Abstract
The role of intracellular ANG II in proximal tubules of the kidney remains poorly understood. We tested the hypothesis that proximal tubule-dominant transfer of AT1a receptors in the cortex mediates intracellular ANG II-induced blood pressure responses in AT1a receptor-deficient (Agtr1a-/-) mice. A GFP-tagged AT1a receptor, AT1aR/GFP, and an enhanced cyan fluorescent intracellular ANG II fusion protein, ECFP/ANG II, were expressed in proximal tubules of Agtr1a-/- mouse kidneys via the adenoviral transfer using a sodium and glucose cotransporter 2 promoter. Transfer of AT1aR/GFP alone or with ECFP/ANG II induced proximal tubule-dominant expression of AT1aR/GFP and/or ECFP/ANG II with a peak response at 2 wk. No significant AT1aR/GFP and/or ECFP/ANG II expression was observed in the glomeruli, medulla, or extrarenal tissues. Transfer of AT1aR/GFP alone, but not ECFP/ANG II, increased systolic blood pressure by 12 ± 2 mmHg by day 14 (n = 9, P < 0.01). However, cotransfer of AT1aR/GFP with ECFP/ANG II increased blood pressure by 18 ± 2 mmHg (n = 12, P < 0.01). Twenty-four hour urinary sodium excretion was decreased by day 7 with proximal tubule-dominant transfer of AT1aR/GFP alone (P < 0.01) or with AT1aR/GFP and ECFP/ANG II cotransfer (P < 0.01). These responses were associated with twofold increases in phosphorylated ERK1/2, lysate, and membrane NHE-3 proteins in freshly isolated proximal tubules (P < 0.01). By contrast, transfer of control CMV-GFP (a recombinant human adenovirus type 5 expresses enhanced green fluorescent protein under the control of a cytomegalovirus (CMV) promoter), ECFP/ANG II, or a scrambled control ECFP/ANG IIc alone in proximal tubules had no effect on all indices. These results suggest that AT1a receptors and intracellular ANG II in proximal tubules of the kidney play an important physiological role in blood pressure regulation.
Keywords: adenoviral gene transfer, hypertension, intracellular angiotensin II, kidney, MAP kinases ERK1/2, proximal tubule, sodium and hydrogen exchanger 3
the kidney undoubtedly plays a critical role in maintaining normal body salt and fluid balance, as well as blood pressure homeostasis, but the precise intrarenal localization and signaling mechanisms involved remain an issue of continuous debates (4, 8, 13). The proximal tubule is the most important tubular segment in the kidney responsible for the reabsorption of ∼65% of the filtered sodium and fluid (48). Proximal tubule sodium and fluid reabsorption are regulated by a complexity of hemodynamic (37) and humoral factors, including ANG II, catecholamines (1, 21), atrial natriuretic peptide (17, 49), and endothelin (18, 23). However, ANG II is well recognized as the key player among these physiological regulators (5, 16, 35, 51). Indeed, all key members of the renin-angiotensin system (RAS) have been localized in proximal tubules of the kidney (2, 19, 36, 51). The expression of the substrate angiotensinogen and key enzymes, renin, and angiotensin I-converting enzyme (ACE) in proximal tubules provides the onsite formation of ANG II independent of the circulating RAS (38). Alternatively, circulating/endocrine, local paracrine, and autocrine ANG II may readily reach proximal tubules through glomerular filtration and peritubular microcirculation, where ANG II is taken up by proximal tubules to act as an intracellular peptide (32, 44, 50). These studies strongly suggest that circulating (endocrine), local tissue (paracrine and autocrine), and intracellular (intracrine) ANG II are all involved in the physiological regulation of proximal tubular sodium and fluid reabsorption and blood pressure homeostasis.
Several groups of investigators have recently investigated the relative contribution of systemic vs. proximal tubule AT1 (AT1a) receptors, and extracellular (endocrine and paracrine) vs. intracellular (intracrine) ANG II in proximal tubules to the regulation of proximal tubular reabsorption and blood pressure (9, 12, 22, 27, 28). Sigmund's group first generated novel transgenic mice with proximal tubule-specific expression of the human angiotensinogen gene using the kidney androgen-regulated protein promoter and found these mice to be hypertensive (9). Sen's group generated two unique strains of mice that express ACE either in vascular smooth endothelial cells (VSMC) or proximal tubules of the kidney and found that only those mice with ACE expression in VSMCs had normal blood pressure and proximal tubular reabsorption (22). By contrast, Bernstein's group produced the so-called ACE 2/2 mice that lack renal brush border-associated ACE and, therefore, are unable to produce ANG II in the proximal tubule (20). Blood pressure was found to be lower in these mice without altering proximal tubular transport (20). Paradoxically, basal blood pressure is also lower in transgenic ACE9/9 mice with target expression of ACE only in proximal tubules, accompanied by urine-concentrating impairment and kidney abnormalities (11). Two recent studies showed that conditional deletion of AT1a receptors in proximal tubules of the kidney was sufficient to decrease proximal tubular reabsorption and lower basal blood pressure (12, 27). However, Le et al. (25) generated transgenic mice in which the AT1a receptor was expressed in proximal tubules under the control of the γ-glutamyl transpeptidase (γGT) promoter, and reported no blood pressure or salt sensitivity-altering effect. Thus, depending on the transgenic models, ANG II and AT1 (AT1a) receptors in proximal tubules of the kidney either play an important role or have no effect on proximal tubular transport and blood pressure.
We have recently reported that intrarenal transfer of an intracellular cyan fluorescent fusion of ANG II, enhanced cyan fluorescent protein (ECFP)/ANG II, selectively in proximal tubules significantly increased blood pressure in Sprague-Dawley rats and C57BL/6J mice (10, 28). In the present study, we further determined the direct role of proximal tubule AT1a receptor in mediating the blood pressure response to ECFP/ANG II transfer in proximal tubules of AT1a receptor-deficient mice (Agtr1a-/-). Specifically, a proximal tubule-specific sodium and glucose cotransporter 2 (sglt2) promoter was used to drive the expression of a GFP-tagged, full-length wild-type AT1a receptor (AT1aR/GFP), with or without ECFP/ANG II, selectively in proximal tubules of Agtr1a-/- mice (28, 42). This approach represents a short-term knocking in or rescue of AT1a receptors selectively in proximal tubules of mice with global deletion of this receptor. The effects of proximal tubule-dominant expression of ECFP/ANG II on systolic blood pressure, 24-h urinary sodium excretion, and activation of MAP kinases ERK1/2 and NHE3 in proximal tubules were then determined.
METHODS
Construction of the ECFP/ANG II or the GFP-tagged AT1a receptor transgene with the proximal tubule-dominant promoter sglt2.
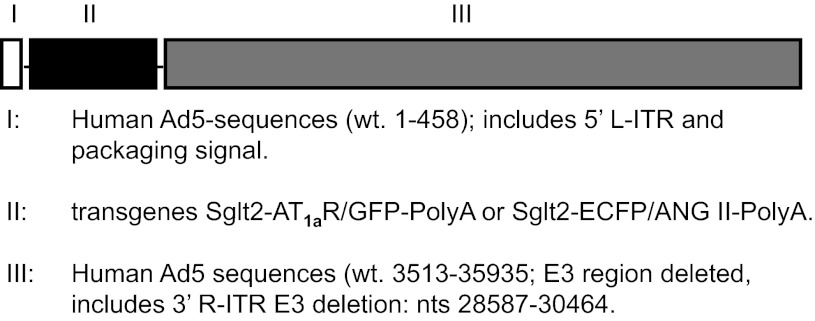
The sglt2 promoter was kindly provided by Drs. Isabelle Rubera and Michel Tauc of University of Nice-Sophia Antipolis, France, and was used to drive the expression of AT1a receptor (Agtr1a) or ECFP/ANG II selectively in proximal tubules of Agtr1a-/- mouse kidney (28, 42). The full-length Agtr1a mouse cDNA ORF clone (NM_177322) was custom made by OriGene (Rockville, MD) in a GFP-expressing vector (pCMV6-AC-GFP), resulting in the expression of a C-terminal GFP-tagged AT1a receptor (AT1aR/GFP). The constructs of the intracellular cyan fluorescent fusion of ANG II and its scrambled counterpart were kindly provided by Dr. Julia Cook of Ochsner Clinic Foundation (New Orleans, LA) (6, 7). To ensure proximal tubule-specific expression of AT1aR/GFP or ECFP/ANG II using the sglt2 promoter, the NotI fragment (2.6 kb) of pGEM-sglt2–5pr-mut was first subcloned into the NotI digested DUAL-Basic vector, and AT1aR/GFP or pECFP/ANG II was then subcloned into XhoI/Klenow digested DUAL-sglt2–5pr-mut (Vector BioLab, Philadelphia, PA). The entire expression cassette was transferred to the adenovirus genome vector and confirmed through restriction mapping with a titer of ∼5 × 1010 pfu/ml. The specific adenoviral vectors used in the present study were Ad-sglt2-AT1aR/GFP with Ad-sglt2-pCMV6 as a control, and Ad-sglt2-ECFP/ANG II with Ad-sglt2-ECFP/ANG IIc as a control (Fig. 1).
Fig. 1.
The construction map of recombinant human adenoviral vectors encoding the green fluorescent protein (GFP)-tagged, full-length angiotensin 1a (AT1a) receptor (AT1aR/GFP), or an intracellular cyan fluorescent fusion of ANG II [enhanced cyan fluorescent protein (ECFP)/ANG II], and the sodium and glucose cotransporter 2 (sglt2) gene promoter.
Expression of AT1aR/GFP and/or ECFP/ANG II in Agtr1a-/- mouse proximal tubule cells.
Agtr1a-/- mouse proximal tubule cells (mPCT) were kindly provided by Dr. Ulrich Hopfer of Case Western Reserve University (45). mPCT cells were cultured to 80% confluence in 6-well plates or on glass coverslips and then were infected with adenovirus encoding the specific transgenes Ad-sglt2-AT1aR/GFP with or without Ad-sglt2-ECFP/ANG II (4 μg/well) for 48 h using the transfection protocol, as we have described previously (29). The expression of AT1aR/GFP or ECFP/ANG II in these cells was visualized using a Nikon-Eclipse TE2000-U inverted fluorescence microscope and a GFP- or CFP-specific filter.
Adenovirus-mediated transfer of AT1aR/GFP with or without ECFP/ANG II selectively in proximal tubules.
Eight groups (n = 6–14 each) of adult male Agtr1a-/- mice were used in the present study. All mice were maintained on a normal rodent chow with free access to tap water. Basal systolic blood pressure, 24-h urine, and urinary sodium excretion were measured before proximal tubule-specific transfer of Ad-sglt2-AT1aR/GFP or Ad-sglt2-ECFP/ANG II was performed. To induce adenoviral AT1aR/GFP and/or ECFP/ANG II transfer, Agtr1a-/- mice were anesthetized and their left renal arteries were temporarily clamped with a fine vessel clip, which briefly interrupted blood flow to the left kidney. Ad-sglt2-AT1aR/GFP with or without Ad-sglt2-ECFP/ANG II were directly and slowly injected into the superficial cortex with six locations (20 μl each) (28). Blood flow to the left kidney was reestablished 5 min after injection of Ad-sglt2-AT1aR/GFP and/or Ad-sglt2-ECFP/ANG II. The control groups of Agtr1a-/- mice received injection of saline, Ad-sglt2-CMV-GFP, or Ad-sglt2-ECFP/ANG IIc as a proper control. In a separate group of Agtr1a-/- mice with AT1aR/GFP and Ad-sglt2-ECFP/ANG II cotransfer, the AT1 receptor blocker losartan (20 mg·kg−1·day−1) was added into the drinking water for 2 wk (n = 14). All animal experiments were approved by the Institutional Animal Care and Use and Recombinant DNA and Biosafety Committees of Henry Ford Health System and University of Mississippi Medical Center.
Measurement of blood pressure and 24-h water intake and urine and urinary electrolyte excretion.
Basal and weekly systolic blood pressure in Agtr1a-/- mice was measured using the indirect BP-2000 Series II tail-cuff blood pressure analysis system (Visitech), as we described previously (28, 34). This noninvasive system was validated by Krege et al. (24) previously and is widely used for measurement of systolic blood pressure in conscious mice. Twenty-four hours of water intake, urine, and urinary sodium and potassium excretion were measured using a metabolic cage in all animals, as we described previously (28, 34).
Measurements of plasma and proximal tubule ANG II levels.
At the end of experiment, Agtr1a-/- mice were killed, and trunk blood samples were collected to measure plasma ANG II levels. One-half of the left kidney was used to isolate fresh proximal tubules from the superficial cortex, as we described (28). Plasma and proximal tubule ANG II were measured using a sensitive ELISA kit (Bachem) (28). Proximal tubule ANG II levels were corrected for the 45% of ANG II degraded during collagenase digestion and sequential separation of proximal tubules (28).
Fluorescent imaging of AT1aR/GFP and ECFP/ANG II expression in proximal tubules.
To confirm the proximal tubule-dominant expression of AT1aR/GFP and ECFP/ANG II in the kidney of Agtr1a-/- mice, fresh and frozen kidney sections (6-μm thick) were cut on a cryostat and thaw-mounted on glass slides. Sections were then briefly counterstained for 5 min with the nuclear marker DAPI (300 nM) to visualize the cell nuclei. Expression of AT1aR/GFP and ECFP/ANG II in the renal superficial cortex was visualized using a Nikon-Eclipse TE2000-U inverted fluorescence microscope (28, 30). A dual DAPI-CFP band-pass excitation filter set (excitation: 440 nm; emission: 495/50 nm) was used for ECFP/ANG II imaging, whereas a dual DAPI-FITC band-pass excitation filter set (excitation: 488 nm; emission: 510/50 nm) was used for AT1aR/GFP imaging, respectively (28, 30). DAPI-stained nuclear images were either blue or converted to red for better differentiation between ECFP or GFP and DAPI. In all fluorescence imaging analyses, the background autofluorescence level was determined in the renal medulla of the same kidney with AT1aR/GFP and/or ECFP/ANG II transfer, or in the superficial cortex of a control Agtr1a-/- mouse kidney that was not transferred with AT1aR/GFP and/or ECFP/ANG II (28, 30).
Colocalization of AT1aR/GFP and/or ECFP/ANG II with megalin expression in the proximal tubule.
To determine the specificity of the sglt2 gene promoter for proximal tubule-dominant transfer of AT1aR/GFP or ECFP/ANG II in the kidney, adjacent cryostat kidney sections with the gene transfer (6 μm) were counterstained for immunofluorescent imaging using a goat polyclonal primary megalin antibody (Santa Cruz Biotechnology) and an Alexa Fluor 594-labeled donkey anti-goat secondary antibody (Life Technologies). Megalin is the multi-ligand endocytic receptor specifically expressed in proximal tubules of the kidney, which plays a crucial role in mediating the uptake of low-molecular-weight proteins (3, 26). Megalin immunofluorescent staining in the superficial cortex was visualized using a fluorescent filter selective for Alexa Fluor 594 (red fluorescence) and was used as a marker for proximal tubules (31).
Western blot analysis of total and phosphorylated MAP kinases ERK1/2, lysate, or membrane NHE-3 proteins in proximal tubules.
To determine the signaling responses to proximal tubule-dominant transfer of AT1aR/GFP and/or ECFP/ANG II in Agtr1a-/- mice, proximal tubules were freshly isolated for Western blot analysis of total (t-ERK1/2) and phosphorylated MAP kinases ERK1/2 (p-ERK1/2) and NHE-3 proteins (t-NHE3 and p-NHE3), as we described previously (28, 34). Additionally, brush border membrane proteins were prepared from proximal tubule samples for Western blot analysis of p-NHE3 proteins (30). The ratios of phosphorylated to total ERK1/2 or NHE-3 proteins were used as an index of MAP kinase ERK1/2 or NHE3 activation (34). The primary antibodies for total and phosphorylated NHE3 were obtained from Chemicon and Santa Cruz Biotechnology, respectively, whereas primary antibodies for total and phosphorylated ERK1/2 were purchased from Cell Signaling, respectively. To ensure equal protein loading, the same membranes were treated with stripping buffer (Pierce) for 20 min, blotted with 5% nonfat dry milk, and reprobed with a mouse anti-β-actin monoclonal antibody at 1:2,000 (Sigma-Aldrich). Western blot signals were detected using enhanced chemiluminescence (Amersham) and analyzed using a Molecular Imager, ChemiDoc XRS+ (Bio-Rad Laboratories) (34).
Statistical analysis.
All data are presented as means ± SE. One-way ANOVA was used to compare the differences in the same parameters between groups of Agtr1a-/- mice. When the P value was less than 0.05, a post hoc Newman-Keuls multiple-comparison test was used to compare two different group means. Additionally, the differences between group means at the same time point(s) were analyzed using unpaired Student's t-test. The significance was set at P < 0.05.
RESULTS
Expression of AT1aR/GFP or ECFP/ANG II in cultured mouse proximal tubule cells.
Figure 2 shows live cell fluorescent imaging of the expression of AT1a/GFP and/or ECFP/ANG II proteins in Agtr1a-/- mouse mPCT cells. The transfection of mPCT cells with Ad-sglt2-AT1a/GFP (Fig. 2A) or Ad-sglt2-ECFP/ANG II (Fig. 2B) for 48 h resulted in intensive expression of both fluorescent proteins in the cytoplasm and perinuclear regions. The expression of AT1a/GFP and ECFP/ANG II appeared to overlap intracellularly in these cells (Fig. 2C).
Fig. 2.
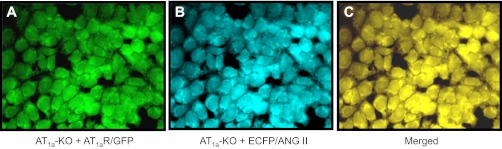
Coexpression of AT1aR/GFP and ECFP/ANG II in cultured Agtr1a-/- mouse proximal tubule cells 48 h after transfection. A: expression of AT1aR/GFP in the cytoplasm and perinuclear region, shown as green fluorescence. B: expression of ECFP/ANG II in the cytoplasm and perinuclear region, shown as blue-green. C: merged image of A and B. Magnification: ×40.
Intrarenal transfer of AT1aR/GFP with or without ECFP/ANG II in proximal tubules of Agtr1a-/- mice.
In Agtr1a-/- mice, adenoviral transfer of AT1aR/GFP or ECFP/ANG II using the sglt2 promoter induced marked expression of the transgenes, green fluorescence for AT1aR/GFP (Fig. 3A) or cyan fluorescence for ECFP/ANG II (Fig. 5A) predominantly in proximal tubules (Figs. 4A and 6A) throughout the superficial cortex. Careful fluorescence microscopic examination of serial longitudinal sections of the kidneys that were transferred with AT1aR/GFP and/or ECFP/ANG II (n = 8) showed that ∼90% of proximal tubules in the superficial cortex expressed these fluorescent proteins. Expression of AT1aR/GFP, ECFP/ANG II, and megalin was largely overlapped in proximal tubules of the superficial cortex (Figs. 3B and 5B). By comparison, AT1aR/GFP (Fig. 3A) or ECFP/ANG II expression (Fig. 5A) was very low but visible in glomeruli and cortical collecting ducts of the cortex, or the medulla of the same kidney. No detectable ectopic expression of either AT1aR/GFP or ECFP/ANG II was visualized in extrarenal tissues, including the heart, blood vessels, or brain (not shown). In freshly isolated proximal tubules, AT1aR/GFP or ECFP/ANG II expression was visualized throughout the tubule wall (Figs. 4A and 6A), whereas insignificant AT1aR/GFP or ECFP/ANG II expression was observed in freshly isolated glomeruli (Figs. 4D and 6D). Furthermore, the expression of ECFP/ANG II and AT1aR/GFP was colocalized in freshly isolated proximal tubules (Fig. 7). AT1aR/GFP was clearly visible in membranes, especially basolateral membranes of freshly isolated proximal tubules (Fig. 7).
Fig. 3.
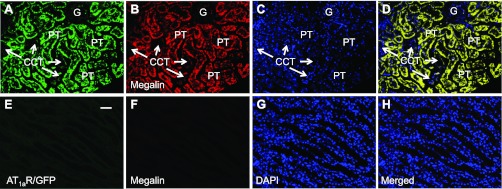
Proximal tubule-dominant expression of AT1aR/GFP in a representative Agtr1a-/- mouse kidney 2 wk after intrarenal adenoviral transfer. A: AT1aR/GFP expression (green) in proximal tubules (PT). B: Alexa Fluor 594-labeled megalin expression (red) in proximal tubules. C: DAPI-stained nuclei (blue) in the same kidney section. D: merged image of A–C, showing the colocalization of AT1aR/GFP and megalin expression (yellow) in proximal tubules. Only very low levels of AT1aR/GFP and megalin expression are visible in the glomerulus (G) and cortical collecting tubules (CCT). E: AT1aR/GFP expression in the outer medulla. F: Alexa Fluor 594-labeled megalin expression in the outer medulla. G: DAPI-stained nuclei in the outer medulla. H: merged image of E–G, showing the lack of AT1aR/GFP and megalin expression in the outer medulla. Magnification: ×40.
Fig. 4.
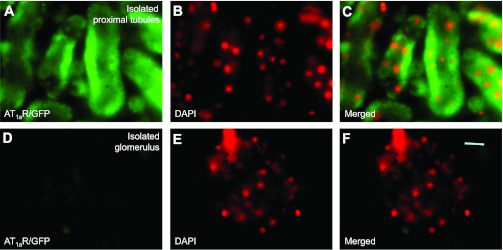
AT1aR/GFP expression in freshly isolated proximal tubules (A–C) or glomerulus (D–F) of a representative Agtr1a-/- mouse kidney 2 wk after intrarenal adenoviral AT1aR/GFP transfer. A: AT1aR/GFP expression. B: DAPI-stained nuclei in the same image of A converted into red for better visualization. C: merged image of A and B. D: lack of AT1aR/GFP expression in a representative, freshly isolated glomerulus. E: DAPI-stained nuclei in the same image of D. F: merged image of D and E. Magnification: ×100.
Fig. 5.
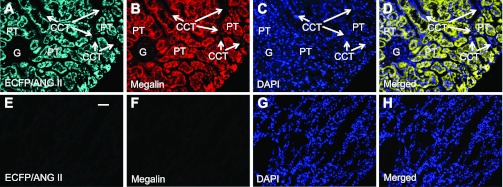
Proximal tubule-dominant expression of ECFP/ANG II in a representative Agtr1a-/- mouse kidney 2 wk after intrarenal adenoviral transfer. A: ECFP/ANG II expression (cyan) in proximal tubules (PT). B: Alexa Fluor 594-labeled megalin expression (red) in proximal tubules (PT). C: DAPI-stained nuclei (blue) in the same kidney section. D: merged image of A–C, showing the colocalization of ECFP/ANG II and megalin expression (yellow) in proximal tubules. Only very low levels of ECFP/ANG II and megalin expression are visible in the glomerulus (G) and cortical collecting tubules (CCT). E: ECFP/ANG II expression in the outer medulla. F: Alexa Fluor 594-labeled megalin expression in the outer medulla. G: DAPI-stained nuclei in the outer medulla. H: merged image of E–G, showing the lack of ECFP/ANG II and megalin expression in the outer medulla. Magnification: ×40.
Fig. 6.

ECFP/ANG II expression in freshly isolated proximal tubules (A–C) or glomerulus (D–F) of a representative Agtr1a-/- mouse kidney 2 wk after intrarenal adenoviral ECFP/ANG II transfer. A: ECFP/ANG II expression. B: DAPI-stained nuclei in the same image of A converted into red for better visualization. C: merged image of A and B. D: lack of ECFP/ANG II expression in a representative, freshly isolated glomerulus. E: DAPI-stained nuclei in the same image of D. F: merged image of D and E. Magnification: ×100.
Fig. 7.
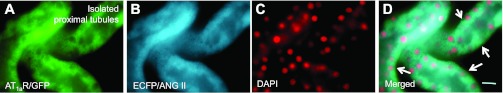
Colocalization of AT1aR/GFP (A) and ECFP/ANG II expression (B) in freshly isolated proximal tubules of a representative Agtr1a-/- mouse kidney 2 wk after intrarenal adenoviral AT1aR/GFP and ECFP/ANG II transfer. C: DAPI-stained nuclei in proximal tubules. D: merged image of A and B, showing the colocalization of AT1aR/GFP and ECFP/ANG II expression in the same proximal tubules. Note that AT1aR/GFP is visualized on the borders of proximal tubules (arrows). Magnification: ×100.
Effects of proximal tubule-dominant transfer of AT1aR/GFP with or without ECFP/ANG II on plasma and proximal tubular ANG II levels in Agtr1a-/- mice.
Table 1 shows that proximal tubule-specific transfer of ECFP/ANG II with or without AT1aR/GFP did not significantly alter plasma or circulating ANG II levels from control Agtr1a-/- mice or Agtr1a-/- mice with transfer of the AT1aR gene only (one-way ANOVA). However, ANG II levels in freshly isolated proximal tubules were significantly increased by >40% in Agtr1a-/- mice with proximal tubule transfer of ECFP/ANG II and AT1aR/GFP (P < 0.01; Table 1).
Table 1.
Effects of proximal tubule-specific transfer of ECFP/ANG II with or without AT1aR/GFP on plasma and proximal tubular ANG II levels in Agtr1a-/- mice
| Parameter | Control (n = 7) | CMV-GFP (n = 8) | AT1aR/GFP (n = 5) | ECFP/ANG II + AT1aR/GFP (n = 8) |
|---|---|---|---|---|
| Plasma ANG II, fmol/ml | 388.9 ± 18.8 | 389.7 ± 12.4 | 327.7 ± 16.1 | 310.5 ± 23.7 |
| Proximal tubular ANG II, pg/mg protein | 31.1 ± 2.7 | 31.6 ± 2.4 | 33.5 ± 3.3 | 44.9 ± 3.4 ** †† |
CMV-GFP, a recombinant human adenovirus type 5 expresses enhanced green fluorescent protein under the control of a cytomegalovirus (CMV) promoter; ECFP, an enhanced cyan fluorescent protein.
P < 0.01 vs. control Agtr1a-/- mice.
P < 0.01 vs. Agtr1a-/- mice transferred with AT1aR alone.
Effects of proximal tubule-dominant transfer of ECFP/ANG II with or without AT1aR/GFP on blood pressure in Agtr1a-/- mice.
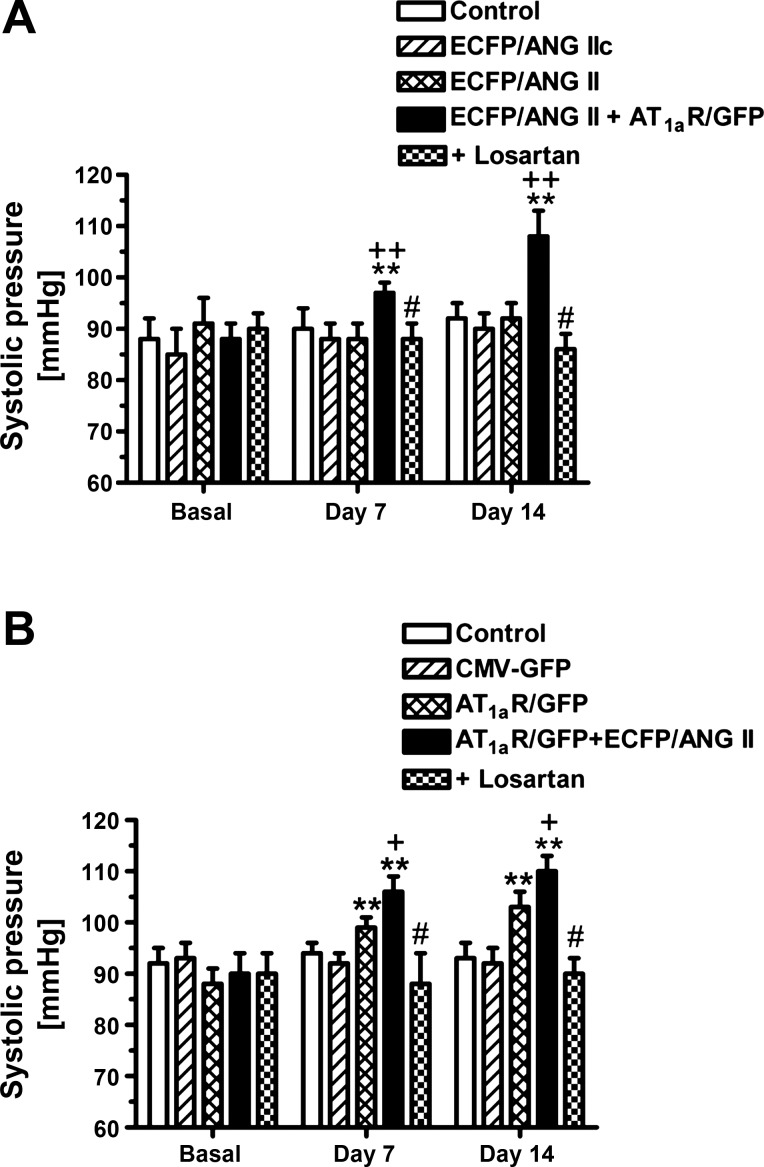
Basal systolic blood pressure was not statistically different in all groups of Agtr1a-/- mice before the AT1aR/GFP and/or ECFP/ANG II gene transfer was performed (Fig. 8). In the sham, time-control Agtr1a-/- mice, blood pressure remained unchanged throughout the experiment for 2 wk. Likewise, proximal tubule-dominant transfer of ECFP/ANG IIc (Fig. 8A), ECFP/ANG II (Fig. 8A), or CMV-GFP alone (Fig. 8B) did not alter blood pressure in Agtr1a-/- mice over a 2-wk period. However, blood pressure was significantly increased in Agtr1a-/- mice with transfer of AT1aR/GFP alone at day 7 (Δ increase of 8 ± 2 mmHg, P < 0.01) and at day 14 after the gene transfer (Δ increase of 12 ± 3 mmHg, P < 0.01), respectively. In Agtr1a-/- mice with cotransfer of AT1aR/GFP and ECFP/ANG II selectively in proximal tubules, blood pressure was increased by 13 ± 2 mmHg at day 7 (P < 0.01), and by 18 ± 2 mmHg after the gene transfer (P < 0.01) (Fig. 8). The effect of AT1aR/GFP transfer alone on blood pressure was statistically smaller than that in Agtr1a-/- mice with the cotransfer of AT1aR/GFP and ECFP/ANG II (P < 0.05, unpaired t-test). Concurrent treatment with losartan prevented the increases in blood pressure induced by proximal tubule-dominant transfer of AT1aR/GFP and ECFP/ANG II in Agtr1a-/- mice (Fig. 8).
Fig. 8.
Effects of proximal tubule-dominant transfer of ECFP/ANG II, AT1aR/GFP or their scrambled control alone, or ECFP/ANG II and AT1aR/GFP cotransfer on systolic blood pressure in Agtr1a-/- mice 1 or 2 wk after the adenoviral gene transfer. Note that losartan blocked blood pressure response to proximal tubule transfer of ECFP/ANG II and AT1aR/GFP. **P < 0.01 vs. basal blood pressure in the same group of Agtr1a-/- mice. +P < 0.05 vs. Agtr1a-/- mice with AT1aR/GFP transfer alone at the same time point (B). ++P < 0.01 vs. Agtr1a-/- mice with ECFP/ANG IIc or ECFP/ANG II transfer alone at the same time point (A). #P < 0.05 vs. Agtr1a-/- mice with proximal tubule cotransfer of ECFP/ANG II and AT1aR/GFP at the same time point.
Effects of proximal tubule-dominant transfer of ECFP/ANG II with or without AT1aR/GFP on systemic and renal responses in Agtr1a-/- mice.
Table 2 summarizes systemic and renal phenotypic responses to proximal tubule-dominant transfer of ECFP/ANG II with or without AT1aR/GFP in Agtr1a-/- mice. At the end of experiment, there were no significant differences in body weight, total blood volume, hematocrit, heart weight, and the heart weight-to-body-weight ratio in all groups of Agtr1a-/- mice (one-way ANOVA). However, in Agtr1a-/- mice with proximal tubule-dominant transfer of ECFP/ANG II with AT1aR/GFP, daily water drinking was significantly increased (P < 0.05). Compared with control Agtr1a-/- mice, total blood volume was also significantly increased (P < 0.05), whereas hematocrit was significantly decreased (P < 0.05), in Agtr1a-/- mice with proximal tubule-dominant transfer of ECFP/ANG II with AT1aR/GFP. Twenty-four hours of urine excretion was decreased by 38% (Table 2, P < 0.01), whereas 24 h of urinary potassium excretion was reduced by 34% at the end of experiment in Agtr1a-/- mice with the cotransfer of ECFP/ANG II with AT1aR/GFP (Table 2, P < 0.01), compared with control Agtr1a-/- mice.
Table 2.
General phenotypic responses to proximal tubule-dominant transfer of ECFP/ANG II with AT1aR/GFP in adult male Agtr1a-/- mice
| Parameter | Control (n = 15) | CMV-GFP (n = 10) | ECFP/ANG IIc (n = 7) | ECFP/ANG II + AT1aR/GFP (n = 13) | + Losartan (n = 14) |
|---|---|---|---|---|---|
| Body wt, g | 24.3 ± 1.3 | 21.7 ± 1.9 | 24.3 ± 1.4 | 26.8 ± 1.3 | 23.7 ± 1.8 |
| Drinking, ml/day | 5.3 ± 0.2 | 5.2 ± 0.4 | 5.2 ± 0.3 | 6.5 ± 0.2* | 6.7 ± 0.2* |
| Blood vol., ml | 0.66 ± 0.04 | 0.70 ± 0.04 | 0.73 ± 0.3 | 0.78 ± 0.02* | 0.72 ± 0.02 |
| Hematocrit, % | 47.8 ± 0.5 | 45.3 ± 0.7 | 46.2 ± 0.5 | 45.1 ± 0.4* | 45.4 ± 0.2* |
| Left kidney wt, g | 0.14 ± 0.01 | 0.13 ± 0.01 | 0.13 ± 0.01 | 0.18 ± 0.01* | 0.14 ± 0.01† |
| Left kidney wt-to-body wt ratio × 100 | 0.60 ± 0.03 | 0.66 ± 0.04 | 0.65 ± 0.04 | 0.69 ± 0.02* | 0.66 ± 0.02 |
| Heart wt, g | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 | 0.14 ± 0.01 |
| Heart wt-to-body wt ratio × 100 | 0.51 ± 0.01 | 0.55 ± 0.02 | 0.54 ± 0.01 | 0.52 ± 0.01 | 0.54 ± 0.02 |
| Heart rate, beats/min | 624 ± 22 | 636 ± 13 | 606 ± 26 | 627 ± 23 | 616 ± 20 |
| Urine, ml/24 h | 3.19 ± 0.38 | 3.43 ± 0.36 | 2.99 ± 0.14 | 1.97 ± 0.41** | 3.08 ± 0.13†† |
| UKV, μmol/24 h | 372.9 ± 6.2 | 369.2 ± 8.1 | 285.8 ± 10.8 | 244.5 ± 5.7** | 389.8 ± 9.2†† |
UKV, urinary potassium excretion.
P < 0.05 or
P < 0.01 vs. sham-control Agtr1a-/- mice.
P < 0.05 or
P < 0.01 vs. ECFP/ANG II + AT1aR/GFP.
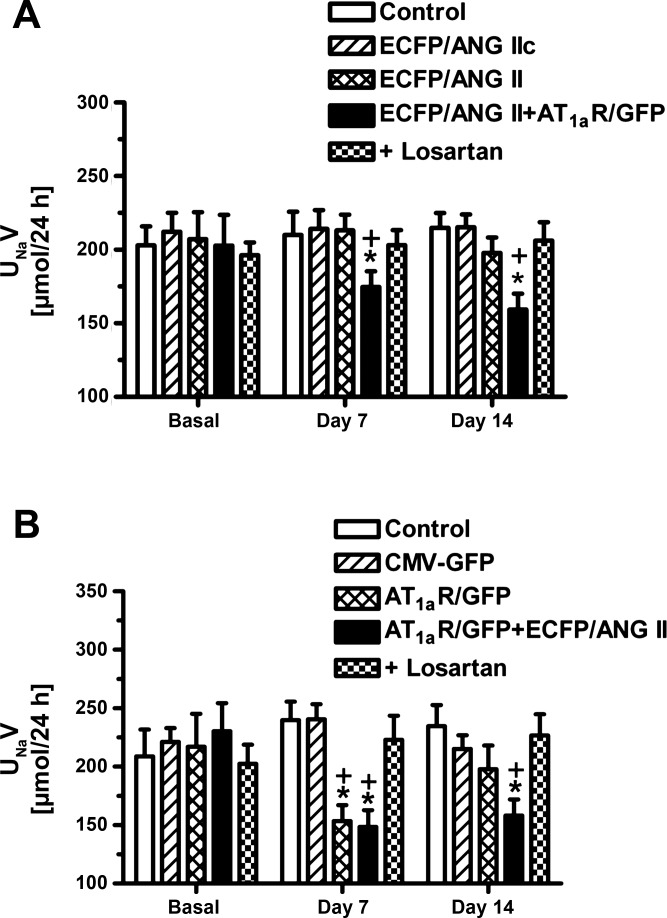
The effects of proximal tubule-dominant transfer of AT1aR/GFP alone or with ECFP/ANG II on 24-h urinary sodium excretion in Agtr1a-/- mice are shown in Fig. 9. Expression of ECFP/ANG IIc, ECFP/ANG II, or CMV-GFP had no effects on 24-h urine or urinary sodium excretion. However, transfer of AT1aR/GFP alone (Fig. 9B) or cotransfer of AT1aR/GFP with ECFP/ANG II (Fig. 9A) significantly decreased 24-h urinary sodium excretion at day 7 and day 14, respectively (P < 0.01 vs. their basal levels or vs. control Agtr1a-/- mice at day 7 or day 14, respectively). Concurrent treatment with losartan reversed the 24-h urinary sodium excretory responses to proximal tubule-dominant transfer of AT1aR/GFP alone or cotransfer with ECFP/ANG II to the levels of control Agtr1a-/- mice (Fig. 9).
Fig. 9.
Effect of proximal tubule-dominant transfer of ECFP/ANG II, AT1aR/GFP, or their scrambled control ECFP/ANG IIc, CMV-GFP alone, or ECFP/ANG II, and AT1aR/GFP cotransfer on 24-h urinary sodium excretion in Agtr1a-/- mice 1 or 2 wk after the adenoviral gene transfer. Note that ECFP/ANG II, ECFP/ANG IIc, or CMV-GFP transfer alone had no effect in Agtr1a-/- mice, but transfer of AT1aR/GFP alone or in combination with ECFP/ANG II did reduce UNaV response by day 7. Losartan blocked sodium excretory response to proximal tubule cotransfer of ECFP/ANG II and AT1aR/GFP. *P < 0.01 vs. basal urinary sodium excretion in the same group of Agtr1a-/- mice. +P < 0.01 vs. Agtr1a-/- mice with ECFP/ANG II or CMV-GFP transfer alone at the same time point.
Effects of proximal tubule-dominant transfer of ECFP/ANG II with or without AT1aR/GFP on MAP kinases ERK 1/2 activation in proximal tubules of Agtr1a-/- mice.
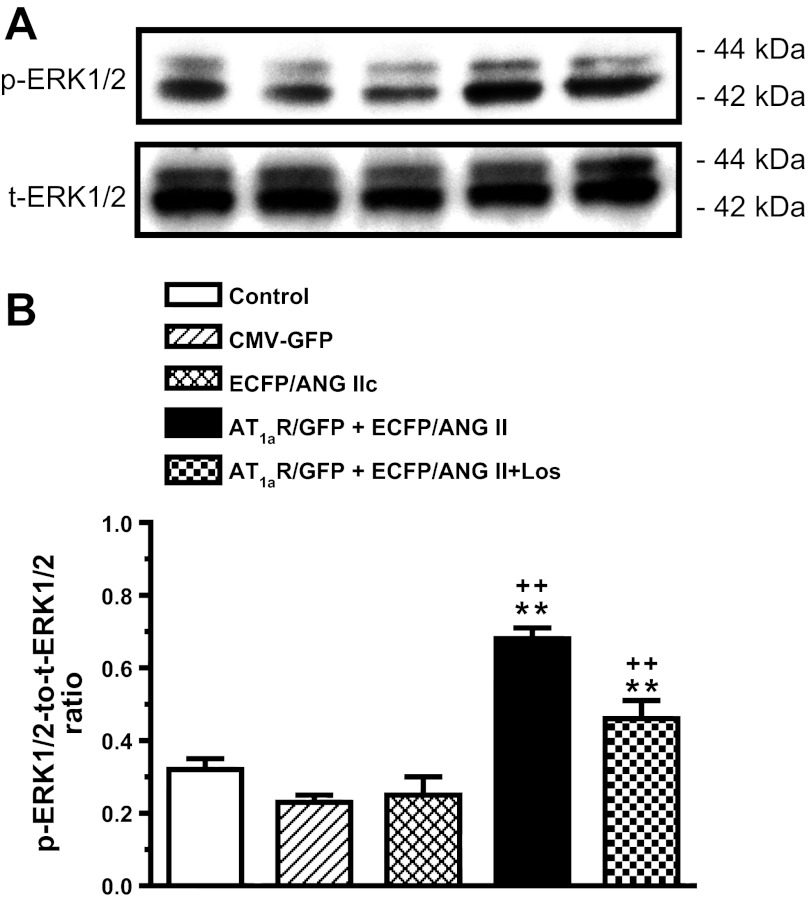
Compared with time-control Agtr1a-/- mice, transfer of ECFP/ANG IIc or ECFP/ANG II alone had no significant effect on MAP kinases ERK 1/2 activation in isolated proximal tubules of Agtr1a-/- mice (time control: 0.32 ± 0.03; ECFP/ANG IIc: 0.28 ± 0.03; and ECFP/ANG II: 0.30 ± 0.05 p-ERK1/2 to t-ERK1/2 ratio, n.s.) (Fig. 10). By contrast, cotransfer of AT1aR/GFP and ECFP/ANG II selectively in proximal tubules of Agtr1a-/- mice increased proximal tubule p-ERK1/2 proteins more than twofold (0.68 ± 0.03 p-ERK1/2 to t-ERK1/2 ratio, P < 0.01 vs. ECFP/ANG II alone). The latter response was largely blocked by losartan (0.42 ± 0.05 p-ERK1/2 to t-ERK1/2 ratio, P < 0.01 vs. ECFP/ANG II+AT1aR/GFP).
Fig. 10.
Effect of proximal tubule-dominant transfer of ECFP/ANG II with or without AT1aR/GFP cotransfer on activation of MAP kinases ERK1/2 in proximal tubules of Agtr1a-/- mice 2 wk after the gene transfer. CMV-GFP or scrambled ECFP/ANG IIc was used as a negative control of AT1aR/GFP or ECFP/ANG II, respectively. A: representative Western blot bands for phosphorylated and total ERK1/2 proteins. B: semiquantitative data from proximal tubule samples of 4-6 animals per group. Losartan partially blocked p-ERK1/2 response to proximal tubule cotransfer of ECFP/ANG II and AT1aR/GFP. **P < 0.01 vs. control Agtr1a-/- mice or Agtr1a-/- mice with ECFP/ANG IIc or CMV-GFP transfer alone. ++P < 0.01 vs. Agtr1a-/- mice with proximal tubule cotransfer of ECFP/ANG II and AT1aR/GFP.
Effects of proximal tubule-dominant transfer of ECFP/ANG II with or without AT1aR/GFP on NHE-3 activation in proximal tubules of Agtr1a-/- mice.
The changes in Western blots showing membrane, lysate, and total NHE3 proteins and the ratio of lysate phosphorylated (p-NHE3) to total NHE3 proteins (NHE3) in freshly isolated proximal tubules are shown in Fig. 11. The ratio of lysate p-NHE3 to NHE3 proteins along with the changes in membrane p-NHE3 were used as an index of the effect of proximal tubule-dominant transfer of AT1aR/GFP and ECFP/ANG II on NHE3 activity response (Fig. 11). The ratios of p-NHE3 to NHE3 proteins in proximal tubules were not statistically different in control Agtr1a-/- mice (0.21 ± 0.03) and mice with proximal tubule-dominant transfer of the control CMV-GFP (0.23 ± 0.02) or ECFP/ANG IIc (0.26 ± 0.05) (Fig. 11B). By contrast, proximal tubule-dominant transfer of AT1aR/GFP and ECFP/ANG II markedly increased lysate and membrane p-NHE3 proteins (Fig. 11A), and the lysate p-NHE3-to-NHE3 protein ratio (Fig. 11B) to 0.83 ± 0.06 (P < 0.01, compared with control, CMV-GFP, or ECFP/ANG IIc, respectively). The membrane and lysate p-NHE3 responses were attenuated by concurrent losartan treatment (0.38 ± 0.06, P < 0.01).
Fig. 11.
Effect of proximal tubule-dominant transfer of ECFP/ANG II with or without AT1aR/GFP cotransfer on phosphorylated lysate and membrane NHE3 proteins in freshly isolated proximal tubules of Agtr1a-/- mice 2 wk after the gene transfer. CMV-GFP or scrambled ECFP/ANG IIc was used as a negative control of AT1aR/GFP or ECFP/ANG II, respectively. Note that losartan largely blocked lysate or membrane p-NHE3 response to proximal tubule cotransfer of ECFP/ANG II and AT1aR/GFP. A: representative Western blot bands for membrane (top) and lysate (middle) phosphorylated NHE3 proteins, or lysate total NHE3 proteins (bottom). B: lysate phosphorylated to total NHE3 ratio. **P < 0.01 vs. control Agtr1a-/- mice or Agtr1a-/- mice with ECFP/ANG IIc or CMV-GFP transfer alone. ++P < 0.01 vs. Agtr1a-/- mice with proximal tubule co-transfer of ECFP/ANG II and AT1aR/GFP.
DISCUSSION
We have recently demonstrated that intrarenal adenoviral transfer of an intracellular cyan fluorescent fusion of ANG II (ECFP/ANG II) predominantly in proximal tubules of the kidney increased blood pressure in rats and mice, which appeared to be mediated by AT1 (AT1a) receptors in the kidney (28, 30). In those studies, we used a proximal tubule-specific sglt2 promoter to drive ECFP/ANG II expression in proximal tubules. Although we did not observe significant ectopic expression in extrarenal tissues, it was still not possible to completely exclude the possibility that the locally expressed ECFP/ANG II may escape from proximal tubules into the circulation and induces blood pressure responses via activation of extrarenal AT1 (AT1a) receptors. The present study was specifically designed to test the hypothesis that AT1a receptors in proximal tubules of the kidney mediate the blood pressure responses to ECFP/ANG II expression in proximal tubules. We demonstrated that proximal tubule-dominant expression of ECFP/ANG II, its scrambled ECFP/ANG IIc fusion protein, or CMV-GFP alone had no effect on blood pressure, urinary sodium excretion, or phosphorylated MAP kinases ERK1/2 and NHE3 proteins in proximal tubules of Agtr1a-/- mice. By contrast, proximal tubule-dominant expression of ECFP/ANG II in the presence of AT1aR/GFP was able to increase systolic blood pressure by an average of 18 mmHg in Agtr1a-/- mice. The latter response was associated with a significant decrease in 24-h urinary sodium excretion, and marked increases in phosphorylated ERK1/2 and lysate and membrane NHE3 proteins in freshly isolated proximal tubules. These results strongly suggest that intracellular ANG II in proximal tubules may play a physiological role in the regulation of blood pressure and that this role is likely mediated by activation of AT1a receptors and NHE3 in proximal tubules of the kidney.
A potential role of AT1a receptors in proximal tubules of the kidney in the long-term regulation of blood pressure by extracellular ANG II has recently been studied by other investigators (12, 27). Gurley et al. (12) used the novel Agtr1aflox approach to generate mutant mice with deficiency of AT1a receptors selectively in proximal tubules of the kidney. These mice had ∼45% reduction of AT1a receptor mRNA expression and ∼40% of decreases in 125I-ANG II radioreceptor binding in nonglomerular cortical region. The proximal tubule-knockout of AT1a receptors in these mice led to about ∼10-mmHg decrease in basal systolic blood pressure and a reduced blood pressure response to systemic infusion of ANG II. Sigmund's group (27) used a proximal tubule-specific, androgen-dependent, promoter construct (KAP2) to generate mice with overexpression or deletion of endogenous AT1a receptors. Androgen-induced overexpression of AT1a receptors in proximal tubules increased basal blood pressure, whereas deletion of proximal tubule AT1a receptors decreased blood pressure (27). However, the latter study found no differences in blood pressure responses to ANG II infusion. Despite these differences, these studies provide evidence that AT1a receptors in proximal tubules actively participate in basal blood pressure regulation.
In the present study, we took a different approach in that we used Agtr1a-/- mice as a template, which are devoid of AT1a receptors in all tissues. We then used the adenovirus-mediated gene transfer approach, as reported recently (28), to express (or knockin) a GFP-tagged, full-length, wild-type AT1a receptor (AT1aR/GFP) in proximal tubules of Agtr1a-/- mice with or without coexpression of the ECFP/ANG II gene. In contrast to the above-mentioned studies (12, 27), in which AT1a receptors were deleted only in proximal tubules of the kidney and the response to circulating ANG II was studied, we expressed AT1a receptors predominantly in proximal tubules of Agtr1a-/- mice to evaluate the role of intracellular ANG II. We and others have previously shown that the expression of ECFP/ANG II was confined exclusively inside the cells and was not secreted or released into extracellular fluid compartments, blood, or urine (7, 28, 30, 41). With this approach, the present study demonstrated that the expression of AT1a receptors alone or with ECFP/ANG II predominantly in proximal tubules of Agtr1a-/- mice increased systolic blood pressure by up to 12 to 18 mmHg above that of control Agtr1a-/- mice (Fig. 8). The extent of blood pressure response to proximal tubule-dominant expression of AT1aR/GFP and ECFP/ANG II is largely consistent to the decreases in basal systolic blood pressure, as reported in mice with proximal tubule-specific deletion of AT1a receptors (12, 27). For example, Gurley et al. (12) found that systolic blood pressure was decreased by about 10 mmHg in mice with proximal tubule-specific deletion of AT1a receptors. Li et al. (27) reported a 13-mmHg difference in basal blood pressure between wild-type control and proximal tubule AT1a receptor knockout mice. Thus, it is likely that the differences in blood pressure responses between the present and above-mentioned studies may be due to the coexpression of ECFP/ANG II predominantly in proximal tubules of the kidney.
Another unique approach in the present study is that we used the sglt2 gene promoter to drive AT1aR/GFP expression in proximal tubules of the kidney, as we described recently for ECFP/ANG II (28). The specificity of this promoter to drive proximal tubule-selective gene transfer or expression in the kidney is not fully understood. However, the sglt2 promoter has recently been used to drive gene expression in proximal tubules of the kidney in a number of studies (40, 42, 43). Sglt2 is a high-capacity, low-affinity sodium and glucose cotransporter, and it is localized almost exclusively in the apical membranes of proximal convoluted tubules (46). Rubera et al. (42) used the sglt2 promoter to drive Cre recombinase overexpression specifically in proximal tubules in transgenic mice. These authors demonstrated that the sglt2-driven Cre expression in proximal tubules was not overlapped with the mTAL marker Tamm-Horsfall protein, nor with the collecting duct marker aquaporin 2 protein. In a recent study, Rae et al. (40) generated transgenic mice in which insulin-like growth factor-1 (1Ea) is overexpressed in proximal tubules of the kidney using the sglt2 promoter. Furthermore, transgenic mice with the sglt2-Cre recombinase expression in proximal tubules have been used to successfully generate proximal tubule-specific knockout of PPARγ coactivator-1α (43) or myocyte enhancer factor 2C (47). In the present study, we first confirmed the effectiveness of the adenovirus-mediated gene expression of AT1a receptors and ECFP/ANG II in cultured Agtr1a-/- mouse proximal tubule cells in vitro (Fig. 2) and then in proximal tubules of Agtr1a-/- mice in vivo (Figs. 3–7). We purposely tagged AT1a receptors with GFP and ANG II fusion protein with ECFP, respectively. This approach has the merit of visualizing the expression of AT1aR/GFP and/or ECFP/ANG II transgenes via direct fluorescent imaging (Figs. 3–7). We found that the expression of AT1aR/GFP and/or ECFP/ANG II was largely overlapped with the antimegalin immunofluorescence-stained tubules in the superficial cortex (Figs. 3 and 5). Megalin is a well-recognized proximal tubule marker because it is expressed almost exclusively in early proximal tubules of the kidney (3, 26). The proximal tubule-dominant expression of AT1aR/GFP and/or ECFP/ANG II was further visualized in freshly isolated proximal tubules, but not in glomeruli (Figs. 4 and 6). However, the use of ECFP and GFP as the reporter of gene expression may also have drawbacks. Like other tissue-specific promoters that have been used for tissue-specific gene deletion or overexpression, the sglt2 promoter-driven transfer of ECFP/ANG II or AT1aR/GFP is not entirely confined to proximal tubules in the cortex. Indeed, very low or insignificant levels of AT1aR/GFP and/or ECFP/ANG II expression may be visible in the glomerular and cortical regions between proximal tubules, probably in cortical collecting tubules (Figs. 3 and 5). This phenomenon may be likely explained by the possibilities of 1) limited expression of these transgenes in the glomeruli and cortical collecting tubules, 2) nonspecific or relatively high levels of autofluorescence in the renal cortex, and 3) intensive levels of ECFP or GFP fluorescence in proximal tubules bleed through or crossover into adjacent glomerulus or collecting ducts.
The precise mechanisms by which proximal tubule expression of AT1aR/GFP and ECFP/ANG II increase blood pressure remain incompletely understood. It is unlikely that systemic factors such as AT1a receptors in extrarenal tissues and/or circulating ANG II play a major role, since AT1a receptors were transferred only in proximal tubules of Agtr1a-/- mice and that plasma ANG II was either decreased or unchanged, rather than increased significantly (Table 1). Furthermore, no cardiac hypertrophy was observed, and heart rate was similar in all groups of Agtr1a-/- mice (Table 2). Li et al. (27) also previously showed that heart rate was not altered by proximal tubule-specific knockout of AT1a receptors. There are some significant systemic and renal responses in the present study, which suggest the possibility of sodium retention and volume expansion as the mechanism in Agtr1a-/- mice with cotransfer of AT1aR/GFP and ECFP/ANG II in proximal tubules of the kidney. Indeed, sodium retention and volume expansion may be supported by significant decreases in 24-h urinary water and sodium excretion, increases in 24-h drinking and blood volume, and a decrease in hematocrit in Agtr1a-/- mice with proximal tubule cotransfer of AT1aR/GFP and ECFP/ANG II (Table 2). A proximal tubule-dependent mechanism is also implicated by more than twofold increases in phosphorylated MAP kinase ERK1/2 signaling proteins (Fig. 10) and lysate and membrane NHE3 proteins (Fig. 11) in freshly isolated proximal tubules of these mice. This interpretation is also consistent with a significant decrease in 24-h urinary lithium excretion, an indirect index of increased whole-kidney proximal tubule reabsorption (28), and an increase in NHE3 activity as determined using 22Na+ uptake in isolated apical membrane vesicles of proximal tubule by ECFP/ANG II transfer (30). Finally, Gurley et al. (12) showed that proximal tubular fluid reabsorption was significantly decreased in mice with proximal tubule deletion of AT1a receptors, accompanied by a decrease in cumulative sodium balance, although basal NHE3 mRNAs or NHE3 proteins were unaltered in the kidney of these mice (12).
Perspectives and Significance
In summary, we used an innovative approach to transiently cotransfer (or knockin) a GFP-tagged full-length, wild-type AT1a receptor and a novel intracellular cyan fluorescent ANG II fusion protein predominantly in proximal tubules of Agtr1a-/- mice. We demonstrate that the transfer of AT1aR/GFP and ECFP/ANG II in proximal tubules of Agtr1a-/- mice led to a significant increase in systolic blood pressure. The mechanism by which the transfer of AT1aR/GFP and ECFP/ANG II in proximal tubules of Agtr1a-/- mice is likely due to increased proximal tubule sodium and water reabsorption and consequently body salt and fluid retention. This interpretation is supported by a number of systemic and renal responses to the transfer of AT1aR/GFP and ECFP/ANG II, which include decreased 24-h urinary sodium excretion, increased 24-h water intake, increased total blood volume, and significant increases in phosphorylated MAP kinase ERK1/2 signaling and lysate and membrane NHE3 proteins in proximal tubules. Since increased blood pressure and signaling responses by proximal tubule-dominant expression of AT1aR/GFP and ECFP/ANG II were blocked by losartan, our results support an important physiological role of proximal tubule AT1a receptors and intracellular ANG II in the long-term regulation of blood pressure. In keeping with this context, functional intracellular and nuclear ANG II receptors have been demonstrated in proximal tubules of the kidney (33, 39). ANG II has been shown to activate these receptors to induce intracellular calcium responses (52); increase transcription of transforming growth factor β1, macrophage chemoattractant protein 1, and NHE3 (10, 33); or increase superoxide production (14, 15). From a clinical perspective, these studies suggest that the long-term genomic effects of intracellular ANG II may be considered regarding whether a renin inhibitor or ACE inhibitor should be prescribed alone, or in combination with an AT1 receptor blocker, to completely block the extracellular and intracellular renin-angiotensin systems.
GRANTS
This work was supported in part by National Institute of Diabetes, Digestive and Kidney Diseases grants (5R01DK067299, 2R56DK067299, and 2R01DK067299–06A2), American Society of Nephrology M. James Scherbenske Grant, and Hearin Foundation Medical Research Scholar Award to Dr. Jia Zhuo.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: X.C.L. and J.L.Z. performed experiments; X.C.L. and J.L.Z. analyzed data; X.C.L. and J.L.Z. interpreted results of experiments; X.C.L. and J.L.Z. prepared figures; X.C.L. and J.L.Z. drafted manuscript; X.C.L. and J.L.Z. edited and revised manuscript; X.C.L. and J.L.Z. approved final version of manuscript; J.L.Z. conception and design of research.
ACKNOWLEDGMENT
We would like to thank Dr. Julie Cook of Ochsner Clinic Foundation for providing the ECFP/ANG II construct, Drs. Isabelle Rubera and Michel Tauc of University of Nice-Sophia Antipolis, France for providing the sglt2 gene promoter construct, and Dr. Ulrich Hopfer of Case Western Reserve University for providing Agtr1a-/- mouse proximal tubule cells. Portions of this work were performed at Henry Ford Health System in Detroit, Michigan, and presented at the 64th Annual Fall Conference and Scientific Session of the American Heart Association Council for High Blood Pressure Research, Washington DC, October 13–16, 2010, and published as an abstract [Hypertension, 56: e64, 2010].
REFERENCES
- 1. Carey RM. Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure. Hypertension 38: 297–302, 2001 [DOI] [PubMed] [Google Scholar]
- 2. Carey RM, Siragy HM. Newly recognized components of the renin-angiotensin system: potential roles in cardiovascular and renal regulation. Endocr Rev 24: 261–271, 2003 [DOI] [PubMed] [Google Scholar]
- 3. Christensen EI, Birn H. Megalin and cubilin: synergistic endocytic receptors in renal proximal tubule. Am J Physiol Renal Physiol 280: F562–F573, 2001 [DOI] [PubMed] [Google Scholar]
- 4. Coffman TM. Under pressure: the search for the essential mechanisms of hypertension. Nat Med 17: 1402–1409, 2011 [DOI] [PubMed] [Google Scholar]
- 5. Cogan MG. Angiotensin II: a powerful controller of sodium transport in the early proximal tubule. Hypertension 15: 451–458, 1990 [DOI] [PubMed] [Google Scholar]
- 6. Cook JL, Mills SJ, Naquin R, Alam J, Re RN. Nuclear accumulation of the AT1 receptor in a rat vascular smooth muscle cell line: effects upon signal transduction and cellular proliferation. J Mol Cell Cardiol 40: 696–707, 2006 [DOI] [PubMed] [Google Scholar]
- 7. Cook JL, Re R, Alam J, Hart M, Zhang Z. Intracellular angiotensin II fusion protein alters AT1 receptor fusion protein distribution and activates CREB. J Mol Cell Cardiol 36: 75–90, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Crowley SD, Gurley SB, Oliverio MI, Pazmino AK, Griffiths R, Flannery PJ, Spurney RF, Kim HS, Smithies O, Le TH, Coffman TM. Distinct roles for the kidney and systemic tissues in blood pressure regulation by the renin-angiotensin system. J Clin Invest 115: 1092–1099, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ding Y, Davisson RL, Hardy DO, Zhu LJ, Merrill DC, Catterall JF, Sigmund CD. The kidney androgen-regulated protein promoter confers renal proximal tubule cell-specific and highly androgen-responsive expression on the human angiotensinogen gene in transgenic mice. J Biol Chem 272: 28142–28148, 1997 [DOI] [PubMed] [Google Scholar]
- 10. Ellis B, Li XC, Miguel-Qin E, Gu V, Zhuo JL. Evidence for a functional intracellular angiotensin system in the proximal tubule of the kidney. Am J Physiol Regul Integr Comp Physiol 302: R494–R509, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gonzalez-Villalobos RA, Billet S, Kim C, Satou R, Fuchs S, Bernstein KE, Navar LG. Intrarenal angiotensin-converting enzyme induces hypertension in response to angiotensin I infusion. J Am Soc Nephrol 22: 449–459, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gurley SB, Riquier-Brison AD, Schnermann J, Sparks MA, Allen AM, Haase VH, Snouwaert JN, Le TH, McDonough AA, Koller BH, Coffman TM. AT1A angiotensin receptors in the renal proximal tubule regulate blood pressure. Cell Metab 13: 469–475, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Guyton AC. Blood pressure control–special role of the kidneys and body fluids. Science 252: 1813–1816, 1991 [DOI] [PubMed] [Google Scholar]
- 14. Gwathmey TM, Alzayadneh EM, Pendergrass KD, Chappell MC. Novel roles of nuclear angiotensin receptors and signaling mechanisms. Am J Physiol Regul Integr Comp Physiol 302: R518–R530, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gwathmey TM, Pendergrass KD, Reid SD, Rose JC, Diz DI, Chappell MC. Angiotensin-(1–7)-angiotensin-converting enzyme 2 attenuates reactive oxygen species formation to angiotensin II within the cell nucleus. Hypertension 55: 166–171, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Harris PJ, Navar LG. Tubular transport responses to angiotensin II. Am J Physiol Renal Fluid Electrolyte Physiol 248: F621–F630, 1985 [DOI] [PubMed] [Google Scholar]
- 17. Harris PJ, Thomas D, Morgan TO. Atrial natriuretic peptide inhibits angiotensin-stimulated proximal tubular sodium and water reabsorption. Nature 326: 697–698, 1987 [DOI] [PubMed] [Google Scholar]
- 18. Harris PJ, Zhuo J, Mendelsohn FA, Skinner SL. Haemodynamic and renal tubular effects of low doses of endothelin in anaesthetized rats. J Physiol 433: 25–39, 1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Harrison-Bernard LM, Zhuo J, Kobori H, Ohishi M, Navar LG. Intrarenal AT1 receptor and ACE binding in ANG II-induced hypertensive rats. Am J Physiol Renal Physiol 282: F19–F25, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hashimoto S, Adams JW, Bernstein KE, Schnermann J. Micropuncture determination of nephron function in mice without tissue angiotensin-converting enzyme. Am J Physiol Renal Physiol 288: F445–F452, 2005 [DOI] [PubMed] [Google Scholar]
- 21. Jose PA, Eisner GM, Felder RA. Role of dopamine receptors in the kidney in the regulation of blood pressure. Curr Opin Nephrol Hypertens 11: 87–92, 2002 [DOI] [PubMed] [Google Scholar]
- 22. Kessler SP, de SSP, Scheidemantel TS, Gomos JB, Rowe TM, Sen GC. Maintenance of normal blood pressure and renal functions are independent effects of angiotensin-converting enzyme. J Biol Chem 278: 21105–21112, 2003 [DOI] [PubMed] [Google Scholar]
- 23. Kohan DE, Rossi NF, Inscho EW, Pollock DM. Regulation of blood pressure and salt homeostasis by endothelin. Physiol Rev 91: 1–77, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Krege JH, Hodgin JB, Hagaman JR, Smithies O. A noninvasive computerized tail-cuff system for measuring blood pressure in mice. Hypertension 25: 1111–1115, 1995 [DOI] [PubMed] [Google Scholar]
- 25. Le TH, Oliverio MI, Kim HS, Salzler H, Dash RC, Howell DN, Smithies O, Bronson S, Coffman TM. A gammaGT-AT1A receptor transgene protects renal cortical structure in AT1 receptor-deficient mice. Physiol Genomics 18: 290–298, 2004 [DOI] [PubMed] [Google Scholar]
- 26. Leheste JR, Rolinski B, Vorum H, Hilpert J, Nykjaer A, Jacobsen C, Aucouturier P, Moskaug JO, Otto A, Christensen EI, Willnow TE. Megalin knockout mice as an animal model of low molecular weight proteinuria. Am J Pathol 155: 1361–1370, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Li H, Weatherford ET, Davis DR, Keen HL, Grobe JL, Daugherty A, Cassis LA, Allen AM, Sigmund CD. Renal Proximal Tubule Angiotensin AT1A Receptors Regulate Blood Pressure. Am J Physiol Regul Integr Comp Physiol 301: R1067–R1077, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li XC, Cook JL, Rubera I, Tauc M, Zhang F, Zhuo JL. Intrarenal transfer of an intracellular cyan fluorescent fusion of angiotensin II selectively in proximal tubules increases blood pressure in rats and mice. Am J Physiol Renal Physiol 300: F1076–F1088, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li XC, Hopfer U, Zhuo JL. AT1 receptor-mediated uptake of angiotensin II and NHE-3 expression in proximal tubule cells through the microtubule-dependent endocytic pathway. Am J Physiol Renal Physiol 297: F1342–F1352, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Li XC, Hopfer U, Zhuo JL. Novel signaling mechanisms of intracellular angiotensin II-induced NHE3 expression and activation in mouse proximal tubule cells. Am J Physiol Renal Physiol 303: F1617–F1628, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Li XC, Zhuo JL. Selective knockdown of AT1 receptors by RNA interference inhibits Val5-Ang II endocytosis and NHE-3 expression in immortalized rabbit proximal tubule cells. Am J Physiol Cell Physiol 293: C367–C378, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Li XC, Zhuo JL. In vivo regulation of AT1a receptor-mediated intracellular uptake of [125I]-Val5-angiotensin II in the kidneys and adrenal glands of AT1a receptor-deficient mice. Am J Physiol Renal Physiol 294: F293–F302, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li XC, Zhuo JL. Intracellular ANG II directly induces in vitro transcription of TGF-β1, MCP-1, and NHE-3 mRNAs in isolated rat renal cortical nuclei via activation of nuclear AT1a receptors. Am J Physiol Cell Physiol 294: C1034–C1045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li XC, Zhuo JL. Phosphoproteomic analysis of AT1 receptor-mediated signaling responses in proximal tubules of angiotensin II-induced hypertensive rats. Kidney Int 80: 620–632, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Navar LG, Carmines PK, Huang WC, Mitchell KD. The tubular effects of angiotensin II. Kidney Int Suppl 20: S81–S88, 1987 [PubMed] [Google Scholar]
- 36. Navar LG, Imig JD, Zou L, Wang CT. Intrarenal production of angiotensin II. Semin Nephrol 17: 412–422, 1997 [PubMed] [Google Scholar]
- 37. Navar LG, Inscho EW, Majid SA, Imig JD, Harrison-Bernard LM, Mitchell KD. Paracrine regulation of the renal microcirculation. Physiol Rev 76: 425–536, 1996 [DOI] [PubMed] [Google Scholar]
- 38. Navar LG, Kobori H, Prieto MC, Gonzalez-Villalobos RA. Intratubular renin-angiotensin system in hypertension. Hypertension 57: 355–362, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pendergrass KD, Averill DB, Ferrario CM, Diz DI, Chappell MC. Differential expression of nuclear AT1 receptors and angiotensin II within the kidney of the male congenic mRen2. Lewis rat. Am J Physiol Renal Physiol 290: F1497–F1506, 2006 [DOI] [PubMed] [Google Scholar]
- 40. Rae FK, Suhaimi N, Li J, Nastasi T, Slonimsky E, Rosenthal N, Little MH. Proximal tubule overexpression of a locally acting IGF isoform, Igf-1Ea, increases inflammation after ischemic injury. Growth Horm IGF Res 22: 6–16, 2012 [DOI] [PubMed] [Google Scholar]
- 41. Redding KM, Chen BL, Singh A, Re RN, Navar LG, Seth DM, Sigmund CD, Tang WW, Cook JL. Transgenic mice expressing an intracellular fluorescent fusion of angiotensin II demonstrate renal thrombotic microangiopathy and elevated blood pressure. Am J Physiol Heart Circ Physiol 298: H1807–H1818, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rubera I, Poujeol C, Bertin G, Hasseine L, Counillon L, Poujeol P, Tauc M. Specific Cre/Lox recombination in the mouse proximal tubule. J Am Soc Nephrol 15: 2050–2056, 2004 [DOI] [PubMed] [Google Scholar]
- 43. Tran M, Tam D, Bardia A, Bhasin M, Rowe GC, Kher A, Zsengeller ZK, Akhavan-Sharif MR, Khankin EV, Saintgeniez M, David S, Burstein D, Karumanchi SA, Stillman IE, Arany Z, Parikh SM. PGC-1α promotes recovery after acute kidney injury during systemic inflammation in mice. J Clin Invest 121: 4003–4014, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. van Kats JP, Schalekamp MA, Verdouw PD, Duncker DJ, Danser AH. Intrarenal angiotensin II: interstitial and cellular levels and site of production. Kidney Int 60: 2311–2317, 2001 [DOI] [PubMed] [Google Scholar]
- 45. Woost PG, Kolb RJ, Finesilver M, Mackraj I, Imboden H, Coffman TM, Hopfer U. Strategy for the development of a matched set of transport-competent, angiotensin receptor-deficient proximal tubule cell lines. In Vitro Cell Dev Biol Anim 42: 189–200, 2006 [DOI] [PubMed] [Google Scholar]
- 46. Wright EM, Loo DD, Hirayama BA. Biology of human sodium glucose transporters. Physiol Rev 91: 733–794, 2011 [DOI] [PubMed] [Google Scholar]
- 47. Xia S, Li X, Johnson T, Seidel C, Wallace DP, Li R. Polycystin-dependent fluid flow sensing targets histone deacetylase 5 to prevent the development of renal cysts. Development 137: 1075–1084, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhuo JL, Li XC. Proximal nephron. Comprehens Physiol In press [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Zhuo JL, Harris PJ, Skinner SL. Atrial natriuretic factor modulates proximal glomerulotubular balance in anesthetized rats. Hypertension 14: 666–673, 1989 [DOI] [PubMed] [Google Scholar]
- 50. Zhuo JL, Imig JD, Hammond TG, Orengo S, Benes E, Navar LG. Ang II accumulation in rat renal endosomes during Ang II-induced hypertension: role of AT1 receptor. Hypertension 39: 116–121, 2002 [DOI] [PubMed] [Google Scholar]
- 51. Zhuo JL, Li XC. New insights and perspectives on intrarenal renin-angiotensin system: Focus on intracrine/intracellular angiotensin II. Peptides 32: 1551–1565, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Zhuo JL, Li XC, Garvin JL, Navar LG, Carretero OA. Intracellular angiotensin II induces cytosolic Ca2+ mobilization by stimulating intracellular AT1 receptors in proximal tubule cells. Am J Physiol Renal Physiol 290: F1382–F1390, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]