Abstract
Efferent renal sympathetic nerves reinnervate the kidney after renal denervation in animals and humans. Therefore, the long-term reduction in arterial pressure following renal denervation in drug-resistant hypertensive patients has been attributed to lack of afferent renal sensory reinnervation. However, afferent sensory reinnervation of any organ, including the kidney, is an understudied question. Therefore, we analyzed the time course of sympathetic and sensory reinnervation at multiple time points (1, 4, and 5 days and 1, 2, 3, 4, 6, 9, and 12 wk) after renal denervation in normal Sprague-Dawley rats. Sympathetic and sensory innervation in the innervated and contralateral denervated kidney was determined as optical density (ImageJ) of the sympathetic and sensory nerves identified by immunohistochemistry using antibodies against markers for sympathetic nerves [neuropeptide Y (NPY) and tyrosine hydroxylase (TH)] and sensory nerves [substance P and calcitonin gene-related peptide (CGRP)]. In denervated kidneys, the optical density of NPY-immunoreactive (ir) fibers in the renal cortex and substance P-ir fibers in the pelvic wall was 6, 39, and 100% and 8, 47, and 100%, respectively, of that in the contralateral innervated kidney at 4 days, 4 wk, and 12 wk after denervation. Linear regression analysis of the optical density of the ratio of the denervated/innervated kidney versus time yielded similar intercept and slope values for NPY-ir, TH-ir, substance P-ir, and CGRP-ir fibers (all R2 > 0.76). In conclusion, in normotensive rats, reinnervation of the renal sensory nerves occurs over the same time course as reinnervation of the renal sympathetic nerves, both being complete at 9 to 12 wk following renal denervation.
Keywords: hypertension, nerve regeneration, substance P, CGRP, renal pelvic wall, renal cortex
in the kidney, the majority of the afferent sensory nerves containing the neuropeptides substance P and calcitonin gene-related peptide (CGRP) are located in the renal pelvic wall (15, 26, 32–34, 40), an ideal location for mechanosensory nerves sensing stretch of the renal pelvic wall. Indeed, these mechanosensory nerves are activated by increases in renal pelvic pressure <5 mmHg; i.e., within the physiological range (17, 36, 47, 54). In healthy normotensive rats fed a normal sodium diet, activation of the renal mechanosensory nerves increases afferent renal nerve activity (ARNA). This in turn leads to decreases in efferent renal sympathetic nerve activity (ERSNA) and a natriuresis, an inhibitory renorenal reflex response (37). A physiological role for the inhibitory renorenal reflexes in the renal control of the homeostatic regulation of arterial pressure and sodium balance was demonstrated in rats in which the afferent renal nerves were removed by dorsal rhizotomy. These rats developed hypertension when fed a high-sodium diet (28, 35).
In contrast to the rather distinct localization of the renal sensory nerves to the renal pelvic wall, the sympathetic nerves innervate both vascular and tubular structures throughout the kidney except in the inner medulla (1, 2). All parts of the renal vasculature are innervated, with the greatest density being along the afferent arterioles. Of the tubular structures, the greatest density of sympathetic nerves is found along the thick ascending limbs and distal convoluted tubules followed by the collecting ducts and proximal tubules.
The sympathetic nerve fibers have also been found in the renal pelvic wall, although much less abundant than the sensory nerves (33, 34, 40). Importantly, where the sympathetic nerve fibers in the pelvic wall were found, they were in close contact with the sensory nerves. Likewise, sympathetic and sensory nerves have been identified in the same nerve bundle close to the renal arterial wall (33, 34). The close anatomical relationship between the renal sympathetic and sensory nerves is associated with a functional interaction between ERSNA and ARNA whereby increases in ERSNA increase ARNA which in turn leads to decreases in ERSNA via activation of the inhibitory renorenal reflexes (33). The reciprocal interaction between ERSNA and ARNA serves as an important mechanism regulating ERSNA during physiological conditions to maintain sodium balance. However, in pathological conditions of sodium retention, the impairment of the inhibitory renorenal reflex control of ERSNA (24, 29, 30) is an inappropriate response that contributes to increased ERSNA and sodium retention (11, 42).
There is considerable evidence for a role of the renal nerves in hypertension (11, 42). Renal denervation has been shown to reduce arterial pressure in many models of experimental hypertension in animals (11) and more recently also in patients with drug-resistant hypertension (14, 38, 39). However, the mechanisms involved in the long-term (>2 yr) reduction in arterial pressure (38) are unclear. The denervation procedures involve most likely interruption of both renal sympathetic and sensory nerves. It has long been known that efferent renal sympathetic nerves reinnervate the kidney following renal denervation (10, 16, 20, 45, 48, 49, 59). Reports of long-term reduction in muscle sympathetic nerve activity after renal denervation (19, 53) suggested that the long-term depressor effect of renal denervation was due to interruption of excitatory reflexes originating in the kidneys and conveyed centrally by the afferent renal nerves. This raised the possibility that the renal sensory nerves do not reinnervate the kidney. Although, there is support for excitatory reflexes originating in diseased kidneys (4, 9, 18, 21, 22, 24), the close anatomical and functional interaction between efferent and afferent renal nerves (33, 34) would suggest a coupling between afferent and efferent renal reinnervation in contrast to a selective lack of sensory reinnervation of the kidney. To date only a few studies have examined the issue of whether renal sensory nerves reinnervate renal tissue following renal denervation. Therefore, we compared the abundance of sensory and sympathetic nerves in the ipsilateral denervated kidney with that in the contralateral innervated kidney at various time points following renal denervation using immunohistochemistry combined with unbiased quantitative methods.
METHODS
The experimental protocols were approved by St. Louis University Animal Care and Use Committee and performed according to the “Guide for the Care and Use of Laboratory Animals” from the National Institutes of Health.
Seventeen male Sprague-Dawley rats (225–275 g) were anesthetized with isoflurane (1.8%, Butler Schein Animal Health, Dublin, OH). The left kidney was exposed by a flank incision. Unilateral left renal denervation was performed by sectioning all visible renal nerves, stripping the renal artery, and painting it with 10% phenol in absolute alcohol (37). The muscle and skin layers were separately sutured, and the rats were treated with carprofen (10 μg/kg im) and enrofloxacin (5 mg/kg im) and returned to their home cages following surgery. This denervation technique results in complete renal denervation, as shown by total abolition of the renal vasoconstrictor response to electrical renal nerve stimulation of the ipsilateral lumbar sympathetic chain and a reduction of the renal tissue norepinephrine content to 3% of that observed in sham renal-denervated rats (12).
Immunohistochemistry.
To determine whether the sensory nerves reinnervate renal tissue following the renal denervation procedure and, if so, whether this occurs with a similar time course as the sympathetic reinnervation, rats were anesthetized with pentobarbital sodium at various time points after left renal denervation 1 (n = 1), 4 (n = 2), 5 (n = 1) days, 1 (n = 2), 2 (n = 2), 3 (n = 3), 4 (n = 1), 6 (n = 2), 9 (n = 2), and 12 (n = 1) wk. In brief, after anesthesia, rats were transcardially perfused with calcium-free Tyrode solution followed by phosphate-buffered (0.1 M, pH 7.4) fixative containing 4% wt/vol paraformaldehyde and 0.2% wt/vol picric acid. The left and right kidneys were quickly dissected, postfixed in fixative for 90 min, and stored in 10% sucrose at 4°C. Fourteen micrometer-thick sections were cut on a cryostat and thaw mounted onto gelatin-coated slides. Renal sections from the left denervated and right innervated kidney were placed on the same slide. The immunohistochemical procedures used to identify renal sensory and sympathetic nerves have been previously described in detail (26, 32–34).
Adjacent slides were incubated with antisera against markers for mechano- and chemosensory nerves (41); substance P [rabbit 1:16,000 (8)] and CGRP [rabbit 1:24,000(50)]; and against markers for sympathetic nerves, neuropeptide Y (NPY) [rabbit, 1:4,000 (57)], and tyrosine hydroxylase (TH) [rabbit, 1:2,000 (43)]. All primary antibodies were incubated for 24 h at 4°C. Immunoreactivity was visualized using the tyramide signal amplification system (TSA-Plus: PerkinElmer Life and Analytical Sciences, Waltham, MA). In addition, sections were counter stained with the nuclear Hoechst 33342 (1:10,000).
Image acquisition.
Whole kidney images where acquired on a Vslide slide scanning microscope (Metasystems, Alltlussheim, Germany) equipped with ×2.5, ×5, × 10, and ×20 objectives and filter sets for DAPI (EX350/50-EM470/40) and FITC (EX493/16-EM527/30). Whole microscope slides were scanned at ×2.5, and tissue was detected based on the Hoechst 33342 signal. After a position map was generated, the left and right kidneys were scanned using ×10 primary objective.
Image processing.
For unbiased quantification of sympathetic and sensory innervation entire cross-sections of the kidney containing pelvis, renal cortex, and medulla were captured. Between 500 and 600 individual field of view images (×10 objective) were stitched together generating high resolution (700 megapixels) channel grayscale images.
For determination of the renal sensory innervation, the renal pelvic wall in the innervated and denervated kidney was outlined and exported to individual images using ImageJ (1.45p, NIH, Bethesda, MD). The sensory nerves in the pelvic wall were then selected based on the optical intensity values of the substance P-immunoreactive (ir) and CGRP-ir fibers (Fig. 1). Renal pelvic wall innervation of substance P-ir and CGRP-ir fibers was calculated as integrated optical density of the substance P-ir/CGRP-ir fibers divided by the integrated optical density of total area of the pelvic wall.
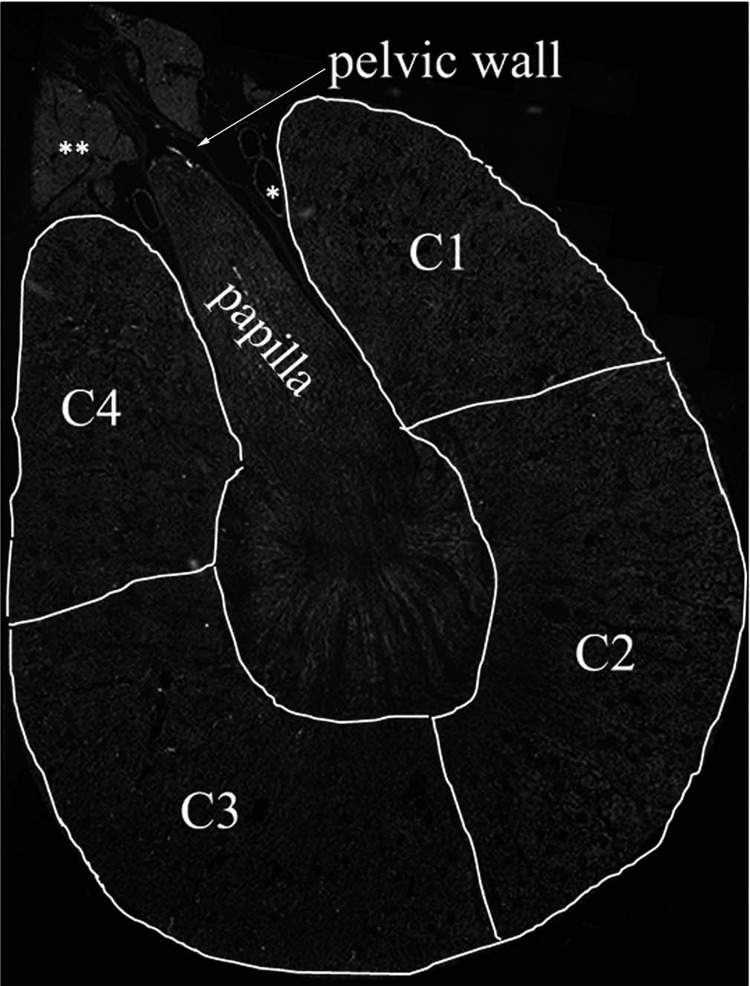
Fig. 1.
For analyses of the optical intensity of the renal sensory nerves, the renal pelvic wall was outlined (arrow) and for analyses of the intensity of the sympathetic nerves four areas of the renal cortex were outlined, C1 through C4. C1 and C4 represent the peripelvic area, and C2 and C3 represent the renal cortical areas more distal from the renal hilus. C2 and C3 also included the outer part of the outer medulla. *Vessels; **fat tissue.
For determination of the renal sympathetic innervation, four sections of the renal cortex + outer medulla were outlined and exported into individual images (Fig. 1). The four individual sections were of similar size in the innervated and the contralateral denervated kidneys. Sections C1 and C4 outlined the peripelvic cortical area; i.e., the cortical area surrounding the renal pelvis. Sections C2 and C3 outlined the cortical area more distal from the renal hilus. C2 and C3 also included the outer part of the outer medulla. The sympathetic nerves in the cortical areas C1 through C4 were selected based on the optical intensity of the NPY-ir and TH-ir fibers. Cortical innervation of NPY-ir and TH-ir fibers in each of the four sections were calculated by dividing the integrated optical density of NPY-ir and TH-ir by the integrated optical density of total area in each cortical section.
Statistical analyses.
The optical density values of the sensory nerves in the renal pelvic wall in the denervated kidneys were compared with those in the contralateral innervated kidney. Likewise, the optical density values of the sympathetic nerves in each region, C1 through C4, in the denervated kidney were compared with those in the contralateral kidney. Linear regression of the ratio of the nerve optical density values in the denervated and the innervated kidneys versus time was calculated and a significance level of 5% chosen (GraphPad Prism 5.03, GraphPad Software, La Jolla, CA).
RESULTS
Sympathetic innervation.
In innervated kidneys, the optical density of the NPY-ir and TH-ir fibers averaged 1.2 ± 0.1% and 1.2 ± 0.2%, respectively, in total renal cortex; i.e., C1 through C4. NPY-ir and TH-ir fibers were observed in the wall of numerous vessels and along tubular structures (Fig. 2, data on TH-ir not shown). NPY-ir and TH-ir fibers were not observed in the inner renal medulla. There were no differences in the optical density of the NPY-ir and TH-ir fibers in the innervated kidneys removed at the various time points after renal denervation of the contralateral kidney.
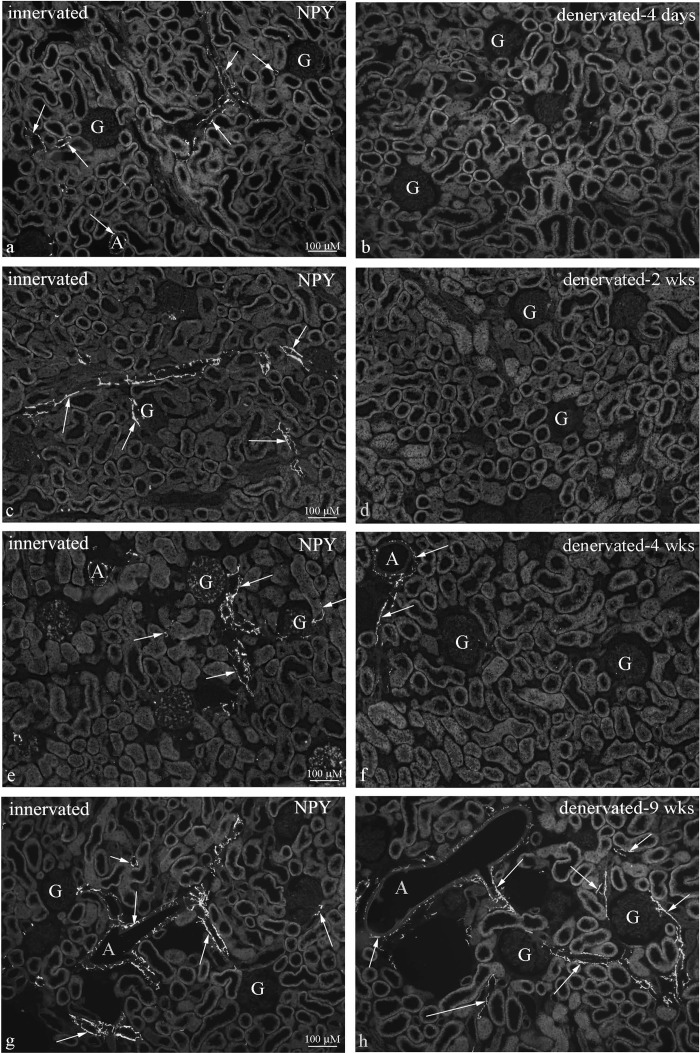
Fig. 2.
Immunohistochemical labeling of renal tissue for neuropeptide Y (NPY) showed NPY-immunoreactive (ir) fibers close to vessels and tubular structures in the peripelvic cortical area in innervated kidneys at all time points (left). In the contralateral denervated kidneys (right), there were no NPY-ir fibers in the peripelvic cortical area 4 days (b) and 2 wk (d) after denervation and markedly reduced numbers 4 wk (f) after denervation. At 9 wk after denervation, the distribution of NPY-ir fibers in the peripelvic cortical are was similar in the innervated and denervated kidneys (g and h). Arrows; nerves, A; arteriole, G; glomerulus.
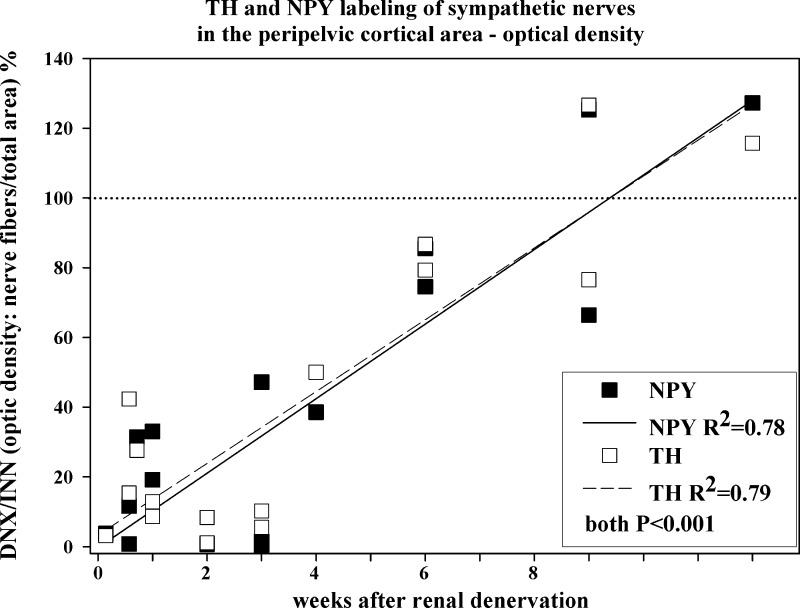
One day after renal denervation, the optical density of the NPY-ir and TH-ir fibers in the peripelvic cortical region, C1 and C4 (Fig. 1), was 4% and 3%, respectively, of that in the contralateral innervated kidney. Over time, there was a gradual increase in the optical density of the NPY-ir and TH-ir fibers in the denervated kidney, being about 40–50% and 100% of that in the contralateral innervated kidney at 4 and 9 to 12 wk, respectively, postrenal denervation (Fig. 3). The gradual increase in the optical density of the NPY-ir and TH-ir fibers in the denervated kidneys followed a similar time course, the slopes, and the y-intercepts of the two regressions lines being similar (Fig. 3).
Fig. 3.
The ratio between the denervated and innervated kidney in each rat of the optical density of NPY/tyrosine hydroxylase (TH)-ir fibers in the renal pelvic wall at various time points following unilateral renal denervation. Closed and open symbols, denervated-to-innervated ratio of the optical density of NPY-ir and TH-ir fibers, respectively; solid and dashed lines, linear regression of the denervated-to-innervated ratio of the optical density of NPY-ir and TH-ir fibers, respectively, vs. time; n = 17.
In the areas more distal from the renal hilus, C2 and C3, the reinnervation of the NPY-ir and TH-ir fibers occurred at a slightly slower pace. At 6 wk after renal denervation, the optical density of the NPY-ir and TH-ir fibers was 25 and 50%, respectively, and at 12 wk 60% and 80%, respectively, of that in the innervated kidneys.
Sensory renal innervation.
In innervated kidneys, the majority of the substance P-ir and CGRP-ir fibers were located in the renal pelvic wall (Fig. 4, data on CGRP-ir not shown), in agreement with previous studies. SP-ir and CGRP-ir fibers were also found in the wall of the artery and vein in the hilus, although the density of the innervation was less than that in the pelvic wall (data not shown). The optical density of substance P-ir and CGRP-ir fibers in the renal pelvic wall averaged 41 ± 2% and 58 ± 1%, respectively, of that in total renal pelvic wall tissue, the difference in optical density being significant (P < 0.01). However, there was no difference in the area occupied by the substance P-ir and CGRP-ir fibers in the renal pelvic wall, 22 ± 2% and 23 ± 1%, respectively, of total pelvic wall area. There were no differences in the optical density of the substance P-ir and CGRP-ir fibers in the innervated kidneys removed at the various time points after renal denervation of the contralateral kidney.
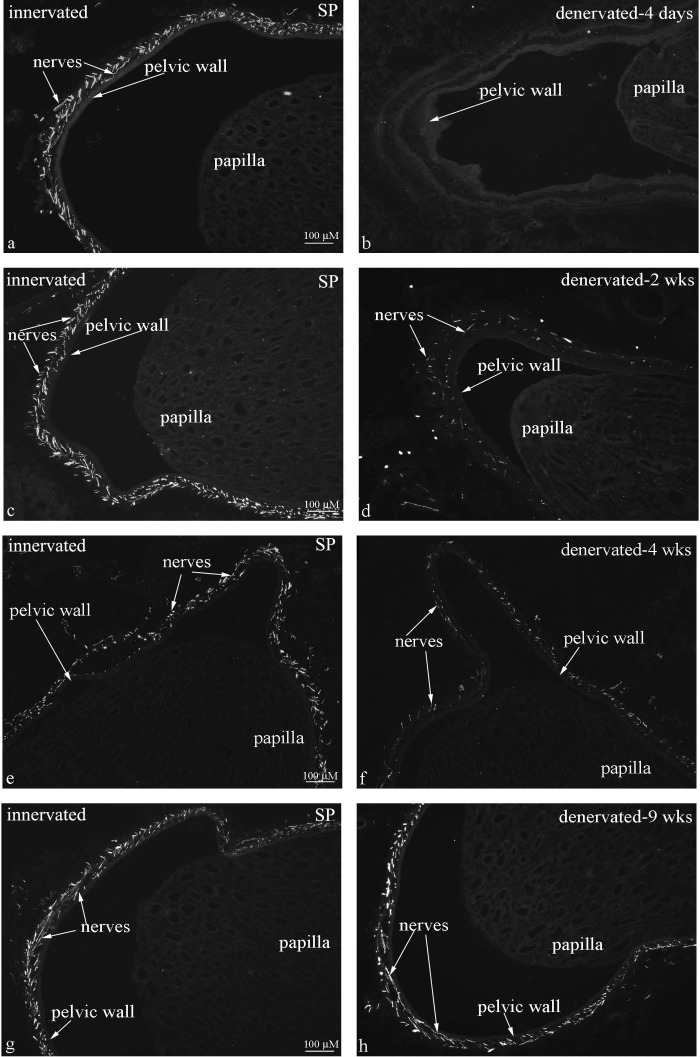
Fig. 4.
Immunohistochemical labeling of renal tissue for substance P (SP) showed dense distribution of SP-ir fibers in the renal pelvic wall in innervated kidneys at all time points (left). In the contralateral denervated kidneys (right), there were no SP-ir fibers in the pelvic wall 4 days after denervation (b) and markedly reduced numbers at 2 and 4 wks after denervation (d, f). At 9 wks after denervation, the distribution of SP-ir fibers in the pelvic wall was similar in the innervated and denervated kidneys (g, h).
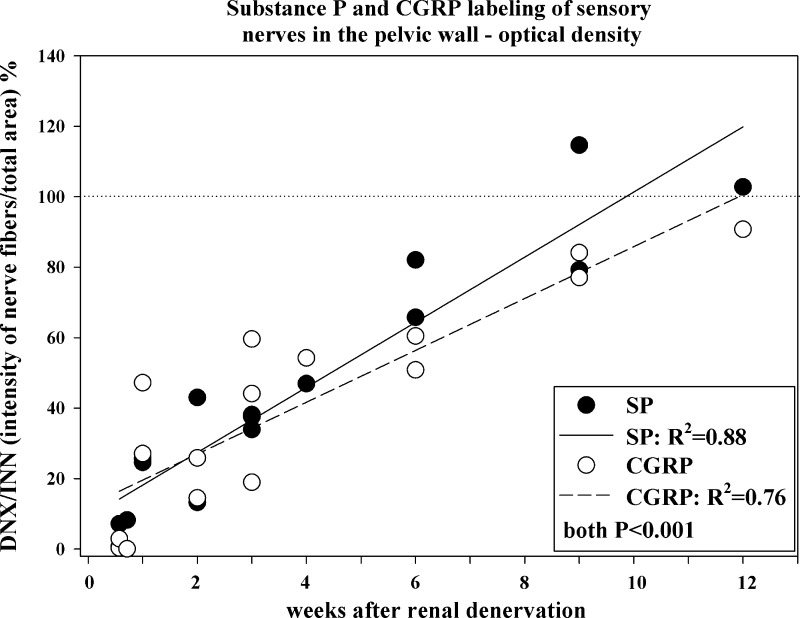
One day after renal denervation, the optical density of the substance P-ir and CGRP-ir fibers in the renal pelvic wall in the denervated kidney was 78% and 94%, respectively, of that in the contralateral innervated kidney (data not shown). However, 4 and 5 days after renal denervation, there was a marked reduction in the optical density of the substance P-ir and CGRP-ir fibers (Figs. 4 and 5) in the denervated kidney. Over time, there was a gradual increase in the optical density of the sensory nerves in the denervated kidney being about 50% and 100% of that in the contralateral innervated kidney at 4 and 9 to 12 wk, respectively, postrenal denervation. The slopes of the two regression lines depicting the relationship between time after renal denervation and the ratio of optical density of the SP-ir and CGRP-ir fibers in the denervated versus innervated kidneys were similar.
Fig. 5.
The ratio between the denervated and innervated kidney in each rat of the optical density of SP/calcitonin gene-related peptide (CGRP)-ir fibers in the renal pelvic wall at various time points following unilateral renal denervation. Closed and open symbols, denervated-to-innervated ratio of the optical density of SP-ir and CGRP-ir fibers, respectively; solid and dashed lines, linear regression of the denervated-to-innervated ratio of the optical density of SP-ir and CGRP-ir fibers, respectively, vs. time; n = 16.
DISCUSSION
With the use of unbiased quantitative histochemical methods, the present study shows that combined surgical-chemical renal denervation resulted in significant reduction in the optical density of the NPY-ir, TH-ir, substance P-ir, and CGRP-ir fibers in renal tissue 4 to 5 days postdenervation. Thereafter, there was a gradual increase in the optical density of the substance P-ir and CGRP-ir fibers in renal pelvic tissue in the denervated kidney. This increase followed a similar time course as that of the NPY-ir and TH-ir fibers in the renal cortical areas surrounding the renal pelvis. At 9 to 12 wk postrenal denervation, the optical density of the substance P-ir and CGRP-ir fibers in renal pelvic tissue and of the NPY-ir and TH-ir fibers in the renal peripelvic cortical tissue was similar in the denervated and innervated kidneys. These findings suggest that reinnervation of the renal sensory nerves occurs over the same time course as reinnervation of the renal sympathetic nerves, both being complete at 9 to 12 wk following renal denervation in normotensive rats.
Denervation procedure.
In the current studies, the renal nerves were denervated by applying a solution of phenol in 95% alcohol on renal vessels and surrounding tissue plus removing all renal nerves visible under a dissecting microscope. It may be argued that this technique is different from the radio frequency energy technique used in patients and results in reinnervation of renal tissue sooner than what may be expected if another technique, like that used in patients, had been applied in the rat. However, it is important to note that the protein denaturation of the neural tissue produced by phenol+alcohol plus surgical removal of the renal nerves resulted in total abolition of immunoreactive NPY and TH in renal tissue as observed 1 day after the denervation procedure. This is consistent with the demonstration by us and many other investigators that this denervation technique reduces renal tissue norepinephrine concentration by more than 90% (11, 37). Thus this technique resulted in more complete renal denervation than that used in patients in which renal vein norepinephrine spillover was reduced by 47% 1 mo after the renal denervation procedure (39). No data are currently available on renal reinnervation following the renal denervation procedure in patients.
Sympathetic renal reinnervation.
Previous studies of the renal sympathetic innervation in rats using radiolabeled norepinephrine showed that 0.6% to 9.6% of the renal vasculature and 2.4% to 6.5% of renal tubular structures are innervated by sympathetic nerves (1, 2). The current findings show that the optical density of the NPY-ir and TH-ir fibers averages 1.2% of total renal cortex in the innervated kidneys suggesting that this method represents a valid evaluation of the sympathetic innervation of renal tissue.
There is considerable evidence for the sympathetic nerves reinnervating renal tissue following renal denervation in both humans and animals. Early histological studies in dogs showed partial reinnervation 3 mo postdenervation (10, 49) with reinnervation being complete at 6 mo after renal denervation (10). Similarly, measurements of renal cortical norepinephrine content in dogs suggested complete reinnervation of the sympathetic nerves 4 mo postrenal denervation (45). Importantly, renal cortical norepinephrine content paralleled the gradual return of the increase in plasma renin activity in response to low-sodium diet over the 4-mo period (45). A study in rats showed partial return of renal cortical norepinephrine content and renal vasoconstrictor responses to electrical renal nerve stimulation at 4 wk following renal denervation (23). The renal vasoconstrictor responses were restored toward control at 8 to 9 wk after renal denervation. Similar findings have been demonstrated in hypertensive rats. Renal denervation delayed the onset of hypertension in both spontaneous hypertensive rats and DOCA-salt hypertensive rats in association with marked decreases in renal tissue norepinephrine content. Arterial pressure increased gradually and was similar to that in sham-denervated hypertensive rats 6 to 7 wk after renal denervation at which time renal tissue norepinephrine content in the denervated kidneys was 60–80% of that in the sham-operated rats (20, 59). Although there are few studies reported in humans, there is anatomical evidence for gradual reinnervation of the human kidney starting 4 wk after renal transplantation with extensive reinnervation at 8 mo (16). Taken together there is considerable evidence, both chemical and functional, for sympathetic reinnervation following denervation in both rats and dogs. Furthermore, available data suggest that there are differences among the species in the time required for sympathetic reinnervation following denervation, weeks in rats, months in dogs, and months-year(s) in humans.
The current findings showed that the optical density of the sympathetic nerves in the denervated kidney was reduced by more than 95% of that in the contralateral innervated kidney 1 day after renal denervation. Thereafter, there was a gradual increase in NPY content in renal sympathetic nerves reaching 40–50% of that in the innervated control kidney at 4 wk and 100% at 9–12 wk after renal denervation. Importantly, similar results were obtained with the NPY and TH antibodies, both of which are well-known markers of the sympathetic nerves. The increased ratio of the optical density of NPY-ir/TH-ir fibers in the denervated and innervated kidneys over time was solely due to an increase in the optical density of the sympathetic nerves in the denervated kidney because no variation in the optical density of the sympathetic nerves in the contralateral innervated kidney was observed at any time point. Taken together, our immunohistochemical findings demonstrating a gradual reinnervation of the sympathetic nerves over time are in agreement with previous histological and physiological studies in rats and dogs (10, 20, 23, 45, 59).
Similarly to what has been reported in human transplanted kidneys (16), the reinnervation of sympathetic nerves into the renal cortical areas further away from the renal hilus, areas C2 and C3 (Fig. 1), occurred at a slower rate compared with the peripelvic renal cortical areas. These data suggest that reinnervation of the renal sympathetic nerves after denervation follow the same pattern as the development of the sympathetic innervation, which starts at the peripelvic structures and reaches the juxtamedullary and cortical areas away from the renal hilus later in the development (40).
Sensory innervation.
Renal denervation in drug-resistant hypertensive patients results in long-term (>2 yr) reduction in arterial pressure (38). Because of the considerable evidence for renal sympathetic reinnervation (vide supra), one of the possible mechanisms suggested to contribute to the long-term reduction in arterial pressure is interruption of excitatory reflexes originating in the kidney. Support for this hypothesis is derived from the findings that bilateral renal denervation reduces arterial pressure in association with reduction in muscle sympathetic nerve activity (19, 53) suggesting interruption of excitatory reflexes originating in the kidneys in these patients. There is evidence to support that when the inhibitory renorenal reflexes are suppressed, excitatory reflexes originating in the kidney prevail (24). Excitatory reflexes originating in diseased/injured kidneys have been demonstrated in various pathological conditions, including renovascular hypertension and renal failure (4, 9, 18, 21, 22). These studies support the notion of excitatory reflexes originating in the kidney and contributing to the increased arterial pressure in drug-resistant hypertensive patients. This has lead to the hypothesis that lack of renal sensory reinnervation contributes to the long-term reduction in arterial pressure following renal denervation.
There have only been few studies examining sensory nerve reinnervation in detail of any organ. There are reports on cardiac transplant patients experiencing chest pain related to cardiac ischemia (56). Also, studies in cardiac autotransplanted dogs have shown normal renal reflex responses to cardiac vagal afferent stimulation 8–12 yr after transplantation (46). These studies would suggest that the afferent nerves may reinnervate cardiac tissue postcardiac transplantation, even if it may be a slow process. A more recent study in rats suggested that the sensory nerves reinnervate the glabrous skin of the rat hind paw 4 to 8 wk after sciatic nerve injury (51). A functional study in dogs examining the pressor responses to intrarenal administration of capsaicin showed the capsaicin-induced pressor responses being absent 2 to 3 wk postkidney autotransplantation and partially restored toward control levels 1–3 years later (52). Further functional support for functional reinnervation of the renal sensory nerves following renal denervation is derived from one-kidney, one-clip hypertensive rats, a model of hypertension characterized by increased afferent renal nerve activity contributing to the increased arterial pressure. Renal denervation of the clipped ischemic kidney resulted in marked decreases in arterial pressure for 3 wk. Five weeks after renal denervation, arterial pressure was similar in the denervated and sham-denervated rats (22). Together, these studies suggest that functional renal sensory reinnervation may occur following renal denervation.
In view of the sympathetic and sensory nerves being located in the same nerve bundles in renal tissue (33, 34, 40) together with the functional interaction between sympathetic and sensory nerves (33, 34), we hypothesized that the sensory nerves may reinnervate the renal tissue and, if so, in a time-dependent fashion similar to that of the sympathetic nerves.
In agreement with previous studies, the majority of the substance P-ir and CGRP-ir fibers in the kidney are located in the renal pelvic wall (15, 26, 32–34, 40). Here we show that the area of the pelvic wall occupied by the substance P-ir and the CGRP-ir fibers was similar. These findings agree with our previous studies that showed colocalization of the two neuropeptides in all sensory nerve fibers in the pelvic wall (32). The higher optical density of the CGRP-ir fibers compared with that of the substance P-ir fibers may reflect a difference in the efficacy of binding between the two antibodies used. However, and more likely, the higher optical density of the CGRP-ir fibers may reflect a higher CGRP content since activation of the renal sensory nerves results in a 10-fold higher release of CGRP than substance P (31). Importantly, the analysis of the ratio of the optical density of the CGRP-ir or substance P-ir fibers between the denervated and innervated kidneys over time were similar to the analysis based on the ratio of the areas occupied by the immunoreactive fibers in the denervated and innervated kidneys.
The current findings showed that 1 day after renal denervation there was only a small reduction in the optical density of the substance P-ir and CGRP-ir in the denervated kidneys, which was not due to a failure of the renal denervation procedure since the optical density of NPY- and TH-immunoreactivities were reduced by over 95% in the same kidney. Instead, these findings may suggest a rather slow release of the neuropeptides during basal conditions in healthy rats. However, 4–5 days after renal denervation there was a marked reduction in the optical density of the substance P-ir and CGRP-ir fibers in the denervated kidney. Thereafter, there was a gradual increase in the optical density of the substance P-ir and CGRP-ir fibers in the denervated kidney over time reaching 50% at 4 wk and 100% at 9 to 12 wk postdenervation. Our findings suggesting a gradual sensory renal reinnervation following renal denervation are indirectly supported by the functional data in one-kidney, one-clip hypertensive rats, which showed a gradual increase in arterial pressure over a similar time period following renal denervation (22, vide supra). There were no differences in the slopes and y-intercepts among the four regression curves depicting the ratio of optical densities of the substance P-ir, CGRP-ir, NPY-ir, and TH-ir fibers in the denervated versus innervated kidneys over time. These findings suggest that the renal sensory nerves reinnervate the renal pelvic wall in a time-dependent fashion similar to the sympathetic nerves in the peripelvic cortical tissue. In agreement with the current findings, data recently presented comparing labeling with antibodies against TH and CGRP in innervated and denervated rat kidneys suggested parallel sympathetic and sensory reinnervation in renal tissue 12 wk following renal denervation (13).
In summary, the substance P/CGRP content in the sensory nerves in the denervated kidney gradually returned toward that of the innervated kidney over a period of 12 wk after renal denervation following a similar time course as the return of NPY/TH content in the sympathetic nerves. It can be speculated that the return of neurotransmitter content demonstrated herein is associated with restoration of function of both efferent sympathetic and afferent sensory nerves. In this regard, then, while removal of both renal sympathetic and renal sensory nerves most likely contributes to the arterial pressure reduction in drug-resistant hypertensive patients following renal denervation, the current results suggest that additional mechanisms are likely to contribute to the long-term arterial pressure reduction observed.
Perspectives and Significance
There is considerable evidence for renal sympathetic nerve activity being inappropriately increased in conditions of hypertension and thereby aggravating the hypertensive process (11, 42). Impairment of the inhibitory renorenal reflexes contributes to the increased ERSNA in pathological conditions characterized by increased activity of the renin angiotensin system, including hypertension (7, 25, 27, 30). When the inhibitory renorenal reflexes are impaired, e.g., in two-kidney, one-clip hypertensive rats, excitatory reflexes originating in the ischemic kidney prevail and contribute to the hypertension (21, 22, 24). Further evidence for excitatory reflexes originating in diseased kidneys is derived from humans and rats with renal failure (4, 9, 18). From these findings, it has been suggested that the fall in arterial pressure in association with decreases in muscle sympathetic nerve activity produced by bilateral renal denervation in drug-resistant hypertensive patients (19, 53) is related, at least in part, to afferent renal denervation removing the influence of excitatory reflexes originating in a diseased kidney.
Removal of both renal sympathetic and renal sensory nerves most likely contributes to the arterial pressure reduction in patients following renal denervation, at least initially. However, the current results show that the renal sensory nerves reinnervate the renal tissue in a similar time-dependent fashion as the sympathetic nerves following renal denervation in normal healthy rats. Although available data suggest that renal reinnervation will take months to year(s) in humans versus weeks in rats, the current findings suggest that additional mechanisms are likely to contribute to the long-term arterial pressure reduction (>2yr) observed in drug-resistant hypertension patients following renal denervation, assuming that the current findings represent functional reinnervation.
Among possible mechanisms contributing to the long-term reduction in arterial pressure initiated by renal denervation may be resetting of central nervous system mechanisms (e.g., neuroplasticity). Afferent renal nerves project to neurons in several brain areas involved in the cardiovascular control (3, 4, 6, 55, 58). There is considerable evidence for increased sympathetic nervous activity in renal failure (4, 5, 44). In rats with renal failure, the decrease in arterial pressure produced by afferent renal denervation was associated with decreases in norepinephrine turnover in posterior and lateral hypothalamus and locus coeruleus (4).
Other mechanisms contributing to the long-term reduction in blood pressure following renal denervation may be related to peripheral resetting involving renal vascular remodeling and/or reduction in the activity of the renin angiotensin system (5, 11, 42). In addition to its beneficial effects on renal function per se, decreases in renal angiotensin would result in disinhibition of the inhibitory renorenal reflexes (25) at a time when the sympathetic and sensory nerves may be reinnervating the kidney. This may lead to restoration of the beneficial reciprocal interaction between ERSNA and ARNA, one of the important mechanisms contributing to maintaining low ERSNA to minimize sodium retention.
GRANTS
This work was supported by grants from the Department of Veterans Affairs, The National Institutes of Health, Heart, Lung, and Blood Institute Grant R01 HL-66068 and Institute on Drug Abuse Grant R01 DA-017371, Swedish Research Council (04X-2887), and Knut and Alice Wallenberg Foundation, Sweden.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: U.C.K. and M.M.K. conception and design of research; U.C.K. and M.M.K. performed experiments; U.C.K. and J.M. analyzed data; U.C.K., J.M., T.H., and M.M.K. interpreted results of experiments; U.C.K. and J.M. prepared figures; U.C.K. drafted manuscript; U.C.K., J.M., T.H., and M.M.K. edited and revised manuscript; U.C.K., J.M., T.H., and M.M.K. approved final version of manuscript.
ACKNOWLEDGMENTS
We thank Laura Willingham and Julie Langasek for technical assistance. We are grateful for the generous supply of substance P and CGRP antisera from Drs. L. Terenius, Center for Molecular Medicine, Karolinska Institutet, Stockholm, Sweden and I. Nylander, Uppsala University, Uppsala, Sweden; TH antisera from the late Dr. M. Goldstein, New York University Medical School, New York; and NPY antisera from the late Dr. J. H. Walsh and Dr. H. C. Wong, The Center for Ulcer Research and Education of the Veterans Affairs/University of California Gastroenteric Biology Center, Los Angeles, CA.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
REFERENCES
- 1. Barajas L, Powers K. Monoaminergic innervation of the rat kidney: a quantitative study. Am J Physiol Renal Fluid Electrolyte Physiol 259: F503–F511, 1990 [DOI] [PubMed] [Google Scholar]
- 2. Barajas L, Powers K, Wang P. Innervation of the renal cortical tubules: a quantitative study. Am J Physiol Renal Fluid Electrolyte Physiol 247: F50–F60, 1984 [DOI] [PubMed] [Google Scholar]
- 3. Calaresu FR, Ciriello J. Renal afferent nerves affect discharge rate of medullary and hypothalamic single units in cat. J Auton Nerv Syst 3: 311–320, 1981 [DOI] [PubMed] [Google Scholar]
- 4. Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension 25: 878–882, 1995 [DOI] [PubMed] [Google Scholar]
- 5. Campese VM, Ku E, Park J. Sympathetic renal innervation and resistant hypertension. Int J Hypertension 2011: 1–6, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ciriello J, Calaresu FR. Central projection of afferent renal fibers in the rat: an anterograde transport study of horseradish peroxidase. J Auton Nerv Syst 8: 273–285, 1983 [DOI] [PubMed] [Google Scholar]
- 7. Chien CT, Chien HF, Cheng YJ, Chen CF, Hsu SM. Renal afferent signaling diuretic response is impaired in streptozotocin-induced diabetic rats. Kidney Int 57: 203–214, 2000 [DOI] [PubMed] [Google Scholar]
- 8. Christensson-Nylander I, Herrera-Marschitz M, Staines W, Hökfelt T, Terenius L, Ungerstedt U, Cuello C, Oertel WH, Goldstein M. Striato-nigral dynorphin and substance P pathways in the rat. Exp Brain Res 64: 169–192, 1986 [DOI] [PubMed] [Google Scholar]
- 9. Converse RL, Jr, Jacobsen TN, Toto RD, Jost CM, Cosentino F, Fouad-Tarazi F, Victor RG. Sympathetic overactivity in patients with chronic renal failure. N Engl J Med 327: 1912–1918, 1992 [DOI] [PubMed] [Google Scholar]
- 10. Couch NP, McBride RA, Dammin GJ, Murray JE. Observations on the nature of the enlargement, the regeneration of the nerves and the function of the canine renal autograft. Br J Exp Pathol 42: 106–113, 1961 [PMC free article] [PubMed] [Google Scholar]
- 11. DiBona GF, Kopp UC. Neural control of renal function. Physiol Rev 77: 75–197, 1997 [DOI] [PubMed] [Google Scholar]
- 12. DiBona GF, Sawin LL. Renal nerves in renal adaptation to dietary sodium restriction. Am J Physiol Renal Fluid Electrolyte Physiol 245: F322–F328, 1983 [DOI] [PubMed] [Google Scholar]
- 13. Ditting T, Christian Fiedler C, Freisinger W, Siegel K, Heinlein S, Schmidt ST, Neuhuber W, Ott C, Schmieder RE, Amann K, Veelken R. Evidence for afferent reinnervation after renal denervation in rats (Abstract). Hypertension 60: A473, 2012 [Google Scholar]
- 14. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Böhm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomized controlled trial. Lancet 376: 1903–1909, 2010 [DOI] [PubMed] [Google Scholar]
- 15. Ferguson M, Bell C. Substance P-immunoreactive nerves in the rat kidney. Neurosci Lett 60: 183–188, 1985 [DOI] [PubMed] [Google Scholar]
- 16. Gazdar AF, Dammin GJ. Neural degeneration and regeneration in human renal transplants. N Engl J Med 283: 222–224, 1970 [DOI] [PubMed] [Google Scholar]
- 17. Genovesi S, Pieruzzi F, Wijnmaalen P, Centonza L, Golin R, Zanchetti A, Stella A. Renal afferents signaling diuretic activity in the cat. Circ Res 73: 906–913, 1993 [DOI] [PubMed] [Google Scholar]
- 18. Hausberg M, Kosch M, Harmelink P, Barenbrock M, Hohage H, Kisters K, Dietl KH, Rahn KH. Sympathetic nerve activity in end-stage renal disease. Circulation 106: 1974–1979, 2002 [DOI] [PubMed] [Google Scholar]
- 19. Hering D, Lambert EA, Marusic P, Walton AS, Krum H, Lambert GW, Esler MD, Schlaich MP. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension 61: 457–464, 2013 [DOI] [PubMed] [Google Scholar]
- 20. Katholi RE, Naftilan AJ, Oparil S. Importance of renal sympathetic tone in the development of DOCA-salt hypertension in the rat. Hypertension 2: 266–273, 1980 [DOI] [PubMed] [Google Scholar]
- 21. Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one-clip Goldblatt hypertension. Hypertension 4: II166–II174, 1982 [PubMed] [Google Scholar]
- 22. Katholi RE, Winternitz SR, Oparil S. Role of the renal nerves in the pathogenesis of one-kidney renal hypertension in the rat. Hypertension 3: 404–409, 1981 [DOI] [PubMed] [Google Scholar]
- 23. Kline RL, Mercer PF. Functional reinnervation and development of supersensitivity to NE after denervation. Am J Physiol Regul Integr Comp Physiol 238: R353–R358, 1980 [DOI] [PubMed] [Google Scholar]
- 24. Kopp UC, Buckley-Bleiler RL. Impaired renorenal reflexes in two-kidney, one clip hypertensive rats. Hypertension 14: 445–452, 1989 [DOI] [PubMed] [Google Scholar]
- 25. Kopp UC, Cicha MZ. Impaired substance P release from renal sensory nerves in SHR involves a pertussis toxin-sensitive mechanism. Am J Physiol Regul Integr Comp Physiol 286: R326–R333, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Kopp UC, Cicha MZ, Nakamura K, Nusing RM, Smith LA, Hökfelt T. Activation of EP4 receptors contributes to prostaglandin E2 mediated stimulation of renal sensory nerves. Am J Physiol Renal Physiol 287: F1269–F1282, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Kopp UC, Cicha MZ, Yorek MA. Impaired responsiveness of renal sensory nerves in streptozotocin-treated rats and obese Zucker diabetic fatty rats: role of angiotensin. Am J Physiol Regul Integr Comp Physiol 294: R858–R866, 2008 [DOI] [PubMed] [Google Scholar]
- 28. Kopp UC, Cicha MZ, Smith LA. Arterial pressure increases in afferent renal denervated rats on high-sodium diet. Hypertension 42: 968–973, 2003 [DOI] [PubMed] [Google Scholar]
- 29. Kopp UC, Cicha MZ, Smith LA. Impaired interaction between efferent and afferent renal nerve activity in SHR involves increased activation of α2-adrenoceptors. Hypertension 57: 640–647, 2011 [DOI] [PubMed] [Google Scholar]
- 30. Kopp UC, Cicha MZ, Smith LA. Impaired responsiveness of renal mechanosensory nerves in heart failure: role of endogenous angiotensin. Am J Physiol Regul Integr Comp Physiol 284: R116–R124, 2003 [DOI] [PubMed] [Google Scholar]
- 31. Kopp UC, Cicha MZ, Smith LA. PGE2 increases release of substance P from renal sensory nerves by activating the cAMP-PKA transduction cascade. Am J Physiol Regul Integr Comp Physiol 282: R1618–R1627, 2002 [DOI] [PubMed] [Google Scholar]
- 32. Kopp UC, Cicha MZ, Smith LA, Hökfelt T. Nitric oxide modulates renal sensory nerve fibers by mechanisms related to substance P receptor activation. Am J Physiol Regul Integr Comp Physiol 281: R279–R290, 2001 [DOI] [PubMed] [Google Scholar]
- 33. Kopp UC, Cicha MZ, Smith LA, Mulder J, Hökfelt T. Renal sympathetic nerve activity modulates afferent renal nerve activity by PGE2-dependent activation of α1- and α2-adrenoceptors on renal sensory nerve fibers. Am J Physiol Regul Integr Comp Physiol 293: R1561–R1572, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Kopp UC, Cicha MZ, Smith LA, Ruohonen S, Scheinin M, Fritz Hokfelt T. Dietary sodium modulates the interaction between efferent and afferent renal nerve activity by altering activation of α2-adrenoceptors on renal sensory nerves. Am J Physiol Regul Integr Comp Physiol 300: R298–R310, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kopp UC, Jones SY, DiBona GF. Afferent renal denervation impairs baroreflex control of efferent renal sympathetic nerve activity. Am J Physiol Regul Integr Comp Physiol 295: R1882–R1890, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kopp UC, Smith LA, Pence AL. Na+-K+-ATPase inhibition sensitizes renal mechanoreceptors activated by increases in renal pelvic pressure. Am J Physiol Regul Integr Comp Physiol 267: R1109–R1117, 1994 [DOI] [PubMed] [Google Scholar]
- 37. Kopp UC, Olson LA, DiBona GF. Renorenal reflex responses to mechano- and chemoreceptor stimulation in the dog and rat. Am J Physiol Renal Fluid Electrolyte Physiol 246: F67–F77, 1984 [DOI] [PubMed] [Google Scholar]
- 38. Krum H, Barman N, Schlaich M, Sobotka P, Esler M, Mahfoud F, Bohm M, Dunlap M. Catheter-based renal sympathetic denervation for resistant hypertension. Durability of blood pressure reduction out to 24 months. Hypertension 57: 911–917, 2011 [DOI] [PubMed] [Google Scholar]
- 39. Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT, Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet 373: 1275–1281, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Liu L, Barajas L. The rat renal nerves during development. Anat Embryol 188: 345–361, 1993 [DOI] [PubMed] [Google Scholar]
- 41. Maggi CA. Tachykinins and calcitonin gene-related peptide (CGRP) as co-transmitters released from peripheral endings of sensory nerves. Progr Neurobiol 45: 1–98, 1995 [DOI] [PubMed] [Google Scholar]
- 42. Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev 90: 513–557, 2009 [DOI] [PubMed] [Google Scholar]
- 43. Markey KA, Kondo H, Shenkman L, Goldstein M. Purification and characterization of tyrosine hydroxylase from a clonal pheochromocytoma cell line. Mol Pharmacol 17: 79–85, 1980 [PubMed] [Google Scholar]
- 44. Masuo K, Lambert GW, Esler MD, Rakugi H, Ogihara T, Schlaich MP. The role of sympathetic nervous activity in renal injury and end-stage renal disease. Hypertens Res 33: 521–528, 2010 [DOI] [PubMed] [Google Scholar]
- 45. Mogil RA, Itskovitz HD, Russell JH, Murphy JJ. Renal innervation and renin activity in salt metabolism and hypertension. Am J Physiol 216: 693–697, 1969 [DOI] [PubMed] [Google Scholar]
- 46. Mohanty PK, Thames MD, Capehart JR, Kawaguchi A, Ballon B, Lower RR. Afferent reinnervation of the autotransplanted heart in dogs. J Am Coll Cardiol 7: 414–418, 1986 [DOI] [PubMed] [Google Scholar]
- 47. Morsing P, Persson AEG. Pelvic pressure and tubuloglomerular feedback in hydronephrosis. Renal Physiol Biochem 13: 181–189, 1990 [DOI] [PubMed] [Google Scholar]
- 48. Norman RA, Dzielak DJ. Role of renal nerves in onset and maintenance of spontaneous hypertension. Am J Physiol Heart Circ Physiol 243: H284–H288, 1982 [DOI] [PubMed] [Google Scholar]
- 49. Norvell JE, Banes RT. Histofluorescence and fluorometric analysis of adrenergic reinnervation in the dog kidney following autotransplantation. Transplantation 27: 69–70, 1979 [PubMed] [Google Scholar]
- 50. Orazzo C, Pieribone VA, Ceccatelli S, Terenius L, Hökfelt T. CGRP-like immunoreactivity in A11 dopamine neurons projecting to the spinal cord and a note on CGRP-CCK cross-reactivity. Brain Res 600: 39–48, 1993 [DOI] [PubMed] [Google Scholar]
- 51. Peleshok JC, Ribeiro-da-Silva A. Delayed reinnervation by nonpeptidergic nociceptive afferents of the glabrous skin of the rat hindpaw in a neuropathic pain model. J Comp Neurol 519: 49–63, 2011 [DOI] [PubMed] [Google Scholar]
- 52. Sankari B, Stowe N, Gavin JP, Satoh S, Nally JV, Novick AC. Studies on the afferent and efferent renal nerves following autotransplantation of the canine kidney. J Urol 148: 206–210, 1992 [DOI] [PubMed] [Google Scholar]
- 53. Schlaich MP, Sobotka PA, Krum H, Lambert E, Esler MD. Renal sympathetic-nerve ablation for uncontrolled hypertension. N Engl J Med 361: 932–934, 2009 [DOI] [PubMed] [Google Scholar]
- 54. Smyth TB, Shortliffe LMD, Constantinou CE. The effect of urinary flow and bladder fullness on renal pelvic pressure in a rat model. J Urol 146: 592–596, 1991 [DOI] [PubMed] [Google Scholar]
- 55. Solano-Flores LP, Rosa-Arellano MP, Ciriello J. Fos induction in central structures after afferent renal stimulation. Brain Res 753: 102–119, 1997 [DOI] [PubMed] [Google Scholar]
- 56. Stark RP, McGinn AL, Wilson RF. Chest pain in cardiac-transplant recipients. Evidence of sensory reinnervation after cardiac transplantation. N Engl J Med 324: 1791–1794, 1991 [DOI] [PubMed] [Google Scholar]
- 57. Theodorsson-Norheim E, Hemsén A, Lundberg JM. Radioimmunoassay for neuropeptide Y (NPY): chromatographic characterization of immunoreactivity in plasma and tissue extracts. Scand J Clin Lab Invest 45: 355–365, 1985 [DOI] [PubMed] [Google Scholar]
- 58. Winternitz SR, Katholi RE, Oparil S. Decrease in hypothalamic norepinephrine content following renal denervation in the one-kidney, one clip Goldblatt hypertensive rat. Hypertension 4: 369–373, 1982 [DOI] [PubMed] [Google Scholar]
- 59. Winternitz SR, Katholi RE, Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest 66: 971–978, 1980 [DOI] [PMC free article] [PubMed] [Google Scholar]