Abstract
AIM: To develop an integrated approach for monitoring gastrointestinal motility and inflammation state suitable for application in long-term spaceflights.
METHODS: Breath tests based on the oral administration of 13C-labeled or hydrogen-producing substrates followed by the detection of their metabolites (13CO2 or H2) in breath were used to measure gastrointestinal motility parameters during the 520-d spaceflight ground simulation within the MARS-500 Project. In particular, the gastric emptying rates of solid and liquid contents were evaluated by 13C-octanoic acid and 13C-acetate breath tests, respectively, whereas the orocecal transit time was assessed by an inulin H2-breath test, which was performed simultaneously with the 13C-octanoic acid breath test. A ready-to-eat, standardized pre-packaged muffin containing 100 mg of 13C-octanoic acid was used in the 13C-octanoic acid breath test to avoid the extemporaneous preparation of solid meals. In addition, a cassette-type lateral flow immunoassay was employed to detect fecal calprotectin, a biomarker of intestinal inflammation. Because no items could be introduced into the simulator during the experiment, all materials and instrumentation required for test performance during the entire mission simulation had to be provided at the beginning of the experiment.
RESULTS: The experiments planned during the simulation of a manned flight to Mars could be successfully performed by the crewmembers without any external assistance. No evident alterations (i.e., increasing or decreasing trends) in the gastric emptying rates were detected using the 13C-breath tests during the mission simulation, as the gastric emptying half-times were in the range of those reported for healthy subjects. In contrast to the 13C-breath tests, the results of the inulin H2-breath test were difficult to interpret because of the high variability of the H2 concentration in the breath samples, even within the same subject. This variability suggested that the H2-breath test was strongly affected by external factors, which may have been related to the diet of the crewmembers or to environmental conditions (e.g., the accumulation of hydrogen in the simulator microenvironment). At least in closed microenvironments such as the MARS-500 simulator, 13C-breath tests should therefore be preferred to H2-breath tests. Finally, the fecal calprotectin test showed significant alterations during the mission simulation: all of the crewmembers were negative for the test at the beginning of the simulation but showed various degrees of positivity in at least one of the subsequent tests, thus indicating the onset of an intestinal inflammation.
CONCLUSION: Breath tests, especially those 13C-based, proved suitable for monitoring gastrointestinal motility in the 520-d isolation experiment within MARS-500 project and can be applied in long-term spaceflights.
Keywords: Breath test, Gastrointestinal inflammation, Gastrointestinal motility, Spaceflight, Stress
INTRODUCTION
A manned mission to Mars is currently starting to garner a consistent level of support, as exploration roadmaps are under study by various space agencies. Nevertheless, several issues related to the health of humans during such a long space mission still must be solved.
Extended-duration space missions expose the crewmembers to microgravity, radiation and a stressful environment due to mission-related factors (e.g., confinement, isolation, anxiety, physiologic stress, sleep deprivation and modifications of their nutrition regimes, circadian rhythms and microbial environments) that affect their physiological status[1]. To properly monitor the crewmembers’ health status during a real space mission, a suitable panel of biochemical tests and related analytical instrumentation should be developed, implemented in the space module and validated for its clinical utility and applicability in spaceflight. These tests should be easily performed onboard by the crewmembers on non-invasively collectable biological samples (e.g., saliva, breath expatriate, urine, or stool) and employing compact devices in a point-of-care format.
Among the alterations that might occur in long-term spaceflights, changes in the gastrointestinal (GI) motility and related gut inflammatory states are of particular relevance. The main factors affecting GI motility are the physical properties of the solid and liquid contents of the stomach and intestine and the functional, hormonal and enzymatic changes in those organs. Spaceflight-related changes in GI function, such as fluid shifts, combined with reduced fluid intake, would tend to decrease GI motility. Although GI motility has not been systematically studied in spaceflight, a significant increase in the mouth-to-caecum transit time has been demonstrated in ground simulations (10 d of -6° head-down bed resting[2,3] and water immersion[4]).
Previous studies have demonstrated that adequate nutritional status is critical to maintaining crew health during extended-duration spaceflight[5-8], and a common cause of reduced dietary intake, especially during the first d of a mission, is space motion sickness[9]. The impact of psychological, physical, and immunological stressors on GI motility, duodenal and biliary secretion, epithelial permeability, and inflammation is currently thoroughly documented, and stress has a major influence on digestive diseases. Gastrointestinal motor dysfunctions, mainly caused by stress conditions, alteration of circadian rhythms and nutritional regimen, may also represent themselves as additional stress factors[10,11]. Decreased GI motility will, in turn, result in delayed intestinal absorption, alterations in the intestinal microflora and decreased bioavailability of orally administered drugs[12]. Such possible alterations must be expeditiously and continuously detected to guide the adoption of the actions necessary to avoid negative consequences to the crewmembers’ health and, more generally, wellness (and thus to the crew’s efficiency).
In this work, we present an integrated approach to the non-invasive monitoring of GI motility and inflammation state that was optimized in the frame of the MARS-500 project. This project was realized by the State Scientific Center of the Russian Federation-Institute of Biomedical Problems of the Russian Academy of Sciences (IBMP), under the auspices of Roscosmos and the Russian Academy of Sciences and in collaboration with the European Space Agency and other space agencies and institutions from all over the world. The project consisted of several isolation experiments, including a final 520-d isolation (the longest spaceflight ground simulation ever conducted) designed to simulate a round-trip manned mission to Mars. The project aimed at obtaining useful information about physical and psychological problems that astronauts might face during a long stay onboard an interplanetary space vehicle and to set up technologies for monitoring their health status with possible application in real space missions.
The integrated approach herein described employed breath tests (BTs) for the evaluation of GI motility. Indeed, 13C- and H2-BTs based on the oral administration of 13C-labeled or hydrogen-producing substrates followed by the detection of the metabolites of these substrates (13CO2 or H2, respectively) in the breath represent a convenient, non-invasive and efficient procedure for obtaining information on motor and organ functions of the GI system. Such tests are routinely used for the detection of alterations in GI motility, bacterial overgrowth, and lactose intolerance, among other issues, and for the diagnosis of infection with Helicobacter pylori[13-15]. We evaluated the gastric emptying rates of solid and liquid content by 13C-octanoic acid and 13C-acetate BT, respectively, whereas the orocecal transit time was assessed by an H2-BT that used inulin as the hydrogen-producing substrate (the latter BT was performed simultaneously with the 13C-octanoic acid BT for the measurement of the gastric emptying rate of solids). In addition, a cassette-type lateral flow immunoassay was employed for detecting fecal calprotectin, a biomarker of intestinal inflammation.
Because they are non-invasive and easily self-performed, BTs are potentially transferrable to the space environment, provided protocol standardization and the development of compact on-board instrumentation. Miniaturized instrumentation based on electrochemical gas sensors is available for H2-BT, whereas compact instrumentation based on non-dispersive infrared spectroscopy (NDIRS) has been developed as an alternative to isotope ratio mass spectrometry (IRMS) for the measurement of 13CO2 in breath[16]. In perspective, miniaturized dedicated analytical instrumentation suitable for on-board operation by the crewmembers will make this integrated approach applicable in real space missions, thus providing a useful tool for the early detection of dysfunctions of the GI system and the adoption of suitable countermeasures, such as diet adjustments or pharmacological interventions.
MATERIALS AND METHODS
Subjects
The crew was composed of six male subjects, who at the beginning of the experiment had a median age of 31 years (range 27-38 years), median body weight of 81 kg (range 74-100 kg), and median body mass index of 26.3 kg/m2 (range 23.6-32.3 kg/m2). During the mission simulation, all of the crewmembers received the same diet, the composition of which was almost identical to that of the diet used in the International Space Station[17].
Ethics
All of the scientific investigations performed in the frame of the MARS-500 experiments were reviewed and approved by the IBMP Committee on Bioethics, and all of the volunteers signed the written informed consent for participation in the experiment.
Materials employed for diagnostic tests
A standard muffin meal (EXPIROGer®, manufactured and packaged by Sofar SpA, Milan, Italy) containing 100 mg of 13C-octanoic acid was employed in the 13C-BT for the measurement of the gastric emptying rate of solid meals. The muffin (weight 100 g) had a 378 kcal (1589 kJ) calorie content and the following composition: 5.5 g of proteins, 57.5 g of carbohydrates, 14.0 g of fats (corresponding to 5.8%, 60.8%, and 33.3% of the total calories, respectively), 1.1 g of dietary fiber and 16.7% moisture. Stable 13C-isotope-labeled sodium acetate (99% isotope purity) was purchased from Cambridge Isotope Laboratories (Andover, MA). Inulin (Beneo™ HP-Gel) with a degree of polymerization of 5-60 was obtained from Orafti (Oreye, Belgium). The enteral nutrition solution Nutrizon standard was manufactured by Otsuka Pharmaceutical (Tokyo, Japan) and had (for 100 mL) a 110 kcal (420 kJ) calorie content, 15% of which were from proteins and 55% from carbohydrates. The semiquantitative rapid immunochromatographic test for the detection of calprotectin in feces (PreventID® Cal Detect®) was produced by Preventis GmbH, Wiesenstr, Germany. The test allowed an easy visual evaluation of fecal calprotectin, providing three degrees of positivity: low (< 15 μg/g), medium (15-60 μg/g), and high (> 60 μg/g).
Assay protocols
Breath tests were performed during the Baseline Data Collection period (BDC; before the start of the simulation) and in three separate experimental sessions at approximately d 100, 240 and 475 of the mission simulation. During each experimental session, different 13C-BTs performed on the same subject were staggered by at least 3 d to allow the washout of the administered substrates and the recovery of basal 13C levels.
The combined 13C- and H2-BT for the measurement of the gastric emptying rate of solids and the orocecal transit time consisted of the simultaneous administration of the EXPIROGer® standard meal and inulin, followed by the measurement of the kinetics of the appearance of 13CO2 and H2 in the breath. In preparation for the test, the crewmembers were requested to refrain from fatty meals or a high intake of dietary fiber the day before the test. Antibiotics, fermented milk products and laxatives were also avoided during the 10-d period preceding the test. After an overnight fast, breath samples were collected to measure the basal levels of 13CO2 and H2. Subsequently, the subjects received the EXPIROGer® standard meal and 5.0 g of inulin dissolved in 200 mL of water. Breath samples for 13CO2 analysis were collected up to 240 min after substrate ingestion in 12-mL glass tubes, which were then transferred outside the simulator for analysis. Samples for the evaluation of breath H2 content were collected in plastic bags up to 440 min after substrate ingestion, and the concentration of H2 was measured on-board immediately after each breath sample had been collected. During the test, the subjects were allowed to drink water and, after 4 h, to resume their usual dietary regimens.
The 13C-BT for the measurement of the gastric emptying rate of liquids consisted of the administration of sodium 13C-acetate followed by the measurement of the kinetics of the appearance of 13CO2 in the breath. After an overnight fast, breath samples were collected to measure the basal level of 13CO2. Subsequently, the subjects orally received 150 mg of sodium 13C-acetate dissolved in 500 mL of Nutrizon enteral nutrition solution, and breath samples were collected up to 240 min after substrate ingestion in 12-mL glass tubes, which were then transferred outside the simulator for analysis. After assuming the substrate, the subjects were requested not to ingest any additional food or drink until the end of the test.
The fecal calprotectin test was performed directly by the crewmembers during the BDC and on day 130, 220, and 475 of the mission simulation following the instructions provided by the manufacturer (the test was repeated twice in each experimental session).
Sample analysis
For the measurement of the 13CO2/12CO2 ratio, breath samples were analyzed using a BreathMAT IRMS (Thermo Finnigan MAT GmbH, Bremen, Germany). The measurement of breath H2 levels was performed on-board by the crewmembers using a portable H2 analyzer equipped with a miniaturized electrochemical cell (Lactotest 102, Medical Electronic Construction R&D sprl, Brussels, Belgium).
Statistical analysis
The 13C-BT results, given as the 13CO2 content of the exhaled CO2 expressed in δ‰ PDB units (zero δ‰ PDB corresponds to 1.12372% 13C atoms), were processed to evaluate the rate of excretion of 13CO2 produced by the metabolism of the 13C-labeled substrate, which was expressed as a percentage of the administered dose per hour. To this purpose, the total expiratory CO2 production of each subject was assumed to be 300 mmol/m2 of body surface/h[18], and the body surface was computed as described by Haycock et al[19]. For the evaluation of the relevant gastric emptying parameters, the excretion kinetics were analyzed by a least-square fitting procedure using a suitable equation[18], and the gastric emptying half-times were calculated from the coefficients of the equation[20].
The H2-BT results, given as the H2 breath concentrations in ppm, were processed for evaluating the enrichment of H2 in the breath over the basal value due to the fermentation of inulin by the intestinal microflora and then plotted as a function of time; the orocecal transit time was assessed as the time at which the breath hydrogen content rose 10 ppm above the basal value[21].
To assess alterations in GI motility, the results of the breath tests performed during the BDC and during the mission simulation were compared by one-way ANOVA for matched data with Dunnett’s post-test using GraphPad Prism version 5.03 (GraphPad Software, San Diego, CA). Values of P < 0.05 were considered to be statistically significant.
RESULTS
Crew health status
The periodic blood biochemical function tests and clinical examinations during the mission simulation did not show any significant pathology or physiological alteration. Comparison of the body weights of the crewmembers during the BDC and at the end of the mission simulation indicated that one subject (B) displayed a significant reduction in weight (-21%), whereas for the other subjects, the reduction was lower (C, D, E and F) or negligible (A). Although no net increases in body weight were observed, subjects A and C experienced a rise in body mass during the first part of the experiment (Table 1).
Table 1.
Dynamics of the body mass (kg) of the crewmembers
|
Crewmember |
||||||
| A | B | C | D | E | F | |
| BDC | 81.5 | 99.5 | 76.6 | 86.9 | 82.5 | 73.5 |
| Exp. session 1 | +3.5 | -2.0 | +4.3 | -1.0 | +0.1 | +0.2 |
| Exp. session 2 | +3.3 | -8.2 | +4.0 | -4.6 | -2.0 | -4.7 |
| Exp. session 3 | +1.5 | -20.4 | -3.8 | -6.9 | -1.2 | -6.8 |
| End of mission simulation | -1.1 | -22.6 | -5.4 | -9.7 | -4.0 | -7.2 |
BDC: Baseline Data Collection.
13C-BT for gastric emptying rate
Figure 1 shows representative 13CO2 excretion kinetic profiles obtained in the 13C-BT for the evaluation of the gastric emptying rates of solids and liquids performed during BDC and in the different experimental sessions during the mission simulation. The gastric emptying half-times obtained for the six crewmembers by analyzing the 13CO2 excretion kinetic profiles using the procedure described in the Statistical Analysis section are reported in Table 2.
Figure 1.
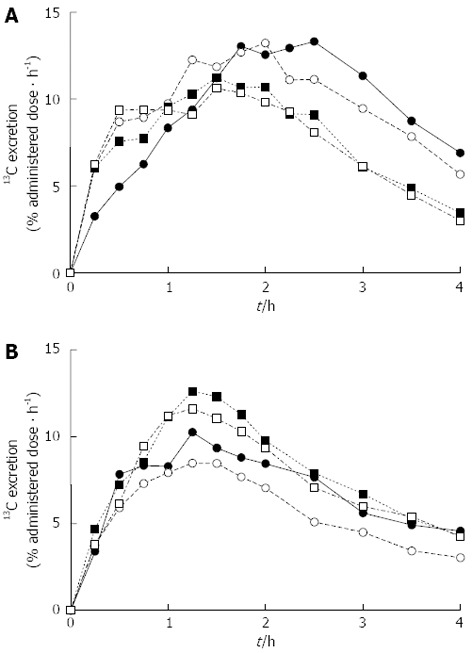
13C-breath test for the evaluation of gastric emptying rates. Representative 13CO2 excretion kinetic profiles obtained in the 13C-breath test for the evaluation of the gastric emptying rates of (A) solids and (B) liquids performed during the Baseline Data Collection period (•) and during the mission simulation (experimental session 1: ◦; experimental session 2: ▪; experimental session 3: ▫).
Table 2.
Gastric emptying half-times (h) evaluated by 13C-breath test
|
Crewmember |
|||||||
| Experimental session | A | B | C | D | E | F | mean ± SD |
| Solids | |||||||
| BDC | 4.4 | 2.8 | 3.3 | 5.0 | 3.2 | 2.8 | 3.5 ± 1.0 |
| Exp. session 1 | 2.7 | 2.7 | 2.2 | 6.2 | 2.9 | 2.9 | 3.2 ± 1.5 |
| Exp. session 2 | 3.7 | 2.2 | 2.9 | 3.5 | 2.8 | 2.5 | 2.8 ± 0.5 |
| Exp. session 3 | 4.9 | 2.2 | 2.7 | 4.8 | 3.4 | 2.6 | 3.3 ± 1.2 |
| Liquids | |||||||
| BDC | 2.6 | 2.3 | 2.4 | 3.0 | 2.8 | 1.9 | 2.5 ± 0.4 |
| Exp. session 1 | 2.6 | 2.0 | 2.2 | 2.8 | 2.6 | 2.5 | 2.5 ± 0.3 |
| Exp. session 2 | 2.6 | 2.1 | 2.7 | 2.6 | 2.6 | 2.5 | 2.5 ± 0.2 |
| Exp. session 3 | 2.9 | 2.0 | 2.6 | 4.0 | 2.8 | 2.6 | 2.8 ± 0.7 |
BDC: Baseline Data Collection.
It can be observed that at the beginning of the simulation (BDC), certain subjects (i.e., A and D) had long gastric emptying half-times of solids (e.g., 4.4 and 5 h for A and D, respectively) and that this behavior was maintained in most of the experimental sessions performed during the mission simulation. As a general rule, long gastric emptying half-times of solids were paralleled (albeit to a lesser extent) by relatively long gastric emptying half-times of liquids, although a large variability in the differences between the two times was observed. Nevertheless, no evident increasing or decreasing trend in the gastric emptying half-times was detected for any crewmember during the mission simulation; most of the measured gastric emptying half-times were in the range of those reported for healthy subjects[18,22], although in certain cases, rather high values were obtained.
H2-BT for orocecal transit time
Figure 2 shows the H2 excretion kinetic profiles obtained in the H2-BT for the evaluation of the orocecal transit time. The H2 breath concentrations showed a large variability, sometimes decreasing below the basal level, which increased the difficulty of identifying the H2 excretion kinetic profiles and evaluating the orocecal transit time by applying the standard criteria reported in the literature (i.e., by identifying the first time at which the breath hydrogen concentration increased by at least 10 ppm above the baseline value).
Figure 2.
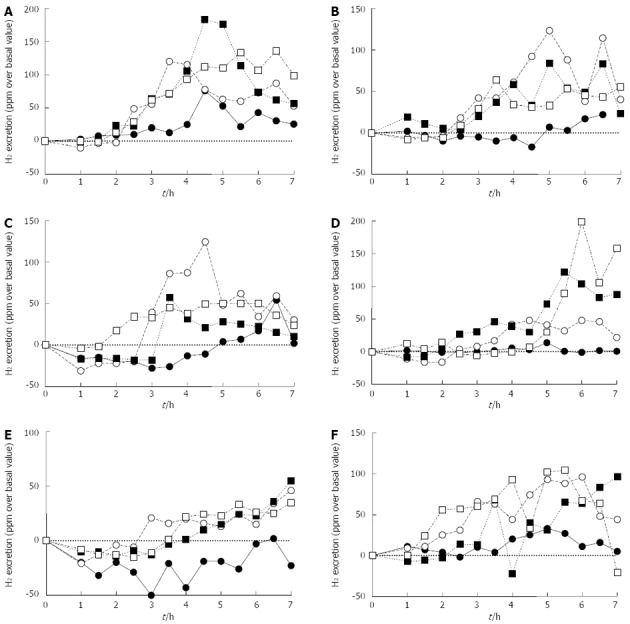
H2-breath test for the evaluation of orocecal transit time. Hydrogen excretion kinetic profiles obtained in the H2-breath test (BT) for the evaluation of the orocecal transit time performed during the Baseline Data Collection period (•) and during the mission simulation (experimental session 1: ◦; experimental session 2: ▪; experimental session 3: ▫). This BT was performed simultaneously with the 13C-octanoic acid BT for the evaluation of the gastric emptying rate of solids (A-F).
Although, in several cases, acceptable H2 excretion profiles were obtained (for example, crewmember D showed high H2 breath concentrations at long times after substrate ingestion, which were paralleled by a delayed gastric emptying of solids), the overall results suggested that the inulin H2-BT was negatively affected by external factors, which may have been related to the simulation environment, such as the closed chamber simulating the space station.
Fecal calprotectin test
Table 3 summarizes the results of the fecal calprotectin test for the evaluation of intestinal inflammation performed during the BDC and during the mission simulation. The results are given as scores according to the semi-quantitative evaluation of calprotectin concentration in fecal samples that was performed with the test. Notably, the crewmembers were negative for the fecal calprotectin test during the BDC, but for all of them positive results were obtained in at least one of the tests performed during the mission simulation. The observed degrees of intestinal inflammation varied from low (in two subjects) to high (in four subjects).
Table 3.
Results of the fecal calprotectin test1
|
Crewmember |
||||||
| Experimental session | A | B | C | D | E | F |
| BDC | - | - | - | - | - | - |
| Day 130 | - | +++ | + | + | - | +++ |
| Day 220 | - | -/+2 | - | - | +++ | -/+2 |
| Day 475 | +++ | -/+++2 | + | - | -/+2 | +++ |
1Legend: (-) negative, (+) low positivity (< 15 μg/g), (++) medium positivity (15-60 μg/g), (+++) high positivity (> 60 μg/g);
The repeated tests gave different results. BDC: Baseline Data Collection.
DISCUSSION
Continuous and non-invasive monitoring of the health status of the crewmembers during space missions requires the development of cutting-edge technologies; their requirements (simple analytical procedures, possibility of self-administration, use of portable point-of-care instrumentation, long shelf-life of reagents) are similar to those faced in critical medicine (e.g., clinical medicine in emergency situations, remote field locations or third-world countries). Thus, new technological solutions that are suitable for the space environment will benefit medical diagnostics for all of us.
In this work, 13C- and H2-BT were employed for the non-invasive monitoring of GI motility during the MARS-500 project. The accuracy of 13C- and H2-BT for the measurement of motor functions of the GI system has been demonstrated by several studies[21,23-25]. However, the application of BT in the space environment still requires certain improvements. For example, the 13C-octanoic acid BT is typically performed using extemporaneously prepared meals (e.g., 13C-octanoic acid is incorporated into egg yolk, which is then pan-cooked and consumed with bread and butter), which makes meal standardization difficult and limits test reproducibility. To overcome this drawback, we employed a ready-to-eat test meal (a muffin containing 100 mg of 13C-octanoic acid) with carbohydrate, lipids, proteins and calorie content optimized for the BT performance. The long-term stability of this test meal and its suitability for the measurement of the gastric emptying rate of solids have been evaluated in a multicenter study[26]. Moreover, the muffin is designed for diagnostics; thus, it is gluten-, lactose- and glucose-free to enable its administration to subjects who are affected by celiac disease, lactose intolerance or diabetes, and the unpleasant taste and odor that are characteristic of short-chain fatty acids are efficiently masked. We also combined the 13C-octanoic acid BT for measuring the gastric emptying rate of a solid meal and the inulin H2-BT for measuring the orocecal transit time into a single test to reduce the number of experimental sessions in the mission simulation and to allow the direct comparison of two different indexes of GI motility, avoiding subject day-to-day variability.
Regarding the instrumentation employed for the analysis of the breath samples, the measurement of the 13CO2/12CO2 ratio was performed by IRMS in an external laboratory. However, NDIRS, which is more amenable to miniaturization, could also be used [17,20]. Work is in progress to develop a miniaturized hybrid analytical device combining the NDIRS technology for 13CO2 measurement with the fuel cell technology for H2 measurement employed in the Lactotest 102 H2 breath analyzer. Such a device will allow the simultaneous onboard measurement of the 13CO2/12CO2 ratio and H2 concentration in a single breath sample, thus avoiding the need for separate breath sample collection in dual BT.
The results obtained during the MARS-500 experiments did not show significant alterations in the gastric emptying rates of solids and liquids (researchers are currently increasingly inclined to use only gastric emptying half-times when reporting the results of the 13C-octanoic acid BT; therefore, we do not discuss other gastric emptying parameters, such as the lag time). Subjects A and D presented long gastric emptying half-times of solids with high variability, but no unambiguous trends were observed. Moreover, it should be taken into account that Choi et al[27,28] suggested that the truncation of the observation period of 13C-octanoic acid BT to four hours could lead to an overestimation of gastric emptying half-times. Therefore, the long half-times measured for subjects A and D could be at least in part ascribed to this factor (indeed, these gastric emptying half-times were close to or even longer than the observation period).
In contrast, the results of the H2-BT for the orocecal transit time, performed simultaneously with the 13C-octanoic acid BT, were difficult to interpret because the high variability of the H2 concentration in the breath samples did not allow a reliable evaluation of the orocecal transit times. Nevertheless, certain results suggested, as expected, a positive correlation with gastric emptying half-times. For example, in subject D, who showed the longest gastric emptying half-times for solids, the highest concentrations of H2 in the breath were often detected at longer times in comparison with the other subjects. These results suggested that the H2-BT was strongly affected by external factors, such as the diet of the crewmembers (hydrogen can be produced by the fermentation of other food sugars and related substances, such as dietary fiber) and the environmental conditions (e.g., the possible accumulation of hydrogen in the simulator microenvironment). Indeed, hydrogen concentrations up to 30-40 ppm were recorded inside the simulator, whereas external values remained below 1.0 ppm. Moreover, the portable H2 analyzer employed in this experiment required manual injection of the breath sample; thus, the reproducibility of the measurement could be improved by implementing automated sample management procedures. Nevertheless, in the absence of further information, it might be concluded that in closed microenvironments, such as the MARS-500 simulator, 13C-BTs should be preferred to H2-based tests. In particular, the lactose 13C-ureide BT, which has been established as a reliable test for the assessment of orocecal transit time[29,30], could represent an alternative to the inulin H2-BT.
In contrast to 13C-BTs, the fecal calprotectin test detected significant alterations during the mission simulation: all of the crewmembers were negative for the test during the BDC but showed various degrees of positivity (from low for subjects C and D to high for subjects A, B, E, and F) in at least one of the tests performed during the mission simulation. Calprotectin is a sensitive fecal marker of intestinal inflammation that is used to differentiate between organic intestinal diseases (e.g., chronic inflammatory diseases, infectious diseases, or colon cancer) and functional intestinal diseases (e.g., irritable bowel syndrome)[31,32]. Application of calprotectin test for screening asymptomatic subjects has also been reported[33,34]. Fecal calprotectin can be determined with high specificity and sensitivity using the CalDetect® lateral flow immunoassay[35]. Because it has been already demonstrated in animal models and humans that stress influences the inflammatory response[36,37], the stress conditions experienced by the crewmembers could be responsible for the observed intestinal inflammation, although external factors related to diet and environment, as well as possible alterations in the intestinal microflora, cannot be excluded.
In conclusion, the results obtained in the MARS-500 mission simulation suggested that the stress level experienced by crewmembers during the mission simulation had no significant impact on the GI motility. Because previous experiments performed in microgravity conditions showed alterations in the GI motility[38,39], it could be concluded that microgravity should have a major impact on GI motor functions, whereas stress-related factors might contribute to the onset of motility alterations but are not the primary cause. Nevertheless, useful information on the possible application of BTs in future isolation experiments or real space missions has been obtained. Due to their simplicity of performance, ability to be performed repeatedly, safety, and non-invasiveness, 13C-BTs represent a promising approach for the monitoring of alterations of motor and/or organ functions of the GI system, thus moving space medicine closer to clinical observation systems used on Earth. In the MARS-500 experiments, 13CO2 analysis in breath samples was performed by IRMS in an external analysis facility, but portable analytical instruments for 13CO2 breath analysis (for example, based on the NDIRS technology) integrated within an informatics framework for data acquisition, analysis, and remote transmission will allow crewmembers to perform such tests autonomously. Regarding H2-BT, suitable portable H2 breath analyzers are already available, but the results suggested that the performance of this BT is strongly affected by external factors; thus, it could be concluded that in this type of application, 13C-BTs should be preferred to H2-based tests. In addition, the measurement of fecal calprotectin by a cassette-type lateral flow immunoassay evidenced a significant degree of intestinal inflammation in all the crewmembers. Although no clinical symptoms associated with intestinal inflammation were reported during the mission simulation, the possibility that a combination of isolation, stress and dietary factors (i.e., prolonged nutrition with canned and preserved foods) could favor the onset of this pathological status should be considered in future mission simulations or real space flights.
ACKNOWLEDGMENTS
Financial contributions from the Italian Space Agency (ASI), the Fondazione del Monte di Bologna e Ravenna (Bologna, Italy), Granarolo SpA (Bologna, Italy), Coswell SpA (Bologna, Italy), Colussi SpA (Milano, Italy), and SOFAR SpA (Milano, Italy) are acknowledged. The corresponding author wishes to thank Dr. Alfonso Labruzzo (SOFAR SpA) for supplying materials for the breath tests and the portable H2 breath analyzer and Dr. Attilio Citrino (SOFAR SpA) for useful scientific discussions about the development of breath test protocols. Inulin was a gift from Orafti. The measurement of the 13CO2/12CO2 ratios of breath samples by IRMS was generously performed by Centro Diagnostico Flegreo (Napoli, Italy).
COMMENTS
Background
Extended-duration space missions expose the crewmembers to microgravity, radiation, stress and other factors that can affect their physiological status. For instance, changes in gastrointestinal motility may result in the reduced intestinal absorption of nutrients, alterations in the intestinal microflora and decreased bioavailability of orally administered drugs. Such possible alterations must be detected expeditiously to avoid negative consequences to the crewmembers’ health and, more generally, wellness.
Research frontiers
The evaluation of the gastrointestinal motility during a real space mission requires biochemical tests that can be easily performed onboard by the crewmembers. Biological samples should be easily collectable in a microgravity environment (e.g., saliva or breath expatriate) and analyzed using compact devices in a point-of-care format. Tests and related analytical instrumentation are to be implemented in the space module and validated for its clinical utility and applicability in spaceflight.
Innovations and breakthroughs
In this study, 13C- and H2-breath tests for the monitoring of gastrointestinal motility have been designed to be self-performed without any external assistance by the subjects participating in the final 520-d isolation experiment in the frame of the MARS-500 project. The reagents for breath test performance have been optimized for long-term storage (no materials could be introduced into the simulator during the isolation period) and minimum preparation required before use; a portable H2 analyzer equipped with a miniaturized electrochemical cell has been provided to allow the onboard measurement of breath H2 levels by the crewmembers. A commercially available cassette-type lateral flow immunoassay was also employed for detecting fecal calprotectin, a biomarker of intestinal inflammation.
Applications
The study suggested that breath tests, especially those based on 13C, could be employed for the monitoring of alterations of motor and/or organ functions of the gastrointestinal system in future isolation experiments or real space missions.
Peer review
The authors present an interesting application of non-invasive gastrointestinal (GI) motility and lower intestinal inflammation tests in a closed-chamber space simulation. Although the results overall reveal no significant change in gastric emptying and require additional confirmation, this study represents an interesting demonstration of how GI monitoring may be achieved with very limited resources. This battery of tests could find application not only in outer space but also in bedside testing in a variety of clinical environments, both inpatient and outpatient.
Footnotes
P- Reviewer Lin J S- Editor Jiang L L- Editor A E- Editor Zhang DN
References
- 1.Hawkey A. Physiological and biomechanical considerations for a human Mars mission. J Br Interplanet Soc. 2005;58:117–130. [PubMed] [Google Scholar]
- 2.Lane HW, LeBlanc AD, Putcha L, Whitson PA. Nutrition and human physiological adaptations to space flight. Am J Clin Nutr. 1993;58:583–588. doi: 10.1093/ajcn/58.5.583. [DOI] [PubMed] [Google Scholar]
- 3.Afonin BV, Goncharova NP. Secretory activity of the stomach during modeling of enhanced filling of abdominal veins. Hum Physiol. 2011;37:832–835. [PubMed] [Google Scholar]
- 4.Afonin BV, Sedova EA, Goncharova NP, Solov'eva AA. [Investigation of the evacuatory function of the gastrointestinal tract in 5-day dry immersion] Aviakosm Ekolog Med. 2011;45:52–57. [PubMed] [Google Scholar]
- 5.Afonin BV, Noskov VB, Polyakov VV. The state of digestive organs during long-term spaceflights. Hum Physiol. 2003;29:561–565. [Google Scholar]
- 6.Smith SM, Zwart SR, Block G, Rice BL, Davis-Street JE. The nutritional status of astronauts is altered after long-term space flight aboard the International Space Station. J Nutr. 2005;135:437–443. doi: 10.1093/jn/135.3.437. [DOI] [PubMed] [Google Scholar]
- 7.Afonin BV, Goncharova NP, Karamyshev IuA. [The functional status of the human stomach in the course of the experiment with antiorthostatic hypokinesia of 4 months duration] Aviakosm Ekolog Med. 2007;41:37–43. [PubMed] [Google Scholar]
- 8.Smith SM, Zwart SR. Nutritional biochemistry of spaceflight. Adv Clin Chem. 2008;46:87–130. doi: 10.1016/s0065-2423(08)00403-4. [DOI] [PubMed] [Google Scholar]
- 9.Lackner JR, Dizio P. Space motion sickness. Exp Brain Res. 2006;175:377–399. doi: 10.1007/s00221-006-0697-y. [DOI] [PubMed] [Google Scholar]
- 10.Riepl RL, Drummer C, Lehnert P, Gerzer R, Otto B. Influence of microgravity on plasma levels of gastroenteropancreatic peptides: a case study. Aviat Space Environ Med. 2002;73:206–210. [PubMed] [Google Scholar]
- 11.Arun CP. The importance of being asymmetric: the physiology of digesta propulsion on Earth and in space. Ann N Y Acad Sci. 2004;1027:74–84. doi: 10.1196/annals.1324.008. [DOI] [PubMed] [Google Scholar]
- 12.Tietze KJ, Putcha L. Factors affecting drug bioavailability in space. J Clin Pharmacol. 1994;34:671–676. doi: 10.1002/j.1552-4604.1994.tb02022.x. [DOI] [PubMed] [Google Scholar]
- 13.Simrén M, Stotzer PO. Use and abuse of hydrogen breath tests. Gut. 2006;55:297–303. doi: 10.1136/gut.2005.075127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Braden B, Lembcke B, Kuker W, Caspary WF. 13C-breath tests: current state of the art and future directions. Dig Liver Dis. 2007;39:795–805. doi: 10.1016/j.dld.2007.06.012. [DOI] [PubMed] [Google Scholar]
- 15.Braden B. Methods and functions: Breath tests. Best Pract Res Clin Gastroenterol. 2009;23:337–352. doi: 10.1016/j.bpg.2009.02.014. [DOI] [PubMed] [Google Scholar]
- 16.Braden B, Caspary WF, Lembcke B. Nondispersive infrared spectrometry for 13CO2/12CO2-measurements: a clinically feasible analyzer for stable isotope breath tests in gastroenterology. Z Gastroenterol. 1999;37:477–481. [PubMed] [Google Scholar]
- 17.Perchonok M, Bourland C. NASA food systems: past, present, and future. Nutrition. 2002;18:913–920. doi: 10.1016/s0899-9007(02)00910-3. [DOI] [PubMed] [Google Scholar]
- 18.Ghoos YF, Maes BD, Geypens BJ, Mys G, Hiele MI, Rutgeerts PJ, Vantrappen G. Measurement of gastric emptying rate of solids by means of a carbon-labeled octanoic acid breath test. Gastroenterology. 1993;104:1640–1647. doi: 10.1016/0016-5085(93)90640-x. [DOI] [PubMed] [Google Scholar]
- 19.Haycock GB, Schwartz GJ, Wisotsky DH. Geometric method for measuring body surface area: a height-weight formula validated in infants, children, and adults. J Pediatr. 1978;93:62–66. doi: 10.1016/s0022-3476(78)80601-5. [DOI] [PubMed] [Google Scholar]
- 20.Kasicka-Jonderko A, Kamińska M, Jonderko K, Setera O, Błońska-Fajfrowska B. Short- and medium-term reproducibility of gastric emptying of a solid meal determined by a low dose of 13C-octanoic acid and nondispersive isotope-selective infrared spectrometry. World J Gastroenterol. 2006;12:1243–1248. doi: 10.3748/wjg.v12.i8.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider AR, Jepp K, Murczynski L, Biniek U, Stein J. The inulin hydrogen breath test accurately reflects orocaecal transit time. Eur J Clin Invest. 2007;37:802–807. doi: 10.1111/j.1365-2362.2007.01862.x. [DOI] [PubMed] [Google Scholar]
- 22.Braden B, Adams S, Duan LP, Orth KH, Maul FD, Lembcke B, Hör G, Caspary WF. The [13C]acetate breath test accurately reflects gastric emptying of liquids in both liquid and semisolid test meals. Gastroenterology. 1995;108:1048–1055. doi: 10.1016/0016-5085(95)90202-3. [DOI] [PubMed] [Google Scholar]
- 23.Mossi S, Meyer-Wyss B, Beglinger C, Schwizer W, Fried M, Ajami A, Brignoli R. Gastric emptying of liquid meals measured noninvasively in humans with [13C]acetate breath test. Dig Dis Sci. 1994;39:107S–109S. doi: 10.1007/BF02300386. [DOI] [PubMed] [Google Scholar]
- 24.Perri F, Bellini M, Portincasa P, Parodi A, Bonazzi P, Marzio L, Galeazzi F, Usai P, Citrino A, Usai-Satta P. (13)C-octanoic acid breath test (OBT) with a new test meal (EXPIROGer): Toward standardization for testing gastric emptying of solids. Dig Liver Dis. 2010;42:549–553. doi: 10.1016/j.dld.2010.01.001. [DOI] [PubMed] [Google Scholar]
- 25.Geboes KP, Luypaerts A, Rutgeerts P, Verbeke K. Inulin is an ideal substrate for a hydrogen breath test to measure the orocaecal transit time. Aliment Pharmacol Ther. 2003;18:721–729. doi: 10.1046/j.1365-2036.2003.01750.x. [DOI] [PubMed] [Google Scholar]
- 26.Perri F, Clemente R, Festa V, Quitadamo M, Niro G, Andriulli A. 13C-octanoic acid breath test: a reliable tool for measuring gastric emptying. Ital J Gastroenterol Hepatol. 1998;30:211–217. [PubMed] [Google Scholar]
- 27.Choi MG, Camilleri M, Burton DD, Zinsmeister AR, Forstrom LA, Nair KS. [13C]octanoic acid breath test for gastric emptying of solids: accuracy, reproducibility, and comparison with scintigraphy. Gastroenterology. 1997;112:1155–1162. doi: 10.1016/s0016-5085(97)70126-4. [DOI] [PubMed] [Google Scholar]
- 28.Choi MG, Camilleri M, Burton DD, Zinsmeister AR, Forstrom LA, Nair KS. Reproducibility and simplification of 13C-octanoic acid breath test for gastric emptying of solids. Am J Gastroenterol. 1998;93:92–98. doi: 10.1111/j.1572-0241.1998.092_c.x. [DOI] [PubMed] [Google Scholar]
- 29.Heine WE, Berthold HK, Klein PD. A novel stable isotope breath test: 13C-labeled glycosyl ureides used as noninvasive markers of intestinal transit time. Am J Gastroenterol. 1995;90:93–98. [PubMed] [Google Scholar]
- 30.Geypens B, Bennink R, Peeters M, Evenepoel P, Mortelmans L, Maes B, Ghoos Y, Rutgeerts P. Validation of the lactose-[13C]ureide breath test for determination of orocecal transit time by scintigraphy. J Nucl Med. 1999;40:1451–1455. [PubMed] [Google Scholar]
- 31.Tibble JA, Sigthorsson G, Foster R, Forgacs I, Bjarnason I. Use of surrogate markers of inflammation and Rome criteria to distinguish organic from nonorganic intestinal disease. Gastroenterology. 2002;123:450–460. doi: 10.1053/gast.2002.34755. [DOI] [PubMed] [Google Scholar]
- 32.Stríz I, Trebichavský I. Calprotectin - a pleiotropic molecule in acute and chronic inflammation. Physiol Res. 2004;53:245–253. [PubMed] [Google Scholar]
- 33.Thjodleifsson B, Sigthorsson G, Cariglia N, Reynisdottir I, Gudbjartsson DF, Kristjansson K, Meddings JB, Gudnason V, Wandall JH, Andersen LP, et al. Subclinical intestinal inflammation: an inherited abnormality in Crohn's disease relatives? Gastroenterology. 2003;124:1728–1737. doi: 10.1016/s0016-5085(03)00383-4. [DOI] [PubMed] [Google Scholar]
- 34.Montalto M, Curigliano V, Santoro L, Armuzzi A, Cammarota G, Covino M, Mentella MC, Ancarani F, Manna R, Gasbarrini A, et al. Fecal calprotectin in first-degree relatives of patients with ulcerative colitis. Am J Gastroenterol. 2007;102:132–136. doi: 10.1111/j.1572-0241.2006.00884.x. [DOI] [PubMed] [Google Scholar]
- 35.Otten CM, Kok L, Witteman BJ, Baumgarten R, Kampman E, Moons KG, de Wit NJ. Diagnostic performance of rapid tests for detection of fecal calprotectin and lactoferrin and their ability to discriminate inflammatory from irritable bowel syndrome. Clin Chem Lab Med. 2008;46:1275–1280. doi: 10.1515/CCLM.2008.246. [DOI] [PubMed] [Google Scholar]
- 36.Caso JR, Leza JC, Menchén L. The effects of physical and psychological stress on the gastro-intestinal tract: lessons from animal models. Curr Mol Med. 2008;8:299–312. doi: 10.2174/156652408784533751. [DOI] [PubMed] [Google Scholar]
- 37.Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62:591–599. [PubMed] [Google Scholar]
- 38.Amidon GL, DeBrincat GA, Najib N. Effects of gravity on gastric emptying, intestinal transit, and drug absorption. J Clin Pharmacol. 1991;31:968–973. doi: 10.1002/j.1552-4604.1991.tb03658.x. [DOI] [PubMed] [Google Scholar]
- 39.Graebe A, Schuck EL, Lensing P, Putcha L, Derendorf H. Physiological, pharmacokinetic, and pharmacodynamic changes in space. J Clin Pharmacol. 2004;44:837–853. doi: 10.1177/0091270004267193. [DOI] [PubMed] [Google Scholar]


