Abstract
Muscle metaboreflex activation (MMA) during submaximal dynamic exercise in normal individuals increases mean arterial pressure (MAP) via increases in cardiac output (CO) with little peripheral vasoconstriction. The rise in CO occurs primarily via increases in heart rate (HR) with maintained or slightly increased stroke volume. When the reflex is sustained during recovery (postexercise muscle ischemia, PEMI), HR declines yet MAP remains elevated. The role of CO in mediating the pressor response during PEMI is controversial. In seven chronically instrumented canines, steady-state values with MMA during mild exercise (3.2 km/h) were observed by reducing hindlimb blood flow by ∼60% for 3–5 min. MMA during exercise was followed by 60 s of PEMI. Control experiments consisted of normal exercise and recovery. MMA during exercise increased MAP, HR, and CO by 55.3 ± 4.9 mmHg, 42.5 ± 6.9 beats/min, and 2.5 ± 0.4 l/min, respectively. During sustained MMA via PEMI, MAP remained elevated and CO remained well above the normal recovery levels. Neither MMA during dynamic exercise nor during PEMI significantly affected peripheral vascular conductance. We conclude that the sustained increase in MAP during PEMI is driven by a sustained increase in CO not peripheral vasoconstriction.
Keywords: circulatory occlusion, exercise pressor reflex, ischemic exercise, contractility, heart rate
skeletal muscle ischemia during or immediately after exercise leads to the accumulation of metabolic by-products that activate group III and IV chemosensitive afferents within the muscle (19, 30, 31, 36, 49, 56, 58–60). Activation of these skeletal muscle afferents causes a reflex increase in sympathetic outflow that generates substantial increases in arterial pressure termed the muscle metaboreflex (2, 5, 12, 24, 39, 43–45, 47, 50, 51, 65). Two different approaches have been employed to study this reflex. Blood flow to the exercising muscle can be reduced before or during a bout of static or dynamic exercise thereby activating the reflex during ischemic exercise (1, 2, 5, 12, 13, 17, 45, 57, 65). In contrast, the reflex can be elicited by inducing ischemia in the muscle immediately before or upon cessation of the exercise [a technique termed postexercise muscle ischemia (PEMI)] (9, 11, 17, 23, 24, 39, 50, 51, 60, 63).
When the reflex is activated during sustained submaximal dynamic exercise in normal subjects, the pressor response occurs primarily via increased cardiac output (CO), which is driven by increased heart rate (HR) coupled with sustained or slightly increased stroke volume (SV) (17, 28, 53, 57, 65). SV is maintained despite this tachycardia due to both enhanced ventricular contractility (14, 16, 45, 53) and maintained or increased ventricular filling pressure via substantial central blood volume mobilization (54). In contrast, during PEMI, whereas mean arterial pressure (MAP) remains elevated for as long as the ischemia is maintained, HR precipitously declines toward resting levels with a time course similar to normal recovery, which led some to speculate that the muscle metaboreflex has little control over the heart (2, 23, 38, 51, 52, 61–63). With HR on the decline during the recovery from exercise with or without PEMI, the role of CO in mediating the pressor response during PEMI remains controversial. Indeed previous studies both support (11, 16–18, 48, 50, 57) and refute (9, 18, 48) any role for CO in mediating the pressor response during PEMI. In the present study, we elicited the muscle metaboreflex during dynamic exercise and sustained this activation during PEMI to compare the mechanisms underlying the muscle metaboreflex-mediated pressor responses in these two distinct settings.
METHODS
Experimental subjects.
Seven adult mongrel canines were selected for the study. All animals were healthy, ∼20–25 kg body wt, of either sex (4 females; 3 males), well adapted to the laboratory environment, and willing to run on a motor-driven treadmill. During experimentation, all animals exercised voluntarily and no negative reinforcement techniques were utilized. The protocols developed and employed in the present study were reviewed and approved by the Institutional Animal Care and Use Committee of Wayne State University and complied with the National Institutes of Health Guide to the Care and Use of Laboratory Animals.
Surgical procedures.
Each animal was completely instrumented with chronic, indwelling cardiovascular devices following two sterile surgical procedures: left thoracotomy and left flank retroperitoneal surgery in that order. The animals recovered a minimum of 10 days before the second surgery and a minimum of 7 days before the first experiment. During preoperative care, the animals were initially sedated with acepromazine (0.4–0.5 mg/kg im). After adequate sedation, the animals were anesthetized with a combined treatment of ketamine and diazepam (5.0 and 0.22 mg/kg iv, respectively). Anesthesia was maintained with isoflurane gas (1–3%) after endotracheal intubation. In addition, the animals received preoperative administration of cefazolin (antibiotic; 30 mg/kg iv), carprofen (analgesic; 4.0 mg/kg iv), buprenorphine (analgesic; 0.01 mg/kg im), and fentanyl [analgesic; 125–175 μg/h, (72h) TDD]. Before the left thoracotomy, animals received selective intercostal nerve blockade with bupivacaine HCl (2.0 mg/kg sq). After each surgical procedure, animals received cefazolin (30 mg/kg iv) and prophylactic cephalexin [antibiotic; 30 mg/kg (bid) po] therapy for the term of the experimental protocol. During the 12-h postoperative period, animals were closely monitored and received buprenorphine and acepromazine (0.05 and 0.5 mg/kg iv, respectively) as needed. For the following 10 days, animals received carprofen [4 mg/kg (opd) po].
In the first surgical procedure, the thoracic cavity was opened via a left thoracotomy (4th intercostal space) approach. The pericardium was cut and reflected to expose the heart. An ultrasonic perivascular flow probe (20PAU, Transonic Systems) was positioned around the ascending aorta to measure CO. Approximately 10 cm caudal to the thoracotomy incision, an implantable telemetry blood pressure transmitter (TA11 PA-D70, Data Sciences International) was tethered subcutaneously. The catheter of the transmitter was tunneled into the thoracic cavity through the seventh intercostal space, and the tip was inserted and secured inside the left ventricle for measuring left ventricular pressure (LVP). For studies unrelated to the present investigation a blood flow transducer was also placed on the left circumflex artery. The pericardium was loosely reapproximated, the cables were tunneled subcutaneously and exteriorized between the scapulae, and the chest was closed in layers.
In the second surgical procedure, an incision was made in the left flank cranial to the iliac crest. The abdominal aorta was exposed and an ultrasonic perivascular flow probe (10PAA, Transonic Systems) was positioned around the terminal aorta for measuring hindlimb blood flow (HLBF). All arterial side branches between the common iliacs and the flow probe were ligated and severed. In addition, two perivascular hydraulic occluders (8–10 mm, DocXS Biomedical Products) were positioned around the terminal aorta (distal to the flow probe) to provide the means to incrementally reduce HLBF. A 19-gauge polyvinyl catheter (S54-HL, Tygon, Norton) was advanced through a ligated lumbar artery and secured into the terminal aorta cranial to the probe and occluders to measure arterial pressure. For studies unrelated to the current investigation a blood flow transducer and vascular occluder were placed on the left renal artery. The cables and vascular occluder tubing were tunneled subcutaneously and exteriorized between the scapulae.
Data acquisition.
After complete postoperative recovery, each animal was brought into the laboratory and allowed to roam freely and acclimate for ∼15–20 min. The animal was then directed onto the treadmill where the instrumention was connected to the data acquisition system (TS420, Transonic Systems Blood Flow Meter, Gould; amplifiers, Data Science International, Telemetry System, LabScribe, iWorx).
Experimental procedures.
All animals performed both control and experimental procedures on separate days, therefore, each animal served as its own control. The experiments began with the animal standing unrestrained on the treadmill until all hemodynamic data were observed to be stable (typically 5–10 min). The treadmill was turned on and the speed was gradually increased to 3.2 km/h at 0% grade [a mild workload for a canine (27)]. Steady state was generally reached within 3–5 min. In the control experiment, after all variables had reached steady state during exercise, the treadmill was abruptly stopped and postexercise (without ischemia) hemodynamic data were collected for 60 s while the animal was standing still. In a separate experiment, the muscle metaboreflex was engaged via partial reductions in HLBF during mild exercise. Once the reflex was strongly engaged, steady-state data were collected for 60 s. The treadmill was then abruptly stopped and the occlusion was sustained for an additional 60 s (PEMI).
Data analysis.
CO, HLBF, LVP, HR, and MAP data were continuously recorded during each experiment. Other hemodynamic parameters were calculated off-line [e.g., SV, dP/dtmax, dP/dtmin, and nonischemic vascular conductance (NIVC)]. NIVC was calculated as (CO − HLBF)/MAP and reflects vascular conductance of all vascular beds except the hindlimbs. Because of technical difficulties, we were only able to obtain LVPs from six animals. One-minute averages of steady-state data were calculated at rest, during exercise, and during metaboreflex activation. Five-second averages were computed for both 60-s postexercise conditions: postexercise (without ischemia) and PEMI. These mean values were then averaged across all animals to obtain the mean values for the entire population of the study. Finally, the last 10 s of recovery were averaged.
Statistical analysis.
Averaged responses for each animal were analyzed via two-way repeated measures ANOVA to compare hemodynamic data for time and/or condition effects. In the event of a significant time-condition interaction, a C-matrix test for simple effects was performed. Data are reported as means ± SE, and statistical significance was ascribed as P < 0.05.
RESULTS
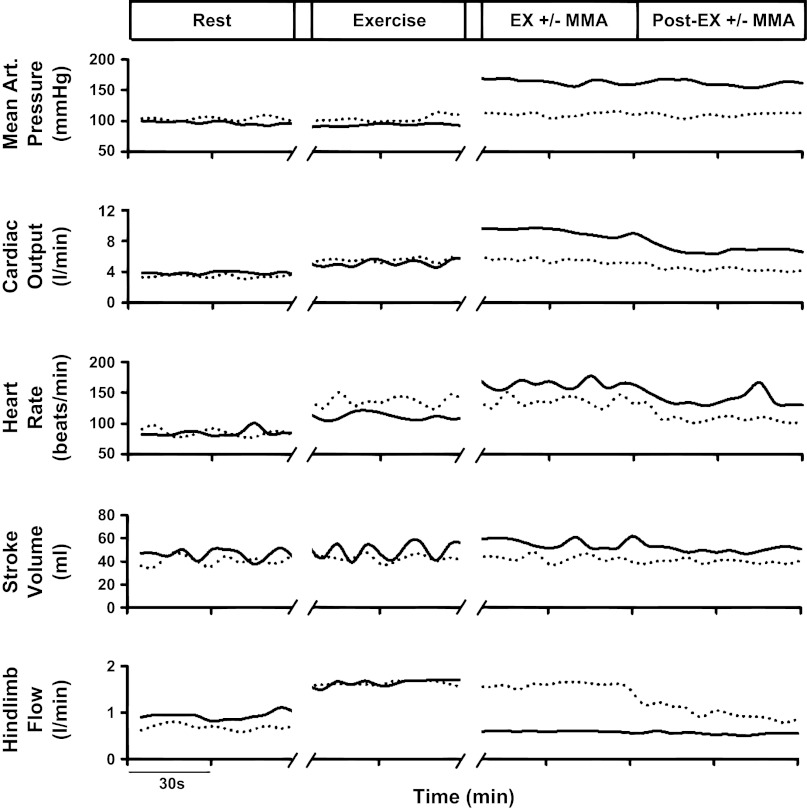
Figure 1 shows the responses in MAP, CO, HR, SV, and HLBF from a control and experimental protocol in one animal. In both protocols, with the transition from rest to exercise, there was a minimal increase in MAP and modest increases in CO and HR concomitant with a substantial rise in HLBF. During the normal recovery from exercise, MAP remained unchanged while CO and HR gradually fell toward resting levels. In the experimental protocol, the muscle metaboreflex was elicited immediately following steady-state exercise and sustained during the postexercise recovery period (PEMI). Muscle metaboreflex activation during the experimental protocol led to marked increases in MAP, CO, and HR and little change in SV. During PEMI, the rise in MAP was sustained. Although CO and HR initially fell during the onset of PEMI, both subsequently plateaued well above their normal recovery levels.
Fig. 1.
Time course of mean arterial pressure (MAP), cardiac output (CO), heart rate (HR), stroke volume (SV), and hindlimb blood flow (HLBF) during control (dotted lines) and experimental (solid lines) protocols in one animal. MMA, muscle metaboreflex activation.
Figure 2 shows the average values of MAP, HR, CO, and NIVC during control and experimental procedures. The 60 s of postexercise recovery (PEMI) are plotted as 5-s averages. The changes in MAP, HR, CO, and NIVC from rest to exercise were not significantly different between the control and experimental protocols. With muscle metaboreflex activation during exercise, MAP, HR, and CO increased substantially.
Fig. 2.
Averaged time course of MAP, HR, CO, and nonischemic vascular conductance (NIVC) during control (open circles) and experimental procedures (filled circles) in 7 animals. Data at rest are the average values from both settings (half-filled circles). *P < 0.05.
During PEMI, the ∼60 mmHg rise in MAP, which was observed with muscle metaboreflex activation, was sustained during the entire 60 s, as every data point during this maneuver was significantly different from control. NIVC fell during the PEMI period with a pattern not significantly different from during the normal recovery from exercise. HR and CO decreased abruptly during the first 15 s of PEMI; however, both of these variables subsequently plateaued significantly above normal recovery levels.
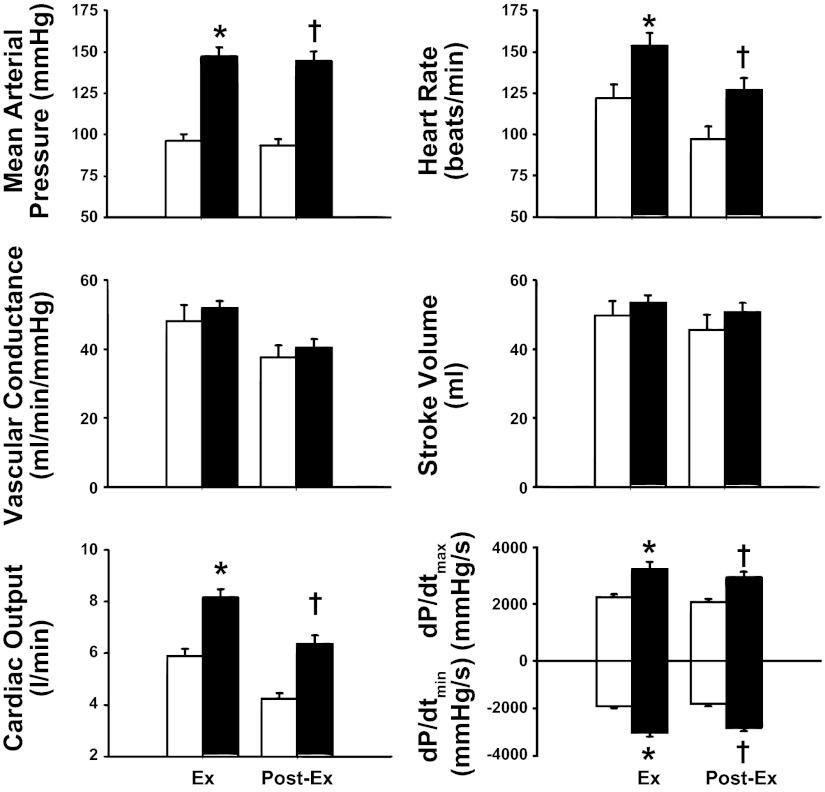
Figure 3 shows the mean hemodynamic responses during exercise and the final 10 s of recovery with and without muscle metaboreflex activation. During the last 10 s of ischemic exercise, there were significant increases in MAP, CO, HR, dP/dtmax, and dP/dtmin compared with the data during exercise without metaboreflex activation.
Fig. 3.
Mean hemodynamic responses during exercise and the final 10 s of recovery with (filled bars) and without (open bars) muscle metaboreflex activation. *P < 0.05 between normal exercise and ischemic exercise; †P < 0.05 between normal recovery from exercise (open bars) and PEMI (filled bars).
During the last 10 s of the recovery from ischemic exercise, MAP, CO, HR, dP/dtmax, and dP/dtmin were all significantly elevated compared with the values during the normal recovery from exercise, whereas there were no significant changes in NIVC or SV.
DISCUSSION
Our major finding is that the mechanisms mediating muscle metaboreflex-induced increases in arterial pressure are similar both when the reflex is activated during dynamic exercise and when the reflex is sustained during the recovery from exercise. In both settings, the pressor response is primarily due to a substantial elevation in CO with little, if any, peripheral vasoconstriction. Moreover, the elevation in CO is driven via an increased HR with a sustained SV. Inasmuch as tachycardia itself can lead to decreases in SV (32, 64), increases in ventricular contractility likely contribute to the sustained SV. The elevated dP/dtmax supports this conclusion. Faster left ventricular relaxation (dP/dtmin) may also aid in the maintenance of SV by increasing filling time. Therefore, our data demonstrate marked muscle metaboreflex-induced chronotropic, inotropic, and lusitropic responses both when the reflex is activated during dynamic exercise and when the reflex is sustained during recovery from exercise.
Muscle metaboreflex activation during exercise: CO versus vasoconstriction.
Muscle metaboreflex activation during exercise evokes large increases in MAP, HR, and CO (2, 3, 21, 40, 44, 45, 65). However, the relative roles of CO versus peripheral vasoconstriction in this pressor response have been unclear. We observed a 45% increase in CO with muscle metaboreflex activation during exercise with no significant peripheral vasoconstriction. We determined the extent of peripheral vasoconstriction by calculating the conductance of all vascular beds with the exception of the hindlimbs (NIVC) (7). Changes in hindlimb conductance must be excluded due to the mechanical effects of the occlusion.
Previous studies have demonstrated a “switch” in the mechanisms of the muscle metaboreflex from a flow-mediated rise in MAP to a vasoconstriction-mediated pressor response when the reflex increase in CO is attenuated. For example, Sheriff et al. (54) and Ichinose et al. (28) demonstrated substantial peripheral vasoconstriction when the metaboreflex-induced increase in CO was either pharmacologically or mechanically prevented. In addition, Augustyniak et al. (8) demonstrated a shift from CO to peripheral vasoconstriction when workload approached maximal levels and further increases in CO were limited. Similar results have been observed in subjects with congestive heart failure, a setting where substantial increases in CO are limited (6, 15, 26, 47). Thus whether or not increased CO or increased vasoconstriction is utilized as a means to raise MAP with metaboreflex activation during dynamic exercise appears dependent on the ability to increase CO.
Muscle metaboreflex activation during PEMI: CO versus vasoconstriction.
Previous studies have come to markedly different conclusions regarding the relative roles of CO versus peripheral vasoconstriction in mediating the pressor response during PEMI [(11, 16–18, 48, 50, 57) versus (9, 18, 48)]. One potential explanation is that a CO response is often seen during imposed PEMI following more intense exercise, whereas PEMI following relatively lower exercise intensities tends to demonstrate little change in CO and the pressor response occurs via peripheral vasoconstriction. We observed a maintained elevation in CO (∼50% above the normal recovery level) during PEMI with little or no change in NIVC. The time course of the recovery of NIVC during PEMI and the normal recovery from exercise are virtually indistinguishable and not significantly different. It should be noted that NIVC contains not only conductance to inactive areas, but a substantial amount of NIVC is skeletal muscle outside of the hindlimbs (25), which also vasodilates in response to the exercise. Thus with exercise NIVC increases and during recovery NIVC falls. To what extent this fall in NIVC during recovery with or without PEMI is neurogenic versus passive vasoconstriction due to reduced metabolic vasodilation in skeletal muscle is not known. Previously, Sheriff et al. (55) has shown that after ganglionic blockade, skeletal muscle vasodilation during exercise markedly exceeds normal levels. Regardless of whether or not tonic sympathetic activity controls the speed of recovery of NIVC, it is clear that the pattern of change in NIVC was not different between the normal recovery and during PEMI.
Role of HR and SV in mediating metaboreflex-induced increases in CO during exercise versus PEMI.
Previous studies in dogs and humans have concluded that HR and CO increase markedly when the muscle metaboreflex is activated during exercise (6–8, 14–17, 20, 26, 28, 45, 65); however, during PEMI the effects on HR are more variable. In general, PEMI elicited from the arm following moderate exercise evokes little sustained tachycardia and any CO response occurs via increased SV (3, 23, 48). In contrast, if PEMI follows leg exercise, HR remains above normal recovery values, and therefore the relative tachycardia contributes to an elevated CO (3, 23, 48). The obversations in humans following leg exercise are similar to those we observed in the present study using canines. Collectively, these studies indicate that either the HR response during PEMI depends on which limb is used or that these differential responses are due to differences in muscle mass.
Previous studies from our laboratory and others have concluded that during PEMI there are sustained increases in sympathetic tone to the heart; however, there are concurrent increases in parasympathetic activity as well (29, 41, 43). This combined activation of both arms of the autonomic nervous system causes bradycardia but sustained increased ventricular contractility (see Fig. 3). The differences in the HR responses between PEMI and metaboreflex activation during dynamic exercise may reflect that during PEMI one is observing responses during the recovery from exercise rather than during exercise per se. Muscle metaboreflex-induced tachycardia occurs primarily via increased sympathetic activity to the heart (22, 43). With the cessation of exercise, parasympathetic activity rises abruptly, which masks the chronotropic effects of sustained sympathetic tone (note that the inotropic effect is well sustained during PEMI; see Fig. 3). The extent to which sympathetic nerve activity remains elevated during PEMI may be dependent on the intensity and type of exercise performed (e.g., isometric vs. isotonic). For example, in canines, the muscle metaboreflex is not tonically active during mild, free-flow treadmill exercise but becomes tonically active as workload increases and/or ventricular function declines (8, 27, 65). In addition, Fisher et al. (22) demonstrated in humans performing static handgrip exercise that “robust” muscle metaboreflex activation (achieved during high-intensity exercise) was required to sustain sympathetic nerve activity during PEMI to a degree such that the prevailing parasympathetic effects on HR were counteracted. However, Amann et al. (4) recently concluded that skeletal muscle afferents contribute to the cardiorespiratory responses during relatively mild exercise in humans.
The increases in CO with metaboreflex activation likely require increased contractility as well as central blood volume mobilization to raise or maintain SV with the elevated afterload and shortened ventricular filling time. Previous studies from our laboratory showed that the muscle metaboreflex can elicit marked increases in central blood volume mobilization (54) as well as ventricular contractility [as evidenced by increased left ventricular maximal elastance, preload recruitable stroke work, as well as dP/dtmax (14, 45, 53)]. Moreover, Little et al. (34, 35) have shown that increases in left ventricular end-diastolic volume per se can lead to increases in dP/dtmax. Thus it is possible that a portion of the rise in contractility with metaboreflex activation may be directly attributable to enhanced central blood volume mobilization.
In the present study, the increase in contractility was sustained during PEMI (Fig. 3). Shoemaker et al. (57) showed a substantial muscle metaboreflex-induced increase in SV during ischemic exercise, and Crisafulli et al. (16) also reported an increase in SV and ventricular contractility with muscle metaboreflex activation during PEMI. These reflex increases in SV were suggested by these authors to be the principal mechanisms behind the increase in CO in these two distinct settings as HR was not affected. We did not observe a significant increase in SV with muscle metaboreflex activation during exercise or PEMI in our studies, and it is possible that these different results are species dependent. For example, there are reports that canines have a limited end-diastolic reserve compared to humans (10). In contrast, other studies have shown that canines possess significant preload reserve (33, 37). Nonetheless, a maintenance or increase in SV during a hemodynamic period in which ventricular filling time is markedly reduced can likely only be achieved if there is an increase in the contractile state and/or an increase in preload.
Perspectives and Significance
Activation of the muscle metaboreflex both during submaximal exercise and during the recovery from exercise elicits substantial increases in ventricular function. The major mechanism of the muscle metaboreflex-mediated pressor response is increased CO with little, if any, peripheral vasoconstriction in both settings. Inasmuch as when workload rises, an increasingly smaller fraction of CO is directed to nonactive vascular beds (e.g., brain, kidneys, and splanchnic organs), even complete vasoconstriction of these beds would cause only a limited increase in arterial pressure (42). In contrast, a metaboreflex-mediated rise in CO generates substantial increases in arterial pressure. Thus, in these settings, the muscle metaboreflex is a flow-raising, pressure-raising reflex. The rise in total systemic blood flow increases systemic perfusion pressure which likely acts to partially restore blood flow to ischemic muscle (46).
GRANTS
This study was supported by National Heart, Lung, and Blood Institute Grants HL-55743 and HL-095819. This study was also funded by the Multidisciplinary Research Group Incubator Program at Wayne State University.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: M.D.S., M.C., J.K., D.S., and D.S.O'L. performed experiments; M.D.S., J.A.S.-M., M.C., J.K., and D.S.O'L. analyzed data; M.D.S., J.A.S.-M., J.K., D.S., R.A.A., and D.S.O'L. interpreted results of experiments; M.D.S., J.A.S.-M., J.K., and D.S.O'L. prepared figures; M.D.S. and D.S.O'L. drafted manuscript; M.D.S., J.A.S.-M., M.C., R.A.A., and D.S.O'L. edited and revised manuscript; R.A.A. and D.S.O'L. approved final version of manuscript; D.S.O'L. conception and design of research.
ACKNOWLEDGMENTS
The authors thank Jody Helme-Day for expert animal care and technical assistance.
REFERENCES
- 1. Adreani CM, Kaufman MP. Effect of arterial occlusion on responses of group III and IV afferents to dynamic exercise. J Appl Physiol 84: 1827–1833, 1998 [DOI] [PubMed] [Google Scholar]
- 2. Alam M, Smirk FH. Observations in man upon a blood pressure raising reflex arising from the voluntary muscles. J Physiol 89: 372–383, 1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Alam M, Smirk FH. Observations in man on a pulse-accelerating reflex from the voluntary muscles of the legs. J Physiol 92: 167–177, 1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Amann M, Runnels S, Morgan DE, Trinity JD, Fjeldstad AS, Wray DW, Reese VR, Richardson RS. On the contribution of group III and IV muscle afferents to the circulatory response to rhythmic exercise in humans. J Physiol 589: 3855–3866, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Asmussen E, Nielsen M. Experiments on nervous factors controlling respiration and circulation during exercise employing blocking of the blood flow. Acta Physiol Scand 60: 103–111, 1964 [DOI] [PubMed] [Google Scholar]
- 6. Augustyniak RA, Ansorge EJ, Kim JK, Sala-Mercado JA, Hammond RL, Rossi NF, O'Leary DS. Cardiovascular responses to exercise and muscle metaboreflex activation during the recovery from pacing-induced heart failure. J Appl Physiol 101: 14–22, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Augustyniak RA, Ansorge EJ, O'Leary DS. Muscle metaboreflex control of cardiac output and peripheral vasoconstriction exhibit differential latencies. Am J Physiol Heart Circ Physiol 278: H530–H537, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Augustyniak RA, Collins HL, Ansorge EJ, Rossi NF, O'Leary DS. Severe exercise alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 280: H1645–H1652, 2001 [DOI] [PubMed] [Google Scholar]
- 9. Bastos BG, Williamson JW, Harrelson T. Left ventricular volumes and hemodynamic responses to postexercise ischemia in healthy humans. Med Sci Sport Exer 32: 1114–1118, 2000 [DOI] [PubMed] [Google Scholar]
- 10. Boettcher DH, Vatner SF, Heyndrickx GR, Braunwald E. Extent of utilization of the Frank-Starling mechanism in conscious dogs. Am J Physiol Heart Circ Physiol 234: H338–H345, 1978 [DOI] [PubMed] [Google Scholar]
- 11. Bonde-Petersen F, Rowell LB, Murray RG, Blomqvist GG, White R, Karlsson E, Campbell W, Mitchell JH. Role of cardiac output in the pressor responses to graded muscle ischemia in man. J Appl Physiol 45: 574–580, 1978 [DOI] [PubMed] [Google Scholar]
- 12. Coote JH, Hilton SM, Perez-Gonzalez JF. The reflex nature of the pressor response to muscular exercise. J Physiol 215: 789–804, 1971 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cornett JA, Herr MD, Gray KS, Smith MB, Yang QX, Sinoway LI. Ischemic exercise and the muscle metaboreflex. J Appl Physiol 89: 1432–1436, 2000 [DOI] [PubMed] [Google Scholar]
- 14. Coutsos M, Sala-Mercado JA, Ichinose M, Li Z, Dawe EJ, O'Leary DS. Muscle metaboreflex-induced coronary vasoconstriction functionally limits increases in ventricular contractility. J Appl Physiol 109: 271–278, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Crisafulli A, Salis E, Tocco F, Melis F, Milia R, Pittau G, Caria MA, Solinas R, Meloni L, Pagliaro P, Concu A. Impaired central hemodynamic response and exaggerated vasoconstriction during muscle metaboreflex activation in heart failure patients. Am J Physiol Heart Circ Physiol 292: H2988–H2996, 2007 [DOI] [PubMed] [Google Scholar]
- 16. Crisafulli A, Scott AC, Wensel R, Davos CH, Francis DP, Pagliaro P, Coats AJS, Concu A, Piepoli MF. Muscle metaboreflex-induced increases in stroke volume. Med Sci Sport Exer 35: 221–228, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Crisafulli A, Piras F, Filippi M, Piredda C, Chiappori P, Melis F, Milia R, Tocco F, Concu A. Role of heart rate and stroke volume during muscle metaboreflex-induced cardiac output increase: differences between activation during and after exercise. J Physiol Sci 61: 385–394, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Crisafulli A, Salis E, Pittau G, Lorrai L, Tocco F, Melis F, Pagliaro P, Concu An. Modulation of cardiac contractility by muscle metaboreflex following efforts of different intensities in humans. Am J Physiol Heart Circ Physiol 291: H3035–H3042, 2006 [DOI] [PubMed] [Google Scholar]
- 19. Darques J, Decherchi P, Jammes Y. Mechanisms of fatigue-induced activation of group IV muscle afferents: the roles played by lactic acid and inflammatory mediators. Neurosci Lett 257: 109–112, 1998 [DOI] [PubMed] [Google Scholar]
- 20. Eiken O, Bjurstedt H. Dynamic exercise in man as influenced by experimental restriction of blood flow in the working muscles. Acta Physiol Scand 131: 339–345, 1987 [DOI] [PubMed] [Google Scholar]
- 21. Euler US, Liljestrand G. The regulation of blood pressure with special reference to muscular work. Acta Physiol Scand 12: 279–300, 1946 [Google Scholar]
- 22. Fisher JP, Seifert T, Hartwich D, Young CN, Secher NH, Fadel PJ. Autonomic control of heart rate by metabolically sensitive skeletal muscle afferents in humans. J Physiol 588: 1117–1127, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Freund PR, Hobbs SF, Rowell LB. Cardiovascular responses to muscle ischemia in man–dependency on muscle mass. J Appl Physiol 45: 762–767, 1978 [DOI] [PubMed] [Google Scholar]
- 24. Freund PR, Rowell LB, Murphy TM, Hobbs SF, Butler SH. Blockade of the pressor response to muscle ischemia by sensory nerve block in man. Am J Physiol Heart Circ Physiol 237: H433–H439, 1979 [DOI] [PubMed] [Google Scholar]
- 25. Hales JRS, Dampney RAL. The redistribution of cardiac output in the dog during heat stress. J Thermal Biol 1: 29–34, 1975 [Google Scholar]
- 26. Hammond RL, Augustyniak RA, Rossi NF, Churchill PC, Lapanowski K, O'Leary DS. Heart failure alters the strength and mechanisms of the muscle metaboreflex. Am J Physiol Heart Circ Physiol 278: H818–H828, 2000 [DOI] [PubMed] [Google Scholar]
- 27. Hammond RL, Augustyniak RA, Rossi NF, Lapanowski K, Dunbar JC, O'Leary DS. Alteration of humoral and peripheral vascular responses during graded exercise in heart failure. J Appl Physiol 90: 55–61, 2001 [DOI] [PubMed] [Google Scholar]
- 28. Ichinose MJ, Sala-Mercado JA, Coutsos M, Li Z, Ichinose TK, Dawe E, O'Leary DS. Modulation of cardiac output alters the mechanisms of the muscle metaboreflex pressor response. Am J Physiol Heart Circ Physiol 298: H245–H250, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Iellamo F, Pizzinelli P, Massaro M, Raimondi G, Peruzzi G, Legramante JM. Muscle metaboreflex contribution to sinus node regulation during static exercise: insights from spectral analysis of heart rate variability. Circulation 100: 27–32, 1999 [DOI] [PubMed] [Google Scholar]
- 30. Kaufman MP. The exercise pressor reflex in animals. Exp Physiol 97: 51–58, 2012 [DOI] [PubMed] [Google Scholar]
- 31. Kniffeki KD, Mense S, Schmidt RF. Responses to group IV afferent units from skeletal muscle to stretch, contraction and chemical stimulation. Exp Brain Res 31: 511–522, 1978 [DOI] [PubMed] [Google Scholar]
- 32. Kumada M, Azuma T, Matsuda K. The cardiac output-heart rate relationship under different conditions. Jpn J Physiol 17: 538–555, 1967 [DOI] [PubMed] [Google Scholar]
- 33. Lee JD, Tajimi T, Patritti J, Ross J., Jr Preload reserve and mechanisms of afterload mismatch in normal conscious dog. Am J Physiol 250: H464–H473, 1986 [DOI] [PubMed] [Google Scholar]
- 34. Little WC. The left-ventricular dP/dtmax-end-diastolic volume relation in closed-chest dogs. Circ Res 56: 808–815, 1985 [DOI] [PubMed] [Google Scholar]
- 35. Little WC, Cheng CP, Mumma M, Igarashi Y, Vinten-Johansen J, Johnston WE. Comparison of measures of left ventricular contractile performance derived from pressure-volume loops in conscious dogs. Circulation 80: 1378–1387, 1989 [DOI] [PubMed] [Google Scholar]
- 36. MacLean DA, Imadojemu VA, Sinoway LI. Interstitial pH, K+, lactate, and phosphate determined with MSNA during exercise in humans. Am J Physiol Regul Integr Comp Physiol 278: R563–R571, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Mahler F, Ross J, Jr, O'Rourke RA, Covell JW. Effects of changes in preload, afterload and inotropic state on ejection and isovolumic phase measures of contractility in the conscious dog. Am J Cardiol 35: 626–634, 1975 [DOI] [PubMed] [Google Scholar]
- 38. Mark AL, Victor RG, Nerhed C, Wallin BG. Microneurographic studies of the mechanisms of sympathetic nerve responses to static exercise in humans. Circ Res 57: 461–469, 1985 [DOI] [PubMed] [Google Scholar]
- 39. McCloskey DL, Mitchell JH. Reflex cardiovascular and respiratory responses originating exercising muscle. J Physiol 224: 173–186, 1972 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Mitchell JH, Kaufman MP, Iwamoto GA. The exercise pressor reflex-its cardiovascular effects, afferent mechanisms, and central pathways. Annu Rev Physiol 45: 229–242, 1983 [DOI] [PubMed] [Google Scholar]
- 41. Nishiyasu T, Tan N, Morimoto K, Nishiyasu M, Yamaguchi Y, Murakami N. Enhancement of parasympathetic cardiac activity during activation of muscle metaboreflex in humans. J Appl Physiol 77: 2778–2783, 1994 [DOI] [PubMed] [Google Scholar]
- 42. O'Leary DS. Regional vascular resistance vs. conductance: which index for baroreflex responses? Am J Physiol Heart Circ Physiol 260: H632–H637, 1991 [DOI] [PubMed] [Google Scholar]
- 43. O'Leary DS. Autonomic mechanisms of muscle metaboreflex control of heart rate. J Appl Physiol 74: 1748–1754, 1993 [DOI] [PubMed] [Google Scholar]
- 44. O'Leary DS. Heart rate control during exercise by baroreceptors and skeletal muscle afferents. Med Sci Sport Exer 28: 210–217, 1996 [DOI] [PubMed] [Google Scholar]
- 45. O'Leary DS, Augustyniak RA. Muscle metaboreflex increases ventricular performance in conscious dogs. Am J Physiol Heart Circ Physiol 275: H220–H224, 1998 [DOI] [PubMed] [Google Scholar]
- 46. O'Leary DS, Sheriff DD. Is the muscle metaboreflex important in control of blood flow to ischemic active skeletal muscle in dogs? Am J Physiol Heart Circ Physiol 268: H980–H986, 1995 [DOI] [PubMed] [Google Scholar]
- 47. O'Leary DS. Altered reflex cardiovascular control during exercise in heart failure: animal studies. Exp Physiol 91: 73–77, 2006 [DOI] [PubMed] [Google Scholar]
- 48. Pawelczyk JA, Pawelczyk RA, Warberg J, Mitchell JH, Secher NH. Cardiovascular and catecholamine responses to static exercise in partially curarized humans. Acta Physiol Scand 160: 23–28, 1997 [DOI] [PubMed] [Google Scholar]
- 49. Rotto DM, Kaufman MP. Effect of metabolic products of muscular contraction on discharge of group III and IV afferents. J Appl Physiol 64: 2306–2313, 1988 [DOI] [PubMed] [Google Scholar]
- 50. Rowell LB, Freund PR, Hobbs SF. Cardiovascular responses to muscle ischemia in humans. Circ Res 48: I37–I47, 1981 [PubMed] [Google Scholar]
- 51. Rowell LB, Hermansen L, Blackmon JR. Human cardiovascular and respiratory responses to graded muscle ischemia. J Appl Physiol 41: 693–701, 1976 [DOI] [PubMed] [Google Scholar]
- 52. Rowell LB, O'Leary DS. Reflex control of the circulation during exercise: chemoreflexes and mechanoreflexes. J Appl Physiol 69: 407–418, 1990 [DOI] [PubMed] [Google Scholar]
- 53. Sala-Mercado JA, Hammond RL, Kim JK, Rossi NF, Stephenson LW, O'Leary DS. Muscle metaboreflex control of ventricular contractility during dynamic exercise. Am J Physiol Heart Circ Physiol 290: H751–H757, 2006 [DOI] [PubMed] [Google Scholar]
- 54. Sheriff DD, Augustyniak RA, O'Leary DS. Muscle chemoreflex-induced increases in right atrial pressure. Am J Physiol Heart Circ Physiol 275: H767–H775, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Sheriff DD, Nelson CD, Sundermann RK. Does autonomic blockade reveal a potent contribution of nitric oxide to locomotion-induced vasodilation? Am J Physiol Heart Circ Physiol 279: H726–H732, 2000 [DOI] [PubMed] [Google Scholar]
- 56. Sheriff DD, Wyss CR, Rowell LB, Scher AM. Does inadequate oxygen delivery trigger pressor response to muscle hypoperfusion during exercise? Am J Physiol Heart Circ Physiol 253: H1199–H1207, 1987 [DOI] [PubMed] [Google Scholar]
- 57. Shoemaker JK, Mattar L, Kerbeci P, Trotter S, Arbeille P, Hughson RL. WISE 2005: stroke volume changes contribute to the pressor response during ischemic handgrip exercise in women. J Appl Physiol 103: 228–233, 2007 [DOI] [PubMed] [Google Scholar]
- 58. Sinoway LI, Rea RF, Mosher TJ, Smith MB, Mark AL. Hydrogen ion concentration is not the sole determinant of muscle metaboreceptor responses in humans. J Clin Invest 89: 1875–1884, 1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sinoway LI, Smith MB, Enders B, Leuenberger U, Dzwonczyk T, Gray K, Whisler S, Moore RL. Role of diprotonated phosphate in evoking muscle reflex responses in cats and humans. Am J Physiol Heart Circ Physiol 267: H770–H778, 1994 [DOI] [PubMed] [Google Scholar]
- 60. Victor RG, Bertocci LA, Pryor SL, Nunnally RL. Sympathetic nerve discharge is coupled to muscle cell pH during exercise in humans. J Clin Invest 82: 1301–1305, 1988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Victor RG, Seals DR. Reflex stimulation of sympathetic outflow during rhythmic exercise in humans. Am J Physiol Heart Circ Physiol 257: H2017–H2024, 1989 [DOI] [PubMed] [Google Scholar]
- 62. Victor RG, Seals DR, Mark AL. Differential control of heart rate and sympathetic nerve activity during dynamic exercise. Insight from intraneural recordings in humans. J Clin Invest 79: 508–516, 1987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Wallin BG, Victor RG, Mark AL. Sympathetic outflow to resting muscles during static handgrip and postcontraction muscle ischemia. Am J Physiol Heart Circ Physiol 256: H105–H110, 1989 [DOI] [PubMed] [Google Scholar]
- 64. White S, Patrick T, Higgins CB, Vatner SF, Franklin D, Braunwald E. Effects of altering ventricular rate on blood flow distribution in conscious dogs. Am J Physiol 221: 1402–1407, 1971 [DOI] [PubMed] [Google Scholar]
- 65. Wyss CR, Ardell JL, Scher AM, Rowell LB. Cardiovascular responses to graded reductions in hindlimb perfusion in exercising dogs. Am J Physiol Heart Circ Physiol 245: H481–H486, 1983 [DOI] [PubMed] [Google Scholar]