Abstract
Persons affected by obstructive sleep apnea (OSA) have increased arterial blood pressure and elevated activity in upper airway muscles. Many cardiorespiratory features of OSA have been reproduced in rodents subjected to chronic-intermittent hypoxia (CIH). We previously reported that, following exposure to CIH, rats have increased noradrenergic terminal density in brain stem sensory and motor nuclei and upregulated expression of the excitatory α1-adrenergic receptors in the hypoglossal motor nucleus. This suggested that CIH may enhance central catecholaminergic transmission. We now quantified c-Fos expression in different groups of pontomedullary catecholaminergic neurons as an indirect way of assessing their baseline activity in rats subjected to CIH or sham treatment (7 AM-5 PM daily for 35 days). One day after the last CIH exposure, the rats were gently kept awake for 2.5 h and then were anesthetized and perfused, and their pontomedullary brain sections were subjected to dopamine β-hydroxylase (DBH) and c-Fos immunohistochemistry. DBH-positive cells were counted in the A1/C1, A2/C2, A5, subcoeruleus (sub-C) and A7 groups of catecholaminergic neurons, and the percentages of those expressing c-Fos were determined. We found that fewer DBH cells expressed c-Fos in CIH- than in sham-treated rats in the medulla (significant in the A1 group). In the pons (rostral A5, sub-C, and A7), c-Fos expression did not differ between the CIH- and sham-treated animals. We suggest that, when measured 20 h after the last CIH exposure, catecholaminergic transmission is enhanced through terminal sprouting and receptor upregulation rather than through increased baseline activity in pontomedullary catecholaminergic neurons.
Keywords: hypertension, locus coeruleus, obstructive sleep apnea, ventrolateral medulla, upper airway
about five percent of adults are affected by the obstructive sleep apnea syndrome (OSA), a disorder caused by a combination of altered upper airway anatomy and insufficient activation of upper airway muscles during sleep (8, 50, 62). These patients experience recurring episodes of hypopnea or complete blockage of the upper airway that often require awakening to resolve and result in repeated periods of systemic hypoxia. Clinical studies show a causal relationship between the severity of OSA and a host of cardiovascular and metabolic derangements, such as arterial hypertension, increased vascular reactivity, and glucose intolerance (34, 35, 51, 61). Studies with rats and mice chronically exposed to episodic hypoxia also often reveal elevated arterial blood pressure, increased sympathetic activity, elevated levels of circulating catecholamines and fatty acids, and impaired ability to maintain proper blood sugar levels (e.g., 4, 12–14, 25, 49), thus generally support the clinical findings.
OSA patients also exhibit elevated levels of activity in upper airway muscles, which is a positive adaptation to the disorder that allows them to maintain an open airway during wakefulness and parts of sleep (27, 43, 59). We previously reported that, following exposure to chronic-intermittent hypoxia (CIH), rats have increased noradrenergic terminal density and upregulated expression of the excitatory α1-adrenergic receptors in the hypoglossal (XII) motor nucleus (46). We also determined that XII nerve activity was more strongly reduced by microinjections of an α1-adrenergic receptor antagonist into the XII nucleus in anesthetized rats previously subjected to CIH than in sham-treated animals (57). Collectively, these data suggested that endogenous noradrenergic excitatory drive to XII motoneurons is enhanced in CIH rats when tested 1 day after the last exposure to CIH, and that central catecholaminergic cell hyperactivity could contribute to a strengthening of the efferent pathways from catecholaminergic neurons. In OSA patients, the motor output from XII motoneurons to the genioglossus and other upper airway muscles would benefit from such an elevated excitatory drive. Indeed, this effect of CIH could offer a mechanistic explanation for the elevated activity during wakefulness in upper airway muscles in OSA patients who experience chronic recurrent nocturnal hypoxia (27, 43, 59).
Although noradrenergic terminal sprouting combined with increased expression of α1-adrenergic receptors could be sufficient to explain the enhancement of central noradrenergic drive, it is also possible that the activity of central noradrenergic neurons is elevated for a prolonged period following exposure to CIH. Investigating this was the goal of our present study. To assess the baseline activity levels in pontomedullary catecholaminergic neurons, we quantified c-Fos expression in neurons of different catecholaminergic groups 1 day after the last exposure to CIH or sham treatment in rats, thus at a time when all acute effects of the last exposure to CIH would have dissipated. Based on prior studies demonstrating that c-Fos expression in noradrenergic, serotonergic, orexinergic, and many other cell groups varies with the level of their spontaneous activity across the sleep-wake states in the absence of any overt stimulation (6, 10, 32, 36, 52), we expected that the baseline c-Fos expression would be increased in brain stem catecholaminergic neurons following exposure to CIH in awake rats. This would be consistent with an elevation of activity in these cells lasting beyond the period of acute effects of the last CIH exposure. Contrary to our expectation, we found that, in the medulla, lower percentages of catecholaminergic neurons expressed c-Fos in rats subjected to CIH than to sham treatment, and there was no difference between the two treatment groups in the pons. A preliminary report has been published (30).
MATERIALS AND METHODS
Animals and administration of CIH.
Twelve adult male Sprague-Dawley rats (305 ± 2 g (SE); range 290–314 g at the beginning of the experiment) were obtained from Charles River Laboratories (Wilmington, MA). All animal procedures followed the American Physiological Society's Guiding Principles in the Care and Use of Vertebrate Animals in Research and Training and were approved by the Institutional Animal Care and Use Committee of the University of Pennsylvania.
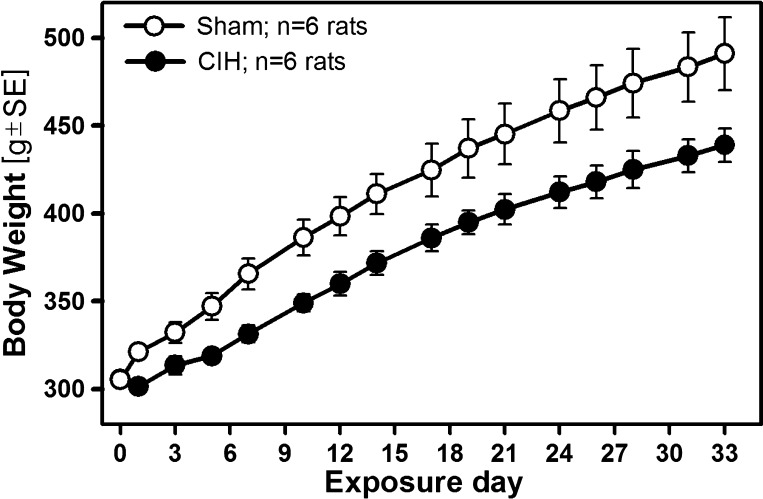
Two or three rats were housed together in standard rat cages that were placed inside 28.5 × 30.0 × 51.5 cm chambers under a 12:12 h light/dark cycle. A standard chow diet (5001/AIN76, Nestle Purina, St. Louis, MO) and water were provided ad libitum. Six rats were subjected to CIH, with O2 levels oscillating between 21% and 10% for 10 h/day (7 AM-5 PM) with 180 s period produced by alternating flows of N2 and O2 (Oxycycler, Biospherix, Redfield, NY), as described and illustrated previously (46). An additional six rats were exposed in adjacent chambers to room air exchanges identically timed to those in the CIH chambers (sham treatment). The exposures lasted 33–38 days (mean: 34.6 ± 0.4 days). The animals were weighed before the first exposure to CIH and then every other day throughout the exposure period. CIH animals did not gain weight during the first 2–3 days of exposure and then gained weight at a slightly slower pace than the sham-treated group (Fig. 1). The rate of body weight gain determined by linear regression between days 3 and 33 of exposure, i.e., between the first day after which both groups steadily gained weight and the last day for which body weight information was available for all animals, was 5.2 ± 0.22 g/day for the sham-treated rats and 4.3 ± 0.17 g/day for CIH rats (P < 0.02, unpaired t-test), similar to our previous studies (12, 46).
Fig. 1.
Time course of body weight changes during the exposure to chronic intermittent hypoxia (CIH) and sham treatments. CIH rats had a transient arrest of body weight gain on the first 2–3 days of exposure, after which they gained weight at a slower rate than the sham-treated group (4.3 vs. 5.2 g/day; see text for details). As a result, the mean body weight of the CIH group was significantly lower than for the sham-treated group throughout the exposure period (P = 0.007–0.05, unpaired t-tests).
Immunohistochemical procedures.
One day postexposure, two rats, one CIH- and one sham-exposed, were placed in a clean cage and brought to the lab at 1 PM. To ensure that varying amounts of sleep before perfusion would not confound the results by superimposing a variability of c-Fos expression in catecholaminergic neurons related to the amounts of prior sleep (32, 40, 47) onto that related to CIH/sham treatments, the animals were gently kept awake for 2.5 h. The animals were continuously observed and, when the novel environment alone was not sufficient to keep them awake and they started to assume a presleep quiet posture, their awake status was maintained by adding a new object to the cage (e.g., a paper towel) or by repositioning the cage. After 2.5 h of wakefulness, the animals were deeply anesthetized with pentobarbital (100 mg/kg ip; Euthasol, Virbac Att, Fort Worth, TX) and transcardially perfused with cold phosphate-buffered saline followed by 4% phosphate-buffered paraformaldehyde (pH 7.4). Brain stems were postfixed in the same fixative, cryoprotected in 30% sucrose, and cut into 35-μm transverse sections, and the sections were collected into six series. During each perfusion session, one pair of rats was processed, with the order of perfusions between the CIH and sham animal varied from one session to another. To minimize any confounding effects of varying efficiency of reagents from one immunostaining procedure to another, series of sections collected from both rats of each pair were combined and together subjected to all subsequent immunohistochemical procedures.
Free-floating sections were subjected to double-labeling for c-Fos and then dopamine-β-hydroxylase (DBH), as described previously for a combination of tyrosine hydroxylase (TH) and c-Fos immunohistochemistry (11, 47). In brief, c-Fos (1:250 or 1:350; Santa Cruz Biotechnology, Santa Cruz, CA; catalog symbol: sc-52) and DBH (1:500, Millipore, Billerica, MA; catalog symbol: MAB308) antibodies were used, and their binding was visualized using biotinylated secondary antibodies tagged with horseradish peroxidase (HRP) (Vectastatin Elite ABC Reagent; Vector, Burlingame, CA). HRP was reacted with 3,3′-diaminobenzidine tetrahydrochloride, with c-Fos stained black by addition of nickel ammonium sulfate to the reaction, and DBH was stained brown due to the omission of the heavy metal salt. Sections from each pair of rats were matched for anteroposterior (A-P) levels, mounted side-by side on glass slides, dehydrated, defatted, coverslipped, and given code names that did not contain information about the treatment.
Cell counting.
Cell counting was conducted using an upright microscope (Leica DML; Wetzlar, Germany) at ×400 magnification. DBH-positive cells were counted bilaterally in the A1/C1, A2/C2, A5, subcoeruleus (sub-C), and A7 cell groups when the labeled cell profile included the cell body with at least one proximal dendrite and when the section contained at least three cells belonging to any of the catecholaminergic groups of interest. Locus coeruleus cells were not counted because their very high density and dark-brown staining precluded accurate counting and reliable recognition of the presence of black-stained neuronal nuclei (c-Fos). Cells within each group were initially counted separately on each side, and the totals per neuronal group were compared between the two sides within each rat and among all rats as a part of a general quality control. Since the counts did not differ between the two sides, bilateral cell counts representing distinct groups were calculated for each rat, and the percentage of those expressing c-Fos was determined. DBH cells were regarded c-Fos positive when they had a clear round or oval aggregation of fully black or distinctly gray nuclear staining within the brown-stained cell body. To ensure that a personal preference did not affect the final results, cells in about one-quarter of the entire material were counted by at least two investigators. The recounts done by two investigators consistently yielded the same sign of the difference in the percentage of DBH cells expressing c-Fos within the compared pairs of rats. Ultimately, the counting of cells within the entire data set done by one person (KBH) was included in this report.
Assignment of DBH-positive cells to different catecholaminergic groups.
The A1/C1 group was divided into its caudal part (mainly noradrenergic A1 neurons) and rostral part (mainly adrenergic C1 neurons) based on previously established landmarks (45). Specifically, DBH cells were regarded as representing mainly the A1 group when they were located caudal to the rostral margin of the lateral reticular nucleus (LRt). As representative of the C1 group, we counted all DBH-positive cells located between the rostral margin of the LRt and the caudal margin of the facial nucleus. A2/C2 group was not divided further because suitable anatomical boundaries for the adrenergic and noradrenergic subgroups have not been established. Within the A5 group, we distinguished between its caudal and rostral parts based on data indicating that the two differently express combinations of enzymes characteristic of catecholaminergic neurons (17). Specifically, A5 neurons identified by DBH immunohistochemistry and located caudal to the level where the facial nerve exits the brain stem show lower levels of TH than those located more rostrally. Accordingly, the A-P level of −9.84 mm from bregma in a rat brain atlas (44), which corresponds to the border between the medulla and the pons, was defined as the borderline between the caudal and rostral parts of the A5 group (A5-c and A5-r, respectively). Two sections closest to this level were excluded from the analysis to enhance any potential contrast between the A5-c and A5-r subgroups. No subgroups were distinguished within the A7 (A-P levels from −9.00 to −8.28 mm from bregma) or sub-C (−9.96 to −8.88 mm from bregma) groups in the pons.
Statistical analysis.
Normality and equal variance of the distributions were verified before application of any subsequent tests (Shapiro-Wilk test and F-test/Homogeneity of variance-test, respectively) (SigmaPlot v. 12; San Jose, CA). Two-way ANOVA with Holm-Sidak correction for multiple comparisons was used to test for the effects of the treatment and cell group (factors), with the data from the two rats whose sections were processed together paired. To uncover common traits among different groups of catecholaminergic neurons, the analysis was applied to various combinations of medullary and pontine cell groups, as defined in the preceding section. A paired Student's t-test of the effect of the treatment was then applied to distinct groups. Variability of the means is characterized by the SE.
RESULTS
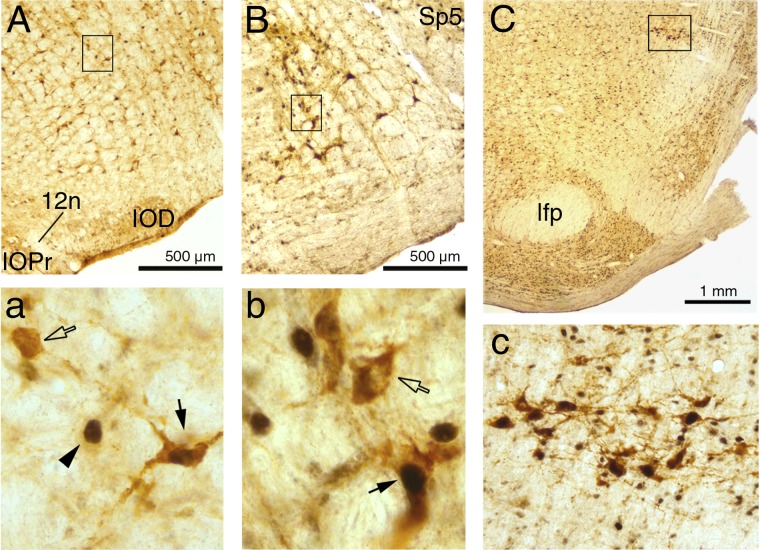
Figure 2 shows examples of DBH-positive cells, with or without c-Fos-positive nuclei, in the A1/C1, A5 and A7 groups. The high-magnification images in Fig. 2, left and middle, include arrows and arrowheads pointing to typical cases of DBH-positive cells with or without c-Fos-positive nuclei or a c-Fos-positive nucleus that belongs to a non-DBH cell. Figure 2, top right, shows the location of the A7 group and the corresponding bottom panel shows an expanded portion of the top panel at a magnification suitable to include all DBH cells present in this section.
Fig. 2.
Examples of dopamine β-hydroxylase (DBH)-positive cells with or without c-Fos-stained nuclei in the A1/C1 (A), A5 (B), and A7 (C) groups. In each column, the top shows a low-magnification image and the bottom shows an enlarged image of the detail framed in the top. The filled and open arrows in the high magnification images for the A1/C1 (a) and A5 (b) groups point to examples of DBH-positive cells with and without c-Fos-stained nuclei, respectively, and the arrowhead in a points to a c-Fos-labeled nucleus in a cell of an unidentified phenotype. The images of the A7 group (C and c) are shown at a lower magnification than in the other panels to visualize the location of the entire A7 group (C) and all its cells in this brain section (c). According to a rat brain atlas (44), the anteroposterior levels closest to these three examples are the following: −13.20 mm (A), −10.56 mm (B), and −8.52 mm (C) relative to bregma. 12n, roots of the hypoglossal nerve; IOD, inferior olive, dorsal division; IOPr, inferior olive, principal division; lfp, longitudinal fasciculus of the pons; Sp5, spinal trigeminal sensory nucleus.
An initial assessment of the percentages of c-Fos-expressing DBH cells across the major cell groups (A1/C1, A2/C2, A5, sub-C, and A7) suggested that c-Fos expression was reduced in CIH rats in some medullary groups but not in the pons. In particular, in each of the six rat pairs, the percentage of cells expressing c-Fos in the combined A1/C1 group was lower in the CIH- than sham-treated rat, with the differences varying from 4.3% to 7.6% in favor of the latter. On the average, 49.9 ± 2.9% of cells in the A1/C1 group were c-Fos positive in CIH rats versus 56.3 ± 2.5% for the sham-treated group (P < 0.001; paired t-test, n = 6 pairs). Trends in the same direction also were present for the A2/C2 group and the entire A5 group, whereas for the sub-C and A7 groups the differences were less than 1.5%.
To explore these differences in more detail relative to the known subdivisions of pontomedullary catecholaminergic neurons, the A1/C1 group was further divided into its predominantly noradrenergic (A1) and adrenergic (C1) subgroups and the A5 group into its caudal (A5-c) and rostral (A5-r) parts (see methods). A two-way ANOVA applied to this data set revealed a significant effect of the treatment (CIH vs. sham) for the medullary groups (A1, C1, A2/C2, and A5-c; F5,3,1=8.97, P = 0.03).
Within the medullary subgroups, post hoc comparisons revealed a significantly lower percentage of DBH cells with c-Fos in the CIH than sham rats in the A1 group (51.2 ± 2.3% vs. 57.4 ± 2.6%, P < 0.04) and a nearly significant difference in the same direction in the A5-c group (51.6 ± 4.6% vs. 57.7% ± 5.2%, P < 0.07). Weak trends toward a lower percentage of c-Fos-positive cells in the CIH than sham rats also were present in the C1 and A2/C2 groups, but the differences were not statistically significant. These percentages are listed in Table 1 together with the average total numbers of DBH-positive cells counted per rat within each of the analyzed subgroups and the average numbers of brain sections per rat in which cells belonging to each of the subgroups were counted. Consistent with the different sizes and rostro-caudal spans of the different catecholaminergic cell groups, the numbers of cells and sections analyzed differed among the groups, but there was no statistical difference in the average cell or section numbers within any of the groups in relation to the treatment.
Table 1.
Comparison of cell counts, percentages of c-Fos-positive cells, and numbers of brain sections in which cells were counted among the different medullary and pontine catecholaminergic cell groups
| c-Fos-Positive DBH Cells per Animal |
All DBH Cells Per Animal |
Percentage of c-Fos-Positive DBH Cells re. All DBH, % |
Brain Sections Analyzed per Animal |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cell Group | CIH | Sham | P | CIH | Sham | P | CIH | Sham | P | CIH | Sham | P |
| Medulla | ||||||||||||
| A1 | 153 ± 31 | 161 ± 30 | 0.55 | 296 ± 55 | 282 ± 49 | 0.70 | 51.2 ± 2.4 | 57.4 ± 2.6 | 0.04 | 12 ± 1 | 11 ± 1 | 0.33 |
| C1 | 75 ± 15 | 68 ± 13 | 0.69 | 152 ± 24 | 130 ± 25 | 0.46 | 49.4 ± 5.2 | 52.8 ± 4.3 | 0.48 | 5 ± 1 | 4 ± 1 | 0.26 |
| A2/C2 | 100 ± 15 | 116 ± 26 | 0.57 | 183 ± 20 | 201 ± 35 | 0.66 | 53.4 ± 2.9 | 56.9 ± 6.0 | 0.57 | 8 ± 1 | 7 ± 1 | 0.34 |
| A5 Caudal | 43 ± 10 | 46 ± 13 | 0.64 | 80 ± 14 | 76 ± 16 | 0.71 | 51.6 ± 4.6 | 57.7 ± 5.2 | 0.07 | 3.1 ± 0.4 | 3.3 ± 0.4 | 0.68 |
| Pons | ||||||||||||
| A5 Rostral | 31 ± 5 | 32 ± 9 | 0.95 | 61 ± 9 | 64 ± 17 | 0.91 | 50.4 ± 4.5 | 46.3 ± 3.9 | 0.32 | 2.3 ± 0.3 | 2.3 ± 0.5 | 0.91 |
| Sub-C | 45 ± 5 | 31 ± 6 | 0.14 | 111 ± 11 | 74 ± 13 | 0.14 | 40.8 ± 2.8 | 40.7 ± 3.3 | 0.95 | 5.5 ± 0.3 | 4.5 ± 0.6 | 0.29 |
| A7 | 21 ± 4 | 20 ± 6 | 0.67 | 42 ± 5 | 39 ± 8 | 0.74 | 50.0 ± 7.6 | 51.4 ± 5.6 | 0.83 | 2.4 ± 0.3 | 1.9 ± 0.2 | 0.20 |
Values are means ± SE. Data are from 6 rats exposed to chronic-intermittent hypoxia (CIH) and 6 rats subjected to sham treatment.The percentage of dopamine β-hydroxylase (DBH) cells expressing c-Fos was consistently lower in CIH rats for the medullary cell groups, with the difference being statistically significant for the A1 group and nearly significant for the caudal A5 group. There was no consistent effect of CIH on the pontine cell groups. Neither the raw cell counts nor the numbers of brain sections differed between the treatment groups. P values show significance levels based on post hoc paired comparisons within each cell group.
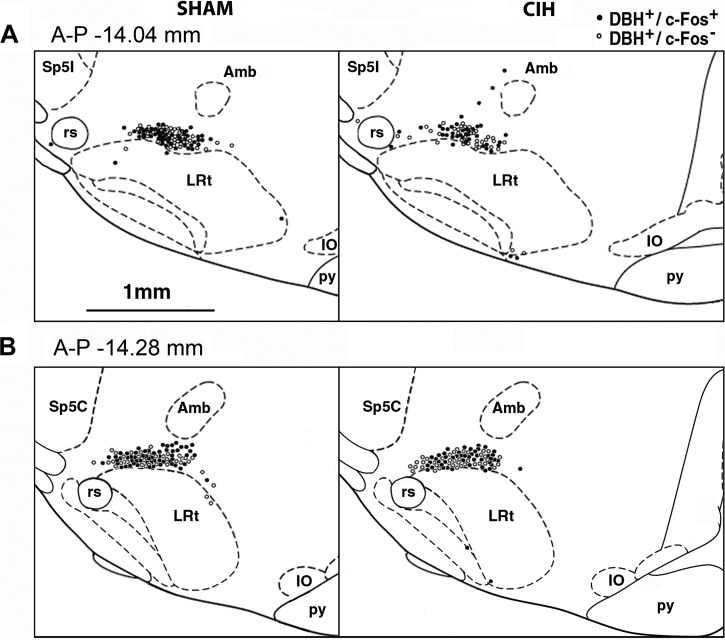
For the A1 group, for which the effect of CIH was most prominent, we examined whether c-Fos-positive and c-Fos-negative DBH cells aggregated within certain mediolateral or dorsoventral locations, and whether the reduced c-Fos expression in CIH rats preferentially occurred in a distinct subregion. To address this, two A-P levels where we typically found the highest numbers of DBH cells per section were selected (−14.28 and −14.04 mm from bregma according to 44), and the positions of all DBH cells found at these two levels in all six CIH and all six sham rats were replotted onto the two corresponding standard brain sections relative to the location of two major local landmarks, the nucleus ambiguus (Amb) and LRt (Fig. 3). At each of these two levels, the proportion of DBH cells that were c-Fos positive was similar to the average for the entire A1 group. However, we found no distinct aggregation of c-Fos-positive or c-Fos-negative cells in any particular subregion of the A1 group in relation to the treatment.
Fig. 3.
Distribution of c-Fos-positive (c-Fos+) and c-Fos-negative (c-Fos−) DBH cells at two anteroposterior (A-P) levels corresponding to the medullary noradrenergic A1 group. Locations of all DBH cells found on one side at these two levels in all 6 sham-treated and 6 CIH-exposed rats were redrawn onto the corresponding standard cross-sections from a rat brain atlas (44). At both levels, the percentage of DBH cells that were c-Fos+ (filled circles) was higher in sham-treated than in CIH-exposed rats, with values similar to the average data for the entire A1 group (58.5% sham vs. 51.9% CIH at A-P −14.04 mm from bregma, and 59.4% sham vs. 55.2% CIH at A-P −14.28 mm from bregma; see Table 1 for the corresponding data for the entire A1 group). There was no obvious aggregation of c-Fos+ or c-Fos− (open circles) DBH cells within the A1 region either within or between the treatment groups. Amb, nucleus ambiguus; IO, inferior olive; LRt, lateral reticular nucleus; py, pyramidal tract; rs, rubrospinal tract; Sp5C, spinal trigeminal sensory nucleus, caudal division; Sp5I, spinal trigeminal sensory nucleus, interpolar division.
For the pontine groups (A5-r, sub-C, and A7), no differences in c-Fos expression in relation to the treatment were revealed by either ANOVA or post hoc comparisons. In the rostral A5 group, the percentage of c-Fos-positive cells tended to be higher in the CIH rats (not significant), whereas in the sub-C and A7, the percentages of c-Fos-expressing cells were nearly identical in the two treatment groups (Table 1).
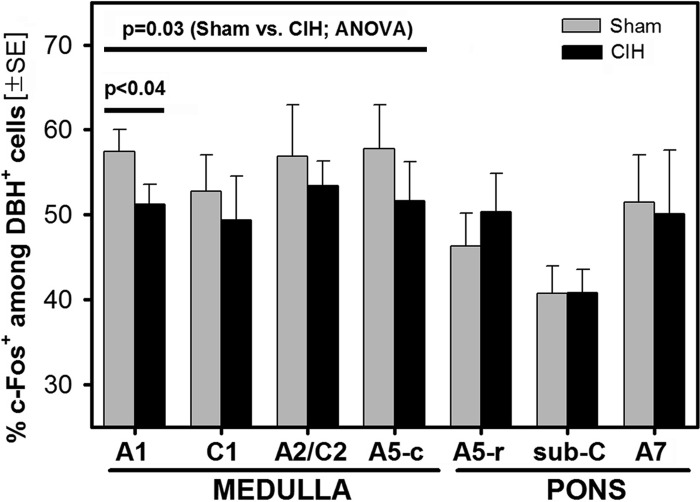
Thus the picture that emerged from our study is that CIH rats had a lower percentage of c-Fos-positive cells than sham rats in the A1 group, and similar trends were present in the remaining medullary groups (C1, A2/C2, and A5-c), whereas in the pons there were no statistical differences between the CIH and sham rats, indicating that CIH had no consistent effect on c-Fos expression in DBH cells in the pons. This is graphically illustrated in Fig. 4.
Fig. 4.
Percentages of DBH cells that had c-Fos-stained nuclei in different groups of pontomedullary catecholaminergic neurons. The percentage of DBH-positive (DBH+) cells that were c-Fos-positive (c-Fos+) was significantly lower, or tended to be lower, in the CIH- than sham-treated rats in all medullary groups [A1, C1, A2/C2, and caudal A5 (A5-c); significant effect of the treatment by two-way ANOVA, 6 rats per treatment group]. The effect was also significant for the A1 group when tested by a paired t-test. In contrast, in the pons [rostral A5 (A5-r), sub-C and A7], CIH, and sham animals did not differ. All animals were perfused at the same time of the day 1 day after 35 days of either daily CIH exposure or sham treatment and with wakefulness maintained before perfusion by exposure to a novel environment.
DISCUSSION
Our main finding is that the baseline levels of c-Fos expression in some of the medullary noradrenergic cells are altered in rats exposed to CIH for 35 days when tested 20 h after cessation of exposures, whereas the baseline c-Fos expression in pontine DBH-expressing cells is not affected. However, contrary to our expectation, we found that c-Fos expression was either significantly reduced or exhibited a downward trend, rather than being increased, in the medullary DBH cells of CIH-exposed rats compared with sham-treated animals. This result may reflect complex changes in transcriptional activity and metabolism in medullary DBH cells that develop during the period of exposure to CIH, but the most direct interpretation of our data would be that medullary catecholaminergic cells have somewhat reduced activity when tested under standardized awake conditions 1 day post-CIH exposure. As such, our data suggest that baseline activity of these cells is not elevated when assessed ∼20 h after an extended period of daily exposures to CIH.
Technical considerations.
Before we discuss our findings and their interpretation, we need to note three important technical issues. First, our rats were all perfused at the same time of the day and only after they were kept awake for 2.5 h. This stabilized the conditions under which both the CIH- and sham-treated rats were compared by eliminating any effects of variable amounts of sleep before perfusion and those related to variability of catecholaminergic cell activity with circadian time. In particular, the amount of sleep before perfusion has a well-established impact on c-Fos expression in catecholaminergic neurons. Unhandled rats perfused during the day have extremely low levels of c-Fos expression in catecholaminergic neurons because, during the lights-on period, they spend most of the time asleep and noradrenergic neurons are silent or nearly silent (32, 40, 41). In contrast, in rats gently kept awake, noradrenergic and other wake-related neurons maintain steady levels of activity and have proportionally elevated c-Fos levels. Indeed, c-Fos levels measured in noradrenergic and other wake-related neurons change in proportion to the levels of activity in these cells under otherwise unstimulated conditions (6, 10, 32, 36, 47). Similarly, in sleep-active neurons, c-Fos levels are proportional to both the amount of prior sleep and the magnitude of accumulated sleep drive even when the rats are kept awake (22, 52). Thus, in many, although not all, types of neurons, c-Fos provides a well-validated surrogate measure of spontaneous levels of their activity. It is also of note that the amount of prior sleep and the related motor activity are not always given the due attention in c-Fos studies. Nevertheless, these effects can be noted in some studies focused on cardiovascular functions of brain stem catecholaminergic neurons. For example, in the study of McCulloch and Panneton (41), the percentage of DBH-positive medullary cells expressing c-Fos was in the 1–8% range in unhandled rats but in 14–40% range in rats that had to swim for 17 s every 5 min during the 2 h before perfusion (the effects of stress were excluded because the animals were well habituated to the experimental procedure). In our study, we kept the animals awake for 2.5 h by placing them in a novel environment and, if necessary, engaging them behaviorally. This ensured that the baseline levels of c-Fos expression in pontomedullary DBH neurons were elevated in both CIH and sham rats to those characteristic of wakefulness.
Second, the amount of c-Fos detected in neurons depends not only on the amount of prior activity (and other metabolic factors) but also on the sensitivity of the assay and the definition as to when a cell is regarded as c-Fos-positive. To ensure that we do not underestimate the baseline c-Fos levels, we used a relatively high concentration of c-Fos antibodies and regarded as c-Fos-positive both those nuclei that were stained to the point of no transparency and those that had semitransparent nuclei but exhibited a well-defined nuclear accumulation of c-Fos staining. As a result, we estimate that our average percentage levels of c-Fos-expressing DBH neurons were elevated by 10–15% compared with counting of only fully blackened nuclei. For example, in one study (32), 37.9% of A5 neurons (recognized using TH immunohistochemistry) were c-Fos positive in awake rats versus 46.3% of rostral A5 neurons labeled for DBH in our sham rats. Collectively, our experimental design was aimed at creating favorable conditions under which to estimate baseline c-Fos expression in awake rats and detect its changes (increases or decreases) in relation to the history of exposure to CIH or sham treatment.
Finally, it needs to be emphasized that acute stimulation of peripheral chemoreceptors is well known to activate pontomedullary catecholaminergic neurons (2, 7, 9, 24), and that activation of these cells contributes to systemic cardiorespiratory activation (1, 39, 58). When such stimulation is applied repeatedly, as in our CIH protocol, it is likely to have long-lasting, or permanent, effects on various aspects of catecholaminergic cell metabolism and physiological processes in which these cells participate. The goal of our study was to assess one aspect of such a long-term change. Specifically, we wanted to assess the level of baseline DBH cell activity, as measured by c-Fos expression, at ∼20 h after the end of last exposure to CIH, thus at a time when most acute effects of CIH would be expected to have dissipated. This experimental design allowed us to obtain new insight into the changes in catecholaminergic cell activity that persist at least for many hours after the last exposure to CIH. It is also of note that a study conducted 35 days after 35 days of CIH exposures yielded no differences between the CIH and sham rats for a number of cardiorespiratory and metabolic parameters (12). This shows that many of the changes elicited by CIH in rodents are likely to gradually normalize during the postexposure period. Thus studying the effects of CIH that persist 1 day after cessation of exposure should help design studies at other, longer and shorter, periods after cessation of CIH exposures.
Effects of CIH on pontomedullary catecholaminergic neurons.
C-Fos expression has been extensively used to assess changes in activity in pontomedullary catecholaminergic neurons in relation to various forms of cardiovascular stress and acute respiratory stimuli (reviewed in Ref. 7). Relatively fewer studies have reported on the effects of CIH on c-Fos expression. In most such studies, c-Fos-positive nuclei were counted in anatomically distinct regions, including the rostral ventrolateral medulla (RVLM) and the nucleus of the solitary tract (NTS), but without a second labeling that would identify the neurochemical phenotype of the studied cells (20, 28, 54). In some of these studies, CIH was found to have a stimulatory effect (20, 54), but in the study of Knight et al. (28) no changes could be detected 1 day post a 7-day exposure to CIH due to the extremely low baseline c-Fos expression (see discussion in the previous section). On the other hand, in the latter study, FosB/ΔFosB was increased in neurochemically unidentified cells located in the RVLM and the NTS and also in DBH-positive cells of the A5 region (28). A similar finding with FosB was reported for neurochemically unidentified cells of the RVLM (31). However, as discussed previously (28), FosB/ΔFosB protein levels decline with a much slower time constant than c-Fos. Consequently, it is possible that the increased FosB/ΔFosB expression found 1 day after termination of exposure to CIH was still the result of a direct stimulatory effect of CIH to which the animals were subjected on the previous day. It is also of note that c-Fos expression in the RVLM, albeit very low, suggested a reduced expression in CIH animals, which would be consistent with our study (cf. Fig. 5 in 28). Thus ours appears to be the first systematic study of c-Fos expression in neurochemically identified pontomedullary catecholaminergic neurons following CIH.
We analyzed all major groups of noradrenergic and adrenergic neurons in the medulla and pons (except the locus coeruleus; see methods) and found significantly reduced c-Fos expression or at least a trend in this direction in relation to CIH exposure in the medullary, but not the pontine, DBH-positive neurons. The reduced c-Fos expression in medullary neurons may be indicative of complex metabolic, transcriptional, and translational changes, but the simplest interpretation would be that the baseline activity of the cells was reduced as a result of many days of exposure to CIH. This may appear to be a counterintuitive result in the face of data pointing to increased arterial blood pressure, heart rate, sympathetic activity, and noradrenergic drive to upper airway motoneurons in rodents exposed to CIH (14, 23, 26, 53, 57) and in the face of the evidence that medullary C1 neurons provide a portion of the endogenous drive to the sympathetic output (1, 38, 39; reviewed in 7, 21). However, as we discussed elsewhere (12), cardiovascular outcomes from studies in which CIH exposures lasted about 30 days appear to depend of the severity of CIH. O2 nadirs lower than 5% often lead to changes typical of the exposure to steady hypoxia, such as pulmonary and systemic hypertension, right ventricular hypertrophy, increased hematocrit (15, 25, 28, 33, 42, 56, 64, 65), and cause an irreversible damage of noradrenergic cells of the locus coeruleus (59). By comparison, erythropoietin levels are only transiently increased in OSA patients, and hematocrit increases are mild and associated with polycythemia only in the most severe OSA (5, 63). This indicates that changes typical of exposure to continuous hypoxia are not a consistent component of OSA pathophysiology. In healthy humans exposed to mild CIH for 14–28 days, arterial blood pressure measured after the exposure was increased by <5 mmHg (16, 60), and a recent longitudinal study found no correlation between OSA and arterial hypertension after correction for age and body mass (3). There are also numerous studies in rodents that report minimal or no changes in baseline cardiovascular parameters after various periods of CIH exposure when measured as early as 1 or 2 days after termination of exposure (19, 23, 29). Within the spectrum of varying severities of OSA and CIH exposures, our protocol produces a moderate level of CIH because our rats do not have significantly increased hematocrit levels or cardiac hypertrophy. On the other hand, they present with trends toward an increased heart rate and/or arterial blood pressure and have significantly suppressed glucose-stimulated insulin release (12). Furthermore, a comparison of the effects of a chronic steady hypoxia and CIH applied for 7 days on TH expression and activity revealed that TH activity was decreased by steady hypoxia and even more so by CIH in medullary tissue, whereas an increase was observed in the cortex (significant only for steady hypoxia) (18). These results are compatible with our findings suggesting a reduced baseline activity in ventrolateral medullary noradrenergic neurons. The differential effect of CIH on medullary and pontine DBH neurons suggested by our study may be related to their different efferent projection patterns (see Ref. 48 for discussion) and correlates with the report that the medullary DBH-positive neurons express the Phox2b protein that is associated with central and peripheral chemoreception, whereas the more rostral A5, A6 (locus coeruleus), and A7 neurons do not (58). The noradrenergic A1 neurons in which we found a statistically significant decline of c-Fos levels in CIH rats have major axonal projections to the NTS, pons, and hypothalamus, are acutely activated by hypotension and hypoxia, and their activation stimulates vasopressin release (see 7, 41, 48 for earlier studies). If they are less active in rats exposed to CIH, they may be in a setpoint that favors their stronger activation in response to their natural excitatory inputs.
Application to cardiorespiratory control in OSA.
The picture that emerges from this and our two earlier studies (46, 57) is that CIH results in significant sprouting of catecholaminergic axon terminals and upregulation of the excitatory α1-adrenergic receptors at least in some regions of the medulla and pons, whereas the baseline levels of activity during wakefulness tend to be reduced, rather than increased, in medullary noradrenergic and adrenergic neurons (significant for the noradrenergic A1 group). This picture may be specific to our level and pattern of CIH administration that, as discussed in the preceding section, does not produce overt signs of concomitant steady hypoxia or lasting hypertension but exerts a significant effect on other metabolic outcomes (reduced body weight gain and suppression of glucose-stimulated insulin release; see Ref. 12 and Fig. 1). Accordingly, in the rats exposed to our CIH protocol, the indices that point to an increased effectiveness of the output from catecholaminergic neurons (terminal sprouting and receptor upregulation) appear to be at least partially counterbalanced by reduced levels of catecholaminergic cell activity. Such a combination may result in minimal net changes in the endogenous contribution of central catecholaminergic neurons to the baseline sympathetic tone or respiratory output at rest following exposure to CIH. However, the same situation may also favor enhanced surges of sympathetic and ventilatory activity at times when central catecholaminergic neurons are stimulated by certain peripheral sensory inputs or emotions. Consistent with this suggestion, a hyperreactivity to immobilization stress has been reported in rats subjected to CIH for 7 days, and it was associated with acutely increased c-Fos expression in locus coeruleus neurons (37). Reflex sympathetic activation is also enhanced following CIH exposure (19, 55). Furthermore, our data showing no changes in c-Fos expression following CIH in pontine DBH-positive cells, combined with the evidence for noradrenergic axon terminal sprouting and α1-adrenergic receptor upregulation in the XII nucleus (46), suggest that CIH exposed rats would be expected to have tonically increased endogenous noradrenergic drive to brain stem motoneurons that innervate upper airway muscles. This is because, among the cell groups that we studied, the pontine A7 group is a major source of noradrenergic excitatory drive to XII motoneurons (11, 48). In support of this, antagonism of α1-adrenergic receptors in the XII nucleus caused larger declines of spontaneous activity of XII motoneurons in rats previously exposed to CIH than in sham-treated rats (57). While c-Fos expression remained unchanged following CIH in A7 neurons, suggesting that prior exposure to CIH did not change their baseline activity during wakefulness, increased α1-adrenergic receptor levels and sprouting of noradrenergic terminals in the XII nucleus would be sufficient to enhance endogenous noradrenergic drive to XII motoneurons.
When the findings in rodents subjected to CIH are applied to cardiorespiratory conditions in OSA patients, it is apparent that CIH may be an important factor responsible for the large surges of sympathetic and respiratory activity that occur in OSA patients during apneic episodes, especially at a time when obstructive episodes are terminated and airway patency is restored. Recurrent hypoxic episodes may also contribute to the tonic hyperactivity of upper airway muscles present in OSA patients during quiet wakefulness (27, 43, 59). Thus the sensitization of the catecholaminergic output by CIH in rodents and the recurrent nocturnal hypoxia in OSA patients may play a positive role as an adaptive mechanism that helps maintain breathing despite the anatomical vulnerability of the upper airway to collapse. However, the same hyperactivity may also have detrimental effects on the cardiovascular system and metabolism by enhancing the vascular system's reactivity and, consequently, contributing to the hypertension, stroke, and diabetes that commonly occur in OSA patients.
Perspectives and Significance
When tested 20 h after 35 days of exposure to CIH, medullary catecholaminergic neurons tend to have reduced c-Fos expression during wakefulness, suggestive of CIH resulting in their reduced baseline activity, especially in the case of noradrenergic A1 and caudal A5 neurons. In contrast, the baseline c-Fos expression is unchanged following CIH in pontine noradrenergic neurons (rostral A5, A7, and sub-C). This suggests heterogeneity in the effects of CIH on anatomically and functionally distinct groups of pontomedullary catecholaminergic neurons, a heterogeneity that may be related to their different efferent projections, cellular biochemistry, and proximity to peripheral input from arterial chemoreceptors. In combination with evidence for catecholaminergic axon terminal sprouting in the brain stem and upregulation of the excitatory α1-adrenergic receptors in rats exposed to CIH, our data suggest that CIH may cause a tonic enhancement of central catecholaminergic transmission at baseline and enhanced responsiveness to external and internal stimuli.
GRANTS
The study was supported by the National Institutes of Health Grant HL-047600 and a research fellowship from the Deutsche Forschungsgemeinschaft (DFG Ste1899/1-1) to G. M. Stettner.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: K.B.H., G.M.S., and L.K. performed experiments; K.B.H., G.M.S., and L.K. analyzed data; K.B.H., G.M.S., and L.K. interpreted results of experiments; K.B.H., G.M.S., and L.K. prepared figures; K.B.H., G.M.S., and L.K. drafted manuscript; K.B.H., G.M.S., and L.K. edited and revised manuscript; K.B.H., G.M.S., and L.K. approved final version of manuscipt; G.M.S. and L.K. conception and design of research.
REFERENCES
- 1. Abbott SB, Stornetta RL, Socolovsky CS, West GH, Guyenet PG. Photostimulation of channelrhodopsin-2 expressing ventrolateral medullary neurons increases sympathetic nerve activity and blood pressure in rats. J Physiol (Lond) 587: 23–31, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Buller KM, Smith DW, Day TA. NTS catecholamine cell recruitment by hemorrhage and hypoxia. Neuro Report 10: 3853–3856, 1999 [DOI] [PubMed] [Google Scholar]
- 3. Cano-Pumarega I, Durán-Cantolla J, Aizpuru F, Miranda-Serrano E, Rubio R, Martínez-Null C, de MJ, Egea C, Cancelo L, Álvarez A, Fernández-Bolaños M, Barbé F. Obstructive sleep apnea and systemic hypertension: longitudinal study in the general population: the Vitoria Sleep Cohort. Am J Respir Crit Care Med 184: 1299–1304, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Carreras A, Kayali F, Zhang J, Hirotsu C, Wang Y, Gozal D. Metabolic effects of intermittent hypoxia in mice: steady versus high-frequency applied hypoxia daily during the rest period. Am J Physiol Regul Integr Comp Physiol 303: R700–R709, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Choi JB, Loredo JS, Norman D, Mills PJ, Ancoli-Israel S, Ziegler MG, Dimsdale JE. Does obstructive sleep apnea increase hematocrit? Sleep Breath 10: 155–160, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science 274: 1211–1215, 1996 [DOI] [PubMed] [Google Scholar]
- 7. Dampney RA, Horiuchi J. Functional organisation of central cardiovascular pathways: studies using c-fos gene expression. Progr Neurobiol 71: 359–384, 2003 [DOI] [PubMed] [Google Scholar]
- 8. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 90: 47–112, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Erickson JT, Millhorn DE. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J Comp Neurol 348: 161–182, 1994 [DOI] [PubMed] [Google Scholar]
- 10. Estabrooke IV, McCarthy MT, Ko E, Chou T, Chemelli RM, Yanagisawa M, Saper CB, Scammell TE. Fos expression in orexin neurons varies with behavioral state. J Neurosci 21: 1656–1662, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fenik VB, Rukhadze I, Kubin L. Inhibition of pontine noradrenergic A7 cells reduces hypoglossal nerve activity in rats. Neuroscience 157: 473–482, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fenik VB, Singletary T, Branconi JL, Davies RO, Kubin L. Glucoregulatory consequences and cardiorespiratory parameters in rats exposed to chronic-intermittent hypoxia: effects of the duration of exposure and losartan. Front Neurol 3: 51, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fletcher EC. Cardiovascular consequences of obstructive sleep apnea: experimental hypoxia and sympathetic activity. Sleep 23: S127–S131, 2000 [PubMed] [Google Scholar]
- 14. Fletcher EC. Invited Review: Physiological consequences of intermittent hypoxia: systemic blood pressure. J Appl Physiol 90: 1600–1605, 2001 [DOI] [PubMed] [Google Scholar]
- 15. Fletcher EC, Lesske J, Qian W, Miller CC, Unger T. Repetitive episodic hypoxia causes diurnal elevations of systemic blood pressure in rats. Hypertension 19: 555–561, 1992 [DOI] [PubMed] [Google Scholar]
- 16. Gilmartin GS, Lynch M, Tamisier R, Weiss JW. Chronic intermittent hypoxia in humans during 28 nights results in blood pressure elevation and increased muscle sympathetic nerve activity. Am J Physiol Heart Circ Physiol 299: H925–H931, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Goodchild AK, Phillips JK, Lipski J, Pilowsky PM. Differential expression of catecholamine synthetic enzymes in the caudal ventral pons. J Comp Neurol 438: 457–467, 2001 [DOI] [PubMed] [Google Scholar]
- 18. Gozal E, Shah ZA, Pequignot JM, Pequignot J, Sachleben LR, Czyżk-Krzeska MF, Li RC, Guo SZ, Gozal D. Tyrosine hydroxylase expression and activity in the rat brain: differential regulation after long-term intermittent or sustained hypoxia. J Appl Physiol 99: 642–649, 2005 [DOI] [PubMed] [Google Scholar]
- 19. Greenberg HE, Sica A, Batson D, Scharf SM. Chronic intermittent hypoxia increases sympathetic responsiveness to hypoxia and hypercapnia. J Appl Physiol 86: 298–305, 1999 [DOI] [PubMed] [Google Scholar]
- 20. Greenberg HE, Sica AL, Scharf SM, Ruggiero DA. Expression of c-fos in the rat brainstem after chronic intermittent hypoxia. Brain Res 816: 638–645, 1999 [DOI] [PubMed] [Google Scholar]
- 21. Guyenet PG. The sympathetic control of blood pressure. Nat Rev Neurosci 7: 335–346, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Gvilia I, Xu F, McGinty D, Szymusiak R. Homeostatic regulation of sleep: a role for preoptic area neurons. J Neurosci 26: 9426–9433, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hinojosa-Laborde C, Mifflin SW. Sex differences in blood pressure response to intermittent hypoxia in rats. Hypertension 46: 1016–1021, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Hirooka Y, Polson JW, Potts PD, Dampney RA. Hypoxia-induced Fos expression in neurons projecting to the pressor region in the rostral ventrolateral medulla. Neuroscience 80: 1209–1224, 1997 [DOI] [PubMed] [Google Scholar]
- 25. Iiyori N, Alonso LC, Li J, Sanders MH, Garcia-Ocana A, O'Doherty RM, Polotsky VY, O'Donnell CP. Intermittent hypoxia causes insulin resistance in lean mice independent of autonomic activity. Am J Respir Crit Care Med 175: 851–857, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Iiyori N, Shirahata M, O'Donnell CP. Genetic background affects cardiovascular responses to obstructive and simulated apnea. Physiol Gen 24: 65–72, 2005 [DOI] [PubMed] [Google Scholar]
- 27. Katz ES, White DP. Genioglossus activity during sleep in normal control subjects and children with obstructive sleep apnea. Am J Respir Crit Care Med 170: 553–560, 2004 [DOI] [PubMed] [Google Scholar]
- 28. Knight WD, Little JT, Carreno FR, Toney GM, Mifflin SW, Cunningham JT. Chronic intermittent hypoxia increases blood pressure and expression of FosB/ΔFosB in central autonomic regions. Am J Physiol Regul Integr Comp Physiol 301: R131–R139, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kraiczi H, Magga J, Sun XY, Ruskoaho H, Zhao X, Hedner J. Hypoxic pressor response, cardiac size, and natriuretic peptides are modified by long-term intermittent hypoxia. J Appl Physiol 87: 2025–2031, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Kubin L, Benincasa Herr K, Stettner GM. In rats exposed to chronic intermittent hypoxia, reduced Fos expression in ventrolateral medullary catecholaminergic A1/C1 neurons contrasts with an upward trend in pontine A7 neurons (Abstract). Neurosci Meeting Planner. Washington, DC: Soc Neurosci, Online Program no. 487.04, 2012 [Google Scholar]
- 31. Kuo TB, Yuan ZF, Lin YS, Lin YN, Li WS, Yang CC, Lai CJ. Reactive oxygen species are the cause of the enhanced cardiorespiratory response induced by intermittent hypoxia in conscious rats. Respir Physiol Neurobiol 175: 70–79, 2011 [DOI] [PubMed] [Google Scholar]
- 32. Leger L, Goutagny R, Sapin E, Salvert D, Fort P, Luppi PH. Noradrenergic neurons expressing Fos during waking and paradoxical sleep deprivation in the rat. J Chem Neuroanat 37: 149–157, 2009 [DOI] [PubMed] [Google Scholar]
- 33. Lesske J, Fletcher EC, Bao G, Unger T. Hypertension caused by chronic intermittent hypoxia–influence of chemoreceptors and sympathetic nervous system. J Hyperten 15: 1593–1603, 1997 [DOI] [PubMed] [Google Scholar]
- 34. Levy P, Bonsignore MR, Eckel J. Sleep, sleep-disordered breathing and metabolic consequences. Eur Respir J 34: 243–260, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Levy P, Tamisier R, Arnaud C, Monneret D, Baguet JP, Stanke-Labesque F, Dematteis M, Godin-Ribuot D, Ribuot C, Pepin JL. Sleep deprivation, sleep apnea and cardiovascular diseases. Front Biosci 4: 2007–2021, 2012 [DOI] [PubMed] [Google Scholar]
- 36. Lu J, Jhou TC, Saper CB. Identification of wake-active dopaminergic neurons in the ventral periaqueductal gray matter. J Neurosci 26: 193–202, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Ma S, Mifflin SW, Cunningham JT, Morilak DA. Chronic intermittent hypoxia sensitizes acute hypothalamic-pituitary-adrenal stress reactivity and Fos induction in the rat locus coeruleus in response to subsequent immobilization stress. Neuroscience 154: 1639–1647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Madden CJ, Stocker SD, Sved AF. Attenuation of homeostatic responses to hypotension and glucoprivation after destruction of catecholaminergic rostral ventrolateral medulla neurons. Am J Physiol Regul Integr Comp Physiol 291: R751–R759, 2006 [DOI] [PubMed] [Google Scholar]
- 39. Madden CJ, Sved AF. Cardiovascular regulation after destruction of the C1 cell group of the rostral ventrolateral medulla in rats. Am J Physiol Heart Circ Physiol 285: H2734–H2748, 2003 [DOI] [PubMed] [Google Scholar]
- 40. Maloney KJ, Mainville L, Jones BE. Differential c-Fos expression in cholinergic, monoaminergic and GABAergic cell groups of the pontomesencephalic tegmentum after paradoxical sleep deprivation and recovery. J Neurosci 19: 3057–3072, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. McCulloch PF, Panneton WM. Activation of brainstem catecholaminergic neurons during voluntary diving in rats. Brain Res 984: 42–53, 2003 [DOI] [PubMed] [Google Scholar]
- 42. McGuire M, Dumbleton M, MacDermott M, Bradford A. Contractile and electrical properties of sternohyoid muscle in streptozotocin diabetic rats. Clin Exp Pharm Physiol 28: 184–187, 2001 [DOI] [PubMed] [Google Scholar]
- 43. Mezzanotte WS, Tangel DJ, White DP. Waking genioglossal electromyogram in sleep apnea patients versus normal controls (a neuromuscular compensatory mechanism). J Clin Invest 89: 1571–1579, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates (6th Ed.) Amsterdam: Elsevier, 2007 [Google Scholar]
- 45. Phillips JK, Goodchild AK, Dubey R, Sesiashvili E, Takeda M, Chalmers J, Pilowsky PM, Lipski J. Differential expression of catecholamine biosynthetic enzymes in the rat ventrolateral medulla. J Comp Neurol 432: 20–34, 2001 [DOI] [PubMed] [Google Scholar]
- 46. Rukhadze I, Fenik VB, Benincasa KE, Price A, Kubin L. Chronic intermittent hypoxia alters density of aminergic terminals and receptors in the hypoglossal motor nucleus. Am J Respir Crit Care Med 182: 1321–1329, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rukhadze I, Fenik VB, Branconi JL, Kubin L. Fos expression in pontomedullary catecholaminergic cells following REM sleep-like episodes elicited by pontine carbachol in urethane-anesthetized rats. Neuroscience 152: 208–222, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rukhadze I, Kubin L. Differential pontomedullary catecholaminergic projections to hypoglossal motor nucleus and viscerosensory nucleus of the solitary tract. J Chem Neuroanat 33: 23–33, 2007 [DOI] [PubMed] [Google Scholar]
- 49. Savransky V, Jun J, Li J, Nanayakkara A, Fonti S, Moser AB, Steele KE, Schweitzer MA, Patil SP, Bhanot S, Schwartz AR, Polotsky VY. Dyslipidemia and atherosclerosis induced by chronic intermittent hypoxia are attenuated by deficiency of stearoyl coenzyme A desaturase. Circ Res 103: 1173–1180, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Schwartz AR, Patil SP, Squier S, Schneider H, Kirkness JP, Smith PL. Obesity and upper airway control during sleep. J Appl Physiol 108: 430–435, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Seicean S, Kirchner HL, Gottlieb DJ, Punjabi NM, Resnick H, Sanders M, Budhiraja R, Singer M, Redline S. Sleep-disordered breathing and impaired glucose metabolism in normal-weight and overweight/obese individuals: the Sleep Heart Health Study. Diab Care 31: 1001–1006, 2008 [DOI] [PubMed] [Google Scholar]
- 52. Sherin JE, Shiromani PJ, McCarley RW, Saper CB. Activation of ventrolateral preoptic neurons during sleep. Science 271: 216–219, 1996 [DOI] [PubMed] [Google Scholar]
- 53. Sica AL, Greenberg HE, Ruggiero DA, Scharf SM. Chronic-intermittent hypoxia: a model of sympathetic activation in the rat. Respir Physiol 121: 173–184, 2000 [DOI] [PubMed] [Google Scholar]
- 54. Sica AL, Greenberg HE, Scharf SM, Ruggiero DA. Chronic-intermittent hypoxia induces immediate early gene expression in the midline thalamus and epithalamus. Brain Res 883: 224–228, 2000 [DOI] [PubMed] [Google Scholar]
- 55. Silva AQ, Schreihofer AM. Altered sympathetic reflexes and vascular reactivity in rats after exposure to chronic intermittent hypoxia. J Physiol (Lond) 589: 6–76, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: cellular and molecular mechanisms. Circ Res 99: 675–691, 2006 [DOI] [PubMed] [Google Scholar]
- 57. Stettner GM, Fenik VB, Kubin L. Effect of chronic intermittent hypoxia on noradrenergic activation of hypoglossal motoneurons. J Appl Physiol 112: 305–312, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Stornetta RL, Moreira TS, Takakura AC, Kang BJ, Chang DA, West GH, Brunet JF, Mulkey DK, Bayliss DA, Guyenet PG. Expression of Phox2b by brainstem neurons involved in chemosensory integration in the adult rat. J Neurosci 26: 10305–10314, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Suratt PM, McTier RF, Wilhoit SC. Upper airway muscle activation is augmented in patients with obstructive sleep apnea compared with that in normal subjects. Am Rev Respir Dis 137: 889–894, 1988 [DOI] [PubMed] [Google Scholar]
- 60. Tamisier R, Pepin JL, Remy J, Baguet JP, Taylor JA, Weiss JW, Levy P. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J 37: 119–128, 2011 [DOI] [PubMed] [Google Scholar]
- 61. Tasali E, Ip MS. Obstructive sleep apnea and metabolic syndrome: alterations in glucose metabolism and inflammation. Proc Am Thor Soc (PATS) 5: 207–217, 2008 [DOI] [PubMed] [Google Scholar]
- 62. White DP. Sleep apnea. Proc Am Thor Soc (PATS) 3: 124–128, 2006 [DOI] [PubMed] [Google Scholar]
- 63. Winnicki M, Shamsuzzaman A, Lanfranchi P, Accurso V, Olson E, Davison D, Somers VK. Erythropoietin and obstructive sleep apnea. Am J Hypert 17: 783–786, 2004 [DOI] [PubMed] [Google Scholar]
- 64. Zhu Y, Fenik P, Zhan G, Mazza E, Kelz M, Aston-Jones G, Veasey SC. Selective loss of catecholaminergic wake active neurons in a murine sleep apnea model. J Neurosci 27: 10060–10071, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Zoccal DB, Bonagamba LG, Oliveira FR, Antunes-Rodrigues J, Machado BH. Increased sympathetic activity in rats submitted to chronic intermittent hypoxia. Exp Physiol 92: 79–85, 2007 [DOI] [PubMed] [Google Scholar]