Abstract
Measurement of exhaled breath condensate (EBC) biomarkers offers a noninvasive means to assess airway disease, but the ability of EBC biomarkers to track longitudinal changes in disease severity remains unproven. EBC was collected from pediatric patients with cystic fibrosis (CF) during regular clinic visits over 1 yr. EBC biomarkers urea, adenosine (Ado), and phenylalanine (Phe) were measured by mass spectrometry, and biomarker ratios were used to control for variable dilution of airway secretions. EBC biomarker ratios were assessed relative to lung function in longitudinal, multivariate models and compared with sputum inflammatory markers and quality of life assessment (CFQ-R). EBC was successfully analyzed from 51 subjects during 184 visits (3.6 ± 0.9 visits per subject). EBC Ado/urea ratio was reproducible in duplicate samples (r = 0.62, P < 0.01, n = 20) and correlated with sputum neutrophil elastase (β = 2.5, P < 0.05). EBC Ado/urea correlated with the percentage predicted of forced expiratory volume in 1 s in longitudinal, multivariate models (β = −2.9, P < 0.01); EBC Ado/Phe performed similarly (β = −2.1, P < 0.05). In contrast, IL-8 and elastase measured in spontaneously expectorated sputum (n = 57 samples from 25 subjects) and the CFQ-R respiratory scale (n = 90 tests from 47 subjects) were not significantly correlated with lung function. EBC was readily collected in a clinic setting from a wide range of subjects. EBC Ado tracked longitudinal changes in lung function in CF, with results similar to or better than established measures.
Keywords: inflammation, purinergic, quality of life, spirometry, sputum
airway disease is the primary cause of morbidity and mortality in cystic fibrosis (CF) (28, 38), and simple and noninvasive biomarkers of airways pathophysiology are needed to monitor disease progression, identify exacerbations, and evaluate the efficacy of novel therapies (39). However, assessing airway disease biomarkers poses considerable challenges, particularly in children. Although bronchoalveolar lavage is considered the gold standard for airway biomarker assessment in CF (41), risks and efforts associated with bronchoscopy limit its routine use (12). Measurement of biomarkers in sputum is less invasive and has been successfully utilized in CF (32, 41). However, many children do not expectorate sputum, even after induction (21, 35). Assessing airway disease biomarkers is particularly challenging in the youngest patients, yet growing evidence (2, 3, 8, 27, 42) suggests a need to initiate therapies early in life to prevent development and progression of lung disease in CF (29).
Many obstacles to assessing airway disease biomarkers can be overcome through use of exhaled breath condensate (EBC) as an airway sample because EBC can be collected simply and noninvasively even in young children (4, 39). EBC contains microaerosols of airway secretions diluted in a larger volume of condensed water vapor (22), and many airway inflammatory biomarkers can be measured in EBC and are elevated in EBC from subjects with CF (39). However, accurate measurement of nonvolatile EBC biomarkers has been hampered by the fact that the fraction of exhaled microaerosols in EBC is very low and highly variable (11, 13). The technical difficulties of measuring both a biomarker and a dilution marker at the low concentrations found in EBC have limited widespread adoption of the EBC approach.
Our previous studies have demonstrated that mass spectrometry can be utilized to perform quantitative analyses of multiple EBC biomarker metabolites, including the adenyl purines adenosine and adenosine monophosphate (AMP) and the dilution marker urea (16). Adenyl purines are released onto the airway surfaces by airway epithelial and inflammatory cells, where they act as signaling molecules to regulate host defenses (5, 41). Inflammatory processes increase release of purines into the extracellular space (5, 25, 34), and elevated airway purine concentrations have been observed in CF and other diseases (10, 14, 30). Using mass spectrometry, we have demonstrated that EBC purines, when measured as ratios to urea to control for dilution, are elevated in subjects with CF (15), asthma (15), and chronic obstructive pulmonary disease (COPD) (17). In addition, we have observed that changes in EBC AMP or EBC adenosine correlate with treatment-related changes in lung function in children with CF (15), similar to observations made for relationships between EBC adenosine and treatment in asthma (23). These findings suggest that EBC concentrations of AMP and adenosine may serve as biomarkers of lung disease severity in CF and other diseases.
To evaluate the potential of EBC purines as longitudinal airway disease biomarkers in CF, EBC samples were collected from children with CF during regular clinic visits over 1 yr and analyzed using established mass spectrometric methods (15–17). EBC measures were compared with lung function values obtained in all children and assessed relative to sputum inflammatory markers and the quality of life measure (CFQ-R) obtained in a subset of subjects.
MATERIALS AND METHODS
Subjects.
Subjects were patients diagnosed with CF (37) and followed in the Pediatric Pulmonology clinic at the University of North Carolina at Chapel Hill. Entrance criteria included age ≥6 yr and the ability to reliably perform spirometry and complete the CFQ-R assessment. All study samples and measures were obtained during scheduled clinic visits. The study was approved by the UNC institutional review board (IRB no. 07-1293).
Sixty-six subjects were recruited, of whom fifty-one completed the study. Eight subjects were omitted from analysis for inability to reliably complete the CFQ-R or attend scheduled clinic visits, and six subjects were omitted due to loss of EBC samples during one mass spectrometric run (excessive background signal). One subject was omitted with EBC biomarker and dilution marker concentrations below detection limits in all samples.
EBC collection and analysis.
EBC was collected and processed using the RTube (Respiratory Research, Charlottesville, VA) as previously described (15). Briefly, subjects exhaled through the RTube for 7 min chilled with a chiller tube held in a cooler pack (measured temperature −10°C) until immediately before collection. Nose clips were not utilized. EBC analyses were performed using previously described mass spectrometric methods (14, 15). Briefly, 300 μl of EBC plus an internal standard solution of isotopically labeled urea, adenosine, AMP, and amino acids was lyophilized to dryness overnight, resuspended in 1:20 volume HPLC grade water, separated by liquid chromatography on an Atlantis T3 C18, 1.8 μm column (Waters, Milford, MA) (16), and then analyzed with TSQ-Quantum Ultra Triple Quadrupole Mass Spectrometer (ThermoScientific, San Jose, CA). Urea, adenosine, AMP, and phenylalanine plus the isotopically labeled internal standard of each compound were assessed using selected reaction monitoring of the ions generated during transition from parent to product ion in tandem mass spectrometry as previously described (15–17).
A total of 242 EBC samples (including duplicates) was analyzed, excluding one mass spectrometric run of 40 samples contaminated by an unexpectedly large background signal for adenosine. AMP was detected in <50% of samples and was not utilized for subsequent analysis. Seventeen EBC samples were excluded as outliers: ten in which two or more biomarkers could not be detected, and seven with two or more biomarker concentrations greater than four standard deviations from the mean, suggesting significant contamination with oral secretions, for an overall success rate of 93%. When available, duplicate values were averaged, including replicate same day samples (n = 20).
Lung function.
Spirometry data were obtained using a Collins Survey III spirometer (Warren E. Collins, Braintree, MA), with percentage of predicted values calculated using published normative data (20). Of the 184 visits in the final analysis, spirometric values were available from the same day for all but five visits. For those visits, spirometric values from the closest visit (≤8 days) were utilized.
Sputum collection and analysis.
Sputum was collected from subjects who could spontaneously expectorate. Samples were processed within 1 h by manually extracting mucus plugs from saliva, vortexing them into 20 ml Hanks Buffered Salt Solution per gram of sputum, and then filtering the mixture through 50-micron nylon mesh. The filtrate was centrifuged for 10 min at 500 g and the supernatant aliquoted and frozen at −80°C. IL-8 concentrations in sputum supernatant were assessed using a commercial kit (Abcam, Cambridge, MA); neutrophil elastase concentrations were determined using the method of Rennard (18).
Quality of life assessment.
Quality of life was assessed using the validated CFQ-R measure (36), which was administered before any clinical assessment. The CFQ-R respiratory scale was utilized for all statistical comparisons. Planned collection of CFQ-R data from all visits was not achieved primarily as the result of 1) subjects arriving in clinic with insufficient time to complete the assessment before the scheduled visit, or 2) vital sign and/or spirometry assessments performed before arrival of study personnel to administer the CFQ-R, precluding valid measures.
Statistical analyses.
Generalized estimating equations were utilized to examine relationships between predictor and outcome variables in both univariate and multivariate models. Correlations are reported as standardized β-values (B divided by standard error) to facilitate comparisons between predictors. Sputum elastase and IL-8 were log transformed before analyses, consistent with previous studies (32). In figures, EBC and sputum values are plotted on log scales to facilitate clarity.
RESULTS
Measurement of EBC biomarkers.
EBC biomarkers were successfully measured using a previously described mass spectrometric technique (15–17) in samples collected from 51 children with CF during 184 clinic visits, with an average of 3.6 ± 0.9 visits per subject (range 2–5) over 11.7 ± 3.7 mo (Fig. 1A). The subject population included a wide range of ages (7.0 to 19.7 yr) and severity of lung disease [31 to 145 percent of predicted forced expiratory volume in 1 s (FEV1)]. Demographic characteristics of the study population are listed in Table 1. Paired sputum samples from spontaneously expectorating subjects were collected during 57 visits from 25 subjects. In addition, CFQ-R quality of life assessments were completed during 90 visits by 47 subjects.
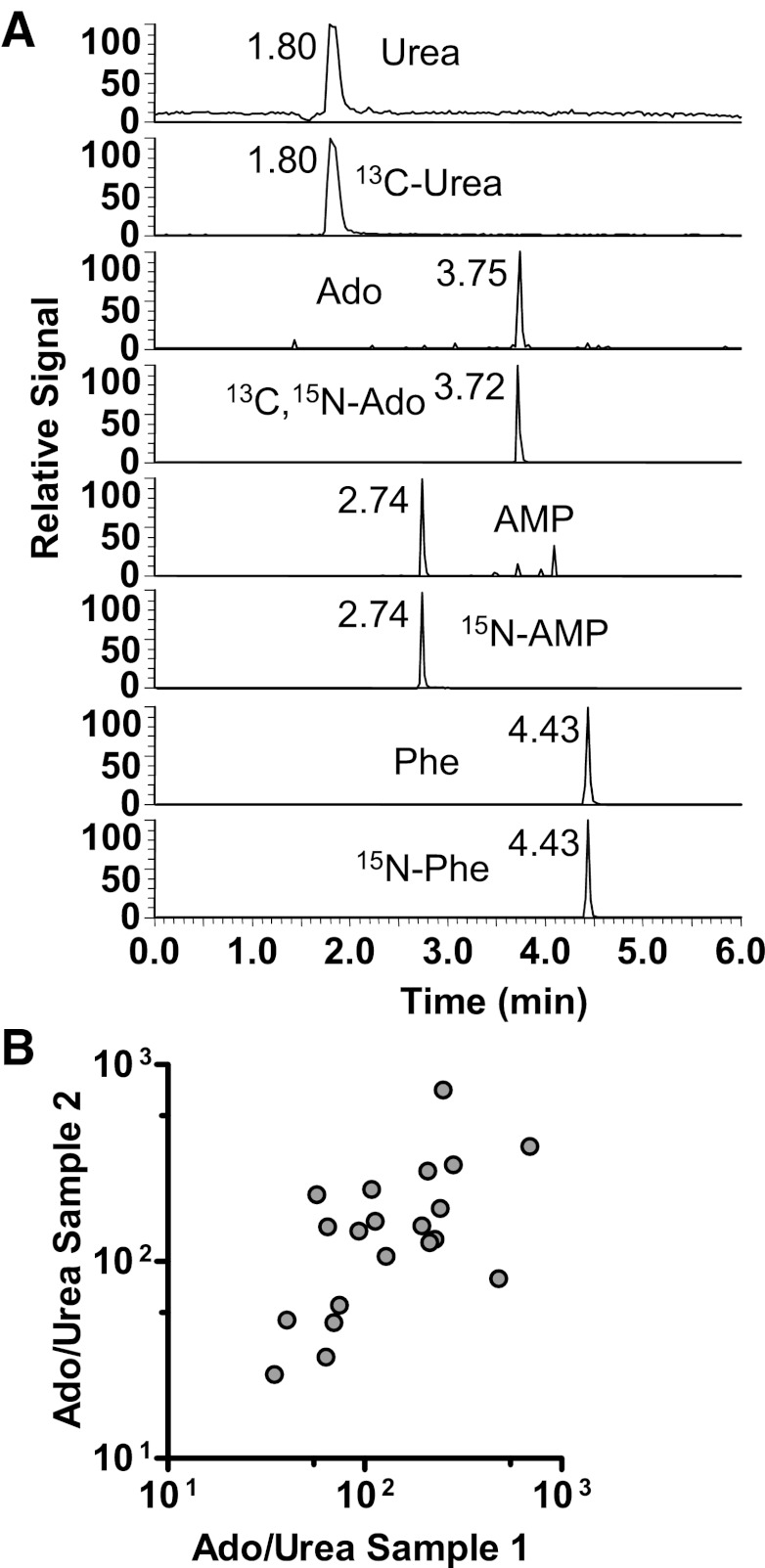
Fig. 1.
A: mass spectrometric chromatogram demonstrating detection of urea, adenosine (Ado), adenosine monophosphate (AMP), and phenylalanine (Phe), as well as stable isotopically labeled (13C- and/or 15N-labeled) internal standards within an exhaled breath condensate (EBC) sample. B: EBC Ado/Urea was reproducible in replicate samples obtained on the same day (r = 0.62, P < 0.01, n = 20).
Table 1.
Study population demographics (n = 51)
| Demographic | Study Values |
|---|---|
| Age, yr (range) | 13.8 ± 3.5 (7.0–19.7) |
| Sex, male (%) | 25 (49) |
| Race, Caucasian (%) | 48 (94) |
| Percentage of Predicted FEV1 (range) | 78 ± 21 (31–145) |
| Any Pseudomonas (%) | 35 (69) |
Applicable values are presented as means ± SE. Any Pseudomonas = Pseudomonas species recovered from any respiratory culture within 6 mo of a visit. FEV, forced expiratory volume in 1 s.
EBC biomarker concentrations were generally lower than those observed in our previous studies (15, 17). EBC adenosine and phenylalanine were detected in samples from all 184 visits and urea in samples from 166 visits. In contrast, EBC AMP was detected in <50% of samples and was not analyzed further. To control for variable dilution of airway secretions in EBC (11, 13), we analyzed the biomarker adenosine as a ratio to urea, an established EBC dilution marker (11, 15). To assess the reliability of this measure, replicate EBC samples obtained 1 h apart during the same clinic visit in 20 subjects were analyzed. Within these paired samples, we observed a statistically significant correlation between the EBC Ado/Urea ratios in the first vs. the second sample, where Ado represents adenosine when referring to significance and figures (Spearman r = 0.59, P < 0.01, Fig. 1B). When EBC adenosine was assessed without using ratios to urea to control for variable dilution, no significant correlation between replicate samples was observed (Spearman r = 0.17, P = 0.40). Assuming that subjects had normal serum urea concentrations of ∼5 mM, estimated dilution factors based on EBC to serum urea ratios were ∼7,800 (IQR 3,400–17,000), similar to those observed in our previous studies (15, 17).
Univariate analysis of EBC biomarkers.
To assess the relationship of EBC biomarkers to lung function and other clinical parameters, we utilized models based on generalized estimating equations that allowed us to account for differences in baseline values as well as relationships over time. EBC Ado/Urea correlated with percentage of predicted FEV1, a well-accepted outcome measure (32), in univariate analyses (β = −2.1, P < 0.05). Similar results were obtained if we utilized the EBC Ado/Phe ratio as an alternative means to control for variable dilution, where Phe stands for phenylalanine when referring to statistics and figures (β = −3.2, P < 0.01), suggesting that the results were more likely related to adenosine than to urea. Neither sputum neutrophil elastase nor sputum IL-8 was significantly correlated with the percentage of predicted FEV1 in univariate analyses (elastase β = −1.7, P = 0.096; IL-8 β = 0.51, P = 0.609), and neither was the CFQ-R Respiratory Scale (β = 1.4, P = 0.167).
Interestingly, we did observe a correlation between EBC Ado/Urea and sputum neutrophil elastase (β = 2.2, P < 0.05, Fig. 2A) with a trend toward correlation for sputum IL-8 (β = 1.4, P = 0.163, Fig. 2B) within the subsets who had both EBC and sputum values. Similarly, EBC Ado/Urea correlated with the CFQ-R Respiratory Scale (β = 3.0, P < 0.01, Fig. 2C).
Fig. 2.
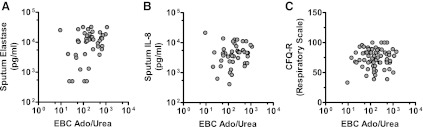
EBC Ado/Urea was compared with sputum neutrophil elastase (A), sputum IL-8 (B), and quality of life assessment (CFQ-R) Respiratory Scale (C) using univariate generalized estimating equation models. EBC Ado/Urea correlated with sputum elastase (β = 2.2, P < 0.05) with a trend toward correlation with sputum IL-8 (β = 1.4, P = 0.163). EBC Ado/Urea also correlated with the CFQ-R Respiratory Scale (β = 3.0, P < 0.01).
Multivariate analyses of EBC biomarkers.
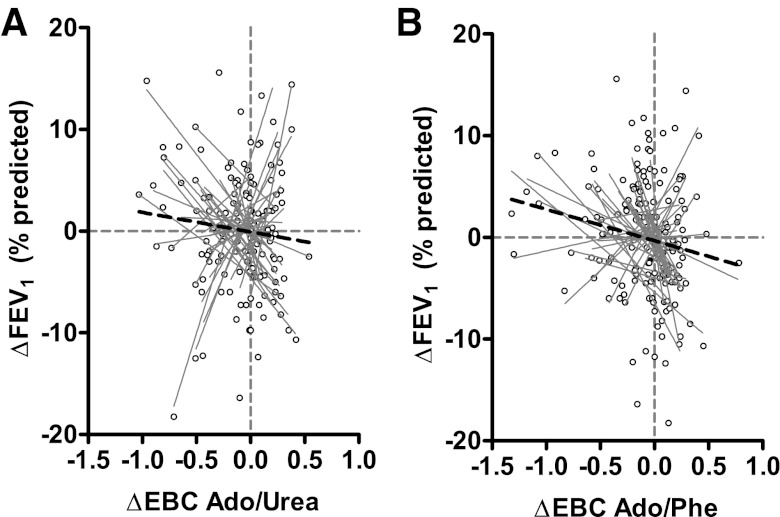
To more fully assess longitudinal relationships between EBC Ado/Urea and lung function, we utilized multivariate models that included age, sex, and presence of Pseudomonas on airway culture within the past 6 mo as potential confounders (9). In the resulting model, EBC Ado/Urea remained significantly negatively correlated with the percentage of predicted FEV1 (β = −2.9, P < 0.01), as were age and Pseudomonas status (Table 2). As before, EBC Ado/Phe was also significantly correlated with the percentage of predicted FEV1 (β = −2.1, P < 0.05) in the multivariate model. These relationships remained significant when analyzed as changes from average values, with significant negative correlations between ΔEBC Ado/Urea and ΔFEV1 (β = −2.3, P < 0.03, Fig. 3A), as well as ΔEBC Ado/Phe and ΔFEV1 (β = −2.4, P < 0.02, Fig. 3B) after controlling for potential confounders.
Table 2.
Multivariate longitudinal analysis of EBC Ado/Urea and other variables vs. percentage of predicted FEV1
| 95% Confidence Interval |
||||||
|---|---|---|---|---|---|---|
| Parameter | B | Standard Error | Lower | Upper | Standardized β | P Value |
| EBC Ado/Urea | −0.006 | 0.0021 | −0.010 | −0.001 | −2.86 | 0.008 |
| Age | −3.11 | 0.73 | −4.55 | −1.67 | −4.24 | <0.001 |
| Sex, female | −6.01 | 3.73 | −13.3 | 1.30 | −1.61 | 0.107 |
| Any Pseudomonas | −3.64 | 1.42 | 0.85 | 6.42 | −2.56 | 0.010 |
EBC, exhaled breath condensate; Ado, adenosine.
Fig. 3.
A: Changes in the percentage of predicted forced expiratory volume in 1 s (FEV1) are plotted against changes in EBC Ado/Urea, with linear regression lines shown for each individual subjects (gray lines) and the dataset as a whole (black dashed line). B: similar representation of changes in the percentage of predicted FEV1 vs. changes in EBC Ado/Phe.
To examine the performance of EBC relative to sputum biomarkers, similar multivariate models were created using sputum IL-8 and neutrophil elastase. In these models, neither sputum IL-8 nor sputum elastase was a significant predictor of percent predicted FEV1 (P values 0.325 and 0.330, respectively). Similarly, CFQ-R Respiratory Scale was not correlated with the percentage of predicted FEV1 in the multivariate model (P = 0.253).
DISCUSSION
This study highlights many of the strengths of the EBC biomarker approach for assessing lung disease in children with CF. EBC was readily collected in the context of routine clinical care, and EBC could be obtained and biomarkers assessed from a wider range of patients than was feasible for sputum biomarkers in this context. More importantly, the measured EBC biomarkers appeared reliable, and the EBC Ado/Urea ratio correlated with the percentage of predicted FEV1 in longitudinal models.
Overall, our findings suggest that EBC adenosine is a potential longitudinal biomarker of pulmonary disease in CF, consistent with previous data suggesting that adenosine and related purinergic signaling pathways play significant roles in inflammatory airway diseases (33, 43). Interestingly, although elevated airway adenosine concentrations have been reported for diseases such as asthma (10, 15, 23, 31) and COPD (17), we have not previously observed elevated concentrations of adenosine in EBC (15), sputum, or bronchoalveolar lavage fluid (14) in subject with CF relative to controls. These findings suggest that changes in airway adenosine from baseline, rather than an absolute elevation, may be the most important factor underlying the longitudinal relationship to disease severity in CF. Indeed, relationships between changes in EBC adenosine and changes in disease severity during treatment of a pulmonary exacerbation were observed in our previous study of children with CF (15), and similar findings have been described for diseases such as asthma (7).
The relationships between EBC adenosine and lung function in CF are similar to those previously described for the sputum inflammatory markers IL-8 and neutrophil elastase (6, 32), suggesting that these markers may function similarly in CF. Although we did not observe statistically significant relationships between sputum markers and lung function in this study, we suspect that this reflects the relatively small number of subjects in our study regularly able to spontaneously expectorate sputum. Applying sputum induction would likely have improved yield (41), but the additional time and resources necessary for induction would have posed challenges within the clinic setting. In contrast, EBC was readily obtained from subjects with a wide range of ages and disease severity, including young children with very mild disease. These advantages are counterbalanced by the need for specialized methods needed to assess EBC biomarkers and dilution markers, in contrast to assays for sputum IL-8 and neutrophil elastase that are technically straightforward and widely available. However, we have demonstrated that it is feasible to address this limitation by analyzing EBC collected and processed at multiple sites for analysis within a central mass spectrometric laboratory (17).
It is also possible that discrepancies between EBC and sputum biomarkers in our study reflect the fact that each sample type assesses different and potentially complementary aspects of airway pathophysiology. Growing evidence suggests that the airway secretion microdroplets incorporated within EBC are generated in the smallest bronchioles (26), suggesting that EBC biomarkers reflect pathophysiology occurring in small airways. This mechanism also implies that EBC samples a large fraction of airways but may undersample highly diseased airways that are completely obstructed with secretions. In contrast, sputum is derived from larger airways (1), and secretions from the most affected airways are likely overrepresented. Therefore, longitudinal variations in EBC and sputum biomarkers may have different clinical implications, with changes in sputum biomarkers reflecting intensification of an existing locus of inflammation, whereas changes in EBC biomarkers reflect a spread of inflammation. Similarly, EBC biomarkers and quality of life measures such as the CFQ-R likely also assess different aspects of disease, which may explain why these measures differed within this relatively modest study.
One limitation of our study was our inability to reliably detect EBC AMP despite previous success measuring this compound (15–17). The reasons for this difficulty are not known but may reflect the changes in our protocol designed to facilitate collections in the clinic setting. Longer collection times, colder chiller tubes, and availability of more sensitive mass spectrometers may improve sensitivity in future studies. Identification of novel biomarkers that are more readily detected via mass spectrometry may also serve to advance the EBC biomarker approach. Furthermore, it would be valuable to identify biomarkers of oral contamination because the most definitive means presently to avoid samples with oral contamination is to perform a separate analytic procedure, e.g., for salivary amylase. Studies suggest that oral contamination of EBC is generally minor but can be significant in a small set of samples (19, 24). Indeed, we eliminated a small number of samples with very high biomarker concentrations based on suspicion that these were grossly contaminated with saliva although the fact that we did not directly assess amylase remains a limitation to our study. Simultaneous measurement of biomarkers, dilution markers, and oral contamination markers is theoretically feasible using mass spectrometry, but identification of the appropriate markers will require further effort.
In summary, we have demonstrated that EBC adenosine, assessed as an EBC Ado/Urea ratio, correlates with lung function measures over time in children with CF. These findings provide further evidence that purinergic signaling pathways are involved in CF disease pathophysiology and suggest that EBC adenosine can be utilized as a biomarker disease severity in CF.
GRANTS
C. Esther Jr. was supported by the Cystic Fibrosis Foundation CFF ESTHER07A0, NIH/NHLBI 1K23HL089708, and NIH/NIEHS P30ES10126. F.-C. Lin and J. Fine were supported by NIH/NCCR UL1RR025747. R. Boucher was supported by NIH/NHLBI HL34322, HL107168, 1-P01-HL08808-01A1, 2-P30-DK065988, 1-P01-HL110873-01, and 5 P50HL 107168-01.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: C.R.E.J., J.F., and R.C.B. conception and design of research; C.R.E.J. and B.M.O. performed experiments; C.R.E.J., F.-C.L., and J.F. analyzed data; C.R.E.J., F.-C.L., J.F., and R.C.B. interpreted results of experiments; C.R.E.J. prepared figures; C.R.E.J. drafted manuscript; C.R.E.J., B.M.O., F.-C.L., J.F., and R.C.B. edited and revised manuscript; C.R.E.J., B.M.O., F.-C.L., J.F., and R.C.B. approved final version of manuscript.
REFERENCES
- 1. Alexis NE, Hu SC, Zeman K, Alter T, Bennett WD. Induced sputum derives from the central airways: confirmation using a radiolabeled aerosol bolus delivery technique. Am J Respir Crit Care Med 164: 1964–1970, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Armstrong DS, Grimwood K, Carlin JB, Carzino R, Gutièrrez JP, Hull J, Olinsky A, Phelan EM, Robertson CF, Phelan PD. Lower airway inflammation in infants and young children with cystic fibrosis. Am J Respir Crit Care Med 156: 1197–1204, 1997 [DOI] [PubMed] [Google Scholar]
- 3. Armstrong DS, Hook SM, Jamsen KM, Nixon GM, Carzino R, Carlin JB, Robertson CF, Grimwood K. Lower airway inflammation in infants with cystic fibrosis detected by newborn screening. Pediatr Pulmonol 40: 500–510, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bodini A, D'Orazio C, Peroni D, Corradi M, Folesani G, Baraldi E, Assael BM, Boner A, Piacentini GL. Biomarkers of neutrophilic inflammation in exhaled air of cystic fibrosis children with bacterial airway infections. Pediatr Pulmonol 40: 494–499, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Chen Y, Corriden R, Inoue Y, Yip L, Hashiguchi N, Zinkernagel A, Nizet V, Insel PA, Junger WG. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science 314: 1792–1795, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Colombo C, Costantini D, Rocchi A, Cariani L, Garlaschi ML, Tirelli S, Calori G, Copreni E, Conese M. Cytokine levels in sputum of cystic fibrosis patients before and after antibiotic therapy. Pediatr Pulmonol 40: 15–21, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Csoma Z, Huszar E, Vizi E, Vass G, Szabo Z, Herjavecz I, Kollai M, Horvath I. Adenosine level in exhaled breath increases during exercise-induced bronchoconstriction. Eur Respir J 25: 873–878, 2005 [DOI] [PubMed] [Google Scholar]
- 8. Davis SD, Fordham LA, Brody AS, Noah TL, Retsch-Bogart GZ, Qaqish BF, Yankaskas BC, Johnson RC, Leigh MW. Computed tomography reflects lower airway inflammation and tracks changes in early cystic fibrosis. Am J Respir Crit Care Med 175: 943–950, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Demko CA, Byard PJ, Davis PB. Gender differences in cystic fibrosis: Pseudomonas aeruginosa infection. J Clin Epidemiol 48: 1041–1049, 1995 [DOI] [PubMed] [Google Scholar]
- 10. Driver AG, Kukoly CA, Ali S, Mustafa SJ. Adenosine in bronchoalveolar lavage fluid in asthma. Am Rev Respir Dis 148: 91–97, 1993 [DOI] [PubMed] [Google Scholar]
- 11. Effros RM, Biller J, Foss B, Hoagland K, Dunning MB, Castillo D, Bosbous M, Sun F, Shaker R. A simple method for estimating respiratory solute dilution in exhaled breath condensates. Am J Respir Crit Care Med 168: 1500–1505, 2003 [DOI] [PubMed] [Google Scholar]
- 12. Effros RM, Dunning MB, III, Biller J, Shaker R. The promise and perils of exhaled breath condensates. Am J Physiol Lung Cell Mol Physiol 287: L1073–L1080, 2004 [DOI] [PubMed] [Google Scholar]
- 13. Effros RM, Hoagland KW, Bosbous M, Castillo D, Foss B, Dunning M, Gare M, Lin W, Sun F. Dilution of respiratory solutes in exhaled condensates. Am J Respir Crit Care Med 165: 663–669, 2002 [DOI] [PubMed] [Google Scholar]
- 14. Esther CR, Jr, Alexis NE, Clas ML, Lazarowski ER, Donaldson SH, Ribeiro CM, Moore CG, Davis SD, Boucher RC. Extracellular purines are biomarkers of neutrophilic airway inflammation. Eur Respir J 31: 949–956, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Esther CR, Jr, Boysen G, Olsen BM, Collins LB, Ghio AJ, Swenberg JW, Boucher RC. Mass spectrometric analysis of biomarkers and dilution markers in exhaled breath condensate reveals elevated purines in asthma and cystic fibrosis. Am J Physiol Lung Cell Mol Physiol 296: L987–L993, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Esther CR, Jr, Jasin HM, Collins LB, Swenberg JA, Boysen G. A mass spectrometric method to simultaneously measure a biomarker and dilution marker in exhaled breath condensate. Rapid Commun Mass Spectrom 22: 701–705, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Esther CR, Jr, Lazaar AL, Bordonali E, Qaqish B, Boucher RC. Elevated airway purines in chronic obstructive pulmonary disease. Chest 140: 954–960, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fujita J, Nelson NL, Daughton DM, Dobry CA, Spurzem JR, Irino S, Rennard SI. Evaluation of elastase and antielastase balance in patients with chronic bronchitis and pulmonary emphysema. Am Rev Respir Dis 142: 57–62, 1990 [DOI] [PubMed] [Google Scholar]
- 19. Gaber F, Acevedo F, Delin I, Sundblad BM, Palmberg L, Larsson K, Kumlin M, Dahlen SE. Saliva is one likely source of leukotriene B4 in exhaled breath condensate. Eur Respir J 28: 1229–1235, 2006 [DOI] [PubMed] [Google Scholar]
- 20. Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general US population. Am J Respir Crit Care Med 159: 179–187, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Ho SA, Ball R, Morrison LJ, Brownlee KG, Conway SP. Clinical value of obtaining sputum and cough swab samples following inhaled hypertonic saline in children with cystic fibrosis. Pediatr Pulmonol 38: 82–87, 2004 [DOI] [PubMed] [Google Scholar]
- 22. Hunt J. Exhaled breath condensate: an evolving tool for noninvasive evaluation of lung disease. J Allergy Clin Immunol 110: 28–34, 2002 [DOI] [PubMed] [Google Scholar]
- 23. Huszar E, Vass G, Vizi E, Csoma Z, Barat E, Molnar Vilagos G, Herjavecz I, Horvath I. Adenosine in exhaled breath condensate in healthy volunteers and in patients with asthma. Eur Respir J 20: 1393–1398, 2002 [DOI] [PubMed] [Google Scholar]
- 24. Ichikawa T, Matsunaga K, Minakata Y, Yanagisawa S, Ueshima K, Akamatsu K, Hirano T, Nakanishi M, Sugiura H, Yamagata T, Ichinose M. Possible impact of salivary influence on cytokine analysis in exhaled breath condensate. Anal Chem Insights 2: 85–92, 2007 [PMC free article] [PubMed] [Google Scholar]
- 25. Idzko M, Hammad H, van Nimwegen M, Kool M, Willart MAM, Muskens F, Hoogsteden HC, Luttmann W, Ferrari D, Di Virgilio F, Virchow JC, Lambrecht BN. Extracellular ATP triggers and maintains asthmatic airway inflammation by activating dendritic cells. Nat Med 13: 913–919, 2007 [DOI] [PubMed] [Google Scholar]
- 26. Johnson GR, Morawska L. The mechanism of breath aerosol formation. J Aerosol Med Pulm Drug Deliv 22: 229–237, 2009 [DOI] [PubMed] [Google Scholar]
- 27. Khan TZ, Wagener JS, Bost T, Martinez J, Accurso FJ, Riches DW. Early pulmonary inflammation in infants with cystic fibrosis. Am J Respir Crit Care Med 151: 1075–1082, 1995 [DOI] [PubMed] [Google Scholar]
- 28. Larsen GL, Holt PG. The concept of airway inflammation. Am J Respir Crit Care Med 162: S2–S6, 2000 [DOI] [PubMed] [Google Scholar]
- 29. Linnane BM, Hall GL, Nolan G, Brennan S, Stick SM, Sly PD, Robertson CF, Robinson PJ, Franklin PJ, Turner SW, Ranganathan SC, AREST-CF Lung function in infants with cystic fibrosis diagnosed by newborn screening. Am J Respir Crit Care Med 178: 1238–1244, 2008 [DOI] [PubMed] [Google Scholar]
- 30. Lommatzsch M, Cicko S, Muller T, Lucattelli M, Bratke K, Stoll P, Grimm M, Durk T, Zissel G, Ferrari D, Di Virgilio F, Sorichter S, Lungarella G, Virchow JC, Idzko M. Extracellular adenosine triphosphate and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 181: 928–934, 2010 [DOI] [PubMed] [Google Scholar]
- 31. Loughlin CE, Esther CR, Jr, Lazarowski ER, Alexis NE, Peden DB. Neutrophilic inflammation is associated with altered airway hydration in stable asthmatics. Respir Med 104: 29–33, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mayer-Hamblett N, Aitken ML, Accurso FJ, Kronmal RA, Konstan MW, Burns JL, Sagel SD, Ramsey BW. Association between pulmonary function and sputum biomarkers in cystic fibrosis. Am J Respir Crit Care Med 175: 822–828, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Mohsenin A, Blackburn MR. Adenosine signaling in asthma and chronic obstructive pulmonary disease. Curr Opin Pulm Med 12: 54–59, 2006 [DOI] [PubMed] [Google Scholar]
- 34. Okada SF, Nicholas RA, Kreda SM, Lazarowski ER, Boucher RC. Physiological regulation of ATP release at the apical surface of human airway epithelia. J Biol Chem 281: 22992–23002, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ordonez CL, Kartashov AI, Wohl ME. Variability of markers of inflammation and infection in induced sputum in children with cystic fibrosis. J Pediatr 145: 689–692, 2004 [DOI] [PubMed] [Google Scholar]
- 36. Quittner AL, Buu A, Messer MA, Modi AC, Watrous M. Development and validation of The Cystic Fibrosis Questionnaire in the United States: a health-related quality-of-life measure for cystic fibrosis. Chest 128: 2347–2354, 2005 [DOI] [PubMed] [Google Scholar]
- 37. Rosenstein BJ, Cutting GR. The diagnosis of cystic fibrosis: a consensus statement. Cystic Fibrosis Foundation Consensus Panel. J Pediatr 132: 589–595, 1998 [DOI] [PubMed] [Google Scholar]
- 38. Rowe SM, Miller S, Sorscher EJ. Cystic fibrosis. N Engl J Med 352: 1992–2001, 2005 [DOI] [PubMed] [Google Scholar]
- 39. Sagel SD. Noninvasive biomarkers of airway inflammation in cystic fibrosis. Curr Opin Pulm Med 9: 516–521, 2003 [DOI] [PubMed] [Google Scholar]
- 41. Sagel SD, Chmiel JF, Konstan MW. Sputum biomarkers of inflammation in cystic fibrosis lung disease. Proc Am Thorac Soc 4: 406–417, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Sly PD, Brennan S, Gangell C, de Klerk N, Murray C, Mott L, Stick SM, Robinson PJ, Robertson CF, Ranganathan SC, Australian Respiratory Early Surveillance Team for Cystic Fibrosis (AREST-CF) Lung disease at diagnosis in infants with cystic fibrosis detected by newborn screening. Am J Respir Crit Care Med 180: 146–152, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Vass G, Horvath I. Adenosine and adenosine receptors in the pathomechanism and treatment of respiratory diseases. Curr Med Chem 15: 917–922, 2008 [DOI] [PubMed] [Google Scholar]