Abstract
Maternal hypoxia inhibits cardiomyocyte proliferation in the heart of fetal and neonatal rats. The present study tested the hypothesis that hypoxia has a direct effect inhibiting cardiomyocyte proliferation via upregulating tissue inhibitors of metalloproteinases (TIMP) in fetal rat hearts. Isolated fetal rat hearts and rat embryonic ventricular myocyte H9c2 cells were treated ex vivo with 20% or 1% O2 for 48 or 24 h, respectively. Hypoxia caused a significant reduction in cardiomyocyte Ki-67 expression and bromodeoxyuridine incorporation in fetal hearts and H9c2 cells. In both fetal hearts and H9c2 cells, hypoxia resulted in a significant decrease in a cell division marker cyclin D2 but an increase in a cell division inhibitor p27. Additionally, hypoxia caused an upregulation of TIMP-3 and TIMP-4 in fetal hearts and H9c2 cells. Knockdown of TIMP-3 in H9c2 cells significantly increased cyclin D2 and Ki-67 and partially blocked the hypoxia-induced inhibition of cyclin D2 and Ki-67 in H9c2 cells. Unlike TIMP-3, TIMP-4 knockdown had no significant effects on the basal levels of cell proliferation but completely abrogated the hypoxia-mediated effects. These findings provide evidence of a novel causal role of TIMP-4 and TIMP-3 in the direct inhibitory effect of hypoxia on cardiomyocyte proliferation in the developing heart.
Keywords: hypoxia, cardiomyocyte, proliferation, TIMP, H9c2
hypoxia is a common stress to fetal development and may occur under many conditions including pregnancy at high altitude, pregnancy with cigarette smoking, drug abuse, anemia, pulmonary disease, and preeclampsia. The adverse effects of fetal hypoxia include intrauterine growth restriction and perinatal morbidity and mortality. The long-term detrimental effects of fetal growth restriction on the development of cardiovascular disease in later adult life have been well established (2, 3, 10, 23). Recent studies in a rat model of maternal hypoxia and fetal growth restriction demonstrated a decrease in cardiomyocyte proliferation and premature transition of mononucleated cells to binucleated cells with increased cell sizes in the fetal heart (1, 38). In rat heart development, the transition of proliferative and hyperplasic growth of mononucleated cells to hypertrophic growth of binucleated cells and terminal differentiation of cardiomyocytes take place within the first 2 wk after birth (5). The hypoxia-mediated premature exit of the cell cycle with disproportional increases in terminally differentiated cardiomyocytes in the heart of growth-restricted fetuses suggests an early morphological indication of cardiomyocyte hypertrophy resulting in fewer but larger cardiomyocytes in the heart of offspring (20, 44). A fetal hypoxia-mediated decrease in cardiomyocyte proliferation was associated with altered expression patterns of matrix metalloproteinases (MMPs) and increased collagen deposition in the heart (38). The functional impact of these changes has been demonstrated in adult offspring in which a sustained reduction of MMP-2 and enhanced collagen accumulation were found in the heart along with left ventricular hypertrophy and stiffening, diastolic dysfunction, and heightened vulnerability to ischemic injury (20, 21, 31, 38, 44–46).
However, it remains unclear whether gestational hypoxia-induced downregulation of cardiomyocyte proliferation in the fetus is a result of hypoxia acting directly on the fetal heart or a result of secondary stress effects induced by maternal hypoxia. Additionally, little is known about the mechanisms linking fetal hypoxia and the inhibition of cardiomyocyte proliferation in the fetal heart. Our recent studies have demonstrated that maternal hypoxia significantly increased the expression levels of tissue inhibitor of metalloproteinase (TIMP)-3 (TIMP-3) and -4 (TIMP-4) in the fetal heart (38). In addition to their roles in modulating MMPs, it has been suggested that both TIMP-3 and TIMP-4 may be involved in inhibiting cardiomyocyte proliferation in rat hearts possibly via a MMP independent and receptor-mediated manner (12, 13, 40). Herein, we present evidence of a novel causal role of TIMP-3 and TIMP-4 in the direct inhibitory effect of hypoxia on cardiomyocyte proliferation in the fetal heart and suggest new insights of molecular mechanisms linking fetal hypoxia and early morphologic indication of cardiomyocyte hypertrophy in the developing heart.
MATERIALS AND METHODS
Experimental animals.
Time-dated pregnant Sprague-Dawley rats were purchased from Charles River Laboratories (Portage, MI). Pregnant rats of gestational day 17 were anesthetized with 75 mg/kg ketamine and 5 mg/kg xylazine injected intramuscularly. The adequacy of anesthesia was determined by the loss of a pedal withdrawal reflex and any other reaction from the animal in response to pinching the toe, tail, or ear of the animal. Additionally, even respiration rate of the animal under anesthesia was closely monitored, and an increased respiration rate was used as a sign that anesthesia was too light. After fetuses were removed, pregnant rats were euthanized by removing the hearts. Day 17 fetal rats were euthanized by decapitation, and hearts were collected for ex vivo studies. For ex vivo treatment, hearts were cultured in M199 (Hyclone, UT) supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin at 37°C in 95% air-5% CO2, as reported previously (31, 32). Hearts were given 24 h of recovery time before being placed in a hypoxic chamber with 1% O2 for 48 h as reported previously (31, 32). All procedures and protocols used in the present study were approved by the Institutional Animal Care and Use Committee of Loma Linda University and followed the guidelines by the NIH Guide for the Care and Use of Laboratory Animals.
Cell culture.
Rat embryonic ventricular myocyte cell line H9c2 was obtained from ATCC (Rockville, MD). Cells were maintained in DMEM and supplemented with 10% FBS and 1% penicillin-streptomycin at 37°C in 95% air-5% CO2. Cells were grown and subcultured in six-well plates with experiments performed between 70% and 80% confluent. For hypoxic studies, cells were treated with 1% or 20% O2, respectively, for 24 h (31, 32).
Immunofluorescence staining.
The expression of Ki-67 and α-sarcomeric actinin (cardiomyocyte marker) was determined in the fetal hearts and H9c2 cells by double immunofluorescence staining visualized with a confocal microscope, as previously described (32, 43). Fetal hearts were fixed and cut into sections (5 μm) transversally at the middle section. The slices were incubated with 0.3% H2O2 for 10 min to block the endogenous peroxidase activity. Antigen retrieval was performed by microwaving sections in a citrate buffer for 10 min before the immunofluorescence staining procedure. H9c2 cells were fixed in acetone for 10 min and treated with 0.3% H2O2 to block the endogenous peroxidase activity. After being blocked with 1% bovine serum albumin for 1 h at room temperature, the samples were incubated with the following primary antibodies: rabbit anti-Ki-67 (Abcam, Cambridge, MA) and mouse anti-α-sarcomeric actinin (Sigma, St. Louis, MO) (1:100) at 4°C overnight. The samples were then incubated with the secondary antibodies: anti-mouse FITC-conjugated and anti-rabbit Texas Red-conjugated antibodies (1:200) at room temperature for 1 h. After three washes, the samples were stained with Hoechst 33258 (5 μg/ml) (Sigma) for 1 min. The immunofluorescence staining was acquired with the Zeiss LSM 710 confocal microscope, and the quantitative analysis of colocalization of Ki-67 and α-sarcomeric actinin-positive cells were carried out with the Image J software in a blinded manner.
Bromodeoxyuridine staining.
The effect of hypoxia on DNA synthesis of fetal hearts was examined with the bromodeoxyuridine (BrdU) staining, as previously described (14, 48). After the hypoxic treatment, fetal hearts were incubated in the M199 media supplemented with the BrdU-labeling reagent (Invitrogen, Camarillo, CA) at 37°C for 6 h. Hearts were then fixed, and transverse sections of 5 μm were prepared from the middle portion of each heart. Immunohistochemical detection of BrdU in fetal hearts was performed using the BrdU staining kit (Invitrogen) according to the instructions of the manufacturer. Nonspecific binding sites were blocked with the blocking solution for 10 min, and the slices were incubated with biotinylated mouse anti-BrdU for 1 h at room temperature. After being rinsed three times in PBS for 5 min each, the samples were exposed to streptavidin-peroxidase and reacted with the diaminobenzidine substrate solution according to the manufacturer's instructions. The immunofluorescence staining of BrdU was viewed with a Zeiss microscope, and the images were acquired with an attached SPOT digital camera imaging system.
Western blot analysis.
Fetal hearts and H9c2 cells were homogenized in a lysis buffer containing 150 mM NaCl, 50 mM Tris·HCl, 10 mM EDTA, 0.1% Tween-20, 1% Triton, 0.1% β-mercaptoethanol, 0.1 mM phenylmethylsulfonyl fluoride, 5 μg/ml leupeptin, and 5 μg/ml aprotinin (pH 7.4) and allowed to incubate for 1 h on ice. Homogenates were then centrifuged at 4°C for 10 min at 10,000 g, and supernatants were collected. Protein concentrations were measured in the supernatants using a protein assay kit (Bio-Rad). Samples with equal amounts of proteins were loaded onto 12% polyacrylamide gel with 0.1% sodium dodecyl sulfate and separated by electrophoresis at 100 V for 1.5 h. Proteins were then transferred onto nitrocellulose membranes. Nonspecific binding sites were blocked for 1 h at room temperature in a Tris-buffered saline solution containing 5% dry milk. The membranes were then probed with primary antibodies against cyclin D2, p27, TIMP-4 (1:1,000 dilution, Abcam), and TIMP-3 (1:1,000 dilution, Millipore, Temecular, CA). To assure equal loading, band intensities were normalized to β2-microglobulin (B2M) (1:10,000, dilution Abcam) or actin (1:5,000 dilution, Sigma). After being washed, membranes were incubated with secondary horseradish peroxidase-conjugated antibodies. Proteins were visualized with enhanced chemiluminescence reagents, and blots were exposed to Hyperfilm. The results were analyzed with the Kodak ID image analysis software.
siRNA transfection.
Small interfering RNA (siRNA) transfection of H9c2 cells was performed as previously described (43). The Silencer-Select Pre-designed siRNAs against rat TIMP-3 and TIMP-4 genes were obtained from Invitrogen (Camarillo, CA). Nontargeting siRNAs (NC) were used as a negative control for the gene-specific siRNAs. The siRNAs were dissolved in nuclease-free water and transfected into H9c2 cells with the siPORT NeoFX agent (Invitrogen), following the manufacturer's instructions. Briefly, H9c2 cells were trypsinized and diluted in normal growth medium and set aside at 37°C. siPORT NeoFX agent was diluted in OPTI-MEM I medium (Invitrogen) and incubated for 10 min at room temperature. siRNAs were diluted in OPTI-MEM I medium and then mixed with the diluted siPORT NeoF Magent. The mixture was incubated for 10 min at room temperature, dispensed with H9c2 cells into six-well plates, and incubated for 24 h before the hypoxia treatment was started.
Statistical analysis.
Data are expressed as means ± SE. Statistical significance (P < 0.05) was determined by analysis of variance (ANOVA) followed by Newman-Keuls post hoc testing or Student's t-test, where appropriate.
RESULTS
Hypoxia directly inhibits cardiomyocyte proliferation.
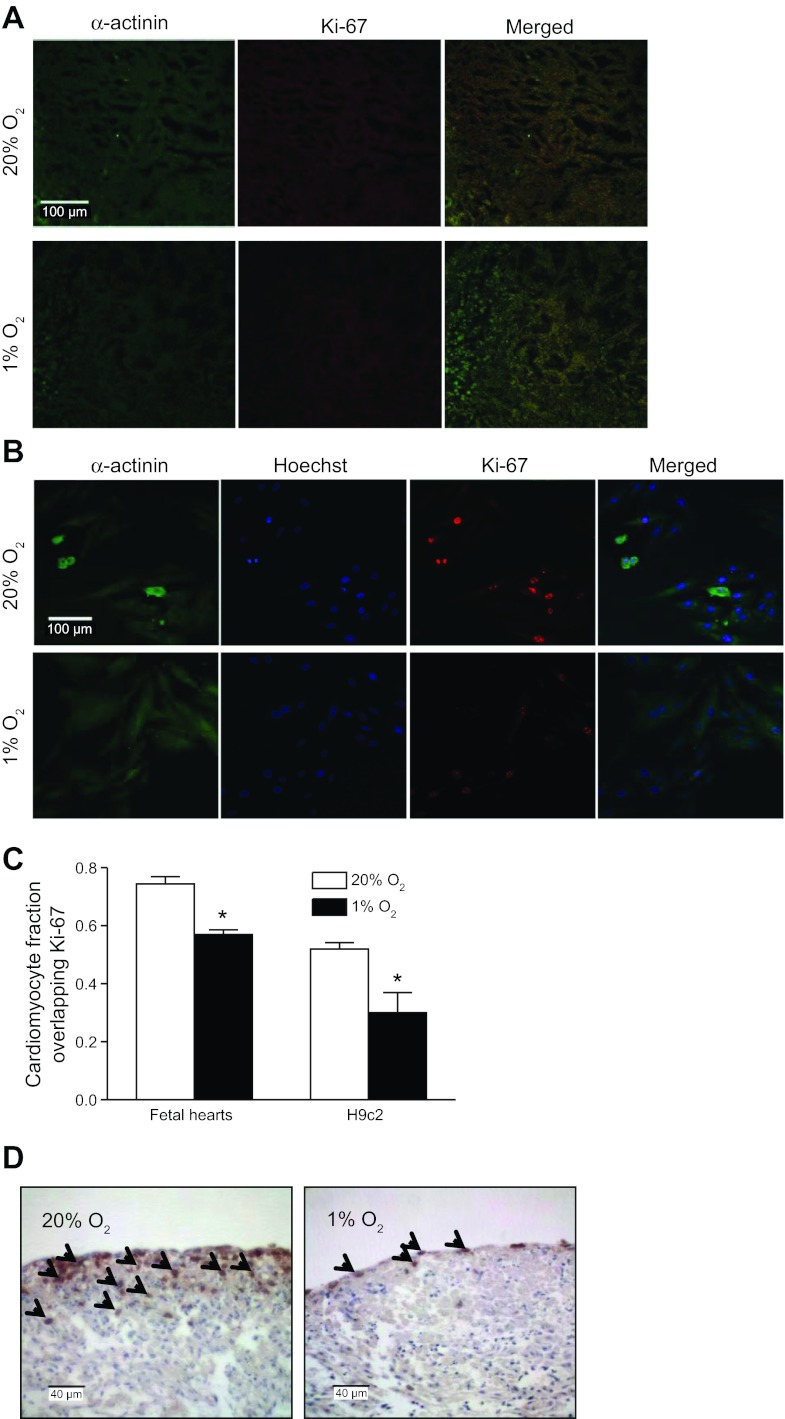
The direct effect of hypoxia on cardiomyocyte proliferation in fetal hearts was determined by examining the double immunofluorescence staining of the nuclear protein Ki-67 and cardiomyocyte marker α-sarcomeric actinin. The Ki-67 expression occurs throughout all phases of the cell cycle, except for the G0 phase (8). As shown in Fig. 1A, double immunofluorescence staining of the fetal heart sections revealed a predominant distribution of Ki-67 in cardiomyocytes. Quantitative analysis of colocalization for Ki-67 and α-sarcomeric actinin demonstrated that hypoxia significantly decreased the percentage of cardiomyocytes that expressed Ki-67 compared with the control condition (Fig. 1C), suggesting the reduced proliferative activity of myocytes in fetal hearts under the hypoxic condition. Whether the cells were arrested at G0 phase permanently or reenter the cell cycle after withdraw of hypoxia remain to be determined. In addition, the direct effect of hypoxia on the DNA synthesis in fetal hearts was determined by BrdU incorporation. As shown in Fig. 1D, the immunostaining of BrdU demonstrated the dark brown dots within the cells that are located at the outer layers of fetal hearts.After the hypoxic treatment, decreased BrdU-positive cells in the fetal heart were observed (Fig. 1D). Given that BrdU is a nonspecific marker for DNA synthesis, in addition to cardiomyocytes, possible BrdU staining of cardiac fibroblasts may not be excluded. The finding of BrdU-positive cells largely located in pericardial area was likely due to the perfusion limitation of BrdU since it was given by addition to the media in intact fetal hearts. Consistent with the findings in fetal hearts, double immunofluorescence staining in H9c2 cells clearly demonstrated that Ki-67 expression was mainly localized within cardiomyocyte nuclei (Fig. 1B). The ratio of Ki-67-positive nuclei to the total number of nuclei in H9c2 cells was significantly reduced by the hypoxic treatment (Fig. 1C), demonstrating the similar response of H9c2 cells as that found in fetal hearts.
Fig. 1.
Hypoxia decreases cardiomyocyte proliferation in fetal hearts and H9c2 cells. Myocyte proliferation was assessed by Ki-67 immunofluorescence staining in fetal rat hearts (A) and H9c2 cells (B) treated with 20% O2 or 1% O2 for 48 and 24 h, respectively. Cardiomyocytes are stained for α-sarcomeric actinin (green) and Ki-67 (red). Nuclei were visualized using Hoechst 33258 (blue). Quantitative data are shown in C. Data are means ± SE. *P < 0.05, 1% O2 vs. 20% O2. n = 6 per group. D shows bromodeoxyuridine (BrdU) incorporation in fetal hearts. Arrows show BrdU-positive nuclei. The images were representatives of 4 control and 5 hypoxic heart samples.
Hypoxia increases p27 and decreases cyclin D2 expression.
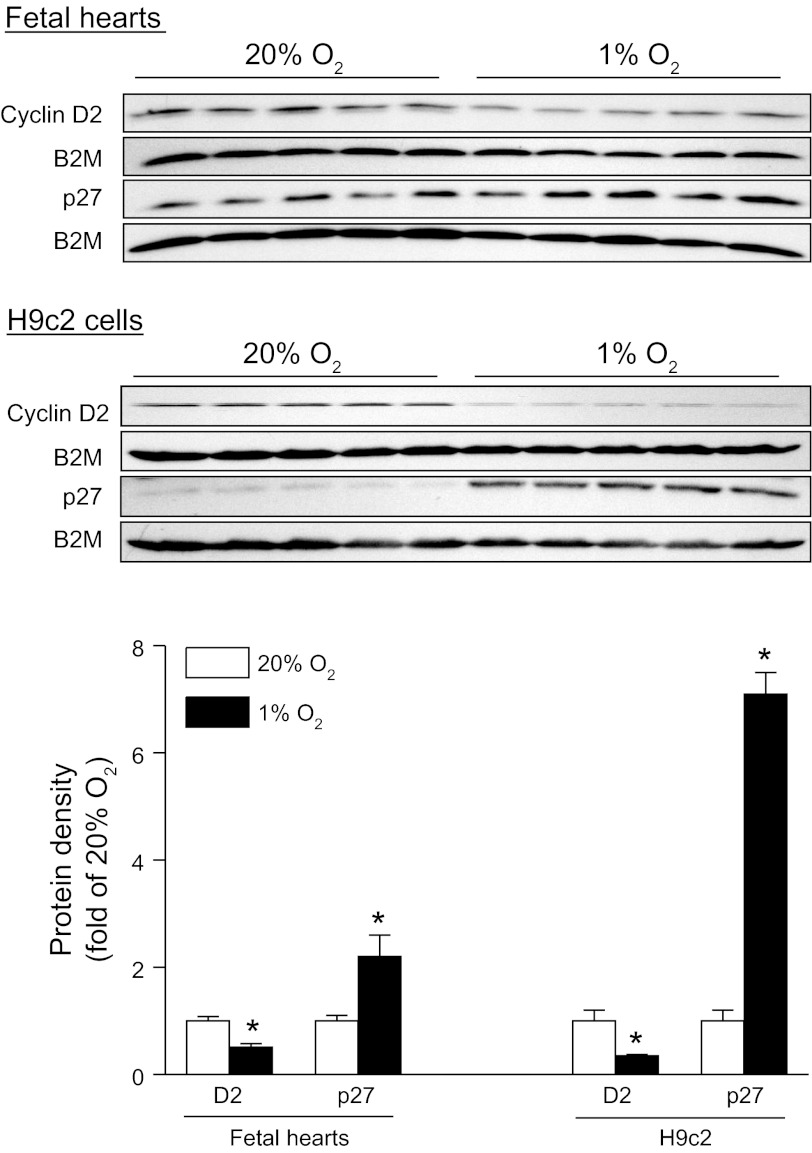
Cyclin D2 is a protein known to be necessary for cardiomyocyte proliferation and serves as a proliferative marker (28). On the other hand, p27 is a cell cycle inhibitor and induces suppression of cell proliferation (28). Consistent with the finding of decreased Ki-67 expression and BrdU incorporation in fetal hearts, Western blot analysis demonstrated that hypoxia significantly decreased the expression of cyclin D2 in the fetal heart (Fig. 2). In contrast, p27 was significantly increased in hypoxic fetal hearts (Fig. 2). Similar findings were obtained in H9c2 cells (Fig. 2).
Fig. 2.
Hypoxia increases p27 and decreases cyclin D2 (D2) in fetal hearts and H9c2 cells. Protein abundance of D2 and p27 was determined by Western blots in fetal rat hearts and H9c2 cells, treated with 20% O2 or 1% O2 for 48 and 24 h, respectively. β2-Microglobulin (B2M) was used as a loading control. Data are means ± SE. *P < 0.05, 1% O2 vs. 20% O2. n = 5 per group.
Hypoxia upregulates TIMP-3 and TIMP-4 expression.
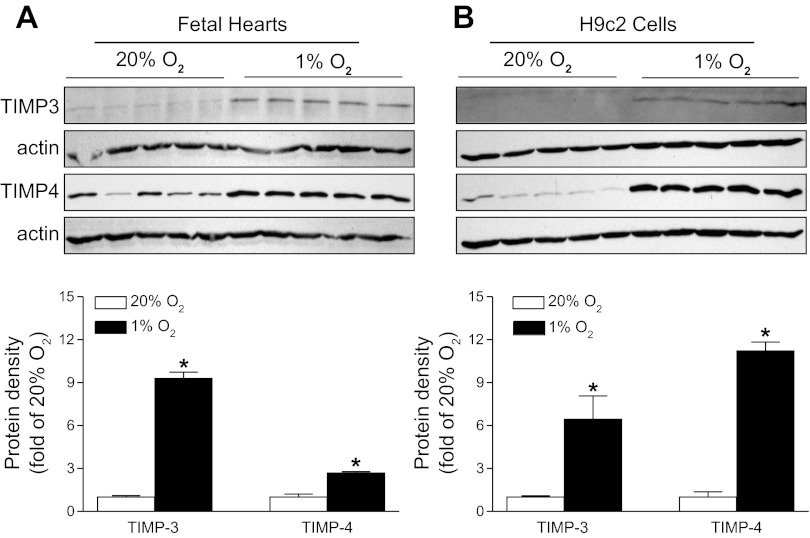
To determine whether potential mediators TIMP-3 and TIMP-4 were involved in the hypoxia-mediated downregulation of cardiomyocyte proliferation, the expression of TIMP-3 and TIMP-4 was measured by Western blot analysis. Consistent with the previous in vivo findings in fetal rat hearts of maternal hypoxia (38), ex vivo hypoxic treatment caused a significant increase in both TIMP-3 and TIMP-4 protein abundance, demonstrating a direct effect of hypoxia on the upregulation of TIMP-3 and TIMP-4 expression in fetal hearts (Fig. 3A). Similar findings were obtained in H9c2 cells (Fig. 3B).
Fig. 3.
Hypoxia upregulates tissue inhibitors of metalloproteinases-3 (TIMP-3) and -4 (TIMP-4) in fetal hearts and H9c2 cells. Protein abundance of TIMP-3 and TIMP-4 was determined by Western blots in fetal rat hearts (A) and H9c2 cells (B) treated with 20% O2 or 1% O2 for 48 and 24 h, respectively. Actin was used as a loading control. Data are means ± SE. *P < 0.05, 1% O2 vs. 20% O2. n = 5 per group.
Knockdown of TIMPs blocks the hypoxia-induced effect.
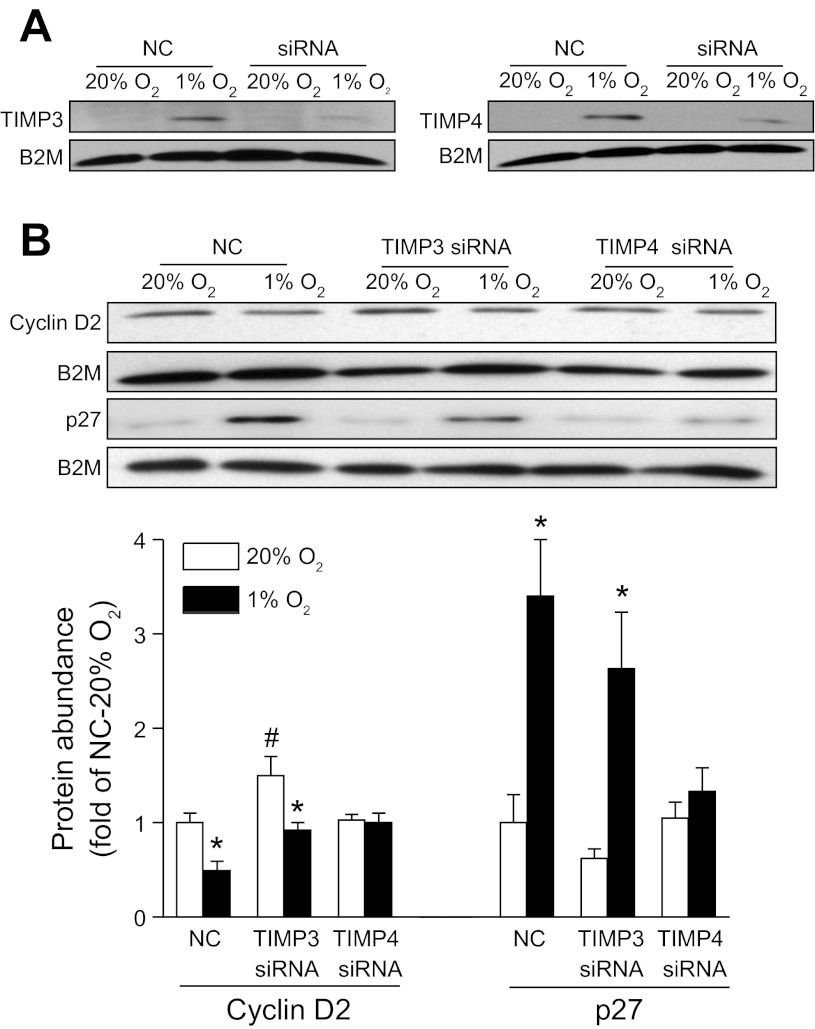
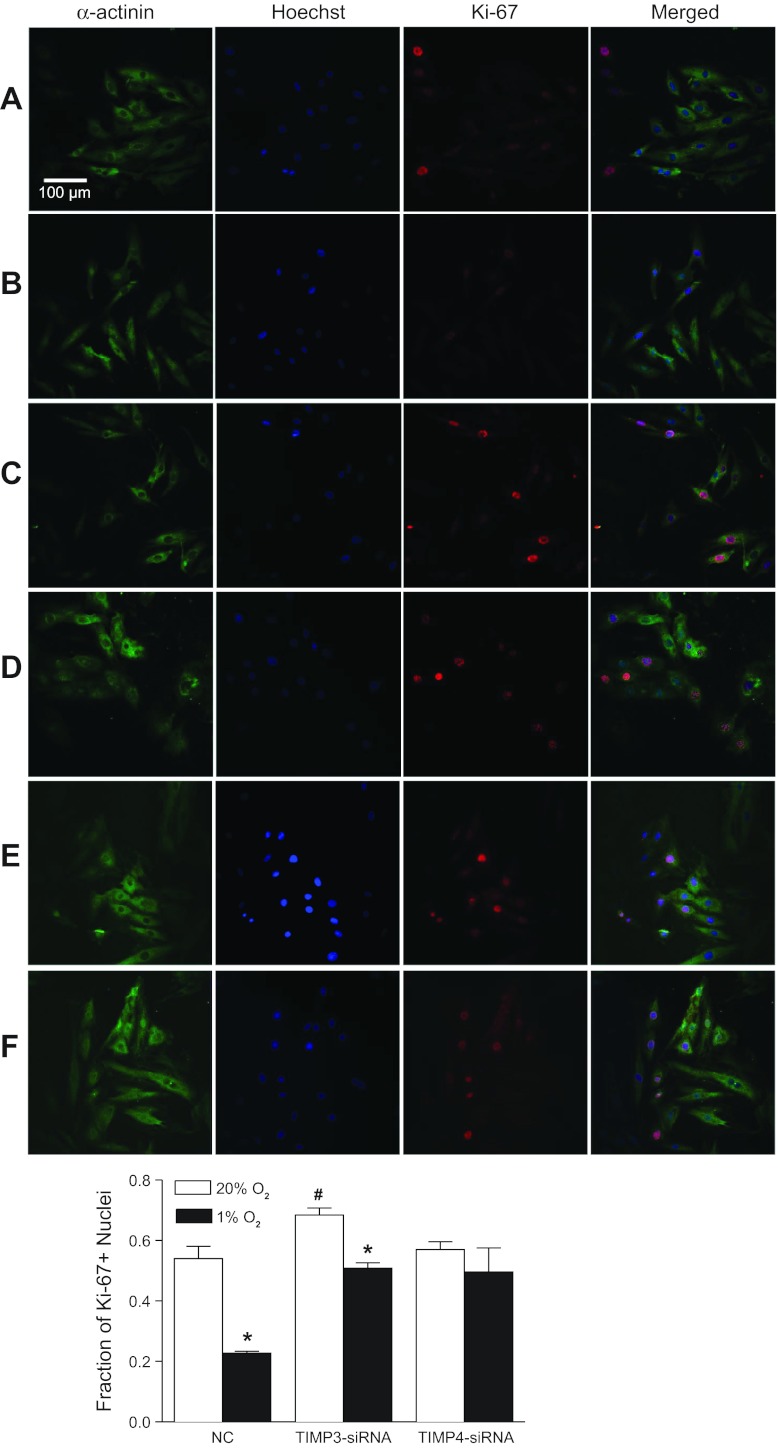
To determine specifically the causal role of TIMP-3 or TIMP-4 in the hypoxia-mediated effect, specific siRNAs were applied to H9c2 cells to silence TIMP-3 and TIMP-4 expression, respectively. As shown in Fig. 4A, both TIMP-3 and TIMP-4 siRNAs efficiently knocked down the hypoxia-induced expression of TIMP-3 and TIMP-4, respectively, in H9c2 cells. We determined further the cause-and-effect relationship of TIMPs knockdown and the hypoxia-induced inhibition of cardiomyocyte proliferation. Knockdown of TIMP-3 significantly increased cyclin D2 expression levels (Fig. 4B) and the Ki-67-positive nuclei (Fig. 5) in H9c2 cells under the control condition and partially blocked the hypoxia-mediated inhibition of cyclin D2 expression (Fig. 4B) and Ki-67-positive nuclei (Fig. 5). Unlike the findings of TIMP-3 knockdown, TIMP-4 knockdown had no significant effect on the expression levels of cyclin D2 and Ki-67-positive nuclei in control H9c2 cells but completely abrogated the hypoxia-induced inhibition of cyclin D2 expression (Fig. 4B) and Ki-67-positive nuclei in H9c2 cells (Fig. 5).
Fig. 4.
Knockdown of TIMP-3 or TIMP-4 inhibits hypoxic effect on cyclin D2 and p27 expression in H9c2 cells. H9c2 cells were transfected with small interfering RNA (siRNA) against TIMP-3 or TIMP-4, respectively, for 24 h followed by the treatment with 20% O2 or 1% O2 for 24 h. Protein abundance of TIMP-3/TIMP-4 (A) and cyclin D2 or p27 (B) was determined by Western blots. B2M was used as a loading control. Data are means ± SE. *P < 0.05, 1% O2 vs. 20% O2; #P < 0.05, siRNA vs. negative control of siRNA (NC). n = 5 per group.
Fig. 5.
Knockdown of TIMP-3 or TIMP-4 blocks hypoxic effect on cardiomyocyte proliferation in H9c2 cells. H9c2 cells were transfected with siRNA against TIMP-3 or TIMP-4, respectively, for 24 h followed by the treatment with 20% O2 or 1% O2 for 24 h. Cardiomyocytes are stained for α-sarcomeric actinin (green) and Ki-67 (red). Nuclei were visualized using Hoechst 33258 (blue). A: negative control of siRNA (NC) at 20% O2; B: NC at 1% O2; C: TIMP-3 siRNA at 20% O2; D: TIMP-3 siRNA at 1% O2; E: TIMP-4 siRNA at 20% O2; F: TIMP-4 siRNA at 1% O2. Data are means ± SE. *P < 0.05, 1% O2 vs. 20% O2; #P < 0.05, TIMP-3 siRNA vs. negative control of siRNA (NC). n = 6 per group.
DISCUSSION
In the present study, we demonstrate for the first time that hypoxia directly inhibits cardiomyocyte proliferation in the fetal heart, which is associated with the increased expression of TIMP-3 and TIMP-4. Additionally, we demonstrate that knockdown of TIMP-4 abrogates the hypoxia-induced inhibition of myocyte proliferation, providing the cause-and-effect evidence of a pivotal role of TIMP-4 in the direct inhibitory effect of hypoxia on cardiomyocyte proliferation in the developing heart.
It has been well documented that prenatal growth of heart depends predominantly on hyperplasia of cardiomyocytes, and the transition of proliferative mononucleated cells to binucleated cells followed by hypertrophic growth of myocytes takes place in late gestation and within the first 2 wk after birth (5, 16). Fetal heart growth patterns may be altered in a number of ways, such as nutrients (including oxygen), hormones, and the hemodynamic factors (9, 35, 37). If the maturation is completed before the appropriate number of myocytes is achieved, the heart development could be compromised at birth with fewer myocytes. It has been shown that thyroid hormone (4), atrial natriuretic peptide (30), experimental reduction in systolic pressure (29), and fetal hypoxia induced by placental restriction (22, 27) reduce the number of cardiomyocytes in the fetal sheep heart. Similar findings were obtained in rats, showing that gestational hypoxia decreased cardiomyocyte proliferation and increased the percentage and size of binucleated myocytes in fetal hearts (1, 38), indicating an early morphology of cardiomyocyte hypertrophy (6).
The present finding that ex vivo hypoxic treatment decreased cardiomyocyte proliferation in isolated intact fetal hearts and H9c2 cells is consistent with the effect of maternal hypoxia on the fetal heart in vivo (38) and demonstrates a primarily direct effect of hypoxia in inhibiting myocyte proliferation in the fetal heart. The intact fetal rat heart model has been well defined and used in previous studies (19, 25, 31, 32, 42, 43, 45). In the present study, the fetal hearts maintained spontaneous contraction and beating throughout the study period. Along with the fetal hearts, embryonic rat ventricular myocyte H9c2 cells have been widely used in the study of cardiomyocyte proliferation and differentiation (11, 17, 26, 33, 36). In the present study, the control of 20% O2 or approximate Po2 of 140 mmHg appeared supra physiological, as fetal blood Po2 is about 25 mmHg. However, it is often difficult to directly compare Po2 values in determining O2 content and the effect of hypoxia between in vivo and in vitro conditions given the absence of hemoglobin in in vitro culture medium, which determines more than 90% of O2 content in the blood. Our previous study in H9c2 cells demonstrated that exposure to 20%, 10.5%, or 3% O2 had no significantly different effects on PKCε expression, but 1% O2 significantly decreased PKCε expression, suggesting a mode of adaptation to oxygen insufficiency and a critical level of hypoxia in fetal cardiomyocytes (31). Consistent with the in vivo study of maternal hypoxia showing increased HIF-1α protein abundance in the fetal heart (1), a marker of tissue hypoxia, the previous study demonstrated that ex vivo treatment of fetal hearts and H9c2 cells with 1% O2 resulted in significant nuclear accumulation of HIF-1α (32). This is in agreement with many previous studies in which hypoxic effects were investigated in fetal tissues or cells under 1% O2 (15, 26, 34, 36, 39). Whereas it is somewhat difficult to make a direct comparison of oxygen consumption by fetal hearts in ex vivo and in vivo conditions, the condition of 1% O2 may cause more severe hypoxia in ex vivo fetal hearts than in vivo hearts. The finding that hypoxia-induced decrease in cardiomyocyte proliferation in fetal hearts in vivo and ex vivo mirrored that found in H9c2 cells suggests a congruent underlying mechanism for each model and provides a comparable model of H9c2 cells in investigating the hypoxic effect.
Consistent with the finding that maternal hypoxia caused an upregulation of TIMP-3 and TIMP-4 in fetal hearts (38), ex vivo hypoxic treatment significantly increased the expression of TIMP-3 and TIMP-4 in isolated fetal hearts and H9c2 cells. No classic HIF-responsive elements were found on either TIMP-3 or TIMP-4 promoters through a computational search of predicted transcription factor binding sites. The possibility that hypoxia-induced upregulation of TIMP-3 and TIMP-4 is mediated by other genes that have HIF-responsive elements remains an intriguing area for further investigation. It is well documented that TIMPs are linked to MMP-independent mechanisms, such as cell proliferation and apoptosis, in addition to their inhibitory effect on specific MMPs (40). Studies in a TIMP-3-deficient mouse model demonstrated an increase in cardiomyocyte proliferation in neonatal hearts (7). Further studies showed that TIMP-3 inhibited neonatal mouse cardiomyocyte proliferation via epidermal growth factor/c-Jun NH2-terminal kinase/sp1 pathway (12). On the other hand, TIMP-4 appears to play dual roles in regulating cell proliferation, and its effect is tissue specific (24). In the present study, the causal effect of TIMP-3 and TIMP-4 in hypoxia-induced inhibition of cardiomyocyte proliferation is further demonstrated by siRNA knockdown experiments in H9c2 cells. Thus we demonstrated that knockdown of TIMP-3 in H9c2 cells significantly increased the expression of cyclin D2 and number of Ki-67-positive nuclei under the normoxic condition, indicating that TIMP-3 plays a pivotal role in controlling the basal levels of myocyte proliferation. Additionally, the previous study demonstrated that cortical cells derived from TIMP-3 knockout mice showed partial resistance against oxygen/glucose deprivation-induced neuronal cell death, implying a proapoptotic role of TIMP-3 in the hypoxic-ischemic condition in vitro (41). In agreement with this finding, the present study provided the evidence that knockdown of TIMP-3 partially blocked the hypoxia-mediated inhibition of proliferation in H9c2 cells. Unlike TIMP-3, TIMP-4 knockdown had no significant effect on baseline myocyte proliferation but completely abrogated the hypoxia-induced inhibition of proliferation in H9c2 cells. Although these results clearly demonstrate the causal effect of TIMP-3 and TIMP-4 in hypoxia-mediated inhibition of cardiomyocyte proliferation, the differential effects of TIMP-3 and TIMP-4 in regulating baseline myocyte proliferation are evident and intriguing. It is possible that the basal expression levels and/or activities of TIMP-3 and TIMP-4 in myocytes are quite different with TIMP-3 having relatively high abundance and activity. The high abundance and activity of TIMP-3 may be important in controlling the basal levels of myocyte proliferation, and further upregulation of TIMP-3 by hypoxia may not produce much greater inhibitory effect on cardiomyocyte proliferation. Unlike TIMP-3, the promoter region of TIMP-4 gene does not contain AP1 (activator protein) binding site that is related to the basal expression of TIMP-3 (47). Thus the basal level of TIMP-4 may be relatively low, and its inhibitory effect on myocyte proliferation is significantly induced by hypoxia. These results collectively suggest that TIMPs comprehensively modulate cardiac tissue remodeling in response to fetal hypoxia. Interestingly, previous studies have demonstrated that genetic manipulation to deplete TIMPs had significant effects on cardiac phenotype. TIMP-3 knockout animals have been shown dilated cardiomyopathy and compromised cardiac contractile performance (7). TIMP-4 deletion mice demonstrate heightened susceptibility to MI but not to pressure overload, and TIMP-4 functions as a metalloproteinase inhibitor after myocardial infarction (18).
Perspectives and Significance
The present study provides the insight into direct inhibitory effect of hypoxia on cardiomyocyte proliferation in the developing heart and demonstrates the causal effect of TIMP-3 and TIMP-4 in the hypoxia-mediated effect. Although it may be difficult to translate directly these findings into humans, finding the key players in hypoxia-induced cardiac remodeling in the developing heart has significance in clinical implications. This is because gestational hypoxia is one of the most important and clinically relevant stresses to the fetus and because large epidemiological studies indicate a link between in utero adverse stimuli during gestation and an increased risk of ischemic heart disease in the adulthood. Recent animal studies have showed that prenatal hypoxia results in collagen accumulation in the heart along with left ventricular hypertrophy and stiffening, diastolic dysfunction, and heightened vulnerability to ischemic injury in offspring (20, 21, 31, 38, 44–46). Further investigation into TIMP-mediated inhibition of cardiomyocyte proliferation in the developing heart is needed to reveal more insights into mechanisms at the molecular level.
GRANTS
A portion of this research used the Loma Linda University School of Medicine Advanced Imaging and Microscopy Core, a facility is supported in part by the National Science Foundation through the Major Research Instrumentation program of the Division of Biological Infrastructure Grant No. 0923559 and the Loma Linda University School of Medicine.
This work was supported in part by the National Institutes of Health (HL-082779, HL-083966, HL-089012, and HL-110125 to L. Zhang).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: W.T. and L.Z. conception and design of research; W.T., F.X., and Y.L. performed experiments; W.T., F.X., Y.L., and L.Z. analyzed data; W.T., F.X., Y.L., and L.Z. interpreted results of experiments; W.T. and L.Z. prepared figures; W.T. drafted manuscript; L.Z. edited and revised manuscript; L.Z. approved final version of manuscript.
REFERENCES
- 1. Bae S, Xiao Y, Li G, Casiano CA, Zhang L. Effect of maternal chronic hypoxic exposure during gestation on apoptosis in fetal rat heart. Am J Physiol Heart Circ Physiol 285: H983–H990, 2003 [DOI] [PubMed] [Google Scholar]
- 2. Barker DJ, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. Lancet 1: 1077–1081, 1986 [DOI] [PubMed] [Google Scholar]
- 3. Bateson P, Barker D, Clutton-Brock T, Deb D, D'Udine B, Foley RA, Gluckman P, Godfrey K, Kirkwood T, Lahr MM, McNamara J, Metcalfe NB, Monaghan P, Spencer HG, Sultan SE. Developmental plasticity and human health. Nature 430: 419–421, 2004 [DOI] [PubMed] [Google Scholar]
- 4. Chattergoon NN, Giraud GD, Louey S, Stork P, Fowden AL, Thornburg KL. Thyroid hormone drives fetal cardiomyocyte maturation. FASEB J 26: 397–408, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Clubb FJ, Jr, Bishop SP. Formation of binucleated myocardial cells in the neonatal rat An index for growth hypertrophy. Lab Invest 50: 571–577, 1984 [PubMed] [Google Scholar]
- 6. Corstius HB, Zimanyi MA, Maka N, Herath T, Thomas W, van der Laarse A, Wreford NG, Black MJ. Effect of intrauterine growth restriction on the number of cardiomyocytes in rat hearts. Pediatr Res 57: 796–800, 2005 [DOI] [PubMed] [Google Scholar]
- 7. Fedak PW, Smookler DS, Kassiri Z, Ohno N, Leco KJ, Verma S, Mickle DA, Watson KL, Hojilla CV, Cruz W, Weisel RD, Li RK, Khokha R. TIMP-3 deficiency leads to dilated cardiomyopathy. Circulation 110: 2401–2409, 2004 [DOI] [PubMed] [Google Scholar]
- 8. Gerdes J, Lemke H, Baisch H, Wacker HH, Schwab U, Stein H. Cell cycle analysis of a cell proliferation-associated human nuclear antigen defined by the monoclonal antibody Ki-67. J Immunol 133: 1710–1715, 1984 [PubMed] [Google Scholar]
- 9. Giraud GD, Louey S, Jonker S, Schultz J, Thornburg KL. Cortisol stimulates cell cycle activity in the cardiomyocyte of the sheep fetus. Endocrinology 147: 3643–3649, 2006 [DOI] [PubMed] [Google Scholar]
- 10. Gluckman PD, Hanson MA, Cooper C, Thornburg KL. Effect of in utero and early-life conditions on adult health and disease. N Engl J Med 359: 61–73, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hamid T, Gu Y, Ortines RV, Bhattacharya C, Wang G, Xuan YT, Prabhu SD. Divergent tumor necrosis factor receptor-related remodeling responses in heart failure: role of nuclear factor-kappaB and inflammatory activation. Circulation 119: 1386–1397, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hammoud L, Burger DE, Lu X, Feng Q. Tissue inhibitor of metalloproteinase-3 inhibits neonatal mouse cardiomyocyte proliferation via EGFR/JNK/SP-1 signaling. Am J Physiol Cell Physiol 296: C735–C745, 2009 [DOI] [PubMed] [Google Scholar]
- 13. Hammoud L, Xiang F, Lu X, Brunner F, Leco K, Feng Q. Endothelial nitric oxide synthase promotes neonatal cardiomyocyte proliferation by inhibiting tissue inhibitor of metalloproteinase-3 expression. Cardiovasc Res 75: 359–368, 2007 [DOI] [PubMed] [Google Scholar]
- 14. Horie N, So K, Moriya T, Kitagawa N, Tsutsumi K, Nagata I, Shinohara K. Effects of oxygen concentration on the proliferation and differentiation of mouse neural stem cells in vitro. Cell Mol Neurobiol 28: 833–845, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Hwang JM, Weng YJ, Lin JA, Bau DT, Ko FY, Tsai FJ, Tsai CH, Wu CH, Lin PC, Huang CY, Kuo WW. Hypoxia-induced compensatory effect as related to Shh and HIF-1alpha in ischemia embryo rat heart. Mol Cell Biochem 311: 179–187, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Jonker SS, Zhang L, Louey S, Giraud GD, Thornburg KL, Faber JJ. Myocyte enlargement, differentiation, and proliferation kinetics in the fetal sheep heart. J Appl Physiol 102: 1130–1142, 2007 [DOI] [PubMed] [Google Scholar]
- 17. Kaneda R, Ueno S, Yamashita Y, Choi YL, Koinuma K, Takada S, Wada T, Shimada K, Mano H. Genome-wide screening for target regions of histone deacetylases in cardiomyocytes. Circ Res 97: 210–218, 2005 [DOI] [PubMed] [Google Scholar]
- 18. Koskivirta I, Kassiri Z, Rahkonen O, Kiviranta R, Oudit GY, McKee TD, Kyto V, Saraste A, Jokinen E, Liu PP, Vuorio E, Khokha R. Mice with tissue inhibitor of metalloproteinases 4 (Timp4) deletion succumb to induced myocardial infarction but not to cardiac pressure overload. J Biol Chem 285: 24487–24493, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lawrence J, Chen M, Xiong F, Xiao D, Zhang H, Buchholz JN, Zhang L. Foetal nicotine exposure causes PKCepsilon gene repression by promoter methylation in rat hearts. Cardiovasc Res 89: 89–97, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li G, Bae S, Zhang L. Effect of prenatal hypoxia on heat stress-mediated cardioprotection in adult rat heart. Am J Physiol Heart Circ Physiol 286: H1712–H1719, 2004 [DOI] [PubMed] [Google Scholar]
- 21. Li G, Xiao Y, Estrella JL, Ducsay CA, Gilbert RD, Zhang L. Effect of fetal hypoxia on heart susceptibility to ischemia and reperfusion injury in the adult rat. J Soc Gynecol Investig 10: 265–274, 2003 [DOI] [PubMed] [Google Scholar]
- 22. Louey S, Jonker SS, Giraud GD, Thornburg KL. Placental insufficiency decreases cell cycle activity and terminal maturation in fetal sheep cardiomyocytes. J Physiol 580: 639–648, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev 85: 571–633, 2005 [DOI] [PubMed] [Google Scholar]
- 24. Melendez-Zajgla J, Del Pozo L, Ceballos G, Maldonado V. Tissue inhibitor of metalloproteinases-4. The road less traveled. Mol Cancer 7: 85, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Meyer K, Zhang H, Zhang L. Direct effect of cocaine on epigenetic regulation of PKCepsilon gene repression in the fetal rat heart. J Mol Cell Cardiol 47: 504–511, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mizukami Y, Ono K, Du CK, Aki T, Hatano N, Okamoto Y, Ikeda Y, Ito H, Hamano K, Morimoto S. Identification and physiological activity of survival factor released from cardiomyocytes during ischaemia and reperfusion. Cardiovasc Res 79: 589–599, 2008 [DOI] [PubMed] [Google Scholar]
- 27. Morrison JL, Botting KJ, Dyer JL, Williams SJ, Thornburg KL, McMillen IC. Restriction of placental function alters heart development in the sheep fetus. Am J Physiol Regul Integr Comp Physiol 293: R306–R313, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Novoyatleva T, Diehl F, van Amerongen MJ, Patra C, Ferrazzi F, Bellazzi R, Engel FB. TWEAK is a positive regulator of cardiomyocyte proliferation. Cardiovasc Res 85: 681–690, 2010 [DOI] [PubMed] [Google Scholar]
- 29. O'Tierney PF, Anderson DF, Faber JJ, Louey S, Thornburg KL, Giraud GD. Reduced systolic pressure load decreases cell-cycle activity in the fetal sheep heart. Am J Physiol Regul Integr Comp Physiol 299: R573–R578, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. O'Tierney PF, Chattergoon NN, Louey S, Giraud GD, Thornburg KL. Atrial natriuretic peptide inhibits angiotensin II-stimulated proliferation in fetal cardiomyocytes. J Physiol 588: 2879–2889, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Patterson AJ, Chen M, Xue Q, Xiao D, Zhang L. Chronic prenatal hypoxia induces epigenetic programming of PKCε gene repression in rat hearts. Circ Res 107: 365–373, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Patterson AJ, Xiao D, Xiong F, Dixon B, Zhang L. Hypoxia-derived oxidative stress mediates epigenetic repression of PKCepsilon gene in foetal rat hearts. Cardiovasc Res 93: 302–310, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Pchejetski D, Kunduzova O, Dayon A, Calise D, Seguelas MH, Leducq N, Seif I, Parini A, Cuvillier O. Oxidative stress-dependent sphingosine kinase-1 inhibition mediates monoamine oxidase A-associated cardiac cell apoptosis. Circ Res 100: 41–49, 2007 [DOI] [PubMed] [Google Scholar]
- 34. Shin EJ, Schram K, Zheng XL, Sweeney G. Leptin attenuates hypoxia/reoxygenation-induced activation of the intrinsic pathway of apoptosis in rat H9c2 cells. J Cell Physiol 221: 490–497, 2009 [DOI] [PubMed] [Google Scholar]
- 35. Sundgren NC, Giraud GD, Stork PJ, Maylie JG, Thornburg KL. Angiotensin II stimulates hyperplasia but not hypertrophy in immature ovine cardiomyocytes. J Physiol 548: 881–891, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tang Y, Jackson M, Qian K, Phillips MI. Hypoxia inducible double plasmid system for myocardial ischemia gene therapy. Hypertension 39: 695–698, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Thornburg KL, Louey S, Giraud GD. The role of growth in heart development. Nestle Nutr Workshop Ser Pediatr Program 61: 39–51, 2008 [DOI] [PubMed] [Google Scholar]
- 38. Tong W, Xue Q, Li Y, Zhang L. Maternal hypoxia alters matrix metalloproteinase expression patterns and causes cardiac remodeling in fetal and neonatal rats. Am J Physiol Heart Circ Physiol 301: H2113–H2121, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Uetani T, Nakayama H, Okayama H, Okura T, Higaki J, Inoue H, Higashiyama S. Insufficiency of pro-heparin-binding epidermal growth factor-like growth factor shedding enhances hypoxic cell death in H9c2 cardiomyoblasts via the activation of caspase-3 and c-Jun N-terminal kinase. J Biol Chem 284: 12399–12409, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Vanhoutte D, Heymans S. TIMPs and cardiac remodeling: “Embracing the MMP-independent-side of the family.” J Mol Cell Cardiol 48: 445–453, 2010 [DOI] [PubMed] [Google Scholar]
- 41. Wetzel M, Li L, Harms KM, Roitbak T, Ventura PB, Rosenberg GA, Khokha R, Cunningham LA. Tissue inhibitor of metalloproteinases-3 facilitates Fas-mediated neuronal cell death following mild ischemia. Cell Death Differ 15: 143–151, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Wildenthal K. Long-term maintenance of spontaneously beating mouse hearts in organ culture. J Appl Physiol 30: 153–157, 1971 [DOI] [PubMed] [Google Scholar]
- 43. Xiong F, Xiao D, Zhang L. Norepinephrine causes epigenetic repression of PKCepsilon gene in rodent hearts by activating Nox1-dependent reactive oxygen species production. FASEB J 26: 2753–2763, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Y, Williams SJ, O'Brien D, Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J 20: 1251–1253, 2006 [DOI] [PubMed] [Google Scholar]
- 45. Xue Q, Dasgupta C, Chen M, Zhang L. Foetal hypoxia increases cardiac AT(2)R expression and subsequent vulnerability to adult ischaemic injury. Cardiovasc Res 89: 300–308, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Xue Q, Zhang L. Prenatal hypoxia causes a sex-dependent increase in heart susceptibility to ischemia and reperfusion injury in adult male offspring: role of protein kinase C epsilon. J Pharmacol Exp Ther 330: 624–632, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Young DA, Phillips BW, Lundy C, Nuttall RK, Hogan A, Schultz GA, Leco KJ, Clark IM, Edwards DR. Identification of an initiator-like element essential for the expression of the tissue inhibitor of metalloproteinases-4 (Timp-4) gene. Biochem J 364: 89–99, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Zhao L, Jiao Q, Chen X, Yang P, Zhao B, Zheng P, Liu Y. mGluR5 is involved in proliferation of rat neural progenitor cells exposed to hypoxia with activation of mitogen-activated protein kinase signaling pathway. J Neurosci Res 90: 447–460, 2012 [DOI] [PubMed] [Google Scholar]