Abstract
Comparative studies of renal structure and function have potential to provide insights into the urine-concentrating mechanism of the mammalian kidney. This review focuses on the tubular transport pathways for water and urea that play key roles in fluid and solute movements between various compartments of the rodent renal inner medulla. Information on aquaporin water channel and urea transporter expression has increased our understanding of functional segmentation of medullary thin limbs of Henle's loops, collecting ducts, and vasa recta. A more complete understanding of membrane transporters and medullary architecture has identified new and potentially significant interactions between these structures and the interstitium. These interactions are now being introduced into our concept of how the inner medullary urine-concentrating mechanism works. A variety of regulatory pathways lead directly or indirectly to variable patterns of fluid and solute movements among the interstitial and tissue compartments. Animals with the ability to produce highly concentrated urine, such as desert species, are considered to exemplify tubular structure and function that optimize urine concentration. These species may provide unique insights into the urine-concentrating process.1
Keywords: desert rodent, renal anatomy, aquaporin, loop of Henle, collecting duct
most mammals produce a concentrated urine, a process that lies at the core of the kidney's role in fluid and solute homeostasis. But, despite decades of research, the production of a corticomedullary osmotic gradient, much of which apparently is built up in the absence of active transport and which underlies the concentrating process, remains largely unexplained. Important recent advances have arisen following the molecular cloning of fluid and solute transporters and channels during the past 20 years. These advances have led to more complete understanding of segmental heterogeneity of fluid and solute transporter and channel expression as well as functional heterogeneity in loops of Henle, collecting ducts (CDs), and blood vessels of the renal medulla. Identification of these transporters and channels has also led to new experimental paradigms for investigating the urine-concentrating mechanism (UCM). Genetically modified animals and cells are providing important and unique insights into transepithelial tubular and vascular fluid and solute flows that are critical for producing the corticomedullary osmotic gradient. This new information has markedly influenced how we perceive the UCM could work. This review surveys water and urea transport pathways of rodent renal medullary nephrons and blood vessels that play key roles in the UCM and considers contributions from comparative studies with emphasis on desert species.
Theories That Attempt to Explain the Urine-Concentrating Mechanism
The UCM has been conceptualized as the countercurrent multiplication of an osmotic pressure difference between descending and ascending limbs of the loops of Henle sustained by active NaCl reabsorption from water-impermeable ascending limbs of Henle's loops (31, 32, 69). This conceptual model has been widely accepted for the outer medulla where active NaCl reabsorption occurs (13, 119). However, countercurrent flow coupled with active solute reabsorption does not explain the concentrating process in the inner medulla, where the steepest corticomedullary osmotic gradient is generated. The ascending thin limbs (ATLs) of the inner medulla, although essentially impermeable to water (16, 43, 154), have no significant active transepithelial transport of NaCl or of any other solute (43, 44, 91, 92). A number of theories have been developed to explain how passive transport in nephrons could produce the inner medullary osmotic gradient, the dominant theory being the passive mechanism hypothesis.
Passive mechanism hypothesis.
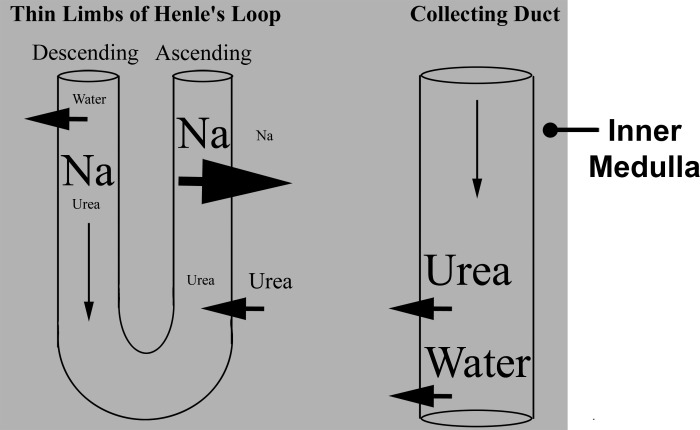
Present understanding of the mechanism by which the corticomedullary osmotic gradient is generated is encapsulated in the widely accepted passive mechanism hypothesis of Kokko and Rector (63), and Stephenson (136). This passive model assumes the inner medullary interstitium has a much higher urea concentration than NaCl, and that fluid in Henle's loops has a much higher NaCl concentration than urea. If the ATL has a sufficiently high NaCl permeability and a sufficiently low urea permeability, then relatively high amounts of NaCl will diffuse passively from the ATL lumen into the interstitium and relatively small amounts of urea will diffuse from the interstitium into the ATL lumen (Fig. 1). If concentration differences can be sustained, the interstitial fluid becomes concentrated while the ATL luminal fluid becomes diluted. The passive hypothesis assumes that these concentration differences are sustained by continuous diffusion of urea into the interstitium from the CD lumen (now known to occur via the urea transporters UT-A1 and UT-A3) and by continuous flow from the descending thin limbs (DTL) into the ATL of tubular fluid having a high NaCl concentration. This delivery has been thought to depend on sufficiently low NaCl and urea permeabilities in the DTL and minimal dissipation of transepithelial concentration gradients along the course of the DTL. We now know that water reabsorption, by way of AQP1 in the DTL, plays a key role in concentrating luminal solutes. As long as water and urea are continually reabsorbed from the CDs [via aquaporin-2 isoform (AQP2), UTA1, and UTA3, respectively], the interstitial urea concentration will remain sufficiently high and the interstitial Na concentration sufficiently low to sustain the concentration gradients that will permit urea secretion into and Na reabsorption from the ATL. The vasa recta are considered to function in the medulla as countercurrent exchangers to delay or prevent the washout of NaCl and urea and prevent the excess accumulation of water during the urine-concentrating process (101). Accordingly, the vasa recta play a critical role in removing fluids from the medulla while simultaneously participating in retention of solutes within the medulla.
Fig. 1.
Passive mechanism hypothesis of urine concentration. Schematic diagram of Na, urea, and water fluxes in inner medullary nephron segments. See text for details.
While the Kokko and Rector and Stephenson passive model represents a useful working model, there are some inconsistencies in biological studies that suggest the model is not correct. Notably, Fenton et al. demonstrated that with knockout of the UTA1/3 urea transporters from the mouse CD, inner medullary NaCl accumulation could still occur within the inner medulla despite the absence of a substantial urea concentration (reviewed in reference 26). If there is a complete disconnect between urea flow and production of a NaCl gradient, then the urea and NaCl separation/mixing tenet of the Kokko and Rector and Stephenson passive model may be incorrect.
Alternative hypotheses.
Other theories that attempt to explain the UCM have been proposed. One of these is a model proposing that an unidentified solute, such as lactate, may play a key role in the concentrating mechanism (37, 50, 141). Another theory relates to the pelvic wall peristaltic contractions that markedly impact tubular and vascular luminal flow dynamics in the medulla. Schmidt-Nielsen and colleagues (116, 117, 125–127) have proposed that these flow dynamics may produce a urine-concentrating effect. Knepper and colleagues (61) proposed that the glycosaminoglycan hyaluronan may serve to mechanically transduce the impact of pelvic wall contractions onto tubular and vascular structures throughout the medulla, and in so doing, influence tubular and vascular fluid flows and the concentrating mechanism.
A more complete understanding of medullary structure and function has led to improved and more refined mathematical models of the UCM (71–73, 142, 151, 152). However, no mathematical model of the UCM simulates production of a corticomedullary osmotic gradient reaching values measured in the antidiuretic rat. Further understanding of the free energy requirements for generating concentrated urine can be gained with an online tool that permits insertion of user-defined values for model parameters (147).
Axial and Lateral Subdivisions of the Renal Medulla
Considerable attention has been placed on understanding the patterned organization of medullary tubules and vessels and their geometrical relationships to each other. The proximity of one structure to another and the expression of segment-specific cell membrane transport pathways influence both axial flows as well as highly organized lateral flows of fluid and solutes that occur between tubules and vessels. These flows are critical for understanding solute exchange, cycling, and sequestration patterns related to the UCM (60, 72, 76, 80, 109, 142, 149), as well as for understanding medullary and renal function more generally. Axial zonation and lateral regionalization of the medulla arising from the geometrical relationships found in the rat, mouse, hamster, chinchilla, kangaroo rat, and others, where noted, are summarized in this section. The sand rat Psamommys produces a high urine osmolality, but this species differs from others in that NaCl, rather than urea, is the dominant osmolyte in the inner medulla. The UCM of Psamommys may therefore have fundamental differences from those species where urea is the dominant osmolyte.
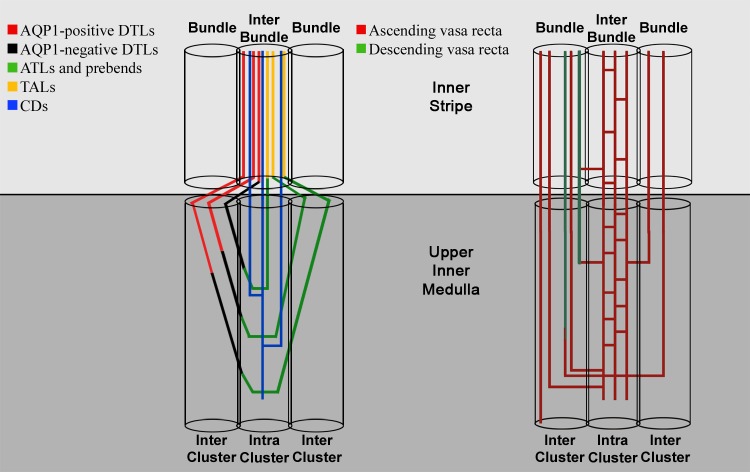
Zonal and regional designations are based on architecture of loops of Henle, CDs, and blood vessels. The outer medulla of most rodents clearly consists of two defined zones in the axial dimension (the outer stripe and the inner stripe) and two regions in the lateral dimension (the vascular bundle and the interbundle regions). A schematic diagram of the inner stripe and upper inner medulla and the nephron and blood vessel segments that are associated with each region is shown in Fig. 2. The inner medullas of the Munich-Wistar rat and kangaroo rat have been shown to consist of two axial zones (an outer zone and inner zone) and two lateral regions (the intracluster and intercluster regions) (48, 110, 144). Similar subdivisions likely occur in other rodents (2, 4, 5, 17, 49, 65, 79). Structural characteristics of the outer and inner medullary zones and regions have been summarized in several reviews (80, 104, 109).
Fig. 2.
Schematic diagram of nephrons and blood vessels in two regions of the inner stripe and two regions of the upper inner medulla. In the inner stripe, the vascular bundle (right, Bundle) consists of descending vasa recta (DVR), nonbranching ascending vas recta (AVR), and short loop descending thin limbs (DTLs; not shown). The interbundle region consists of long-loop DTLs, thick ascending limbs, collecting ducts (CDs), and interconnecting capillaries (cross-hatched AVR). Bundle and interbundle regions are shown in a transverse tissue section in Fig. 3. In the upper two-thirds of the inner medulla, the intracluster region consists of CDs and ascending thin limb (ATLs) (left) and interconnecting capillaries (right). The intercluster region consists of DTLs and ATLs (left) and nonbranching AVR (right). Cluster and intercluster regions are shown in transverse tissue sections in Fig. 4. DTLs of nephrons that form their bends within 1 mm below the outer medullary-inner medullary boundary express no detectable aquaporin-1 isoforms (AQP1) in their inner medullary segments and lie near the interface of the intercluster and intracluster regions; their ATLs lie within the intracluster region. DTLs of loops that form their bends deeper than 1 mm below the outer medullary-inner medullary boundary pass from the intercluster region into the intracluster region above the prebend segment (the terminal portion of the DTL), and their ATLs exit into the intercluster region above the equivalent postbend length. Structural details are based on the following references: 2, 4, 5, 48, 49, 55, 65, 79, 109, 110, 144, 150, 156. Figure was modified from Pannabecker (104).
Subdivisions of the outer medulla.
In transverse sections of the outer medulla, the vascular bundle can be viewed as a central feature around which other segments are arranged in an organized fashion (80) (Figs. 2 and 3). This is true for both the outer stripe, where straight proximal tubule segments make the most abundant contribution in terms of tissue mass, and for the inner stripe, where the thick ascending limbs are the dominant tissue. The vascular bundles contain descending and ascending vasa recta (DVR and AVR), and, in the inner stripe of most rodents, short-loop DTLs (Fig. 3). The DVR and AVR within these bundles undergo little or no branching. Thus the inner stripe consists of many vascular bundles running parallel to each other along the corticopapillary axis, and each bundle consists of vessels carrying countercurrent flows. The vascular bundles are separated from each other in the lateral dimension by interbundle regions (4, 65). The interbundle region contains DTLs of long loops of Henle, thick ascending limbs of the loops of Henle, CDs, and interconnecting capillaries (Figs. 2 and 3).
Fig. 3.
Immunolocalization of tubules and vessels in the inner stripe of the Munich-Wistar rat outer medulla. Bundle regions (two are circled) consist of DVR and short loop DTLs. Interbundle regions consist of long-loop DTLs, thick ascending limbs, and CDs. Unbranched AVR of the bundle region and interconnecting capillaries of the interbundle region are not shown. Scale bar: 250 μm. Figure was modified from Pannabecker (104).
Upon entering the inner stripe, DTLs of short-loop nephrons of the rat approach the vascular bundle and then descend alongside the perimeter of the bundle (65, 68). In the mouse, DTLs of short-loop nephrons approach and then enter the vascular bundle, whereupon they continue their descent within the bundle. The close proximity between the DTLs of short-loop nephrons and the DVR and AVR that is brought about by this repositioning within the inner stripe is considered to serve as a significant functional adaptation that contributes to the relatively high urine-concentrating ability of rodents (4, 60, 80, 148). The outer medullary vascular bundles are arranged with the shortest DVR (those destined for the outer medulla) positioned at the periphery of the bundle. DVR destined to terminate in the inner medulla lie near to the central core of the bundle. Thus outer medullary vessels peel off from the bundle at all intervals in their progressive descent and feed into capillary plexuses, with the longest DVR remaining within the bundle at the outer medullary-inner medullary boundary. These long DVR continue to descend through the outer inner medulla, still arranged within loosely organized vascular bundles through approximately the initial third, and as they descend, the vessels begin to fan out from each other (65, 94, 156).
Subdivisions of the inner medulla.
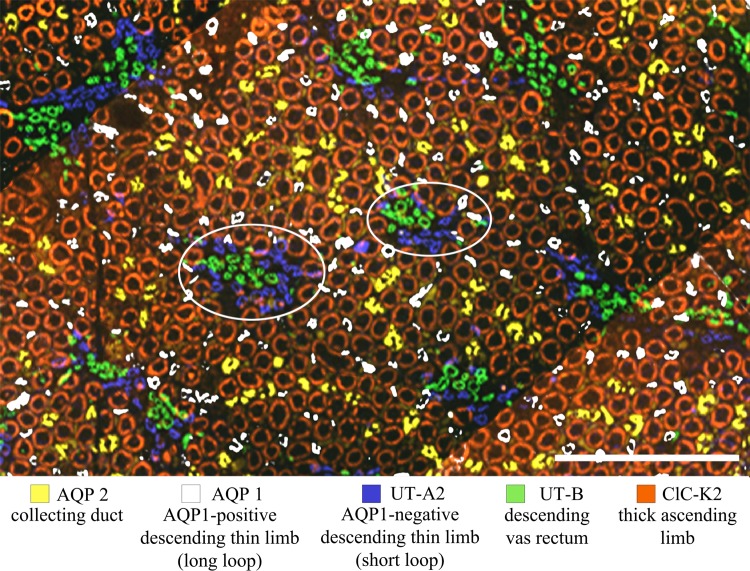
In the inner medulla, the CDs coalesce as they descend the corticopapillary axis. In the rat, about 7,300 CDs enter into the inner medulla and coalesce into about 6–13 CDs at the tip of the papilla, where they form the ducts of Bellini. The CDs form distinct clusters, which can be viewed as the central organizing feature of the initial two-thirds of the inner medulla. CD clusters define two lateral regions. These two regions have been termed the intracluster and the intercluster regions (48, 110, 144) (Fig. 4). The “intracluster” region includes the area encompassed by a group of coalescing CDs, and the “intercluster” region includes the area that separates adjacent CD clusters. These two lateral regions are very distinct in the initial two-thirds of the inner medulla but become less distinct in the terminal third. Distinguishing features of the intracluster region are the four or more symmetrically arranged capillaries that abut and run alongside every CD in the corticopapillary direction (Fig. 5). DTLs consist of an upper AQP1-positive segment followed by a lower segment that expresses undetectable AQP1 (this segmentation is discussed in Segmentation of Medullary Nephrons and Blood Vessels). In the transverse dimension, inner medullary AQP1-positive DTLs of the Munich-Wistar rat have been shown quantitatively to be distributed nonuniformly, whereas ATLs are distributed in a much more uniform pattern (108). DTLs and ATLs of the kangaroo rat are distributed in a similar fashion (Fig. 6). Three-dimensional reconstructions of rat and kangaroo rat inner medulla, produced from serial sections as in Fig. 4, have shown that the AQP1-positive DTLs lie outside CD clusters in the intercluster region. AQP1-positive DTLs are therefore anatomically separated from CDs, lying in a spatially distinct compartment (Fig. 2). Each AQP1-negative segment makes a gradual move toward the intracluster region, with the terminal DTL (the prebend segment) and the bend abutting CDs. The prebend and bend therefore lie within the intracluster region. The lower segment of each ATL, just after the bend, lies within the intracluster region and then gradually moves out toward the intercluster region (150). Because loops form bends at all axial levels, ATLs are distributed almost uniformly at all levels in transverse sections. Architecture of the mouse inner medulla has been shown to resemble that of the rat and kangaroo rat (158), including the positioning of AQP1-positive DTLs within the intercluster region (T. L. Pannabecker, unpublished observations).
Fig. 4.
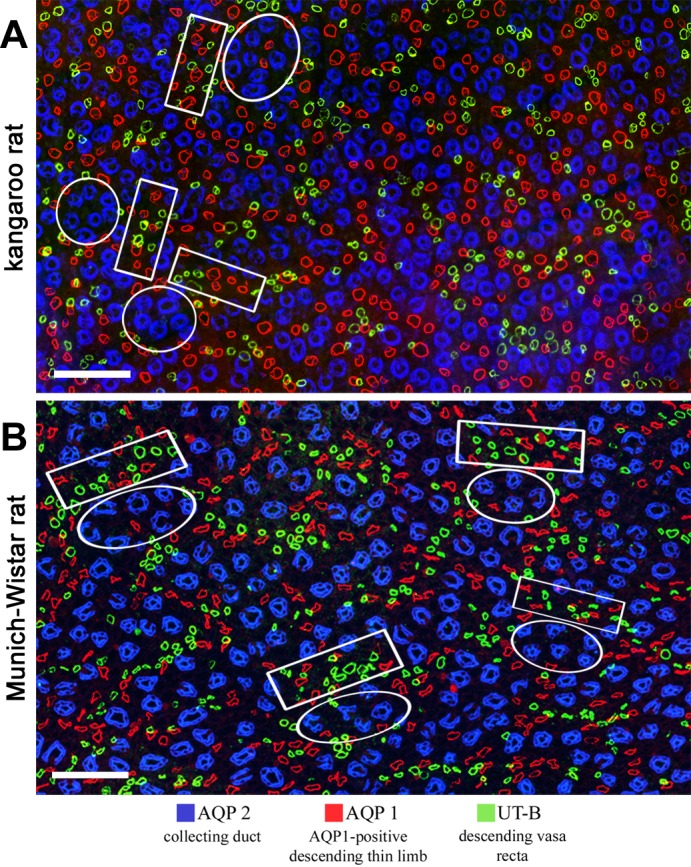
Immunolocalization of tubules and vessels in the upper inner medulla. A: kangaroo rat; B: Munich-Wistar rat. Intracluster regions (circles) consist predominantly of CDs. Intercluster regions (rectangles) consist of DTLs and DVR. Interconnecting capillaries and ATLs of the intracluster region and ATLs and nonbranching AVR of the intercluster region are not shown. Both images are overlays of two sections no more than 3 μm apart. Transverse sections are from ∼900 μm below the outer medulla. Scale bars: 100 μm. Figure was modified from Issaian et al. (48).
Fig. 5.
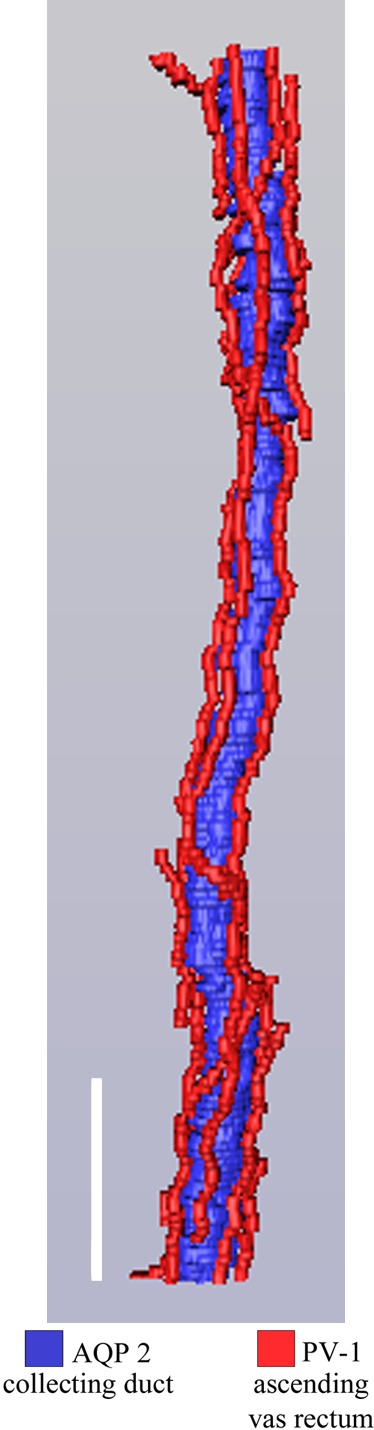
Three-dimensional reconstruction of single CD segment of Munich-Wistar rat inner medulla with multiple AVR butted up against it. Scale bar: 100 μm. Figure was modified from Pannabecker and Dantzler (107).
Fig. 6.
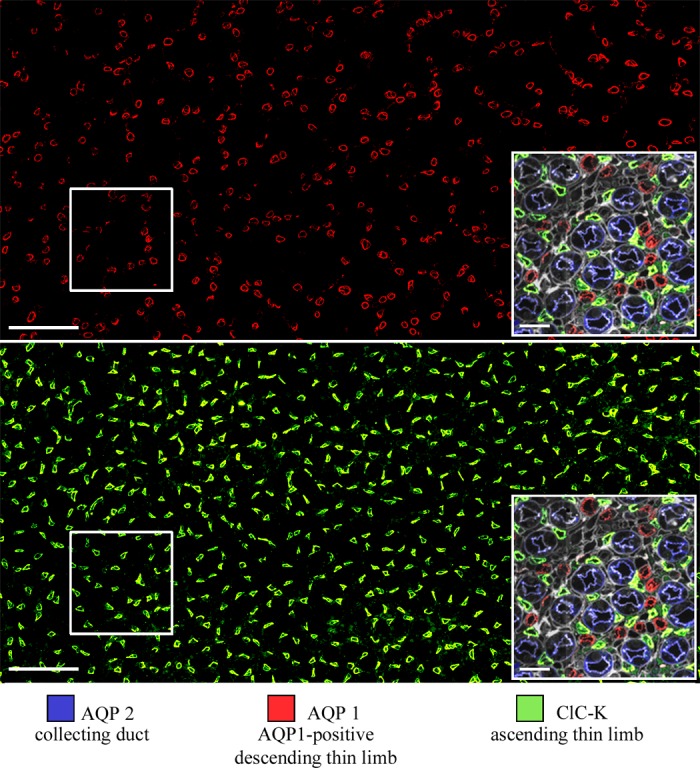
AQP1 and ClC-K1 expression in kangaroo rat inner medulla. Transverse section from 2,000 μm below the outer medulla. Scale bars: 100 μm and 20 μm (inset). Boxes are enlarged in right corner insets. Figure was modified from Urity et al. (144).
In the inner medulla of the Munich-Wistar rat and kangaroo rat, an outer zone consisting of the initial two-thirds of the IM and an inner zone consisting of the terminal third can be distinguished. Comparable zonation occurs in the hamster, mouse, Psammomys, and chinchilla. Distinct CD clusters dominate the architecture of the outer zone, with clusters much less apparent in the inner zone. Types 2 and 3 long-loop DTLs are apparent in the outer zone, with predominantly type 3 DTLs in the inner zone. A greater proportion of fenestrated over nonfenestrated vessels in the inner zone has been reported for Munich-Wistar rat and kangaroo rat (48, 55, 106, 107, 156) (T. L. Pannabecker, unpublished). A large fraction of loops exhibit substantial transverse-running segments at loop bends, lying in close association with the very large diameter CDs that exist at the papilla tip, has been reported only for the Munich-Wistar rat (106, 109). NaCl reabsorption from these transverse-running segments could amplify salt build-up within the papilla tip, with consequent amplification of water reabsorption from the CDs in this terminal zone. The unique papillary loop of Henle segment in the chinchilla (17) and a comparable segment hypothesized to exist in Psammomys obesis lie in the inner zone (7, 17). Transverse-running loop bends of the rat terminal papilla and the unique papillary loop of the chinchilla lie in a region that is not frequently investigated, and possibly these segment types are more common among other species than the literature would indicate.
Segmentation of Medullary Nephrons and Blood Vessels
Loops of Henle.
Medullary nephrons are categorized into two types determined by the depths at which the loops of Henle form their bends. These two types are the short-loop nephrons, which, in most mammalian species, form their bends in the outer medulla, and the long-loop nephrons, which form their bends in the inner medulla (Fig. 7). In the most highly concentrating species, the number of short loops is always greater than the number of long loops (65). The ratio of short-loop nephrons to long-loop nephrons is about 82:18 in mice (67, 158), 70:30 in rats (133), and 66:34 in Psammomys (52).
Fig. 7.
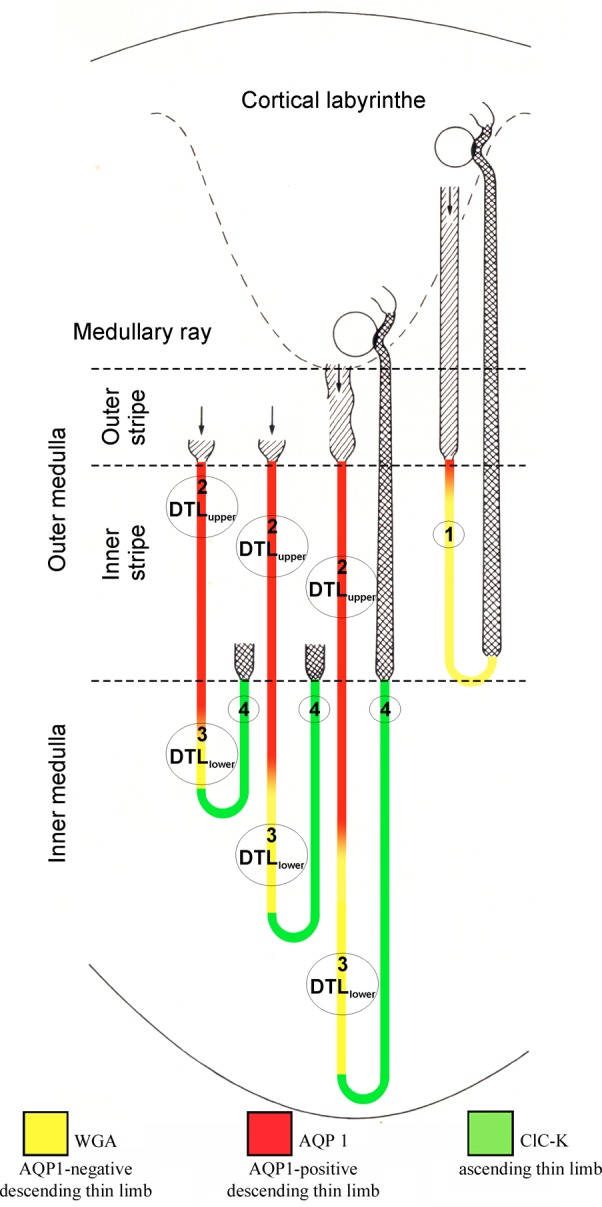
Segmentation of rat thin limbs of the loop of Henle. The short-loop nephron (right, belonging to a superficial glomerulus) has a DTL (pars recta of proximal tubule; hatched), a DTL (turning back near the outer medullary-inner medullary boundary), and a thick ascending limb (cross-hatched), which passes into the distal convoluted tubule a short distance beyond the macula densa (shown in black). The long-loop nephron (second from right, belonging to a juxtamedullary glomerulus) contains a DTL subdivided into two parts, type 2 and type 3 epithelium; and an ATL, type 4 epithelium; the bend is located in the inner medulla. Two additional long-loop nephrons (incompletely drawn) demonstrate heterogeneity among long-loop nephrons, which turn back at different levels within the inner medulla. Numbers 1–4 refer to type of epithelium encountered in corresponding thin limb part: type 1, DTL of short loops; type 2, upper part of DTL of long loops; type 3, lower part of DTL of long loops; type 4 (beginning short distance before bend), ATL. WGA, wheat germ agglutinin. Figure based on data from Refs. 17, 66, 105, 159. From Pannabecker (104).
Short-loop nephrons have a thin descending segment, whereas their ascending segment consists only of the thick ascending limb of the loop of Henle. The bends of the short loops of most species lie at approximately the same axial level of the inner stripe, at the outer medullary-inner medullary boundary. Long-loop nephrons have a thin descending segment and, in the inner medulla, a thin ascending segment, the latter becoming the thick ascending limb at the outer medullary-inner medullary boundary. The bends of long loops form at all levels of the inner medulla in a cascading fashion (Fig. 7). As a result, the number of loops at progressively deeper transverse levels declines in number; this decline can be fit to an exponential rate (59, 78, 110). About 1,500 of the 10,000 long loops entering the inner medulla of rat kidney reach the second half of the inner medulla, and only about 250 reach the terminal 1 mm of the papilla (65). The decreasing loop population as a function of inner medullary depth is considered to contribute to urine hypertonicity (78).
The ultrastructural characteristics of cells of thin limbs of the loop of Henle have been reviewed in detail (2, 17, 49, 53, 65, 129, 130, 158). Short-loop DTLs consist of a single segment, the type 1 segment. The long-loop DTLs of most species that have been studied (rat, chinchilla, mouse, kangaroo rat, Psammomys, rabbit, hamster) are known to consist of two morphologically distinct segments (53). These two segments are designated as the type 2 segment (DTLupper), for those descending segments lying in the inner stripe and outer inner medulla, and the type 3 segment (DTLlower), for those segments lying in the innermost region of the inner medulla. The type 3 segment continues almost to the bend of the loop of Henle for all species studied, except for the chinchilla. Most long DTLs in the outer half of the inner stripe consist of type 2 epithelia; however, a clearly heterogeneous and species-specific distribution of types 2 and 3 DTLs exists at deeper levels of the medulla. In Psammomys and mouse, most long DTLs at the beginning of the inner medulla consist of type 2 epithelia (53), whereas in the rat most DTLs at this level are lined with type 3 epithelia (130). The transition from type 2 to type 3 DTL occurs relatively abruptly (16, 130). The transition from type 2 to type 3 segments corresponds to the transition between AQP1-positive and AQP1-negative segments, as discussed below in this section. Type 4 epithelia are found in the prebend segment that lies at the terminal portion of the descending segment and in the entire ATL.
In nearly all species that have been investigated to date, the long-looped DTL terminates with a prebend segment that has ultrastructural characteristics of the ATL. Prebend segments with these characteristics have a mean length of about 700 μm in the mouse (158) and range from about 50 to 140 μm in the Sprague-Dawley rat (130) and from 0 to 140 μm in rabbit (54). In the Munich-Wistar rat, the length of expression of the Cl channel ClC-K1 along the prebend in the outer inner medullary zone is about 190 μm, whereas in the kangaroo rat the length of ClC-K1 expression is only about 100 μm (144). In the chinchilla, a unique papillary segment beginning ≤2,000 μm before the bend and terminating some distance after the bend is morphologically distinct from the ATL (17). A segment exhibiting some similarity to the chinchilla papillary segment is considered to be present in the desert sand rat Psammomys (7, 17), a species that can produce a urine having a maximal osmolality similar to that of the mouse and chinchilla (about 6,000 mosmol/kgH2O) (8).
In most species, the bends of long-loop nephrons resemble U-shaped hairpin bends. However, in the terminal 500-μm region of the Munich-Wistar rat papilla, some loop bends exhibit 5- to 10-fold greater transverse length than the average transverse length of hairpin bends (106).
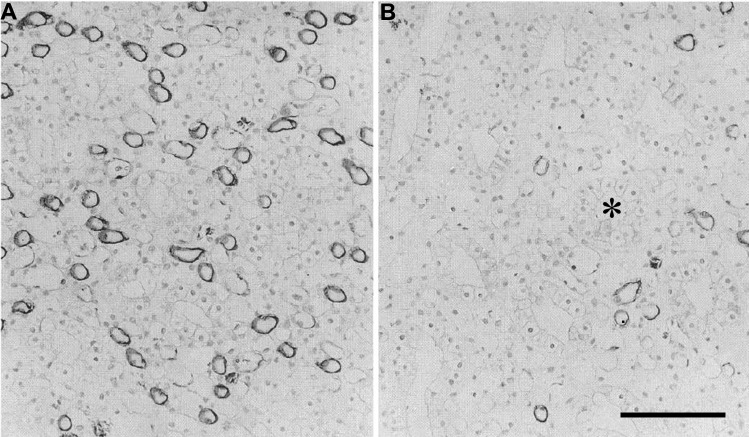
DTLs that express undetectable AQP1 in their inner medullary segments and reach to no more than about 1 mm into the Munich-Wistar rat inner medulla lie at the periphery of CD clusters in transverse sections (108) (Fig. 2). Loops of the Munich-Wistar rat kidney that reach more than 1 mm into the inner medulla label for AQP1 only in the upper 40% of their DTLs; the lower 60% does not express detectable AQP1. In contrast, loops of Henle from the kangaroo rat that make their bends at depths equal to those of the Munich-Wistar rat, label for AQP1 in the upper 60–70% of their DTLs. This suggests that loops of the kangaroo rat have a proportionately longer water-permeable epithelium than those of the Munich-Wistar rat (Fig. 7). Whether this reflects merely an anatomical distinction, possibly related to the longer papillary length, or, on the contrary, leads to significant functional impact is not clear. In any case, the greater length of AQP1 expression in the kangaroo rat DTL could permit osmotic equilibration between the luminal and interstitial fluids for a longer length of each loop, possibly leading to greater luminal solute concentration (144). Detectable AQP1 levels are relatively low in all DTL segments in the final 2.0–2.5 mm of the rat, kangaroo rat, and chinchilla inner medulla (Fig. 8) (17, 106, 144). Apparently, a lower density of AQP1 water channels is involved in osmotic equilibration between DTL luminal fluid and interstitial fluid in the type 3 AQP1-negative segments. It is possible that osmotic equilibration requires very low water flux in the deepest DTL segments due to abundant solute secretion in this region (90, 111). Solute secretion potentially leads to elevation of luminal NaCl concentrations sufficiently high to enable NaCl reabsorption by the deepest ATL segments (73, 77).
Fig. 8.
Immunohistochemical localization of AQP1 water channel protein in chinchilla inner medulla. A: section from 60–75% of the way along the inner medullary axis, demonstrating heavy labeling of numerous thin limbs. B: section from papillary tip. Few thin limbs were labeled in this region. *A collecting duct. Scale bar: ∼100 μm. Figure was modified from Chou et al. (17).
The transition from the DTL of short-loop nephrons to the thick ascending limb (TAL) occurs before or after the loop bend, following species-dependent patterns (2, 53, 158). The transition from ATL of long-loop nephrons to TAL occurs fairly abruptly at the outer medullary-inner medullary boundary in most species (53), and in fact, this transition defines the outer medullary-inner medullary boundary. The TAL of the inner stripe is morphologically distinct from the TAL of the outer stripe and cortex, exhibiting greater cell height, more abundant mitochondria, and deeper and more extensive and complex invaginations of the basal plasmalemma (64).
The outer medullary interstitial fluid osmolality is raised by NaCl reabsorption from the TAL. This NaCl reabsorption occurs by way of secondary active transport at the apical membrane, chiefly by NKCC2, and active transport at the basolateral membrane by Na-K-ATPase (95). TALs of the inner stripe of rat kidney exhibit greater Na-K-ATPase activity than those of the outer stripe and cortex (29), and vasopressin increases NaCl reabsorption in the medullary but not cortical TAL (35). An outer medullary-inner medullary mathematical model of the UCM predicts that a higher active transport rate in TAL of the inner stripe, relative to that of the outer stripe, can produce a higher urine osmolality for a given energy cost (72). Mitochondria occupy a greater percentage of medullary TAL cell volume in smaller mammals, and mitochondria are more densely packed with cristae (1). It is therefore reasonable to hypothesize that the greater concentrating capability of some smaller animals such as some desert rodents may arise, in part, from greater TAL Na-K-ATPase activity.
Collecting ducts.
CDs descend through the outer medulla largely as unbranched segments. In the inner medulla, near the outer medullary-inner medullary boundary, CDs begin to coalesce, becoming larger in diameter in their descent. In the rat about 7,000 CDs enter the inner medulla (99, 124) and, in the Munich Wistar rat, just prior to their forming the ducts of Bellini where CD fluid exits the kidney, have coalesced variably into about 10–15 CDs (106).
The outer medullary CDs consist of two cell types, intercalated and principal cells. The inner medullary CDs of the initial one-third of the inner medulla (IMCD1) also consist of intercalated and principal cells (about 10% of IMCD1 cells are intercalated); however, CDs of the terminal two-thirds of the inner medulla (IMCD2 and IMCD3) consist of a structurally and functionally distinct cell type, the IMCD cell (21, 88). Urea and water permeabilities, and their regulation by vasopressin vary among IMCD1, -2, and -3. A number of reviews focus on the intricacies of rat and mouse CD tubular and cellular functionality (11, 27).
Vasa recta.
The renal medulla is perfused by blood vessels called vasa recta. Vasa recta consist of two types: those with a continuous endothelium (DVR) and those with a discontinuous, fenestrated, endothelium (AVR). Vasa recta arise from efferent arterioles of juxtamedullary glomeruli. The efferent arterioles enter the medulla, where they give rise to the DVR. DVR descend a distance before giving rise to the AVR, which then ascend through the medulla. The DVR are defined by the absence of the one to three layers of smooth muscle cells (containing smooth muscle α-actin) in the efferent arteriole wall (101). There is a gradual transition along the vessel to a point where pericytes completely replace the smooth muscle cells. This point also corresponds to the loss of accompanying nerves, which are then absent throughout the deeper medulla. The DVR express the urea transporter UT-B; the water channel AQP1 is expressed in apical and basolateral membranes of some, but not all, DVR (see Water and urea transport in the descending and ascending vasa recta).
In AVR, the endothelial fenestrae in the Sprague-Dawley rat measure 500 to 800 Å in diameter and are bridged by single, electron-dense membranes, or diaphragms, that are ∼40 Å thick and 65.4 nm in diameter (86, 128). Fenestrae are arranged in plaques with a mean interfenestral distance of about 115 nm (at 1,800 μm above the papilla tip). The protein PV-1 is expressed in the fenestral diaphragm (135). The fenestrae of medullary AVR are considered to have very high permeability to water and small solutes as shown for fenestrae of vessels in other tissues (87, 93, 101, 102). A type of AVR commonly referred to as “interconnecting” or “communicating” capillaries exhibits no known structural or functional distinctions but is considered to be a population of vessels carrying plasma in an ascending direction that is spatially resolved from nonbranching vessels (101).
DVR and AVR exhibit little or no branching, whereas interconnecting capillaries generally exhibit repeated branching, thereby forming sparse networks (94, 101, 120). All of the DVR carry blood in a descending direction, and AVR and interconnecting (fenestrated) capillaries are generally considered to carry blood in an ascending direction. However, functional studies of Zimmerhackl and colleagues (160) suggested that 10–15% of Munich-Wistar rat DVR are fenestrated segments that carry blood in a descending direction. This was confirmed with three-dimensional reconstructions that showed for the outer zone of the Munich-Wistar rat inner medulla that all DVR have a terminal fenestrated (PV-1-positive) segment that partially overlaps with the UT-B-positive segment. This fenestrated segment descends a distance equal to about 15% of the length of the connecting UT-B-positive DVR (156).
The DVR descend through the outer and inner medulla and terminate at all levels of the medulla. Each terminal DVR feeds into one or several interconnecting capillary networks (branching AVR) that ascend and undergo extensive branching (55, 94, 120). These capillaries join with capillaries that arise from the termini of other DVR. The DVR, collectively, thus feed into a complex network of capillaries, also referred to as the vascular plexus. Distinct capillary plexuses of the outer medulla arise chiefly from DVR terminating in the outer medulla; capillary plexuses of the inner medulla arise from DVR terminating in the inner medulla (94, 120). Outer medullary plexuses are significantly denser than the sparse plexuses of the inner medulla.
In the outer medulla, the capillary plexuses lie in the interbundle region. In the inner medulla, the capillary plexuses lie in the intracluster region where they form close physical associations with the CDs (see Inner Medullary Interstitial Microdomains). At various levels, interconnecting capillaries of the plexuses join AVR that are grouped with DVR in vascular bundles. These AVR then ascend along the corticopapillary axis. The dominant pathway for AVR flow from the inner medulla into the outer medulla occurs via the vascular bundles; however, a fraction of inner medullary capillaries exit directly into the interbundle region (55, 94).
Water and Urea Transport Proteins of the Renal Medulla
Several key membrane proteins of medullary nephrons and blood vessels that are involved with transepithelial fluid and urea flux have been cloned and expressed in heterologous systems. These proteins include members of the aquaporin water channel and UT urea transporter families (113, 155). Medullary aquaporins that are associated with the UCM include AQP1, AQP2, AQP3, and AQP4. The urea transporters include UT-A1, UT-A2, UT-A3, and UT-B. Medullary nephrons in which these transporters are expressed are shown in Fig. 9. Additional isoforms for both protein families are expressed in renal and nonrenal tissue (96, 122); these are not discussed further. Functional studies of these proteins have provided some important insights into long-standing, but poorly understood, issues of nephron and vessel function related to the UCM of rat and mouse.
Fig. 9.
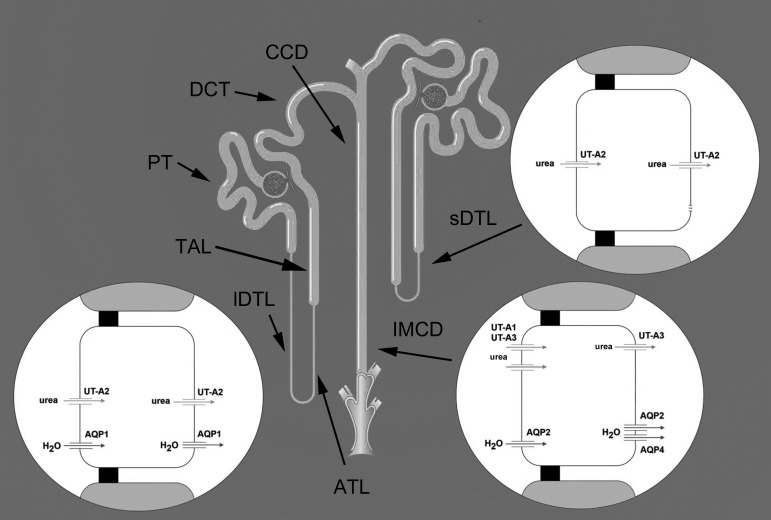
Aquaporin and urea transporters in descending thin limb of loop of Henle and inner medullary collecting duct (IMCD). In addition, but not shown, AQP1 is expressed in the initial ∼10% of the short-loop DTL (159) and has been reported to be expressed in the basolateral membrane of the thick ascending limb (TAL) (14). PT, proximal tubule; lDTL, long-loop descending thin limb; ATL, ascending thin limb; sDTL, short-loop descending thin limb; DCT, distal convoluted tubule; CCD, cortical collecting duct. The apical membrane is shown on the left and basal membrane on the right of tubule cell structures. Tight junctions are shown as black squares. See text for further details. Figure was modified from Fenton and Knepper (26).
Water Channels
Aquaporin 1.
A specific pathway for transmural osmotic water flux in DTLs and DVR was identified with the cloning of the first water channel AQP1 (reviewed in Ref. 96). In the short-loop DTLs of the rat and mouse this constitutively active water channel is weakly expressed in both apical and basolateral plasma membranes but only along the initial 10% immediately subsequent to the proximal tubule (159). In contrast, it is abundantly expressed in cells of long-loop DTLs, also in both the apical and basolateral plasma membranes (97), consistent with a role for AQP1 in the movement of water across both surfaces of the cells.
Cells of the type 2 DTL epithelium express a high density of intramembranous particles (IMPs) in the apical and basolateral membranes of thin limb segments from Wistar rat kidney (39) and Sprague-Dawley rat kidney (129). Freeze-fracture electron microscopy studies of rat and mouse kidney have shown that the IMPs in inner medullary long-loop DTLs are predominantly AQP1 water channels (19, 146). The density of IMPs in DTLs extending through the outer medulla and into the initial third of the inner medulla is quantitatively greater than the density of IMPs in DTLs from the lower two-thirds of the inner medulla (130). The density of IMPs in DTLs from the lower two-thirds of inner medullary DTLs is quantitatively similar to the density of inner medullary ATL IMPs. These studies are consistent with a significant reduction in abundance of water channels in the lower two-thirds of inner medullary DTLs. In the ATL, IMPs likely reflect expression of proteins unrelated to AQP1 (146). Intramembranous particles are also abundant in plasma membranes of DVR endothelial plasma membranes and sparse in AVR (128). Some of these particles represent AQP1 water channels in DVR, as well as other proteins in both DVR and AVR.
Aquaporin 2.
AQP2 is expressed in the apical and basolateral membranes and in subapical vesicles of medullary CDs. Similar patterns of AQP2 expression in medullary CDs are seen in kidneys of rats and mice (26, 96), chinchilla (T. L. Pannabecker, unpublished), kangaroo rat (48, 144), musk shrew (89), and degus (10). Vasopressin regulates total AQP2 protein abundance within the cell and polarized protein targeting to the plasma membrane (11). In the short term, elevated vasopressin leads to protein sorting from endosomes to apical membrane, whereas in the longer term, elevated vasopressin leads to greater abundance of total AQP2 protein within the cell.
Hypertonicity can influence preferential targeting of AQP2 to either basolateral or apical membrane in the rat. After in vitro or in vivo exposure to pericellular hypertonic media, vasopressin increased the basolateral-to-apical AQP2 protein expression ratio in the CDs of the outer zone of the inner medulla (145). The physiological implications of an increased basolateral-to-apical expression ratio are poorly understood; however, recent studies suggest that AQP2 is constitutively sorted to the basolateral membrane and, following vasopressin treatment, is sorted to the apical membrane (157). In the context of hydration-dehydration cycles, the hydropenic kidney reabsorbs its greatest proportion of filtered fluid in CDs that lie outside of the inner medulla. The relative significance of inner medullary CD basolateral and apical AQP2 expression (and membrane fluid permeabilities) in the process of transepithelial water flows needs clarification. Whether or not, and which genomic modifications occur in response to hypertonicity, and that modify vasopressin-regulated AQP2 targeting in medullary CDs are not known. As AQP2 is the dominant water transport pathway in the CD, investigations into variable AQP2 regulatory pathways among species with high inner medullary interstitial osmolality and high urine-concentrating capacity may provide clues to the overall physiological roles of AQP2 membrane sorting.
Aquaporin 3 and aquaporin 4.
AQP4 protein is expressed in the S3 segment of proximal tubules of the mouse but not in rat proximal tubules (38). AQP4 protein is also absent in proximal tubules of kangaroo rat and musk shrew (38, 89); although it is expressed in the basolateral membrane of medullary CDs of the rat and mouse (26, 96) and musk shrew (89). On the other hand, AQP4 protein is absent from the inner medullary CD of the kangaroo rat (Dipodomys merriami) (38). Although AQP4 mRNA is expressed in the CD of the kangaroo rat, there is complete absence of protein expression, indicating that protein expression of AQP4 in the kangaroo rat CD varies at the transcriptional or translational level.
In the mouse and rat, AQP4 is considered to be the principal basolateral membrane pathway for CD water flux. The isolated initial IMCD from the AQP4 knockout mouse exhibits a fourfold reduction in transepithelial water permeability (in vitro) compared with that of the wild type (both with 100 pM peritubular vasopressin) (20). On the other hand, with ad-lib water access in vivo, urine osmolality is no different between wild type and AQP4 knockout, and only a mild urine-concentrating defect (∼20% below that of the wild type) occurs after 36 h water deprivation (with or without intraperitoneal DDAVP) (85). Thus a relatively large reduction in IMCD transepithelial water permeability appears to have a relatively minor effect on overall in vivo urine-concentrating ability. These data suggest that AQP4 is a relatively unimportant player in the UCM. Possibly another aquaporin, such as AQP3, plays a proportionately greater role in transepithelial water flux of kangaroo rat CDs (38).
Urea Transporters
UT-A1 and UT-A3.
Studies have shown UT-A1 and UT-A3 protein expression in the cytoplasm and apical membrane of the IMCD of the rat (9, 98, 140) and UT-A3 protein expression in the basolateral membrane of the rat (9) and mouse (137). UT-A1 and UT-A3 IMCD expression varies with dietary protein levels, so species-dependent expression may follow patterns that reflect diet and habitat. The crystal structure of a bacterial homologue of the mammalian UT-A1 and UT-A3 channels shows that the urea transporter operates by a channel-like mechanism that exhibits selective permeation consistent with that of UT urea transporters (82). The slot-like structure at the opening of the protein pore is considered to serve as a selectivity barrier to permeation.
The distribution of UT-A1 and UT-A3 throughout the renal medulla, the short- and long-term regulation of their expression and function, and their roles in the UCM are the focus of many recent reviews (6, 26, 121, 122) and are not discussed further.
UT-A2.
In the kidney, the urea transporter UT-A2 is expressed only in the DTL. Expression of mRNA for UT-A2 was observed in the lower portion of type 1 epithelia of the Sprague-Dawley rat short-loop DTL and in the long-loop DTL (131). The abundance of the UT-A2 transcript is increased in Sprague-Dawley rats in response to water restriction (132) and is increased in Brattleboro rats (a strain with impaired vasopressin synthesis and release) by dDAVP infusion (114).
Strong immunohistochemical labeling for UT-A2 protein was observed in the apical and basolateral plasma membranes, as well as the cytoplasm of the lower portion of type 1 epithelia of the rat short-loop DTL. Weak labeling was observed in the outer and inner medullary segments of rat long-loop DTLs close to the outer medullary-inner medullary boundary (56, 83, 98, 148). UT-A2 is also expressed in the mouse short-loop and long-loop DTL (28, 148) and is expressed in the kangaroo rat short DTL (T. L. Pannabecker, unpublished observations).
In Brattleboro rats, UT-A2 antibody labels the lower portion of descending limbs from short-loop nephrons, and this labeling is increased in response to treatment with the vasopressin analog dDAVP (148). Increased labeling with dDAVP administration is also seen in Brattleboro rat descending limbs of long-loop nephrons near the outer medullary-inner medullary boundary. Tissue levels of total UT-A2 protein, both in the outer and inner medulla, were increased with dDAVP infusion, as determined with immunoblotting. Further studies are needed to determine whether the increased levels of UT-A2 expression with dDAVP reflect an increased length of expression along the entire tubule axis. Water restriction for 3 days increased UT-A2 immunoreactivity intensities in the plasma membrane and cytoplasm of rat loop of Henle in the initial inner medulla, and UT-A2 expression decreased in hydrated rats given 3% sucrose in water for 3 days (83) and in rats treated with furosemide for up to 7 days (81). These studies suggested that changes in hydration status over a 3- to 7-day period can affect urea transporter protein expression without changing its subcellular distribution.
UT-B.
In the kidney, UT-B is expressed only in the medullary DVR endothelial cells (115, 143). Urea countercurrent exchange between the highly fenestrated AVR and the DVR is considered important for cycling urea into the inner medulla (6). The mild urine-concentrating effect seen in the UT-B knockout mouse indicates a role for UT-B in the UCM (57, 153).
Water and Urea Transport in Medullary Nephrons and Blood Vessels
Water and urea transport in the loops of Henle.
In all thin limb segments, transepithelial flux of major solutes is generally considered to occur primarily by diffusion. There is very little evidence that active transport contributes to substantial, transcellular solute flux in any of the thin limbs of the loop of Henle (15, 40, 43, 138).
Short DTLs (type 1) of the hamster were reported to have substantial water permeability in the single published study of short loop water permeability (42). However, in this study Imai and colleagues acknowledged the possibility of failing to distinguish between types 1 and 2 segments, so the high water permeability values of short loop DTLs may be significantly lower than those reported from that study. The absence of detectable AQP1 in short loop DTLs of the mouse, rat, and human, determined with immunohistochemistry (159), as well as a low but significant osmotic water permeability in long-loop DTLs of AQP1 knockout mice (discussed below), suggest the possible existence of alternative pathways for transepithelial water flux.
The water permeability of type 1 segments is lower than the very high permeabilities measured in segments of the long-loop DTLupper (type 2) from the hamster (41, 42, 45–47), rabbit (62), chinchilla (16), rat (22, 40), and mouse (19). The osmotic water permeability along the axis of long-loop DTLs declines significantly with increased depth below the outer medullary-inner medullary boundary in the rat and chinchilla, the only species for which measurements are available for both upper and lower DTL segments.
The long-loop DTLs are functionally heterogeneous; tubule segments composed of cells with structural characteristics typical of types 2 and 3 epithelia exhibit high and low osmotic water permeability, respectively, in the chinchilla (17). In the Munich-Wistar rat, high and low osmotic permeability correlate with the presence and absence of AQP1 protein expression, respectively (22, 105, 108). Chinchilla DTLs have an additional papillary segment intervening between types 3 and 4 (17). The water permeability of isolated, perfused papillary type thin limbs of chinchilla exhibit very low but nonzero water permeability (68 ± 9 μm/s). Variations in the imposed osmotic driving force in isolated tubule experiments (at least within the range of several hundred mosmol/kgH2O) do not significantly alter the very low fluid permeability from DTLs of the deep papilla of chinchilla (16).
It has been shown for the moderately antidiuretic rat and hamster that luminal fluid at the bend of the loops that lie near the tip of the papilla is in approximate osmotic equilibrium with capillary fluid (31, 51, 91, 92, 112) and therefore is likely in equilibrium with interstitial fluid. Transepithelial water permeability data for the rat, hamster, and chinchilla thin limbs suggest that osmotic equilibration by water flux could occur by relatively high volume flux in the upper DTL. In contrast, for the lower DTL, studies with the rat and chinchilla suggest that if osmotic equilibration occurs at all, it involves relatively little volume flux. Equilibration may therefore occur by solute flux, to varying degrees, in both the upper and lower DTL of the rat, hamster, chinchilla, and Psammomys. A role for solute secretion has been captured in mathematical models of the UCM (77). The collective histotopography of inner medullary AQP1-positive DTLs, on one hand, and AQP1-negative DTLs on the other, and their different water permeabilities are likely related to distinct contributions of these segments to production of the axial osmotic gradient of the inner medulla (76).
ATLs from all mammalian species investigated have repeatedly been shown to have little or no transepithelial osmotic water permeability (16, 22, 40, 43, 84, 154) and lack plasma membrane expression of any of the known aquaporins (96).
Micropuncture studies have shown a large net secretion of urea into rat and hamster short-loop and long-loop DTLs (70, 90, 111). The UT-A2 urea transporter is one likely pathway for urea secretion into the DTL, at least for those DTL segments lying near the outer medullary-inner medullary boundary. The combination of low transepithelial water permeability and significant transepithelial urea permeability in the terminal portions of DTLs of short-loop nephrons have been shown to support urea cycling in mathematical models of the UCM (75).
In the Munich-Wistar rat (25) and chinchilla (15, 16) long-loop DTL, urea permeability increases with increasing depth below the outer medullary-inner medullary boundary, qualitatively opposite to the axial decline in water permeability. These transepithelial urea fluxes in inner medullary DTL segments appear to be nonsaturable and are not inhibited by phloretin, and therefore are unlikely to be mediated by any of the known phloretin-sensitive renal urea transporters (UT-A1, UT-A2, UT-A3) (122). Two hypothetical pathways for these urea movements include an unknown urea transporter and/or the paracellular pathway.
The chinchilla ATL (15) and Munich-Wistar rat ATL (25) have been found to have very high transepithelial urea permeabilities, substantially higher than the urea permeabilities published over 30 years ago for ATLs from hamster and rat (40). As in the DTL, the transepithelial urea fluxes shown to occur in chinchilla ATL are not inhibited by phloretin and therefore are unlikely to be mediated by any of the known phloretin-sensitive renal urea transporters. Also, as in the DTL, two hypothetical pathways for these urea movements include an unknown urea transporter and/or the paracellular pathway. The high urea permeability of chinchilla ATL, combined with an even greater NaCl permeability, would enable rapid osmotic equilibration of luminal loop of Henle fluid to occur as fluid ascends toward the outer medulla. Although conforming to the passive mechanism principle that Na reabsorption exceed urea secretion (18) (Fig. 1), a high urea permeability potentially prevents dilution of ATL luminal fluid. A high ATL urea permeability could therefore oppose buildup of the inner medullary solute gradient if urea flux is secretory. However, if urea is secretory in the lower ATL and reabsorptive in the upper ATL, as has been suggested (150), then the rapid equilibration could produce a more dilute ATL fluid and more concentrated interstitium, thereby sustaining the medullary axial solute gradient. In any case, the urea passive flux is dependent on the urea concentration in tubular and interstitial compartments and some uncertainty of these concentrations remains.
The TAL consists of a tight epithelium that actively transports NaCl and exhibits very low transepithelial osmotic permeability; thus a dilute luminal fluid is produced by this segment (119). The TAL cells undergo volume regulation (33, 34, 36), and AQP1 water channels in the basolateral membrane are believed to play a role in fluid flows associated with volume regulation (14). The rat and rabbit outer medullary TAL exhibits very low transepithelial urea permeability (58, 118) and expresses no known urea transporters.
Water and urea transport in the collecting duct.
Water and urea reabsorption by medullary CDs are key to the UCM. Transepithelial water and urea permeabilities increase at distinct levels below the outer medulla by way of aquaporins and urea transporters that are expressed in the principal cells, as noted above. Intercalated cells are devoid of known channels or transporters for water and urea transport. AQP2 membrane expression levels are considered to be rate limiting for transepithelial water permeability in medullary CDs (96). Vasopressin acting via the cAMP signaling pathway is the key regulator of CD water and urea permeability. In addition, a number of primary and secondary signaling pathways and extracellular fluid tonicity regulate total aquaporin and urea transporter protein expression levels and polarity within the cell (3, 11, 123).
High basal levels of plasma vasopressin may contribute to the high basal urine osmolality of desert rodents, possibly through direct effects on water and urea transporter membrane expression levels. Kangaroo rat basal plasma vasopressin concentrations during normal hydration were found to be two- to threefold higher than plasma vasopressin concentrations of Sprague-Dawley rat (134). Kangaroo rats were also found to exhibit a greater capacity for sustaining elevated plasma vasopressin for prolonged periods.
Water and urea transport in the descending and ascending vasa recta.
Osmotic water flow across the medullary DVR endothelium, driven by a NaCl gradient, occurs via the water channel AQP1 (100). Urea flux across the medullary DVR occurs via the urea transporter UTB (101). The AVR have been shown by in vivo micropuncture studies to have very high transendothelial permeabilities to both water and urea. All medullary AVR are highly fenestrated, and these pores are thought to underlie the high fluid and solute permeabilities. All fenestrated vessels of the medulla have a diaphragm; the only known protein associated with the diaphgragm is PV-1 (135). The terminal segments of the DVR in the inner medulla of Munich-Wistar rat and kangaroo rat lack expression of AQP1 and UTB, and, in the rat, show positive expression of PV-1 along variable axial distances (156). These segments are therefore fenestrated and are assumed to be highly permeable to water and all small-molecular-weight solutes. There have been no studies of isolated, perfused inner medullary vasa recta, so there remains some uncertainty about transendothelial permeabilities (101).
Most fenestrated vessels exit the inner medulla by way of vascular bundles (101); however, in Munich-Wistar rat, inner medullary capillaries (branching AVR) that lie distant from vascular bundles ascend directly from the outer zone of the inner medulla into the inner stripe interbundle region (55). These capillaries ascend from specialized interstitial compartments or microdomains in the inner medulla (see Inner Medullary Interstitial Microdomains May Serve as Solute Mixing Chambers). Urea may be sequestered within these microdomains (76), raising the possibility that capillaries exiting these regions could carry significant amounts of urea into the interbundle region of the outer medulla. As noted above, long-loop DTLs of the rat and chinchilla exhibit significant transepithelial (phloretin-insensitive) urea permeability, and, as suggested by modeling studies, the apparent outer medullary transepithelial concentration gradients could support urea secretion into long-loop DTLs (76). Therefore, these segments may serve as countercurrent exchangers with interbundle capillaries. The targeted delivery of urea to outer medullary DTLs, by way of branching AVR that originate in the inner medulla, and subsequent return of urea to the deeper inner medulla, may represent a significant urea recycling pathway (55).
Most papillary vessels are fenestrated and express PV-1 (86, 106), although vessels with continuous endothelium do exist in the terminal papilla of rat (128) and kangaroo rat (T. L. Pannabecker, unpublished observations). Whereas all DVR of the rat outer medulla express UT-B, only those of the outer half of the inner medulla express detectable UT-B (56, 106, 156). Similarly, reduced levels of UT-B are expressed in the inner half of the kangaroo rat inner medulla (T. L. Pannabecker, unpublished observations). Therefore, UTB-mediated countercurrent urea exchange between descending and ascending vessels lying near to each other is a feature primarily of the outer zone of the inner medulla (12, 86, 128), and it appears that this is limited to regions outside the CD clusters (48, 107). Solute recycling that occurs between descending and ascending vessels in the inner zone of the IM likely involves primarily fenestrated vessels. Countercurrent exchange between DVR and AVR in the outer zone of the IM may therefore be more selective for urea compared with the inner zone of the IM where it may more permissively include NaCl and urea, though both solutes are exchanged to some degree throughout the IM (101). Variation in vascular architecture further supports the concept that the inner and outer zones play functionally distinct roles in the urine-concentrating mechanism (109, 110), a concept based, in part, on the gradual disappearance of distinct intracluster and intercluster regions in the inner zone.
Inner Medullary Interstitial Microdomains May Serve as Solute Mixing Chambers
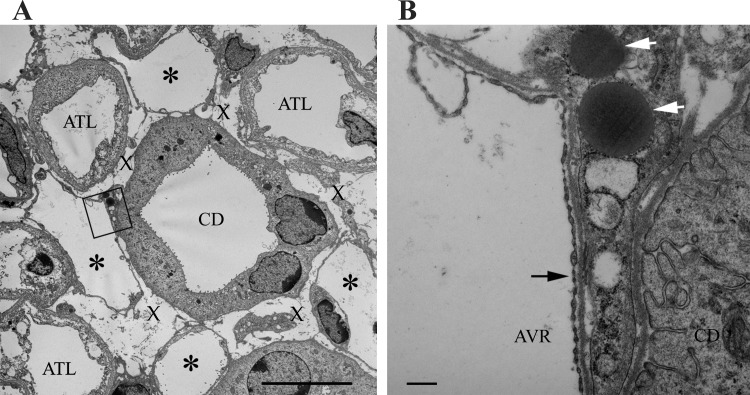
CDs in combination with some of the AVR and ATLs, when viewed in transverse sections, are arranged so as to form interstitial compartments or microdomains (Fig. 10). In the rat (107) and kangaroo rat (48), these have been referred to as interstitial nodal spaces (INSs). The INSs are bordered on one side by a CD, on the opposite side by one or more prebend segments or ATLs, and on the other two sides by ascending vasa recta or capillaries. INSs are arrayed at structured intervals throughout the inner medulla, within CD clusters, and could play an important role in generating the corticopapillary osmotic gradient, possibly by serving as compartmentalized solute mixing chambers (74, 76). The entire length of each prebend and postbend equivalent length ATL segment lies in contact with one or more INSs (103). Inclusion of NaCl-permeable loop bends as components of interstitial nodal spaces may function specifically to raise the osmolality of CD tubular fluid by facilitating the targeted delivery of NaCl from thin limbs to the CD clusters (109). In so doing, NaCl from thin limbs and urea from CDs preferentially mix together within a defined compartment, in accordance with the Kokko-Rector and Stephenson model of urine concentration as described in the introduction (63, 136). Reabsorbed solutes may be carried to higher levels at which point they may diffuse into INS compartments that are more dilute and provide a concentrating effect that leads to water reabsorption at progressively higher levels.
Fig. 10.
Ultrastructure of interstitial nodal spaces in a transverse ultrathin section from kangaroo rat inner medulla. A: interstitial nodal spaces are marked with an X. Fenestrated blood vessels (AVR) are marked with asterisks. B: magnification of boxed area in A. Black arrow identifies fenestrations of AVR endothelium. An interstitial cell containing lipid droplets (white arrows) lies juxtaposed with the AVR and CD. Section is from midway between the outer medullary-inner medullary boundary and papilla tip. Scale bars, 10 μm (A); 500 nm (B). From Issaian et al. (48).
Perspectives and Significance
Advanced understanding of structural and functional diversity of renal water channel and urea transporter expression, and of water and urea membrane permeabilities of nephron and vascular segments in a greater variety of species will widen our understanding of the UCM. This may also lead to better understanding of the diverse regulatory mechanisms associated with water channel and urea transporter function and the ways in which the urine-concentrating mechanism is tied in with nonrenal functions. In vivo studies have highlighted the efficiency of the spiny mouse kidney in filtering and excreting very high concentrations of NaCl, relative to C57BL/6 mouse (24). To accomplish this, the C57BL/6 mouse requires larger volumes of water, suggesting that species-specific AQP regulation mechanisms are involved. These and other studies are contributing to the understanding that, even among those species with high urine-concentrating capability, there may be significantly different mechanisms associated with concentrating and diluting urine (23, 30, 139).
GRANTS
This review was supported by the National Institute of Diabetes and Digestive and Kidney Diseases Grant DK-083338 and by the National Science Foundation Grants IOS-0952885 and IOS-1137275.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: T.L.P. conception and design of research; T.L.P. performed experiments; T.L.P. analyzed data; T.L.P. interpreted results of experiments; T.L.P. prepared figures; T.L.P. drafted manuscript; T.L.P. edited and revised manuscript; T.L.P. approved final version of manuscript.
REFERENCES
- 1. Abrahams S, Greenwald L, Stetson DL. Contribution of renal medullary mitochondrial density to urinary concentrating ability in mammals. Am J Physiol Regul Integr Comp Physiol 261: R719–R726, 1991 [DOI] [PubMed] [Google Scholar]
- 2. Bachmann S, Kriz W. Histotopography and ultrastructure of the thin limbs of the loop of Henle in the hamster. Cell Tissue Res 225: 111–127, 1982 [DOI] [PubMed] [Google Scholar]
- 3. Bagnasco SM. Role and regulation of urea transporters. Pflügers Arch 450: 217–226, 2005 [DOI] [PubMed] [Google Scholar]
- 4. Bankir L, De Rouffignac C. Urinary concentrating ability: insights from comparative anatomy. Am J Physiol Regul Integr Comp Physiol 249: R643–R666, 1985 [DOI] [PubMed] [Google Scholar]
- 5. Bankir L, Kaissling B, Rouffignac CD, Kriz W. Vascular organization of the kidney of Psammomys Obesus. Anat Embryol (Berl) 155: 149–160, 1979 [DOI] [PubMed] [Google Scholar]
- 6. Bankir L, Yang B. New insights into urea and glucose handling by the kidney, and the urine concentrating mechanism. Kidney Int 81: 1179–1198, 2012 [DOI] [PubMed] [Google Scholar]
- 7. Barrett JM, Kriz W, Kaissling B, De Rouffignac C. The ultrastructure of the nephrons of the desert rodent (Psammonys obesus) kidney. II. Thin limbs of Henle of long-looped nephrons. Am J Anat 151: 499–514, 1978 [DOI] [PubMed] [Google Scholar]
- 8. Beuchat CA. Structure and concentrating ability of the mammalian kidney: correlations with habitat. Am J Physiol Regul Integr Comp Physiol 271: R157–R179, 1996 [DOI] [PubMed] [Google Scholar]
- 9. Blount MA, Klein JD, Martin CF, Tchapyjnikov D, Sands JM. Forskolin stimulates phosphorylation and membrane accumulation of UT-A3. Am J Physiol Renal Physiol 293: F1308–F1313, 2007 [DOI] [PubMed] [Google Scholar]
- 10. Bozinovic F, Gallardo PA, Visser GH, Cortes A. Seasonal acclimatization in water flux rate, urine osmolality and kidney water channels in free-living degus: molecular mechanisms, physiological processes and ecological implications. J Exp Biol 206: 2959–2966, 2003 [DOI] [PubMed] [Google Scholar]
- 11. Brown D, Bouley R, Paunescu TG, Breton S, Lu HA. New insights into the dynamic regulation of water and acid-base balance by renal epithelial cells. Am J Physiol Cell Physiol 302: C1421–C1433, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Bulger RE, Trump BF. Fine structure of the rat renal papilla. Am J Anat 118: 685–722, 1966 [DOI] [PubMed] [Google Scholar]
- 13. Burg MB, Green N. Function of thick ascending limb of Henle's loop. Am J Physiol 224: 659–668, 1973 [DOI] [PubMed] [Google Scholar]
- 14. Cabral PD, Herrera M. Membrane-associated aquaporin-1 facilitates osmotically driven water flux across the basolateral membrane of the thick ascending limb. Am J Physiol Renal Physiol 303: F621–F629, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: urea and NaCl permeabilities. Am J Physiol Renal Fluid Electrolyte Physiol 264: F337–F343, 1993 [DOI] [PubMed] [Google Scholar]
- 16. Chou CL, Knepper MA. In vitro perfusion of chinchilla thin limb segments: segmentation and osmotic water permeability. Am J Physiol Renal Fluid Electrolyte Physiol 263: F417–F426, 1992 [DOI] [PubMed] [Google Scholar]
- 17. Chou CL, Nielsen S, Knepper MA. Structural-functional correlation in chinchilla long loop of Henle thin limbs: a novel papillary subsegment. Am J Physiol Renal Fluid Electrolyte Physiol 265: F863–F874, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Chou CL, Knepper MA, Layton HE. Urinary concentrating mechanism - the role of the inner medulla. Semin Nephrol 13: 168–181, 1993 [PubMed] [Google Scholar]
- 19. Chou CL, Knepper MA, Van Hoek AN, Brown D, Ma T, Verkman AS. Reduced water permeability and altered ultrastructure in thin descending limb of Henle in aquaporin-1 null mice. J Clin Invest 103: 491–496, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chou CL, Ma TH, Yang BX, Knepper MA, Verkman S. Fourfold reduction of water permeability in inner medullary collecting duct of aquaporin-4 knockout mice. Am J Physiol Cell Physiol 274: C549–C554, 1998 [DOI] [PubMed] [Google Scholar]
- 21. Clapp WL, Madsen KM, Verlander JW, Tisher CC. Morphologic heterogeneity along the rat inner medullary collecting duct. Lab Invest 60: 219–230, 1989 [PubMed] [Google Scholar]
- 22. Dantzler WH, Evans KK, Pannabecker TL. Osmotic water permeabilities in specific segments of rat inner medullary thin limbs of Henle's loops. FASEB J 23: 970–973, 2009 [Google Scholar]
- 23. De Rouffignac C, Morel F. Micropuncture study of water, electrolytes, and urea movements along the loops of Henle in Psammomys. J Clin Invest 48: 474–486, 1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Dickinson H, Moritz K, Wintour EM, Walker DW, Kett MM. A comparative study of renal function in the desert-adapted spiny mouse and the laboratory-adapted C57BL/6 mouse: response to dietary salt load. Am J Physiol Renal Physiol 293: F1093–F1098, 2007 [DOI] [PubMed] [Google Scholar]
- 25. Evans K, Pannabecker TL, Dantzler WH. Urea permeabilities in defined segments of rat renal inner medullary thin limbs of Henle's loops. FASEB J. In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Fenton RA, Knepper MA. Mouse models and the urinary concentrating mechanism in the new millennium. Physiol Rev 87: 1083–1112, 2007 [DOI] [PubMed] [Google Scholar]
- 27. Fenton RA, Praetorius J. Molecular physiology of the medullary collecting duct. In: Comprehensive Physiology, edited by Terjung RL. Bethesda, MD: Am. Physiol. Soc., 2011, p. 1031–1056 [DOI] [PubMed] [Google Scholar]
- 28. Fenton RA, Stewart GS, Carpenter B, Howorth A, Potter EA, Cooper GJ, Smith CP. Characterization of mouse urea transporters UT-A1 and UT-A2. Am J Physiol Renal Physiol 283: F817–F825, 2002 [DOI] [PubMed] [Google Scholar]
- 29. Garg LC, Mackie S, Tisher CC. Effect of low potassium-diet on Na-K-ATPase in rat nephron segments. Pflügers Arch 394: 113–117, 1982 [DOI] [PubMed] [Google Scholar]
- 30. Gordge L, Roberts JR. Kidney function in the Spinifex hopping mouse, Notomys alexis. Comp Biochem Physiol A Mol Integr Physiol 150: 90–101, 2008 [DOI] [PubMed] [Google Scholar]
- 31. Gottschalk CW, Mylle M. Micropuncture study of the mammalian urinary concentrating mechanism: evidence for the countercurrent hypothesis. Am J Physiol 196: 927–936, 1959 [DOI] [PubMed] [Google Scholar]
- 32. Hai MA, Thomas S. The time-course of changes in renal tissue composition during lysine vasopressin infusion in the rat. Pflügers Arch 310: 297–319, 1969 [DOI] [PubMed] [Google Scholar]
- 33. Hebert SC. Hypertonic cell volume regulation in mouse thick limbs. I. ADH Dependency and Nephron Heterogeneity. Am J Physiol Cell Physiol 250: C907–C919, 1986 [DOI] [PubMed] [Google Scholar]
- 34. Hebert SC. Hypertonic cell volume regulation in mouse thick limbs. II. Na+-H+ and Cl−-HCO3− exchange in basolateral membranes. Am J Physiol Cell Physiol 250: C920–C931, 1986 [DOI] [PubMed] [Google Scholar]
- 35. Hebert SC, Culpepper RM, Andreoli TE. NaCl transport in mouse medullary thick ascending limbs. I. Functional nephron heterogeneity and ADH-stimulated NaCl cotransport. Am J Physiol Renal Fluid Electrolyte Physiol 241: F412–F431, 1981 [DOI] [PubMed] [Google Scholar]
- 36. Hebert SC, Sun A. Hypotonic cell volume regulation in mouse medullary thick ascending limb: effects of ADH. Am J Physiol Renal Fluid Electrolyte Physiol 255: F962–F969, 1988 [DOI] [PubMed] [Google Scholar]
- 37. Hervy S, Thomas SR. Inner medullary lactate production and urine-concentrating mechanism: a flat medullary model. Am J Physiol Renal Physiol 284: F65–F81, 2003 [DOI] [PubMed] [Google Scholar]
- 38. Huang Y, Tracy R, Walsberg GE, Makkinje A, Fang P, Brown D, Van Hoek AN. Absence of aquaporin-4 water channels from kidneys of the desert rodent Dipodomys merriami merriami. Am J Physiol Renal Physiol 280: F794–F802, 2001 [DOI] [PubMed] [Google Scholar]
- 39. Humbert F, Pricam C, Perrelet A, Orci L. Freeze-fracture differences between plasma-membranes of descending and ascending branches of rat Henles thin loop. Lab Invest 33: 407–411, 1975 [PubMed] [Google Scholar]
- 40. Imai M. Function of the thin ascending limb of Henle of rats and hamsters perfused in vitro. Am J Physiol Renal Fluid Electrolyte Physiol 232: F201–F209, 1977 [DOI] [PubMed] [Google Scholar]
- 41. Imai M. Functional heterogeneity of the descending limbs of Henle's loop. II. Interspecies differences among rabbits, rats, and hamsters. Pflügers Arch 402: 393–401, 1984 [DOI] [PubMed] [Google Scholar]
- 42. Imai M, Hayashi M, Araki M. Functional heterogeneity of the descending limbs of Henle's loop. I. Internephron heterogeneity in the hamster kidney. Pflügers Arch 402: 385–392, 1984 [DOI] [PubMed] [Google Scholar]
- 43. Imai M, Kokko JP. Sodium chloride, urea, and water transport in the thin ascending limb of Henle. Generation of osmotic gradients by passive diffusion of solutes. J Clin Invest 53: 393–402, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Imai M, Kusano E. Effects of arginine vasopressin on the thin ascending limb of Henle's loop of hamsters. Am J Physiol Renal Fluid Electrolyte Physiol 243: F167–F172, 1982 [DOI] [PubMed] [Google Scholar]
- 45. Imai M, Taniguchi J, Yoshitomi K. Transition of permeability properties along the descending limb of long-loop nephron. Am J Physiol Renal Fluid Electrolyte Physiol 254: F323–F328, 1988 [DOI] [PubMed] [Google Scholar]
- 46. Imai M, Yasoshima K, Yoshitomi K. Mechanism of water transport across the upper portion of the descending thin limb of long-looped nephron of hamsters. Pflügers Arch 415: 630–637, 1990 [DOI] [PubMed] [Google Scholar]
- 47. Imai M, Yoshitomi K. Heterogeneity of the descending thin limb of Henle's loop. Kidney Int 38: 687–694, 1990 [DOI] [PubMed] [Google Scholar]
- 48. Issaian T, Urity VB, Dantzler WH, Pannabecker TL. Architecture of vasa recta in the renal inner medulla of the desert rodent Dipodomys merriami: potential impact on the urine concentrating mechanism. Am J Physiol Regul Integr Comp Physiol 302: R748–R756, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jamison RL, Kriz W. Urinary Concentrating Mechanism. New York: Oxford University Press, 1982 [Google Scholar]
- 50. Jen JF, Stephenson JL. Externally driven countercurrent multiplication in a mathematical model of the urinary concentrating mechanism of the renal inner medulla. Bull Math Biol 56: 491–514, 1994 [DOI] [PubMed] [Google Scholar]
- 51. Johnston PA, Battilana CA, Lacy FB, Jamison RL. Evidence for a concentration gradient favoring outward movement of sodium from the thin loop of Henle. J Clin Invest 59: 234–240, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Kaissling B, De Rouffignac C, Barrett JM, Kriz W. The structural organization of the kidney of the desert rodent Psammomys obesus. Anat Embryol 148: 121–143, 1975 [DOI] [PubMed] [Google Scholar]
- 53. Kaissling B, Kriz W. Morphology of the loop of Henle, distal tubule, and collecting duct. In: Handbook of Physiology. Renal Physiology, edited by Windhager EE. Bethesda, MD: Am. Physiol. Soc., 1992, sect. 8, vol. I, chapt. 3, p. 109–167 [Google Scholar]
- 54. Kaissling BWK. Structural analysis of the rabbit kidney. Adv Anat Embryol Cell Biol 56: 1–123, 1979 [DOI] [PubMed] [Google Scholar]
- 55. Kim J, Pannabecker TL. Two-compartment model of inner medullary vasculature supports dual modes of vasopressin-regulated inner medullary blood flow. Am J Physiol Renal Physiol 299: F273–F279, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kim YH, Kim DU, Han KH, Jung JY, Sands JM, Knepper MA, Madsen KM, Kim J. Expression of urea transporters in the developing rat kidney. Am J Physiol Renal Physiol 282: F530–F540, 2002 [DOI] [PubMed] [Google Scholar]
- 57. Klein JD, Sands JM, Qian L, Wang X, Yang B. Upregulation of urea transporter UT-A2 and water channels AQP2 and AQP3 in mice lacking urea transporter UT-B. J Am Soc Nephrol 15: 1161–1167, 2004 [DOI] [PubMed] [Google Scholar]
- 58. Knepper MA. Urea transport in isolated thick ascending limbs and collecting ducts from rats. Am J Physiol Renal Fluid Electrolyte Physiol 245: F634–F639, 1983 [DOI] [PubMed] [Google Scholar]
- 59. Knepper MA, Danielson RA, Saidel GM, Post RS. Quantitative analysis of renal medullary anatomy in rats and rabbits. Kidney Int 12: 313–323, 1977 [DOI] [PubMed] [Google Scholar]
- 60. Knepper MA, Roch-Ramel F. Pathways of urea transport in the mammalian kidney. Kidney Int 31, 1987 [DOI] [PubMed] [Google Scholar]
- 61. Knepper MA, Saidel GM, Hascall VC, Dwyer T. Concentration of solutes in the renal inner medulla: interstitial hyaluronan as a mechano-osmotic transducer. Am J Physiol Renal Physiol 284: F433–F446, 2003 [DOI] [PubMed] [Google Scholar]
- 62. Kokko JP. Sodium chloride and water transport in the descending limb of Henle. J Clin Invest 49: 1838–1846, 1970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kokko JP, Rector FC. Countercurrent multiplication system without active transport in inner medulla. Kidney Int 2: 214–223, 1972 [DOI] [PubMed] [Google Scholar]
- 64. Kone BC, Madsen KM, Tisher CC. Ultrastructure of the thick ascending limb of Henle in the rat kidney. Am J Anat 171: 217–226, 1984 [DOI] [PubMed] [Google Scholar]
- 65. Kriz W. Structural organization of the renal medulla: comparative and functional aspects. Am J Physiol Regul Integr Comp Physiol 241: R3–R16, 1981 [DOI] [PubMed] [Google Scholar]
- 66. Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: The Kidney: Physiology and Pathophysiology (2nd ed), edited by Seldin DW, Giebisch G. New York: Raven, 1992, p. 707–777 [Google Scholar]
- 67. Kriz W, Koepsell H. Structural organization of mouse kidney. Z Anat Entwicklungsgesch 144: 137–163, 1974 [DOI] [PubMed] [Google Scholar]
- 68. Kriz W, Schnermann J, Koepsell H. The position of short and long loops of Henle in the rat kidney. Z Anat Entwicklungsgesch 138: 301–319, 1972 [DOI] [PubMed] [Google Scholar]
- 69. Kuhn W, Ryffel K. Herstellung konzentrierter Losüngen aus verdünten durch blosse Membranwirkung: ein Modellversuch zur Funktion der Niere. Hoppe-Seylers Z Physiol Chem 276: 145–178, 1942 [Google Scholar]
- 70. Lassiter WE, Gottschalk CW, Mylle M. Micropuncture study of net transtubular movement of water and urea in nondiuretic mammalian kidney. Am J Physiol 200: 1139–1147, 1961 [DOI] [PubMed] [Google Scholar]
- 71. Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 300: F356–F371, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Layton AT. A mathematical model of the urine concentrating mechanism in the rat renal medulla. II. Functional implications of three-dimensional architecture. Am J Physiol Renal Physiol 300: F372–F384, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Layton AT, Dantzler WH, Pannabecker TL. Urine concentrating mechanism: impact of vascular and tubular architecture and a proposed descending limb urea-Na+ cotransporter. Am J Physiol Renal Physiol 302: F591–F605, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Layton AT, Gilbert RL, Pannabecker TL. Isolated interstitial nodal spaces may facilitate preferential solute and fluid mixing in the rat renal inner medulla. Am J Physiol Renal Physiol 302: F830–F839, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Layton AT, Layton HE. A region-based mathematical model of the urine concentrating mechanism in the rat outer medulla. I. Formulation and base-case results. Am J Physiol Renal Physiol 289: F1346–F1366, 2005 [DOI] [PubMed] [Google Scholar]
- 76. Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Functional implications of the three-dimensional architecture of the rat renal inner medulla. Am J Physiol Renal Physiol 298: F973–F987, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Layton AT, Pannabecker TL, Dantzler WH, Layton HE. Two modes for concentrating urine in rat inner medulla. Am J Physiol Renal Physiol 287: F816–F839, 2004 [DOI] [PubMed] [Google Scholar]
- 78. Layton HE. Distribution of Henle's loops may enhance urine concentrating capability. Biophys J 49: 1033–1040, 1986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Lemley KV, Kriz W. Anatomy of the renal interstitium. Kidney Int 39: 370–381, 1991 [DOI] [PubMed] [Google Scholar]
- 80. Lemley KV, Kriz W. Cycles and separations: the histotopography of the urinary concentrating process. Kidney Int 31: 538–548, 1987 [DOI] [PubMed] [Google Scholar]
- 81. Leroy C, Basset G, Gruel G, Ripoche P, Trinh-Trang-Tan MM, Rousselet G. Hyperosmotic NaCl and urea synergistically regulate the expression of the UT-A2 urea transporter in vitro and in vivo. Biochem Biophys Res Commun 271: 368–373, 2000 [DOI] [PubMed] [Google Scholar]
- 82. Levin EJ, Quick M, Zhou M. Crystal structure of a bacterial homologue of the kidney urea transporter. Nature 462: 757–761, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lim SW, Han KH, Jung JY, Kim WY, Yang CW, Sands JM, Knepper MA, Madsen KM, Kim J. Ultrastructural localization of UT-A and UT-B in rat kidneys with different hydration status. Am J Physiol Regul Integr Comp Physiol 290: R479–R492, 2006 [DOI] [PubMed] [Google Scholar]
- 84. Liu W, Morimoto T, Kondo Y, Iinuma K, Uchida S, Imai M. “Avian-type” renal medullary tubule organization causes immaturity of urine-concentrating ability in neonates. Kidney Int 60: 680–693, 2001 [DOI] [PubMed] [Google Scholar]
- 85. Ma T, Yang B, Gillespie A, Carlson EJ, Epstein CJ, Verkman AS. Generation and phenotype of a transgenic knockout mouse lacking the mercurial-insensitive water channel aquaporin-4. J Clin Invest 100: 957–962, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. MacPhee PJ. Fluid uptake by the renal medullary vasa recta: An estimate based on a quantitative analysis of the distribution of fenestrae in the vasa recta of young Sprague-Dawley rats. Exp Physiol 83: 23–34, 1998 [DOI] [PubMed] [Google Scholar]
- 87. MacPhee PJ, Michel CC. Fluid uptake from the renal medulla into the ascending vasa recta in anaesthetized rats. J Physiol 487: 169–183, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Madsen KM, Clapp WL, Verlander JW. Structure and function of the inner medullary collecting duct. Kidney Int 34: 441–454, 1988 [DOI] [PubMed] [Google Scholar]
- 89. Maeda S, Kuwahara S, Ito H, Tanaka K, Hayakawa T, Seki M. Expression and localization of aquaporins in the kidney of the musk shrew (Suncus murinus). J Histochem Cytochem 56: 67–75, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Marsh DJ. Solute and water flows in thin limbs of Henle's loop in the hamster kidney. Am J Physiol 218: 824–831, 1970 [DOI] [PubMed] [Google Scholar]
- 91. Marsh DJ, Azen SP. Mechanism of NaCl reabsorption by hamster thin ascending limbs of Henle's loop. Am J Physiol 228: 71–79, 1975 [DOI] [PubMed] [Google Scholar]
- 92. Marsh DJ, Solomon S. Analysis of electrolyte movement in thin Henle's loops of hamster papilla. Am J Physiol 208: 1119–1128, 1965 [DOI] [PubMed] [Google Scholar]
- 93. Michel CC, Curry FE. Microvascular permeability. Physiol Rev 79: 703–761, 1999 [DOI] [PubMed] [Google Scholar]
- 94. Moffat DB, Fourman J. The vascular pattern of the rat kidney. J Anat 97: 543–553, 1963 [PMC free article] [PubMed] [Google Scholar]
- 95. Mount DB. Transport of sodium, chloride, and potassium. In: Brenner and Rector's The Kidney (9th ed.). Philadelphia, PA: Elsevier, 2012, p. 158–201 [Google Scholar]
- 96. Nielsen S, Frokiaer J, Marples D, Kwon TH, Agre P, Knepper MA. Aquaporins in the kidney: from molecules to medicine. Physiol Rev 82: 205–244, 2002 [DOI] [PubMed] [Google Scholar]
- 97. Nielsen S, Smith BL, Christensen EI, Knepper MA, Agre P. Chip28 water channels are localized in constitutively water-permeable segments of the nephron. J Cell Biol 120: 371–383, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Nielsen S, Terris J, Smith CP, Hediger MA, Ecelbarger CA, Knepper MA. Cellular and subcellular localization of the vasopressin- regulated urea transporter in rat kidney. Proc Natl Acad Sci USA 93: 5495–5500, 1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Oliver JA. Nephrons and Kidneys: A Quantitative Study of Developmental and Evolutionary Mammalian Renal Architectonics. New York: Harper and Row, 1968 [Google Scholar]
- 100. Pallone TL, Edwards A, Ma T, Silldorff EP, Verkman AS. Requirement of aquaporin-1 for NaCl-driven water transport across descending vasa recta. J Clin Invest 105: 215–222, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pallone TL, MR T, Edwards A, Jamison RL. Countercurrent exchange in the renal medulla. Am J Physiol Regul Integr Comp Physiol 284: R1153–R1175, 2003 [DOI] [PubMed] [Google Scholar]
- 102. Pallone TL, Work J, Myers RL, Jamison RL. Transport of sodium and urea in outer medullary descending vasa recta. J Clin Invest 93: 212–222, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Pannabecker TL. Loop of Henle interaction with interstitial nodal spaces in the renal inner medulla. Am J Physiol Renal Physiol 295: F1744–F1751, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Pannabecker TL. Structure and function of the thin limbs of the loops of Henle. In: Comprehensive Physiology, edited by Terjung RL. Bethesda, MD: Am. Physiol. Soc., 2012, p. 2063–2086 [DOI] [PubMed] [Google Scholar]
- 105. Pannabecker TL, Abbott DE, Dantzler WH. Three-dimensional functional reconstruction of inner medullary thin limbs of Henle's loop. Am J Physiol Renal Physiol 286: F38–F45, 2004 [DOI] [PubMed] [Google Scholar]
- 106. Pannabecker TL, Dantzler WH. Three-dimensional architecture of collecting ducts, loops of Henle, and blood vessels in the renal papilla. Am J Physiol Renal Physiol 293: F696–F704, 2007 [DOI] [PubMed] [Google Scholar]
- 107. Pannabecker TL, Dantzler WH. Three-dimensional architecture of inner medullary vasa recta. Am J Physiol Renal Physiol 290: F1355–F1366, 2006 [DOI] [PubMed] [Google Scholar]
- 108. Pannabecker TL, Dantzler WH. Three-dimensional lateral and vertical relationships of inner medullary loops of Henle and collecting ducts. Am J Physiol Renal Physiol 287: F767–F774, 2004 [DOI] [PubMed] [Google Scholar]
- 109. Pannabecker TL, Dantzler WH, Layton HE, Layton AT. Role of three-dimensional architecture in the urine concentrating mechanism of the rat renal inner medulla. Am J Physiol Renal Physiol 295: F1271–F1285, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Pannabecker TL, Henderson C, Dantzler WH. Quantitative analysis of functional reconstructions reveals lateral and axial zonation in the renal inner medulla. Am J Physiol Renal Physiol 294: F1306–F1314, 2008 [DOI] [PubMed] [Google Scholar]
- 111. Pennell JP, Lacy FB, Jamison RL. An in vivo study of the concentrating process in the descending limb of Henle's loop. Kidney Int 5: 337–347, 1974 [DOI] [PubMed] [Google Scholar]
- 112. Pennell JP, Sanjana V, Frey NR, Jamison RL. The effect of urea infusion on the urinary concentrating mechanism in protein-depleted rats. J Clin Invest 55: 399–409, 1975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Preston GM, Carroll TP, Guggino WB, Agre P. Appearance of water channels in Xenopus oocytes expressing red-cell Chip28 protein. Science 256: 385–387, 1992 [DOI] [PubMed] [Google Scholar]
- 114. Promeneur D, Bankir L, Hu MC, Trinh-Trang-Tan M-M. Renal tubular and vascular urea transporters: influence of antidiuretic hormone on messenger RNA expression in Brattleboro rats. J Am Soc Nephrol 9: 1359–1366, 1998 [DOI] [PubMed] [Google Scholar]
- 115. Promeneur D, Rousselet G, Bankir L, Bailly P, Cartron JP, Ripoche P, Trinh-Trang-Tan MM. Evidence for distinct vascular and tubular urea transporters in the rat kidney. J Am Soc Nephrol 7: 852–860, 1996 [DOI] [PubMed] [Google Scholar]
- 116. Pruitt MEC, Knepper MA, Graves B, Schmidt-Nielsen B. Effect of peristaltic contractions of the renal pelvic wall on solute concentrations of the renal inner medulla in the hamster. Am J Physiol Renal Physiol 290: F892–F896, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Reinking LN, Schmidt-Nielsen B. Peristaltic flow of urine in the renal papillary collecting ducts of hamsters. Kidney Int 20: 55–60, 1981 [DOI] [PubMed] [Google Scholar]
- 118. Rocha AS, Kokko JP. Permeability of medullary nephron segments to urea and water: effect of vasopressin. Kidney Int 6: 379–387, 1974 [DOI] [PubMed] [Google Scholar]
- 119. Rocha AS, Kokko JP. Sodium chloride and water transport in the medullary thick ascending limb of Henle. Evidence for active chloride transport. J Clin Invest 52: 612–623, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Rollhauser H, Kriz W, Heinke W. Das Gefass-system der Rattenniere. Zeitschrift fur Zellforschung 64: 381–403, 1964 [PubMed] [Google Scholar]
- 121. Sands JM, Layton HE. The physiology of urinary concentration: an update. Semin Nephrol 29: 178–195, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Sands JM, Layton HE. The urine concentrating mechanism and urea transporters. In: The Kidney: Physiology and Pathophysiology, edited by Alpern RJ, Hebert SC. Philadelphia, PA: Elsevier, 2007, p. 1143–1177 [Google Scholar]
- 123. Sands JM, Layton HE, Fenton RA. Urine concentration and dilution. In: Brenner and Rector's The Kidney (9th ed.), edited by Taal MW, Chertow GM, Marsden PA, Skorecki KL, Yu ASL, Brenner BM. Philadelphia, PA: Elsevier, 2012, p. 326–352 [Google Scholar]
- 124. Saxen L. Organogenesis of the Kidney. Cambridge, UK: Cambridge University Press, 1987 [Google Scholar]
- 125. Schmidt-Nielsen B. The renal concentrating mechanism in insects and mammals: A new hypothesis involving hydrostatic pressures. Am J Physiol Regul Integr Comp Physiol 268: R1087–R1100, 1995 [DOI] [PubMed] [Google Scholar]
- 126. Schmidt-Nielsen B, Churchill M, Reinking LN. Occurrence of renal pelvic refluxes during rising urine flow rate in rats and hamsters. Kidney Int 18: 419–431, 1980 [DOI] [PubMed] [Google Scholar]
- 127. Schmidt-Nielsen B, Graves B. Changes in fluid compartments in hamster renal papilla due to peristalsis in the pelvic wall. Kidney Int 22: 613–625, 1982 [DOI] [PubMed] [Google Scholar]
- 128. Schwartz MM, Karnovsky MJ, Venkatachalam M. Ultrastructural differences between rat inner medullary descending and ascending vasa recta. Lab Invest 35: 161–170, 1976 [PubMed] [Google Scholar]
- 129. Schwartz MM, Karnovsky MJ, Venkatachalam MA. Regional membrane specialization in the thin limbs of Henle loops as seen by freeze-fracture electron-microscopy. Kidney Int 16: 577–589, 1979 [DOI] [PubMed] [Google Scholar]
- 130. Schwartz MM, Venkatachalam MA. Structural differences in thin limbs of Henle: physiological implications. Kidney Int 6: 193–208, 1974 [DOI] [PubMed] [Google Scholar]
- 131. Shayakul C, Knepper MA, Smith CP, DiGiovanni SR, Hediger MA. Segmental localization of urea transporter mRNAs in rat kidney. Am J Physiol Renal Physiol 272: F654–F660, 1997 [DOI] [PubMed] [Google Scholar]
- 132. Smith CP, Lee WS, Martial S, Knepper MA, You GF, Sands JM, Hediger MA. Cloning and regulation of expression of the rat-kidney urea transporter (Rut2). J Clin Invest 96: 1556–1563, 1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Sperber I. Studies on the mammalian kidney. Zoologiska Bidrag Fran Uppsala 22: 249–435, 1944 [Google Scholar]
- 134. Stallone JN, Braun EJ. Regulation of plasma antidiuretic hormone in the dehydrated kangaroo rat (Dipodomys spectabilis M.). Gen Comp Endocrinol 69: 119–127, 1988 [DOI] [PubMed] [Google Scholar]
- 135. Stan RV, Kubitza M, Palade GE. PV-1 is a component of the fenestral and stomatal diaphragms in fenestrated endothelia. Proc Natl Acad Sci USA 96: 13203–13207, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136. Stephenson JL. Concentration of urine in a central core model of the renal counterflow system. Kidney Int 2: 85–94, 1972 [DOI] [PubMed] [Google Scholar]
- 137. Stewart GS, Fenton RA, Wang W, Kwon TH, White SJ, Collins VM, Cooper G, Nielsen S, Smith CP. The basolateral expression of mUT-A3 in the mouse kidney. Am J Physiol Renal Physiol 286: F979–F987, 2004 [DOI] [PubMed] [Google Scholar]
- 138. Takahashi N, Kondo Y, Fujiwara I, Ito O, Igarashi Y, Abe K. Characterization of Na+ transport across the cell membranes of the ascending thin limb of Henle's loop. Kidney Int 47: 789–794, 1995 [DOI] [PubMed] [Google Scholar]
- 139. Takei Y, Bartolo RC, Fujihara H, Ueta Y, Donald JA. Water deprivation induces appetite and alters metabolic strategy in Notomys alexis: unique mechanisms for water production in the desert. Proc Biol Sci 279: 2599–2608, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140. Terris JM, Knepper MA, Wade JB. UT-A3: localization and characterization of an additional urea transporter isoform in the IMCD. Am J Physiol Renal Physiol 280: F325–F332, 2001 [DOI] [PubMed] [Google Scholar]
- 141. Thomas SR. Inner medullary lactate production and accumulation: a vasa recta model. Am J Physiol Renal Physiol 279: F468–F481, 2000 [DOI] [PubMed] [Google Scholar]
- 142. Thomas SR, Wexler AS. Inner medullary external osmotic driving force in a 3-D model of the renal concentrating mechanism. Am J Physiol Renal Fluid Electrolyte Physiol 269: F159–F171, 1995 [DOI] [PubMed] [Google Scholar]
- 143. Tsukaguchi H, Shayakul C, Berger UV, Tokui T, Brown D, Hediger MA. Cloning and characterization of the urea transporter UT3. Localization in rat kidney and testis. J Clin Invest 99: 1506–1515, 1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144. Urity VB, Issaian T, Braun EJ, Dantzler WH, Pannabecker TL. Architecture of kangaroo rat inner medulla: segmentation of descending thin limb of Henle's loop. Am J Physiol Regul Integr Comp Physiol 302: R720–R726, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145. van Balkom BW, van Raak M, Breton S, Pastor-Soler N, Bouley R, van der Sluijs P, Brown D, Deen PM. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J Biol Chem 278: 1101–1107, 2003 [DOI] [PubMed] [Google Scholar]
- 146. Verbavatz JM, Brown D, Sabolic I, Valenti G, Ausiello DA, Vanhoek AN, Ma T, Verkman AS. Tetrameric assembly of Chip28 water channels in liposomes and cell-membranes-a freeze-fracture study. J Cell Biol 123: 605–618, 1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Vilbig RL, Sarkar A, Zischkau J, Knepper MA, Pisitkun T. An online tool for calculation of free-energy balance for the renal inner medulla. Am J Physiol Renal Physiol 303: F366–F372, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148. Wade JB, Lee AJ, Liu C, Ecelbarger C, Mitchell C, Bradford AD, Terris J, Kim GH, Knepper MA. UT-A2: a 55-kDa urea transporter in thin descending limb whose abundance is regulated by vasopressin. Am J Physiol Renal Physiol 278: F52–F62, 2000 [DOI] [PubMed] [Google Scholar]
- 149. Wang X, Thomas SR, Wexler AS. Outer medullary anatomy and the urine concentrating mechanism. Am J Physiol Renal Physiol 274: F413–F424, 1998 [DOI] [PubMed] [Google Scholar]
- 150. Westrick KY, Serack BJ, Dantzler WH, Pannabecker TL. Axial compartmentation of descending and ascending thin limbs of Henle's loops. Am J Physiol Renal Physiol 304: F308–F376, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151. Wexler AS, Kalaba RE, Marsh DJ. Three-dimensional anatomy and renal concentrating mechanism. I. Modeling results. Am J Physiol Renal Fluid Electrolyte Physiol 260: F368–F383, 1991 [DOI] [PubMed] [Google Scholar]
- 152. Wexler AS, Kalaba RE, Marsh DJ. Three-dimensional anatomy and renal concentrating mechanism. II. Sensitivity results. Am J Physiol Renal Fluid Electrolyte Physiol 260: F384–F394, 1991 [DOI] [PubMed] [Google Scholar]
- 153. Yang B, Bankir L, Gillespie A, Epstein CJ, Verkman AS. Urea-selective concentrating defect in transgenic mice lacking urea transporter UT-B. J Biol Chem 277: 10633–10637, 2002 [DOI] [PubMed] [Google Scholar]
- 154. Yool AJ, Brokl OH, Pannabecker TL, Dantzler WH, Stamer WD. Tetraethylammonium block of water flux in aquaporin-1 channels expressed in kidney thin limbs of Henle's loop and a kidney-derived cell line. BMC Physiol 2: 4, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155. You G, Smith CP, Kanai Y, Lee WS, Stelzner M, Hediger MA. Cloning and characterization of the vasopressin-regulated urea transporter. Nature 365: 844–847, 1993 [DOI] [PubMed] [Google Scholar]
- 156. Yuan J, Pannabecker TL. Architecture of inner medullary descending and ascending vasa recta: pathways for countercurrent exchange. Am J Physiol Renal Physiol 299: F265–F272, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157. Yui N, Lu HA, Chen Y, Nomura N, Bouley R, Brown D. Basolateral targeting and microtubule dependent transcytosis of the aquaporin-2 water channel. Am J Physiol Cell Physiol 304: C38–C48, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158. Zhai XY, Thomsen JS, Birn H, Kristoffersen IB, Andreasen A, Christensen EI. Three-dimensional reconstruction of the mouse nephron. J Am Soc Nephrol 17: 77–88, 2006 [DOI] [PubMed] [Google Scholar]
- 159. Zhai XY, Fenton RA, Andreasen A, Thomsen JS, Christensen EI. Aquaporin-1 is not expressed in descending thin limbs of short-loop nephrons. J Am Soc Nephrol 18: 2937–2944, 2007 [DOI] [PubMed] [Google Scholar]
- 160. Zimmerhackl B, Robertson CR, Jamison RL. Fluid uptake in the renal papilla by vasa recta estimated by two methods simultaneously. Am J Physiol Renal Fluid Electrolyte Physiol 248: F347–F353, 1985 [DOI] [PubMed] [Google Scholar]