measurements of the decreasing concentration of exogenous active free TGF-β1 in the explant. The initial rate of free TGF-β1 loss, dCBath/dt, was determined and used to calculate the quantity Ntkf through Eq. 4.
Finite-element analysis
The open-source finite-element program FEBio (www.febio.org) (30,31) was used to model the one-dimensional (1D) transport of active TGF-β1 at 10 ng/mL from the articular surface into a cylindrical cartilage explant (∅4 × 2.5 mm). The code was extended to account for reversible binding kinetics according to the relation of Eq. 1. The model employed the values of Nt, KD, and kf obtained from the above experimental measurements. The diffusivity D and partition coefficient κ of the 25 kDa active TGF-β in cartilage were estimated at values of 21.3 μm2/s and 0.09, respectively, from the interpolation of data from other proteins and polysaccharides in the tissue (32). To account for the loss of active TGF-β in the bath as it accumulates in the cartilage explant, the bathing solution was modeled with a finite TGF-β content and with a volume of 10 mL as utilized in experiments. The finite-element solution provided the spatiotemporal responses of free (CF) and bound (CB) active TGF-β1 in the explant. For comparison purposes, an additional simulation was conducted to examine the 1D uptake of active TGF-β1 in the absence of binding interactions (Nt = 0).
Statistical analysis
A two-way analysis of variance (ANOVA, α = 0.05) was performed to determine the effect of TGF-β exposure and tissue depth on cartilage TGF-β1 levels during the active TGF-β penetration test (study 2), with statistical significance set at p < 0.05. One-way ANOVAs were performed to detect differences in latent TGF-β1 synthesis rates between different tissue types and TGF-β3 supplementation levels (study 1), and in the uptake ratio, RU, for different TGF-β bath concentrations in the equilibrium binding test (study 3). Tukey’s post hoc test was used to detect differences in the means. For comparison between SLC concentrations in explants at the 100 ng/mL and 1000 ng/mL bath concentrations, an unpaired Student’s t-test (two-tailed) was used.
Results
Study 1: TGF-β synthesis rate
The concentrations of TGF-β1 released by samples are reported based on the volume of the explant rather than the volume of the conditioned media. Cartilage explants possessed high levels of endogenous TGF-β1 in their ECM, and all tissues continued to synthesize and release TGF-β1 throughout the culture period as described here. All tissues exhibited constant TGF-β1 synthesis rates, as illustrated by linear relationships in cumulative secretion over time (mean R2 = 0.99 ± 0.01; Fig. 1 A). Active TGF-β1 was not detected in the conditioned medium of any samples, indicating that TGF-β1 was released predominantly in the latent form. In the absence of exogenous TGF-β, synovium and cartilage explants (all tissue zones) released latent TGF-β1 at similar rates, whether considered on the basis of explant volume or cell content (Table 1). The supplementation of exogenous TGF-β3 significantly increased the latent TGF-β1 synthesis rate (p < 0.001; Fig. 1 B). No significant cell death was observed in these samples as indicated by viability staining (results not shown).
Figure 1.
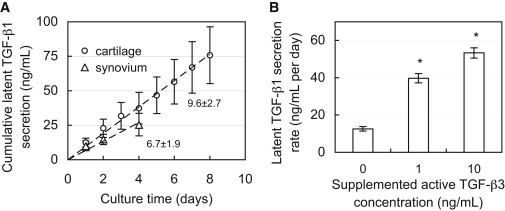
(A) Cumulative secretion of latent TGF-β1 from cultured synovium and middle-zone cartilage tissue explants. The slope represents the secretion rate from tissue (ng/mL/day on tissue volume basis). (B) Secretion rate of latent TGF-β1 from SZ cartilage explants during exposure to varying levels of exogenous active TGF-β3. ∗p < 0.001 indicates significant increase above control (0 ng/mL TGF-β3) secretion rate.
Table 1.
Secretion rate of latent TGF-β1 from explants of synovium and different tissue zones (superficial, middle, and deep) of articular cartilage
| Latent TGF-β1 secretion rate |
||
|---|---|---|
| Per tissue volume (ng/mL per day) | Per cell number (ng/million cells per day) | |
| Superficial zone | 6.7 ± 0.8 | 0.05 ± 0.03 |
| Middle zone | 9.6 ± 2.7 | 0.07 ± 0.04 |
| Deep zone | 8.3 ± 0.9 | 0.08 ± 0.01 |
| Synovium | 6.7 ± 1.9 | 0.04 ± 0.03 |
Large concentrations of latent TGF-β1 were measured in the ECM of SZ cartilage (82.7 ± 11.6 ng/mL). These levels did not change after 12 days of culture (75.1 ± 21.1 ng/mL, p = 0.51). Further, devitalized cartilage explants released near-zero levels of TGF-β1 into conditioned media (0.34 ± 0.17 ng/mL/day).
Study 2: Experimental rate of penetration of TGF-β1 into articular cartilage
Active TGF-β1
Endogenous levels of TGF-β1 varied throughout the depth of devitalized explants, decreasing from 207 ± 20 ng/mL near the articular surface (80 μm deep) to 118 ± 41 ng/mL at 880 μm deep (Fig. 2 A). At all positions throughout the depth, endogenous levels remained unchanged after 40 h of incubation (p = 1.0). Upon exposure to exogenous active TGF-β1, its concentration near the articular surface increased significantly, reaching 556 ± 283 ng/mL after 20 h (p < 0.001, relative to 40 h control; Fig. 2 B). However, no significant increase above endogenous levels was detected below 240 μm into the tissue (p > 0.99), indicating that active TGF-β1 did not reach this depth even after 40 h of exposure. This 35-fold increase above the external bath concentration (total levels minus endogenous levels, divided by bath concentration) suggests that active TGF-β1 binds substantially to the cartilage ECM.
Figure 2.
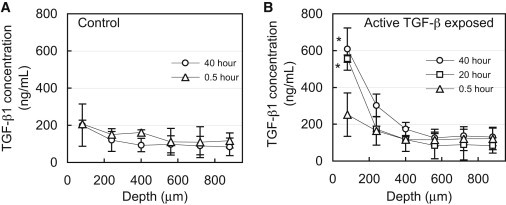
1D uptake of active TGF-β1 into articular cartilage. (A) Depth-dependent variation of endogenous levels of latent TGF-β1 in cartilage explants after incubation in TGF-β-free solution. (B) Depth-dependent levels of TGF-β1 (endogenous latent + exogenous active) after exposure of the articular surface to a bath of 10 ng/mL active exogenous TGF-β1. ∗p < 0.001 indicates significant increase above corresponding endogenous levels. Both 20 and 40 h uptake values were compared with 40 h endogenous control.
Latent TGF-β1
After 24 h, the ratio of the SLC concentration in cartilage explants, normalized to the bath concentration, was 0.04 ± 0.01 (for 100 ng/mL bath), indicating that the SLC is substantially partitioned from the tissue ECM. At the higher SLC bath concentration (1000 ng/mL), the normalized ratio did not change significantly (0.05 ± 0.02, p = 0.51).
Study 3: Theoretical analysis of active TGF-β1 uptake into articular cartilage
Experiments
Transient binding of TGF-β1
The bath concentration of free active TGF-β1 decreased monotonically, from 6.80 ± 0.31 ng/mL to 4.86 ± 0.17 ng/mL after 2 h (Fig. 3), due to binding with the cartilage explant. The initial slope, evaluated from the first two time points (0 and 10 min), was calculated (dCbath/dt = −3.05 ng/mL/h) and used with Eq. 4 to produce Ntkf ≈ 1.8 min−1.
Figure 3.
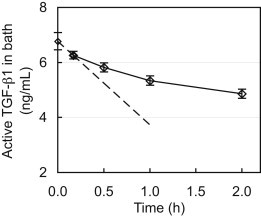
Transient decrease of the exogenous active TGF-β1 concentration in a bathing solution due to TGF-β1 binding to the ECM of a submerged cartilage explant. The dashed line represents the initial slope, which was used to determine the product Ntkf according to Eq. 4.
Equilibrium binding of TGF-β1
Measurement of the transient uptake of active TGF-β1 in cartilage explants confirmed that the 24 h exposure period was indeed sufficient to reach equilibrium in the tissue (Fig. 4 A). TGF-β1 levels in explants increased linearly with bath concentration (R2 = 0.94; Fig. 4 B) at a slope of 63.2, indicating that on average the concentration was 63-fold higher in the tissue relative to the surrounding bath. When it was normalized, the uptake ratio averaged RU = 57.3 ± 14.3 for all groups and exhibited no statistical decrease with increasing TGF-β1 bath concentration (Fig. 5). According to Eq. 3, this constant ratio is equal to the value κ(Nt/KD + 1). However, the lack of a sigmoidal transition point in the data indicates that even at the highest TGF-β1 bath concentration employed here (CBath = 450 ng/mL), the binding sites did not undergo saturation. Therefore, this experiment was unable to yield definitive values for Nt or KD. According to Eq. 3, the earliest this sigmoidal transition can occur is immediately after a bath concentration of 450 ng/mL, as indicated by the theoretical curve in Fig. 5 (case 1), for which Nt = 4 × 105 ng/mL (given κ = 0.09). Therefore, the understanding that this transition will occur at or beyond this point yields the constraint Nt ≥ 4 × 105 ng/mL. To examine the impact of this uncertainty, we implemented three hypothetical binding cases in the finite-element simulations, which examine parametric variations in Nt. These cases consisted of further delayed saturation transition points (Fig. 5) corresponding to Nt = 4 × 105 (case 1), 40 × 105 (case 2), or 400 × 105 (case 3) ng/mL. The remaining governing binding parameters were computed based on the experimentally determined constraints, Nt/KD = 636 (given RU = 57.3 and κ = 0.09) and Ntkf = 1.8 min−1, and are summarized in Table 2.
Figure 4.
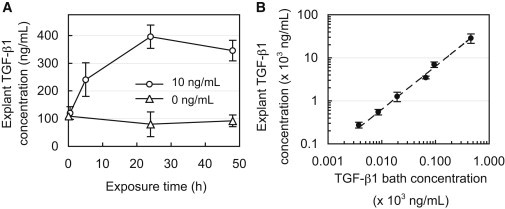
(A) Concentration of endogenous and exogenous (bound + free) TGF-β1 in cartilage matrix after exposure to 10 ng/mL of exogenous active TGF-β1 or a TGF-β1-free solution (0 ng/mL) for up to 48 h. (B) Concentration of exogenous (bound + free) TGF-β1 in cartilage matrix after exposure to varying levels of exogenous active TGF-β1 (4–450 ng/mL) for 24 h.
Figure 5.
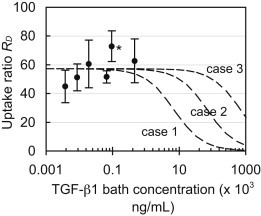
Normalized equilibrium uptake ratio, RU, of exogenous active TGF-β1 in the cartilage matrix versus exposed bath concentration. According to Eq. 3, as the bath concentration approaches zero, this concentration ratio is equal to the value κ(Nt/KD + 1). Dashed curves represent theoretical cases of Eq. 3 based on parameters summarized in Table 2. Each curve reflects a different binding site transition point. ∗p < 0.01 indicates significant increase above the value at the lowest TGF-β1 bath concentration.
Table 2.
Experimentally determined binding parameters implemented in finite-element theoretical simulations
| Case # | Nt/KD | Binding site density, Nt [ × 105 ng/mL] | Dissociation constant, KD [× 105 ng/mL] | Forward rate constant, kf [× 10−6 min.ng/mL−1] | Reverse rate constant, kr [min−1] | Partition coefficient, κ | TGF-β diffusivity, D [μm2/s] |
|---|---|---|---|---|---|---|---|
| 1 | 636 | 4 | 0.0063 | 4.6 | 0.03 | 0.09 | 21.3 |
| 2 | 636 | 40 | 0.063 | 0.46 | 0.03 | 0.09 | 21.3 |
| 3 | 636 | 400 | 0.63 | 0.046 | 0.03 | 0.09 | 21.3 |
Finite-element analysis
Theoretical predictions of the 1D (through-the-depth) uptake of active TGF-β1, based on the experimentally determined binding parameters (Table 2), were generated and plotted for multiple time points (Fig. 6 A). For consistency with the presentation of experimental results in Fig. 2 B, theoretically predicted active TGF-β1 concentrations in the tissue were superposed over the (smoothed) experimentally measured endogenous levels (Fig. 2 A). The theoretical uptake prediction for parametric case 1 (Nt = 4 × 105 ng/mL) may be compared with the experimentally determined results (Fig. 2 B). Predictions show that the concentration of active exogenous TGF-β1 at the articular surface reaches levels 40-fold higher than the bath concentration, consistent with experimental results. Furthermore, predictions show that active exogenous TGF-β1 penetrates very slowly into the tissue and is not present below 240 μm into the tissue even after 40 h of exposure, also in agreement with experimental results. No significant differences were observed in the theoretical predictions between any of the three parametric cases for Nt, indicating that the theoretical prediction was insensitive to the uncertainty in this parameter for the given conditions (i.e., for Nt ≥ 4 × 105 ng/mL and testing durations ≤ 40 h). Further simulations demonstrate that this 800 μm thick section of tissue will not equilibrate throughout its depth, even after 1 year of exposure time. In contrast, simulations in the absence of binding indicate that the concentration of active TGF-β1 should become nearly uniform throughout this section after only 40 h, at a value equal to κCBath (Fig. 6 B).
Figure 6.
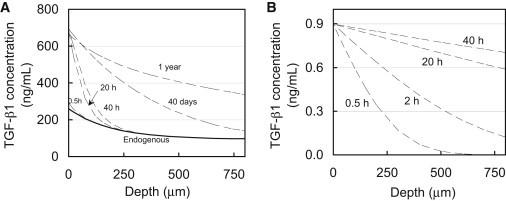
Theoretical simulation of 1D depth-dependent uptake of exogenous active TGF-β1 (CBath = 10 ng/mL) in a cartilage explant. (A) Simulations based on binding and transport properties summarized in Table 2. The solid curve represents the level of endogenous latent TGF-β1 throughout the depth of the tissue based on experimental measures (Fig. 2A). (B) Simulation of a diffusing 25 kDa molecule in the absence of binding interactions with a partition coefficient of κ = 0.09. The concentration in the tissue does not exceed the value of κCBath and reaches a nearly uniform value throughout the thickness after only 40 h.
Discussion
Traditionally, it has been understood that TGF-β plays an important role in the development and maintenance of articular cartilage (4). However, few studies have addressed the physiologic extracellular events that regulate chondrocyte access to TGF-β in the tissue, including its secretion, activation, and binding to the ECM. The results of this study advance two important concepts: 1), large amounts of latent TGF-β1 are continuously synthesized and secreted from both the synovium and articular cartilage; and 2), upon activation, TGF-β1 binds substantially to and accumulates in the SZ of articular cartilage.
To our knowledge, this is the first study to explicitly compare the rates of latent TGF-β1 secretion from articular cartilage and synovium tissue explants. The elevated, constant secretion rates demonstrated in this study (Fig. 1) strongly suggest that these surrounding tissues of the synovial joint are capable of supplying and continuously replenishing a large amount of latent TGF-β1 to SF. The demonstrations that: 1), endogenous TGF-β1 levels remain constant in cartilage explants throughout the culture period; and 2), TGF-β1 is not secreted from devitalized explants indicate that tissues are in fact synthesizing latent TGF-β1 rather than releasing it from their matrix. Furthermore, results indicate that all cartilage tissue zones contribute to this supply, and that synthesis in cartilage is autoinduced by active TGF-β, as observed in isolated chondrocytes (16) and other tissues (33–36). Considering the typical volume of SF (5 mL (37)) and cartilage tissue (20 mL (38)) in a human knee joint, these results suggest that on the order of 40 ng/mL of latent TGF-β1 is supplied to the SF per day.
Although it is well understood that TGF-β can bind to a large variety of ECM constituents, our results demonstrate that these interactions can significantly influence the transport of active TGF-β into articular cartilage, as evidenced by the 35-fold concentration enhancement in the SZ and substantially delayed penetration further into the tissue (Fig. 2). In contrast, according to Fick’s law of diffusion, the concentration of a freely diffusing (nonbinding) 25 kDa molecule should not exceed the value of κCBath (∼1 ng/mL), and should reach a nearly uniform distribution throughout the entire 800 μm depth of the explant after only 40 h (Fig. 6 B). The ability of the theoretical binding-transport model to faithfully describe the actual response (Fig. 6 A) confirms that these observations can be mostly attributed to the nonspecific binding of active TGF-β1 to the cartilage ECM, rather than to experimental artifacts or a complex heterogeneous distribution of binding sites. It is interesting to note that according to these measurements, the extensive binding response is not due to a particularly high affinity of TGF-β1 to binding sites. The dissociation constant of active TGF-β1 in cartilage implemented in theoretical case 1 (KD = 25.2 nM) is greater than that of IGF-1 to its specific binding protein (KD =5.1 nM (39)), indicating a lower binding affinity. Rather, it results from the vast concentration of binding sites in the tissue; the concentration of binding sites Nt is at least 6 orders of magnitude greater than the 10 ng/mL exogenous bath concentration. From a physical perspective, as active TGF-β begins to diffuse into the tissue, it must first occupy and fill an overwhelming supply of binding sites before it progresses further into the tissue. Even in the absence of cellular biological processes, it would take more than a year for the concentration of active TGF-β to equilibrate throughout the depth of the tissue (Fig. 6 A). It is highly likely that the active TGF-β will have been internalized by chondrocytes or degraded by proteases well before this state is reached.
The results of this study complement our previous characterization of mechanical, shear-induced TGF-β activation (13). Together, these results strongly suggest a sequential physiologic mechanism through which SZ chondrocytes obtain access to active TGF-β (Fig. 7). Articular chondrocytes (throughout the entire depth of the tissue) and synoviocytes constitutively synthesize and secrete large quantities of latent TGF-β into the SF, producing large stores of soluble growth factor. Upon physiologic joint motion, SF is subjected to high levels of mechanical shearing, which in turn induces the rapid activation of TGF-β (13). As this newly activated TGF-β diffuses into the cartilage ECM, it binds and accumulates in the SZ of the tissue to perform an important functional role. Our previous finding that latent TGF-β in healthy SF does not undergo spontaneous activation in the absence of shearing (e.g., via chemical mediators) strongly suggests that shear-induced activation is the dominant physiologic mechanism through which SZ cartilage obtains access to active TGF-β.
Figure 7.
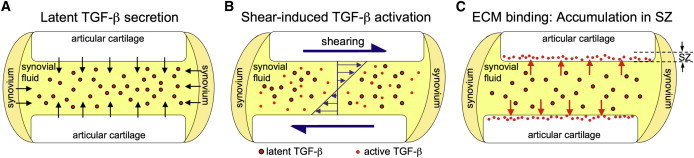
Sequential mechanistic illustration of physiologic accumulation of active TGF-β in the SZ of articular cartilage. (A) Large amounts of latent TGF-β are constitutively synthesized and secreted from the cartilage and synovium into SF. (B) TGF-β undergoes rapid activation from the high levels of SF shearing induced by physiologic joint motion (13). (C) Active TGF-β accumulates in the SZ of the cartilage tissue due to the overwhelming presence of nonspecific binding sites in the ECM.
Furthermore, the observed significant degree of partitioning of the SLC (partition coefficient of 0.04–0.05) suggests that although latent TGF-β can transport into or out of the tissue, it first must undergo activation in SF before it can enter the cartilage ECM at substantial levels. This level of partitioning in cartilage is routinely observed for large proteins that do not bind to the ECM (32). In the native synovial joint, latent TGF-β may be present in both the SLC and LLC (40). Although the LLC in SF may bind to the cartilage ECM, due to its molecular weight, which is considerably larger than that of the SLC, it is also unlikely to enter the tissue from SF at appreciable levels.
Based on the well-characterized influence of TGF-β on chondrocyte biosynthesis, this mechanism is likely to play an important role in the metabolic activity of SZ cartilage. Inhibition of TGF-β signaling promotes cartilage degradation with effects that are most pronounced in the SZ of the tissue (41). TGF-β exposure has routinely been shown to enhance the synthesis of a host of ECM structural proteins, such as proteoglycans (6,7,42), type II collagen (8), cartilage oligomeric matrix protein (COMP) (43), and other signaling molecules (44). Further, the induction of chondrocyte biosynthesis by active TGF-β often exhibits a biphasic concentration-dependent response, suggesting that the tight regulation of TGF-β activity in SF is crucial for maintaining cartilage metabolism. Interestingly, for a variety of proteins (e.g., proteoglycans (7) and SZ protein (SZP) (45)) maximal induction occurs at the levels of active TGF-β induced by SF shearing (1–10 ng/mL). In particular, this mechanism may play a critical role in the regulation of SZP, a molecule that is secreted exclusively by SZ chondrocytes and is believed to reduce friction or wear at the articular surface of cartilage (46). It is of particular interest to note that SZ chondrocytes do not synthesize SZP in the absence of active TGF-β (45,47,48). Therefore, shear-induced activation may be required for SZP production and serve as a self-regulating mechanism whereby prolonged joint sliding motion can induce a replenishment mechanism for this tribologically beneficial protein.
Although the importance of TGF-β is clear, its activity may also play a role in the development and progression of pathological conditions. High active TGF-β levels have been shown to induce pathological effects associated with osteoarthritis, such as inflammation, synovial fibrosis, and the formation of osteophytes (49–51). Furthermore, high steady-state levels of active TGF-β have been observed in pathological SF samples (10,11). Investigators have questioned whether these growth-factor states contribute to or counteract pathologic conditions (52,53). Control of TGF-β activation through physiologic joint shearing may provide the active TGF-β levels required for chondrocyte metabolic activity while preventing the excessive levels that are associated with a pathologic response.
It remains unclear which specific ECM molecules are responsible for the binding of active TGF-β and accumulation in the SZ. Active TGF-β has been demonstrated to bind to a large variety of ECM molecules, such as the core protein of proteoglycans (decorin, biglycan, and fibromodulin (20)), glycosaminoglycans (hyaluronan, chondroitin sulfate, heparin, and heparin sulfate (21,22)), COMP (25), and possibly type II collagen (23). The specificity of these interactions may be an additional factor in the role of TGF-β, since TGF-β’s interactions with protein can stimulate or inhibit its activity (54,55). Although activated TGF-β can physically reassociate with free LAP, given the low affinity of this interaction and physiologic levels of these two molecules, appreciable amounts of reassociation are unlikely to occur (56).
Further, high levels of endogenous latent TGF-β1 are present in the cartilage ECM, as observed in the absence of active TGF-β exposure (Fig. 2 A). Although these high TGF-β levels were previously reported in articular cartilage (2), to our knowledge, this is the first study to demonstrate that their concentration is highest at the articular surface and decreases through the depth of the tissue. Conventionally, in a variety of tissues, latent TGF-β is understood to bind to the ECM through interactions with proteins, such as elastin (57), fibrillin (58), and fibronectin, via the LTBP. Interestingly, these ECM constituents are present at higher levels in the SZ of the tissue (59–62) and thus may be responsible for the observed heterogeneous distribution of latent TGF-β.
An additional implication of this study is that nonspecific binding interactions make it highly unlikely that a significant amount of active TGF-β from the SF will reach the middle and deep zones of the tissue. In spite of this limitation, it has been clearly demonstrated that TGF-β is required to maintain the biochemical integrity of these deeper cartilage regions (41). Therefore, it is likely that the deeper zones access active TGF-β from an alternative source, most likely the large endogenous stores of matrix-bound latent TGF-β (Fig. 2 A). It has been suggested that cartilage matrix-bound TGF-β may undergo activation from a variety of chemical mediators, such as matrix metalloproteinases (63–65), plasmin (40), transglutaminase (66), and lysophospholipids (67). Alternatively, the direct mechanical compression that cartilage experiences during joint motion may activate this matrix-bound latent TGF-β, akin to the shear-induced activation of the soluble TGF-β in SF. Future studies will be required to elucidate the contributions of each of these potential mechanisms.
Overall, the results of this study provide a novel (to our knowledge) insight into the likely dominant mechanism that regulates active TGF-β levels in SZ cartilage. This analysis can potentially lead to an understanding of the factors that lead to deviations in TGF-β activity and their potential role in the progression of pathological conditions of the synovial joint.
Acknowledgments
This work was supported by grants R01AR060361 and R01AR43628 from the National Institute of Arthritis and Musculoskeletal and Skin Diseases. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Arthritis and Musculoskeletal and Skin Diseases or the National Institutes of Health.
Supporting Material
References
- 1.Roberts A.B., Sporn M.B. Transforming growth factor β. Adv. Cancer Res. 1988;51:107–145. [PubMed] [Google Scholar]
- 2.Morales T.I., Joyce M.E., Roberts A.B. Transforming growth factor-β in calf articular cartilage organ cultures: synthesis and distribution. Arch. Biochem. Biophys. 1991;288:397–405. doi: 10.1016/0003-9861(91)90212-2. [DOI] [PubMed] [Google Scholar]
- 3.Gelb D.E., Rosier R.N., Puzas J.E. The production of transforming growth factor-β by chick growth plate chondrocytes in short term monolayer culture. Endocrinology. 1990;127:1941–1947. doi: 10.1210/endo-127-4-1941. [DOI] [PubMed] [Google Scholar]
- 4.Malemud C.J. The role of growth factors in cartilage metabolism. Rheum. Dis. Clin. North Am. 1993;19:569–580. [PubMed] [Google Scholar]
- 5.Chandrasekhar S., Harvey A.K. Transforming growth factor-β is a potent inhibitor of IL-1 induced protease activity and cartilage proteoglycan degradation. Biochem. Biophys. Res. Commun. 1988;157:1352–1359. doi: 10.1016/s0006-291x(88)81024-6. [DOI] [PubMed] [Google Scholar]
- 6.Hiraki Y., Inoue H., Suzuki F. Effect of transforming growth factor β on cell proliferation and glycosaminoglycan synthesis by rabbit growth-plate chondrocytes in culture. Biochim. Biophys. Acta. 1988;969:91–99. doi: 10.1016/0167-4889(88)90092-4. [DOI] [PubMed] [Google Scholar]
- 7.O’Keefe R.J., Puzas J.E., Rosier R.N. Effects of transforming growth factor-β on matrix synthesis by chick growth plate chondrocytes. Endocrinology. 1988;122:2953–2961. doi: 10.1210/endo-122-6-2953. [DOI] [PubMed] [Google Scholar]
- 8.Redini F., Galera P., Pujol J.P. Transforming growth factor β stimulates collagen and glycosaminoglycan biosynthesis in cultured rabbit articular chondrocytes. FEBS Lett. 1988;234:172–176. doi: 10.1016/0014-5793(88)81327-9. [DOI] [PubMed] [Google Scholar]
- 9.Hyytiäinen M., Penttinen C., Keski-Oja J. Latent TGF-β binding proteins: extracellular matrix association and roles in TGF-β activation. Crit. Rev. Clin. Lab. Sci. 2004;41:233–264. doi: 10.1080/10408360490460933. [DOI] [PubMed] [Google Scholar]
- 10.Fava R., Olsen N., Pincus T. Active and latent forms of transforming growth factor β activity in synovial effusions. J. Exp. Med. 1989;169:291–296. doi: 10.1084/jem.169.1.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brennan F.M., Chantry D., Feldmann M. Detection of transforming growth factor-β in rheumatoid arthritis synovial tissue: lack of effect on spontaneous cytokine production in joint cell cultures. Clin. Exp. Immunol. 1990;81:278–285. doi: 10.1111/j.1365-2249.1990.tb03331.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miossec P., Naviliat M., Banchereau J. Low levels of interleukin-4 and high levels of transforming growth factor β in rheumatoid synovitis. Arthritis Rheum. 1990;33:1180–1187. doi: 10.1002/art.1780330819. [DOI] [PubMed] [Google Scholar]
- 13.Albro M.B., Cigan A.D., Ateshian G.A. Shearing of synovial fluid activates latent TGF-β. Osteoarthritis Cartilage. 2012;20:1374–1382. doi: 10.1016/j.joca.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lafyatis R., Thompson N.L., Wilder R.L. Transforming growth factor-β production by synovial tissues from rheumatoid patients and streptococcal cell wall arthritic rats. Studies on secretion by synovial fibroblast-like cells and immunohistologic localization. J. Immunol. 1989;143:1142–1148. [PubMed] [Google Scholar]
- 15.Studer R.K., Georgescu H.I., Evans C.H. Inhibition of transforming growth factor β production by nitric oxide-treated chondrocytes: implications for matrix synthesis. Arthritis Rheum. 1999;42:248–257. doi: 10.1002/1529-0131(199902)42:2<248::AID-ANR6>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 16.Villiger P.M., Lotz M. Differential expression of TGF β isoforms by human articular chondrocytes in response to growth factors. J. Cell. Physiol. 1992;151:318–325. doi: 10.1002/jcp.1041510213. [DOI] [PubMed] [Google Scholar]
- 17.Lafeber F.P., Vander Kraan P.M., Bijlsma J.W. Osteoarthritic human cartilage is more sensitive to transforming growth factor β than is normal cartilage. Br. J. Rheumatol. 1993;32:281–286. doi: 10.1093/rheumatology/32.4.281. [DOI] [PubMed] [Google Scholar]
- 18.Le M., Gohr C.M., Rosenthal A.K. Transglutaminase participates in the incorporation of latent TGFβ into the extracellular matrix of aging articular chondrocytes. Connect. Tissue Res. 2001;42:245–253. doi: 10.3109/03008200109016839. [DOI] [PubMed] [Google Scholar]
- 19.Rider C.C. Heparin/heparan sulphate binding in the TGF-β cytokine superfamily. Biochem. Soc. Trans. 2006;34:458–460. doi: 10.1042/BST0340458. [DOI] [PubMed] [Google Scholar]
- 20.Hildebrand A., Romarís M., Ruoslahti E. Interaction of the small interstitial proteoglycans biglycan, decorin and fibromodulin with transforming growth factor β. Biochem. J. 1994;302:527–534. doi: 10.1042/bj3020527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hintze V., Miron A., Scharnweber D. Sulfated hyaluronan and chondroitin sulfate derivatives interact differently with human transforming growth factor-β1 (TGF-β1) Acta Biomater. 2012;8:2144–2152. doi: 10.1016/j.actbio.2012.03.021. [DOI] [PubMed] [Google Scholar]
- 22.Lyon M., Rushton G., Gallagher J.T. The interaction of the transforming growth factor-βs with heparin/heparan sulfate is isoform-specific. J. Biol. Chem. 1997;272:18000–18006. doi: 10.1074/jbc.272.29.18000. [DOI] [PubMed] [Google Scholar]
- 23.Qi W.N., Scully S.P. Extracellular collagen modulates the regulation of chondrocytes by transforming growth factor-β 1. J. Orthop. Res. 1997;15:483–490. doi: 10.1002/jor.1100150402. [DOI] [PubMed] [Google Scholar]
- 24.Paralkar V.M., Vukicevic S., Reddi A.H. Transforming growth factor β type 1 binds to collagen IV of basement membrane matrix: implications for development. Dev. Biol. 1991;143:303–308. doi: 10.1016/0012-1606(91)90081-d. [DOI] [PubMed] [Google Scholar]
- 25.Haudenschild D.R., Hong E., Di Cesare P.E. Enhanced activity of transforming growth factor β1 (TGF-β1) bound to cartilage oligomeric matrix protein. J. Biol. Chem. 2011;286:43250–43258. doi: 10.1074/jbc.M111.234716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Murphy-Ullrich J.E., Schultz-Cherry S., Höök M. Transforming growth factor-β complexes with thrombospondin. Mol. Biol. Cell. 1992;3:181–188. doi: 10.1091/mbc.3.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang L., Gardiner B.S., Grodzinsky A.J. On the role of diffusible binding partners in modulating the transport and concentration of proteins in tissues. J. Theor. Biol. 2010;263:20–29. doi: 10.1016/j.jtbi.2009.11.023. [DOI] [PubMed] [Google Scholar]
- 28.Reisenbichler H., Jirtle R.L. BSA treatment of plasticware reduces TGF β binding. Biotechniques. 1994;17:675–676. [PubMed] [Google Scholar]
- 29.McGowan K.B., Kurtis M.S., Sah R.L. Biochemical quantification of DNA in human articular and septal cartilage using PicoGreen and Hoechst 33258. Osteoarthritis Cartilage. 2002;10:580–587. doi: 10.1053/joca.2002.0794. [DOI] [PubMed] [Google Scholar]
- 30.Ateshian G.A., Albro M.B., Weiss J.A. Finite element implementation of mechanochemical phenomena in neutral deformable porous media under finite deformation. J. Biomech. Eng. 2011;133:081005. doi: 10.1115/1.4004810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maas S.A., Ellis B.J., Weiss J.A. FEBio: finite elements for biomechanics. J. Biomech. Eng. 2012;134:011005. doi: 10.1115/1.4005694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Maroudas A. Distribution and diffusion of solutes in articular cartilage. Biophys. J. 1970;10:365–379. doi: 10.1016/S0006-3495(70)86307-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roberts A.B. Molecular and cell biology of TGF-β. Miner. Electrolyte Metab. 1998;24:111–119. doi: 10.1159/000057358. [DOI] [PubMed] [Google Scholar]
- 34.Dallas S.L., Park-Snyder S., Bonewald L.F. Characterization and autoregulation of latent transforming growth factor β (TGF β) complexes in osteoblast-like cell lines. Production of a latent complex lacking the latent TGF β-binding protein. J. Biol. Chem. 1994;269:6815–6821. [PubMed] [Google Scholar]
- 35.Bascom C.C., Wolfshohl J.R., Moses H.L. Complex regulation of transforming growth factor β 1, β 2, and β 3 mRNA expression in mouse fibroblasts and keratinocytes by transforming growth factors β 1 and β 2. Mol. Cell. Biol. 1989;9:5508–5515. doi: 10.1128/mcb.9.12.5508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Obberghen-Schilling E., Roche N.S., Roberts A.B. Transforming growth factor β 1 positively regulates its own expression in normal and transformed cells. J. Biol. Chem. 1988;263:7741–7746. [PubMed] [Google Scholar]
- 37.Kraus V.B., Stabler T.V., McDaniel G. Measurement of synovial fluid volume using urea. Osteoarthritis Cartilage. 2007;15:1217–1220. doi: 10.1016/j.joca.2007.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Faber S.C., Eckstein F., Reiser M. Gender differences in knee joint cartilage thickness, volume and articular surface areas: assessment with quantitative three-dimensional MR imaging. Skeletal Radiol. 2001;30:144–150. doi: 10.1007/s002560000320. [DOI] [PubMed] [Google Scholar]
- 39.Garcia A.M., Szasz N., Frank E.H. Transport and binding of insulin-like growth factor I through articular cartilage. Arch. Biochem. Biophys. 2003;415:69–79. doi: 10.1016/s0003-9861(03)00215-7. [DOI] [PubMed] [Google Scholar]
- 40.Pedrozo H.A., Schwartz Z., Boyan B.D. Potential mechanisms for the plasmin-mediated release and activation of latent transforming growth factor-β1 from the extracellular matrix of growth plate chondrocytes. Endocrinology. 1999;140:5806–5816. doi: 10.1210/endo.140.12.7224. [DOI] [PubMed] [Google Scholar]
- 41.Chen C.G., Thuillier D., Alliston T. Chondrocyte-intrinsic Smad3 represses Runx2-inducible matrix metalloproteinase 13 expression to maintain articular cartilage and prevent osteoarthritis. Arthritis Rheum. 2012;64:3278–3289. doi: 10.1002/art.34566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales T.I., Roberts A.B. Transforming growth factor β regulates the metabolism of proteoglycans in bovine cartilage organ cultures. J. Biol. Chem. 1988;263:12828–12831. [PubMed] [Google Scholar]
- 43.Recklies A.D., Baillargeon L., White C. Regulation of cartilage oligomeric matrix protein synthesis in human synovial cells and articular chondrocytes. Arthritis Rheum. 1998;41:997–1006. doi: 10.1002/1529-0131(199806)41:6<997::AID-ART6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 44.Shi S., Mercer S., Trippel S.B. Growth factor regulation of growth factors in articular chondrocytes. J. Biol. Chem. 2009;284:6697–6704. doi: 10.1074/jbc.M807859200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niikura T., Reddi A.H. Differential regulation of lubricin/superficial zone protein by transforming growth factor β/bone morphogenetic protein superfamily members in articular chondrocytes and synoviocytes. Arthritis Rheum. 2007;56:2312–2321. doi: 10.1002/art.22659. [DOI] [PubMed] [Google Scholar]
- 46.Swann D.A., Sotman S., Brooks C. The isolation and partial characterization of the major glycoprotein (LGP-I) from the articular lubricating fraction from bovine synovial fluid. Biochem. J. 1977;161:473–485. doi: 10.1042/bj1610473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schmidt T.A., Gastelum N.S., Sah R.L. Differential regulation of proteoglycan 4 metabolism in cartilage by IL-1α, IGF-I, and TGF-β1. Osteoarthritis Cartilage. 2008;16:90–97. doi: 10.1016/j.joca.2007.05.009. [DOI] [PubMed] [Google Scholar]
- 48.Neu C.P., Khalafi A., Reddi A.H. Mechanotransduction of bovine articular cartilage superficial zone protein by transforming growth factor β signaling. Arthritis Rheum. 2007;56:3706–3714. doi: 10.1002/art.23024. [DOI] [PubMed] [Google Scholar]
- 49.van Beuningen H.M., van der Kraan P.M., van den Berg W.B. Transforming growth factor-β 1 stimulates articular chondrocyte proteoglycan synthesis and induces osteophyte formation in the murine knee joint. Lab. Invest. 1994;71:279–290. [PubMed] [Google Scholar]
- 50.Elford P.R., Graeber M., MacKenzie A.R. Induction of swelling, synovial hyperplasia and cartilage proteoglycan loss upon intra-articular injection of transforming growth factor β-2 in the rabbit. Cytokine. 1992;4:232–238. doi: 10.1016/1043-4666(92)90061-u. [DOI] [PubMed] [Google Scholar]
- 51.Allen J.B., Manthey C.L., Wahl S.M. Rapid onset synovial inflammation and hyperplasia induced by transforming growth factor β. J. Exp. Med. 1990;171:231–247. doi: 10.1084/jem.171.1.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Westacott C.I., Sharif M. Cytokines in osteoarthritis: mediators or markers of joint destruction? Semin. Arthritis Rheum. 1996;25:254–272. doi: 10.1016/s0049-0172(96)80036-9. [DOI] [PubMed] [Google Scholar]
- 53.van den Berg W.B. Growth factors in experimental osteoarthritis: transforming growth factor β pathogenic? J. Rheumatol. Suppl. 1995;43:143–145. [PubMed] [Google Scholar]
- 54.Takeuchi Y., Kodama Y., Matsumoto T. Bone matrix decorin binds transforming growth factor-β and enhances its bioactivity. J. Biol. Chem. 1994;269:32634–32638. [PubMed] [Google Scholar]
- 55.Hausser H., Gröning A., Kresse H. Selective inactivity of TGF-β/decorin complexes. FEBS Lett. 1994;353:243–245. doi: 10.1016/0014-5793(94)01044-7. [DOI] [PubMed] [Google Scholar]
- 56.Böttinger E.P., Factor V.M., Sporn M.B. The recombinant proregion of transforming growth factor β1 (latency-associated peptide) inhibits active transforming growth factor β1 in transgenic mice. Proc. Natl. Acad. Sci. USA. 1996;93:5877–5882. doi: 10.1073/pnas.93.12.5877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Karonen T., Jeskanen L., Keski-Oja J. Transforming growth factor β 1 and its latent form binding protein-1 associate with elastic fibres in human dermis: accumulation in actinic damage and absence in anetoderma. Br. J. Dermatol. 1997;137:51–58. [PubMed] [Google Scholar]
- 58.Raghunath M., Unsöld C., Meuli M. The cutaneous microfibrillar apparatus contains latent transforming growth factor-β binding protein-1 (LTBP-1) and is a repository for latent TGF-β1. J. Invest. Dermatol. 1998;111:559–564. doi: 10.1046/j.1523-1747.1998.00339.x. [DOI] [PubMed] [Google Scholar]
- 59.Yu J., Urban J.P. The elastic network of articular cartilage: an immunohistochemical study of elastin fibres and microfibrils. J. Anat. 2010;216:533–541. doi: 10.1111/j.1469-7580.2009.01207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mansfield J., Yu J., Winlove P. The elastin network: its relationship with collagen and cells in articular cartilage as visualized by multiphoton microscopy. J. Anat. 2009;215:682–691. doi: 10.1111/j.1469-7580.2009.01149.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yeh A.T., Hammer-Wilson M.J., Peavy G.M. Nonlinear optical microscopy of articular cartilage. Osteoarthritis Cartilage. 2005;13:345–352. doi: 10.1016/j.joca.2004.12.007. [DOI] [PubMed] [Google Scholar]
- 62.Balazs E.A. The role of hyaluronan in the structure and function of the biomatrix of connective tissues. Struct. Chem. 2009;20:233–243. [Google Scholar]
- 63.Maeda S., Dean D.D., Boyan B.D. The first stage of transforming growth factor β1 activation is release of the large latent complex from the extracellular matrix of growth plate chondrocytes by matrix vesicle stromelysin-1 (MMP-3) Calcif. Tissue Int. 2002;70:54–65. doi: 10.1007/s002230010032. [DOI] [PubMed] [Google Scholar]
- 64.Dangelo M., Sarment D.P., Pacifici M. Activation of transforming growth factor β in chondrocytes undergoing endochondral ossification. J. Bone Miner. Res. 2001;16:2339–2347. doi: 10.1359/jbmr.2001.16.12.2339. [DOI] [PubMed] [Google Scholar]
- 65.Maeda S., Dean D.D., Boyan B.D. Activation of latent transforming growth factor β1 by stromelysin 1 in extracts of growth plate chondrocyte-derived matrix vesicles. J. Bone Miner. Res. 2001;16:1281–1290. doi: 10.1359/jbmr.2001.16.7.1281. [DOI] [PubMed] [Google Scholar]
- 66.Rosenthal A.K., Gohr C.M., Le M. Participation of transglutaminase in the activation of latent transforming growth factor β1 in aging articular cartilage. Arthritis Rheum. 2000;43:1729–1733. doi: 10.1002/1529-0131(200008)43:8<1729::AID-ANR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 67.Gay I., Schwartz Z., Boyan B.D. Lysophospholipid regulates release and activation of latent TGF-β1 from chondrocyte extracellular matrix. Biochim. Biophys. Acta. 2004;1684:18–28. doi: 10.1016/j.bbalip.2004.04.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


