Abstract
Regional variation in sweating over the human body is widely recognized yet variation in vasomotor responses and mechanisms causing this variation remain unclear. This study aimed to explore the relation between regional sweating rates (RSR) and skin blood flow (SkBF) responses to thermal and pharmacological stimuli in young, healthy subjects. In nine subjects (23 ± 3 yr), intradermal microdialysis (MD) probes were inserted into the ventral forearm, abdomen, thigh, and lower back and perfused with lactated Ringer solution. RSR over each MD membrane were measured using ventilated capsules with a laser Doppler probe housed in each capsule for measurement of red cell flux (laser Doppler flux, LDF) as an index of SkBF. Subjects completed a whole body heating protocol to 1°C rise in oral temperature and an acetylcholine dose response (ACh 1 × 10−7-0.1 M; mean skin temperature 34°C). Maximal LDF were obtained at the end of both protocols (50 mM sodium nitroprusside).During heating RSR varied among sites (P < 0.0001) and was greater on the back versus other sites (P < 0.05), but LDF was similar between sites (P = 0.343). RSR and SkBF showed a strong relation during initial (arm: r = 0.77 ± 0.09, thigh: r = 0.81 ± 0.08, abdomen: r = 0.89 ± 0.04, back: r = 0.86 ± 0.04) but not latter stages of heating. No differences in RSR (P = 0.160) or SkBF (LDF, P = 0.841) were observed between sites during ACh perfusion. Taken together, these data suggest that increases in SkBF are necessary to initiate and increase sweating, but further rises in RSR are not fully dependent on SkBF in a dose-response manner. Furthermore, RSR cannot be explained by cholinergic sensitivity or variation in SkBF.
Keywords: sweating, skin blood flow, regional, laser Doppler flowmetry, microdialysis
in young, healthy individuals, challenges to thermoregulatory homeostasis elicit significant increases in skin blood flow (SkBF) and eccrine sweating. Cutaneous vasodilation provides the heat required to evaporate sweat from the skin surface and is partially reflected by skin temperature. Regional variation in sweating over the body is well documented (3, 5, 6, 19, 23–25, 28, 38), however, cannot be explained by regional eccrine sweat gland density (28, 37) or regional skin temperature (Tsk) (3). This discrepancy may result from variations in individual sweat gland output and/or sudomotor sensitivity (23–25).Variation in SkBF between regions is present both at a basal level and during pharmacologically induced maximal dilation (7). Arterial occlusion of a limb causes a decrease in sweating rate during either whole body heating or administration of sudorific agents (2, 4, 30). More recently, manipulation of local skin temperature has been shown to alter local sweating rate by independent effects of SkBF and local skin temperature, supporting a relation between the two responses (40).
Thermoregulatory reflex cutaneous vasodilation is mediated via the corelease of ACh, acting to stimulate sweating by stimulating sweat gland muscarinic receptors, and an unknown cotransmitter from sympathetic cholinergic nerves that mediates cutaneous vasodilation (15). Putative neurotransmitters include vasoactive intestinal peptide (1), substance P (41), and calcitonin gene-related peptide (31), acting in part through nitric oxide-dependent cGMP mechanisms (16) to mediate reflex cutaneous vasodilation. A functional relationship between cutaneous vasodilation and eccrine sweating has long been suggested (21), with observations of vasodilation and augmented sweating upon corelease of vasoactive intestinal peptide (VIP) and ACh from cholinergic secretomotor nerves (42). However, a complete understanding of the mechanisms of reflex cutaneous vasodilation and its relationship with eccrine sweating remain unclear.
The present study was conducted largely as a follow up from sweat mapping data thath reported highly detailed sweating patterns over large surface areas of the human body (34, 35). The significant variation in regional sweating rates (RSR) over the body could not be explained by variation in skin temperature. In consideration of the reported effects of local SkBF (40), skin temperature, and arterial occlusion on local sweating rates, the present study aimed to explore the relation between regional sweating and SkBF responses to both thermal and pharmacological stimuli in young, healthy subjects. We hypothesized that regions of highest sweating rate would coincide with regions of highest SkBF during whole body heating. We further hypothesized that body regions with the greatest sweat rates and skin blood flows would show the greatest cholinergic sensitivity.
METHODS
Subjects
Experimental protocols were approved by the Institutional Review Board at The Pennsylvania State University and conformed to the guidelines set forth by the Declaration of Helsinki. Verbal and written consent were voluntarily obtained from all subjects before participation. Nine young (23 ± 2 yr, 5 men and 4 women) subjects participated in the study. Subjects underwent a complete medical screening, including blood chemistry, complete lipid, renal, and liver enzyme profile evaluation (Quest Diagnostics Nichol Institute, Chantilly, VA), resting electrocardiogram, and physical examination. All subjects were screened for the presence of cardiovascular, dermatological, and neurological disease. Subjects were nonsmokers, nondiabetic, normally active (neither sedentary nor highly exercise trained), and were not taking medications including antihypertensives or other drugs that may affect the cardiovascular system, including antioxidants, hormone replacement therapy, or oral contraceptives. All young women were normally menstruating and were tested during the early follicular phase (days 1–7) of their menstrual cycle. All subjects completed a graded exercise test (Bruce protocol) on a semirecumbent bike to determine V̇o2 peak in a room maintained at 23°C, 40% relative humidity. Subjects underwent a dual X-ray absorptiometry (DEXA) scan for assessment of regional body composition. Subject characteristics are presented in Table 1.
Table 1.
Participant characteristics
| Subjects (Male, Female) | Age, yr | BMI, kg/m2 | AD, m2 | Total Body fat, % | Systolic BP, mmHg | Diastolic BP, mmHg | MAP, mmHg | HbA1c, mmol/mol | HDL, mg/dl | LDL, mg·dl | V̇o2max, ml·kg−1·min−1 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (5, 4) | 23 ± 3 | 25 ± 4 | 1.95 ± 0 | 27 ± 6 | 108 ± 10 | 69 ± 8 | 82 ± 8 | 35 ± 2 | 66 ± 21 | 81 ± 27 | 35 ± 9 |
Values are means ± SD.
BMI. body mass index; AD, DuBois body surface area; BP, blood pressure; MAP, mean arterial pressure; HbA1c, plasma glucose concentration; HDL, high-density lipoprotein; LDL, low-density lipoprotein.
Instrumentation
Protocols were performed in a thermoneutral laboratory with the subjects in a supine position. Four intradermal microdialysis (MD) fibers (MD 2000, Bioanalytical Systems) (10 mm, 20-kDa cutoff membrane) were inserted as previously described (8), into the ventral forearm, abdomen, thigh, and lower back. Foam was placed underneath the subject to prevent pressure being placed on the equipment and measurement site at the lower back. Fibers were inserted following temporary anesthesia of each site using ethyl chloride spray (Gebauer, Cleveland, OH) (11). Sites were selected based on high and low sweat regions from whole body sweat maps and using similar anatomical measurements to determine MD fiber placement (34, 35). Briefly, the abdomen site was identified on the left side of the body as midway between the anterior superior iliac spine and the navel; the thigh site was calculated as 0.6 times the upper leg length (distance from anterior superior iliac spine to the proximal edge of the patella); the lower back site was identified on the left side of the body at the height of the anterior superior iliac spine, 5–10 cm lateral to the vertebrae. Initial insertion trauma was allowed to subside for 60–90 min, during which time lactated Ringer solution was perfused through all fibers at a rate of 2 μl/min (Bioanylitical Systems Bee hive and Baby Bee microinfusion pumps, West Lafayette, IN). Arterial blood pressure was measured via brachial auscultation every 5 min following hyperemia and throughout the protocol. Mean arterial pressure (MAP) was calculated as [1/3 systolic blood pressure + (2/3 diastolic blood pressure)]. SkBF was expressed as laser-Doppler flux (LDF) as an indication of absolute flow.
Whole body heating was achieved using a water-perfused suit that covered the entire body with exception to the head, hands, feet, and a distal portion of the experimental arm. Incisions were made in the water-perfused suit at each MD fiber site except the arm to prevent the sweat capsule and LDF probes being covered and to maintain contact between the skin and suit surrounding each site. Local skin temperature (Tsk) was continuously measured at six sites using copper-constantan thermocouples (calf, thigh, abdomen, chest, upper arm, and upper back) and an unweighted mean skin temperature (Tsk mean) was calculated. Oral temperature (Tor) was measured as an index of body core temperature (Tcore) using a thermistor placed in the sublingual sulcus throughout baseline and whole body heating. All thermocouples and the sublingual thermistor were calibrated in a heated water bath against a glass, mercury thermometer before each experiment. The sublingual probe was inserted and positioned at the site of highest temperature at the sublingual sulcus, before being taped in place. Subjects were instructed to maintain a closed mouth for the duration of the baseline and heating protocol, during which time Tor was closely monitored. Mean body temperature (Tbody) was calculated as Tbody = (0.9 × Tor) + (0.1 × mean Tsk). An index of SkBF was measured using laser-Doppler flowmetry probes (MoorLAB, Moor Instruments) placed over each MD site measuring cutaneous red blood cell flux, which was recorded continuously during the experiment.
Total body sweating rate was determined from the change in body mass during whole body heating using a scale accurate to ± 10 g (SECA model 770 1321143). Values were not corrected for metabolic and respiratory losses. Local sweating rates were measured using ventilated capsules with compressed medical grade nitrogen used as the perfusion gas (20, 32) specifically manufactured so that sweating rate and LDF could be measured simultaneously. Sweat capsules (4.46 cm2 surface area) were positioned over the center of the membrane portion of each MD fiber. The temperature and humidity of the air flowing out of the capsules were measured using capacitance hygrometers that were calibrated by the manufacturer and regularly calibrated using reference solutions of known temperature and humidity (model HMT330, Vaisala, Helsinki, Finland). Sweating rate was calculated based on the change in relative humidity of the air as it passed through the capsule (Δrh), the airflow rate (AF), the density of saturated steam at the given temperature (D), and the capsules surface area (SA), using the following equation: SR = [AF × (Δrh/100) × D]/SA.
Experimental Protocols
Acetylcholine dose response.
After MD fiber insertion and resolution of insertion trauma, baseline data were collected for 20 min. Immediately after baseline measurement, Tsk was clamped at 34°C during perfusion of seven ascending concentrations of ACh at a rate of 2 μl/min for 10 min each: 1 × 10−7 to 1 × 10−1 M dissolved in Ringer solution in 10-fold increments. This amount of time allowed for a plateau in SkBF at each concentration of ACh. After completion of the ACh dose response, 50 mM sodium nitroprusside (SNP) was perfused through all sites at a rate of 4 μl/min to induce maximal cutaneous vasodilation (LDF) (10, 14).
Whole body heating.
Upon arrival to the laboratory subjects provided a urine sample for assessment of hydration status via urine specific gravity and osmolality, and body mass was recorded. Protocols were performed on the same body regions as the ACh dose response and separated by at least 1 wk to allow skin to fully heal between trials. After MD fiber insertion and resolution of insertion trauma, baseline data were collected for 20 min with mean Tsk maintained at thermoneutral using a water-perfused suit (34°C). After collection of baseline data, 50°C water was perfused through the suit to elevate Tor by 1°C, after which Tor was clamped for 5 min by reducing the temperature of the water perfusing the suit. After 10 min of steady-state LDF values, water perfusing the suit was returned to 34°C and 50 mM SNP (Nitropress; Abott Laboratories, Chicago, IL) was perfused through each MD site at a rate of 4 μl/min to obtain maximal cutaneous vasodilation (LDF) values (9, 26).
Data and Statistical Analysis
Data were digitalized at 40 Hz, recorded, and stored for offline analysis using Windaq software and Dataq data acquisition system (Windaq; Dataq Instruments, Akron, OH). Baseline values were determined as the last 5 min before commencing whole body heating or ACh dose response. Local sweating and SkBF data are presented as mean values over 60 s at each 0.1°C rise in oral temperature throughout the heating protocol or for each ACh dose. The relation between SkBF and RSR was considered in two stages, defined as early (BL to 0.5°CΔTor) and late (0.6 to 1.0°CΔTor) phases of whole body heating. Dose-response curves for LDF and RSR at each site during the ACh dose-response protocol were constructed using a nonlinear fitting technique, with a Hill slope of 1, from which the effective concentration eliciting 50% of the maximal response (EC50) was calculated (Sigma Plot 11.0) (39). Maximal SkBF values were averaged over a stable 60-s plateau during perfusion of 50 mM SNP. Sweating and cutaneous vasodilation thresholds for the heating protocol were determined by two independent investigators who were blinded during analysis. LDF and sweating data for each protocol were analyzed using a two-way, mixed model, repeated measures ANOVA [site × heating phase (ΔTor) or ACh dose; proc mix SAS 9.2]. Specific planned comparisons were performed when appropriate to determine where differences between groups and sites occurred with appropriate Bonferroni correction. The level of significance was set at α = 0.05. Values are presented as means ± SE.
RESULTS
Acetylcholine Dose Response
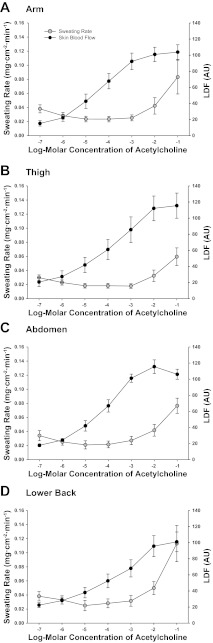
No differences in SkBF (LDF, P = 0.841) or sweating rate (P = 0.160) were observed between sites during the ACh dose-response protocol (Fig. 1). To examine the relation between absolute SkBF and RSR, LDF values are presented. When calculated as %CVCmax no differences in the pattern of SkBF between sites were observed from that of the LDF data, therefore the normalized data are not presented. Both sweating rate and SkBF increased similarly between sites with ascending doses of ACh (site × phase interaction: RSR, P = 0.418; SkBF, P = 0.574). Vasodilation occurred at a lower ACh concentration compared with sweating at all sites, and no correlation was present between RSR and SkBF within any site. The EC50 of the ACh dose-response curves (Table 2) for SkBF differed between regional sites (P = 0.027), being lower on the thigh and back compared with the arm (P < 0.05), and greater on the thigh versus the abdomen (P < 0.05). EC50 values were not calculated for RSR due to the highest dose of ACh not eliciting a maximal sweating rate. No differences in RSR or SkBF were present between sites during perfusion of SNP (Table 2).
Fig. 1.
Regional sweating rates (mg·min−1·cm−2) and laser-Doppler flux (LDF) during local administration of 10−7 to 10−1 M acetylcholine at ventral forearm (A), thigh (B), abdomen (C), and lower back (D).
Table 2.
Relative thresholds (ΔTor from baseline, °C) for the onset of cutaneous vasodilation and sweating, and maximal vasodilation (LDF) during infusion of 50 Mm SNP at four sites in young subjects
| Relative Thresholds (°C Above Baseline Tor) During Heating Protocol |
Maximal LDF Values (50 mM SNP) |
||||
|---|---|---|---|---|---|
| Site | Vasodilation | Sweating | LDF EC50 Values (Log-Molar Concentration), ACh Dose Response | Heating protocol | ACh dose response |
| Arm (ventral forearm) | 0.2 ± 0.1 | 0.3 ± 0.1 | −4.39 ± 0.31 | 112 ± 10† | 104 ± 8 |
| Abdomen | 0.2 ± 0.1 | 0.2 ± 0.0 | −3.47 ± 0.22§ | 139 ± 9‡ | 106 ± 9 |
| Thigh | 0.2 ± 0.1 | 0.2 ± 0.0* | −4.11 ± 0.21* | 113 ± 15 | 107 ± 6 |
| Lower back | 0.1 ± 0.0 | 0.1 ± 0.0* | −3.38 ± 0.32* | 79 ± 7 | 112 ± 14 |
Values are means ± SE. LDF, laser Doppler flux; SNP, sodium nitroprusside. Values significantly different from arm site,
P < 0.05; significantly different from back site,
P < 0.05,
P < 0.001; significantly different from thigh,
P < 0.05.
Passive Heating
Absolute Tor increased from 36.75 ± 0.09°C to 37.77 ± 0.09°C throughout the heating protocol (P < 0.001), and mean Tsk increased from 35.24 ± 0.26°C to 38.78 ± 0.25°C (P < 0.001). MAP did not change significantly during the heating protocol (MAP: baseline 73 ± 4 mmHg, 1°CΔTor 77 ± 4 mmHg, P = 0.177). The duration of passive heating to 1°CΔTor was 88 ± 9 min and did not correlate strongly with total lean mass, total body fat, body surface area, or V̇o2max. Total sweat loss during the whole body heating protocol was 767 ± 84 g·m−2·h−1. Within-subject analysis indicated strong correlation coefficients for SkBF and RSR at all sites during the early stage (BL-0.5°CΔTor) of heating (arm: r = 0.77 ± 0.09, thigh: r = 0.81 ± 0.08, abdomen: r = 0.89 ± 0.04, lower back: r = 0.86 ± 0.04) and only at the arm during the latter stage (0.6–1°CΔTor) of heating (arm: r = 0.70 ± 0.07, thigh: r = 0.23 ± 0.21, abdomen: r = 0.03 ± 0.22, lower back: r = 0.39 ± 0.19).
Within-subject analysis of the relation between RSR, SkBF, and body composition data (Table 3) at 0.5 and 1.0°CΔTor showed considerable variation, with a strong correlation between regional lean mass versus RSR only (0.5°CΔTor r = 0.79, 1.0°CΔTor r = 0.64).
Table 3.
Regional body composition corresponding to skin blood flow and sweating rate measurement sites
| Regional Body Composition |
|||
|---|---|---|---|
| Site | Fat, kg | Lean, kg | Fat, % |
| Left Arm | 0.99 ± 0.11 | 2.63 ± 0.30 | 27 ± 4 |
| Trunk | 8.71 ± 1.20 | 26.09 ± 1.44 | 24 ± 2 |
| Right Leg | 4.46 ± 0.46 | 8.58 ± 0.60 | 33 ± 3 |
| Total Body | 20.52 ± 2.24 | 52.51 ± 3.36 | 27 ± 2 |
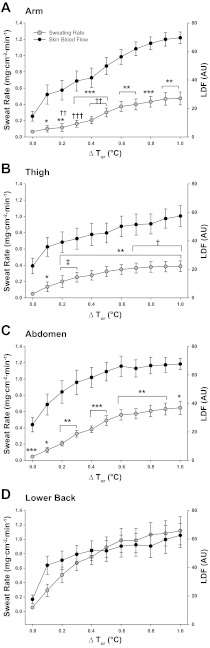
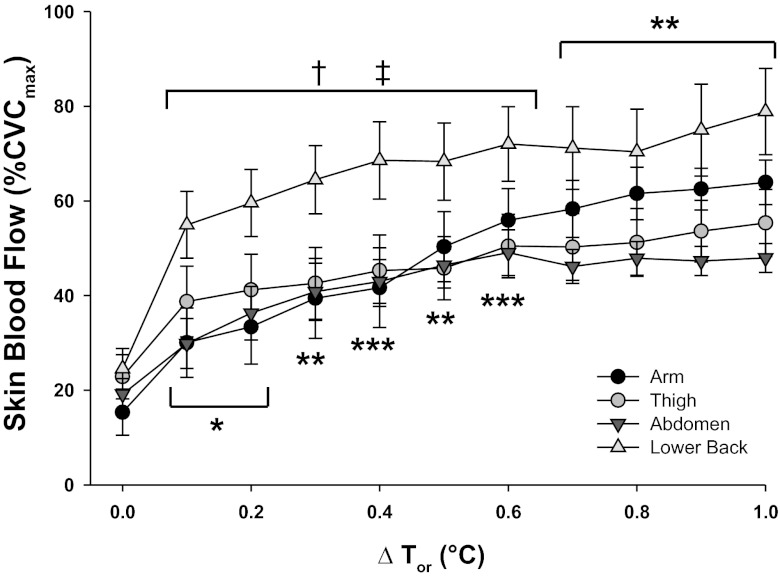
Figure 2 illustrates mean RSR and LDF as an index of SkBF during the heating protocol. Since MAP did not change significantly throughout the protocol, presentation of absolute SkBF data are shown as LDF units for comparison with regional sweating rates. RSR varied between sites (P < 0.0001) throughout the heating protocol, but no differences were present at baseline. RSR was greater on the lower back compared with the other sites throughout heating (all sites P < 0.05). Differences in RSR between the remaining sites varied with increasing Tor, showing greater RSR on 1) the thigh versus arm during the initial stages of heating (0.2–0.3°C ΔTor), 2) abdomen versus arm from 0.2 to 0.5°C ΔTor, and 3) the abdomen versus thigh during the latter stages of heating (0.7–1.0°C ΔTor). The sweating threshold on the arm (0.3°C ΔTor from baseline) was higher than the thigh and the lower back (Table 2); however, no differences in cutaneous vasodilation thresholds were evident between sites. Unlike the delay in sweating response observed upon exposure to whole body heating, cutaneous vasodilation occurred at the onset of the heating stimulus and continued to increase with elevations in Tor (heating phase, P < 0.001). SkBF reached a plateau at 0.6°C ΔTor at the abdomen compared with a steadier rise at the arm, thigh, and lower back, although no differences in LDF were observed between sites (site, P = 0.343). Maximal SkBF elicited from perfusion of 50 mM SNP was significantly lower on the back compared with the abdomen (P < 0.001) and the arm (P < 0.05) (Table 2). When normalized to maximal values (Fig. 3), SkBF showed similar patterns to those observed in the absolute data (Fig. 2), with exception to the lower back. Because of the low maximal SkBF on the back, the normalized SkBF values were significantly higher on the back throughout heating compared with the other sites. No differences in maximal SkBF were present between the whole body heating and ACh dose-response protocols at any site.
Fig. 2.
Regional sweating rates (mg·min−1·cm−2) and LDF during whole body heating to 1°C rise in oral temperature (Tor) at ventral forearm (A), thigh (B), abdomen (C), and lower back (D). Regional sweating values significantly different from lower back, *P < 0.05, **P < 0.01, ***P < 0.001; significantly different from abdomen site, †P < 0.05, ††P < 0.01, †††P < 0.001; significantly different from arm, ‡P < 0.05, following Bonferroni correction to adjust for multiple comparisons.
Fig. 3.
Regional skin blood flow (%CVCmax) during whole body heating to 1°C rise in Tor at ventral forearm (A), thigh (B), abdomen (C), and lower back (D). Values significantly different from thigh, *P < 0.05, **P < 0.01, ***P < 0.001; significantly different from abdomen site, †P < 0.05, ††P < 0.01; significantly different from arm, ‡P < 0.05, following Bonferroni correction to adjust for multiple comparisons.
DISCUSSION
The major new findings of the present study were that 1) RSR differed between body regions in response to thermal but not pharmacological stimuli, 2) elevations in absolute SkBF in response to either passive heating or ACh did not differ between regions, 3) RSR correlated strongly with absolute regional SkBF during the initial but not the latter stages of whole body heating, and 4) no significant correlation was observed between regional sweating rate and SkBF with ascending ACh doses.
The present data identify considerable regional differences in sweating rates at different body sites during passive heating, which are consistent with patterns of distribution identified in models of both passive and exercise-induced hyperthermia (13, 24, 35), using varied measurement techniques. Specifically, RSR were highest on the lower back and lowest on the arm and thigh, supporting observations from recent studies of high versus low sweating rates on central versus peripheral body regions, respectively (23, 24, 34, 35). Despite wide recognition of these regional differences, the physiological underpinnings remain unclear. The most likely explanations underlying this phenomenon include variation in sweat gland output, neural innervation (sympathetic nerve activity), and local glandular sensitivity. The present study examined regional differences in sweat gland cholinergic sensitivity via local delivery of ACh in a dose-dependent manner. No differences in RSR were observed between the four sites at any ACh concentration, suggesting similar cholinergic sensitivities at the regions measured. The RSR elicited by the ACh dose-response protocol are low compared with literature values using similar techniques (18, 33). This may be accounted for by the skin surface area covered by the capsule (4.46 cm−2) compared with the 0.5-cm2 capsules used over MD fibers by other authors (18). When set volumes of distilled water (5–20 μl) are injected into both capsules, the area under the curve is similar (unpublished pilot data). When adjusted for surface area (multiply by 8.9), the RSR values in the present study are comparable with those of other studies using similar sites (12, 18, 32). One potential explanation for regional differences in sweating over the body includes differences in heat-activated sweat gland (HASG) output. Inoue and Shibasaki (12) observed little variation in HASG densities during passive heating of young, healthy males but found local sweat gland output to correspond closely with RSR. The authors observed similar RSR values on the back, thigh, and forearm compared with the present data during a 60-min passive heating protocol, providing support for sweat gland output as a likely explanation for the regional variation in the present sweating data.
One of the primary aims of this study was to examine the relation between RSR and SkBF during both thermal and pharmacological stimuli. In doing so, we were interested in quantified sweating rates versus quantified SkBF, which may be examined using absolute data (Fig. 2). Since MAP did not vary throughout the heating protocol, we have presented the regional SkBF data in arbitrary LDF units, as similarly presented by other authors (12). Contrary to our hypothesis, absolute SkBF (LDF) was similar between sites throughout passive heating. We originally hypothesized that there would be greater cutaneous vasodilation in regions of higher sweating rates. Cutaneous vasodilation is the initial response to elevations in skin and/or core body temperature, acting to increase convective heat loss to the environment. When elevations in core temperature persist, sweat is produced in the secretory coil of eccrine sweat glands from an ultrafiltrate of blood plasma before being secreted onto the skin surface to allow evaporative cooling. It therefore seemed logical that areas of greatest sweating would require a higher blood flow for ultrafiltrate production. Inoue and colleagues (13) similarly observed discrepancies between regions of high RSR and SkBF in young, healthy subjects. Notably, despite similarities in RSR compared with the present study, these authors observed regional differences in SkBF during the heating protocol. They found the greatest SkBF on the thigh, followed by the forearm, and the lowest values on the back and chest. It is unclear why there is such a discrepancy compared with the present data. Inoue and Shibasaki (13) normalized regional SkBF as percent baseline (%LDFbaseline) in conjunction with RSR and noted differences between sites, which may reflect potential differences in baseline values. The present data illustrated small differences in baseline SkBF, although these values were not significant following correction for multiple comparisons. Similar discrepancies with the present data are observed by Inoue and Shibasaki (12) in an earlier study examining age-related differences in sweating and SkBF in which data are presented in absolute terms (LDF). Although SkBF data are typically normalized to maximum or baseline values when examining vascular control, in the present study it is more pertinent to express values of flow when considering the functional relation between sweating and SkBF. The presentation of both LDF units and %CVCmax in the present study negate data normalization as an issue for comparison with the literature. It would therefore seem likely that methodological issues may contribute to such differences. In particular, the use of a water-perfused suit in the present study elicits a greater mean Tsk compared with heating the lower legs in a water bath (12, 13). Modification of local sweating rate and SkBF by alterations in local skin temperature are widely recognized and may help explain some of the discrepancies with the literature (27, 29, 40).
A further consideration is the use of both males and females in the present data, although this was not an aim of the study, and we are underpowered to statistically examine sex differences in RSR and SkBF. When considered separately, females appear to have slightly higher sweating rates at the abdomen and back and slightly delayed sweating thresholds at the arm and leg compared with the males during heating. No differences were present in absolute flow between sexes, and only a slightly higher normalized blood flow (%CVCmax) was observed on the lower back in females. Both SkBF and RSR followed similar regional patterns in both males and females during passive heating and are consistent with regional patterns observed in recent literature examining RSR variation over the body (24, 34, 35). Despite V̇o2max values being similar between sexes, males and females were not matched for factors such as anthropometric characteristics. Making a direct comparison between sexes may therefore be misleading and is beyond the scope of this study.
In the present study, regional sweating and SkBF cholinergic sensitivity were investigated to compliment the physiologically relevant whole body heating data. A number of studies have investigated cutaneous blood flow and sweating responses to local administration of ACh, methacholine (MCh), and in combination with acetylcholinesterase, utilizing the dorsal or ventral forearm as a measurement site (17, 18, 33). Consistent with literature on the forearm (18), in the present study increases in SkBF were elicited at lower concentrations of ACh compared with sweating at all sites. No differences in absolute SkBF were observed between sites; however, differences in EC50 were present between sites. The differences in EC50 suggest regional variation in vascular cholinergic sensitivity. Taken together with the whole body heating data, the earlier cutaneous vasodilation versus sweating thresholds may be explained by differences in cholinergic sensitivity between the two responses. This still fails to explain the regional relation between sweating and SkBF upon exposure to a thermal stimulus. No correlation was present between SkBF and RSR during the ACh dose response, yet the two responses were strongly correlated at all sites during the initial phase of whole body heating. It would therefore appear that increases in SkBF are necessary to increase sweating, but that the two responses diverge with greater elevations in Tor. Taken together, these physiological and pharmacological data may indicate possible differences in control mechanisms, particularly in the latter stages of heating. Cutaneous vasodilation stimulated via cholinergic nerves is partially mediated through release of an unknown cotransmitter (15), and by putative vasodilator peptides, such as VIP, released upon sudomotor nerve activation (36). The release of cotransmitters and other vasoactive substances in addition to ACh may help explain some of the regional variation in vasomotor and sudomotor responses during heat stress; however, additional studies are necessary to explore the precise neurovascular signaling.
Limitations
The pairing of the MD technique with sweat capsules may have directly affected the regional sweat rate being measured. The MD fiber may potentially block a number of sweat glands in the skin under the capsule, causing the RSR to be lower. There is no way of identifying if this has occurred. Data from the present study are consistent with literature values using sweat capsules without MD fibers, and with other techniques, suggesting the effect of the fibers on RSR to be minimal or at least relatively consistent across sites. Second, the depth of MD fiber insertion was kept as consistent as possible. The depth on the abdomen was slightly deeper than the other sites, although we do not believe this significantly affected the outcome of the data. Maximal sweating rates may not have been achieved using a 100 mM ACh concentration during the present dose-response protocol, with regional differences in sensitivity being missed at the higher concentrations. Other authors have obtained maximal sweat rates on the ventral forearm with a 1 M concentration of ACh (18). It does however seem unlikely that such large variation in RSR would emerge between the present data and perfusion of 1 M ACh concentration to explain the observed variation between sites during exposure to thermal stimuli.
The use of sublingual temperature to estimate core temperature thresholds for sweating may not be the most precise measure. However, regional comparisons were made between sites within each subject (repeated measures), therefore, any lag time or limitations in the measurement would similarly affect each site. Furthermore, the threshold values in the present study are consistent with literature values (22, 40). Finally, despite the present study including both male and female data, it was not the aim of this study to examine sex differences and is underpowered to do so. Considerable debate surrounds the presence of sex differences in thermoregulatory function, which although beyond the scope of this paper, certainly warrants future investigation.
Perspectives and Significance
When exposed to a thermoregulatory challenge, vasodilation and eccrine sweating are stimulated to maintain core body temperature. These responses are not uniform over the body and do not show a uniform dysfunction with aging and a number of pathological conditions. Few studies have addressed regional variation in sweating and SkBF, typically using a single body site to represent whole body responses. The results from the present study contribute novel data to improve our understanding of regional variation in vasomotor and sudomotor responses to pharmacological and physiological stimuli. Collectively they suggest that regional variation in sweating and SkBF is unlikely to be attributable to differences in cholinergic sensitivity and is more likely due to the role of a putative cotransmitter and due to variation in output per gland for sweating responses. Understanding these responses and their putative relation in a young, healthy population forms the basis for a greater understanding of mechanisms of dysfunction in disease states. This is particularly pertinent to the increasingly aging population and disease states such as diabetes mellitus. Research has demonstrated significant age-related and pathology-related decrements in thermoregulatory responses during heat stress, for example, during heat waves, increasing susceptibility to heat-related illness and injury.
GRANTS
This study was supported by National Institutes of Health Grant R01-AG007004-22 (to W. L. Kenney) and American College of Sports Medicine Foundation Research Endowment 2012 (C. J. Smith).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: C.J.S., W.L.K., and L.M.A. conception and design of research; C.J.S. performed experiments; C.J.S. analyzed data; C.J.S., W.L.K., and L.M.A. interpreted results of experiments; C.J.S. prepared figures; C.J.S. drafted manuscript; C.J.S., W.L.K., and L.M.A. edited and revised manuscript; C.J.S., W.L.K., and L.M.A. approved final version of manuscript.
ACKNOWLEDGMENTS
The authors thank Jane Pierzga for her IRB application preparation and Sue Slimak for her assistance with data collection.
REFERENCES
- 1.Bennett LA, Johnson JM, Stephens DP, Saad AR, Kellogg DL., Jr Evidence for a role for vasoactive intestinal peptide in active vasodilatation in the cutaneous vasculature of humans. J Physiol 552: 223–232, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins KJ, Sargent F, Weiner JS. The effect of arterial occlusion on sweat-gland responses in the human forearm. J Physiol 148: 615–624, 1959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cotter JD, Patterson MJ, Taylor NA. The topography of eccrine sweating in humans during exercise. Eur J Appl Physiol Occup Physiol 71: 549–554, 1995 [DOI] [PubMed] [Google Scholar]
- 4.Elizondo RS. Local control of eccrine sweat gland function. Fed Proc 32: 1583–1587, 1973 [PubMed] [Google Scholar]
- 5.Havenith G, Fogarty A, Bartlett R, Smith CJ, Ventenat V. Male and female upper body sweat distribution during running measured with technical absorbents. Eur J Appl Physiol 104: 245–255, 2008 [DOI] [PubMed] [Google Scholar]
- 6.Hertzman AB. Individual differences in regional sweating. J Appl Physiol 10: 242–248, 1957 [DOI] [PubMed] [Google Scholar]
- 7.Hertzman AB, Randall WC. Regional differences in the basal and maximal rates of blood flow in the skin. J Appl Physiol 1: 234–241, 1948 [DOI] [PubMed] [Google Scholar]
- 8.Holowatz LA, Kenney WL. Local ascorbate administration augments NO- and non-NO-dependent reflex cutaneous vasodilation in hypertensive humans. Am J Physiol Heart Circ Physiol 293: H1090–H1096, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Holowatz LA, Kenney WL. Up-regulation of arginase activity contributes to attenuated reflex cutaneous vasodilatation in hypertensive humans. J Physiol 581: 863–872, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holowatz LA, Thompson CS, Minson CT, Kenney WL. Mechanisms of acetylcholine-mediated vasodilatation in young and aged human skin. J Physiol 563: 965–973, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Howe HR, III, Heidal K, Choi MD, Kraus RM, Boyle K, Hickner RC. Increased adipose tissue lipolysis after a 2-week high-fat diet in sedentary overweight/obese men. Metabolism 60: 976–981, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inoue Y, Shibasaki M. Regional differences in age-related decrements of the cutaneous vascular and sweating responses to passive heating. Eur J Physiol 74: 84, 1996 [DOI] [PubMed] [Google Scholar]
- 13.Inoue Y, Shibasaki M, Hirata K, Araki T. Relationship between skin blood flow and sweating rate, and age related regional differences. Eur J Appl Physiol 79: 17–23, 1998 [DOI] [PubMed] [Google Scholar]
- 14.Johnson JM, O'Leary DS, Taylor WF, Kosiba W. Effect of local warming on forearm reactive hyperaemia. Clin Physiol 6: 337–346, 1986 [DOI] [PubMed] [Google Scholar]
- 15.Kellogg DL, Jr, Pergola PE, Piest KL, Kosiba WA, Crandall CG, Grossmann M, Johnson JM. Cutaneous active vasodilation in humans is mediated by cholinergic nerve cotransmission. Circ Res 77: 1222–1228, 1995 [DOI] [PubMed] [Google Scholar]
- 16.Kellogg DL, Zhao JL, Wu Y, Johnson JM. Antagonism of soluble guanylyl cyclase attenuates cutaneous vasodilation during whole body heat stress and local warming in humans. J Appl Physiol 110: 1406–1413, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kenney WL, Fowler SR. Methylcholine-activated eccrine sweat gland density and output as a function of age. J Appl Physiol 65: 1082–1086, 1988 [DOI] [PubMed] [Google Scholar]
- 18.Kimura K, Low DA, Keller DM, Davis SL, Crandall CG. Cutaneous blood flow and sweat rate responses to exogenous administration of acetylcholine and methacholine. J Appl Physiol 102: 1856–1861, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuno Y. Human Perspiration. Springfield: Thomas, 1956, p. 190–204 [Google Scholar]
- 20.Lorenzo S, Minson CT. Human cutaneous reactive hyperaemia: role of BKCa channels and sensory nerves. J Physiol 585: 295–303, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Love AHG, Shanks RG. The relationship between the onset of sweating and vasodilation in the forearm during body heating. J Physiol 162: 121–128, 1962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynn AG, Gagnon D, Binder K, Boushel RC, Kenny GP. Divergent roles of plasma osmolality and the baroreflex on sweating and skin blood flow. Am J Physiol Regul Integr Comp Physiol 302: R634–R642, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Machado-Moreira CA, Caldwell JN, Mekjavic IB, Taylor NA. Sweat secretion from palmar and dorsal surfaces of the hands during passive and active heating. Aviat Space Environ Med 79: 1034–1040, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Machado-Moreira CA, Smith FM, van den Heuvel AM, Mekjavic IB, Taylor NA. Sweat secretion from the torso during passively-induced and exercise-related hyperthermia. Eur J Appl Physiol 104: 265–270, 2008 [DOI] [PubMed] [Google Scholar]
- 25.Machado-Moreira CA, Wilmink F, Meijer A, Mekjavic IB, Taylor NA. Local differences in sweat secretion from the head during rest and exercise in the heat. Eur J Appl Physiol 104: 257–264, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Minson CT, Holowatz LA, Wong BJ, Kenney WL, Wilkins BW. Decreased nitric oxide- and axon reflex-mediated cutaneous vasodilation with age during local heating. J Appl Physiol 93: 1644–1649, 2002 [DOI] [PubMed] [Google Scholar]
- 27.Nadel ER, Bullard RW, Stolwijk JA. Importance of skin temperature in the regulation of sweating. J Appl Physiol 31: 80–87, 1971 [DOI] [PubMed] [Google Scholar]
- 28.Ogata K. Functional variations in human sweat glands, with remarks upon the regional difference of the amount of sweat. J Oriental Med 23: 98–101, 1935 [Google Scholar]
- 29.Ogawa T, Asayama M. Quantitative analysis of the local effect of skin temperature on sweating. Jpn J Physiol 36: 417–422, 1986 [DOI] [PubMed] [Google Scholar]
- 30.Randall WC, Deering R, Dougherty I. Reflex sweating and the inhibition of sweating by prolonged arterial occlusion. J Appl Physiol 1: 53–59, 1948 [DOI] [PubMed] [Google Scholar]
- 31.Savage MV, Brengelmann GL, Buchan AM, Freund PR. Cystic fibrosis, vasoactive intestinal polypeptide, and active cutaneous vasodilation. J Appl Physiol 69: 2149–2154, 1990 [DOI] [PubMed] [Google Scholar]
- 32.Shibasaki M, Crandall CG. Effect of local acetylcholinesterase inhibition on sweat rate in humans. J Appl Physiol 90: 757–762, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Shibasaki M, Crandall CG. Effect of local acetylcholinesterase inhibition on sweat rate in humans. J Appl Physiol 90: 757–762, 2001 [DOI] [PubMed] [Google Scholar]
- 34.Smith CJ, Havenith G. Body mapping of sweating patterns in athletes: a sex comparison. Med Sci Sports Exerc 44: 2350–2361, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Smith CJ, Havenith G. Body mapping of sweating patterns in male athletes in mild exercise-induced hyperthermia. Eur J Appl Physiol 111: 1391–1404, 2011 [DOI] [PubMed] [Google Scholar]
- 36.Sugenoya J, Ogawa T, Jmai K, Ohnishi N, Natsume K. Cutaneous vasodilatation responses synchronize with sweat expulsions. Eur J Appl Physiol Occup Physiol 71: 33–40, 1995 [DOI] [PubMed] [Google Scholar]
- 37.Szabo G. The number of eccrine sweat glands in human skin. In: Advances in Biology of the Skin, edited by Montagna W, Ellis RA, Silver AF. London: Pergamon, 1962, p. 1–5 [Google Scholar]
- 38.Taylor NAS, Caldwell FN, Mekjavic IB. The sweating foot: local differences in sweat secretion during exercise-induced hyperthermia. Aviat Space Envir Md 77: 1020–1027, 2006 [PubMed] [Google Scholar]
- 39.Wenner MM, Wilson TE, Davis SL, Stachenfeld NS. Pharmacological curve fitting to analyze cutaneous adrenergic responses. J Appl Physiol 111: 1703–1709, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wingo JE, Low DA, Keller DM, Brothers RM, Shibasaki M, Crandall CG. Skin blood flow and local temperature independently modify sweat rate during passive heat stress in humans. J Appl Physiol 109: 1301–1306, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong BJ, Tublitz NJ, Minson CT. Neurokinin-1 receptor desensitization to consecutive microdialysis infusions of substance P in human skin. J Physiol 568: 1047–1056, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yamashita Y, Ogawa T, Ohnishi N, Imamura R, Sugenoya J. Local effect of vasoactive intestinal polypeptide on human sweat-gland function. Jpn J Physiol 37: 929–936, 1987 [DOI] [PubMed] [Google Scholar]