Abstract
AIM: To evaluate the efficacy of transarterial chemoembolization (TACE) using a suspension of a fine-powder formulation of cisplatin (DDPH) in lipiodol (LPD) in the treatment of hepatocellular carcinoma (HCC).
METHODS: The subjects were 262 HCC patients treated with TACE using a DDPH-LPD suspension. The DDPH-LPD suspension was prepared by mixing 50 mg of DDPH into 10 mL of LPD. TACE was repeated when treated lesions relapsed and/or new hepatic lesions were detected. These patients received additional TACE using the same agent. TACE was repeated until complete regression of the tumor was obtained. The primary efficacy endpoint of the current study was the objective early response rate. Secondary efficacy endpoints were progression-free survival (PFS) and overall survival.
RESULTS: The objective early response rate was 43.6%. Cumulative PFS rates were 56.7% at 6 mo, 23.1% at 12 mo, 13.4% at 18 mo, and 10.5% at 24 mo. The median PFS was 6.6 mo. Cumulative survival rates were 90.6% at 6 mo, 81.9% at 12 mo, 70.5% at 24 mo, and 58.8% at 36 mo. Median survival time was 46.6 mo. All adverse reactions were controllable by temporary suspension of treatment. No serious complications or treatment-related deaths were observed.
CONCLUSION:TACE using a suspension of DDPH in LPD may be a useful treatment for HCC.
Keywords: Cisplatin, DDPH, Hepatocellular carcinoma, Portal vein tumor thrombosis, Transarterial chemoembolization
INTRODUCTION
Hepatocellular carcinoma (HCC) is a common primary liver cancer with a rising incidence worldwide[1]. In Japan, more than 30 000 people die of HCC each year[2]. Curative therapies such as resection, liver transplantation, and local ablative treatments may offer a chance of improved life expectancy, but these treatment modalities are applicable to only a small proportion of HCC patients. As a result, in patients with advanced HCC who are not eligible for these curative therapies, transarterial chemoembolization (TACE) has been the mainstay treatment option with proven survival benefits[3,4]. Many studies of TACE have been reported; a method using lipiodol (LPD), an oily contrast medium used as a drug delivery system, is now widely used, and anticancer drugs such as doxorubicin (ADM), epirubicin, and other anthracyclines are often used[5,6]. However, the tumors have a high frequency of recurrence after TACE[5,7]. Moreover, HCC is not necessarily sensitive to these drugs[8,9]. Therefore, the therapeutic results of TACE for HCC should improve as anticancer drugs become more effective.
Cisplatin (CDDP), a platinum compound, is an effective anticancer agent used in the treatment of various malignancies[10]. Researchers have reported that TACE using a suspension of CDDP powder in LPD may be more effective against unresectable HCC than TACE using an ADM-LPD emulsion[11,12]. However, only a few institutions have used this for TACE because it is difficult to refine the CDDP powder. Since 2004, a fine-powder formulation of CDDP (DDPH, IA-call; Nippon Kayaku, Tokyo, Japan) has been available as a therapeutic agent for intra-arterial infusion in Japan. As a result, TACE using DDPH has become widespread in Japanese institutions. We have already used TACE with DDPH for HCC patients and reported favorable results[13]. The aim of this study was to elucidate the efficacy of this therapy by analyzing the clinical results of 262 HCC patients treated in this manner.
MATERIALS AND METHODS
Study design and patient eligibility
This clinical investigation was approved by the ethics committee of our institution, and informed consent was obtained from all patients. The study was designed as a single-institution, open clinical study. The primary efficacy endpoint of the current study was the objective response rate. Secondary efficacy endpoints were progression-free survival (PFS) and overall survival (OS).
Eligibility criteria were as follows: (1) Eastern Cooperative Oncology Group performance status of 0-2; (2) age over 20 years; (3) diagnosis of HCC based on imaging or histological findings; (4) no indication for surgical resection or local ablation therapy such as radiofrequency ablation (RFA); (5) bidimensionally measurable hepatic lesions; (6) adequate hepatic function (serum total bilirubin < 3.0 mg/dL), and adequate renal function (serum creatinine < the upper normal limit); (7) no extrahepatic metastasis; (8) no tumor thrombus in the main trunk of the portal vein; or (9) no HCC treatment for 4 wk before study entry.
Enrolled were patients with HCC suitable for curative treatments such as surgical resection and local ablation therapy but who were of high risk for these therapies. A total of 262 consecutive patients who were to undergo TACE using DDPH between January 2006 and May 2011 were enrolled. All of the enrolled patients met the inclusion criteria.
Diagnosis
The diagnostic criteria for HCC via imaging were based on hyperattenuation in the arterial phase and hypoattenuation in the portal phase on dynamic computed tomography (CT) or magnetic resonance imaging (MRI), and tumor stain on angiography. When HCC could not be diagnosed by imaging alone, fine-needle biopsy using abdominal ultrasonography (US) was performed to obtain histological proof. Further assessment of HCC was conducted by measuring levels of α-fetoprotein (AFP) and des-γ-carboxy prothrombin (DCP).
Liver function was evaluated according to the Child-Pugh classification[14]. Tumor stage was assessed based on the tumor node metastasis (TNM) staging system of the Liver Cancer Study Group of Japan[15]. Portal vein tumor thrombosis (PVTT) grade was classified as follows: Vp0, no invasion of the portal vein; Vp1, invasion of the third or more distal branch of the left or right portal vein; Vp2, invasion of the second branch of the portal vein; Vp3, invasion of the first branch of the portal vein; and Vp4, invasion of the trunk of the portal vein.
Preparation of the agents for TACE
DDPH was mixed with LPD (iodized oil, Lipiodol Ultra-Fluide; Andre Guerget, Aulnay-sous-Bois, France). The DDPH-LPD suspension was prepared by mixing 50 mg of DDPH into 10 mL of LPD. The dosage of DDPH-LPD suspension was adjusted depending on the tumor size, number of tumors, degree of liver impairment, and renal function, but the maximum dose of DDPH-LPD suspension was not allowed to exceed 10 mL.
Treatment procedures
In all TACE procedures, hepatic angiography was performed by the femoral approach using a 4-Fr catheter and a 1.8-Fr to 2.4-Fr microcatheter. After confirming the hepatic arteries supplying the target tumor, a catheter was selectively inserted into the hepatic artery supplying the target tumor, and the DDPH-LPD suspension was injected. In patients with several tumors in the liver, superselective catheterization was performed for each lesion. If superselective catheterization was not possible, the DDPH-LPD suspension was injected into the right and left main hepatic arteries distal to the origin of the cystic artery. After the injection, arterioembolization was performed used porous gelatin particles (Gelpart; Nippon Kayaku, Tokyo, Japan) mixed with contrast medium.
All patients were followed up with US, CT, and/or MRI after 1 mo and then every 3 mo thereafter. Treatment was repeated by TACE alone when treated lesions relapsed and/or new hepatic lesions were detected. These patients received additional TACE using the same agent during the follow-up period unless the tumors progressed. TACE was repeated until complete regression of the tumor was obtained.
Evaluation of therapeutic efficacy
Tumor response was assessed by US, CT, and/or MRI at 1 mo from the start of treatment and every 3 mo thereafter. The response was classified according to the modified RECIST (mRECIST) criteria[16], which take into account only the viable (arterially enhancing) component of the target tumors, and grade tumor response as follows: complete response (CR)-disappearance of any intratumoral arterial enhancement in all target lesions; partial response (PR)-at least a 30% decrease in the sum of diameters of viable target lesions, taking as reference the baseline sum of the diameters of target lesions; progressive disease (PD)-increase (at least 20%) in the sum of the diameters of viable target lesions, taking as reference the smallest sum of the diameters of viable (enhancing) target lesions recorded since treatment started, or appearance of new lesions; stable disease (SD)-all other cases.
Toxicity was evaluated using the National Cancer Institute-Common Terminology Criteria for Adverse Events, version 3.0 (CTCAE v3.0).
Statistical analysis
Baseline data are expressed as means ± SD or as medians and range. Statistical analysis was performed in September 2011. The cumulative survival rate and PFS were calculated from the date of therapy initiation and assessed by the Kaplan-Meier life-table method, and differences were evaluated using the log-rank test. Univariate analysis of predictors for survival of patients was assessed using the Kaplan-Meier life-table method, and differences were evaluated using the log-rank test. Multivariate analysis of predictors for survival was assessed by the Cox proportional hazards model. Significance was accepted at P < 0.05. All analyses were performed using SPSS version 11 software (SPSS, Chicago, IL, United States).
RESULTS
Patient characteristics
The characteristics of the 262 patients are listed in Table 1. There were 176 male and 86 female patients, ranging in age from 32 to 92 years (median age, 70 years). There were 147 (56.1%), 93 (35.5%), and 22 (8.4%) patients with Child-Pugh Stages A, B, and C, respectively. The median diameter of the largest tumor was 32.5 mm (range, 8-300 mm). Serum AFP levels were > 10 ng⁄mL in 182 patients, and 146 patients were DCP-positive (> 40 mAU⁄mL).
Table 1.
Baseline characteristics of the 262 patients
| Characteristics | ||
| Enrolled patients | 262 | |
| Age (yr) | Median (range) | 70 (32-92) |
| Sex | Male/female | 176/86 |
| Etiology | HBV/HCV/NBNC | 30/170/62 |
| Child Pugh classification | A/B/C | 147/93/22 |
| Number of tumors | < 10/≥ 10 | 114/148 |
| Maximum tumor size (mm) | Median (range) | 32.5 (8.0-300.0) |
| Stage1 | I/II/III/IV | 17/45/136/64 |
| PVTT grade | Vp0/Vp1-2/Vp3 | 202/27/33 |
| Total bilirubin (mg/dL) | mean ± SD | 1.0 ± 0.7 |
| Albumin (g/dL) | mean ± SD | 3.4 ± 0.6 |
| Prothrombin time (%) | mean ± SD | 91.1 ± 8.4 |
| Platelet count (× 104/L) | mean ± SD | 8.9 ± 5.0 |
| AFP (ng/mL) | median (range) | 31.6 (1.0-1 000 000) |
| AFP-L3 (ng/mL) | median (range) | 4.0 (0-91.8) |
| DCP (mAU/mL) | median (range) | 60 (0-928 900) |
| Previous treatment | Yes/no | 107/155 |
Data are expressed as median values with ranges, mean ± SD, or number of patients.
According to the modified RECIST (mRECIST) criteria. HBV: Hepatitis B virus; HCV: Hepatitis C virus; NBNC: Negative for hepatitis B surface antigen and HCV antibody; PVTT: Portal vein tumor thrombosis; AFP: α-fetoprotein; AFP-L3: Lens culinaris agglutinin-reactive fraction of α-fetoprotein; DCP: Des-γ-carboxy prothrombin.
Clinical efficacy
The median duration of follow-up was 17.0 mo (range, 2.0-64.0 mo). A total of 682 TACE procedures were performed in 262 patients. The median number of TACE procedures was 2 cycles (range, 1-13 cycles). Early response status in the 262 patients was assessed after the first course of therapy. As a result, 34 patients (13.0%) had CR, 80 patients (30.6%) had PR, 69 patients (26.3%) had SD, and 79 patients (30.1%) had PD [response rate (CR + PR/all cases) = 43.6%]. The disease control rate (CR + PR + SD/all cases) was 69.9%.
PFS
The median PFS was 6.6 mo. The PFS rates at 6, 12, 18, and 24 mo were 56.7%, 23.1%, 13.4%, and 10.5%, respectively (Figure 1).
Figure 1.
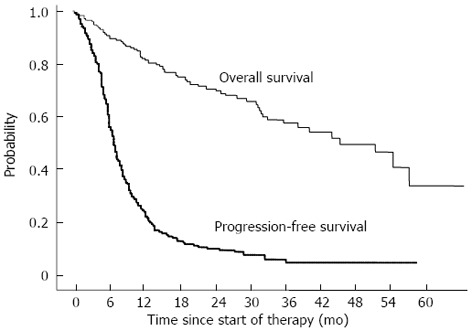
Overall survival and progression-free survival curves of 262 patients treated with transarterial chemoembolization using DDPH.
Survival
The median survival time (MST) was 46.6 mo. The cumulative survival rates at 6, 12, 24, and 36 mo were 90.6%, 81.9%, 70.5%, and 58.8%, respectively (Figure 1).
Cumulative survival rates of patients with no PVTT were 96.3% at 6 mo, 90.4% at 12 mo, 79.7% at 24 mo. On the other hand, cumulative survival rates of patients with PVTT were 68.6% at 6 mo, 49.0% at 12 mo, 33.7% at 24 mo. The survival rate was significantly higher in patients with no PVTT than in patients with PVTT (P < 0.001, Figure 2A).
Figure 2.
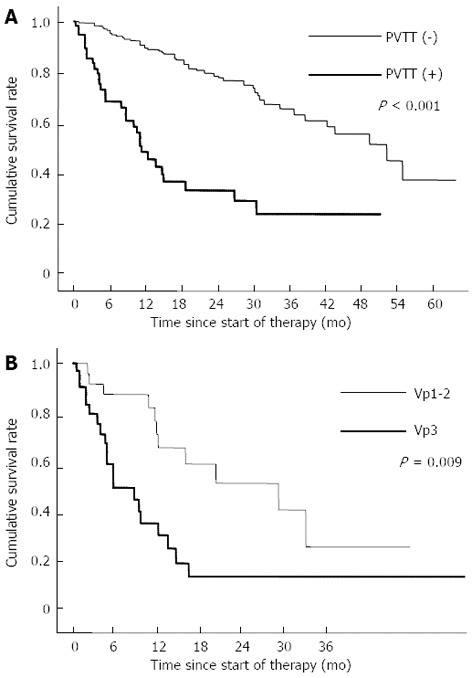
Cumulative survival rates. A: Comparison of cumulative survival rates between patients with no portal vein tumor thrombosis and those with portal vein tumor thrombosis; B: Comparison of the cumulative survival rates between patients with Vp1-2 and patients with Vp3. PVTT: Portal vein tumor thrombosis.
Moreover, cumulative survival rates were determined by PVTT grade in 60 patients with PVTT. Cumulative survival rates of patients with Vp1-2 were 87.6% at 6 mo, 67.0% at 12 mo, 52.8% at 24 mo. On the other hand, cumulative survival rates of patients with Vp3 were 51.0% at 6 mo, 32.5% at 12 mo, 16.2% at 24 mo. The survival rate was significantly higher in patients with Vp1-2 than in patients with Vp3 (P = 0.009, Figure 2B).
Prognostic factors affecting patient survival were analyzed by examining 17 potential parameters (Table 2). Univariate analysis revealed 12 significant prognostic factors related to survival: stage (P < 0.001), Child Pugh classification (P = 0.005), JIS score (P < 0.001), total bilirubin (P = 0.031), albumin (P = 0.012), number of tumors (P < 0.001), maximum tumor size (P < 0.001), PVTT grade (P < 0.001), tumor distribution (P < 0.001), AFP (P < 0.001), DCP (P < 0.001), and therapeutic effect (P < 0.001).
Table 2.
Univariate and multivariate analysis of predictors of survival
| Variable | Hazard ratio | 95%CI | P value |
| Univariate analysis of predictors of survival | |||
| Age (≤ 65 vs > 65 yr) | 1.085 | 0.772-1.525 | 0.638 |
| Gender (M vs F) | 0.861 | 0.607-1.222 | 0.403 |
| Previous treatment (no vs yes) | 1.258 | 0.911-1.738 | 0.164 |
| HCV antibody (negative vs positive) | 0.951 | 0.673-1.344 | 0.776 |
| Stage (I, II, III vs IV) | 2.705 | 1.919-3.814 | < 0.001 |
| Child Pugh classification (A vs B or C) | 1.584 | 1.149-2.184 | 0.005 |
| JIS score (0-2 vs 3-5) | 2.285 | 1.638-3.189 | < 0.001 |
| Total bilirubin (≤ 1.5 mg/dL vs > 1.5 mg/dL) | 1.578 | 1.044-2.385 | 0.031 |
| Albumin (> 3.5 mg/dL vs ≤ 3.5 mg/dL) | 1.527 | 1.100-2.119 | 0.012 |
| Number of tumors (< 10 vs ≥ 10) | 1.920 | 1.378-2.675 | < 0.001 |
| Maximum tumor size (≤ 50 mm vs > 50 mm) | 2.052 | 1.404-2.998 | < 0.001 |
| PVTT grade (Vp0, 1, 2 vs Vp3) | 4.142 | 2.754-6.230 | < 0.001 |
| Tumor distribution (Unilateral vs Bilateral) | 2.237 | 1.464-3.420 | < 0.001 |
| AFP (≤ 100 ng/mL vs > 100 ng/mL) | 2.131 | 1.539-2.949 | < 0.001 |
| AFP-L3 (≤ 50% vs > 50%) | 1.664 | 0.957-2.894 | 0.071 |
| DCP (≤ 100 mAU/mL vs > 100 mAU/mL) | 2.201 | 1.588-3.051 | < 0.001 |
| Therapeutic effect (CR + PR vs SD + PD) | 3.419 | 2.382-4.909 | < 0.001 |
| Multivariate analysis of predictors of survival | |||
| PVTT grade (Vp0, 1, 2 vs Vp3) | 2.310 | 1.217-4.384 | 0.010 |
| AFP (≤ 100 ng/mL vs > 100 ng/mL) | 1.856 | 1.265-2.724 | 0.002 |
| Therapeutic effect (CR + PR vs SD + PD) | 3.392 | 2.240-5.135 | < 0.001 |
The JIS score is obtained by simply adding both scores for the tumor, lymph node, metastasis (TNM) stage and Child-Turcotte-Pugh stage. HCV: Hepatitis C virus; JIS: Japan integrated staging; PVTT: Portal vein tumor thrombosis; AFP: α-fetoprotein; DCP: Des-γ-carboxy prothrombin; CR: Complete response; PR: Partial response; SD: Stable disease; PD: Progressive disease.
Multivariate analysis showed 3 significant prognostic factors related to survival: PVTT grade (P = 0.010), AFP (P = 0.002), and therapeutic effect (P < 0.001).
Adverse effects
Table 3 summarizes the adverse effects. No treatment-related deaths occurred, and no grade 4 treatment-related adverse effects were noted. Fever and nausea were seen transiently in 50% or more patients, but both were mild. Leucopenia and thrombocytopenia occurred in 15 (5.7%) and 18 (6.9%) patients, respectively; these were also mild and transient. Although grade 2 or higher liver abscess and hepatic/renal failure were observed in 4 (1.4%) and 1 (0.4%) patients, respectively, these adverse reactions were controllable by medical treatment. In addition, hepatic arterial damage (HAD) after TACE was observed in one patient. Although one patient was observed to have slight wall irregularity of the hepatic artery, HAD associated with TACE did not interfere with catheterization at the next TACE session.
Table 3.
Adverse effects among the 262 patients n (%)
| Adverse effect | Grade 1 | Grade 2 | Grade 3 | Grade 4 |
| Nausea/vomiting | 160 (61.1) | 48 (18.3) | - (-) | - (-) |
| General fatigue | 28 (10.7) | 17 (6.5) | 1 (0.4) | - (-) |
| Fever | 168 (64.1) | 27 (10.3) | - (-) | - (-) |
| Abdominal pain | 121 (46.1) | 54 (20.6) | - (-) | - (-) |
| Leucopenia | 13 (4.9) | 2 (0.7) | - (-) | - (-) |
| Thrombocytopenia | 16 (6.1) | 2 (0.7) | - (-) | - (-) |
| AST/ALT | 154 (58.8) | 46 (17.6) | - (-) | - (-) |
| Liver abscess | - (-) | 2 (0.7) | 2 (0.7) | - (-) |
| Hepatic/Renal failure | - (-) | - (-) | 1 (0.4) | - (-) |
Data are expressed as number of patients with percentages in parentheses. AST: Aspartate aminotransferase; ALT: Alanine aminotransferase.
DISCUSSION
TACE plays a crucial role in the treatment of HCC without surgical resection or RFA. The survival benefit of TACE has also been confirmed by randomized, controlled trials and a meta-analysis[3,4]. The most commonly used agent used in TACE for HCC treatment is ADM-LPD emulsion, followed by embolization with a gelatin sponge[12,13,17,18]. The MST ranged from 18 to 34 mo with the use of TACE with ADM-LPD emulsion, but there is no clear evidence identifying the best chemotherapeutic agent for TACE.
CDDP is an effective anticancer agent used in the treatment of various malignancies[10]. CDDP has been reported to exert its actions by binding to the DNA in cancer cells, inhibiting DNA synthesis and subsequent cellular division. The antitumor activity of CDDP is closely associated with the serum concentration of the drug[19].
The key point of intra-arterial infusion chemotherapy is the selective retention of anticancer drugs at a high concentration in the HCC for a long time. LPD shows very selective deposition within HCC, in which it remains for several months after intra-arterial injection, whereas it disappears more rapidly from the nontumorous parenchyma[20]. Consequently, augmented antitumor efficacy and milder side effects were expected with the use of this substance for TACE. In fact, Morimoto et al[21] investigated the pharmacological advantages of TACE using DDPH for hypervascular hepatic tumors in animal experiments. They reported that the tumor concentration of the platinum agent in the DDPH-LPD-TACE group was about 14 times higher than that in the DDPH-hepatic arterial infusion (HAI) group. In addition, they reported that the plasma concentrations of the platinum agent were lower in the DDPH-LPD-TACE group than in the DDPH-HAI group.
Ono et al[12] reported that TACE using a suspension of CDDP powder in LPD was more effective against unresectable HCC than that using ADM-LPD emulsion. However, because CDDP was only available as a solution, it was difficult to prepare a high-dose CDDP suspension using LPD.
A fine-powder formulation of CDDP, namely DDPH, for intra-arterial infusion has been available for HCC treatment since 2004 in Japan. Dispensing of CDDP powder improved with the development of DDPH, and DDPH has now come to replace CDDP powder. Since DDPH-LPD suspension for TACE in HCC patients was expected to yield better therapeutic outcomes, TACE using DDPH-LPD suspension became widespread in Japanese institutions. We have already used TACE with DDPH-LPD suspension for HCC patients and reported favorable results[13]. This article focused on the efficacy of this therapy by analyzing the clinical results of 262 HCC patients treated in this manner.
The MST in the current study was 46.6 mo. The cumulative survival rates at 6, 12, 24, and 36 mo were 90.6%, 81.9%, 70.5% and 58.8%, respectively. The outcome in the present study was superior to previous trials of TACE using ADM, epirubicin, and other anthracyclines[5,6,13]. This could be explained as being due to the fact that TACE with ADM cannot be repeated as required because of the high frequency of adverse effects of ADM, such as leucopenia, severe vascular changes, and hepatic artery occlusion[12,13,22]. In the current study, leucopenia and HAD were observed in only 15 (5.7%) and 1 (0.4%) patients, respectively. Considering that TACE is often repeated in most patients, longer patency of the hepatic artery is preferable for properly deploying the lipiodol mixture and embolic agents into the tumor. In addition, we concluded that anthracyclines such as ADM may be relatively less effective against HCC; this is because of the high expression level of P-glycoprotein, which transports antitumor agents such as anthracyclines or vinca alkaloids from cells with a high active efflux mechanism in HCC tumors[23].
Moreover, survival in the present study was superior to previous trials of TACE using drug-eluting beads[24-29]. In these outcomes of previous trials of TACE using drug-eluting beads, the response rates were superior to the current study. Nevertheless, the cumulative survival rates of the patients in the current study were higher than those of the patients in the previous trials. Drug-eluting beads are known to give more distal vessel occlusion for a long-term period[30]. Therefore, it is possible that TACE with drug-eluting beads could have a greater embolizant effect than TACE with DDPH-LPD suspension, and this would lead to increased tumor growth factor release in response to hypoxia, with a consequent probability of recurrence and reduced overall survival.
The presence of PVTT has traditionally been considered a contraindication for transarterial therapy[31]. However, a recent study has revealed that TACE for patients with PVTT had survival benefits over conservative treatment[32]. Compared with this recent study, cumulative survival rates of patients with PVTT in the present study were better. On the other hand, cumulative survival rates of patients with Vp3 in subgroup analysis of the present study were 51.0% at 6 mo, 32.5% at 12 mo, and 16.2% at 24 mo. In our previous study of hepatic arterial infusion chemotherapy (HAIC) with 5-fluorouracil (5-FU) and pegylated IFN-α2b (PEG-IFNα-2b) for HCC patients with Vp3/4, cumulative survival rates were 83.8% at 6 mo, 77.8% at 12 mo, 55.6% at 24 mo[33]. Although it is impossible to compare the results of TACE using a suspension of DDPH in LPD and HAIC using 5-FU and PEG-IFNα-2b for HCC patients with Vp3, we think that a randomized controlled study comparing these therapies in patients with Vp3/4 will be needed in the future.
The prognosis of HCC patients depends on many factors, such as tumor stage and liver function. In the current study, the prognostic factors in patients treated with TACE with DDPH-LPD suspension were investigated. Among the variables examined, PVTT grade (Vp0-2), AFP (≤ 100 ng/mL), and therapeutic effect (CR+PR) were identified as being significantly associated with longer survival times on multivariate analysis. These results were similar to the result of a nationwide prospective cohort study by Takayasu et al[34], which was performed in 8510 patients with unresectable HCC who underwent TACE using an emulsion of lipiodol and anticancer agents followed by gelatin sponge particles.
Considering these facts, we conclude that TACE using DDPH-LPD suspension could be a useful treatment strategy for HCC patients. To confirm these results, randomized controlled trials comparing TACE using DDPH-LPD suspension with TACE using ADM-LPD emulsion or TACE using drug-eluting beads for patients with HCC are mandatory. Moreover, we think that a randomized controlled study comparing these therapies and HAIC for HCC patients with PVTT will be needed in the future.
ACKNOWLEDGMENTS
The authors wish to thank Ms. Kouko Motodate for preparing serum samples.
COMMENTS
Background
In recent years, transcatheter arterial chemoembolization (TACE) using an emulsion of doxorubicin (ADM) with lipiodol (LPD) (ADM-LPD emulsion) followed by embolization with a gelatin sponge has been commonly employed for hepatocellular carcinoma (HCC) treatment. However, HCC is not necessarily sensitive to these drugs.
Research frontiers
Cisplatin, a platinum compound, is an effective anticancer agent used in the treatment of various malignancies. Recently, a fine-powder formulation of cisplatin (DDPH, IA-call; Nipponkayaku, Tokyo, Japan) has also been available since 2004 as a therapeutic agent for intra-arterial infusion in Japan. Researchers have recently reported that TACE using a suspension of cisplatin powder in LPD may be more effective against unresectable HCC as compared with that using ADM-LPD emulsion. Therefore, TACE using DDPH has become widespread in Japanese institutions.
Innovations and breakthroughs
In this article, the authors evaluated the effectiveness of TACE using DDPH-LPD for 262 HCC patients. The objective early response rate was 43.6%. Cumulative survival rates were 90.6% at 6 mo, 81.9% at 12 mo, 70.5% at 24 mo, and 58.8% at 36 mo. Median survival time was 46.6 mo. All adverse reactions were controllable by temporary suspension of treatment. No serious complications or treatment-related deaths were observed. The outcome in the present study was superior to previous trials of TACE using ADM-LPD. Moreover, survival in the present study was superior to previous trials of TACE using drug-eluting beads.
Applications
Although randomized, controlled trials comparing TACE using DDPH-LPD suspension with TACE using ADM-LPD emulsion or TACE using drug-eluting beads for patients with HCC are mandatory, the authors conclude that TACE using DDPH-LPD suspension could be a useful treatment strategy for HCC patients.
Terminology
TACE is a minimally invasive medical procedure to restrict a tumor’s blood supply. TACE is an interventional radiology procedure. The procedure involves gaining percutaneous access to the hepatic artery. When a blood vessel supplying tumor has been selected, alternating aliquots of the chemotherapy dose and of embolic particles, or particles containing the chemotherapy agent, are injected through the catheter. CDDP is a chemotherapy drug. It was the first member of a class of platinum-containing anti-cancer drugs that now also includes carboplatin and oxaliplatin. These platinum complexes react in vivo, binding to and causing crosslinking of DNA, which ultimately triggers apoptosis.
Peer review
This paper is well written. The clinical results are appropriately described. The authors present clinical evaluation of TACE of DDPH in lipiodol in the treatment of HCC. The data indicate that the treatment in HCC patients resulted in significantly better early response rate, overall survival, progression free survival and cumulative survival rates.
Footnotes
P- Reviewer Pandey VN S- Editor Song XX L- Editor Logan S E- Editor Zhang DN
References
- 1.Jemal A, Clegg LX, Ward E, Ries LA, Wu X, Jamison PM, Wingo PA, Howe HL, Anderson RN, Edwards BK. Annual report to the nation on the status of cancer, 1975-2001, with a special feature regarding survival. Cancer. 2004;101:3–27. doi: 10.1002/cncr.20288. [DOI] [PubMed] [Google Scholar]
- 2.Umemura T, Ichijo T, Yoshizawa K, Tanaka E, Kiyosawa K. Epidemiology of hepatocellular carcinoma in Japan. J Gastroenterol. 2009;44 Suppl 19:102–107. doi: 10.1007/s00535-008-2251-0. [DOI] [PubMed] [Google Scholar]
- 3.Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: Chemoembolization improves survival. Hepatology. 2003;37:429–442. doi: 10.1053/jhep.2003.50047. [DOI] [PubMed] [Google Scholar]
- 4.Llovet JM, Real MI, Montaña X, Planas R, Coll S, Aponte J, Ayuso C, Sala M, Muchart J, Solà R, et al. Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial. Lancet. 2002;359:1734–1739. doi: 10.1016/S0140-6736(02)08649-X. [DOI] [PubMed] [Google Scholar]
- 5.Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, Hasegawa H, Hirohashi S. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987;163:345–351. doi: 10.1148/radiology.163.2.3031724. [DOI] [PubMed] [Google Scholar]
- 6.Uchida H, Ohishi H, Matsuo N, Nishimine K, Ohue S, Nishimura Y, Maeda M, Yoshioka T. Transcatheter hepatic segmental arterial embolization using lipiodol mixed with an anticancer drug and Gelfoam particles for hepatocellular carcinoma. Cardiovasc Intervent Radiol. 1990;13:140–145. doi: 10.1007/BF02575465. [DOI] [PubMed] [Google Scholar]
- 7.Kawai S, Okamura J, Ogawa M, Ohashi Y, Tani M, Inoue J, Kawarada Y, Kusano M, Kubo Y, Kuroda C. Prospective and randomized clinical trial for the treatment of hepatocellular carcinoma--a comparison of lipiodol-transcatheter arterial embolization with and without adriamycin (first cooperative study). The Cooperative Study Group for Liver Cancer Treatment of Japan. Cancer Chemother Pharmacol. 1992;31 Suppl:S1–S6. doi: 10.1007/BF00687096. [DOI] [PubMed] [Google Scholar]
- 8.Bokemeyer C, Kynast B, Harstrick A, Laage E, Schmoll E, von Wussow P, Schmoll HJ. No synergistic activity of epirubicin and interferon-alpha 2b in the treatment of hepatocellular carcinoma. Cancer Chemother Pharmacol. 1995;35:334–338. doi: 10.1007/BF00689454. [DOI] [PubMed] [Google Scholar]
- 9.Lai CL, Wu PC, Chan GC, Lok AS, Lin HJ. Doxorubicin versus no antitumor therapy in inoperable hepatocellular carcinoma. A prospective randomized trial. Cancer. 1988;62:479–483. doi: 10.1002/1097-0142(19880801)62:3<479::aid-cncr2820620306>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 10.Uchiyama N, Kobayashi H, Nakajo M, Shinohara S. Treatment of lung cancer with bronchial artery infusion of cisplatin and intravenous sodium thiosulfate rescue. Acta Oncol. 1988;27:57–61. doi: 10.3109/02841868809090319. [DOI] [PubMed] [Google Scholar]
- 11.Kamada K, Nakanishi T, Kitamoto M, Aikata H, Kawakami Y, Ito K, Asahara T, Kajiyama G. Long-term prognosis of patients undergoing transcatheter arterial chemoembolization for unresectable hepatocellular carcinoma: comparison of cisplatin lipiodol suspension and doxorubicin hydrochloride emulsion. J Vasc Interv Radiol. 2001;12:847–854. doi: 10.1016/s1051-0443(07)61510-3. [DOI] [PubMed] [Google Scholar]
- 12.Ono Y, Yoshimasu T, Ashikaga R, Inoue M, Shindou H, Fuji K, Araki Y, Nishimura Y. Long-term results of lipiodol-transcatheter arterial embolization with cisplatin or doxorubicin for unresectable hepatocellular carcinoma. Am J Clin Oncol. 2000;23:564–568. doi: 10.1097/00000421-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Kasai K, Ushio A, Sawara K, Miyamoto Y, Kasai Y, Oikawa K, Kuroda H, Takikawa Y, Suzuki K. Transcatheter arterial chemoembolization with a fine-powder formulation of cisplatin for hepatocellular carcinoma. World J Gastroenterol. 2010;16:3437–3444. doi: 10.3748/wjg.v16.i27.3437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Child CG, Turcotte JG. Surgery and portal hypertension. Major Probl Clin Surg. 1964;1:1–85. [PubMed] [Google Scholar]
- 15.Japan LCSGo. The General Rules for the Clinical and Pathological Study of Primary Liver Cancer. 5th ed. Tokyo: Kanehara; 2009. [Google Scholar]
- 16.Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52–60. doi: 10.1055/s-0030-1247132. [DOI] [PubMed] [Google Scholar]
- 17.Shimamura Y, Gunvèn P, Takenaka Y, Shimizu H, Shima Y, Akimoto H, Arima K, Takahashi A, Kitaya T, Matsuyama T. Combined peripheral and central chemoembolization of liver tumors. Experience with lipiodol-doxorubicin and gelatin sponge (L-TAE) Cancer. 1988;61:238–242. doi: 10.1002/1097-0142(19880115)61:2<238::aid-cncr2820610206>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 18.Takayasu K, Suzuki M, Uesaka K, Muramatsu Y, Moriyama N, Yoshida T, Yoshino M, Okazaki N, Hasegawa H. Hepatic artery embolization for inoperable hepatocellular carcinoma; prognosis and risk factors. Cancer Chemother Pharmacol. 1989;23 Suppl:S123–S125. doi: 10.1007/BF00647257. [DOI] [PubMed] [Google Scholar]
- 19.Takahashi K, Ebihara K, Honda Y, Nishikawa K, Kita M, Oomura M, Shibasaki C. [Antitumor activity of cis-dichlorodiammineplatinum(II) and its effect on cell cycle progression] Gan To Kagaku Ryoho. 1982;9:624–631. [PubMed] [Google Scholar]
- 20.Nakakuma K, Tashiro S, Hiraoka T, Uemura K, Konno T, Miyauchi Y, Yokoyama I. Studies on anticancer treatment with an oily anticancer drug injected into the ligated feeding hepatic artery for liver cancer. Cancer. 1983;52:2193–2200. doi: 10.1002/1097-0142(19831215)52:12<2193::aid-cncr2820521203>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 21.Morimoto K, Sakaguchi H, Tanaka T, Yamamoto K, Anai H, Hayashi T, Satake M, Kichikawa K. Transarterial chemoembolization using cisplatin powder in a rabbit model of liver cancer. Cardiovasc Intervent Radiol. 2008;31:981–985. doi: 10.1007/s00270-008-9367-8. [DOI] [PubMed] [Google Scholar]
- 22.Doroshow JH, Locker GY, Myers CE. Experimental animal models of adriamycin cardiotoxicity. Cancer Treat Rep. 1979;63:855–860. [PubMed] [Google Scholar]
- 23.Itsubo M, Ishikawa T, Toda G, Tanaka M. Immunohistochemical study of expression and cellular localization of the multidrug resistance gene product P-glycoprotein in primary liver carcinoma. Cancer. 1994;73:298–303. doi: 10.1002/1097-0142(19940115)73:2<298::aid-cncr2820730211>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 24.Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Prognostic factors for survival in patients with unresectable hepatocellular carcinoma undergoing chemoembolization with doxorubicin drug-eluting beads: a preliminary study. HPB (Oxford) 2010;12:174–180. doi: 10.1111/j.1477-2574.2009.00138.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dhanasekaran R, Kooby DA, Staley CA, Kauh JS, Khanna V, Kim HS. Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC) J Surg Oncol. 2010;101:476–480. doi: 10.1002/jso.21522. [DOI] [PubMed] [Google Scholar]
- 26.Martin RC, Rustein L, Pérez Enguix D, Palmero J, Carvalheiro V, Urbano J, Valdata A, Kralj I, Bosnjakovic P, Tatum C. Hepatic arterial infusion of doxorubicin-loaded microsphere for treatment of hepatocellular cancer: a multi-institutional registry. J Am Coll Surg. 2011;213:493–500. doi: 10.1016/j.jamcollsurg.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 27.Seki A, Hori S, Kobayashi K, Narumiya S. Transcatheter arterial chemoembolization with epirubicin-loaded superabsorbent polymer microspheres for 135 hepatocellular carcinoma patients: single-center experience. Cardiovasc Intervent Radiol. 2011;34:557–565. doi: 10.1007/s00270-010-9975-y. [DOI] [PubMed] [Google Scholar]
- 28.Osuga K, Hori S, Hiraishi K, Sugiura T, Hata Y, Higashihara H, Maeda N, Tomoda K, Nakamura H. Bland embolization of hepatocellular carcinoma using superabsorbent polymer microspheres. Cardiovasc Intervent Radiol. 2008;31:1108–1116. doi: 10.1007/s00270-008-9369-6. [DOI] [PubMed] [Google Scholar]
- 29.Scartozzi M, Baroni GS, Faloppi L, Paolo MD, Pierantoni C, Candelari R, Berardi R, Antognoli S, Mincarelli C, Risaliti A, et al. Trans-arterial chemo-embolization (TACE), with either lipiodol (traditional TACE) or drug-eluting microspheres (precision TACE, pTACE) in the treatment of hepatocellular carcinoma: efficacy and safety results from a large mono-institutional analysis. J Exp Clin Cancer Res. 2010;29:164. doi: 10.1186/1756-9966-29-164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stampfl S, Stampfl U, Rehnitz C, Schnabel P, Satzl S, Christoph P, Henn C, Thomas F, Richter GM. Experimental evaluation of early and long-term effects of microparticle embolization in two different mini-pig models. Part II: liver. Cardiovasc Intervent Radiol. 2007;30:462–468. doi: 10.1007/s00270-005-0350-3. [DOI] [PubMed] [Google Scholar]
- 31.Lladó L, Virgili J, Figueras J, Valls C, Dominguez J, Rafecas A, Torras J, Fabregat J, Guardiola J, Jaurrieta E. A prognostic index of the survival of patients with unresectable hepatocellular carcinoma after transcatheter arterial chemoembolization. Cancer. 2000;88:50–57. doi: 10.1002/(sici)1097-0142(20000101)88:1<50::aid-cncr8>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 32.Luo J, Guo RP, Lai EC, Zhang YJ, Lau WY, Chen MS, Shi M. Transarterial chemoembolization for unresectable hepatocellular carcinoma with portal vein tumor thrombosis: a prospective comparative study. Ann Surg Oncol. 2011;18:413–420. doi: 10.1245/s10434-010-1321-8. [DOI] [PubMed] [Google Scholar]
- 33.Kasai K, Ushio A, Kasai Y, Sawara K, Miyamoto Y, Oikawa K, Kuroda H, Takikawa Y, Suzuki K. Combination therapy of intra-arterial 5-fluorouracil and systemic pegylated interferon α-2b for advanced hepatocellular carcinoma. Int J Clin Oncol. 2011;16:221–229. doi: 10.1007/s10147-010-0151-9. [DOI] [PubMed] [Google Scholar]
- 34.Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M, et al. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461–469. doi: 10.1053/j.gastro.2006.05.021. [DOI] [PubMed] [Google Scholar]


