Abstract
Evidence indicates that skeletal muscle lipid droplet-associated proteins (PLINs) regulate lipolysis through protein-protein interactions on the lipid droplet surface. In adipocytes, PLIN1 is thought to regulate lipolysis by directly interacting with comparative gene identification-58 (CGI-58), an activator of adipose triglyceride lipase (ATGL). Upon lipolytic stimulation, PLIN1 is phosphorylated, releasing CGI-58 to fully activate ATGL and initiate triglyceride breakdown. The absence of PLIN1 in skeletal muscle leads us to believe that other PLIN family members undertake this role. Our purpose was to examine interactions between PLIN2, PLIN3, and PLIN5, with ATGL and its coactivator CGI-58 at rest and following contraction. Isolated rat solei were incubated for 30 min at rest or during 30 min of intermittent tetanic stimulation [150-ms volleys at 60 Hz with a train rate of 20 tetani/min (25°C)] to maximally stimulate intramuscular lipid breakdown. Results show that the interaction between ATGL and CGI-58 increased 128% following contraction (P = 0.041). Further, ATGL interacts with PLIN2, PLIN3, and PLIN5 at rest and following contraction. The PLIN2-ATGL interaction decreased significantly by 21% following stimulation (P = 0.013). Both PLIN3 and PLIN5 coprecipitated with CGI-58 at rest and following contraction, while there was no detectable interaction between PLIN2 and CGI-58 in either condition. Therefore, our findings indicate that in skeletal muscle, during contraction-induced muscle lipolysis, ATGL and CGI-58 strongly associate and that the PLIN proteins work together to regulate lipolysis, in part, by preventing ATGL and CGI-58 interactions at rest.
Keywords: adipocyte differentiation-related protein, adipophilin, OXPAT, MLDP, TIP47, ABHD5
fatty acids (fa) released from intramuscular triglycerides (IMTG) during lipolysis provide an important source of energy during muscle contraction. In skeletal muscle, IMTGs are packaged into lipid droplets that possess a unique coat of proteins associated with the surrounding phospholipid monolayer. This protein coat provides an interface for specific processes, such as transport, lipogenesis, and lipolysis (10, 34). Perilipins (PLINs) are the most recognized family of lipid droplet proteins and are the most likely to be involved in the regulation of lipogenesis and lipolysis in skeletal muscle (31).
Our understanding of PLIN proteins in skeletal muscle is limited; however, studies in other tissues and in cell culture indicate that PLIN proteins are key regulators of lipid metabolism, as they appear to be directly involved with how cells and tissues store, mobilize, and utilize fatty acids (8, 12, 15, 34, 35, 62). The PLIN family consists of five members, PLIN1 (perilipin), PLIN2 (adipocyte differentiation-related protein, ADRP), PLIN3 (tail-interacting protein of 47 kDa, TIP47), PLIN4 (S3–12), and PLIN5 (OXPAT, MLDP, and LSDP5) (41). Each PLIN has a unique tissue distribution and possibly a unique physiological function. To date, PLIN1 is the only protein of this family for which a distinct role has been established, regulating triglyceride storage and lipolysis (for review, see Refs. 4, 69, and 74). PLIN1 is primarily expressed in adipose tissue and controls adipocyte lipolysis by directly regulating the activity of the lipases surrounding the droplet (11, 42, 55, 60). Lipolysis is regulated by three lipases, adipose triglyceride lipase (ATGL), the rate-limiting lipase that initiates lipolysis by hydrolyzing the first ester bond releasing the first fatty acid; hormone-sensitive lipase (HSL), which has a high affinity for diacylglycerol as a substrate; and, finally, monoacylglycerol lipase. To fully activate ATGL in adipose tissue, it must be associated with the protein comparative gene identification-58 (CGI-58) (51). However, in adipocytes, under basal conditions, CGI-58 is bound to PLIN1, thus preventing full ATGL activity (56, 72). Adipocyte lipolysis is initiated when catecholamines bind to β-adrenergic receptors on the cell surface. Through the action of a stimulatory G protein, adenylate cyclase is activated, leading to increased intracellular cAMP and activation of cAMP-dependent PKA. PKA phosphorylates both PLIN1 and HSL (11, 18, 26, 42, 59, 60). It is the PKA-dependent phosphorylation of PLIN1 that causes the release of CGI-58, leading to the initiation of lipolysis by activating ATGL (4, 18–21). Phosphorylation of PLIN1 and HSL promotes the translocation and docking of HSL to PLIN1 on the lipid droplet surface (42, 43, 53, 58, 66).
Evidence suggests that skeletal muscle lipolysis is regulated by PLIN protein interactions occurring on the lipid droplet surface in a manner similar to that observed in adipose tissue (6, 8, 15). However, there are a few distinct differences between adipose tissue and skeletal muscle lipolysis. Although adipose tissue lipolysis aims to deliver FAs to other tissues for use, the goal of skeletal muscle lipolysis is to directly provide FAs to the mitochondria for oxidation and energy production. Further, while hormonal regulation of skeletal muscle lipolysis is similar to adipose tissue lipolysis, the full activation of ATGL and HSL in skeletal muscle is likely more complex, involving contraction-mediated routes (39). PLIN1 is not expressed in skeletal muscle, but PLIN2, PLIN3, and PLIN5 are all expressed and may be involved in regulating contraction-induced lipolysis (26, 71). Previous studies in skeletal muscle have focused on the role of HSL in regulating lipolysis, and there have been few studies investigating PLIN proteins in skeletal muscle. Interestingly, there have been no studies to date investigating the interactions between PLIN proteins, ATGL, and/or CGI-58, in response to contraction in skeletal muscle.
PLIN2 is one of the predominant PLIN proteins found in skeletal muscle (9, 27, 48). PLIN2 is believed to form a protective coat restricting lipolysis through interactions with lipolytic enzymes (3, 33, 48, 52). Other studies have supported a role for PLIN2 in limiting the interaction of the lipases with the triglycerides within the lipid droplet (as reviewed in Ref. 5), but this has yet to be investigated in whole skeletal muscle. In support of this theory, recent work in human skeletal muscle found that PLIN2-associated lipid droplets are preferentially depleted over those lipid droplets not associated with PLIN2 (54). Further, work in isolated rat soleus indicates that HSL is recruited to lipid droplets and PLIN2 with β-adrenergic stimulation or electrically stimulated contraction (48). Together, these results suggest that PLIN2 expression in skeletal muscle may be related to an enhanced IMTG utilization during contraction.
PLIN3 is one of the least studied of this family of proteins, but the homology of PLIN3 is similar to PLIN2 (44), and PLIN3 compensates for the loss of PLIN2 in Plin2−/−- mice (57). It is speculated that PLIN3 prevents cell death by maintaining mitochondrial membrane potential, although the mechanisms are not fully understood (29). It is possible that through interactions with skeletal muscle lipases, PLIN3 regulates the delivery of FAs to the mitochondria, thus preventing an overload. It is not presently known whether CGI-58 or ATGL bind to PLIN3 in skeletal muscle.
PLIN5 is unique in that its distribution is restricted to tissues that undergo high rates of lipid oxidation, such as skeletal muscle (specifically type I fibers), cardiac muscle, liver, and brown adipose tissue (8, 44, 47). Because of this unique distribution, it has been hypothesized that PLIN5 facilitates lipolysis and the oxidation of intracellular lipids in these tissues. In support of this hypothesis, skeletal muscle PLIN5 protein expression is increased under conditions that increase FA oxidation, such as fasting and insulin deficiency (13, 71). Further, PLIN5 content increases in response to endurance training (47). Recent work in Plin5−/− mice found that the hearts lacked detectable lipid droplets and contain less TG and FA compared with wild-type controls, and perfusion of these hearts with an inhibitor of ATGL recovers the lipid droplet content, suggesting an interaction between PLIN5 and ATGL in this tissue (32). These findings indicate that PLIN5 limits lipase actions; however, whether PLIN5 interacts with either ATGL and/or CGI-58 in whole skeletal muscle has yet to be determined.
The precise mechanisms regulating contraction-induced lipolysis in skeletal muscle are poorly understood. Therefore, the purpose of this study was to first determine whether there are any interactions between PLIN2, PLIN3, and PLIN5 with ATGL and/or CGI-58 at rest, and second, to evaluate the effects of lipolytic muscle contraction on these interactions in an isolated muscle preparation. We hypothesized that PLIN proteins would contribute to the regulation of skeletal muscle lipolysis by preventing the interaction of ATGL and CGI-58 at rest.
METHODS
Animals.
A total of 20 male Long-Evans rats (4–6 wk old, body mass 101 ± 7 g) were used in this study. Animals were housed in groups within the Brock University Animal Facility, where they were maintained on a 12:12-h light-dark cycle at 22°C. The rats were fed a standard rodent diet and had ad libitum access to food and water. All experimental procedures and protocols were approved by the Brock University Animal Care and Utilization Committee and conformed to all Canadian Council on Animal Care guidelines.
Muscle preparation.
Animals were anesthetized via intraperitoneal injection of pentobarbital sodium (6 mg/100 g body wt), and then the left and right soleus muscles were removed and placed in organ baths, where they were assigned to one of two experimental groups: 1) rest or 2) electrically stimulated contraction (36). To briefly summarize the preparation, each soleus muscle was dissected from tendon to tendon, sutures were tied in-situ, and the muscle was then removed and immediately placed in an organ bath (Radnoti Glass Technology, Monrovia, CA), which contained 15 ml of fully oxygenated liquid Sigma medium 199 (M 4530; Sigma-Aldrich, Oakville, Ontario, Canada) and suspended at a resting tension of 1 g. The incubation medium was continuously gassed with 95% O2-5% CO2, and temperature was maintained at 25°C (2). All muscles were allowed to equilibrate at rest for 30 min. After the initial incubation, the muscles were assigned to either the rest or stimulated group. The soleus was chosen for this set of experiments because it is primarily oxidative in nature (∼80% type I fibers) and has previously shown the greatest reliance on lipid metabolism (14, 17, 46). Further, while PLIN5 is expressed in all skeletal muscles, its expression is highest in more oxidative muscles containing more type I fibers, such as the soleus (71). These characteristics make the soleus an ideal muscle for investigating the role of PLIN proteins in skeletal muscle lipolysis. Further, the stimulation protocol that was utilized in this study and a previously published study (36) was designed and developed to elicit maximal rates of triglyceride use in the soleus muscle (16).
Stimulation protocol.
Following the equilibration period, the muscles remained at rest or were stimulated to contract for 30 min, as previously reported by our laboratory (36). Initially, optimal stimulus voltage was determined by assessing force responses (Grass Telefactor force transducer, West Warwick, RI) to single electrical pulses (Grass model FT03 with P11T amplifier). Stimulus intensity was increased from 10 V in 10-V increments, until a plateau in twitch force was reached, after which stimulus voltage was increased to ∼1.25 of this level. During the 30-min stimulus protocol, muscles received repeated volleys of brief (150 ms) but high-frequency (60 Hz) trains at a train rate of 20 tetani/min (muscles were suspended at 1 g of resting tension throughout). This protocol was previously proven to elicit maximal rates of triglyceride pool turnover and rates of TAG oxidation without the development of fatigue (16). Throughout this period, muscle force was recorded using Grass Polyview Data Acquisition and Analysis System (Astro-Med, West Warwick, RI) and analyzed using the Polyview Reviewer (Grass Polyview Data Acquisition and Analysis System; Astro-Med).
Coimmunoprecipitation.
Soleus muscles were homogenized in Griffin lysis buffer (150 mM NaCl, 50 mM Tris·HCl, 1 mM EGTA) using a 1:25 dilution of muscle to buffer with added protease (11836170001; Roche Diagnostics, Laval, QC, Canada) and phosphatase inhibitor tablets (04906845001; Roche Diagnostics). Protein concentration of the total homogenates was determined using a Bradford assay. Sample homogenates were immunoprecipitated (IP) with 5 μl of the appropriate antibody and then immunoblotted (IB) for the corresponding protein. Specifically, 500–1,000 μg of protein from each sample was incubated for 2 h with 5 μl of the desired primary antibody at 4°C. Following this, 20 μl of Protein G or A-agarose beads (sc-2001, sc-2002; Santa Cruz Biotechnology, Santa Cruz, CA) was added to each sample for overnight incubation at 4°C. The pellet of each sample was then collected by centrifugation at 130 rpm for 5–10 s. Pellets were washed 3 times in PBS and resuspended in 40 μl of 2× sample buffer. To test for antibody interference in the samples, a blank sample containing only the precipitating antibody and lysis buffer was prepared in exactly the same manner as the experimental samples. For interactions in which antibody interference occurred, a secondary that only detects native antibodies was used (Clean Blot IP Detection Reagent; Thermo Scientific, Waltham, MA). All samples were then boiled and separated using 8 or 10% SDS-PAGE. As validation that all of our protein of interest precipitated from the sample, pilot work was done in which the supernatant leftover from the IP procedure was Western blotted for the protein of interest.
Western blot analysis.
SDS-PAGE (8 or 10% separating; 4% stacking) was used to separate proteins (CGI-58, PLIN2, PLIN3, and PLIN5) at 120 V for 1.5 h, and proteins were electroblotted onto polyvinylidene difluoride membranes (Amersham Biosciences, Piscataway, NJ) for 1 h at 100 V followed by blocking in 2, 3, or 5% fat-free milk in TBST. Primary antibodies for coprecipitated proteins were diluted 1:1,000 in 2 or 3% fat-free milk in TBST and incubated overnight at 4°C. Secondary antibodies were diluted 1:10,000–20,000 in 2 or 3% milk and incubated for 1 h. Blots of specific proteins were visualized with enhanced chemiluminescence (Amersham Biosciences). The densities of the individual bands were integrated using ImageJ software (http://rsbweb.nih.gov/ij/). Each blot had loaded whole soleus homogenate as a positive control for the coprecipitated protein. Blots were normalized to total protein loaded determined by Ponceau S staining (M530; Sigma-Aldrich), and results are reported as the ratio of the density of the target protein to the density of the loaded protein in arbitrary units (50).
Antibodies.
The following antibodies were used and have been used previously: PLIN2 (52 kDa) mouse monoclonal antibody (cat. no. 610102; Progen Biotechnik, Heidelberg, Germany) (47), PLIN3 (47 kDa) (cat. no. 3883; ProSci, Poway, CA) (47), PLIN5 (52 kDa) guinea pig polyclonal antibody (cat. nos. GP34 and GP31; Progen Biotechnik) (7, 40, 47), ATGL (54 kDa) (rabbit monoclonal antibody no. 2439; Cell Signaling Technology, Danvers, MA) (1), and CGI-58 (42 kDa) rabbit polyclonal antibody (NB110-41576; Novus Biologicals, Oakville, ON, Canada) (1, 63).
Statistics.
Differences in protein interactions between rest and stimulated muscles were evaluated using two-tailed unpaired t-tests. Statistical significance was set at P < 0.05. All data are expressed as means ± SE.
RESULTS
Muscle force.
Adequate oxygenation and muscle viability were assessed by the ability to maintain force production over the duration of the stimulation protocol (30 min), as previously demonstrated (16, 36, 49). In our hands, this type of nonfatiguing contraction did not deplete muscle ATP concentrations (36). The initial isometric force normalized to soleus mass was 86.8 ± 4.1 g/g wet wt (n = 19). During stimulation, force output was recorded at 5-min intervals, and this was noted not to vary by more than 5% of initial isometric force at any point during the protocol. Accordingly, little or no fatigue or muscle degradation was evident in our results, indicating that the muscles remained stable and viable during the entire protocol.
Association between ATGL and CGI-58.
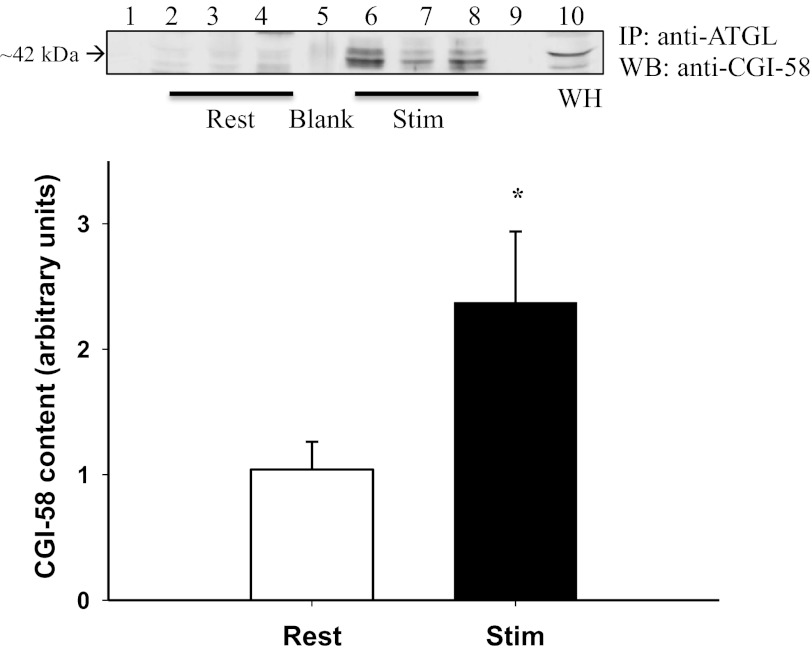
ATGL coimmunoprecipitated with CGI-58 at rest and following stimulated contraction. However, following stimulated contraction the amount of CGI-58 protein that coimmunoprecipitated with ATGL significantly increased by 128% (P = 0.041) (Fig. 1).
Fig. 1.
Adipose triglyceride lipase (ATGL)-comparative gene identification-58 (CGI-58) interaction at rest and following stimulated contraction. CGI-58 protein content (arbitrary units) in ATGL immunoprecipitated (IP) samples at rest and following contraction. Representative Western blot (WB) for ATGL-CGI-58 protein interaction, shown as IP ATGL and Western blot for CGI-58. Lane 1, standard; lanes 2–4, rest samples; lane 5, blank (absent antibody interference), lanes 6–8, stimulated samples, lane 9, empty, lane 10, soleus whole homogenate (WH) used as a positive control. *Significant increase in ATGL-CGI-58 interaction following stimulated contraction (P = 0.041).
Association between PLIN2, PLIN3, and PLIN5 with ATGL.
PLIN2, PLIN3, and PLIN5 all coimmunoprecipitated with ATGL at rest and following stimulated contraction. PLIN2 protein content in ATGL immunoprecipitated samples significantly decreased by 21% following stimulated contraction (P = 0.028) (Fig. 2A).
Fig. 2.
ATGL-skeletal muscle lipid droplet proteins (PLIN) interactions at rest and following stimulated contraction. A: PLIN2 protein content (arbitrary units) in ATGL immunoprecipitated samples at rest and following contraction. Representative Western blot for ATGL-PLIN2 protein interaction, shown as IP ATGL and Western blot for PLIN2. Lane 1, standard, lanes 2–4 rest samples, lane 5 blank, lanes 6–8, stimulated samples; lane 9, empty; lane 10, soleus whole homogenate used as a positive control. *Significant decrease in ATGL-PLIN2 association following stimulated contraction (P = 0.028). B: PLIN3 protein content (arbitrary units) in ATGL-immunoprecipitated samples at rest and following contraction. Representative Western blot for ATGL-PLIN3 protein interaction, shown as IP ATGL and Western blot for PLIN3. Lane 1, standard, lanes 2–4, rest samples, lane 5, blank (Note IgG band at ∼50 kDa); lanes 6–8, stimulated samples; lane 9, empty; lane 10, whole soleus homogenate. No significant difference in ATGL-PLIN3 association following stimulated contraction (P = 0.266). C: PLIN5 protein content (arbitrary units) in ATGL immunoprecipitated samples at rest and following contraction. Representative Western blot for ATGL-PLIN5 protein interaction, shown as IP ATGL and Western blot for PLIN5. Lane 1, standard; lanes 2–4, rest samples; lane 5, blank (absent antibody interference); lanes 6–8, stimulated samples; lane 9, soleus whole homogenate used as positive control. No significant difference in ATGL-PLIN5 association following stimulated contraction (P = 0.591).
Similarly, PLIN3 protein content in ATGL-immunoprecipitated samples decreased by 27% following stimulated contraction; however, this decrease was not significant (P = 0.266) (Fig. 2B). Finally, PLIN5 protein content in ATGL immunoprecipitated samples also decreased by 19% following stimulated contraction; however, this decrease was not significant (P = 0.59) (Fig. 2C).
Association between PLIN2, PLIN3, and PLIN5 with CGI-58.
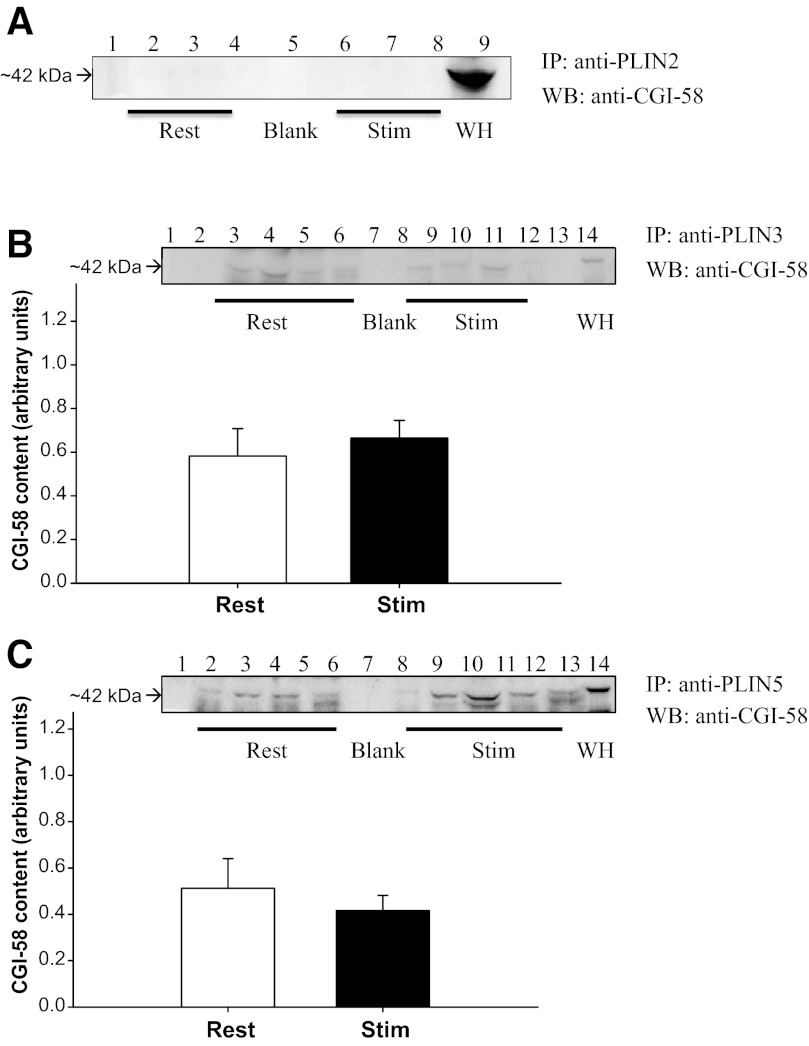
There was no detectable interaction found between PLIN2 and CGI-58 in PLIN2-immunoprecipitated samples blotted for CGI-58 (Fig. 3A).
Fig. 3.
PLIN-CGI-58 at rest and following stimulated contraction. A: representative Western blot of CGI-58 in immunoprecipitated PLIN2 samples. Lane 1, standard; lanes 2–4, rest samples; lane 5, blank (absent antibody interference); lanes 6–8, stimulated samples; lane 9, soleus whole homogenate used as a positive control. No detectable interaction between PLIN2 and CGI-58. B:CGI-58 protein content (arbitrary units) in PLIN3-immunoprecipitated samples at rest and following contraction. Representative Western blot of CGI-58 in IP PLIN3 samples. Lanes 1, standard; lanes 2–5, rest samples; lane 6, blank (absent antibody interference); lanes 7–10, stimulated samples, lane 12, soleus whole homogenate used as positive control. No significant difference in PLIN3-CGI-58 association following stimulated contraction (P = 0.58). C: CGI-58 protein content (arbitrary units) in PLIN5-immunoprecipitated samples at rest and following contraction. Representative Western blot of CGI-58 in IP PLIN5 samples. Lane 1, standard; lanes 2–5, rest samples; lane 6, blank (absent antibody interference); lanes 7–10, stimulated samples; lane 11, soleus whole homogenate used as positive control. No significant difference in PLIN5-CGI-58 association following stimulated contraction (P = 0.50).
CGI-58 immunoprecipitated with both PLIN3 and PLIN5 at rest and following stimulated contraction. However, there was no change following contraction for either PLIN proteins (P = 0.08 and P = 0.42, respectively) (Fig. 3, B and C).
DISCUSSION
This is the first study to examine the interactions between PLIN proteins, ATGL, and CGI-58 in whole skeletal muscle at rest and following stimulated contraction in the absence of adrenergic stimulation. Using an isolated muscle technique, we examined interactions of ATGL and its coactivator, CGI-58, with each other as well as PLIN2, PLIN3, and PLIN5. We found that in skeletal muscle, the interaction between ATGL and CGI-58 increases twofold (128%) after 30 min of stimulating contraction. Further novel results from this study show that PLIN2, PLIN3, and PLIN5 all interact with ATGL at rest and following contraction and that only PLIN3 and PLIN5 interact with CGI-58. Further, the interaction between PLIN2 and ATGL is significantly decreased following stimulated contraction, although similar decreases in the interaction of PLIN3 and PLIN5 with ATGL were not significant. These findings support the hypothesis that PLIN proteins may regulate skeletal muscle lipolysis by sequestering ATGL and CGI-58, and it is possible that in skeletal muscle, these PLIN proteins work together in concert to regulate lipolysis.
The stimulation protocol used in this study was chosen because it elicits maximal rates of lipolysis in isolated soleus muscle, and we have previously shown that this method leads to a significant decline in IMTGs (16, 36). This is the first study to show that during lipolytic stimulating contraction in skeletal muscle ATGL and CGI-58 interact. This interaction increased 128% postcontraction consistent with an increased rate of lipolysis during this period (16). We found that PLIN2, PLIN3, and PLIN5 all interact with ATGL at rest and following stimulated contraction, but the PLIN2-ATGL interaction was the only one to show a significant decrease poststimulation. However, this decrease in the association between PLIN2 and ATGL (21%) does not fully account for the large increase in the association between ATGL and CGI-58 (128%) following contraction. With more than one PLIN protein interacting with ATGL and/or CGI-58, perhaps in skeletal muscle, the PLIN proteins work together as a complex. The results of this study indicate that the roles of PLIN proteins in regulating skeletal muscle lipolysis are much more complex than what is currently understood in adipose tissue.
Cell culture studies provide evidence that both PLIN2 and PLIN5 compete with lipases at the lipid droplet surface to lower basal lipolytic rates (3, 33, 65, 67). Specifically, increasing the expression of PLIN2 and/or PLIN5 in human embryonic kidney cells or AML12 liver cells reduces the rate of basal and PKA-stimulated lipolysis, potentially by interacting with ATGL (3, 33, 65). Our finding that PLIN2 is associated with ATGL at rest is consistent with previous work indicating that PLIN2 encourages lipid accumulation by inhibiting lipolysis. In general, PLIN2 is found on the lipid droplet surface from the beginning of synthesis and is upregulated in parallel with stored lipid during lipid droplet formation (9, 30, 68). Cell culture studies (murine fibroblasts, human embryonic kidney cells, McA-RH7777 cells, and primary rat hepatocytes) have also demonstrated that overexpression of PLIN2 encourages lipid accumulation (30, 33, 37), whereas downregulation of PLIN2 results in elevated rates of basal lipolysis (3). Further, in PLIN1-null mice, PLIN2 replaces PLIN1 on the lipid droplets (61), but PLIN2 appears to be a less robust barrier to lipases than PLIN1 (38, 61). Although PLIN2 is similar to PLIN1 in sequence homology, it has been demonstrated that, unlike PLIN1, PLIN2 is not phosphorylated by PKA (59, 61). Since PLIN2 was the only PLIN protein in the present study to show a significant decline in ATGL association following contraction, it is possible that this interaction is regulated by a contraction-mediated route rather than a hormonal one. The exact mechanisms regulating the interaction between PLIN2 and ATGL in skeletal muscle needs further study. Our finding that PLIN2 does not interact with CGI-58 is in agreement with a previous study using Chinese hamster ovary (CHO) cells that found that lipid droplets coated with PLIN2 did not recruit CGI-58 to the lipid droplets as effectively as either PLIN1 or PLIN5 (64). However, there are other studies with conflicting results. Yamaguchi et al. (73) investigated the functions of PLIN2 and performed yeast two-hybrid screening to find any functional partners, finding that CGI-58 can interact with PLIN2. Therefore, it is possible that the lack of interaction may be cell/tissue-specific.
Because of PLIN5 being highly expressed in oxidative tissues, it seems likely that this PLIN protein is involved in the regulation of skeletal muscle lipid droplet dynamics. In PLIN5-null mice, lipid droplets are undetectable in hearts; however, lipid droplets are observed in other tissues, including skeletal muscle suggesting that the role(s) of PLIN5 may differ depending on the individual cell/tissue type. There was no significant difference in TG content in soleus muscle from PLIN5-null mice compared with wild-type controls (small decrease); however, this could be due to the other skeletal muscle PLIN proteins compensating for the loss, or the possibility that more than one PLIN protein is required to fully regulate skeletal muscle lipolysis. Previous work investigating possible interaction partners for PLIN5 has been done with a cell culture model. Using CHO cells, Wang et al. (65) determined that PLIN5 directly binds to ATGL and that PLIN5 is a substrate for PKA, suggesting that phosphorylation of PLIN5 enables lipolysis. The lack of a significant decline in the PLIN5-ATGL interaction seen in the present study may be due to our stimulation protocol, which did not include any adrenergic stimulation to activate PKA. Recently, it was demonstrated that PLIN5 facilitates lipolysis by promoting the colocalization and functional interaction of CGI-58 and ATGL (23, 25). The same PLIN5 molecule does not bind both at the same time (24) and appears to be responsible for directing CGI-58 to the droplet surface to increase ATGL activity (22). In the present study, we found that PLIN5 interacts with both ATGL and CGI-58; however, it is not known what proportion of PLIN5 is bound to either ATGL or CGI-58, or whether PLIN5 interacts with both ATGL and CGI-58 at the same time.
Previous to the current work, it was unknown whether PLIN3 interacted with either ATGL or CGI-58. Our results suggest that PLIN3 is also involved in reducing lipolysis through interactions with both ATGL and CGI-58. It has been suggested that PLIN3 plays a role in lipid droplet formation/growth synthesis by inhibiting lipolysis. This is supported by work using immunofluorescence microscopy in 3T3-L1 cells treated with oleate, glucose, and insulin (designed in increase TG synthesis) finding that PLIN3 moved from the cytosol to the lipid droplet (70). Moreover, in PLIN2-null mice, PLIN2 on the LD is replaced with PLIN3 (57). To further support a role for PLIN3 in preventing lipolysis and promoting TG synthesis, siRNA knockdown of PLIN3 in PLIN2 knockout mice resulted in reduced number of lipid droplets, reduced incorporation of oleate into TG, and increased incorporation of oleate to phospholipids, where knockout of PLIN2 alone resulted in no change (15). Interestingly, the sequence of amino acids 191–437 of PLIN3 is similar to the amino acid sequence 200–463 of PLIN5, which has been found to be the binding site for both ATGL and CGI-58 on PLIN5 (28). Our finding that both PLIN3 and PLIN5 interact with ATGL and CGI-58 in skeletal muscle is consistent with these findings. However, as PLIN3 and PLIN5 are found in the cytosol, as well as on the lipid droplet, it is unknown which population of these proteins is bound to either ATGL and/or CGI-58. Further study in this area is needed to determine these interactions.
Perspectives and Significance
This study examined the interactions of the rate-limiting lipase, ATGL, its coactivator, CGI-58, and three skeletal muscle PLIN proteins at rest and following contraction. This is the first study to demonstrate that in isolated skeletal muscle the interaction between ATGL and CGI-58 is significantly increased following a contraction protocol that lacks adrenergic stimulation. Further, this study provides evidence that in skeletal muscle, both ATGL and CGI-58 are potentially regulated by more than one protein of the PLIN family. Taken together, these data suggest that, the skeletal muscle PLIN proteins, ATGL and CGI-58, may not ever exist in a single complex at rest or during contraction but may instead represent a network of proteins interacting with one another at different times for different purposes. Evidence indicates that all three of these PLIN proteins are associated with mitochondria (7, 29, 45, 67). It is possible that in skeletal muscle these PLIN proteins work together to control the rate of lipolysis and thus of FA entry into the mitochondria during lipolysis. Understanding the mechanisms by which PLIN proteins contribute to these processes will help to clarify both the physiology of healthy cells and tissues, as well as the pathophysiological basis of some important metabolic diseases. Further research is needed to elucidate the specific roles of skeletal muscle PLIN proteins in regulating lipolysis and FA entry into the mitochondria.
GRANTS
This research is supported by Natural Sciences and Engineering Research Council of Canada (NSERC) grants to S. J. Peters, R. Vandenboom, and B. D. Roy. Laboratory infrastructure support was provided by the Canadian Foundation for Innovation, the Ontario Innovation Trust, and NSERC. R. MacPherson is the recipient of an Ontario Graduate Scholarship in Science and Technology, as well as a NSERC Postgraduate Scholarship.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
Author contributions: R.E.M., R.V., B.D.R., and S.J.P. conception and design of research; R.E.M. and S.R. performed experiments; R.E.M. and S.R. analyzed data; R.E.M., S.R., R.V., B.D.R., and S.J.P. interpreted results of experiments; R.E.M. and S.R. prepared figures; R.E.M. drafted manuscript; R.E.M., S.R., R.V., B.D.R., and S.J.P. edited and revised manuscript; R.E.M., S.R., R.V., B.D.R., and S.J.P. approved final version of manuscript.
REFERENCES
- 1. Alsted TJ, Nybo L, Schweiger M, Fledelius C, Jacobsen P, Zimmermann R, Zechner R, Kiens B. Adipose triglyceride lipase in human skeletal muscle is upregulated by exercise training. Am J Physiol Endocrinol Metab 296: E445–E453, 2009 [DOI] [PubMed] [Google Scholar]
- 2. Antolic A, Harrison R, Farlinger C, Cermak NM, Peters SJ, LeBlanc P, Roy BD. Effect of extracellular osmolality on cell volume and resting metabolism in mammalian skeletal muscle. Am J Physiol Regul Integr Comp Physiol 292: R1994–R2000, 2007 [DOI] [PubMed] [Google Scholar]
- 3. Bell M, Wang H, Chen H, McLenithan JC, Gong DW, Yang RZ, Yu D, Fried SK, Quon MJ, Londos C, Sztalryd C. Consequences of lipid droplet coat protein downregulation in liver cells: abnormal lipid droplet metabolism and induction of insulin resistance. Diabetes 57: 2037–2045, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bezaire V, Langin D. Regulation of adipose tissue lipolysis revisited. Proc Nutr Soc 68: 350–360, 2009 [DOI] [PubMed] [Google Scholar]
- 5. Bickel PE, Tansey JT, Welte MA. PAT proteins, an ancient family of lipid droplet proteins that regulate cellular lipid stores. Biochim Biophys Acta 1791: 419–440, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Blanchette-Mackie EJ, Dwyer NK, Barber T, Coxey RA, Takeda T, Rondinone CM, Theodorakis JL, Greenberg AS, Londos C. Perilipin is located on the surface layer of intracellular lipid droplets in adipocytes. J Lipid Res 36: 1211–1226, 1995 [PubMed] [Google Scholar]
- 7. Bosma M, Minnaard R, Sparks LM, Schaart G, Losen M, de Baets MH, Duimel H, Kersten S, Bickel PE, Schrauwen P, Hesselink MK. The lipid droplet coat protein perilipin 5 also localizes to muscle mitochondria. Histochem Cell Biol 137: 205–216, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Brasaemle DL. Thematic review series: adipocyte biology. The perilipin family of structural lipid droplet proteins: stabilization of lipid droplets and control of lipolysis. J Lipid Res 48: 2547–2559, 2007 [DOI] [PubMed] [Google Scholar]
- 9. Brasaemle DL, Barber T, Wolins NE, Serrero G, Blanchette-Mackie EJ, Londos C. Adipose differentiation-related protein is an ubiquitously expressed lipid storage droplet-associated protein. J Lipid Res 38: 2249–2263, 1997 [PubMed] [Google Scholar]
- 10. Brasaemle DL, Dolios G, Shapiro L, Wang R. Proteomic analysis of proteins associated with lipid droplets of basal and lipolytically stimulated 3T3–L1 adipocytes. J Biol Chem 279: 46835–46842, 2004 [DOI] [PubMed] [Google Scholar]
- 11. Brasaemle DL, Rubin B, Harten IA, Gruia-Gray J, Kimmel AR, Londos C. Perilipin A increases triacylglycerol storage by decreasing the rate of triacylglycerol hydrolysis. J Biol Chem 275: 38486–38493, 2000 [DOI] [PubMed] [Google Scholar]
- 12. Brown DA. Lipid droplets: proteins floating on a pool of fat. Curr Biol 11: R446–R449, 2001 [DOI] [PubMed] [Google Scholar]
- 13. Dalen KT, Dahl T, Holter E, Arntsen B, Londos C, Sztalryd C, Nebb HI. LSDP5 is a PAT protein specifically expressed in fatty acid oxidizing tissues. Biochim Biophys Acta 1771: 210–227, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Delp MD, Duan C. Composition and size of type I, IIA, IID/X, and IIB fibers and citrate synthase activity of rat muscle. J Appl Physiol 80: 261–270, 1996 [DOI] [PubMed] [Google Scholar]
- 15. Ducharme NA, Bickel PE. Lipid droplets in lipogenesis and lipolysis. Endocrinology 149: 942–949, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Dyck DJ, Bonen A. Muscle contraction increases palmitate esterification and oxidation and triacylglycerol oxidation. Am J Physiol Endocrinol Metab 275: E888–E896, 1998 [DOI] [PubMed] [Google Scholar]
- 17. Dyck DJ, Peters SJ, Glatz J, Gorski J, Keizer H, Kiens B, Liu S, Richter EA, Spriet LL, van der Vusse GJ, Bonen A. Functional differences in lipid metabolism in resting skeletal muscle of various fiber types. Am J Physiol Endocrinol Metab 272: E340–E351, 1997 [DOI] [PubMed] [Google Scholar]
- 18. Egan JJ, Greenberg AS, Chang MK, Londos C. Control of endogenous phosphorylation of the major cAMP-dependent protein kinase substrate in adipocytes by insulin and β-adrenergic stimulation. J Biol Chem 265: 18769–18775, 1990 [PubMed] [Google Scholar]
- 19. Granneman JG, Moore HP. Location, location: protein trafficking and lipolysis in adipocytes. Trends Endocrinol Metab 19: 3–9, 2008 [DOI] [PubMed] [Google Scholar]
- 20. Granneman JG, Moore HP, Granneman RL, Greenberg AS, Obin MS, Zhu Z. Analysis of lipolytic protein trafficking and interactions in adipocytes. J Biol Chem 282: 5726–5735, 2007 [DOI] [PubMed] [Google Scholar]
- 21. Granneman JG, Moore HP, Krishnamoorthy R, Rathod M. Perilipin controls lipolysis by regulating the interactions of AB-hydrolase containing 5 (Abhd5) and adipose triglyceride lipase (Atgl). J Biol Chem 284: 34538–34544, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J Biol Chem 284: 3049–3057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Granneman JG, Moore HP, Mottillo EP, Zhu Z. Functional interactions between Mldp (LSDP5) and Abhd5 in the control of intracellular lipid accumulation. J Biol Chem 284: 3049–3057, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J Biol Chem 286: 5126–5135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Granneman JG, Moore HP, Mottillo EP, Zhu Z, Zhou L. Interactions of perilipin-5 (Plin5) with adipose triglyceride lipase. J Biol Chem 286: 5126–5135, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Greenberg AS, Egan JJ, Wek SA, Garty NB, Blanchette-Mackie EJ, Londos C. Perilipin, a major hormonally regulated adipocyte-specific phosphoprotein associated with the periphery of lipid storage droplets. J Biol Chem 266: 11341–11346, 1991 [PubMed] [Google Scholar]
- 27. Heid HW, Moll R, Schwetlick I, Rackwitz HR, Keenan TW. Adipophilin is a specific marker of lipid accumulation in diverse cell types and diseases. Cell Tissue Res 294: 309–321, 1998 [DOI] [PubMed] [Google Scholar]
- 28. Hickenbottom SJ, Kimmel AR, Londos C, Hurley JH. Structure of a lipid droplet protein; the PAT family member TIP47. Structure 12: 1199–1207, 2004 [DOI] [PubMed] [Google Scholar]
- 29. Hocsak E, Racz B, Szabo A, Mester L, Rapolti E, Pozsgai E, Javor S, Bellyei S, Gallyas F, Jr, Sumegi B, Szigeti A. TIP47 protects mitochondrial membrane integrity and inhibits oxidative-stress-induced cell death. FEBS Lett 584: 2953–2960, 2010 [DOI] [PubMed] [Google Scholar]
- 30. Imamura M, Inoguchi T, Ikuyama S, Taniguchi S, Kobayashi K, Nakashima N, Nawata H. ADRP stimulates lipid accumulation and lipid droplet formation in murine fibroblasts. Am J Physiol Endocrinol Metab 283: E775–E783, 2002 [DOI] [PubMed] [Google Scholar]
- 31. Kimmel AR, Brasaemle DL, McAndrews-Hill M, Sztalryd C, Londos C. Adoption of PERILIPIN as a unifying nomenclature for the mammalian PAT-family of intracellular lipid storage droplet proteins. J Lipid Res 51: 468–471, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kuramoto K, Okamura T, Yamaguchi T, Nakamura TY, Wakabayashi S, Morinaga H, Nomura M, Yanase T, Otsu K, Usuda N, Matsumura S, Inoue K, Fushiki T, Kojima Y, Hashimoto T, Sakai F, Hirose F, Osumi T. Perilipin 5, a lipid droplet-binding protein, protects heart from oxidative burden by sequestering fatty acid from excessive oxidation. J Biol Chem 287: 23852–23863, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Listenberger LL, Ostermeyer-Fay AG, Goldberg EB, Brown WJ, Brown DA. Adipocyte differentiation-related protein reduces the lipid droplet association of adipose triglyceride lipase and slows triacylglycerol turnover. J Lipid Res 48: 2751–2761, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Liu P, Ying Y, Zhao Y, Mundy DI, Zhu M, Anderson RG. Chinese hamster ovary K2 cell lipid droplets appear to be metabolic organelles involved in membrane traffic. J Biol Chem 279: 3787–3792, 2004 [DOI] [PubMed] [Google Scholar]
- 35. Londos C, Sztalryd C, Tansey JT, Kimmel AR. Role of PAT proteins in lipid metabolism. Biochimie 87: 45–49, 2005 [DOI] [PubMed] [Google Scholar]
- 36. Macpherson RE, Herbst EA, Reynolds EJ, Vandenboom R, Roy BD, Peters SJ. Subcellular localization of skeletal muscle lipid droplets and PLIN family proteins OXPAT and ADRP at rest and following contraction in rat soleus muscle. Am J Physiol Regul Integr Comp Physiol 302: R29–R36, 2012 [DOI] [PubMed] [Google Scholar]
- 37. Magnusson B, Asp L, Bostrom P, Ruiz M, Stillemark-Billton P, Linden D, Boren J, Olofsson SO. Adipocyte differentiation-related protein promotes fatty acid storage in cytosolic triglycerides and inhibits secretion of very low-density lipoproteins. Arterioscler Thromb Vasc Biol 26: 1566–1571, 2006 [DOI] [PubMed] [Google Scholar]
- 38. Martinez-Botas J, Anderson JB, Tessier D, Lapillonne A, Chang BH, Quast MJ, Gorenstein D, Chen KH, Chan L. Absence of perilipin results in leanness and reverses obesity in Lepr(db/db) mice. Nat Genet 26: 474–479, 2000 [DOI] [PubMed] [Google Scholar]
- 39. Meex RC, Schrauwen P, Hesselink MK. Modulation of myocellular fat stores: lipid droplet dynamics in health and disease. Am J Physiol Regul Integr Comp Physiol 297: R913–R924, 2009 [DOI] [PubMed] [Google Scholar]
- 40. Minnaard R, Schrauwen P, Schaart G, Jorgensen JA, Lenaers E, Mensink M, Hesselink MK. Adipocyte differentiation-related protein and OXPAT in rat and human skeletal muscle: involvement in lipid accumulation and type 2 diabetes mellitus. J Clin Endocrinol Metab 94: 4077–4085, 2009 [DOI] [PubMed] [Google Scholar]
- 41. Miura S, Gan JW, Brzostowski J, Parisi MJ, Schultz CJ, Londos C, Oliver B, Kimmel AR. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J Biol Chem 277: 32253–32257, 2002 [DOI] [PubMed] [Google Scholar]
- 42. Miyoshi H, Souza SC, Zhang HH, Strissel KJ, Christoffolete MA, Kovsan J, Rudich A, Kraemer FB, Bianco AC, Obin MS, Greenberg AS. Perilipin promotes hormone-sensitive lipase-mediated adipocyte lipolysis via phosphorylation-dependent and -independent mechanisms. J Biol Chem 281: 15837–15844, 2006 [DOI] [PubMed] [Google Scholar]
- 43. Mottagui-Tabar S, Ryden M, Lofgren P, Faulds G, Hoffstedt J, Brookes AJ, Andersson I, Arner P. Evidence for an important role of perilipin in the regulation of human adipocyte lipolysis. Diabetologia 46: 789–797, 2003 [DOI] [PubMed] [Google Scholar]
- 44. Murphy S, Martin S, Parton RG. Lipid droplet-organelle interactions; sharing the fats. Biochim Biophys Acta 1791: 441–447, 2009 [DOI] [PubMed] [Google Scholar]
- 45. Nakamura N, Fujimoto T. Adipose differentiation-related protein has two independent domains for targeting to lipid droplets. Biochem Biophys Res Commun 306: 333–338, 2003 [DOI] [PubMed] [Google Scholar]
- 46. Peters SJ, Dyck DJ, Bonen A, Spriet LL. Effects of epinephrine on lipid metabolism in resting skeletal muscle. Am J Physiol Endocrinol Metab 275: E300–E309, 1998 [DOI] [PubMed] [Google Scholar]
- 47. Peters SJ, Samjoo IA, Devries MC, Stevic I, Robertshaw HA, Tarnopolsky MA. Perilipin family (PLIN) proteins in human skeletal muscle: the effect of sex, obesity, and endurance training. Appl Physiol Nutr Metab 37: 724–735, 2012 [DOI] [PubMed] [Google Scholar]
- 48. Prats C, Donsmark M, Qvortrup K, Londos C, Sztalryd C, Holm C, Galbo H, Ploug T. Decrease in intramuscular lipid droplets and translocation of HSL in response to muscle contraction and epinephrine. J Lipid Res 47: 2392–2399, 2006 [DOI] [PubMed] [Google Scholar]
- 49. Rennie MJ, Holloszy JO. Inhibition of glucose uptake and glycogenolysis by availability of oleate in well-oxygenated perfused skeletal muscle. Biochem J 168: 161–170, 1977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Romero-Calvo I, Ocon B, Martinez-Moya P, Suarez MD, Zarzuelo A, Martinez-Augustin O, de Medina FS. Reversible Ponceau staining as a loading control alternative to actin in Western blots. Anal Biochem 401: 318–320, 2010 [DOI] [PubMed] [Google Scholar]
- 51. Schweiger M, Schreiber R, Haemmerle G, Lass A, Fledelius C, Jacobsen P, Tornqvist H, Zechner R, Zimmermann R. Adipose triglyceride lipase and hormone-sensitive lipase are the major enzymes in adipose tissue triacylglycerol catabolism. J Biol Chem 281: 40236–40241, 2006 [DOI] [PubMed] [Google Scholar]
- 52. Shaw CS, Sherlock M, Stewart PM, Wagenmakers AJ. Adipophilin distribution and colocalization with lipid droplets in skeletal muscle. Histochem Cell Biol 131: 575–581, 2009 [DOI] [PubMed] [Google Scholar]
- 53. Shen WJ, Patel S, Miyoshi H, Greenberg AS, Kraemer FB. Functional interaction of hormone-sensitive lipase and perilipin in lipolysis. J Lipid Res 50: 2306–2313, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Shepherd SO, Cocks M, Tipton KD, Ranasinghe AM, Barker TA, Burniston JG, Wagenmakers AJ, Shaw CS. Preferential utilization of perilipin 2-associated intramuscular triglycerides during 1 h of moderate-intensity endurance-type exercise. Exp Physiol 97: 970–980, 2012 [DOI] [PubMed] [Google Scholar]
- 55. Souza SC, Muliro KV, Liscum L, Lien P, Yamamoto MT, Schaffer JE, Dallal GE, Wang X, Kraemer FB, Obin M, Greenberg AS. Modulation of hormone-sensitive lipase and protein kinase A-mediated lipolysis by perilipin A in an adenoviral reconstituted system. J Biol Chem 277: 8267–8272, 2002 [DOI] [PubMed] [Google Scholar]
- 56. Subramanian V, Rothenberg A, Gomez C, Cohen AW, Garcia A, Bhattacharyya S, Shapiro L, Dolios G, Wang R, Lisanti MP, Brasaemle DL. Perilipin A mediates the reversible binding of CGI-58 to lipid droplets in 3T3–L1 adipocytes. J Biol Chem 279: 42062–42071, 2004 [DOI] [PubMed] [Google Scholar]
- 57. Sztalryd C, Bell M, Lu X, Mertz P, Hickenbottom S, Chang BH, Chan L, Kimmel AR, Londos C. Functional compensation for adipose differentiation-related protein (ADFP) by Tip47 in an ADFP null embryonic cell line. J Biol Chem 281: 34341–34348, 2006 [DOI] [PubMed] [Google Scholar]
- 58. Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161: 1093–1103, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Sztalryd C, Xu G, Dorward H, Tansey JT, Contreras JA, Kimmel AR, Londos C. Perilipin A is essential for the translocation of hormone-sensitive lipase during lipolytic activation. J Cell Biol 161: 1093–1103, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Tansey JT, Huml AM, Vogt R, Davis KE, Jones JM, Fraser KA, Brasaemle DL, Kimmel AR, Londos C. Functional studies on native and mutated forms of perilipins. A role in protein kinase A-mediated lipolysis of triacylglycerols. J Biol Chem 278: 8401–8406, 2003 [DOI] [PubMed] [Google Scholar]
- 61. Tansey JT, Sztalryd C, Gruia-Gray J, Roush DL, Zee JV, Gavrilova O, Reitman ML, Deng CX, Li C, Kimmel AR, Londos C. Perilipin ablation results in a lean mouse with aberrant adipocyte lipolysis, enhanced leptin production, and resistance to diet-induced obesity. Proc Natl Acad Sci USA 98: 6494–6499, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Tansey JT, Sztalryd C, Hlavin EM, Kimmel AR, Londos C. The central role of perilipin a in lipid metabolism and adipocyte lipolysis. IUBMB Life 56: 379–385, 2004 [DOI] [PubMed] [Google Scholar]
- 63. Timmers S, de Vogel-van den Bosch J, Hesselink MK, van Beurden D, Schaart G, Ferraz MJ, Losen M, Martinez-Martinez P, De Baets MH, Aerts JM, Schrauwen P. Paradoxical increase in TAG and DAG content parallel the insulin sensitizing effect of unilateral DGAT1 overexpression in rat skeletal muscle. PLos One 6: e14503, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Wang H, Bell M, Sreenevasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem 286: 15707–15715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wang H, Bell M, Sreenevasan U, Hu H, Liu J, Dalen K, Londos C, Yamaguchi T, Rizzo MA, Coleman R, Gong D, Brasaemle D, Sztalryd C. Unique regulation of adipose triglyceride lipase (ATGL) by perilipin 5, a lipid droplet-associated protein. J Biol Chem 286: 15707–15715, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Wang H, Hu L, Dalen K, Dorward H, Marcinkiewicz A, Russell D, Gong D, Londos C, Yamaguchi T, Holm C, Rizzo MA, Brasaemle D, Sztalryd C. Activation of hormone-sensitive lipase requires two steps, protein phosphorylation and binding to the PAT-1 domain of lipid droplet coat proteins. J Biol Chem 284: 32116–32125, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wang H, Sreenevasan U, Hu H, Saladino A, Polster BM, Lund LM, Gong DW, Stanley WC, Sztalryd C. Perilipin 5, a lipid droplet-associated protein, provides physical and metabolic linkage to mitochondria. J Lipid Res 52: 2159–2168, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wang SM, Hwang RD, Greenberg AS, Yeo HL. Temporal and spatial assembly of lipid droplet-associated proteins in 3T3–L1 preadipocytes. Histochem Cell Biol 120: 285–292, 2003 [DOI] [PubMed] [Google Scholar]
- 69. Watt MJ, Hoy AJ. Lipid metabolism in skeletal muscle: generation of adaptive and maladaptive intracellular signals for cellular function. Am J Physiol Endocrinol Metab 302: E1315–E1328, 2012 [DOI] [PubMed] [Google Scholar]
- 70. Wolins NE, Quaynor BK, Skinner JR, Schoenfish MJ, Tzekov A, Bickel PE. S3–12, Adipophilin, and TIP47 package lipid in adipocytes. J Biol Chem 280: 19146–19155, 2005 [DOI] [PubMed] [Google Scholar]
- 71. Wolins NE, Quaynor BK, Skinner JR, Tzekov A, Croce MA, Gropler MC, Varma V, Yao-Borengasser A, Rasouli N, Kern PA, Finck BN, Bickel PE. OXPAT/PAT-1 is a PPAR-induced lipid droplet protein that promotes fatty acid utilization. Diabetes 55: 3418–3428, 2006 [DOI] [PubMed] [Google Scholar]
- 72. Yamaguchi T, Omatsu N, Matsushita S, Osumi T. CGI-58 interacts with perilipin and is localized to lipid droplets. Possible involvement of CGI-58 mislocalization in Chanarin-Dorfman syndrome. J Biol Chem 279: 30490–30497, 2004 [DOI] [PubMed] [Google Scholar]
- 73. Yamaguchi T, Omatsu N, Omukae A, Osumi T. Analysis of interaction partners for perilipin and ADRP on lipid droplets. Mol Cell Biochem 284: 167–173, 2006 [DOI] [PubMed] [Google Scholar]
- 74. Zechner R, Kienesberger PC, Haemmerle G, Zimmermann R, Lass A. Adipose triglyceride lipase and the lipolytic catabolism of cellular fat stores. J Lipid Res 50: 3–21, 2009 [DOI] [PubMed] [Google Scholar]